Abstract
Purpose
Babble Boot Camp (BBC) is a package of proactive activities and routines designed to prevent speech and language disorders in infants at predictable risk. It is implemented via parent training and currently undergoing clinical trial in children with a newborn diagnosis of classic galactosemia (CG), a metabolic disease with high risk of speech and language disorders. The purpose of this study is to provide updates to a previous pilot study and to present the first set of post-intervention results.
Method
The intervention and data collection occurred during child ages < 6–24 months, with follow-up assessments of speech and language at ages 2.5 and 3.5 years. Treatment targets included earliest vocalization rates, babble complexity, speech production accuracy, and vocabulary and syntactic growth. The oldest 15 children with CG (including three untreated controls) completed the first set of follow-up assessments. Aggregate data up to 10 months were available for 17 treated children with CG, six untreated children with CG, and six typical controls.
Results
At ages 7–9 months, babbling complexity, as measured with mean babbling level, was higher in the treated children with CG than in the untreated children with CG and the typical controls. Prior to 24 months of age, the treated children with CG had greater expressive but not receptive vocabulary sizes than an untreated control. Follow-up testing showed typical language scores for all 12 treated children with CG and typical articulation scores for 11 of these, whereas one of three untreated children with CG had low articulation and expressive language scores.
Conclusions
The BBC appears to be a viable intervention to support the speech and expressive language development of children with GC. Future studies will evaluate the relative contributions of the earliest and later BBC components to outcomes.
Childhood speech and language disorders are not rare. Approximately 4% of first graders in the United States have a diagnosis of speech sound disorder (SSD; Campbell et al., 2003; Law et al., 2000; Shriberg et al., 1999), defined as difficulty with producing the speech sounds in their language correctly by the expected milestones (Campbell et al., 2003; Law et al., 2000; Shriberg et al., 1999). Developmental language disorder (DLD), defined as difficulty with formulating and comprehending language, is diagnosed in 7%–10% of children in the United States (Tomblin et al., 1997). Both of these disorders exert a heavy toll on children with SSD or DLD and their families. Generally, SSDs are associated with the frustration of not being understood (Lousada et al., 2014), negative perceptions and bullying by peers (Hall, 1991; Hitchcock et al., 2015; Lindsay et al., 2008; McCormack et al., 2009), reading disabilities (Peterson et al., 2009), and, if left untreated or undertreated, social and work-related difficulties in adulthood (Allard & Williams, 2008; Culton, 1986; Mitchell et al., 2005). Treatment can be lengthy and costly (Baker & McLeod, 2011; Campbell, 1999; Vieland et al., 1995). Similarly, children with DLD are at risk for educational and social disadvantages (Young et al., 2002) and increased health costs (Cronin et al., 2017). Thus, SSDs and DLDs present formidable challenges not only in terms of the high numbers of affected children but also in terms of the associated personal and societal costs.
Speech and language disorders cannot be diagnosed reliably on behavioral grounds until children are old enough to show evidence of delays. Most standardized assessment tools are available for child ages at which sufficient speech and language abilities have typically emerged, for instance, ≥ 24 months for speech (Goldman & Fristoe, 2015) and ≥ 36 months for language (Semel et al., 2004). Only after the initial diagnosis has been made can intervention begin. This time course presents a problem for very early and preventive interventions. Whereas children with autism spectrum disorder can be identified and begin to benefit from early intervention as young as 12 months of age (Dawson et al., 2010; Rogers et al., 2014), most children with SSD and DLD are toddlers and preschoolers when they first have the opportunity to benefit from interventions. If the risk for SSD and DLD could be predicted on biological grounds instead of identified on behavioral grounds, this would motivate the development of very early and preventive interventions. The idea to prevent, rather than remediate, SSD and DLD draws on at least two concepts: leveraging the brain plasticity of very young infants and avoiding the need to unlearn faulty behavior patterns (Teulier et al., 2015).
The general question whether young children's speech and language skills can be positively influenced by strategic parental input has been addressed in a few studies. For instance, in a sample of typical infants, parents were coached in using “parentese” (speaking style with higher pitch, slower speed, and enhanced intonational contours) and turn-taking techniques when interacting with their children when they were 6, 10, and 14 months old. Compared to a control group, the treatment group had higher rates of using the interaction techniques, and these metrics were correlated with the children's language growth rates (Ferjan Ramirez et al., 2020). A randomized controlled trial of adolescent mothers and their infants, 20% of whom were born preterm, showed that parent coaching during the infants' first year of life was associated with higher infant vocalization and turn-taking rates, compared to the control group (Hoffman et al., 2020). In a series of three studies of infants born at very low birth weight and normal birth weight (Landry et al., 2006, 2008, 2012), parent coaching in responsive parenting techniques when the infants were 6–13 months old was associated with increased emotionally supportive behaviors in the mothers and broad benefits for child development. A second round of coaching when the children were toddlers was associated with the mothers' increased cognitive response behaviors. These results were seen in both groups of children, those born at very low and normal birth weight. These findings support a beneficial effect of parent training on the language development of typical children and children born at very low birth weight.
To investigate whether SSD and DLD can be prevented in infants born with a known and predictable risk for these disorders, Babble Boot Camp (BBC) was designed and implemented in a pilot sample of infants with a newborn diagnosis of classic galactosemia (CG). This disease is a recessively inherited inborn error of metabolism characterized by defective conversion of galactose to glucose due to a near absence of the enzyme galactose-1-phosphate uridyl transferase. In the United States, CG is diagnosed via newborn screening. Annual incidence rates in the United States range from one in 30,000 to one in 60,000 (Fridovich-Keil, 2008), whereas among individuals of Irish descent, the incidence rate is higher, one in 16,000 (Coss et al., 2013). The inability to metabolize galactose causes an accumulation of galactose in the blood, which has toxic and potentially lethal effects if not treated with a lactose-restricted diet. However, despite early detection and strict adherence to lactose-restricted diets (Berry, 2011; Demirbas et al., 2018; Rubio-Gozalbo et al., 2019; Ryan et al., 2013), children with CG are at high risk not only for fine and gross motor deficits and learning disabilities (Antshel et al., 2004; Karadag et al., 2013; Potter et al., 2013) but also for DLD (Lewis et al., 2013a, 2013b; Potter et al., 2008; Timmers et al., 2011, 2012; Waggoner et al., 1990) and SSD (Waisbren et al., 1983), especially a severe form called childhood apraxia of speech (CAS; C. D. Nelson et al., 1991; D. Nelson, 1995; Shriberg et al., 2011; Webb et al., 2003). Approximately 40%–85% of individuals with CG were reported to have SSDs (Hughes et al., 2009; Rubio-Gozalbo et al., 2019; Waggoner et al., 1990), compared to 4% among young school-age children in the United States generally (Shriberg et al., 1999), and 24%–63% of children with CG were reported to have CAS (Shriberg et al., 2011; Waggoner et al., 1990; Waisbren et al., 2012; Webb et al., 2003), compared to 0.1% among children generally (Shriberg et al., 1997). Approximately 50%–78% of children with CG were reported to have DLD (Potter et al., 2008; Rubio-Gozalbo et al., 2019; Waggoner et al., 1990), compared to 7%–10% among children generally (Tomblin et al., 1997). Expressive language abilities are affected more frequently by CG than receptive abilities. Specifically, expressive language delays were mainly seen in children with CG who had typical cognitive abilities, whereas mixed expressive/receptive language delays were seen in children with CG who had concomitant cognitive delays (Potter et al., 2008). Importantly, children with CG qualify for speech and language services based on observed deficits and typically begin therapy before 4 years of age; however, despite treatment, difficulties with speech and language persist in many cases (Potter et al., 2008, 2013).
Children with CG were selected for the clinical trial of the BBC because of the known genotype–phenotype associations. If BBC proves to be effective in this population, this will motivate new clinical trials in other populations of very young children at genetic risk. Genetics of communication disorders is an emerging field, from the discovery of the role of the FOXP2 gene in a family with familial CAS (Lai et al., 2001, 2003) to more recent genetic (Hildebrand et al., 2020) and chromosomal (Fedorenko et al., 2016; Peter et al., 2014, 2017) findings in individuals and families with various forms of disorders of spoken and written language, as recently reviewed (Guerra & Cacabelos, 2019). Advances in understanding genotype–phenotype associations in addition to those in CG will facilitate early identification of infants at risk who may benefit from proactive interventions such as BBC. BBC may also be suitable for trialing with children with other known risk factors, such as preterm birth (Hillman et al., 2019; Vohr, 2014) and craniofacial disorders (Scherer et al., 1999; note that our team has initiated pilot trialing of the BBC in infants born preterm). Tailoring proactive interventions based on individual predictable risk factors is an approach that leverages aspects in precision medicine (Goetz & Schork, 2018), translated here into the realm of behavioral traits, specifically communication abilities.
The BBC is a bundle of routines and activities designed to stimulate and foster the earliest signals of communication, prespeech and speech sound production, receptive and expressive language skills, and communicative competency. As the first comprehensive proactive program for infants at predictable risk for SSD and DLD, BBC is currently undergoing a randomized controlled trial (ClinicalTrials.gov NCT03838016).
The basic concept underlying the BBC implementation is parent training (see Figure 1). A pediatric speech-language pathologist (SLP) trains parents to use strategies designed to support and increase their child's communication skills, and the parents implement these activities and routines on a daily basis. Fidelity checks (reliability and validity) are conducted at two levels: the SLP's training methods and the parents' implementation.
Figure 1.
Babble Boot Camp conceptualization of parents receiving and implementing the training. SLP = speech-language pathologist.
The first infants in the BBC project were enrolled in 2017, and initial results were reported for the oldest five children with CG who participated in the clinical trial (Peter et al., 2019). Four of these children had participated in the active treatment arm, and one was an untreated control. The following metrics were analyzed and reported: Complexity of babble productions as measured with the mean babbling level (MBL; Stoel-Gammon, 1989); complexity of meaningful speech productions as measured with the syllable structure level (SSL; Paul & Jennings, 1992); receptive and expressive vocabulary size as measured with the MacArthur–Bates Communicative Development Inventories–Second Edition (CDI; Fenson et al., 2007); and general development of fine and gross motor, personal–social communication, and problem solving as measured with the Ages & Stages Questionnaires–Third Edition (Squires & Bricker, 2009). Of the four children in the treatment arm, all had higher MBL scores than the untreated control child, three had higher SSL scores, two had higher expressive vocabularies, and three had higher communication and personal–social skills.
Since the first report on the BBC (Peter et al., 2019), the participant sample has grown in age and number. Here, we provide updates on an expanded sample of 12 treated participants with CG and an untreated control child with CG, all of whom had provided continuous data since infancy, and for the first time, we provide results from follow-up assessment at age 2.5 or 3.5 years. Here, the focus is on earliest speech sound productions in babble and speech and vocabulary size during the intervention, as well as follow-up articulation and language outcomes after the end of the intervention phase. In addition, data are included on younger untreated children with CG and typical controls.
The following research questions (RQ) and hypotheses (H) were addressed:
RQ1: Do children with CG who are undergoing the BBC intervention show better speech sound production skills, as measured with MBL and SSL, than the untreated controls during BBC's active phase (child ages ≥ 2–24 months)?
RQ1H: In the BBC, parents learn to support quantity and quality of their children's vocalizations with respect to not only babble but also meaningful speech. If the SLP's' training and the parents' implementation of this treatment target are effective, the MBL and SSL scores in the treated children will be higher than those in the untreated controls.
RQ2: Do children with CG who are undergoing the BBC intervention show higher expressive and receptive vocabulary sizes, compared to the untreated control during the BBC active phase?
RQ2H: If vocabulary size aligns with the observation that expressive language skills are more frequently affected by CG than receptive language skills (Potter et al., 2008) and if the intervention is effective on both levels (SLP training and parent implementation), we expect that the untreated control with CG will have lower expressive vocabulary skills, compared to the treated children with CG, but that the untreated control's receptive vocabulary skills do not differ from those in the treated children with CG.
RQ3: Do children who underwent the BBC intervention have better articulation skills at follow-up, compared to untreated controls?
RQ3H: If early professional support during the first 2 years of life had a beneficial effect on the children's developing articulation skills, we expect this benefit to persist, and we predict higher articulation skills at follow-up in the treatment group, compared to the untreated controls. Under that same hypothesis, we expect a close association between speech production skills as measured during the treatment phase and speech production skills at follow-up.
RQ4: Are the language skills as measured at follow-up higher in the treated group, compared to the untreated controls?
RQ4H: Similarly to RQ3H, we expect the treatment effects on language, regardless of whether they are based on the known associations between early speech sound production skills and later language skills, on the direct language-based treatment targets, or both, to persist to follow-up. Based on previous observations that expressive language is more often affected by CG than receptive language (Potter et al., 2008), treatment effects on expressive language are expected to be greater than those on receptive language. We predict close associations between early language measures and language measures at follow-up.
RQ5: Are early measures of speech sound production associated with articulation and language skills later on?
RQ5H: Previous research showed that babble complexity is associated with speech and language abilities later on (American Speech-Language-Hearing Association, 2007; DePaolis et al., 2013; McCune & Vihman, 2001; Oller et al., 1998, 1999; Stoel-Gammon, 1989, 2011; Vihman & Greenlee, 1987; Vihman et al., 1985). If these early skills lay down the foundation for later skills in speech and language and if the BBC intervention is effective in boosting babble skills, then the association between early babble skills and later articulation and language skills should persist, even if the early babble skills were enhanced by the intervention. Alternatively, an association between early babble skills and later articulation and language skills could be explained by an environmental factor underlying both skills, such as parental modeling and feedback on all levels from babble to expressive and receptive language. This study is not designed to disambiguate these two possibilities, as it lacks a sufficiently large sample of children with CG who did and did not receive intervention during the babble stage.
In either case, if we find that babble complexity predicts speech and language abilities later on in children who are receiving an intervention during all stages of babble, meaningful speech, and language during the first 2 years of life, this confirms previous observations of the predictive relationship between these early and later skills, but here in the context of an intentional intervention. If we do not find evidence of a predictive relationship between the early and later skills, the intervention may have had different effects on the children's skills at various ages.
Method
The Appendix contains the Template for Intervention Description and Replication checklist with the location of the core components of the clinical trial in this document.
Participants
This study was conducted with the approval of the institutional review board at Arizona State University. Adults gave written consent for their own participation and written permission for their children to participate. Families were recruited via online announcements on the Galactosemia Foundation website (https://static1.squarespace.com/static/551b5c96e4b00eb2216e7c74/t/5fac2de41ef47e6e87ee3b55/1605119465763/BBC+CG+Flyer20200918_Approved.pdf) and in social media and by referral by health care providers. Recruitment and enrollment are ongoing.
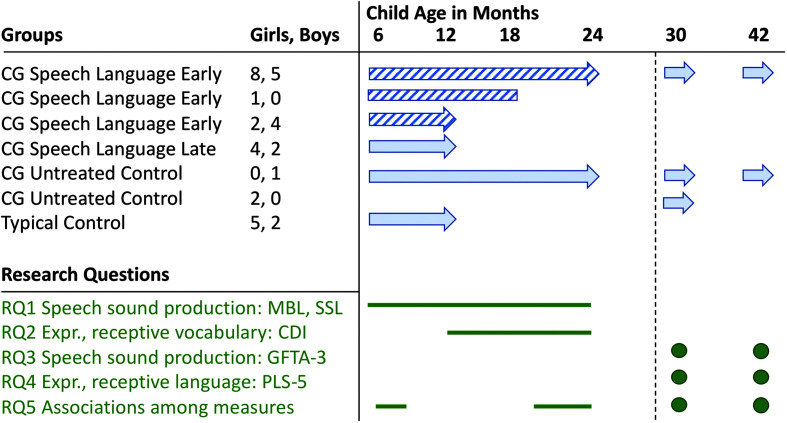
Infants with CG and their parents were randomized into one of two groups, speech-language early (SLE) intervention starting at child age 2–5 months or delayed entry into the speech and language intervention starting later (SLL), at child age 15 months (see Figure 2). In the initial phase of the project, a control group for infants with CG who did not receive any part of the BBC's speech and language intervention was established; however, retention in the untreated group was problematic. The delayed entrance into the speech and language intervention was established to take the place of the untreated control group. Two other participant groups were typically developing (TD) infants who entered the study at 2–5 months of age, and toddlers and preschoolers with CG who were already too old to participate in the BBC speech and language intervention and who participated in annual standardized assessments at ages 2.5 years and older.
Figure 2.
Summary of available data for the participating children by subgroup and health status. Diagonal pattern fill: treatment and data collection. Solid fill: data collection only. Rightward arrow: continues in the study. Rightward vertical line: withdrew from the study. Dashed line: start of follow-up testing. CDI = MacArthur–Bates Communicative Development Inventories–Second Edition; CG = classic galactosemia; Expr. = expressive; GFTA-3 = Goldman-Fristoe Test of Articulation–Third Edition; MBL = mean babbling level; PLS-5 = Preschool Language Scales–Fifth Edition; RQ = research question; SSL = syllable structure level.
Inclusionary criteria for participation in the two BBC speech and language intervention groups were as follows: Boys and girls in any region of the United States or abroad were eligible to participate as long as their primary language in the home was English. All racial and ethnic groups were eligible. At least one parent with at least an eighth-grade education had to be available to participate. Parents could be biological, foster, or adoptive parents. To be included in one of the CG treatment groups, the child had to have a newborn diagnosis of CG, be free of any other health impairment that could confound the results of the study, and be between 2 and 5 months of age at entry into the study. The same criteria applied to the toddler/preschool group with CG, except that their age at entry into the study was < 2.5 years. To participate in the TD group, children had to be free of CG and any health impairment that could confound the results of the study, and they had to be 2–5 months old when they entered the study.
At the time of this writing, 45 families are enrolled in the project. For the purposes of this study, data from the oldest children with CG who provided continuous data up to 24 months of age and underwent follow-up testing at 2.5 or 3.5 years of age are presented. These were 12 children with CG who received the speech and language intervention from < 6 to 24 months of age and one untreated child with CG. One additional child with CG in the treatment group left the study at 18 months of age; only those metrics applicable up to that age were included in the study. Two children with CG joined the study as untreated toddlers and provided speech and language assessment data at 2.5 years of age. Data up to 10 months of age were available for six untreated children with CG who were waiting to start the speech and language intervention at 15 months of age and seven TD controls. In total, 26 of 28 children with CG were described by their parents as White, and two were described as “more than one race.” This high prevalence of White children among the participants with CG is consistent with the high CG incidence rates in individuals of Irish descent (Coss et al., 2013). Of seven typical children, five were described as White, and two were described as “more than one race.” Figure 2 summarizes the participant groups and time points for which data were available. Table 1 provides parents' highest educational attainment in percent per group.
Table 1.
Parents' highest educational achievement in percent per group.
| Education | CG speech-language early |
CG speech-language late |
CG untreated controls |
Typical controls |
||||
|---|---|---|---|---|---|---|---|---|
| Mothers | Fathers | Mothers | Fathers | Mothers | Fathers | Mothers | Fathers | |
| Completed college (%) | 76 | 76 | 83 | 67 | 67 | 100 | 67 | 100 |
| Some college (%) | 18 | 12 | 17 | 0 | 67 | 0 | 33 | 0 |
| Completed high school (%) | 6 | 12 | 0 | 17 | 0 | 0 | 0 | 0 |
| Some high school (%) | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 0 |
Note. CG = classic galactosemia.
Procedure
The intervention was implemented by a pediatric SLP, co-author J. D., who met with the families on a regular basis. To train the parents, the SLP used the teach–model–coach–review approach (Roberts et al., 2014). A key principle underlying most activities is the zone of proximal development (ZPD; Vygotsky, 1979), also referred to as scaffolding, where parents provide speech and language models that bridge what the child can already do and what is slightly beyond the child's skill set: Within the ZPD are skills that the child can do with help.
The intervention begins with an orientation meeting during which the SLP provides an overview of the BBC's components. During the active intervention phase (child ages either < 6–24 months or 15–24 months, when the intervention is implemented and data are collected on a frequent and regular basis), the SLP meets with the parents once per week for approximately 20 min. Prior to the weekly meeting, parents send the SLP two brief home videos, each maximally 2 min long. One of these videos shows the parent and child engaged in the activity or routine that is presently being implemented; the other video shows the child during a typical daily activity as deemed representative of the child skill by the parent. The building blocks of the program are 17 activities and routines designed to support children's communication abilities. These include intentional eye contact to support bonding and modeling, responding to infant vocalizations to increase vocalization behaviors and to build dyadic interactions, eliciting and reinforcing babble to increase babble complexity, labeling objects to expand the child's vocabulary, modeling word productions to increase the child's phonemic inventory, and recasting and expanding simple sentences slightly to build syntactic complexity within the ZPD. A detailed description of these activities and routines is available at the Open Science Framework entry for the BBC (https://osf.io/yzht4/) and in our previous publication (Peter et al., 2019).
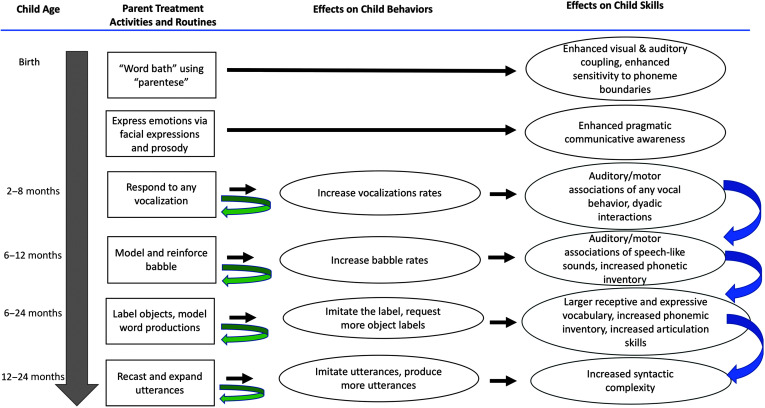
The BBC model (see Figure 2) incorporates several intended direct and indirect treatment effects and feedback loops. For example, when parents talk to their young infant frequently, using child-directed speech patterns, the “intended direct effects” on the child are enhanced visual and auditory coupling in the child's perception of the speech signal and enhanced sensitivity to phonemic boundaries. As the children's vocalization rates at all levels (coo, babble, and meaningful speech) increase, presumably at least in part due to the parents' eliciting and reinforcing strategies, parents have more opportunities to provide responses and expansions. This increase in child behaviors thus represents a “feedback loop” that potentiates the treatment effect on child speech production and language skills. An example of a potential “indirect treatment effect” is influencing speech and language skills later on by fostering babble complexity during the prespeech stage. As mentioned, previous research on typical children has shown that speech sound production skills in babble are associated with speech and language abilities later on (American Speech-Language-Hearing Association, 2007; DePaolis et al., 2013; McCune & Vihman, 2001; Oller et al., 1998, 1999; Stoel-Gammon, 1989, 2011; Vihman & Greenlee, 1987; Vihman et al., 1985). If phonetic complexity in babble predicts articulation and language skills later on, it is possible that intentionally increasing a child's babble complexity via BBC activities provides the foundation for advanced speech and language abilities later on. As mentioned, however, if parents not only provide strong support during the prespeech stage but also during verbal development, enhanced speech and language skills in toddlers could result from the support during the prespeech stage, support during verbal development, or both. Figure 3 is a schematic of selected direct and indirect treatment effects and feedback loops.
Figure 3.
Selected treatment activities and routines and their intended effects on child behaviors and skills. Curved arrows pointing back to the element of origin under “Parent Treatment Activities and Routines”: feedback loops. Curved arrows pointing to a different element under “Effects on Child Skills”: indirect treatment effects.
When the children turn 24 months old, the active phase ends. Follow-up assessments of speech and language skills, along with other metrics of health, development, and quality of life are conducted once yearly at 2.5, 3.5, and 4.5 years of age. Regarding the children in the present report, co-author N. P. oversaw these follow-up assessments.
For the children described in this study, all intervention activities were conducted online using telepractice software that is compatible with Health Insurance Portability and Accountability. This online approach made it possible for families to participate regardless of place of residence. Prior to March 2020, follow-up testing was conducted face-to-face by local SLPs. After this date, due to the coronavirus disease 2019 pandemic, follow-up testing was seamlessly converted to virtual testing using telepractice tools by co-author N. P., an SLP with extensive pediatric telepractice and assessment experience. To date, participants include families living in the United States, Canada, and the United Kingdom.
While enrolled in the BBC, all children 24 months of age and younger were closely monitored by team members who did not include the treating SLP, using a variety of tools. Sets of questionnaires were collected every 3 months to generate metrics covering child language development, general health and development, and quality of life. Here, we report on child language development in terms of expressive and receptive vocabulary, as measured with the CDI (Fenson et al., 2007). Parents filled out the questionnaires by checking the “understands” or “understands and says” options next to specific words in lists of common words in children's vocabularies. The CDI provides expressive vocabulary percentiles, convertible to standard scores, for ages 12, 15, 18, 21, and 24 months, whereas receptive vocabulary data are only available for 12 and 15 months.
Close monitoring also included a day-long audio recording once a month, using the Language Environment Analysis (LENA) recording system (LENA Research Foundation). On the day of the recording, parents activated the LENA recording device and placed it into the front pocket of a specially designed vest that the child wore throughout the day. When the recording was complete at the end of the day, parents either uploaded the recording to a secure cloud where it was accessed by the research team or they mailed the device back to the research team. For the participants in this report, the average recording length was 13.25 hr (SD = 2.17 hr). To obtain sufficient material for analyzing child utterances, the .wav file produced by the LENA device was digitally segmented to obtain the three 5-min segments with the highest number of infant vocalizations, following the methods of a large public corpus of child speech samples (VanDam, 2021; VanDam et al., 2016).
Team members with advanced training and experience in phonetics, all undergraduate or graduate students of Speech and Hearing Science at Arizona State University, transcribed the infant vocalizations into the International Phonetic Alphabet beginning with child age 6 months. Over time, 11 different transcribers were involved, typically two or three in any given semester. All were blind to the status of the child (treated or untreated CG, untreated typical).
For the purposes of this study, child speech sound productions were analyzed using the MBL for nonmeaningful (babbled) utterances (Stoel-Gammon, 1989) and the SSL for meaningful (words and word attempts) utterances (Paul & Jennings, 1992). A meta-analysis of six studies of the MBL and SSL showed that low MBL levels were predictive of lack of meaningful speech at 24 months of age and that children with language impairment obtained lower SSL scores than typical peers, supporting the reliability and clinical validity of these measures (Morris, 2010). MBL calculations were based on the first 50 nonmeaningful utterances in the three 5-min sections with highest infant vocalization rates identified by the LENA software. In cases where fewer than 50 utterances could be transcribed, no MBL score was reported. Per MBL guidelines (Stoel-Gammon, 1989), each nonmeaningful utterance was scored on a 3-point system, where a score of 1 is assigned for simple utterances consisting of a vowel, a syllabic consonant, or a consonant–vowel or vowel–consonant sequence where the consonant is a glide, a glottal stop, or a glottal fricative, which are not considered to be true consonants. Examples of Level 1 utterances are [a] and [wawa]. A score of 2 is assigned to utterances containing at least one consonant–vowel or vowel–consonant sequence with a true consonant, for example, [ba] or [ip]. The same score of 2 is assigned where a true consonant occurs in a cluster with a glide, for example, [bwa]. In utterances with two or more syllables, the consonants may be the same ones or differ only in voicing. Examples are [bapa] and [dida]. A score of 3 is assigned to utterances containing at least two true consonants produced in different parts of the mouth and/or with different manner of articulation. Examples are [bama] and [ədap]. Scores were averaged to arrive at the MBL for that child and month. The guidelines for scoring the SSL are similar to those for the MBL. One difference is that consonant clusters receive a score of 4. Computing an SSL score requires a minimum of 10 meaningful utterances (defined as consisting of recognizable words, as opposed to babbled utterances). In cases where fewer utterances were available, no SSL score was recorded.
During the annual follow-up testing, speech sound accuracy was measured with the Goldman-Fristoe Test of Articulation–Third Edition (GFTA-3; Goldman & Fristoe, 2015). Here, we report the standard scores from the Sounds in Words subtest. Expressive and receptive language skills were measured with the Preschool Language Scales–Fifth Edition (PLS-5; Zimmerman et al., 2011).
Reliability and Validity
Implementation fidelity was ensured in several ways. All sessions were video-recorded. The same SLP provided the interventions for all the children in this report. The SLP checked that all components of the teach–model–coach–review model (Roberts et al., 2014) were implemented. During sessions where parents learned new routines or activities, the SLP provided feedback to ensure correct implementation, and she checked the weekly home videos for correct implementation. The families described in this study attended the available sessions at an average rate of 95% (SD = 3%). Furthermore, compliance was measured with a point system where 1 point was awarded for each of the following components: submitting the home videos before the session, showing evidence of follow-through in at least one of the videos or in the discussion, and attending the scheduled session. The average score for the families described here was 2.73 (SD = 0.19). One additional measure of implementation fidelity is the regularity with which monthly LENA audio recordings were provided by the parents. Out of a total of 373 recordings expected, 331 were completed and provided to the team, an overall compliance rate of 89%.
MBL and SSL transcribers were blind to the group assignment of the participants. Of the 227 sets of sound recordings from children ≥ 6 months of age transcribed to obtain MBL and SSL values for this study, 10% were retranscribed by a different team member, and an average discrepancy between the two sets of transcriptions was calculated. For each double-transcribed recording, the absolute difference between the two scores was calculated relative to the mean of the two scores, as follows:
| (1) |
The average discrepancy was 0.04 (SD = 0.05). This low discrepancy rate indicates a high degree of intertranscriber reliability, not necessarily with respect to the exact phonetic transcriptions, but certainly with respect to the MBL and SSL values.
All MBL and SSL scores were checked for correct calculations and correct data entry. Any errors detected during this quality control step were corrected.
Each CDI questionnaire was scored by one team member and rescored and entered into the database by another team member. Any differences between the two sets of scores were resolved by consensus.
The follow-up testing with the GFTA-3 and PLS-5 was video-recorded. The child's responses were noted on the test protocol during the testing and checked later by a second team member based on the recording. Any score differences were resolved by consensus. The assessment team (co-authors N. P. and D. W.) was blind to the group status (treated CG, untreated CG, or TD) of the child.
Statistical Analysis
RQ1 (Differences Between Treated and Untreated Children With CG Regarding Speech Sound Production Skills During the BBC Active Phase)
For children who had reached 24 months of age, growth of MBL and SSL was measured in terms of the trajectory slope over time. Children were only included if at least two data points were available for each half of the captured age range. One treated child's SSL data were excluded because this metric could only be measured at 20, 21, and 23 months. To compare the untreated control child to the 12 treated children, a z test was performed. Adjusted statistical significance for these two z tests is 0.025.
The fact that, at < 15 months of age, the SLL group had not yet received any speech and language treatment allowed us to compare some available data from these untreated children with CG to data from treated children with CG. To compare MBL scores in the treated CG children (data available for 17 children), children with CG still awaiting treatment (data available for seven children), and TD children (data available for six children), scores were averaged across ages 7–9 months, and between-groups effect sizes were computed using Cohen's d, where the difference between two group means is expressed relative to the pooled standard deviations of the two groups. A Cohen's d of 0.2 indicates a small effect size, 0.5 indicates a medium effect size, and ≥ 0.8 indicates a large effect size (Cohen, 1992). Because SSL scores cannot be measured until meaningful speech emerges, they could not be compared across these treated and untreated groups.
RQ2 (Differences Between Treated and Untreated Children With CG With Respect to Expressive and Receptive Vocabulary Size During the BBC Active Phase)
CDI data from the oldest treated children with CG were compared to those from the untreated control with respect to expressive and receptive vocabularies. To capture maximum potential treatment effects, expressive vocabulary percentiles, converted to z scores, were averaged for the two data points near the end of the intervention, at ages 21 and 24 months. Receptive vocabulary percentiles, converted to z scores, were averaged for the only two available data points at 12 and 15 months. A z test of equality of means was carried out for both measures of vocabulary size. The adjusted alpha for two z tests is .025.
RQ3 (Differences in Articulation Skills Between Treated and Untreated Children With CG at Follow-Up) and RQ4 (Differences in Language Skills Between Treated and Untreated Children With CG at Follow-Up)
Speech and language outcomes at follow-up (GFTA-3, PLS-5 Expressive, and PLS-5 Receptive) were tested against expectations under random conditions, using Fisher's exact test. Expectations were conservatively based on 60% of children with CG being at risk for speech disorders and language disorders (Hughes et al., 2009; Potter et al., 2008; Rubio-Gozalbo et al., 2019; Waggoner et al., 1990). Outcomes were classified as typical or below expectation using −1.5 SD (< 7th percentile) as a cutoff (Bryan et al., 2015; Villagomez et al., 2019). With three measures tested against expectations, adjusted α = .0167.
RQ5 (Associations Between Early Measures of Speech Sound Production and Articulation and Language Skills at Follow-Up)
Correlations between each of two measures of speech sound production (the averaged MBL at ages 7–9 months and the averaged MBL and SSL at 19–24 months) and each of three follow-up assessments (articulation, expressive language, and receptive language) were calculated. Adjustment for multiple testing leads to α = .0083.
Due to the lack of independence among the data points, both in terms of inherent similarities in the variables and longitudinal data from the same children, adjusting for multiple testing is highly conservative. Therefore, nominal statistical significance at .05 can be considered, as well.
Results
MBL
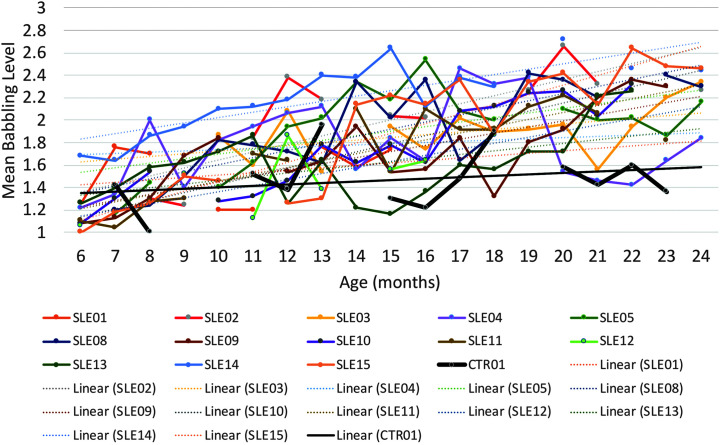
MBL scores up to 24 months of age were available for 12 children with CG who had completed the BBC intervention and close monitoring phase (monthly LENA recordings and quarterly questionnaires) at 24 months of age and one control child with CG who had completed the close monitoring but had not received the intervention. Where MBL data points were missing, the main reason was that the child did not produce enough nonmeaningful utterances to calculate an MBL score. This occurred primarily in the older children with high SSL scores. MBL slopes were distributed with skewness of −.08 and kurtosis of 1.87. All children showed growth in their MBL scores, as indicated by a positive slope of their trendlines. The untreated control child with CG obtained the second lowest slope (0.0126), whereas the mean (and standard deviation) of the treated children's scores were 0.05 (0.02), ranging from 0.0108 to 0.086. The inequality between the slope of the untreated control and the treated children with CG was statistically significant (z = 5.95, one-tailed p < .0001). Figure 4 shows MBL scores as a function of child age in months for the 13 children who completed the BBC at 24 months of age, of whom one was an untreated control. Note the variability from month to month and the overall rising trajectories in the treated CG group.
Figure 4.
Mean babbling level scores for 12 children with classic galactosemia (CG) who received the Babble Boot Camp (BBC) intervention and one child with CG who did not receive the BBC intervention (bolded black line). CTR = untreated control with CG; SLE = speech-language early intervention.
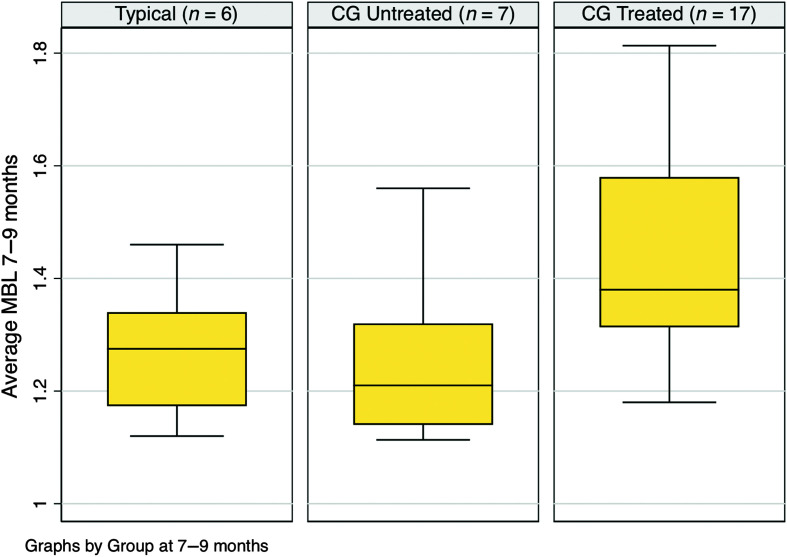
For purposes of comparing MBL scores of the children with CG in the treatment group to typical controls and untreated children with CG, average MBL scores for ages 7–9 months were consulted. Data from the older untreated CG control and six children with CG who had not yet started the intervention were combined and compared to data from six TD children and 17 children with CG in the treated group. This variable was distributed with skewness of .74 and kurtosis of 2.61. Highest MBL scores were seen in the CG treatment group (M = 1.46, SD = 0.20), followed by the TD (M = 1.27, SD = 0.12) and untreated CG (M = 1.25, SD = 0.16) groups. The effect size (Cohen's d) between the CG treatment and TD groups was 0.99, and between the CG treated and untreated groups, it was 1.08. Figure 5 shows per-group box plots.
Figure 5.
Averaged mean babbling level (MBL) scores across ages 7–9 months in six typically developing children, 17 children with classic galactosemia (CG) who underwent the Babble Boot Camp (BBC) intervention, and five children with CG who did not undergo the BBC intervention during this age span.
SSL
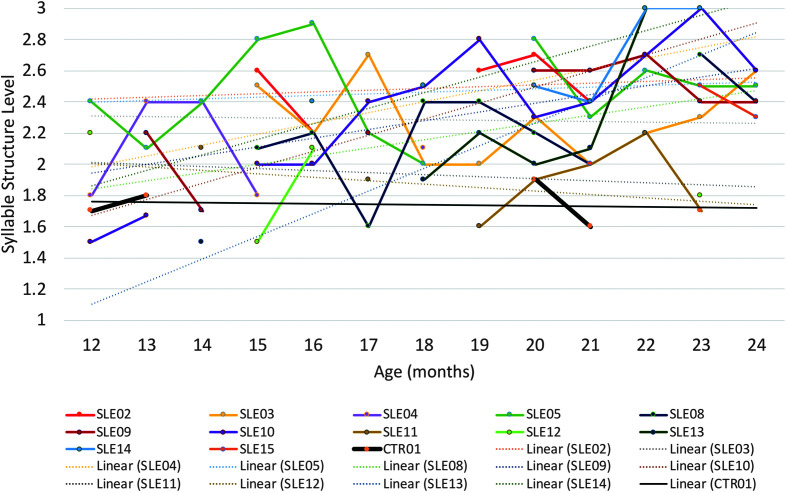
SSL scores for ages 12–24 months were available for 11 children with CG who were receiving the BBC speech and language intervention and one untreated child with CG. When SSL scores were not available, the main reason was that the child was not producing enough meaningful speech during the selected transcription segments to calculate an SSL score. This occurred primarily in younger children in the treatment group and multiple times during ages 12–24 months in the untreated control. This variable was distributed with skewness of .54 and kurtosis of 2.16. The treated children had higher growth slopes (M = 0.05, SD = 0.05, range: −0.2 to 0.15) than the untreated control (0), z = 3.32, one-tailed p = .0005. Figure 6 shows SSL scores as a function of child age in months.
Figure 6.
Syllable structure level scores for 12 children with classic galactosemia (CG) who received the Babble Boot Camp (BBC) intervention and one child (CTR01) with CG who did not receive the BBC intervention. CTR = untreated control with CG; SLE = speech-language early intervention.
Expressive and Receptive Vocabulary Size
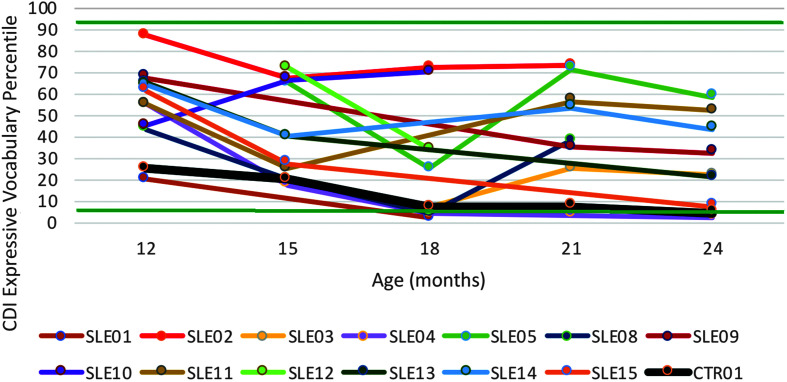
Expressive vocabulary sizes, measured with CDI (Fenson et al., 2007) scores, fell below −1.5 SD (seventh percentile) for one treated child with CG at ages 18, 21, and 24 months and the untreated control with CG at 24 months of age. All other scores were above this level. Based on the average scores for 21 and 24 months of age, the untreated control with CG ranked 10th of 11 in the expressive vocabulary size. A z test based on z scores derived from the percentiles, averaged for ages 21 and 24 months (distributed with skewness = −0.25 and kurtosis = 1.92), was significant (z = 4.40, p < .0001).
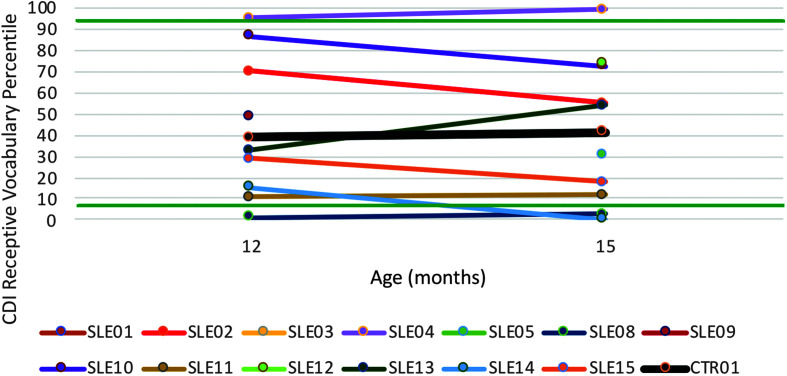
Receptive vocabulary scores fell below −1.5 SD (seventh percentile) for one treated child with CG at both 12 and 15 months and another treated child at 15 months. The untreated control child with CG obtained percentile rankings well above this cutoff at both time points, with a ranking as seventh of 13 based on the averaged receptive vocabulary scores for 12 and 15 months. A z test based on z scores derived from the percentiles, averaged for ages 12 and 15 months (distributed with skewness = −.31 and kurtosis = 2.61), was not significant (z = 0.19, p = .5736). Figures 7 and 8 show percentile rankings for the participants as a function of age for expressive and receptive vocabulary sizes, respectively.
Figure 7.
Expressive vocabulary size based on MacArthur–Bates Communicative Development Inventories–Second Edition (CDI) percentiles in 13 children with classic galactosemia (CG) who underwent the Babble Boot Camp (BBC) intervention and one child with CG who did not. Horizontal lines at the seventh and 93rd percentiles = ±1.5 SDs. CTR = untreated control with CG; SLE = speech-language early intervention.
Figure 8.
Receptive vocabulary size based on MacArthur–Bates Communicative Development Inventories–Second Edition (CDI) percentiles in 13 children with classic galactosemia (CG) who underwent the Babble Boot Camp (BBC) intervention and one child with CG who did not. Horizontal lines at the seventh and 93rd percentiles = ±1.5 SDs. CTR = untreated control with CG; SLE = speech-language early intervention.
Follow-Up Testing
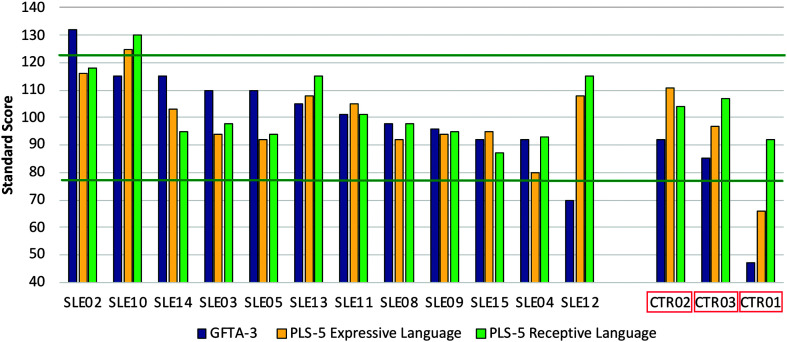
Standardized test scores at follow-up age 2.5 years (3.5 years of age in two cases, SLE05 and CTR01) were available for the oldest 12 children with CG who underwent the BBC intervention and three control children with CG who did not undergo the intervention. Of the treated children with CG, 11 had articulation scores of > −1.5 SD (GFTA-3 standard score > 78) and one obtained a lower score (GFTA-3 standard score = 70). Conservatively estimating that speech disorders are seen in 60% of children with CG, finding one of 12 children with disordered articulation and no children with disordered language is above expectation under random conditions (Fisher's exact p = .014). Note that one child had an articulation standard score that exceeded +1.5 SDs. Two untreated controls with CG had articulation scores of > −1.5 SD, but one untreated control (CTR01) had articulation standard score of 47, which placed him below the first percentile.
All of the treated children with CG had expressive and receptive language scores of > −1.5 SD. Under the conservative expectation that 60% of children with CG have language disorders, finding no children with disordered language is above expectation under random conditions (Fisher's exact p = .002). One of the treated children had a receptive language score that exceeded +1.5 SDs. Two untreated controls with CG had expressive and receptive language scores of > −1.5 SD. One untreated control, the same one whose articulation score fell below the first percentile, had a receptive language score of > −1.5 SD, but his expressive language corresponded to the first percentile.
For nine of the 12 treated children, receptive language scores were higher than expressive language scores, and the same was true for two of the three untreated controls. Figure 9 is a bar graph of the obtained standard scores for the treated and untreated children with CG, sorted by GFTA-3 scores.
Figure 9.
Follow-up test scores for articulation, expressive language, and receptive language for 12 children with classic galactosemia (CG) who completed the early speech and language intervention (SLE) and three untreated children with CG (CTR), ordered by Goldman-Fristoe Test of Articulation–Third Edition (GFTA-3) standard score. Horizontal lines at standard scores 78 and 123 = ±1.5 SDs. PLS-5 = Preschool Language Scales–Fifth Edition.
For purposes of calculating correlations among metrics of early speech sound production and follow-up speech and language testing, two metrics of speech sound production during the intervention period were calculated. The earliest measure was an averaged MBL score for ages 7–9 months. To capture general speech sound complexity in babble and meaningful speech, MBL and SSL scores were averaged, and to further capture maximum possible treatment effects near the end of the intervention period, these combined scores were averaged for ages 19–24 months. The earliest MBL measure was not statistically significantly correlated with any of the three follow-up measures of articulation and language. The combined MBL/SSL scores at ages 19–24 months were correlated with the follow-up measures of articulation (GFTA-3; r = .79, p = .0013) and expressive language (PLS-5; r = .58, p = .0354), but not with receptive language (PLS-5; r = .19, p = .5413).
Discussion
This study follows an initial study of the effects of a preventive intervention (BBC) for children with CG who are at predictable risk for speech and language disorders (Peter et al., 2019). In this expanded sample of participants, continued suggestive evidence of beneficial treatment effects is presented. For the first time, we report on outcomes at follow-up testing.
RQ1 asked whether children with CG achieve higher speech sound production skills, as measured with MBL and SSL, compared to untreated controls. This question was answered positively in two ways. First, the overall growth trajectories for MBL and SSL were greater for 12 (11 for SSL) treated children with CG, compared to those in the untreated control who provided data throughout the study. The trajectories show considerable variability from month to month, but overall growth in the treated children with CG (see Figure 4). Second, for ages 7–9 months, where data were available for a total of 17 treated children with CG and, additionally, six untreated children with CG who were awaiting treatment at 15 months of age and seven children with TD, MBL scores in the group of treated children with CG were greater than those in the other two groups. This is consistent with treatment effects on babble complexity that provided a boost to the treated children, compared to even the children with TD.
RQ2 asked if children with CG in the treatment group achieved higher expressive and receptive vocabulary sizes during the intervention, compared to the untreated control. Consistent with the hypothesis that the BBC intervention is beneficial for the expressive language skills of children with CG because CG affects expressive language to a greater degree than receptive language (Potter et al., 2008), the untreated control obtained the second lowest ranking of the expressive percentile scores, scoring significantly lower than the treated group on average. Again, consistent with the observation that receptive language is affected to a lesser degree than expressive language, the untreated control child with CG ranked near the middle of the receptive vocabulary percentiles, and his scores did not differ from those in the treated group. Note that at 12 months of age, all children had expressive vocabulary percentiles above 7, but some children obtained scores below that cutoff later on. This reflects the fact that at 12 months of age, having no or few words is not uncommon; the CDI shows that at the 50th percentile, girls have seven words and boys have two words. To keep up with typical development, children need to add many words quickly; the CDI shows 50th percentile averages of 91 words for girls and 81 words for boys at 18 months of age and 346 words for girls and 252 for boys at 24 months of age. By 24 months of age, the untreated control and one treated child with CG, both boys, had fallen below the 7th percentile.
RQ3 asked whether children with CG in the treatment group had higher articulation scores at follow-up than what would be expected based on the literature of children receiving conventional treatment. Only one of the 12 treated children obtained an articulation score below −1.5 SD, fewer than would be expected under random conditions. One of three untreated controls with CG had, by far, the lowest articulation score. These findings are consistent with a beneficial effect of the BBC on articulation that were sustained at follow-up.
RQ4 asked whether the treated children with CG had higher language abilities at follow-up, compared to untreated children with CG as reported in the literature. Children with CG who are receiving conventional treatment typically have difficulty with expressive language; only in the presence of cognitive delays, both expressive and receptive language would be expected to be disordered (Potter et al., 2008). All children in the treated group with CG had typical expressive language scores, which is above expectation given the published rates of expressive language disorders in children with CG. One of three untreated controls had a very low expressive language score. Together, these findings are consistent with a beneficial effect of the BBC on expressive language.
Receptive language scores at follow-up were above −1.5 SD for all children with CG in this study, whether they had received treatment or not. Prior to 24 months of age, the single untreated control for whom data were available for that age range had typical receptive vocabulary skills, whereas two of 13 treated children had low receptive language skills. These two observations are consistent with the previously published findings that CG affects receptive language skills to a lesser degree than expressive language skills (Potter et al., 2008).
RQ5 addressed possible associations among early measures of speech production during nonmeaningful speech and later measures of articulation and language. The most significant correlation was found between the combined MBL/SSL measure of speech sound complexity at ages 19–24 months and the follow-up measure of articulation. A nominally significant correlation was found for this same measure and expressive language at follow-up. The observation that the average MBL score for ages 7–9 months was clearly elevated in the treatment group, compared to the untreated controls with CG and the typical controls, suggests a possible treatment effect at that age. However, this measure was not correlated with any of the follow-up measures of articulation and language, a first indication that perhaps the earliest treatment effects are not causally related to the outcomes. Speech sound complexity near the end of the intervention, as measured with averaged MBL and SSL scores, was correlated with articulation scores at follow-up, suggesting that these metrics address the same construct and that MBL and SSL scores near the end of the intervention predict articulation skills later on.
Conclusions
Overall, the findings from this report are consistent with a beneficial effect of the BBC intervention on early speech sound production skills and early expressive vocabulary skills during the intervention and a sustained benefit on articulation and expressive language skills at follow-up. This is consistent with the intended change of parent behaviors that mediated changes in the children, although parent behaviors were not explicitly included in this study. In future studies, we will report direct measures of changes in parent behaviors.
Not all children with CG develop speech and language disorders. It is possible that a proportion of the treated children with CG would not have developed these disorders but that the intervention gave them a boost in these areas. This may explain why two treated children with CG obtained some speech and language scores above +1.5 SDs at follow-up. Similarly, the MBL scores at ages 7–9 months were higher in the treatment group than the untreated and even the typical children. This may represent a boost in speech sound complexity skills at that age.
Limitations and Future Studies
The small sample size and some missing data are clear limitations of this study. Nonetheless, statistically significant results were found, even with conservative adjustments for multiple testing. Because the project is ongoing, new participants are added continually, and the enrolled participants generate new data on a monthly basis. Future publications will report on increasingly larger data sets.
As mentioned, high babble complexity levels predict high speech and language skills later on in typical children (American Speech-Language-Hearing Association, 2007; DePaolis et al., 2013; McCune & Vihman, 2001; Oller et al., 1998, 1999; Stoel-Gammon, 1989, 2011; Vihman & Greenlee, 1987; Vihman et al., 1985). Here, we show evidence that the intervention focus on earliest speech production during babble was associated with higher complexity scores in the treated children with CG, compared to untreated children with and without CG. We also show that the treated children with CG obtained average expressive language skills at follow-up, an unexpected result under random conditions. Whether the observed growth in language skills is influenced by the focus on prespeech skills or driven mainly by the direct focus on language skills later on will be investigated in future studies based on more extensive data, comparing the results in children who started the intervention prior to 6 months of age to those in children who started at 15 months of age. Future studies will also address many additional aspects of the BBC intervention, for instance, child health and development across many domains, parent and child quality of life as a function of the children's progress in communication skills, treatment effects on cognitive development, and the role of CG genotype and gender in treatment response.
Acknowledgments
This study was supported by National Institute of Child Health and Human Development Grant 5R01HD098253 to B. Peter, N. Potter, and M. VanDam; Arizona State University Institute for Social Science Research Seed Grant to B. Peter; Arizona State University New Faculty Startup Fund to B. Peter; NSF-SBE RIDIR-1539133 to M. VanDam; and the Washington Research Foundation to M. VanDam.
Appendix
The TIDieR (Template for Intervention Description and Replication) Checklist*
*We strongly recommend using this checklist in conjunction with the TIDieR guide (see BMJ 2014;348:g1687), which contains an explanation and elaboration for each item.
The focus of TIDieR is on reporting details of the intervention elements (and where relevant, comparison elements) of a study. Other elements and methodological features of studies are covered by other reporting statements and checklists and have not been duplicated as part of the TIDieR checklist. When a randomized trial is being reported, the TIDieR checklist should be used in conjunction with the CONSORT statement (see www.consort-statement.org) as an extension of Item 5 of the CONSORT 2010 Statement. When a clinical trial protocol is being reported, the TIDieR checklist should be used in conjunction with the SPIRIT statement as an extension of Item 11 of the SPIRIT 2013 Statement (see www.spirit-statement.org). For alternate study designs, TIDieR can be used in conjunction with the appropriate checklist for that study design (see www.equator-network.org).
**Authors – use N/A if an item is not applicable for the intervention being described. Reviewers – use “?” if information about the element is not reported/not sufficiently reported.
†If the information is not provided in the primary article, give details of where this information is available. This may include locations such as a published protocol or other published articles (provide citation details) or a website (provide the URL).
ǂIf completing the TIDieR checklist for a protocol, these items are not relevant to the protocol and cannot be described until the study is complete.
Funding Statement
This study was supported by National Institute of Child Health and Human Development Grant 5R01HD098253 to B. Peter, N. Potter, and M. VanDam; Arizona State University Institute for Social Science Research Seed Grant to B. Peter; Arizona State University New Faculty Startup Fund to B. Peter; NSF-SBE RIDIR-1539133 to M. VanDam; and the Washington Research Foundation to M. VanDam.
References
- Allard, E. R. , & Williams, D. F. (2008). Listeners' perceptions of speech and language disorders. Journal of Communication Disorders, 41(2), 108–123. https://doi.org/10.1016/j.jcomdis.2007.05.002 [DOI] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association. (2007). Childhood apraxia of speech [Position statement] . http://www.asha.org/policy
- Antshel, K. M. , Epstein, I. O. , & Waisbren, S. E. (2004). Cognitive strengths and weaknesses in children and adolescents homozygous for the galactosemia Q188R mutation: A descriptive study. Neuropsychology, 18(4), 658–664. https://doi.org/10.1037/0894-4105.18.4.658 [DOI] [PubMed] [Google Scholar]
- Baker, E. , & McLeod, S. (2011). Evidence-based practice for children with speech sound disorders: Part 1. Narrative review. Language, Speech, and Hearing Services in Schools, 42(2), 102–139. https://doi.org/10.1044/0161-1461(2010/09-0075) [DOI] [PubMed] [Google Scholar]
- Berry, G. T. (2011). Erratum to: Is prenatal myo-inositol deficiency a mechanism of CNS injury in galactosemia? Journal of Inherited Metabolic Disease, 34(2), 555–555. https://doi.org/10.1007/s10545-011-9310-z [DOI] [PubMed] [Google Scholar]
- Bryan, K. , Garvani, G. , Gregory, J. , & Kilner, K. (2015). Language difficulties and criminal justice: The need for earlier identification. International Journal of Language & Communication Disorders, 50(6), 763–775. https://doi.org/10.1111/1460-6984.12183 [DOI] [PubMed] [Google Scholar]
- Campbell, T. F. (1999). Functional treatment outcomes in young children with motor speech disorders. In Caruso A. J. & Strand E. A. (Eds.), Clinical management of motor speech disorders in children (pp. 385–396). Thieme. [Google Scholar]
- Campbell, T. F. , Dollaghan, C. A. , Rockette, H. E. , Paradise, J. L. , Feldman, H. M. , Shriberg, L. D. , Sabo, D. L. , & Kurs-Lasky, M. (2003). Risk factors for speech delay of unknown origin in 3-year-old children. Child Development, 74(2), 346–357. https://doi.org/10.1111/1467-8624.7402002 [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. https://doi.org/10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Coss, K. P. , Doran, P. P. , Owoeye, C. , Codd, M. B. , Hamid, N. , Mayne, P. D. , Crushell, E. , Knerr, I. , Monavari, A. A. , & Treacy, E. P. (2013). Classical galactosaemia in Ireland: Incidence, complications and outcomes of treatment. Journal of Inherited Metabolic Disease, 36(1), 21–27. https://doi.org/10.1007/s10545-012-9507-9 [DOI] [PubMed] [Google Scholar]
- Cronin, P. , Reeve, R. , McCabe, P. , Viney, R. , & Goodall, S. (2017). The impact of childhood language difficulties on healthcare costs from 4 to 13 years: Australian longitudinal study. International Journal of Speech-Language Pathology, 19(4), 381–391. https://doi.org/10.1080/17549507.2016.1216599 [DOI] [PubMed] [Google Scholar]
- Culton, G. L. (1986). Speech disorders among college freshmen. Journal of Speech and Hearing Disorders, 51(1), 3–7. https://doi.org/10.1044/jshd.5101.03 [DOI] [PubMed] [Google Scholar]
- Dawson, G. , Rogers, S. , Munson, J. , Smith, M. , Winter, J. , Greenson, J. , Donaldson, A. , & Varley, J. (2010). Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics, 125(1), e17–e23. https://doi.org/10.1542/peds.2009-0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirbas, D. , Coelho, A. I. , Rubio-Gozalbo, M. E. , & Berry, G. T. (2018). Hereditary galactosemia. Metabolism: Clinical and Experimental, 83, 188–196. https://doi.org/10.1016/j.metabol.2018.01.025 [DOI] [PubMed] [Google Scholar]
- DePaolis, R. A. , Vihman, M. M. , & Nakai, S. (2013). The influence of babbling patterns on the processing of speech. Infant Behavior & Development, 36(4), 642–649. https://doi.org/10.1016/j.infbeh.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Fedorenko, E. , Morgan, A. , Murray, E. , Cardinaux, A. , Mei, C. , Tager-Flusberg, H. , Fisher, S. E. , & Kanwisher, N. (2016). A highly penetrant form of childhood apraxia of speech due to deletion of 16p11.2. European Journal of Human Genetics, 24(2), 302–306. https://doi.org/10.1038/ejhg.2015.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson, L. , Marchman, V. A. , Thal, D. J. , Dale, P. S. , Reznick, J. S. , & Bates, E. (2007). MacArthur–Bates Communicative Development Inventories–Second Edition: User's guide and technical manual. Brookes. [Google Scholar]
- Ferjan Ramirez, N. , Lytle, S. R. , & Kuhl, P. K. (2020). Parent coaching increases conversational turns and advances infant language development. Proceedings of the National Academy of Sciences of the United States of America, 117(7), 3484–3491. https://doi.org/10.1073/pnas.1921653117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil, J. L. (2008). Galactosemia. In Valle D., Beaudet A. L., Vogelstein B., Kinzler K., Antonarakis S. E., & Ballabio A. (Eds.), The online metabolic & molecular bases of inherited disease. McGraw Hill. https://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225081023 [Google Scholar]
- Goetz, L. H. , & Schork, N. J. (2018). Personalized medicine: Motivation, challenges, and progress. Fertility and Sterility, 109(6), 952–963. https://doi.org/10.1016/j.fertnstert.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, R. , & Fristoe, M. (2015). Goldman-Fristoe Test of Articulation–Third Edition. Pearson. [Google Scholar]
- Guerra, J. , & Cacabelos, R. (2019). Genomics of speech and language disorders. Journal of Translational Genetics and Genomics, 3(9). https://doi.org/10.20517/jtgg.2018.03 [Google Scholar]
- Hall, B. J. C. (1991). Attitudes of fourth and sixth graders toward peers with mild articulation disorders. Language, Speech, and Hearing Services in Schools, 22(1), 334–340. https://doi.org/10.1044/0161-1461.2201.334 [Google Scholar]
- Hildebrand, M. S. , Jackson, V. E. , Scerri, T. S. , Van Reyk, O. , Coleman, M. , Braden, R. O. , Turner, S. , Rigbye, K. A. , Boys, A. , Barton, S. , Webster, R. , Fahey, M. , Saunders, K. , Parry-Fielder, B. , Paxton, G. , Hayman, M. , Coman, D. , Goel, H. , Baxter, A. , … Morgan, A. T. (2020). Severe childhood speech disorder: Gene discovery highlights transcriptional dysregulation. Neurology, 94(20), e2148–e2167. https://doi.org/10.1212/WNL.0000000000009441 [DOI] [PubMed] [Google Scholar]
- Hillman, L. S. , Day, L. S. , Hoffman, H. J. , & Stockbauer, J. W. (2019). Poorer outcomes of all low birth weight groups at age 10: Missouri statewide case-control study. Early Human Development, 136, 60–69. https://doi.org/10.1016/j.earlhumdev.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Hitchcock, E. R. , Harel, D. , & Byun, T. M. (2015). Social, emotional, and academic impact of residual speech errors in school-aged children: A survey study. Seminars in Speech and Language, 36(4), 283–294. https://doi.org/10.1055/s-0035-1562911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, L. , Hersey, A. , Tucker, R. , & Vohr, B. (2020). Randomised control language intervention for infants of adolescent mothers. Acta Paediatrica, 109(12), 2604–2613. https://doi.org/10.1111/apa.15261 [DOI] [PubMed] [Google Scholar]
- Hughes, J. , Ryan, S. , Lambert, D. , Geoghegan, O. , Clark, A. , Rogers, Y. , Hendroff, U. , Monavari, A. , Twomey, E. , & Treacy, E. P. (2009). Outcomes of siblings with classical galactosemia. Journal of Pediatrics, 154(5), 721–726. https://doi.org/10.1016/j.jpeds.2008.11.052 [DOI] [PubMed] [Google Scholar]
- Karadag, N. , Zenciroglu, A. , Eminoglu, F. T. , Dilli, D. , Karagol, B. S. , Kundak, A. , Dursun, A. , Hakan, N. , & Okumus, N. (2013). Literature review and outcome of classic galactosemia diagnosed in the neonatal period. Clinical Laboratory, 59(9–10), 1139–1146. https://doi.org/10.7754/Clin.Lab.2013.121235 [DOI] [PubMed] [Google Scholar]
- Lai, C. S. , Fisher, S. E. , Hurst, J. A. , Vargha-Khadem, F. , & Monaco, A. P. (2001). A forkhead-domain gene is mutated in a severe speech and language disorder. Nature, 413(6855), 519–523. https://doi.org/10.1038/35097076 [DOI] [PubMed] [Google Scholar]
- Lai, C. S. , Gerrelli, D. , Monaco, A. P. , Fisher, S. E. , & Copp, A. J. (2003). FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain, 126(Pt. 11), 2455–2462. https://doi.org/10.1093/brain/awg247 [DOI] [PubMed] [Google Scholar]
- Landry, S. H. , Smith, K. E. , & Swank, P. R. (2006). Responsive parenting: Establishing early foundations for social, communication, and independent problem-solving skills. Developmental Psychology, 42(4), 627–642. https://doi.org/10.1037/0012-1649.42.4.627 [DOI] [PubMed] [Google Scholar]
- Landry, S. H. , Smith, K. E. , Swank, P. R. , & Guttentag, C. (2008). A responsive parenting intervention: The optimal timing across early childhood for impacting maternal behaviors and child outcomes. Developmental Psychology, 44(5), 1335–1353. https://doi.org/10.1037/a0013030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry, S. H. , Smith, K. E. , Swank, P. R. , Zucker, T. , Crawford, A. D. , & Solari, E. F. (2012). The effects of a responsive parenting intervention on parent–child interactions during shared book reading. Developmental Psychology, 48(4), 969–986. https://doi.org/10.1037/a0026400 [DOI] [PubMed] [Google Scholar]
- Law, J. , Boyle, J. , Harris, F. , Harkness, A. , & Nye, C. (2000). Prevalence and natural history of primary speech and language delay: Findings from a systematic review of the literature. International Journal of Language & Communication Disorders, 35(2), 165–188. https://doi.org/10.1080/136828200247133 [DOI] [PubMed] [Google Scholar]
- Lewis, F. M. , Coman, D. J. , Syrmis, M. , Kilcoyne, S. , & Murdoch, B. E. (2013a). Charting a seven-year trajectory of language outcomes for a child with galactosemia. Journal of Developmental and Behavioral Pediatrics, 34(6), 414–418. https://doi.org/10.1097/DBP.0b013e31829a7be1 [DOI] [PubMed] [Google Scholar]
- Lewis, F. M. , Coman, D. J. , Syrmis, M. , Kilcoyne, S. , & Murdoch, B. E. (2013b). Differential phonological awareness skills in children with classic galactosemia: A descriptive study of four cases. Journal of Inherited Metabolic Disease Reports, 10, 45–52. https://doi.org/10.1007/8904_2012_200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, G. , Dockrell, J. E. , & Mackie, C. (2008). Vulnerability to bullying in children with a history of specific speech and language difficulties. European Journal of Special Needs Education, 23(1), 1–16. https://doi.org/10.1080/08856250701791203 [Google Scholar]
- Lousada, M. , Jesus, L. M. T. , Hall, A. , & Joffe, V. (2014). Intelligibility as a clinical outcome measure following intervention with children with phonologically based speech-sound disorders. International Journal of Language & Communication Disorders, 49(5), 584–601. https://doi.org/10.1111/1460-6984.12095 [DOI] [PubMed] [Google Scholar]
- McCormack, J. , McLeod, S. , McAllister, L. , & Harrison, L. J. (2009). A systematic review of the association between childhood speech impairment and participation across the lifespan. International Journal of Speech-Language Pathology, 11(2), 155–170. https://doi.org/10.1080/17549500802676859 [Google Scholar]
- McCune, L. , & Vihman, M. M. (2001). Early phonetic and lexical Development. Journal of Speech, Language, and Hearing Research, 44(3), 670–684. https://doi.org/10.1044/1092-4388(2001/054) [DOI] [PubMed] [Google Scholar]
- Mitchell, P. , McMahon, B. , & McKee, D. (2005). Speech impairment and workplace discrimination: The national EEOC ADA research project. Journal of Vocational Rehabilitation, 23(3), 163–169. [Google Scholar]
- Morris, S. R. (2010). Clinical application of the mean babbling level and syllable structure level. Language, Speech, and Hearing Services in Schools, 41(2), 223–230. https://doi.org/10.1044/0161-1461(2009/08-0076) [DOI] [PubMed] [Google Scholar]
- Nelson, C. D. , Waggoner, D. D. , Donnell, G. N. , Tuerck, J. M. , & Buist, N. R. (1991). Verbal dyspraxia in treated galactosemia. Pediatrics, 88(2), 346–350. https://www.ncbi.nlm.nih.gov/pubmed/1861938 [PubMed] [Google Scholar]
- Nelson, D. (1995). Verbal dyspraxia in children with galactosemia. European Journal of Pediatrics, 154(7, Suppl. 2), S6–S7. https://doi.org/10.1007/BF02143795 [DOI] [PubMed] [Google Scholar]
- Oller, D. K. , Eilers, R. E. , Neal, A. R. , & Cobo-Lewis, A. B. (1998). Late onset canonical babbling: A possible early marker of abnormal development. American Journal of Mental Retardation, 103(3), 249–263. https://doi.org/10.1352/0895-8017(1998)103<0249:LOCBAP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Oller, D. K. , Eilers, R. E. , Neal, A. R. , & Schwartz, H. K. (1999). Precursors to speech in infancy. Journal of Communication Disorders, 32(4), 223–245. https://doi.org/10.1016/S0021-9924(99)00013-1 [DOI] [PubMed] [Google Scholar]
- Paul, R. , & Jennings, P. (1992). Phonological behavior in toddlers with slow expressive language development. Journal of Speech and Hearing Research, 35(1), 99–107. https://doi.org/10.1044/jshr.3501.99 [DOI] [PubMed] [Google Scholar]
- Peter, B. , Lancaster, H. , Vose, C. , Fares, A. , Schrauwen, I. , & Huentelman, M. (2017). Two unrelated children with overlapping 6q25.3 deletions, motor speech disorders, and language delays. American Journal of Medical Genetics Part A, 173(10), 2659–2669. https://doi.org/10.1002/ajmg.a.38385 [DOI] [PubMed] [Google Scholar]
- Peter, B. , Matsushita, M. , Oda, K. , & Raskind, W. (2014). De novo microdeletion of BCL11A is associated with severe speech sound disorder. American Journal of Medical Genetics Part A, 164(8), 2091–2096. https://doi.org/10.1002/ajmg.a.36599 [DOI] [PubMed] [Google Scholar]
- Peter, B. , Potter, N. , Davis, J. , Donenfeld-Peled, I. , Finestack, L. , Stoel-Gammon, C. , Lien, K. , Bruce, L. , Vose, C. , Eng, L. , Yokoyama, H. , Olds, D. , & VanDam, M. (2019). Toward a paradigm shift from deficit-based to proactive speech and language treatment: Randomized pilot trial of the Babble Boot Camp in infants with classic galactosemia. F1000Research, 8, 271. https://doi.org/10.12688/f1000research.18062.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, R. L. , Pennington, B. F. , Shriberg, L. D. , & Boada, R. (2009). What influences literacy outcome in children with speech sound disorder? Journal of Speech, Language, and Hearing Research, 52(5), 1175–1188. https://doi.org/10.1044/1092-4388(2009/08-0024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, N. L. , Lazarus, J. A. C. , Johnson, J. M. , Steiner, R. D. , & Shriberg, L. D. (2008). Correlates of language impairment in children with galactosaemia. Journal of Inherited Metabolic Disease, 31(4), 524–532. https://doi.org/10.1007/s10545-008-0877-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, N. L. , Nievergelt, Y. , & Shriberg, L. D. (2013). Motor and speech disorders in classic galactosemia. Journal of Inherited Metabolic Disease Reports, 11, 31–41. https://doi.org/10.1007/8904_2013_219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, M. Y. , Kaiser, A. P. , Wolfe, C. E. , Bryant, J. D. , & Spidalieri, A. M. (2014). Effects of the teach-model-coach-review instructional approach on caregiver use of language support strategies and children's expressive language skills. Journal of Speech, Language, and Hearing Research, 57(5), 1851–1869. https://doi.org/10.1044/2014_JSLHR-L-13-0113 [DOI] [PubMed] [Google Scholar]
- Rogers, S. J. , Vismara, L. , Wagner, A. L. , McCormick, C. , Young, G. , & Ozonoff, S. (2014). Autism treatment in the first year of life: A pilot study of infant start, a parent-implemented intervention for symptomatic infants. Journal of Autism and Developmental Disorders, 44(12), 2981–2995. https://doi.org/10.1007/s10803-014-2202-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Gozalbo, M. E. , Haskovic, M. , Bosch, A. M. , Burnyte, B. , Coelho, A. I. , Cassiman, D. , Couce, M. L. , Dawson, C. , Demirbas, D. , Derks, T. , Eyskens, F. , Forga, M. T. , Grunewald, S. , Haberle, J. , Hochuli, M. , Hubert, A. , Huidekoper, H. H. , Janeiro, P. , Kotzka, J. , … Berry, G. T. (2019). The natural history of classic galactosemia: Lessons from the GalNet registry. Orphanet Journal of Rare Diseases, 14(1), 86. https://doi.org/10.1186/s13023-019-1047-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, E. L. , Lynch, M. E. , Taddeo, E. , Gleason, T. J. , Epstein, M. P. , & Fridovich-Keil, J. L. (2013). Cryptic residual GALT activity is a potential modifier of scholastic outcome in school age children with classic galactosemia. Journal of Inherited Metabolic Disease, 36(6), 1049–1061. https://doi.org/10.1007/s10545-012-9575-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, N. J. , D'Antonio, L. L. , & Kalbfleisch, J. H. (1999). Early speech and language development in children with velocardiofacial syndrome. American Journal of Medical Genetics, 88(6), 714–723. https://doi.org/10.1002/(SICI)1096-8628(19991215)88:6<714::AID-AJMG24>3.0.CO;2-B [PubMed] [Google Scholar]
- Semel, E. , Wiig, E. , & Secord, W. (2004). Clinical Evaluation of Language Fundamentals Preschool–Second Edition. Pearson. [Google Scholar]
- Shriberg, L. D. , Aram, D. M. , & Kwiatkowski, J. (1997). Developmental apraxia of speech. Journal of Speech, Language, and Hearing Research, 40(2), 273–285. https://doi.org/10.1044/jslhr.4002.273 [DOI] [PubMed] [Google Scholar]
- Shriberg, L. D. , Potter, N. L. , & Strand, E. A. (2011). Prevalence and phenotype of childhood apraxia of speech in youth with galactosemia. Journal of Speech, Language, and Hearing Research, 54(2), 487–519. https://doi.org/10.1044/1092-4388(2010/10-0068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg, L. D. , Tomblin, J. B. , & McSweeny, J. L. (1999). Prevalence of speech delay in 6-year-old children and comorbidity with language impairment. Journal of Speech, Language, and Hearing Research, 42(6), 1461–1481. https://doi.org/10.1044/jslhr.4206.1461 [DOI] [PubMed] [Google Scholar]
- Squires, J. , & Bricker, D. (2009). Ages & Stages Questionnaires–Third Edition. Brookes. [Google Scholar]
- Stoel-Gammon, C. (1989). Prespeech and early speech development of two late talkers. First Language, 9(6), 207–223. https://doi.org/10.1177/014272378900900607 [Google Scholar]
- Stoel-Gammon, C. (2011). Relationships between lexical and phonological development in young children. Journal of Child Language, 38(1), 1–34. https://doi.org/10.1017/S0305000910000425 [DOI] [PubMed] [Google Scholar]
- Teulier, C. , Lee, D. K. , & Ulrich, B. D. (2015). Early gait development in human infants: Plasticity and clinical applications. Developmental Psychobiology, 57(4), 447–458. https://doi.org/10.1002/dev.21291 [DOI] [PubMed] [Google Scholar]
- Timmers, I. , Jansma, B. M. , & Rubio-Gozalbo, M. E. (2012). From mind to mouth: Event related potentials of sentence production in classic galactosemia. PLOS ONE, 7(12), e52826. https://doi.org/10.1371/journal.pone.0052826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers, I. , van den Hurk, J. , Di Salle, F. , Rubio-Gozalbo, M. E. , & Jansma, B. M. (2011). Language production and working memory in classic galactosemia from a cognitive neuroscience Perspective: Future research directions. Journal of Inherited Metabolic Disease, 34(2), 367–376. https://doi.org/10.1007/s10545-010-9266-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin, J. B. , Records, N. L. , Buckwalter, P. , Zhang, X. , Smith, E. , & O'Brien, M. (1997). Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research, 40(6), 1245–1260. https://doi.org/10.1044/jslhr.4006.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDam, M. (2021). VanDam Public 5-Minute Corpus. https://homebank.talkbank.org/access/Public/VanDam-5minute.html
- VanDam, M. , Warlaumont, A. S. , Bergelson, E. , Cristia, A. , Soderstrom, M. , De Palma, P. , & MacWhinney, B. (2016). HomeBank: An online repository of daylong child-centered audio recordings. Seminars in Speech and Language, 37(2), 128–142. https://doi.org/10.1055/s-0036-1580745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieland, V. J. , Merette, C. , Goodman, D. , & Rouillard, E. (1995). Identification and mapping of Mendelian subtypes of disease. Genetic Epidemiology, 12(6), 819–824. https://doi.org/10.1002/gepi.1370120648 [DOI] [PubMed] [Google Scholar]
- Vihman, M. M. , & Greenlee, M. (1987). Individual differences in phonological development. Journal of Speech and Hearing Research, 30(4), 503–521. https://doi.org/10.1044/jshr.3004.503 [DOI] [PubMed] [Google Scholar]
- Vihman, M. M. , Macken, M. A. , Miller, R. , Simmons, H. , & Miller, J. (1985). From babbling to speech: A re-assessment of the continuity issue. Language, 61(2), 397–445. https://doi.org/10.2307/414151 [Google Scholar]
- Villagomez, A. N. , Munoz, F. M. , Peterson, R. L. , Colbert, A. M. , Gladstone, M. , MacDonald, B. , Wilson, R. , Fairlie, L. , Gerner, G. J. , Patterson, J. , Boghossian, N. S. , Burton, V. J. , Cortes, M. , Katikaneni, L. D. , Larson, J. C. G. , Angulo, A. S. , Joshi, J. , Nesin, M. , Padula, M. A. , … Brighton Collaboration Neurodevelopmental Delay Working, Group. (2019). Neurodevelopmental delay: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine, 37(52), 7623–7641. https://doi.org/10.1016/j.vaccine.2019.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohr, B. (2014). Speech and language outcomes of very preterm infants. Seminars in Fetal and Neonatal Medicine, 19(2), 78–83. https://doi.org/10.1016/j.siny.2013.10.007 [DOI] [PubMed] [Google Scholar]
- Vygotsky, L. S. (1979). The development of higher forms of attention in childhood. Soviet Psychology, 18(1), 67–115. https://doi.org/10.2753/RPO1061-0405180167 [Google Scholar]
- Waggoner, D. D. , Buist, N. R. , & Donnell, G. N. (1990). Long-term prognosis in galactosaemia: Results of a survey of 350 cases. Journal of Inherited Metabolic Disease, 13(6), 802–818. https://doi.org/10.1007/BF01800204 [DOI] [PubMed] [Google Scholar]
- Waisbren, S. E. , Norman, T. R. , Schnell, R. R. , & Levy, H. L. (1983). Speech and language deficits in early-treated children with galactosemia. Journal of Pediatrics, 102(1), 75–77. https://doi.org/10.1016/S0022-3476(83)80292-3 [DOI] [PubMed] [Google Scholar]
- Waisbren, S. E. , Potter, N. L. , Gordon, C. M. , Green, R. C. , Greenstein, P. , Gubbels, C. S. , Rubio-Gozalbo, E. , Schomer, D. , Welt, C. , Anastasoaie, V. , D'Anna, K. , Gentile, J. , Guo, C. Y. , Hecht, L. , Jackson, R. , Jansma, B. M. , Li, Y. , Lip, V. , Miller, D. T. , … Berry, G. T. (2012). The adult galactosemic phenotype. Journal of Inherited Metabolic Disease, 35(2), 279–286. https://doi.org/10.1007/s10545-011-9372-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, A. L. , Singh, R. H. , Kennedy, M. J. , & Elsas, L. J. (2003). Verbal dyspraxia and galactosemia. Pediatric Research, 53(3), 396–402. https://doi.org/10.1203/01.PDR.0000049666.19532.1B [DOI] [PubMed] [Google Scholar]
- Young, A. R. , Beitchman, J. H. , Johnson, C. , Douglas, L. , Atkinson, L. , Escobar, M. , & Wilson, B. (2002). Young adult academic outcomes in a longitudinal sample of early identified language impaired and control children. The Journal of Child Psychology and Psychiatry, 43(5), 635–645. https://doi.org/10.1111/1469-7610.00052 [DOI] [PubMed] [Google Scholar]
- Zimmerman, I. , Steiner, V. , & Pond, R. (2011). Preschool Language Scales–Fifth Edition. Pearson. [Google Scholar]



 This work is licensed under a
This work is licensed under a