Abstract
Plastic pollution in various forms has emerged as the most severe environmental threat. Small plastic chunks, such as microplastics and nanoplastics derived from primary and secondary sources, are a major concern worldwide due to their adverse effects on the environment and public health. Several years have been spent developing robust spectroscopic techniques that should be considered top-notch; however, researchers are still trying to find efficient and straightforward methods for the analysis of microplastics but have yet to develop a viable solution. Because of the small size of these degraded plastics, they have been found in various species, from human brains to blood and digestive systems. Several pollution-controlling methods have been tested in recent years, and these methods are prominent and need to be developed. Bacterial degradation, sunlight-driven photocatalyst, fuels, and biodegradable plastics could be game-changers in future research on plastic pollution control. However, recent fledgling steps in controlling methods appear insufficient due to widespread contamination. As a result, proper regulation of environmental microplastics is a significant challenge, and the most equitable way to manage plastic pollution. Therefore, this paper discusses the current state of microplastics, some novel and well-known identification techniques, strategies for overcoming microplastic effects, and needed solutions to mitigate this planetary pollution. This review article, we believe, will fill a void in the field of plastic identification and pollution mitigation research.
Keywords: Microplastics, Pollution, Bacterial degradations, Plastic mitigation
Background
In 2019, around 368 million metric tons (MMT) of plastics were manufactured worldwide, with half of them produced in Asia (Tiseo 2021). The prevailing estimate suggests that, by 2050, approximately 12 billion metric tons of plastic waste will be in landfills or could spill over to the natural habitat which will be more than 250% compared to 4.9 billion metric tons (60% of all plastic ever produced) produced in 2015 (Geyer et al. 2017). In 2020, this upward trend was disrupted, with production falling to around 367 MMT, a 0.3 percent decrease due to COVID-19 (Plastics Europe 2022). However, this increasing trend is going sky-high after the COVID due to the overproduction of facial masks. Customers’ preferences for “single-use” packaging, which is more efficient and chemically stable, have hastened and contaminated various ecosystems, harming the environment and posing health risks (Lusher 2015). With more time, rather than biodegrading, plastics are crumbled into smaller and smaller chunks, resulting in micro- to nano-sized fragments (Allan et al. 2021; Hartmann et al. 2019; The Lancet Planetary Health 2017). If we are to comprehend the fate of plastics, we must first consider the mechanism by which plastics enter nature. In particular, littering, dumping of plastic waste, and waste collection are all ways plastics end up in the environment. The mode and amount of plastic dispersion into various environmental components is a critical issue requiring further research (Lambert et al. 2014; Yin et al. 2019). Furthermore, the potential effects of degraded small chunks on the human body and the environment are of global concern (Wagner and Reemtsma 2019). Even nanoplastics derived from a single microplastic particle are expected to be a more complex issue around the world due to their small size, which allows them to pass through biological membranes easily (Hernandez et al. 2017; Ter et al. 2017; Yee et al. 2021). In turn, researchers must devise efficient methods for detecting these particles in fractions of a second to microseconds. Consequently, future research should concentrate on this, and here, we have summarized some techniques that can assist in the identification of MPs; however, these methods do not provide sufficient solutions.
Several studies have been conducted that have demonstrated that MPs have a significant negative impact on public and environmental health. It has been a long-standing issue due to the large number of MPs, and it must be addressed as soon as possible. This review will cover how MPs have evolved, their global challenges, emerging techniques for MPs monitoring, a potential route to environment penetration, impact on human health, and current environmental hazards in a global context. In addition, this review summarizes the most recent rules and regulations enacted concerning plastic issues, international collaborations, and potential alternative solutions adopted by many countries. We hope that this review will inspire researchers working in MPs management and plastics mitigation to come up with some innovative ideas.
Plastics type, origin, and sources
With the massive development of plastic materials, fragmented plastics have been adopted and named based on their size, origination, and process of fragmentations. Several researchers have begun to consider sub-scale plastics fragmentation, also known as “nano-plastics,” and various studies have set their upper size limits of 1000 nm or 100 nm. Further, small chunks of degrading plastic of 1–5000 μm in length are known as MPs in general. They were first discovered in German beer brands (Liebezeit and Liebezeit 2014), water samples, and air samples (The Lancet Planetary Health 2017). Cosmetics, polythene bags and plastic containers, electrical appliances, goods packaging, glass, and many other items are significant sources of MPs. When using sources for further research and mitigation, the distinction between primary and secondary sources must be considered.
Plastic pellets in manufacturing industries, scrubbers, commercial cleaning abrasives, plastic resin flakes, plastic powder or fluff used to produce plastic goods (Andrady 2011), along with volatile particulate contaminants such as micro-polyester, nano Fe3O4, and SiO2 from printing toners are the potential sources of primary MPs (Jujun et al. 2013).
Likewise, secondary MPs originate from the breakdown of larger plastics subsequently into nano-, micro-, and macrosizes. Before being discharged into the environment due to weathering, such as exposure to wind abrasion, wave action, photodegradation, biodegradation, hydrolysis, and ultraviolet radiation from sunlight are the potential routes to generate secondary MPs (Gewert et al. 2015; Picó and Barceló 2019; Rogers 2020). Also, the fragmentation process, which emphasizes routes to generate secondary MPs, resulting from the gradual degradation of plastics in water, consists of three mechanisms: bio-fragmentation, assimilation, and biodeterioration showing emphasized, impactful MPs generation pathways (Emadian et al. 2017). The primary and secondary MPs sources are evolved from the different ways of plastic degradation, as depicted in Fig. 1.
Fig. 1.
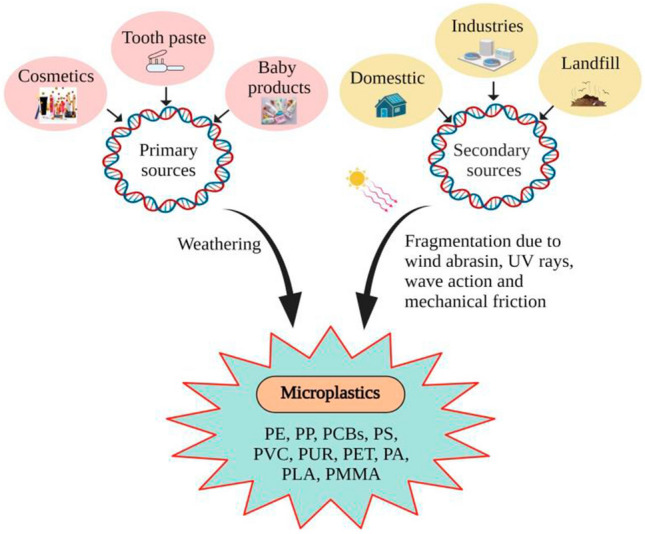
Types, sources, and the way of formation of primary and secondary MPs (GESAMP 2015)
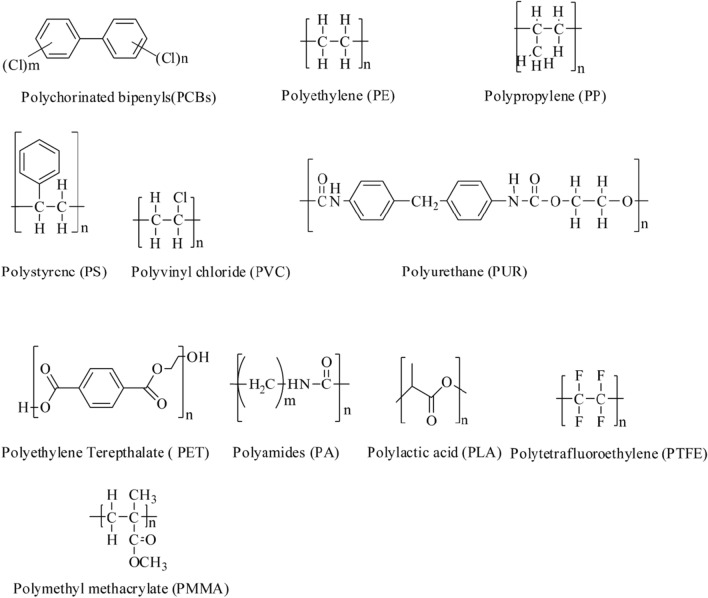
Moreover, small fragmented plastics called microbeads (size 10–500 mm) are also patented as ingredients in personal care products for exfoliating skin in hand and facial scrubs and used as an increasing viscosity in toothpaste (Anagnosti et al. 2021). The high demand and gradual environmental deterioration of plastic materials have become a significant global threat. If we talk about forms of plastics or group types of polymers used in plastics, they have been classified as various forms. However, the industries are dominated by six plastics groups, namely polyethylene (PE) (high and low density), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), polyurethane (PUR), and polyethylene terephthalate (PET) (Kole et al. 2017). Some major plastic types found in MPs and microbeads are shown in Fig. 2.
Fig. 2.
Some major polymer products found in MPs
The recent data showed that packaging and construction are the two major consumer end-use markets, and the automotive industry is the third-largest consumer. Most of these plastics comprise PE and PP (Plastics-the Facts 2020). These plastics products are mainly synthetic ones; however, such plastics can also be extracted naturally from trees (latex), plants (cellulose), animals (horn and milk), insects (shellac) (Science of Plastics 2016).
Detection techniques of MPs
Rapid separation and characterization of primary and secondary MPs from aquatic and terrestrial environments have risen to the top of the research agenda. Using visual and a combination of visual and analytical tools, many studies have been conducted to identify and quantify MPs (Lusher et al. 2017; Shim et al. 2017). MPs can be identified in general using two techniques: physical characterization (microscopy) followed by chemical characterization (spectroscopic) for plastics confirmation (Shim et al. 2017). The four most preliminary steps, such as density separation, filtration, sieving, and visual sorting of MPs, are required before identification. These four initial techniques can easily identify the morphology (shape, size, and color) of larger MP fragments (Hidalgo-Ruz et al. 2012). Furthermore, fluorescence combined with density separation provides a sensitive and simple method for highlighting the most common plastic polymer fragments in marine sediments (Maes et al. 2017). Besides this, Wagner et al. used various techniques and came up with a unified result, which could be the best knowledge in the field of detection techniques, as evidenced by high accuracy (Wagner et al. 2019). Hence, techniques that can identify these MPs and their small fragments are essential for the identifying process, and renowned techniques are highlighted below.
Visual techniques
The naked eye or an optical microscope with objectives ranging from 10 to 50 times magnification is used for visual identification. Image-analysis software such as Histolab and Olympus stream were sometimes used in conjunction with the microscope (Elkhatib and Oyanedel-Craver 2020). Visual identification is used in the majority (79%) of MP characterization studies (Picó and Barceló 2019) because it is simple and easy to use. However, it is not reliable for identifying MPs because non-plastic particles such as cellulose, keratin, viscose rayon, coal/fly ash, and paint chips can interfere with this approach, resulting in false-positive and also this approach has difficulties in identifying particles smaller than 100 µm and translucent particles (Elkhatib and Oyanedel-Craver 2020; Picó and Barceló 2019). In turn, to optimize the techniques for the digestion of human hair, cotton clothing fibers, and cigarette filters, the wet peroxide oxidation (WPO) and staining method (rapid screening of MPs done by using Nile Red dye) can be used (Erni-Cassola et al. 2017). Another unique approach includes an expedited digestion step that uses a mixture of sodium hydroxide (NaOH) and nitric acid (HNO3) to digest all organic material in one hour, as well as a separate separation step that uses sodium iodide (NaI) to minimize mineral residues in samples when needed. Except for polyamide, this approach provided an MPs recovery rate of 95%, and all studied polymer types were recovered with only slight changes in weight, size, and color (Roch and Brinker 2017). Further, if we add a scanning electron microscope (SEM), which visualizes the surface properties of particles, it would be an easy task for research purposes. By scanning the surface of a material with a concentrated electron beam, this approach produces high-resolution pictures. In addition, particle distinction is possible due to the fine sample pictures (> 0.5 nm), however, the polymer’s composition is not determined by SEM (Elkhatib and Oyanedel-Craver 2020). As a result, for polymer compositions, we should identify and focus on other techniques that provide both.
Spectroscopic techniques
While the prominent MPs can be easily seen in visual techniques with the help of chemical methods, small fragments of particles are difficult to identify. Hence, such particles can be easily identified through spectroscopy techniques (Möller et al. 2020). The most frequent analytical procedures used to determine the composition of MPs, such as PE, PP, PS, or PVC, were Raman spectroscopy and Fourier transform infrared spectroscopy (FTIR) (Hidalgo-Ruz et al. 2012). Spectroscopic techniques are used to analyze the molecules of the samples, resulting in a characteristic polymer spectrum that can be identified using a reference spectra library (Elkhatib and Oyanedel-Craver 2020). Moreover, for small plastic fragments, spectroscopy (FTIR spectroscopy, near-infrared spectroscopy, and Raman spectroscopy) is strongly suggested since it can reliably establish the chemical composition of unknown plastic fragments (Munno et al. 2020). However, Cabernard et al. proved that Raman spectroscopy is aloof better than the FTIR technique for quantification and analyzing the data of MPs (Cabernard et al. 2018). Sometimes we falsely measure the MPs fragments in the sample by other techniques; however, spectroscopic techniques give us the reliable result whether this plastic-type is real or fake like sodium dodecyl sulfate can give spurious results in MPs’ analysis (Witzig et al. 2020). The recent study by Hu et al. identified several types of MPs deploying different techniques (Fig. 3), showing various morphology, FTIR wave numbers of Fig. 1 molecules of functional groups of plastic types.
Fig. 3.

FTIR (A–D) and images (E–H) of the most prevalent types of MPs found in samples; adopted from (Hu et al. 2018); copyright 2018 American Chemical Society
Moreover, these techniques required sample preparation and laborious work in the laboratory to perform; thus, spectroscopic techniques are not fulfilling the desires of analytical techniques, but they could have the potential to be a landmark step in the identification field of MPs if they are overcome by some limitations (Shim et al. 2017). Recently, Rennel et al. gave the solution to a time-consuming task in spectroscopy data analysis. In his research, his team shows about 98% correct identification of plastics types by matching with the spectrum of the library within a concise period. This paper further added that this is mainly working by the algorithm and is limited to FTIR techniques and applied to other spectroscopic techniques. Some of the MPs identification using spectroscopic techniques are summarized in Table 1.
Table 1.
MPs occurrence and detection techniques
| Identification techniques | Items | MPs range | References |
|---|---|---|---|
| Visual techniques | |||
| Dissection microscope at 30 × magnification | Honey and sugar |
Colored fibers: 166 ± 147/kg of honey Fragments: 9 ± 9/kg of honey Colored fibers: 217 ± 123/kg of sugar 32 ± 7/kg of sugars |
Liebezeit and Liebezeit (2013) |
| Visual microscopy | Seawater | 452 fibers and 827 particles, later confirmed by Raman spectra | Lenz et al. (2015) |
| Stereomicroscopy | Air | 2–355 particles/m2/day | Dris et al. (2017) |
| SEM | Atmospheric fallout | 175–313 particles/m2/day | Cai et al. (2017) |
| Fluorescence microscopy | Atmospheric deposition | 136.5–512.0 MPs particles per m2/day | Klein and Fischer (2019) |
| Optical Microscopy/SEM/EDS | Freshwater sport Fish | 16 MPs particles were identified in the 30 fish, and the sizes of MPs fragments ranged from 50 to 1500 μm | Wagner et al. (2019) |
| Spectroscopic techniques | |||
| FTIR and Raman spectroscopy | Raw and treated drinking water |
Raw: 1473 ± 34 to 3605 ± 497 particles/L Treated: 338 ± 76 to 628 ± 28 particles/L |
Pivokonsky et al. (2018) |
| Micro-Raman spectroscopy | Tap water | 440 ± 275 particles/L | Tong et al. (2020) |
| FTIR | Surface water and Australian freshwater Paratya australiensis |
Surface water: 0.40 ± 0.27 items/L Shrimp: 0.52 ± 0.55 items/and (24 ± 31 items/g) |
Nan et al. (2020) |
| Soil sample | 0.34 ± 0.36 particles per kilogram dry weight of soil | Piehl et al. (2018) | |
| Ocean trawl and fish gut | Of the 46 trawl particles, 20 were MPs. All 28 particles extracted from GI tracts were MPs | Wagner et al. (2017) | |
| Sea-surface water | 110 particles/m3 | Kosore et al. (2018) | |
| Alpine glacier | 74.4 ± 28.3/kg | Ambrosini et al. (2019) | |
| Table salt products | 9.77 MPs particles/kg | Lee et al. (2019) | |
| Chromatographic techniques | |||
| Liquid chromatography–tandem mass spectrometry | Pet foods |
Cat foods: < 1500 ng/g to 12,000 ng/g Dog foods: < 1500 to 4600 ng/g |
Zhang et al. (2019a, b) |
| Microfiltration | Honey, milk, soft drinks, and beer | On average, 40 MPs/L | Diaz-Basantes et al. (2020) |
| MFs-Millipore™ 0.45 µm pore size mixed cellulose esters membrane filters | Himalayan surface water | ≤ 5 mm to ≥ 250 µm in three one-liter surface water | Simpson (2019) |
| Pyrolysis–gas chromatography–mass spectrometry (Py-GC/MS) | Lake sediments | 43 plastics debris/16 sediments | Castelvetro et al. (2021) |
| WWTP sample | 0.003 to 0.060 mg PS/m3 | Funck et al. (2020) | |
| Liquid Chromatography–Tandem Mass Spectrometry | Indoor dust; Clams digestive residues | 246 and 430 mg/kg PC and PET type MP; 63.7 mg/kg of PC and 127 mg/kg of PET MPs | Wang et al. (2017) |
Chromatographic techniques
This technique involves identifying individual polymer types of MPs that work in conjunction with extraction techniques and can identify the MPs qualitatively and quantitatively (Möller et al. 2020). In chromatographic procedures, progress is made sequentially, allowing MPs identification to be made quickly. The series of advancements include high-temperature gel permeation chromatography (HT-GPC), liquid extraction with size-exclusion chromatography (SEC), and pyrolysis gas chromatography–mass spectrometry (Pyr GC–MS). Pyr GC–MS is a sensitive and well-proven technology for mass quantification and characterization. Pyr GC–MS is well suited for detecting MPs in environmental samples of numerous polymer types and their organic additives when used in conjunction with attenuated total reflectance (ATR-FTIR) spectroscopy (Möller et al. 2020). For instance, pyrolysis coupled with gas chromatography/mass spectrometry (Py-GC/MS) is used to obtain information on the composition of MPs (Hermabessiere et al. 2018). Thermal decomposition of materials is used in pyr-GC/MS, and the decomposition products are separated using gas chromatography and analyzed using mass spectrometry (Witzig et al. 2020). Although much research has been carried out to identify and quantify MPs, very few and efficient techniques are available to analyze them. The identification techniques recently used for different MPs containing samples are summarized in Table 1.
Although, having the several advantages of existing detection techniques described above, they are not sufficient to detect MPs in a second to a few minutes. Several preliminary techniques should be replaced with efficient and fast methods. For example, using castor oil to separate MPs could be the ideal technique to replace the existing density separation method (Mani et al. 2019). The hyperspectral image (HSI) could also be the future of MPs identification because of the significant reduction in reagent consumption, which saves time and thus provides a rapid and efficient method for MPs analysis (Zhang et al. 2019a, b). Similarly, Fenton’s reagent (a mixture of H2O2 and ferrous ion, Fe2+) was used to isolate MPs from organic‐rich wastewater, reduce the time during the sample preparation and identification process as well as offer a simple, high‐speed, and low‐cost method for processing MPs present within environmental samples (Tagg et al. 2017).
Moreover, the visual techniques are insufficient to provide MPs type and other information. Similarly, spectroscopic technologies could not solve the problem adequately due to time-consuming and efficient sample preparation methods that need to be implied. In such cases, FTIR and Raman spectroscopy alone cannot facilitate the scrutiny process of MPs in different samples. For instance, Zhang et al. provided an efficient technique to overcome these circumstances, and a custom-made portable Pyr-MS has been developed. This device measures the MPs particles in a wide range and is not limited by the shape, size, and color like FTIR and Raman do. Furthermore, it avoids the complex extraction and separation procedures of the pyrolysis/thermogravimetric–gas chromatography–mass spectrometry (Pyr/TGA-GC-MS). It realizes the rapid analysis of MPs in 5 min (Zhang et al. 2020a, b). Another novel method related to spectroscopy was developed called Raman Tweezers (RTs), namely optical tweezers combined with Raman spectroscopy, as an analytical tool for the study of MPs and nanoplastics in seawater; could have the potential to strongly impact future research on micro- and nanoplastics environmental pollution (Gillibert et al. 2019).
Life cycle of MPs
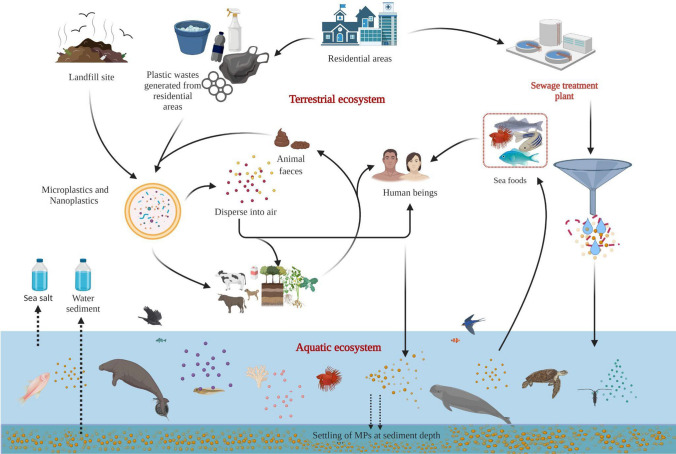
MPs are introduced to the environment through atmospheric deposition, land-based sources, fertilizers, artificial turf, road, landfill and air transportation, textiles, tourism activities, marine vessels, and aquaculture (Lambert et al. 2014). In addition, degradations such as physicochemical activity, UV, and bacteria degrade plastic that enters the environment into micro-nanosizes (Lee et al. 2014); however, the rate of fragmentation is dependent on environmental conditions. Terrestrial ecosystems are among the most commonplace where MPs are prevalent due to improper waste management and are found to highly deteriorate the quality of soil (Machado et al. 2018). They then build up in the deep sea, virgin polar regions, and ice sheets. They are ingested by live species and impact their feeding, digesting excretion, and reproduction processes (Amelia et al. 2021); however, the marine-based contribution is still considerable and underappreciated.
As mentioned in the background section, marine organisms can swallow plastic either directly from the seawater or through the ingestion of an organism that has already been exposed to it. As a result, plastic waste has been found in seafood intended for human consumption and fish and shellfish purchased from markets (Woods et al. 2021). In this way, MPs get into human foods (Barboza et al. 2018) and drinking water (Schymanski et al. 2018) and are among the most common intake pathways of MPs into the human body, causing negative impacts on health. The more detailed MPs origin, separation, and segregation cycle are depicted in Fig. 4.
Fig. 4.
Life cycle of MPs (from origin to disposal)
Effects of MPs on the environment
The MPs fragments have been found in the environment and pose a significant problem in different ecology sectors. Other studies found that MPs reached the top of the world (Mount Everest) (Napper et al. 2020) to the deep ocean (Bergmann et al. 2017; Cunningham et al. 2020). Almost 80% of MPs originated from land, and less than 20% from water. The mortality and injury of aquatic birds, fish, mammals, and reptiles caused by plastic aggregation and digestion are among the effects of MPs on the environment (Sana et al. 2020). The primary environmental concern to terrestrial, aquatic, and public health have been conspicuous topics in recent decades, and the detailed impacts on different environment sectors are discussed below.
Impact on the terrestrial ecosystem
MPs are a scourge to the environment, reflecting how plasticized our lives have become and can have potential adverse effects on the terrestrial ecosystem (Alberts 2020). While MPs in marine environments have been extensively studied, research on MPs in terrestrial ecosystems is just starting to gain momentum (He et al. 2020; Machado et al. 2018). MPs are more likely to interact with the biota in terrestrial systems, potentially affecting the geochemistry and biophysical environment and producing environmental toxicity. This section presents new insights into MPs as a global change stressor in terrestrial systems, especially in soil and air environments.
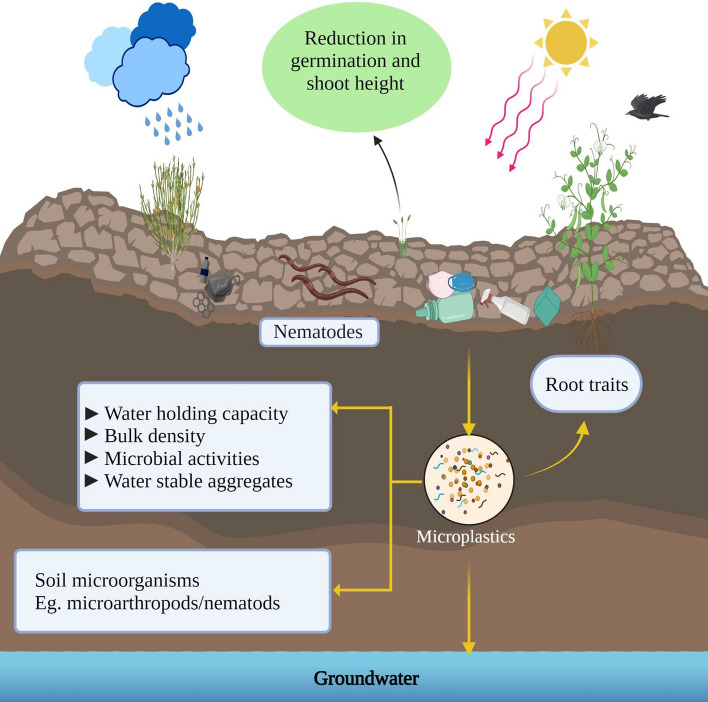
MPs are found in soils worldwide, especially in agricultural soils (Kumar et al. 2020; Li et al. 2020; Möller et al. 2020; van den Berg et al. 2020). They enter the soil environment in diverse ways, such as irrigation, sewage sludge, littering, and atmospheric deposition (van den Berg et al. 2020; Yang et al. 2021a, b). MP’s vertical and horizontal mobility within the soil is regulated by various factors, including soil biota and soil characteristics. When MPs are integrated into soil aggregates, they alter the structure of the soil (Guo et al. 2020). Because of soils’ low light and oxygen conditions, MPs may survive for decades. As a result, MPs may interact with soil fauna by altering their biophysical environment, affecting their fitness and soil function. They can, of course, be uptaken by plants and transported along the food chain once they accumulate in the soil. For instance, impacts of MPs (biodegradable polylactic acid (PLA), conventional high-density polyethylene (HDPE), and MPs clothing fibers) have been observed on the germination of seeds that are exposed to fibers or PLA MPs along with a reduction in shoot height (Boots et al. 2019). Lwanga et al. observed the decline in growth, as well as mortality among Lumbricus terrestris (Oligochaeta, Lumbricidae), exposed to MPs (PE, < 150 μm) in different concentrations (Huerta Lwanga et al. 2016). MPs affected the bulk density, water holding capacity, and the functional relationship between microbial activity and water-stable aggregates (de Souza Machado et al. 2019). Machado et al. identified MPs effects on soil health and performance of spring onion (Allium fistulosum), changes in plant biomass, elemental tissue composition, root traits, and soil microbial activities (de Souza Machado et al. 2019). Further, soil microorganisms like earthworms (Lumbricus terrestris) can easily ingest MPs and accumulate via the intestine in casts. Burrows may cause long-term ecological effects to not only its species but also other different organisms as it forms the bases for many food chains (Huerta Lwanga et al. 2016). Similarly, Lin et al. studied the impact of MPs on soil organisms and found that with the increment in MPs, worms and microarthropods populations decreased (Lin et al. 2020). Also, the insertion of polyethylene fragments in the field significantly affected the composition and abundance of microarthropod and nematode communities. The impact of MPs on the terrestrial ecosystem is depicted in Fig. 5.
Fig. 5.
Conceptual diagram showing the various mechanisms via which MPs could affect the terrestrial ecosystem
Likewise, the widespread use of face masks during the COVID-19 emergency provides proof of the environmental disorder in both the terrestrial and aquatic world and that the global pandemic has not diminished the threat of ecological plastic contamination (Acharya et al. 2021; Aragaw 2020). The surgical face masks, which have been used to control COVID-19, can easily show the effect on the higher organism, which will affect the food chain and ultimately chronic health problems to humans and the environment (Aragaw 2020; Fadare and Okoffo 2020). However, researchers suggested that further study of its potential effects on human health is needed.
MPs have also been observed in atmospheric fallouts, as well as in indoor and outdoor environments. However, there still exist questions regarding the occurrence, fate, transport, and effect of atmospheric MPs due to limited physical analysis and lack of standardized sampling and identification methods (Gasperi et al. 2018; Zhang et al. 2020a, b). A study, for the first time, investigated the MPs fibers in indoor and outdoor air (Dris et al. 2017). They showed that the indoor concentrations ranged between 1.0 and 60.0 fibers/m3 and outdoor concentrations were significantly lower as they vary between 0.3 and 1.5 fibers/m3. Allen et al. observed atmospheric MPs in the Pristine mountain watershed. They analyzed samples collected over five months that represent atmospheric wet and dry deposition, identifying 249 fragments, 73 films, and 44 fibers per square meter deposited on the catchment daily (Allen et al. 2019). Likewise, Dris et al. and Cai et al. highlighted the range between 2 and 355 particles/m2/day and 175–313 particles/m2/day in the atmospheric fallout, respectively (Cai et al. 2017; Dris et al. 2017). The number of MPs in the air could vary widely between different areas in the same environment. Similarly, one study detected 136.5–512.0 MP particles per m2/day, showing high concentrations in rural sites of the Metropolitan area of Hamburg, Germany (Klein and Fischer 2019). Some natural phenomena such as wind speed, direction, convection, and turbulence affect MPs’ transportation and deposition. These phenomena result in the transport of MPs particles to ocean surface air and even remote sites.
Impact on the aquatic ecosystem
MPs are prevalent in the marine environment due to hydrodynamic processes and wind and ocean currents transportation, which has contributed to exponential scientific concern in recent decades. Approximately 70% of marine plastic debris is deposited in sediments, 15% floats in coastal areas, and the remainder floats on the surface seawater. Due to their tiny sizes, MPs can be ingested accidentally by marine organisms such as fish, mussels, zooplankton, sea birds, and so on (Cole et al. 2013; Wieczorek et al. 2019; Yang et al. 2021a, b). Table 2 depicts some experimental scenarios that demonstrate the presence and effects of MPs on oceanic species.
Table 2.
Experimental designs for detecting impacts of MPs in aquatic organisms
| S. No | Organisms | Plastic types | Concentrations | Exposed duration | Results | References |
|---|---|---|---|---|---|---|
| 1 | Mytilus edulis | PE, Polyhydroxy butyrate(PHB) | Dispersed in 5 ml of 0.1% Tween80 solution | 96 h | Decreased activity levels of CAT and GST in gills, SOD in digestive glands, and SeGPx in both tissues | Magara et al. (2019) |
| 2 | Ciona intestinalis | PS | 50 mg particles/ml, was diluted 1:1000 in filtered seawater (FSW) to produce a stock suspension of 50 µg/ml | 8 days | Delayed development due to lower food intake and insufficient energy supply | Barnes et al. (2009) |
| 3 | Ruditapes philippinarum | PET | 0.125 or 12.5 µg/ml | 7 days | No histological effects | Messinetti et al. (2019) |
| 4 |
Daphnia Magna |
PE |
20; 40; 80; 160 and 320 mg/L |
96 h | No toxic effects | Castro et al. (2020) |
| 5 | Caenorhabditis elegans | PS | 1.0 mg/L | 3 days | The lowest survival rate, the most significant decrease in body length, and the shortest average life span | Lei et al. (2018a, b) |
| 6 | Tubifex tubifex | Microfibers/MPs fragments |
56–2544 particles kg-1 |
– | Trophic transfer and biomagnification of MPs up the aquatic food chain | Hurley et al. (2017) |
| 7 | Mytilus galloprovincialis | PS alone or mixture with carbamazepine (Cbz) | PS (from 0.05 up to 50 mg/ L), to Cbz (6.3 μg/L) alone and to the mixture of PS + Cbz (0.05 mg/L + 6.3 μg/L) | 96 h | Increased total antioxidant capacity, genotoxicity, and lipid peroxidation | Brandts et al. (2018) |
| 8 | Tadpole | PES & PP | 0 to 2.73 items individual − 1 | Hu et al. (2018) |
MPs have been detected in various organisms, from large mammals to small molluscs and their effects have been explored. For instance, the ingestion of debris by three benthic-foraging fish species in Sydney Harbour, Australia, has been reported (Halstead et al. 2018). They investigated that debris ingestion at the time of sampling ranged from 21 to 64% for the three species, and the debris number ranged from 0.2 to 4.6 items per fish for the different species, with ∼ 53% of debris being MPs. Lu et al. found that 5 μm and 70 nm polystyrene (PS) MPs caused inflammation and lipid accumulation in the liver of Danio rerio (Lu et al. 2016). MPs can also exert size-dependent toxicity. The moderate size of polystyrene particles, i.e., 1.0 µm, resulted in the most prominent toxicity on surviving, development, and motor-related neurons in Caenorhabditis elegans (Lei et al. 2018a, b). MPs may also serve as a carrier for harmful elements such as dichlorodiphenyltrichloroethane (DDT) and hexachlorobenzene and ultimately end up in the living organism that consumes them (Laskar and Kumar 2019). It can alter the feeding capacity of the Calanus helgolandicus, a key trophic link between primary producers and higher trophic marine organisms (Cole et al. 2013, 2015).
According to the study, MPs and their smaller fragments, NPs are easily ingested by some aquatic species. They tend to acquire gastrointestinal toxicity, liver toxicity, neurotoxicity, and reproductive toxicity (Chang et al. 2020). On the exposure of 70 μm polyamide, PE, PP, PVC, and PS particle exposure, species such as zebrafish and nematode suffered from villi cracking and splitting of enterocytes due to gastrointestinal toxicity (Lei et al. 2018a, b). Furthermore, studies have explored that 0.5 μm PS microplastics induced dysbiosis, microbiota, and inflammation in the gut of adult zebrafish (Jin et al. 2018) and causes significant histological changes and a strong inflammatory response to the ingestion of PE microplastics in the blue mussel Mytilus edulis L. (von Moos et al. 2012). Upon MPs ingestion, oxidative stress was observed in the liver of Eriocheir sinensis (Yu et al. 2018a, b). Lu et al. studied that PS MPs prompted oxidative stress in zebrafish, causing the change in metabolic paths, leading to disrupted lipid and energy metabolism (Lu et al. 2016).
Heavy metal toxicity of plastic in aquatic ecosystems is another significant concern to scientific circles. Plastic contains different types of heavy metals used for the manufacturing process and ultimately go to an environment and pollute the environment. Metals like cadmium, zinc, and lead have been used for heat stabilizers and slip agents in plastic manufacturing. These metals, which comprise up to 3% of the polymer composition, can show detrimental effects (Munier and Bendell 2018). Moreover, this study indicated that PVC is likely one of the main culprits of heavy metal contamination from plastic waste in our oceans, based on 144 samples analyzed.
Microplastics as a public health concern
With recent advancements in tools that enabled the characterization of MPs in food, water, and air, we have seen colossal data sets generating strong evidence concerning MPs nature, chemical composition, reactivity, and structures (Kik et al. 2020; Lo Brutto et al. 2021; Ripken et al. 2021).
In the last few years, MPs effect on human health and small vertebrates are a concerning topic to the researchers and have seen many epidemic examples where MPs are found in their organisms and affect their life. Few studies already set the landmark step and showed that MPs are easily excess to the different (small to large) parts of the body; however, significant problems due to these are very low. To date, many studies have classified various possible diseases that are caused due to suspected MPs and need to do more research in the future. Yan et al. recently discovered microplastics in the feces of people with inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, and discovered significant links between these two. They claim that MPs can cause intestinal inflammation, gut microbiome disruptions, and other issues in animals (Yan et al. 2022). According to the researchers, it is still unknown whether this exposure causes or contributes to IBD or whether people with IBD accumulate more faecal microplastics due to their disease. Although it was once thought that microplastics passed harmlessly through the gastrointestinal tract and out of the body, new research suggests that even the tiniest pieces can cross cell membranes and enter circulation. Microplastic exposure can cause cell death, inflammation, and metabolic disorders in cells and laboratory animals.
Similarly, plastics particles are recently found in human blood samples where polyethylene terephthalate, polyethylene, and polymers of styrene are the major ones, while polypropylene is in below the limits to quantification in number (Leslie et al. 2022). This groundbreaking human biomonitoring study demonstrated that plastic particles are bioavailable for uptake into the human bloodstream and the associated risk. We believe these particles cause some other malfunction in the body, which has yet to be identified. Likewise, in one study conducted by Yong et al., cellular physiology was influenced to varying degrees by MPs, and NPs concentrations found it would be a sort of cellular stress (Yong et al. 2020).
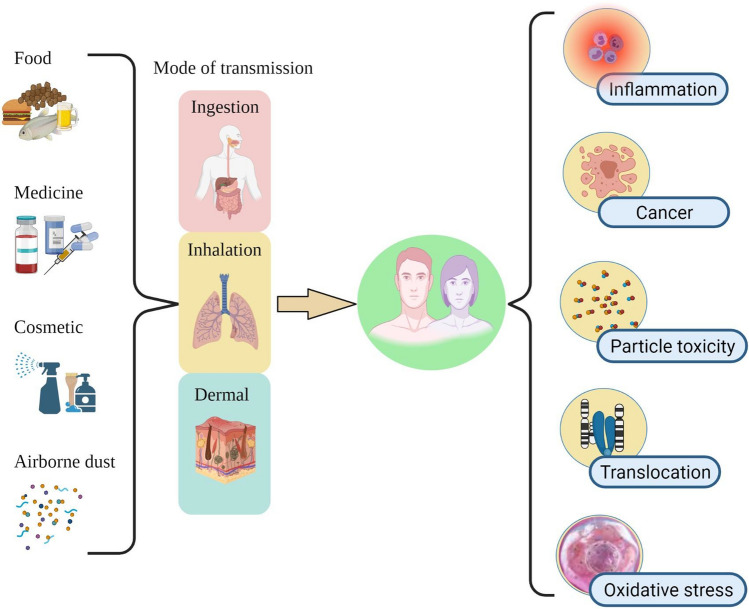
Furthermore, Gastric exposure, Pulmonary exposure, and Dermal exposure are one of the main routes by which microplastics and nanoplastics enter the human body and cause negative impacts on human health (Table 2). However, the exposure pathway of human cells depends on the particles’ size and the surface’s chemistry (Yee et al. 2021). Since the explosive effect of MPs on human health is not conclusive to date, researchers have started the experiment on the mammalian model to predict MPs toxicity to relate it to humans. On the exposure of PS MPs to mice for about 28 days, it was found to be accumulated in the kidney, guts, and liver leading to the problems like liver inflammation and lipid metabolism disorder (Deng et al. 2017). In vitro study on PS NPs revealed cationic polystyrene nanoparticles were found to cause reactive oxygen species (ROS) generation and endoplasmic reticulum (ER) stress in mice leading to apoptosis of macrophage (RAW 264.7) and epithelial (BEAS-2B) cells (Xia et al. 2008). One of the studies found that PS nanoplastics reduced the viability of human gastric adenocarcinoma cells and were reported to induce the expression of inflammatory genes such as IL‐6 and IL‐8 (Forte et al. 2016). Aside from that, transcriptome results revealed that prolonged MPs exposure altered the transcription levels of gut-related genes and several essential metabolic pathways and life processes (Wu et al. 2020). Hwang and team members investigated that PS and PP particles were potential immune stimulants to cause health problems by inducing the production of cytokines from immune cells (Hwang et al. 2019, 2020). Evidence showed the different types of effects on human beings due to the over-exposure of MPs as portrayed in Fig. 6 (Table 3).
Fig. 6.
Potential threat to human health due to environmental exposure to MPs
Table 3.
Some of the potentially toxic effects of MPs and NPs on human health are listed below in the table
| Toxicity effect | Plastics | Size of plastics | Results | References |
|---|---|---|---|---|
| Oxidative stress |
PVC PMMA (poly methyl methacrylate) |
120 nm 140 nm |
Increases reactive oxygen species (ROS), and reduce cell feasibility | Mahadevan and Valiyaveettil (2021) |
| Cationic PS NPs | 60 nm | Increases reactive oxygen species (ROS) generation and endoplasmic reticulum (ER) stress | Chiu et al. (2015) | |
| Gastrointestinal effect | PS NPs | 50 nm and 200 nm | Alter intestinal ion transport | Mahler et al. (2012) |
| PS MPs | 0.5 µm and 5 µm | Increased metabolic disorder risk in the offspring | Luo et al. (2019) | |
| PS MPs | 0.5 and 50 μm | Induce mouse hepatic lipid disorder | Lu et al. 2018 | |
| PS MPs | 5 µm | Reduces intestinal mucus secretion and induce gut microbiota dysbiosis | Jin et al. (2019) | |
| Neurotoxicity | PS MPs | 5 and 20 μm | Increase in AChE activity in the liver, and may lead to the reduction in cholinergic neurotransmission efficiency | Deng et al. (2017) |
| PS NPs | 38.92 nm | Decreased locomotor activity | Rafiee et al. (2018) |
However, most studies that have established the associative link between specific diseases and MPs either have poor study design (McCormick et al. 2014) or have a paucity of data to back the asserted claim (Fournier et al. 2020). Despite the insufficiency of reliable clinical data, some biological experiments, at least in vitro, have reported the detrimental effect of MPs exposure on the living system (Chen et al. 2021; Fournier et al. 2020; Magrì et al. 2021; Mahadevan and Valiyaveettil 2021). Again, the relevance of these in vitro studies, which utilize single cell type; almost always with immortal cell line, makes it non-ideal evidence to relate to human complexities with multi-organelle functionality. Nevertheless, phagocytic cell uptake of MPs has gained some evidence in supporting the removal through the cellular excretory pathway relevant to in vivo (Ramsperger et al. 2020). As expected, MPs without any biological traces are most often ignored by the human immune surveillance; however, other additive effects as blood vessel dilation, infiltration, and congestion as a result of MPs structural architecture is another expected effect of MPs as shown in an in-vivo model of small animal (Araújo et al. 2020); however, it has not yet been reported in humans.
In contrast to MPs ingested through food and water, occupational exposure to airborne MPs has been documented, and its amount is correlative to the pathology observed among MP exposed human workers (Araújo et al. 2020; Atis 2005; Barroso et al. 2002; Eschenbacher et al. 1999; Kern et al. 2000). MPs have also been shown to be associated with increased chronic bronchial constriction and asthma-like clinical features, thus yielding an overall decrease in quality of life among workers who have had prolonged exposure to MPs (Kern et al. 2000). Although exact pathophysiology is not known, chronic airway irritants including some form of MPs might disassemble the existing immune tolerance in the respiratory tract, which at a time would certainly be dose-dependent along with numerous confounding human physiological factors that would influence the outcome such as human genetics (Powell et al. 2007).
In summary, the pathophysiology, spectrum of illness, and long-term effect due to prolonged exposure to MP have yet to be elucidated, evidence of which must be derived from well-designed clinical-epidemiological studies. Nevertheless, based on data on small mammals, invertebrates, and in-vitro human cell toxicity, we can partially assume that MPs do have the potential to exacerbate human physiology and homeostasis, but the evidence to assert this claim is very weak; thus more evidence is needed in this topic.
Challenges to control the MPs
Although several detrimental effects of MPs are on the various environmental sectors, its management is an arduous job; it seems that every step toward the MPs inspection, including sampling, extraction, isolation, and detection, implies a hurdle to large-scale surveillance. Some of the significant challenges of MPs in terms of their identification, quantification, and management are listed as follows:
One of the significant challenges is comprehending the physicochemical properties of MPs that are heterogeneous and may possess an ecotoxicity effect (Lambert et al. 2014).
MPs management becomes more challenging as public perceptions, attitudes, and behavioral preferences toward MPs remain underexplored (Deng et al. 2020).
The lack of reliable analytical techniques, proper identification, and quantification of MPs in complex matrices such as food products is another matter of concern (Hermsen et al. 2018).
The policy formulated for the resistance to the intervention of banning the use of plastics got breached once it got implemented (Sharp et al. 2010).
Owing to the lack of standard established policies and procedures for natural environmental datasets, it is hard to assess MPs, or nanoplastics (Yu et al. 2018a, b).
The lack of a specialized database that comprises distinct spectra of polymeric materials has limited plastics modification studies. The infrared spectra of different polymers change when they interact with the environment (Fotopoulou and Karapanagioti 2019).
The real issues of plastic recycling are indeed aligned with the level of plastic purity. Plastics are produced by more than one polymer type or may be fused with an additive to improve strength; however, extracting desired plastic materials is complicated. In addition, PE consists of a linear and highly stable carbon–carbon (C–C) backbone, making it resistant to degradation and creating a significant challenge for plastic waste biodegradation (Gao and Sun 2021).
It is difficult to collect adequate quantities of MPs particles for chemical analysis in complex samples, coupled with low detection frequencies and high detection limits for the tiny MPs. Therefore, multi-residual analytical tools aim to resolve these pragmatic challenges (Hong et al. 2017).
Controlling measures of MPs and global strategies
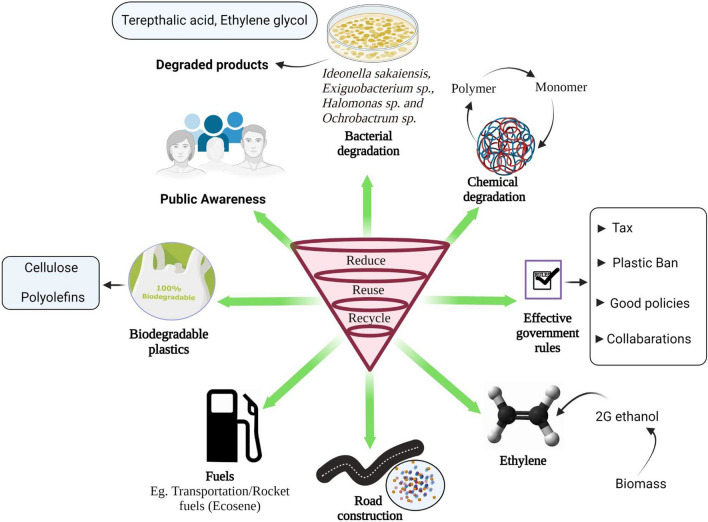
Production of the lavish amount of plastic worldwide poses great challenges to control. Source control is the most acceptable method to control MPs pollution (Ruan et al. 2018). Society needs to limit unnecessary single-use of plastic items like water bottles, plastic shopping bags, straws, and utensils in the momentary term. The government should focus on garbage collection and recycling systems to reduce waste in the environment. Standardized analytical methods for the reliable identification and quantification of MPs and nanoplastics in different matrices should be developed (Andrady 2011). Environmentally friendly and economical substitutes for plastics must be promoted (Zhang et al. 2018); in the long term, researchers need to conceive ways to break down its basic units, which can be remodeled into new materials (Thompson 2018). Researchers have discovered the mutant enzyme that takes a few days to break down the plastic drinks bottle and is far faster than the centuries it takes in oceans (Carrington 2018). These are the common steps we can put forward when we will inhibit plastic problems.
Some studies have highlighted the effectiveness of plastic degrading bacteria in the most recent years. In turn, Ideonella sakaiensis is a bacterium from the genus Ideonella and the family Comamonadaceae, which can break down and consume plastic polyethylene terephthalate (PET) as a sole carbon and energy source (Yoshida et al. 2016). These bacteria help recycle plastic. They use two enzymes sequentially to break down PET into terephthalic acid and ethylene glycol; the two substances from which it is manufactured are not harmful to the environment (Bornscheuer 2016). Similarly, Gao and sun isolated three types of bacteria genera named Exiguobacterium sp., Halomonas sp., Ochrobactrum sp.; however, notably, SEM observations on their research indicated the mixture of Exiguobacterium sp., Halomonas sp. and Ochrobactrum sp. had a greater degradation efficiency on both PET and PE films as compared to single isolates (Gao and Sun 2021). Moreover, for the first time, photocatalytic robots were able to efficiently degrade different synthetic microplastics using an active photocatalytic degradation procedure based on intelligent visible-light-driven microrobots capable of capturing and degrading microplastics. Polylactic acid and polycaprolactone, in particular, were degraded using microrobots with hybrid wireless capabilities, demonstrating for the first time the possibility of efficient degradation of ultra-small plastic particles in confined complex spaces, which has implications for microplastic treatment research (Beladi-Mousavi et al. 2021).
In another part of the same scenario, heavy metals found on aquatic environmental samples coming from plastics are also an inevitable problem in recent years which have been burgeoning day by day. Different heavy metals are detected during the experiment of different samples, and some heavy metals are coming from plastics additives and their fillers. Several studies have been carried out to detect the heavy metal in aquatic and other biota and removing them is an essential step. Awual et al. studied the simultaneous detection and removal of Pb (II) ion in a naked-eye manner by organic ligand functionalized composite material employing a simple one-step capture operation. The composite material was successfully fixed onto mesoporous silica, and the Pb(II) ion was quantified in a simple, rapid, repeatable, sensitive, and selective manner (Awual et al. 2020). Similarly, lead can be effectively removed by ligand-based composite materials; cesium metal can be removed from wastewater using bio-slag effectively (Khandaker et al. 2020); Cu(II) ions can be removed from environmental samples using ligand supported mesoporous silica. Furthermore, heavy metals that severely affect aquatic environments can be removed by several methods, and various research has been conducted.
Moreover, the omnipresence of plastic demands an alternate solution that could be renewable, environmentally sustainable, and biodegradable. Unregulated and mismanaged bioplastics could cause another environmental mayhem, close to traditional plastics. Therefore, it is a critical moment to leverage the force of legislation to set relevant criteria with a high threshold for the classification of bioplastics that can be aspired to by firms and trusted by consumers (Bhagwat et al. 2020). Biodegradable plastics based on cellulose and polyolefins should be promoted due to their low cost, high mechanical strength, and easy decomposition in the environment (Ammala et al. 2011). Recently, researchers developed a plant-based, scalable material that may be used to replace single-use plastics in a variety of consumer products and a polymer film that mimicked the qualities of spider silk. The new material is as robust as several popular plastics on the market today and might be used to replace plastic in various everyday items.
Furthermore, the material was developed utilizing a novel method for combining plant proteins into molecularly similar materials to silk. The energy-efficient technology produces a plastic-like free-standing film that can be manufactured on an industrial scale using sustainable components (Kamada et al. 2021). Further, other bioplastics require industrial composting facilities to break down; however, this material can be composted at home. In addition, the Cambridge-developed substance does not require any chemical alterations to its natural building components, allowing it to break down safely in most natural settings. It is an alternative to single-use plastic and MPs will commercialize this product.
Synthetic plastics, which have been used widely due to their physiochemical properties and good economic feasibilities, are mostly derived from petroleum products that show resistance to biodegradation (Rendón-Villalobos et al. 2016). Thus, the synthetic plastics that release hazardous substances and cause several pollutions are attributed to their low biodegradability and are, therefore, one of the challenging issues. To resolve the issue, biopolymers originating from plants, animals, or microbes should be sustainably used as some of the biopolymers like polylactic acid has been found naturally biodegradable and been applicable in the fields like packaging, disposable goods, bottles, goods with high durability along with nano-medicine, surgical, drug delivery, and therapeutic applications, and hi-tech fields (Kabir et al. 2020; Vink et al. 2004). The utilization of biopolymers has been shown to have much potential for preserving natural ecosystems and preventing further environmental deterioration (Hall and Geoghegan 2018). Though the high cost of biopolymers is a baring issue to compete with synthetic polymers, further research on technological advancement and innovative efforts is required to make it more feasible for customers worldwide users.
In addition, Brazil has recently been practicing producing bio-ethylene from 1G bioethanol which has the same physical and chemical properties as petrochemical ethylene. A recent study claim: the prospect of manufacturing bio ethylene from 2G bioethanol catalytic dehydration is a major challenge that needs further study and optimization of the processes involved, such as the proper use of well-stabilized catalysts capable of achieving large conversions of ethanol and ethylene selectivity. Moreover, the conversion of bio ethylene to bio-polyethylene does not present an apparent complexity until the latter is completed. However, bioplastics should be synthesized from biomass of the second generation instead of the first generation. Due to their inherited smaller carbon footprint, they should replace petro-plastics in as many applications as possible. In applications needing superior properties of petro-plastics, the petro-plastic itself should be extracted from renewable materials instead of substituting them for mechanically inferior bioplastic (Mendieta et al. 2020). Moreover, waste plastic has potential use in bituminous road construction as its addition in small doses (about 5–10% by weight of bitumen) helps in substantial improvement of stability, strength, fatigue life, and other desirable properties of bituminous mixes, leading to the improved longevity and pavement performance (Kalantar et al. 2012). The vacuum gasification condensation technique should be adopted for controlling the MPs and pollutants from printing toner (Ruan et al. 2018). The outline of recent solutions that could be the best strategies for plastic problems is shown in Fig. 7.
Fig. 7.
Pathways of MPs controlling techniques
It is imperative to introduce specific legislative solid rules and policies that could monitor the excessive use of plastics; otherwise, the ecosystem’s health will worsen over time. Efficient management, recycling, and an environmentally friendly disposal system would help make the environment free of plastics. Substantial policies are formulated in developing countries against plastics and their products, such as a complete ban on plastic bags and plastic bottles, a fine imposed on plastics. (Gopinath et al. 2020). Over the last three decades, legislation has been developed worldwide to address the dangers and consequences of plastics and increasing plastic waste (Table 4). Several strategies to reduce the use of MPs and microbeads have been promptly translated into international and national regulatory directives by international and intergovernmental policymakers. The following are some of these acts summarized:
Table 4.
Different strategies to combat the MPs all around the globe by different countries
| Year | Country/organization | Strategies/Act | Goal | References |
|---|---|---|---|---|
| 2012 | Netherland/The Dutch Plastic Soup Foundation | ‘Beat the Microbead’ campaign | To remove plastic microbeads from personal care products | Dauvergne (2018) |
| 2015 | US | The Microbead-Free Waters Act of 2015 | To forbid the deliberate production and selling of non-biodegradable plastic microbeads in rinse items for personal care | McDevitt et al. (2017) |
| 2015 | United Nations (UN) | Transforming Our World: The 2030 Agenda for Sustainable Development | To minimize the adverse impact on human health and the environment due to loss of plastics, chemicals, and waste materials in the atmosphere | Rosa (2017) |
| 2016 | Canada | Canadian Environmental Protection Act (CEPA) | To prohibit the import and export of personal care exfoliating goods containing microbeads | Pettipas et al. (2016); Zuzek (2016) |
| 2016 | World Economic Forum (WEF) | The New plastics Economy | To minimize plastic waste leakage into a natural environment and recycle reuse, and control plastics material biodegradation | The New Plastics Economy (2016) |
| 2017 | UK | United Kingdom Department for Environment Food and Rural Affairs, 2016 | To ban microbeads in cosmetic and personal care goods | Xanthos and Walker (2017) |
| 2017 | China | Ban the import of 32 kinds of solid wastes, including plastic waste | To achieve global environmental sustainability by realizing the transition from export to domestic management and from landfilling to recycling | Wen et al. (2021) |
| 2018 | Japan | Recycle and reuse all plastics, including electronic appliances and automobile parts | Reducing disposable plastic waste by 25 percent by 2030 | The Japan Times (2018) |
| 2018 | Canada/G7 | Strategy on zero plastic waste/Ocean Plastics Charter | To take action on resource-efficient life cycle management approach to plastics in the economy | CCME (2018) |
| 2016/2018/2021 | India | Ban single plastic bags/ Banned plastic bags below 50 μm | India Proposes Phase-Out of Single-Use Plastic Items by 2022 | Laskar and Kumar (2019); “The National Law Review” (2021) |
| 2011–2021 | Nepal | Plastic Bag Regulation and Control Directive 2011; Solid Waste Management (SWM) | Single plastic banned; banned < 40 micron thickness plastics | Bhardwaj et al. (2020); UNDP (2021) |
Future perspectives
MPs have the potential to be exposed to human beings via soil, water, food and are globally dispersed in every ecosystem. Current researchers are actively working on potential risks of MPs in public health and the environment. There are numerous examples throughout history of reducing the threat of MPs, which might be considered landmark developments in MPs management. Alternatives to plastics have been the subject of numerous studies, but none have proven effective.
To mitigate the impacts of MPs, very thin plastic, plastic shopping bags with fewer than 40 microns should be banned, and an efficient recycling system for plastic bags should be implemented. Furthermore, effective law enforcement, different strategies/acts have been put forward, and various countries are actively doing better work. Plastic litter is already dumped in non-manageable ways, and disposal through waste-to-energy will be crucial in minimizing the environmental impact of plastics. Alternative solutions like bioplastic, fuel research, biopolymer, chemical degradation techniques, bacterial degradation techniques are actively involved in research topics. However, effective and potential steps that can completely replace plastic products in the future are arduous jobs now.
Moreover, future research should assess whether microbial enzymes involved in plastic degradation could be utilized for minimizing MPs pollution to the environment. Additionally, the adequately designed human study is of a dire need to establish appropriate evidence on MPs’ exposure and associated health risk. Specifically, a long-term follow-up study will be particularly valuable for insight into chronic exposure to MPs. Evidence of such shall be helpful to inform policy and thus to design intervention needed to reduce the MPs exposure to humans and its impact on health. Besides, awareness-raising through schools, colleges, universities, various governmental and non-governmental organizations, and networks regarding the chronic effects of MPs pollution through programs and campaigns and educating individuals responsibility to minimize plastic by choosing to reject, reduce, reuse, and recycle plastics will help attenuate the MPs pollution.
In conclusion, if management processes are not handled appropriately, MP’s pollution will impact the animal population and disrupt the ecosystem’s balance, as we already discussed. Consequently, it might trigger an ecological imbalance that would make a precarious environment for survival. Therefore, solid policies and efficient management are much more required in future perspectives. More scientific innovation should also be encouraged, facilitating the production of environmentally friendly derivatives instead of plastics.
Abbreviations
- MMT
Million metri ton
- MPs
Microplastics
- LDPE
Low density polyethylene
- MFs
Microfibers
- PE
Polyethylene
- PP
Polypropylene
- PVC
Polyvinylchlorde
- PS
Polystyrene
- PUR
Polyurethane
- PET
Polyethyleneterepthalate
- WHO
World health organizations
- SEM
Scanning electron microscopy
- FTIR
Fourier transform infrared spectroscopy
- SEC
Size exclusion chromatography
- GC-MS
Gas chromatography-Mass spectroscopy
- HT-GPC
High temperature gel permeation chromatography
- HSI
Hyperspetral image
- ROS
Reactive oxygen species
Author’s Contributions
Ganesh Lamichhane (GL) conceptualized the manuscript; GL, Ashis Acharya (AA1), Rishab Marahatta (RM), Bindu Modi (BM), Rajesh Paudel (RP), Anurag Adhikari (AA2), Bimal Kumar Raut (BKR), and Sagar Aryal (SA) significantly contributed on literature review. GL and AA1 have equally contributed to editing the manuscript and Niranjan Parajuli (NP) supervised the project.
Funding
None.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Acharya A, Bastola G, Modi B, Marhatta A, Belbase S, Lamichhane G, Gyawali N, Dahal RK. The impact of COVID-19 outbreak and perceptions of people towards household waste management chain in nepal. Geoenviron Disaster. 2021;8(1):14. doi: 10.1186/s40677-021-00188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts EC (2020) Our life is plasticized: new research shows microplastics in our food, water, and air. Mongabay Environmental News. https://news.mongabay.com/2020/07/our-life-is-plasticized-new-research-shows-microplastics-in-our-food-water-air/
- Allan J, Belz S, Hoeveler A, Hugas M, Okuda H, Patri A, Rauscher H, Silva P, Slikker W, Sokull-Kluettgen B, Tong W, Anklam E. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul Toxicol Pharmacol. 2021;122:104885. doi: 10.1016/j.yrtph.2021.104885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Allen D, Phoenix VR, Roux GL, Jiménez PD, Simonneau A, Binet S, Galop D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat Geosci. 2019 doi: 10.1038/s41561-019-0335-5. [DOI] [Google Scholar]
- Ambrosini R, Azzoni RS, Pittino F, Diolaiuti G, Franzetti A, Parolini M. First evidence of microplastic contamination in the supraglacial debris of an alpine glacier. Environ Pollut. 2019;253:297–301. doi: 10.1016/j.envpol.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Amelia TSM, Khalik WMAWM, Ong MC, Shao YT, Pan H-J, Bhubalan K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog Earth Planet Sci. 2021;8(1):12. doi: 10.1186/s40645-020-00405-4. [DOI] [Google Scholar]
- Ammala A, Bateman S, Dean K, Petinakis E, Sangwan P, Wong S, Yuan Q, Yu L, Patrick C, Leong KH. An overview of degradable and biodegradable polyolefins. Prog Polym Sci. 2011;36(8):1015–1049. doi: 10.1016/j.progpolymsci.2010.12.002. [DOI] [Google Scholar]
- Anagnosti L, Varvaresou A, Pavlou P, Protopapa E, Carayanni V. Worldwide actions against plastic pollution from microbeads and microplastics in cosmetics focusing on European policies. Mar Pollut Bull. 2021;162:111883. doi: 10.1016/j.marpolbul.2020.111883. [DOI] [PubMed] [Google Scholar]
- Andrady AL. Microplastics in the marine environment. Mar Pollut Bull. 2011;62(8):1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Aragaw TA. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar Pollut Bull. 2020;159:111517. doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atis S. The respiratory effects of occupational polypropylene flock exposure. Eur Respir J. 2005;25(1):110–117. doi: 10.1183/09031936.04.00138403. [DOI] [PubMed] [Google Scholar]
- Awual MdR, Hasan MdM, Iqbal J, Islam A, Islam MdA, Rahman AAM, MM Naked-eye lead(II) capturing from contaminated water using innovative large-pore facial composite materials. Microchem J. 2020;154:104585. doi: 10.1016/j.microc.2019.104585. [DOI] [Google Scholar]
- Barboza LGA, Vethaak AD, Lavorante BRBO, Lundebye AK, Guilhermino L. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar Pollut Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- Barnes DKA, Galgani F, Thompson RC, Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B Biol Sci. 2009;364(1526):1985–1998. doi: 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso E, Ibañez MD, Aranda FI, Romero S. Polyethylene flock-associated interstitial lung disease in a Spanish female. Eur Respir J. 2002;20(6):1610–1612. doi: 10.1183/09031936.02.00030102. [DOI] [PubMed] [Google Scholar]
- Beladi-Mousavi SM, Hermanová S, Ying Y, Plutnar J, Pumera M. A maze in plastic wastes: autonomous motile photocatalytic microrobots against microplastics. ACS Appl Mater Interface. 2021;13(21):25102–25110. doi: 10.1021/acsami.1c04559. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Wirzberge V, Krumpen T, Lorenz C, Primpke S, Tekman MB, Gerdts G. High quantities of microplastic in arctic deep-sea sediments from the hausgarten observatory. Environ Sci Technol. 2017;51(19):11000–11010. doi: 10.1021/acs.est.7b03331. [DOI] [PubMed] [Google Scholar]
- Bhagwat G, Gray K, Wilson SP, Muniyasamy S, Vincent SGT, Bush R, Palanisami T. Benchmarking bioplastics: a natural step towards a sustainable future. J Polym Environ. 2020;28(12):3055–3075. doi: 10.1007/s10924-020-01830-8. [DOI] [Google Scholar]
- Bhardwaj N, Kumar B, Verma P. Microwave-assisted pretreatment using alkali metal salt in combination with orthophosphoric acid for generation of enhanced sugar and bioethanol. Biomass Convers Biorefin. 2020 doi: 10.1007/s13399-020-00640-1. [DOI] [Google Scholar]
- Boots B, Russell CW, Green DS. Effects of microplastics in soil ecosystems: above and below ground. Environ Sci Technol. 2019;53(19):11496–11506. doi: 10.1021/acs.est.9b03304. [DOI] [PubMed] [Google Scholar]
- Bornscheuer UT. Feeding on plastic. Science. 2016;351(6278):1154–1155. doi: 10.1126/science.aaf2853. [DOI] [PubMed] [Google Scholar]
- Brandts I, Teles M, Gonçalves AP, Barreto A, Franco-Martinez L, Tvarijonaviciute A, Martins MA, Soares AMVM, Tort L, Oliveira M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci Total Environ. 2018;643:775–784. doi: 10.1016/j.scitotenv.2018.06.257. [DOI] [PubMed] [Google Scholar]
- Cabernard L, Roscher L, Lorenz C, Gerdts G, Primpke S. Comparison of raman and fourier transform infrared spectroscopy for the quantification of microplastics in the aquatic environment. Environ Sci Technol. 2018;52(22):13279–13288. doi: 10.1021/acs.est.8b03438. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang J, Peng J, Tan Z, Zhan Z, Tan X, Chen Q. Characteristic of microplastics in the atmospheric fallout from dongguan city, China: preliminary research and first evidence. Environ Sci Pollut Res. 2017;24(32):24928–24935. doi: 10.1007/s11356-017-0116-x. [DOI] [PubMed] [Google Scholar]
- Carrington D (2018) Scientists accidentally create a mutant enzyme that eats plastic bottles. The Guardian. https://www.theguardian.com/environment/2018/apr/16/scientists-accidentally-create-mutant-enzyme-that-eats-plastic-bottles
- Castelvetro V, Corti A, Biale G, Ceccarini A, Degano I, La Nasa J, Lomonaco T, Manariti A, Manco E, Modugno F, Vinciguerra V. New methodologies for the detection, identification, and quantification of microplastics and their environmental degradation by-products. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-12466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro GB, Bernegossi AC, Felipe MC, Corbi JJ. Is the development of Daphnia magna neonates affected by short-term exposure to polyethylene microplastics? J Environ Sci Health A. 2020;55(8):935–946. doi: 10.1080/10934529.2020.1756656. [DOI] [PubMed] [Google Scholar]
- CCME (2018) Canadian Council of Ministers of the Environment. http://extwprlegs1.fao.org/docs/pdf/can189817.pdf
- Chang X, Xue Y, Li J, Zou L, Tang M. Potential health impact of environmental micro- and nanoplastics pollution. J Appl Toxicol. 2020;40(1):4–15. doi: 10.1002/jat.3915. [DOI] [PubMed] [Google Scholar]
- Chen W, Chu Q, Ye X, Sun Y, Liu Y, Jia R, Li Y, Tu P, Tang Q, Yu T, Chen C, Zheng X. Canidin-3-glucoside prevents nano-plastics induced toxicity via activating autophagy and promoting discharge. Environ Pollut. 2021;274:116524. doi: 10.1016/j.envpol.2021.116524. [DOI] [PubMed] [Google Scholar]
- Chiu HW, Xia T, Lee YH, Chen CW, Tsai JC, Wang YJ. Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress. Nanoscale. 2015;7(2):736–746. doi: 10.1039/C4NR05509H. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. Microplastic ingestion by zooplankton. Environ Sci Technol. 2013;47(12):6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod calanus helgolandicus. Environ Sci Technol. 2015;49(2):1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- Cunningham EM, Ehlers SM, Dick JTA, Sigwart JD, Linse K, Dick JJ, Kiriakoulakis K. High abundances of microplastic pollution in deep-sea sediments: evidence from antarctica and the Southern ocean. Environ Sci Technol. 2020;54(21):13661–13671. doi: 10.1021/acs.est.0c03441. [DOI] [PubMed] [Google Scholar]
- da Costa Araújo AP, Gomes AR, Malafaia G. Hepatotoxicity of pristine polyethylene microplastics in neotropical physalaemus cuvieri tadpoles (Fitzinger, 1826) J Hazard Mater. 2020;386:121992. doi: 10.1016/j.jhazmat.2019.121992. [DOI] [PubMed] [Google Scholar]
- Dauvergne P. The power of environmental norms: marine plastic pollution and the politics of microbeads. Environ Polit. 2018;27(4):579–597. doi: 10.1080/09644016.2018.1449090. [DOI] [Google Scholar]
- de Machado AAS, KloasZarflHempelRillig WCSMC. Microplastics as an emerging threat to terrestrial ecosystems. Glob Change Biol. 2018;24(4):1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Machado AA, Lau CW, Kloas W, Bergmann J, Bachelier JB, Faltin E, Becker R, Görlich AS, Rillig MC. Microplastics can change soil properties and affect plant performance. Environ Sci Technol. 2019;53(10):6044–6052. doi: 10.1021/acs.est.9b01339. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;7(1):46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Cai L, Sun F, Li G, Che Y. Public attitudes towards microplastics: perceptions, behaviors and policy implications. Resour Conserv Recycl. 2020;163:105096. doi: 10.1016/j.resconrec.2020.105096. [DOI] [Google Scholar]
- Diaz-Basantes MF, Conesa JA, Fullana A. Microplastics in honey, beer, milk and refreshments in ecuador as emerging contaminants. Sustainability. 2020;12(14):5514. doi: 10.3390/su12145514. [DOI] [Google Scholar]
- Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut. 2017;221:453–458. doi: 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Elkhatib D, Oyanedel-Craver V. A critical review of extraction and identification methods of microplastics in wastewater and drinking water. Environ Sci Technol. 2020;54(12):7037–7049. doi: 10.1021/acs.est.9b06672. [DOI] [PubMed] [Google Scholar]
- Emadian SM, Onay TT, Demirel B. Biodegradation of bioplastics in natural environments. Waste Manage. 2017;59:526–536. doi: 10.1016/j.wasman.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA. Lost, but found with nile red: a novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ Sci Technol. 2017;51(23):13641–13648. doi: 10.1021/acs.est.7b04512. [DOI] [PubMed] [Google Scholar]
- Eschenbacher WL, Kreiss K, Lougheed MD, Pransky GS, Day B, Castellan RM. Nylon flock-associated interstitial lung disease. Am J Respir Crit Care Med. 1999;159(6):2003–2008. doi: 10.1164/ajrccm.159.6.9808002. [DOI] [PubMed] [Google Scholar]
- Fadare OO, Okoffo ED. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci Total Environ. 2020;737:140279. doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte M, Iachetta G, Tussellino M, Carotenuto R, Prisco M, De Falco M, Laforgia V, Valiante S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol in Vitro. 2016;31:126–136. doi: 10.1016/j.tiv.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Fotopoulou KN, Karapanagioti HK (2019) Degradation of various plastics in the environment. In: Hazardous chemicals associated with plastics in the marine environment, Springer, Cham
- Fournier SB, D’Errico JN, Adler DS, Kollontzi S, Goedken MJ, Fabris L, Yurkow EJ, Stapleton PA. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol. 2020;17(1):55. doi: 10.1186/s12989-020-00385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funck M, Yildirim A, Nickel C, Schram J, Schmidt TC, Tuerk J. Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC–MS. MethodX. 2020;7:100778. doi: 10.1016/j.mex.2019.100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Sun C. A marine bacterial community capable of degrading poly(ethylene terephthalate) and polyethylene. J Hazard Mater. 2021;416:125928. doi: 10.1016/j.jhazmat.2021.125928. [DOI] [PubMed] [Google Scholar]
- Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, Langlois V, Kelly FJ, Tassin B. Microplastics in air: are we breathing it in? Curr Opin Environ Sci Health. 2018;1:1–5. doi: 10.1016/j.coesh.2017.10.002. [DOI] [Google Scholar]
- GESAMP (2015) Sources, fate and effects of microplastics in the marine environment: a global assessment (No. 90; p. 96). https://ec.europa.eu/environment/marine/good-environmental-status/descriptor-10/pdf/GESAMP_microplastics%20full%20study.pdf
- Gewert B, Plassmann MM, MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impact. 2015;17(9):1513–1521. doi: 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillibert R, Balakrishnan G, Deshoules Q, Tardivel M, Magazzù A, Donato MG, Maragò OM, Lamy de La Chapelle M, Colas F, Lagarde F, Gucciardi PG. Raman tweezers for small microplastics and nanoplastics identification in seawater. Environ Sci Technol. 2019;53(15):9003–9013. doi: 10.1021/acs.est.9b03105. [DOI] [PubMed] [Google Scholar]
- Gopinath KP, Nagarajan VM, Krishnan A, Malolan R. A critical review on the influence of energy, environmental and economic factors on various processes used to handle and recycle plastic wastes: development of a comprehensive index. J Clean Prod. 2020;274:123031. doi: 10.1016/j.jclepro.2020.123031. [DOI] [Google Scholar]
- Guo JJ, Huang XP, Xiang L, Wang YZ, Li YW, Li H, Cai QY, Mo CH, Wong MH. Source, migration and toxicology of microplastics in soil. Environ Int. 2020;137:105263. doi: 10.1016/j.envint.2019.105263. [DOI] [PubMed] [Google Scholar]
- Hall AR, Geoghegan M. Polymers and biopolymers at interfaces. Rep Prog Phys. 2018;81(3):036601. doi: 10.1088/1361-6633/aa9e9c. [DOI] [PubMed] [Google Scholar]
- Halstead JE, Smith JA, Carter EA, Lay PA, Johnston EL. Assessment tools for microplastics and natural fibres ingested by fish in an urbanised estuary. Environ Pollut. 2018;234:552–561. doi: 10.1016/j.envpol.2017.11.085. [DOI] [PubMed] [Google Scholar]
- Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M, Herrling MP, Hess MC, Ivleva NP, Lusher AL, Wagner M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol. 2019;53(3):1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- He D, Bristow K, Filipović V, Lv J, He H. Microplastics in terrestrial ecosystems: a scientometric analysis. Sustainability. 2020;12(20):8739. doi: 10.3390/su12208739. [DOI] [Google Scholar]
- Hermabessiere L, Himber C, Boricaud B, Kazour M, Amara R, Cassone AL, Laurentie M, Paul-Pont I, Soudant P, Dehaut A, Duflos G. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal Bioanal Chem. 2018;410(25):6663–6676. doi: 10.1007/s00216-018-1279-0. [DOI] [PubMed] [Google Scholar]
- Hermsen E, Mintenig SM, Besseling E, Koelmans AA. Quality criteria for the analysis of microplastic in biota samples: a critical review. Environ Sci Technol. 2018;52(18):10230–10240. doi: 10.1021/acs.est.8b01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Yousefi N, Tufenkji N. Are there nanoplastics in your personal care products? Environ Sci Technol Lett. 2017;4(7):280–285. doi: 10.1021/acs.estlett.7b00187. [DOI] [Google Scholar]
- Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol. 2012;46(6):3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- Hong SH, Shim WJ, Hong L. Methods of analysing chemicals associated with microplastics: a review. Anal Method. 2017;9(9):1361–1368. doi: 10.1039/C6AY02971Jz. [DOI] [Google Scholar]
- Hu L, Chernick M, Hinton DE, Shi H. Microplastics in small waterbodies and tadpoles from yangtze river delta China. Environ Sci Technol. 2018;52(15):8885–8893. doi: 10.1021/acs.est.8b02279. [DOI] [PubMed] [Google Scholar]
- Huerta Lwanga E, Gertsen H, Gooren H, Peters P, Salánki T, van der Ploeg M, Besseling E, Koelmans AA, Geissen V. Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (oligochaeta, lumbricidae) Environ Sci Technol. 2016;50(5):2685–2691. doi: 10.1021/acs.est.5b05478. [DOI] [PubMed] [Google Scholar]
- Hurley RR, Woodward JC, Rothwell JJ. Ingestion of microplastics by freshwater tubifex worms. Environ Sci Technol. 2017;51(21):12844–12851. doi: 10.1021/acs.est.7b03567. [DOI] [PubMed] [Google Scholar]
- Hwang J, Choi D, Han S, Choi J, Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci Total Environ. 2019;684:657–669. doi: 10.1016/j.scitotenv.2019.05.071. [DOI] [PubMed] [Google Scholar]
- Hwang J, Choi D, Han S, Jung SY, Choi J, Hong J. Potential toxicity of polystyrene microplastic particles. Sci Rep. 2020;10(1):7391. doi: 10.1038/s41598-020-64464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Xia J, Pan Z, Yang J, Wang W, Fu Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ Pollut. 2018;235:322–329. doi: 10.1016/j.envpol.2017.12.088. [DOI] [PubMed] [Google Scholar]
- Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- Jujun R, Jia L, Zhenming X. Improvements of the recovery line of waste toner cartridges on environmental and safety performasnces. Environ Sci Technol. 2013;47(12):6457–6462. doi: 10.1021/es305311k. [DOI] [PubMed] [Google Scholar]
- Kabir E, Kaur R, Lee J, Kim KH, Kwon EE. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J Clean Prod. 2020;258:120536. doi: 10.1016/j.jclepro.2020.120536. [DOI] [Google Scholar]
- Kalantar ZN, Karim MR, Mahrez A. A review of using waste and virgin polymer in pavement. Constr Build Mater. 2012;33:55–62. doi: 10.1016/j.conbuildmat.2012.01.009. [DOI] [Google Scholar]
- Kamada A, Rodriguez-Garcia M, Ruggeri FS, Shen Y, Levin A, Knowles TPJ. Controlled self-assembly of plant proteins into high-performance multifunctional nanostructured films. Nat Commun. 2021;12(1):3529. doi: 10.1038/s41467-021-23813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern DG, Kuhn C, Ely EW, Pransky GS, Mello CJ, Fraire AE, Müller J. Flock worker’s lung. Chest. 2000;117(1):251–259. doi: 10.1378/chest.117.1.251. [DOI] [PubMed] [Google Scholar]
- Khandaker S, Toyohara Y, Saha GC, Awual MdR, Kuba T. Development of synthetic zeolites from bio-slag for cesium adsorption: Kinetic, isotherm and thermodynamic studies. J Water Process Eng. 2020;33:101055. doi: 10.1016/j.jwpe.2019.101055. [DOI] [Google Scholar]
- Kik K, Bukowska B, Sicińska P. Polystyrene nanoparticles: sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ Pollut. 2020;262:114297. doi: 10.1016/j.envpol.2020.114297. [DOI] [PubMed] [Google Scholar]
- Klein M, Fischer EK. Microplastic abundance in atmospheric deposition within the metropolitan area of hamburg, Germany. Sci Total Environ. 2019;685:96–103. doi: 10.1016/j.scitotenv.2019.05.405. [DOI] [PubMed] [Google Scholar]
- Kole PJ, Löhr AJ, Van Belleghem F, Ragas A. Wear and tear of tyres: a stealthy source of microplastics in the environment. Int J Environ Res Public Health. 2017;14(10):1265. doi: 10.3390/ijerph14101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosore C, Ojwang L, Maghanga J, Kamau J, Kimeli A, Omukoto J, Ngisiag’e N, Mwaluma J, Ong’ada H, Magori C, Ndirui E. Occurrence and ingestion of microplastics by zooplankton in Kenya’s marine environment: first documented evidence. Afr J Mar Sci. 2018;40(3):225–234. doi: 10.2989/1814232X.2018.1492969. [DOI] [Google Scholar]
- Kumar M, Xiong X, He M, Tsang DCW, Gupta J, Khan E, Harrad S, Hou D, Ok YS, Bolan NS. Microplastics as pollutants in agricultural soils. Environ Pollut. 2020;265:114980. doi: 10.1016/j.envpol.2020.114980. [DOI] [PubMed] [Google Scholar]
- Lambert S, Sinclair C, Boxall A. Occurrence, degradation, and effect of polymer-based materials in the environment. In: Whitacre DM, editor. Reviews of environmental contamination and toxicology. Cham: Springer; 2014. pp. 1–53. [DOI] [PubMed] [Google Scholar]
- Laskar N, Kumar U. Plastics and microplastics: a threat to the environment. Environ Technol Innov. 2019;14:100352. doi: 10.1016/j.eti.2019.100352. [DOI] [Google Scholar]
- Lee H, Shim WJ, Kwon JH. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci Total Environ. 2014;470–471:1545–1552. doi: 10.1016/j.scitotenv.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Lee H, Kunz A, Shim WJ, Walther BA. Microplastic contamination of table salts from Taiwan, including a global review. Sci Rep. 2019;9(1):10145. doi: 10.1038/s41598-019-46417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Liu M, Song Y, Lu S, Hu J, Cao C, Xie B, Shi H, He D. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ Sci Nano. 2018;5(8):2009–2020. doi: 10.1039/C8EN00412A. [DOI] [Google Scholar]
- Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish danio rerio and nematode caenorhabditis elegans. Sci Total Environ. 2018;619–620:1–8. doi: 10.1016/j.scitotenv.2017.11.103. [DOI] [PubMed] [Google Scholar]
- Lenz R, Enders K, Stedmon CA, Mackenzie DMA, Nielsen TG. A critical assessment of visual identification of marine microplastic using raman spectroscopy for analysis improvement. Mar Pollut Bull. 2015;100(1):82–91. doi: 10.1016/j.marpolbul.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Leslie HA, van Velzen MJ, Brandsma SH, Vethaak D, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022 doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- Li W, Luo Y, Pan X. Microplastics in agricultural soils. In: He D, Luo Y, editors. Microplastics in terrestrial environments. Cham: Springer; 2020. pp. 63–76. [Google Scholar]
- Liebezeit G, Liebezeit E. Non-pollen particulates in honey and sugar. Food Addit Contam A. 2013;30(12):2136–2140. doi: 10.1080/19440049.2013.843025. [DOI] [PubMed] [Google Scholar]
- Liebezeit G, Liebezeit E. Synthetic particles as contaminants in German beers. Food Addit Contam A. 2014;31(9):1574–1578. doi: 10.1080/19440049.2014.945099. [DOI] [PubMed] [Google Scholar]
- Lin D, Yang G, Dou P, Qian S, Zhao L, Yang Y, Fanin N. Microplastics negatively affect soil fauna but stimulate microbial activity: insights from a field-based microplastic addition experiment. Proc R Soc B Biol Sci. 2020;287(1934):20201268. doi: 10.1098/rspb.2020.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Brutto S, Iaciofano D, Lo Turco V, Potortì AG, Rando R, Arizza V, Di Stefano V. First assessment of plasticizers in marine coastal litter-feeder fauna in the mediterranean sea. Toxics. 2021;9(2):31. doi: 10.3390/toxics9020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol. 2016;50(7):4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Lu L, Wan Z, Luo T, Fu Z, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Luo T, Zhang Y, Wang C, Wang X, Zhou J, Shen M, Zhao Y, Fu Z, Jin Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ Pollut. 2019;255:113122. doi: 10.1016/j.envpol.2019.11312210.1016/j.envpol.2019.113122. [DOI] [PubMed] [Google Scholar]
- Lusher AL, Welden NA, Sobral P, Cole M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal Method. 2017;9(9):1346–1360. doi: 10.1039/C6AY02415G. [DOI] [Google Scholar]
- Lusher A. Microplastics in the marine environment: distribution, interactions and effects. In: Bergmann M, Gutow L, Klages M, editors. Marine anthropogenic litter. Cham: Springer; 2015. pp. 245–307. [Google Scholar]
- Maes T, Jessop R, Wellner N, Haupt K, Mayes AG. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with nile red. Sci Rep. 2017;7(1):44501. doi: 10.1038/srep44501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magara G, Khan FR, Pinti M, Syberg K, Inzirillo A, Elia AC. Effects of combined exposures of fluoranthene and polyethylene or polyhydroxybutyrate microplastics on oxidative stress biomarkers in the blue mussel (Mytilus edulis) J Toxicol Environ Health A. 2019;82(10):616–625. doi: 10.1080/15287394.2019.1633451. [DOI] [PubMed] [Google Scholar]
- Magrì D, Veronesi M, Sánchez-Moreno P, Tolardo V, Bandiera T, Pompa PP, Athanassiou A, Fragouli D. PET nanoplastics interactions with water contaminants and their impact on human cells. Environ Pollut. 2021;271:116262. doi: 10.1016/j.envpol.2020.116262. [DOI] [PubMed] [Google Scholar]
- Mahadevan G, Valiyaveettil S. Understanding the interactions of poly(methyl methacrylate) and poly(vinyl chloride) nanoparticles with BHK-21 cell line. Sci Rep. 2021;11(1):2089. doi: 10.1038/s41598-020-80708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler GJ, Esch MB, Tako E, Southard TL, Archer SD, Glahn RP, Shuler ML. Oral exposure to polystyrene nanoparticles affects iron absorption. Nat Nanotechnol. 2012;7(4):264–271. doi: 10.1038/nnano.2012.3. [DOI] [PubMed] [Google Scholar]
- Mani T, Frehland S, Kalberer A, Burkhardt-Holm P. Using castor oil to separate microplastics from four different environmental matrices. Anal Method. 2019;11(13):1788–1794. doi: 10.1039/C8AY02559B. [DOI] [Google Scholar]
- McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol. 2014;48(20):11863–11871. doi: 10.1021/es503610r. [DOI] [PubMed] [Google Scholar]
- McDevitt JP, Criddle CS, Morse M, Hale RC, Bott CB, Rochman CM. Addressing the issue of microplastics in the wake of the microbead-free waters act–a new standard can facilitate improved policy. Environ Sci Technol. 2017;51(12):6611–6617. doi: 10.1021/acs.est.6b05812. [DOI] [PubMed] [Google Scholar]
- Mendieta CM, Vallejos ME, Felissia FE, Chinga-Carrasco G, Area MC. Review: bio-polyethylene from wood wastes. J Polym Environ. 2020;28(1):1–16. doi: 10.1007/s10924-019-01582-0. [DOI] [Google Scholar]
- Messinetti S, Mercurio S, Scarì G, Pennati A, Pennati R. Ingested microscopic plastics translocate from the gut cavity of juveniles of the ascidian Ciona intestinalis. Eur Zool J. 2019;86(1):189–195. doi: 10.1080/24750263.2019.1616837. [DOI] [Google Scholar]
- Möller JN, Löder MGJ, Laforsch C. Finding microplastics in soils: a review of analytical methods. Environ Sci Technol. 2020;54(4):2078–2090. doi: 10.1021/acs.est.9b04618. [DOI] [PubMed] [Google Scholar]
- Munier B, Bendell LI. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLoS ONE. 2018;13(2):e0191759. doi: 10.1371/journal.pone.0191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munno K, De Frond H, O’Donnell B, Rochman CM. Increasing the accessibility for characterizing microplastics: introducing new application-based and spectral libraries of plastic particles (SLoPP and SLoPP-E) Anal Chem. 2020;92(3):2443–2451. doi: 10.1021/acs.analchem.9b03626. [DOI] [PubMed] [Google Scholar]
- Nan B, Su L, Kellar C, Craig NJ, Keough MJ, Pettigrove V. Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria. Aust Environ Pollut. 2020;259:113865. doi: 10.1016/j.envpol.2019.113865. [DOI] [PubMed] [Google Scholar]
- Napper IE, Davies BFR, Clifford H, Elvin S, Koldewey HJ, Mayewski PA, Miner KR, Potocki M, Elmore AC, Gajurel AP, Thompson RC. Reaching new heights in plastic pollution—preliminary findings of microplastics on mount everest. One Earth. 2020;3(5):621–630. doi: 10.1016/j.oneear.2020.10.020. [DOI] [Google Scholar]
- Pettipas S, Bernier M, Walker TR. A Canadian policy framework to mitigate plastic marine pollution. Mar Policy. 2016;68:117–122. doi: 10.1016/j.marpol.2016.02.025. [DOI] [Google Scholar]
- Picó Y, Barceló D. Analysis and prevention of microplastics pollution in water: current perspectives and future directions. ACS Omega. 2019;4(4):6709–6719. doi: 10.1021/acsomega.9b00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl S, Leibner A, Löder MGJ, Dris R, Bogner C, Laforsch C. Identification and quantification of macro- and microplastics on agricultural farmland. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-36172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivokonsky M, Cermakova L, Novotna K, Peer P, Cajthaml T, Janda V. Occurrence of microplastics in raw and treated drinking water. Sci Total Environ. 2018;643:1644–1651. doi: 10.1016/j.scitotenv.2018.08.102. [DOI] [PubMed] [Google Scholar]
- Plastics- the facts (2022) https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf
- Powell JJ, Thoree V, Pele LC. Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract. Br J Nutr. 2007;98(S1):S59–S63. doi: 10.1017/S0007114507832922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee M, Dargahi L, Eslami A, Beirami E, Jahangiri-rad M, Sabour S, Amereh F. Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure. Chemosphere. 2018;193:745–753. doi: 10.1016/j.chemosphere.2017.11.076. [DOI] [PubMed] [Google Scholar]
- Ramsperger AFRM, Narayana VKB, Gross W, Mohanraj J, Thelakkat M, Greiner A, Schmalz H, Kress H, Laforsch C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci Adv. 2020;6(50):eabd1211. doi: 10.1126/sciadv.abd1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendón-Villalobos R, Ortíz-Sánchez A, Tovar-Sánchez E, Flores-Huicochea E. The role of biopolymers in obtaining environmentally friendly materials. In: Poletto M, editor. Composites from renewable and sustainable materials. United States: InTech; 2016. [Google Scholar]
- Ripken C, Kotsifaki DG, Nic Chormaic S. Analysis of small microplastics in coastal surface water samples of the subtropical island of okinawa, Japan. Sci Total Environ. 2021;760:143927. doi: 10.1016/j.scitotenv.2020.143927. [DOI] [PubMed] [Google Scholar]
- Roch S, Brinker A. Rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes. Environ Sci Technol. 2017;51(8):4522–4530. doi: 10.1021/acs.est.7b00364. [DOI] [PubMed] [Google Scholar]
- Rogers K (2020) Microplastics | Definition, properties, & plastic pollution. encyclopedia britannica. https://www.britannica.com/technology/microplastic
- Rosa W. A new era in global health. United States: Springer; 2017. Transforming our world: the 2030 agenda for sustainable development. [Google Scholar]
- Ruan J, Qin B, Huang J. Controlling measures of micro-plastic and nano pollutants: a short review of disposing waste toners. Environ Int. 2018;118:92–96. doi: 10.1016/j.envint.2018.05.038. [DOI] [PubMed] [Google Scholar]
- Sana SS, Dogiparthi LK, Gangadhar L, Chakravorty A, Abhishek N. Effects of microplastics and nanoplastics on marine environment and human health. Environ Sci Pollut Res. 2020;27(36):44743–44756. doi: 10.1007/s11356-020-10573-x. [DOI] [PubMed] [Google Scholar]
- Schymanski D, Goldbeck C, Humpf HU, Fürst P. Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154–162. doi: 10.1016/j.watres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Science of Plastics (2016) Science History Institute. https://www.sciencehistory.org/science-of-plastics
- Sharp A, Høj S, Wheeler M. Proscription and its impact on anti-consumption behaviour and attitudes: the case of plastic bags. J Consum Behav. 2010;9(6):470–484. doi: 10.1002/cb.335. [DOI] [Google Scholar]
- Shim WJ, Hong SH, Eo SE. Identification methods in microplastic analysis: a review. Anal Method. 2017;9(9):1384–1391. doi: 10.1039/C6AY02558G. [DOI] [Google Scholar]
- Simpson M (2019) Exploratory study of microplastics in the Eastern Himalayas of Nepal. GSA Annual Meeting in Phoenix, Arizona, USA - 2019. https://gsa.confex.com/gsa/2019AM/webprogram/Paper336514.html
- Tagg AS, Harrison JP, Ju-Nam Y, Sapp M, Bradley EL, Sinclair CJ, Ojeda JJ. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem Commun. 2017;53(2):372–375. doi: 10.1039/C6CC08798A. [DOI] [PubMed] [Google Scholar]
- Ter HA, Jeanneau L, Martignac M, Jardé E, Pedrono B, Brach L, Gigault J. Nanoplastic in the North atlantic subtropical gyre. Environ Sci Technol. 2017;51(23):13689–13697. doi: 10.1021/acs.est.7b03667. [DOI] [PubMed] [Google Scholar]
- The Lancet Planetary Health Microplastics and human health–an urgent problem. Lancet Planet Health. 2017;1(7):e254. doi: 10.1016/S2542-5196(17)30121-3. [DOI] [PubMed] [Google Scholar]
- Thompson A (2018) Solving microplastic pollution means reducing, recycling and fundamental rethinking. Scientific American. https://www.scientificamerican.com/article/solving-microplastic-pollution-means-reducing-recycling-mdash-and-fundamental-rethinking1/
- The Japan Times (2018) The Japan Times: https://www.japantimes.co.jp/news/2018/10/14/national/japan-eyes-reducing-amount-plastic-waste-much-25-2030/
- Tiseo L (2021) Global plastic production 1950–2018. Statista. https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/
- Tong H, Jiang Q, Hu X, Zhong X. Occurrence and identification of microplastics in tap water from China. Chemosphere. 2020;252:126493. doi: 10.1016/j.chemosphere.2020.126493. [DOI] [PubMed] [Google Scholar]
- UNDP (2021) https://www.np.undp.org/content/nepal/en/home/library/crisis_prevention_and_recovery/exploring-the-avenues-for-plastic-waste-management.html
- van den Berg P, Huerta-Lwanga E, Corradini F, Geissen V. Sewage sludge application as a vehicle for microplastics in Eastern Spanish agricultural soils. Environ Pollut. 2020;261:114198. doi: 10.1016/j.envpol.2020.114198. [DOI] [PubMed] [Google Scholar]
- Vink ETH, Rábago Karl R, Glassner DA, Springs B, O’Connor RP, Kolstad J, Gruber PR. The sustainability of natureworksTM polylactide polymers and ingeoTM polylactide fibers: an update of the future. Macromol Biosci. 2004;4(6):551–564. doi: 10.1002/mabi.200400023. [DOI] [PubMed] [Google Scholar]
- von Moos N, Burkhardt-Holm P, Köhler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L after an experimental exposure. Environ Sci Technol. 2012;46(20):11327–11335. doi: 10.1021/es302332w. [DOI] [PubMed] [Google Scholar]
- Wagner S, Reemtsma T. Things we know and don’t know about nanoplastic in the environment. Nat Nanotechnol. 2019;14(4):300–301. doi: 10.1038/s41565-019-0424-z. [DOI] [PubMed] [Google Scholar]
- Wagner J, Wang ZM, Ghosal S, Rochman C, Gassel M, Wall S. Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Anal Method. 2017;9(9):1479–1490. doi: 10.1039/C6AY02396G. [DOI] [Google Scholar]
- Wagner J, Wang ZM, Ghosal S, Murphy M, Wall S, Cook AM, Robberson W, Allen H. Nondestructive extraction and identification of microplastics from freshwater sport fish stomachs. Environ Sci Technol. 2019;53(24):14496–14506. doi: 10.1021/acs.est.9b05072. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang J, Hou S, Sun H. A simple method for quantifying polycarbonate and polyethylene terephthalate microplastics in environmental samples by liquid chromatography-tandem mass spectrometry. Environ Sci Technol Lett. 2017;4(12):530–534. doi: 10.1021/acs.estlett.7b00454. [DOI] [Google Scholar]
- Wen Z, Xie Y, Chen M, Dinga CD. China’s plastic import ban increases prospects of environmental impact mitigation of plastic waste trade flow worldwide. Nat Commun. 2021;12(1):425. doi: 10.1038/s41467-020-20741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek AM, Croot PL, Lombard F, Sheahan JN, Doyle TK. Microplastic ingestion by gelatinous zooplankton may lower efficiency of the biological pump. Environ Sci Technol. 2019;53(9):5387–5395. doi: 10.1021/acs.est.8b07174. [DOI] [PubMed] [Google Scholar]
- Witzig CS, Földi C, Wörle K, Habermehl P, Pittroff M, Müller YK, Lauschke T, Fiener P, Dierkes G, Freier KP, Zumbülte N. When good intentions go bad—false positive microplastic detection caused by disposable gloves. Environ Sci Technol. 2020;54(19):12164–12172. doi: 10.1021/acs.est.0c03742. [DOI] [PubMed] [Google Scholar]
- Woods JS, Verones F, Jolliet O, Vázquez-Rowe I, Boulay AM. A framework for the assessment of marine litter impacts in life cycle impact assessment. Ecol Ind. 2021;129:107918. doi: 10.1016/j.ecolind.2021.107918. [DOI] [Google Scholar]
- World Economic Forum (2016) The new plastics economy https://www.weforum.org/reports/the-new-plastics-economy-rethinking-the-future-of-plastics/
- Wu S, Wu M, Tian D, Qiu L, Li T. Effects of polystyrene microbeads on cytotoxicity and transcriptomic profiles in human Caco-2 cells. Environ Toxicol. 2020;35(4):495–506. doi: 10.1002/tox.22885. [DOI] [PubMed] [Google Scholar]
- Xanthos D, Walker TR. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): a review. Mar Pollut Bull. 2017;118(1–2):17–26. doi: 10.1016/j.marpolbul.2017.02.048. [DOI] [PubMed] [Google Scholar]
- Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2(1):85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- Yan Z, Liu Y, Zhang T, Zhang F, Ren H, Zhang Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ Sci Technol. 2022;56(1):414–421. doi: 10.1021/acs.est.1c03924. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen G, Wang J. Microplastics in the marine environment: sources, fates. Impact Microb Degrad Toxics. 2021;9(2):41. doi: 10.3390/toxics9020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang Y, Kang S, Wang Z, Wu C. Microplastics in soil: a review on methods, occurrence, sources, and potential risk. Sci Total Environ. 2021;780:146546. doi: 10.1016/j.scitotenv.2021.146546. [DOI] [PubMed] [Google Scholar]
- Yee MSL, Hii LW, Looi CK, Lim WM, Wong SF, Kok YY, Tan BK, Wong CY, Leong CO. Impact of microplastics and nanoplastics on human health. Nanomaterials. 2021;11(2):496. doi: 10.3390/nano11020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Jiang C, Wen X, Du C, Zhong W, Feng Z, Long Y, Ma Y. Microplastic pollution in surface water of urban lakes in changsha, China. Int J Environ Res Public Health. 2019;16(9):1650. doi: 10.3390/ijerph16091650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong C, Valiyaveettil S, Tang B. Toxicity of microplastics and nanoplastics in mammalian systems. Int J Environ Res Public Health. 2020;17(5):1509. doi: 10.3390/ijerph17051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K. A bacterium that degrades and assimilates poly(ethylene terephthalate) Science. 2016;351(6278):1196–1199. doi: 10.1126/science.aad6359. [DOI] [PubMed] [Google Scholar]
- Yu P, Liu Z, Wu D, Chen M, Lv W, Zhao Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol. 2018;200:28–36. doi: 10.1016/j.aquatox.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhou D, Li Z, Zhu C. Advancement and challenges of microplastic pollution in the aquatic environment: a review. Water Air Soil Pollut. 2018;229(5):140. doi: 10.1007/s11270-018-3788-z. [DOI] [Google Scholar]
- Zhang K, Shi H, Peng J, Wang Y, Xiong X, Wu C, Lam PKS. Microplastic pollution in China’s inland water systems:a review of findings, methods, characteristics, effects, and management. Sci Total Environ. 2018;630:1641–1653. doi: 10.1016/j.scitotenv.2018.02.300. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang L, Kannan K. Polyethylene terephthalate and polycarbonate microplastics in pet food and feces from the United States. Environ Sci Technol. 2019;53(20):12035–12042. doi: 10.1021/acs.est.9b03912. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Shan J, Zhao J, Zhang W, Liu L, Wu F. Hyperspectral imaging based method for rapid detection of microplastics in the intestinal tracts of fish. Environ Sci Technol. 2019;53(9):5151–5158. doi: 10.1021/acs.est.8b07321. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang H, Yu K, Li N, Liu Y, Liu X, Zhang H, Yang B, Wu W, Gao J, Jiang J. Rapid monitoring approach for microplastics using portable pyrolysis-mass spectrometry. Anal Chem. 2020;92(6):4656–4662. doi: 10.1021/acs.analchem.0c00300. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kang S, Allen S, Allen D, Gao T, Sillanpää M. Atmospheric microplastics: a review on current status and perspectives. Earth Sci Rev. 2020;203:103118. doi: 10.1016/j.earscirev.2020.103118. [DOI] [Google Scholar]
- Zuzek P (2016) Coastal zone canada association. The zone 16. https://gallery.mailchimp.com/2a4b8bcd7085564c6496079df/files/THEZONE_FALL_2016.pdf