Abstract
For epidemiological studies of Campylobacter infections, molecular typing methods that can differentiate campylobacters at the strain level are needed. In this study we used a recently developed genotyping method, amplified fragment length polymorphism (AFLP), which is based on selective amplification of restriction fragments of chromosomal DNA, for genetic typing of Campylobacter jejuni and Campylobacter coli strains derived from humans and poultry. We developed an automated AFLP fingerprinting method in which restriction endonucleases HindIII and HhaI were used in combination with one set of selective PCR primers. This method resulted in evenly distributed band patterns for amplified fragments ranging from 50 to 500 bp long. The discriminatory power of AFLP was assessed with a C. jejuni strain, an isogenic flagellin mutant, and distinct C. jejuni strains having known pulsed-field gel electrophoresis and fla PCR-restriction fragment length polymorphism genotypes. Unrelated C. jejuni strains produced heterogeneous patterns, whereas genetically related strains produced similar AFLP patterns. Twenty-five Campylobacter strains obtained from poultry farms in The Netherlands grouped in three C. jejuni clusters that were separate from a C. coli cluster. The band patterns of 10 C. jejuni strains isolated from humans were heterogeneous, and most of these strains grouped with poultry strains. Our results show that AFLP analysis can distinguish genetically unrelated strains from genetically related strains of Campylobacter species. However, desirable genetically related strains can be differentiated by using other genotyping methods. We concluded that automated AFLP analysis is an attractive tool which can be used as a primary method for subtyping large numbers of Campylobacter strains and is extremely useful for epidemiological investigations.
Thermophilic Campylobacter jejuni and Campylobacter coli are important human pathogens and are common causes of gastrointestinal diseases in both developed and developing countries (21). Complications of C. jejuni infections, such as reactive arthritis and pancreatitis, have been described, and clinical evidence strongly suggests that infection with C. jejuni may be a precipitating factor for the development of polyneuropathies, such as Guillain-Barré syndrome (13). Campylobacter is widespread in nature and can be isolated from the gastrointestinal tracts of many animal species, as well as from freshwater. The major infection route for humans supposedly is consumption of contaminated poultry products, although epidemiological data suggest that there are other sources of infection (23). In order to better understand the epidemiology of Campylobacter infections in both poultry and humans, reproducible typing methods which can distinguish individual strains are necessary. Preferably, methods that also determine genetic distances between different but related strains should be developed in order to obtain valuable information concerning the spread and stability of bacterial populations.
Several phenotypic methods for typing C. jejuni and C. coli have been described; these methods include serotyping, phage typing, and biotyping. However, they are not generally available due to a lack of specific reagents. Some other disadvantages of phenotypic methods are that they have restricted differentiation powers and a high proportion of strains are nontypeable. Recently, researchers have developed molecular techniques for genetic subtyping; these methods include pulsed-field gel electrophoresis (PFGE), fla PCR-restriction fragment length polymorphism (RFLP) analysis, ribotyping, and randomly amplified polymorphic DNA analysis (2, 6, 11, 15, 19). The advantage of these genotypic methods is that they are more generally available and applicable; however, the methods that have been described often lack adequate discriminatory power, and the reproducibility of some is poor (19). Consequently, there is an increasing need for highly sensitive and reliable genomic typing methods for Campylobacter strains.
Amplified fragment length polymorphism (AFLP) is a recently developed method for genotyping (28). This method is based on selective amplification of restriction fragments generated from total genomic DNA. AFLP fingerprinting has been shown to have potential for strain identification, as well as high-resolution differentiation of genetically related bacterial strains (5, 8–10, 22). This technique can be easily automated, which allows standardization and high throughput of strains in epidemiological investigations. Moreover, digitization of the data results in easy storage, cross-referencing, and exchange of data between laboratories. Therefore, we investigated whether AFLP could be adapted for fingerprinting and epidemiological analysis of Campylobacter isolates.
The parameters that determine the discriminatory power of AFLP are the restriction enzymes and selective amplification primers used. The enzymes and primers were selected and optimized in this study by using a set of genetically defined C. jejuni strains. The method was then used with randomly obtained Campylobacter strains isolated from poultry and from human patients with gastroenteritis. Reproducible AFLP fingerprints were obtained and used to identify individual strains and to determine genetic distances. Our results show that AFLP fingerprinting is a genotyping method that is very suitable for epidemiological studies of Campylobacter spp.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All of the strains used in this study are listed in Table 1. Strains 81116, 81116R3, 302a, 303a, 331a, 318a, 307a, and 130c have been described previously (29, 31). Twenty-five Campylobacter strains were randomly selected in time and place from a longitudinal study of poultry farms in The Netherlands in 1993 (7). H. P. Endtz (Department of Clinical Microbiology, University Hospital Dijkzigt, Rotterdam, The Netherlands) kindly donated 10 C. jejuni strains obtained from patients with epidemiologically unrelated human enteritis. C. jejuni ATCC 29429 and C. coli ATCC 33559 were included as reference strains. Bacteria were grown on blood agar plates supplemented with 5% sheep blood and incubated 48 h at 37°C under microaerophilic conditions.
TABLE 1.
Campylobacter strains used in this study
| Strain no. | Strain designation | Species | Source of isolationa | fla PCR typeb | PFGE typeb | Reference |
|---|---|---|---|---|---|---|
| 1 | 81116 | C. jejuni | Laboratory reference strain | 18 | ||
| 2 | 81116R3 | C. jejuni | flaB mutant of 81116c | 29 | ||
| 3 | 331 | C. jejuni | Poultry meat | 1, 12 | 23 | 31 |
| 4 | 318 | C. jejuni | Poultry meat | 1, 1 | 22 | 31 |
| 5 | 307 | C. jejuni | Poultry meat | 8, 17 | 21 | 31 |
| 6 | 302 | C. jejuni | Poultry meat | 3, 4 | 20 | 31 |
| 7 | 303 | C. jejuni | Poultry meat | 1, 4 | 20 | 31 |
| 8 | 130c | C. jejuni | Poultry meat | 6, 13 | 7 | 31 |
| 9 | C144 | C. jejuni | Chicken | 7 | ||
| 10 | C350 | C. jejuni | Chicken | 7 | ||
| 11 | C356 | C. jejuni | Chicken | 7 | ||
| 12 | C591 | C. jejuni | Chicken | 7 | ||
| 13 | C626 | C. coli | Chicken | 7 | ||
| 14 | C2505 | C. coli | Chicken | 7 | ||
| 15 | C2143 | C. coli | Chicken | 7 | ||
| 16 | C2146 | C. coli | Chicken | 7 | ||
| 17 | C2150 | C. coli | Chicken | 7 | ||
| 18 | C2155 | C. coli | Chicken | 7 | ||
| 19 | C2450 | C. jejuni | Chicken | 7 | ||
| 20 | C2246 | C. jejuni | Chicken | 7 | ||
| 21 | C2264 | C. jejuni | Chicken | 7 | ||
| 22 | C2345 | C. jejuni | Chicken | 7 | ||
| 23 | C2355 | C. coli | Chicken | 7 | ||
| 24 | C2360 | C. jejuni | Chicken | 7 | ||
| 25 | C2362 | C. jejuni | Chicken | 7 | ||
| 26 | C2375 | C. coli | Chicken | 7 | ||
| 27 | C2380 | C. coli | Chicken | 7 | ||
| 28 | C2385 | C. coli | Chicken | 7 | ||
| 29 | C2390 | C. coli | Chicken | 7 | ||
| 30 | C2400 | C. coli | Chicken | 7 | ||
| 31 | C2412 | C. jejuni | Chicken | 7 | ||
| 32 | C2436 | C. coli | Chicken | 7 | ||
| 33 | C2446 | C. jejuni | Chicken | 7 | ||
| 34 | 19971322 | C. jejuni | Humand | |||
| 35 | 19980464 | C. jejuni | Human | |||
| 36 | 19971423 | C. jejuni | Human | |||
| 37 | 19971591 | C. jejuni | Human | |||
| 38 | 19980411 | C. jejuni | Human | |||
| 39 | 19980569 | C. jejuni | Human | |||
| 40 | 19980623 | C. jejuni | Human | |||
| 41 | 19980624 | C. jejuni | Human | |||
| 42 | 19980706 | C. jejuni | Human | |||
| 43 | 19980652 | C. jejuni | Human | |||
| 44 | ATCC 29429 | C. jejuni | Reference strain | |||
| 45 | ATCC 33559 | C. coli | Reference strain |
Most strains were isolated in The Netherlands; the exceptions were strains 331 and 318, which were isolated from poultry meat that originated from France (31).
Genotypes have been described previously (31).
Strain 81116R3 is a laboratory-derived mutant of 81116 containing a kanamycin resistance cassette in flaB (29).
Human C. jejuni isolates were randomly isolated from enteritis patients and were obtained from H. P. Endtz, Department of Clinical Microbiology, University Hospital Dijkzigt Rotterdam, The Netherlands.
Epidemiological and genetic relationships of strains.
Several strains included in this study could be grouped on the basis of their epidemiological or genetic relationships. Strains 81116 and 81116R3 are genetically related, since the latter is a mutant of 81116 in which the flaB gene is inactivated by introduction of a kanamycin resistance cassette (29). Strains 302a and 303a were isolated from the same batch of poultry meat and were found to have identical phenotypic characteristics, such as serotype, biotype, and phagetype; they had identical PFGE genotypes, but their fla genotypes differed (31). Strains 331a, 318a, 307a, and 130c, which were isolated from poultry meat, were epidemiologically unrelated and did not share genetic characteristics, as determined by PFGE and fla PCR-RFLP analysis. The chicken isolates (strain no. 9 to 33) were not epidemiologically related to each other or to the randomly chosen human isolates (strain no. 34 to 43).
Identification of C. jejuni and C. coli.
In order to confirm species identities, a multiprimer PCR method was used as described previously (25). The primers used were based on the nucleotide sequences of species-specific probes selected from C. jejuni and C. coli DNA fragment libraries (26). This PCR analysis was kindly performed by J. van der Plas, TNO Nutrition and Food Research, Zeist, The Netherlands.
Isolation of chromosomal DNA.
Cells were scraped from the plates and washed with 1 ml of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) (approximately 109 cells/ml). DNA was prepared by using a Wizard genomic DNA purification kit (Promega, Madison, Wis.). DNA concentrations were determined with a spectrophotometer and were standardized at 0.1 μg/μl. DNA integrity was checked by agarose gel electrophoresis, and DNA preparations were stored at −20°C.
AFLP reactions.
The AFLP analysis was performed by using a protocol adapted from the AFLP microbial fingerprinting protocol of PE Applied Biosystems (Perkin-Elmer, Norwalk, Conn.) (20). Briefly, 5 to 10 ng of chromosomal DNA was digested with 5 U of HindIII and either 5 U of HhaI, 5 U of MseI, or 5 U of TaqI and was subsequently ligated to HindIII and HhaI (or MseI or TaqI) restriction site-specific adapters for 2 h at 37°C. A preselective PCR was carried out in a 20-μl (final volume) mixture containing 4 μl of the restriction-ligation mixture, 5 pmol of preselective HindIII primer, 50 pmol of preselective HhaI (or MseI or TaqI) primer, 2 μl of 10× PCR buffer II (Perkin-Elmer), 2 μl of 10 mM MgCl2 (Perkin-Elmer), 200 μM dATP, 200 μM dGTP, 200 μM dCTP, 200 μM dTTP, and 1.25 U of AmpliTaq DNA polymerase (Perkin-Elmer). After amplification the PCR sample was diluted 20:1 with TE. For the selective PCR 1.5 μl of the resulting diluted PCR sample was amplified in a 10-μl (final volume) mixture under the reaction conditions described above by using 1 pmol of fluorescently labelled HindIII-selective primer and 5 pmol of HhaI-selective primer (or MseI-selective primer or TaqI-selective primer). The following selective primers were tested: a HindIII primer containing an adjacent A (HindA) or C (HindC) selective nucleotide; a HhaI primer containing an adjacent A (HhaA) or adjacent A and T (HhaAT) selective nucleotide; and MseI primer with a selective T (MseT), and a TaqI primer with a selective A (TaqA) or C (TaqC). PCR were performed according the AFLP microbial fingerprinting protocol by using a model 9600 GeneAmp PCR system. The final products were diluted 1:1 with TE, and 1 μl, together with a GENESCAN-500 internal lane standard (PE Applied Biosystems), was analyzed on a 7.3% denaturing sequencing gel by using a model ABI 373A automated DNA sequencer. Gels were routinely prepared by using the ABI protocols and were electrophoresed for 5 h.
Testing the reproducibility of band patterns.
The reproducibility of AFLP band patterns was investigated by using eight C. jejuni strains from which chromosomal DNA was isolated five times; the strains used for this were 81116, 81116R3, 331a, 318a, 307a, 302a, 303a, and 130c.
Data analysis.
After electrophoresis, the AFLP patterns were collected with the GeneScan software (PE Applied Biosystems). Densitometric values were transferred to the GelCompar v4.1 software (Applied Maths, Kortrijk, Belgium), and gels were normalized by using an internal size standard that was added to each lane. A similarity matrix was created by determining the Pearson product-moment correlation coefficient (r). The unweighted pair group method using average linkage (UPGMA) was used to cluster the patterns (27).
RESULTS
AFLP conditions.
To obtain the optimal length distribution of DNA fragments, several combinations of restriction endonucleases were tested. HindIII together with the 4-bp-cutting endonuclease MseI resulted in many (>80) small bands (lengths, 50 to 200 bp). The combination of HindIII and TaqI resulted in 40 to 65 bands ranging from 50 to 500 bp long. These bands were evenly distributed and were obtained with all of the selective primers tested. However, after this combination of enzymes was used repeatedly, unacceptable variation occurred in the high-molecular-weight portions of the AFLP fingerprints. This was probably due to irreproducible digestion of chromosomal DNA by TaqI. Restriction enzyme HhaI was found to be a suitable alternative when it was combined with HindIII. Using HindIII and HhaI together with selective primers HindA and HhaA, we obtained 40 to 50 evenly distributed and highly reproducible bands that ranged from 50 to 450 bp long (Fig. 1). When an extra selective nucleotide (HindA combined with HhaAT) was added, the number of bands was reduced to 20 to 30, and the level of discrimination between strains decreased considerably (data not shown). When HindC was used as a selective primer, fewer evenly distributed bands were obtained (data not shown). Therefore, the combination consisting of HindA and HhaA was used for further AFLP analysis.
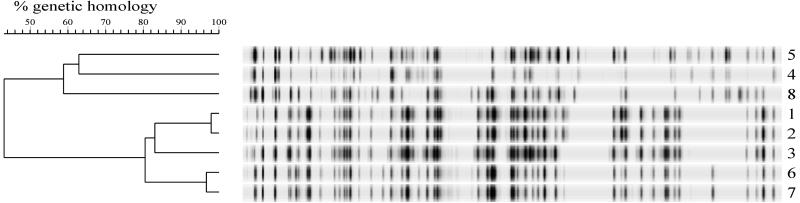
FIG. 1.
AFLP analysis of fluorescently labelled fingerprints from genetically defined C. jejuni strains. The numbers on the right are strain numbers (Table 1). AFLP fingerprints were generated from chromosomal DNA digested with HindIII and HhaI. The specific primers used were HindA and HhaA. The dendrogram was constructed by using UPGMA. The scale indicates percentages of similarity, as determined with the Pearson product-moment correlation coefficient (Gelcompar cluster analysis).
Reproducibility of AFLP analysis.
The reproducibility of the AFLP method was assessed by performing five successive DNA isolations and AFLP analyses with eight C. jejuni strains. Minor variations in the background intensity in consecutive analyses were observed. After normalization of individual gels obtained from independent duplicate experiments, band patterns that exhibited 90 to 98% homology were obtained. Slight variations in peak heights, which in exceptional cases introduced ambiguities when patterns were compared, were sometimes observed. This finding emphasized the conclusion that a standardized AFLP procedure (as described in Materials and Methods) should be used in order to reduce gel-to-gel variation. Furthermore, an analysis of multiple samples on different gels revealed that the clustering of strains was the same irrespective of the 2 to 10% band differences (data not shown). We used a cut-off similarity value of 90% as the cut-off identity level (i.e., isolates whose AFLP patterns were >90% identical were assumed to be closely related genetically).
AFLP analysis of genetically related C. jejuni strains.
The discriminatory power of the AFLP analysis was determined with a set of C. jejuni strains that were genetically characterized previously (29, 31). A laboratory strain and an isogenic mutant with a kanamycin resistance cassette in the flaB gene were included as representatives of closely related isolates (strain no. 1 and 2) (Table 1) (29). Five poultry meat isolates were included as unrelated strains (strain no. 3 through 6 and 8) (Table 1) (31). In addition, two strains that were derived from the same meat source and had almost identical genotypic characteristics were included (strain no. 6 and 7) (Table 1) (31). These strains were analyzed and used to construct a UPGMA dendrogram (Fig. 1). The reference laboratory strain and the mutant (strain no. 1 and 2) produced highly homologous AFLP fingerprints (level of homology, 97%) (Fig. 1). Strain no. 1 through 6 and 8 were genetically unrelated, produced heterogeneous AFLP patterns, and were clearly differentiated by the AFLP analysis (Fig. 1) (31). Epidemiologically related strain no. 6 and 7, which exhibited minor differences in the fla PCR-RFLP analysis but produced identical PFGE patterns, produced indistinguishable AFLP patterns with an intralinkage homology level of 96%.
AFLP analysis of randomly isolated Campylobacter poultry strains.
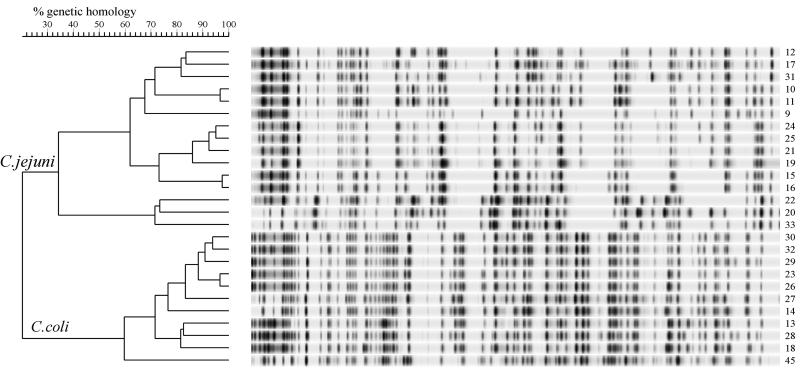
The AFLP method was next used to determine the genetic variation in 25 randomly isolated poultry strains (strain no. 9 through 33 [Table 1]). Two groups that produced distinct band patterns were identified (Fig. 2). Using a species-specific multiplex PCR (data not shown), we identified the strains belonging to one cluster as C. coli strains, whereas the other strains were identified as C. jejuni strains. Large differences in band patterns between the two species were apparent, and therefore the linkage level was only 22%. The C. jejuni and C. coli strains obtained from the American Type Culture Collection (strain no. 44 and 45, respectively) grouped within the species cluster. Three heterogeneous groups with a minimal level of linkage between groups of 32% were identified within the C. jejuni cluster. Some C. jejuni strains were genetically related; strain no. 10 and 11 were related, strain no. 15 and 16 were related, and strain no. 24, 25, and 21 were related. A more closely related group with a homology linkage level of 60% was found in the C. coli cluster. The levels of genetic homology between strain no. 23 and 26 and among strain no. 30, 32, and 29 were more than 90%. These results showed that AFLP analysis can be used to identify individual strains of two Campylobacter species.
FIG. 2.
Dendrogram based on fluorescently labelled AFLP patterns of 25 Campylobacter poultry strains. For the experimental and analytical conditions used see the legend to Fig. 1. The clusters of C. coli and C. jejuni strains are indicated.
AFLP fingerprinting of human Campylobacter strains.
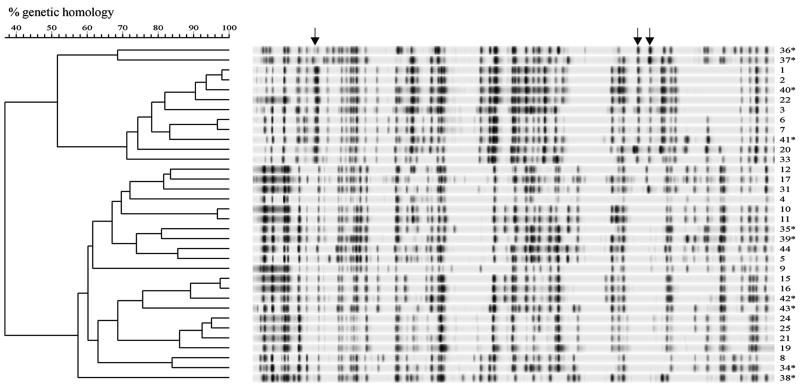
Next, we analyzed 10 C. jejuni strains obtained from Dutch patients with enteritis that had no epidemiological association with each other. Ten distinct AFLP fingerprints were produced by these strains (Fig. 3). The highest level of homology observed between C. jejuni strains obtained from humans was 80%, and the lowest level of homology was 42%. On a combined dendrogram containing all of the C. jejuni strains isolated from poultry, human patients, and poultry meat, there were three main clusters containing both human and poultry strains (Fig. 4). No separate clusters of human-specific or chicken-specific strains were present. However, human strain no. 36 and 37 grouped separately, and strain no. 38 was separated because of a very distinct AFLP pattern. There was no apparent epidemiological link among strain no. 40 (a human isolate), strain no. 22 (a chicken isolate), strain no. 1 (a laboratory-adapted strain that was originally isolated from an outbreak in the United Kingdom [18]), and strain no. 2 (a flaB mutant of strain no. 1 [29]); however, these four isolates appeared to be genetically related as they produced similar AFLP patterns (Fig. 4). The other human strains were distributed in the three groups, and these groups corresponded with the clusters obtained when only poultry isolates were analyzed (Fig. 2 and 4). These results show that there was an overlap between strains from poultry and strains from human patients. In one cluster of strains, cluster-specific bands were detected. In addition, in other smaller groups of strains specific bands were found that could be used for identification of strain-specific (group-specific) genetic markers (Fig. 4).
FIG. 3.
Dendrogram based on fluorescently labelled AFLP fingerprints of 10 human strains of C. jejuni. For the experimental and analytical conditions used see the legend to Fig. 1.
FIG. 4.
Combined dendrogram based on AFLP band patterns of C. jejuni human, poultry, and poultry meat isolates. Human strains are indicated with asterisks. Group-specific AFLP bands are indicated by arrows. For the experimental and analytical conditions used see the legend to Fig. 1.
DISCUSSION
A number of genotypic strain identification methods have been used in attempts to determine the epidemiology of Campylobacter infections (1, 2, 6, 19). All of the previously described methods have limitations and, with the possible exception of the flagellin PCR-RFLP and PFGE methods, still need to prove their value in epidemiological studies. We looked for another genotyping method suitable for Campylobacter spp. for several reasons. First, the most widely used genotyping method, the flagellin PCR-RFLP method, detects a single genomic locus in which recombination has been shown to occur (3, 30). Moreover, since Campylobacter cells are naturally competent to take up and incorporate DNA, any genotyping technique based on a single genetic locus may provide inadequate results. Methods that produce complete genomic fingerprints are probably more reliable for this organism. One of these methods, PFGE, is better than the randomly amplified polymorphic DNA method because of its greater reproducibility and sensitivity. However, recent evidence has suggested that PFGE patterns can also vary, presumably due to the genetic variability of Campylobacter strains (16, 31). Moreover, Campylobacter PFGE patterns comprise fewer bands than the PFGE patterns obtained with other organisms, so that application of the Tenover criteria for interpretation of patterns is questionable (24). We investigated the suitability of AFLP as a genotyping method for Campylobacter strains for these reasons.
In this study, we identified optimal AFLP conditions for Campylobacter fingerprinting. The AFLP restriction endonucleases that are usually used (8, 28) were not useful, since Campylobacter chromosomal DNA was not digested with EcoRI, MseI digestion resulted in many small fragments, and TaqI digestion was often incomplete. Restriction endonuclease HindIII combined with the 4-bp-cutting endonuclease HhaI resulted in reproducible and highly informative band patterns containing 40 to 70 bands evenly distributed in length. The resolutions of the different selective primers tested varied, but HindA combined with HhaA produced highly discriminatory evenly distributed bands.
To obtain reproducible AFLP fingerprints, a standardized protocol for AFLP analysis and computer-based analysis was required. Despite standardization, minor differences in the fingerprints of identical samples were observed. These differences may have resulted from differences in PCR amplification efficiencies and gel-to-gel variations. For this reason, 2 to 10% “noise” should be ignored when AFLP data are interpreted. The data presented in this paper suggest that this level of noise does not affect analysis of C. jejuni strains from various sources since the band patterns obtained are sufficiently heterogeneous. Genetically related strains produced homologous patterns and grouped together, whereas unrelated strains were separated on the dendrogram. However, other genotypic analyses, such as PFGE, ribotyping, and flagellin PCR-RFLP analysis, can be performed to obtain additional information concerning related strains.
The advantage of AFLP over other techniques is that multiple bands are derived from all over the genome. This prevents overinterpretation or misinterpretation due to point mutations or single-locus recombinations, which may affect other genotypic characteristics. For example, two strains that had different flagellin genotypes but produced identical PFGE patterns also produced indistinguishable AFLP patterns. These strains are clearly genetically closely related despite the fact that their flagellin genotypes are different.
PFGE analysis detected variation that occurred in genetically related C. jejuni or C. coli strains (16, 31). It has been suggested that such variation results from large-scale genomic recombinations. Whether the large number of small fragments obtained with AFLP fingerprinting is affected by these recombinational events will have to be established in the future.
Two groups comprising strains belonging to C. jejuni and C. coli were identified when strains randomly obtained from poultry were examined. Distinction of these two species based on biochemical tests is often doubtful. On the basis of hybridization studies and multilocus enzyme electrophoresis typing, C. coli and C. jejuni were identified as separate species that exhibit 25 to 49% homology (1, 4). This is consistent with the genetic homology value determined by AFLP analysis (22%). The C. coli strains from poultry seemed to be more closely related to each other than to the C. jejuni strains, and this finding suggests that C. coli poultry strains are more clonal than C. jejuni poultry strains. C. coli and C. jejuni strains produced very distinct AFLP band patterns; however, the use of AFLP for identification of Campylobacter spp. is currently under investigation.
Common types have been identified in human and poultry strains by serotyping, multilocus enzyme electrophoresis typing, and PFGE typing (1, 14, 17). Clusters containing both human and poultry strains, as well as some strains that were closely related genetically, were also obtained when AFLP analysis was used. This is another indication that poultry products are a likely source of human infections. An interesting observation is that cluster-specific bands were found in AFLP fingerprints. Further analysis of these bands may result in identification of specific strain characteristics. Clearly, AFLP analysis of more C. jejuni strains from different sources is needed to elucidate relationships between strains and sources and to correlate fingerprints with virulence or other strain characteristics.
An important advantage of AFLP analysis is that fingerprints can be analyzed with an automated sequencer; thus, a database can be compiled automatically, and fingerprint data can be compared and exchanged. Although the AFLP method is relatively elaborate and requires purification of intact double-stranded DNA, as well as specialized equipment and software, the resulting fingerprints are more informative than gene sequencing data, which have the same limitations (12).
Use of the AFLP fingerprinting method resulted in a high degree of discrimination and identification of C. jejuni and C. coli strains and was found to be useful and practical. However, further analysis is needed to determine the discriminative power and taxonomic significance of AFLP analysis for differentiating strains of the genus Campylobacter.
ACKNOWLEDGMENTS
We thank Ellard Kruijt for useful discussions concerning analysis of AFLP data, Wilma Jacobs-Reitsma for isolation of the poultry strains, and Jan van der Plas for PCR identification of strains. We also thank Hilde Smith for critically reading the manuscript.
This study was initiated during a visit of Trudy Wassenaar to the ID-DLO in Lelystad, The Netherlands, supported by Cost Action 97.
REFERENCES
- 1.Aeschbacher M, Piffaretti J. Population genetics of human and animal enteric Campylobacter strains. Infect Immun. 1989;57:1432–1437. doi: 10.1128/iai.57.5.1432-1437.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibson J R, Fitzgerland C, Owen J. Comparison of PFGE, ribotyping and phage-typing in the epidemiological analysis of Campylobacter jejuni serotype HS2 infections. Epidemiol Infect. 1995;115:215–225. doi: 10.1017/s0950268800058349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington C S, Thomson-Carter F M, Carter P E. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J Clin Microbiol. 1997;35:2386–2392. doi: 10.1128/jcm.35.9.2386-2392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey S M, Greenwood J R. Relationships among catalase-positive campylobacters determined by deoxyribonucleic acid-deoxyribonucleic acid hybridization. Int J Syst Bacteriol. 1983;33:275–284. [Google Scholar]
- 5.Huys G, Coopman R, Janssen P, Kersters K. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int J Syst Bacteriol. 1996;46:572–580. doi: 10.1099/00207713-46-2-572. [DOI] [PubMed] [Google Scholar]
- 6.Jackson C J, Fox A J, Wareing D R A, Hutchinson D N, Jones D M. The application of genotyping techniques to the epidemiological analysis of Campylobacter jejuni. Epidemiol Infect. 1996;117:233–244. doi: 10.1017/s0950268800001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs-Reitsma W, Bolder N M, Mulder R W A W. Caecal carriage of Campylobacter and Salmonella in Dutch broiler flocks at slaughter: a one-year study. Poult Sci. 1994;73:1260–1266. doi: 10.3382/ps.0731260. [DOI] [PubMed] [Google Scholar]
- 8.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 9.Janssen P, Maquelin K, Coopman R, Tjernberg R, Bouvet P, Kersters K, Dijkshoorn L. Discrimination of Acinetobacter genomic species by AFLP fingerprinting. Int J Syst Bacteriol. 1997;47:1179–1187. doi: 10.1099/00207713-47-4-1179. [DOI] [PubMed] [Google Scholar]
- 10.Kiem P, Kalif A, Schupp J, Hill K, Travis S E, Richmond K, Adair D M, Hugh-Jones M, Kuske C R, Jackson P. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J Bacteriol. 1997;179:818–824. doi: 10.1128/jb.179.3.818-824.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazurier S, van der Giessen A, Heuvelman K, Wernars K. RAPD analysis of Campylobacter isolates: DNA fingerprinting without the need to purify DNA. Lett Appl Microbiol. 1992;14:260–262. doi: 10.1111/j.1472-765x.1992.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 12.Meinersmann R J, Helsel L O, Fields P I, Hiett K L. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J Clin Microbiol. 1997;35:2810–2814. doi: 10.1128/jcm.35.11.2810-2814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishu B, Ilyas A A, Koski C L, Vriesendorp F, Cook S D, Mithen F A, Blaser M J. Serologic evidence of previous Campylobacter jejuni infection in patients with the Guillain-Barré syndrome. Ann Intern Med. 1993;118:947–953. doi: 10.7326/0003-4819-118-12-199306150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Møller Nielsen E, Engberg J, Madsen M. Distribution of serotypes of Campylobacter jejuni and C. coli from Danish patients, poultry, cattle and swine. FEMS Immunol Med Microbiol. 1997;19:47–56. doi: 10.1111/j.1574-695X.1997.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 15.Nachamkin I, Bohachick K, Patton C M. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J Clin Microbiol. 1993;31:1531–1536. doi: 10.1128/jcm.31.6.1531-1536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.On S L W. In vitro genotypic variation of Campylobacter coli documented by pulsed-field gel electrophoretic DNA profiling: implications for epidemiological studies. FEMS Microbiol Lett. 1998;165:341–346. doi: 10.1111/j.1574-6968.1998.tb13167.x. [DOI] [PubMed] [Google Scholar]
- 17.On S L W, Nielsen E M, Engberg J, Madsen M. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol Infect. 1998;120:231–237. doi: 10.1017/s0950268898008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer S R, Gully P R, White J M, Pearson A D, Suckling W G, Jones D M, Rawes J C, Penner J L. Water-borne outbreak of Campylobacter gastroenteritis. Lancet. 1982;i:287–290. doi: 10.1016/s0140-6736(83)91698-7. [DOI] [PubMed] [Google Scholar]
- 19.Patton C M, Wachsmuth I K, Evins G M, Kiehlbauch J A, Plikaytis N, Troup L, Tompkins L, Lior H. Evaluation of 10 methods to distinguish epidemic-associated Campylobacter isolates. J Clin Microbiol. 1991;29:680–688. doi: 10.1128/jcm.29.4.680-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkin-Elmer Corporation. Microbial AFLP fingerprinting protocol. Norwalk, Conn: Perkin-Elmer Corp.; 1996. [Google Scholar]
- 21.Skirrow M B, Blaser M J. Clinical and epidemiological considerations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 3–9. [Google Scholar]
- 22.Sloos J H, Janssen P, van Boven C P A, Dijkshoorn L. AFLP typing of Staphylococcus epidermidis in multiple sequential blood cultures. Res Microbiol. 1998;149:221–228. doi: 10.1016/s0923-2508(98)80082-x. [DOI] [PubMed] [Google Scholar]
- 23.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized countries. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 24.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Giessen A W, Tilburg J J H C, Ritmeester W S, van der Plas J. Reduction of Campylobacter infections in broiler flocks by application of hygiene measures. Epidemiol Infect. 1998;121:57–66. doi: 10.1017/s0950268898008899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Plas J, Hofstra H, Huis in ’t veld J H J. DNA probe assays for detection, identification and typing of Campylobacter species and Helicobacter pylori. Microb Ecol Health Dis. 1993;4:S60. [Google Scholar]
- 27.Vauterin L A, Vauterin P. Computer-aided objective comparison of electrophoresis patterns for grouping and identification of microorganisms. Eur Microbiol. 1992;1:37–41. [Google Scholar]
- 28.Vos P, Hogers R, Bleeker M, Reijans M, van der Lee T, Hames M, Frijters A, Pot J, Peleman J, Kuiper J, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1996;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassenaar T M, Bleumink-Pluym N M C, van der Zeijst B A M. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wassenaar T M, Fry B N, van der Zeijst B A M. Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer. Microbiology. 1995;141:95–101. doi: 10.1099/00221287-141-1-95. [DOI] [PubMed] [Google Scholar]
- 31.Wassenaar T M, Geilhausen B, Newell D G. Evidence of genomic instability in Campylobacter jejuni isolated from poultry meat. Appl Environ Microbiol. 1998;64:1816–1821. doi: 10.1128/aem.64.5.1816-1821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]