Abstract
Introduction:
Adults with mild traumatic brain injury (mTBI) are at risk for communication disorders, yet studies exploring cognitive-communication performance are currently lacking.
Aims:
This aim of this study was to characterize discourse-level performance by adults with mTBI on a standardized elicitation task and compare it to (a) healthy adults, (b) adults with orthopedic injuries (OIs), and (c) adults with moderate to severe TBI.
Method:
This study used a cross-sectional design. The participants included mTBI and OI groups recruited prospectively from an emergency medicine department. Moderate to severe TBI and healthy data were acquired from TalkBank. One-way analyses of variance were used to compare mean linguistic scores.
Results:
Seventy participants across all groups were recruited. Groups did not differ on demographic variables. The study found significant differences in both content and productivity measures among the groups. Variables did not appear sensitive to differentiate between mTBI and OI groups.
Discussion:
Cognitive and language performance of adults with mTBI is a pressing clinical issue. Studies exploring language with carefully selected control groups can influence the development of sensitive measures to identify individuals with cognitive-communication deficits.
Traumatic brain injury (TBI) is a public health concern, and it is estimated that 69.0 million people worldwide will incur TBI (Dewan et al., 2018), with 70%–90% of those being classified as mild traumatic brain injury (mTBI; Voss et al., 2015). After the acute stages of injury, individuals with TBI often report neurologically based symptoms such as cognitive difficulties (Raskin et al., 1998), sleep problems (Wiseman-Hakes et al., 2013), and disturbances of physical and emotional behavior such as headaches, anxiety, nausea, and depression (Karr et al., 2014). Although there are numerous studies centering on the negative effects of TBI on cognitive function, there is limited available literature on functions known to be dependent on cognition, such as expressive and receptive communication (Blyth et al., 2012). Research exploring communication, particularly language performance after TBI, has primarily focused on those with moderate to severe injuries (Coelho et al., 2013). This research has shed light on TBI-specific language deficits and has distinguished these deficits from other clinical adult populations such as stroke or progressive disease (Coelho, Grela, et al., 2005; Coelho, Ylvisaker, & Turkstra, 2005; Mozeiko et al., 2011).
Undoubtedly, language research in persons with moderate to severe TBI has progressed over the past 3 decades. However, research centered on mTBI, also known as concussion, which accounts for 87% of the total TBI population in the United States (Faul et al., 2010), is in the early stages of study. Clinical practice guidelines for treating communication disorders after mTBI have yet to be developed despite the knowledge that mTBI is a known risk factor for these disorders (Norman et al., 2013, 2020). In order to be able to develop these clinical recommendations for assessment and treatment, a rich understanding of the underlying mechanisms of these disorders and how they present and persist beyond the acute stages of injury is necessary.
Many individuals with TBI report cognitive-communication deficits, defined as “impairments of verbal and non-verbal language resulting from the disruption of cognitive skills” (American Speech-Language-Hearing Association, 1993, 2004). Success in modern day society is highly dependent on the ability to maintain interpersonal relationships through expressive and receptive language use; however, these cognitive-communication deficits affect everyday activities such as employment, education, and social interactions (Ruben, 2000). Deficits in attention, working memory, executive function, and speed of processing have been identified in adults post mTBI (Dean et al., 2012; Frencham et al., 2005; Miotto et al., 2010; Tombaugh et al., 2007; Walhovd et al., 2014). Research suggests that these underlying cognitive problems influence language use (Galetto et al., 2013). Speed of information processing critical to communication has been correlated with expressive and receptive language performance (Norman et al., 2019a, 2019b). Specific characterization of language performance after mTBI is not only an important research objective but a pressing clinical need, as early identification of deficits following mTBI has been linked with more favorable outcomes following injury (LeBlanc et al., 2006, 2014).
Clinical Gaps
Evidence-based treatment is dependent on accurate assessment. Understanding cognitive-communication disorders, at this time, is seriously limited due to insufficient clinical knowledge and a paucity of research. There are many challenges in the assessment of TBI-related cognitive-communication disorders, including ensuring the use of quality instruments and clinician confidence, and this is particularly significant in mTBI where there are no available clinical practice guidelines to inform assessment practices (Hardin & Kelly, 2019). Language assessment tools currently used by speech-language pathologists (SLPs) lack both sensitivity and specificity, and clinicians often utilize aphasia batteries, which present significant issues when these instruments fail to detect the subtle deficits specific to mTBI (Duff et al., 2002). Thus, SLPs have reported a reliance on neuropsychological measures or informal, nonstandardized measures to diagnose cognitive-communication disorders after mTBI (Krug & Turkstra, 2015).
As suggested earlier, what is known about the language performance of individuals with mTBI is limited. Adults with mTBI have demonstrated deficits in word retrieval and category naming (Barrow et al., 2003, 2006; Crawford et al., 2007; King et al., 2006; Tucker & Hanlon, 1998). Unfortunately, these studies have suffered from limitations in design and rigor, including limited demographic information about the study sample, variability in injuries, lack of well-matched controls, and the use of published assessments standardized for other populations (i.e., aphasia).
Discourse and TBI
Examining performance in connected speech, specifically at the level of discourse (e.g., narrative, argument, conversation), shows some promise. Discourse measures capture the relationship between cognitive and linguistic processes and present a realistic sample of an individual's connected speech (Chapman et al., 2001). By employing a tool that has been validated and shown to be sensitive to TBI-related language deficits in individuals with moderate to severe TBI (Bogart et al., 2012; Coelho et al., 2013; Power et al., 2020), researchers and, ultimately, clinicians could potentially shed light on the unique characteristics of mTBI-related communication problems and develop treatments addressing these problems.
Conversational discourse in mTBI during the acute stages of injury has been studied by one research group (LeBlanc et al., 2014, 2020). These studies have primarily focused on the relationship between cognitive-communication and rehabilitation as well as long-term outcomes. The “Protocole Montreal d'évaluation de la communication” (MEC), consisting of a 10-min conversation on two different topics with the examiner that is then rated on a 17-point checklist, was used to evaluate conversational discourse in adults with mild–severe TBI. The MEC checklist is composed of 17 communication behaviors, including word finding problems, no self-correction of word errors, imprecise expression of ideas, production of inappropriate or unexpected comments, and inappropriate topic switches, which have been previously identified as general cognitive-communication impairments in the literature (LeBlanc et al., 2014). The study team found that participants with TBI had scores below the 10th percentile on the Discourse subtest of the MEC. Notably, participants with mTBI showed errors in word-finding, self-correction, and idea expression. Speech rate (a measure of efficiency) was also a problem in 27% of the sample. While these studies have contributed much needed general knowledge about the importance of measuring communication competence in the acute stages of mTBI, the use of a checklist limits the depth of linguistic analyses and the implications of any language problems beyond broad communication terms. What remains unclear is how individuals with mTBI in the postacute stage of recovery perform on a widely used, standardized elicitation discourse task that has been shown to be sensitive to TBI-related communication deficits in more severe injury categories. Because little is known about language performance in mTBI, and the use of aphasia tests (Duff et al., 2002), such as the Boston Diagnostic Aphasia Examination or Western Aphasia Battery, is a predominant clinical practice by SLPs, there is also the need to carefully describe the nature of discourse production in this population relative to other populations.
Understanding the discourse characteristics inherent to moderate to severe TBI populations can inform these initial efforts in mTBI research. Several studies examining discourse deficits of the moderate and severe TBI population have associated performance with the recruitment of microlinguistic (defined as within sentence measures by Coelho, 2007) and macrolinguistic processes, defined as thematic elements that overlap over one discourse act (Davis & Coelho, 2004; Marini et al., 2014; Peach, 2013). These processes are representative of lexical and syntactic procedures and the cognitive integration of cohesion, coherence, and grammar to maintain contextual organization, respectively (Coelho, Grela, et al., 2005). A study by Coelho, Ylvisaker, and Turkstra (2005) demonstrated that individuals with TBI produced fewer propositions, demonstrating less complexity, organization, and clarity of discourse.
Cognitive functions are also implicated in studies exploring “story goodness,” defined as completeness plus organization (Lê et al., 2011). The use of both measures, completeness and goodness, to assess organization and content of discourse production distinguished participants with TBI from healthy controls in Lê et al. (2011). Findings from indices that represent the complex interchange between cognitive and linguistic functions support the previous notion of multilevel analyses to discriminate discourse production for the TBI population.
In addition to narrative discourse performance, researchers have also explored the implications of multilevel interactions for persuasive and procedural discourse in moderate and severe TBI. Ghayoumi et al. (2015) found a significant difference in persuasive discourse abilities of participants with TBI when compared to healthy controls. In this study, participants were asked to formulate an opinion and provide supporting rationale for the use of public transport or a private vehicle. Each response was assessed across four different linguistic measures: productivity, sentential complexity, maze, and cohesion. Individuals with moderate and severe TBI demonstrated significant differences across all measures during persuasive discourse production exhibited by reduced complexity of ideas and sentence structure, increased repetitions, revisions, abandoned utterances, nonlinguistic vocalizations, and audible pauses, and an overall difficulty linking ideas by lexical and grammatic relation.
Likewise, Stubbs et al. (2018) evaluated the productivity (i.e., total number of meaningful words, total number of utterances, speaking time, speech rate, words per utterance) and macrostructure (essential, optional, and low content information) of procedural discourse at 3 and 6 months postinjury. Participants with severe TBI exhibited “impoverished discourse” due to reduced linguistic productivity, and increased low content elements signifying repeated, tangential, or irrelevant information when asked to describe the essential steps for making a cheese and Vegemite sandwich. Results of both Ghayoumi et al. (2015) and Stubbs et al. (2018) underscore the importance of acknowledging underlying cognitive skills such as executive function when interpreting productivity performance in discourse of adults with TBI.
In some studies, however, discourse deficits have been found to be more domain-general rather than a resulting dysfunction of discrete cognitive functions. Byom and Turkstra (2017) explored the role and relationship between theory of mind and executive functions by quantifying mental state terms (MSTs), dysfluencies, and speech rate during discussions of controversial issues. The conditions changed throughout the discourse task to challenge mental flexibility and cognitive demand. Findings from this study demonstrated group effects exhibited by a decreased use of MSTs, meaning words demonstrating thoughts, feelings, and desires, and slower speaking rate for participants with TBI. However, when compared across conditions, both participants with TBI and control participants demonstrated both a decreased use of MSTs and a slower speaking rate. These results led researchers to believe that the cognitive demand of communication tasks, particularly in discourse, may contribute to social communication impairments in individuals with TBI and, consequently, may negatively impact the way these individuals are perceived by communication partners.
Based on knowledge gleaned from previous research, it is quite possible that the cognitive processes (e.g., attention, working memory, executive function, and speed of information processing) that have been implicated in discourse performance in severe TBI can also be affected in mTBI. Discourse tasks often require adequate timing of semantic and syntactic processing, organization, and inhibition skills. Measures that require a high level of coordination of cognitive and linguistic processes could be potentially be manipulated in discourse studies examining mTBI. Therefore it is a promising area of study with clinical relevance.
In summary, research in the use of discourse measures in moderate to severe TBI indicate that at least two distinct levels of measurement are necessary: those that tap into language content and meaning and those that measure elements related to productivity and efficiency such as words per minute, total words, duration of the language sample, and whether speech is fluent and understandable (Galski et al., 1998). Measures that tap into areas of cognition that are known to be impaired in mTBI are imperative to explore in language research. Norman et al. (2019a, 2019b) explored the effect of speed of information processing on language performance and found speed-based deficits at the word and sentence level of production and comprehension by manipulating language demands via speeded and unspeeded conditions. Therefore, in moving this research forward and examining connected speech in the mTBI population, it is important to not only include content measures but productivity and time-based measures as well to accurately assess how individuals will eventually perform in real-time conversational discourse. Understanding this interplay between cognition and language is at the center of understanding language performance after mTBI.
Aims
The purpose of this study was to characterize discourse-level language performance of adults with mTBI on a standardized elicitation task, the “Cinderella” story from the TBIBank (MacWhinney et al., 2011) protocol, and to determine whether their performance was significantly different from (a) demographically matched healthy adults, (b) adults with orthopedic injuries (OI), and (c) adults with moderate to severe TBI. We focused outcome measures on two general areas: content and productivity. These areas of interest are based on existing TBI research as well as our knowledge that cognitive areas such as information processing could significantly influence linguistic productivity. We predicted that individuals with mTBI would demonstrate lower language content as defined by total number of main concepts produced on the “Cinderella” story.
A secondary aim was to determine whether the groups were statistically different from each other on language productivity measures (see Table 1 for productivity measures of interest and their definitions) and to quantify the degree of that difference from each other. For both analyses, we predicted that results would show a graded effect such that participants with mTBI and moderate to severe TBI would produce relatively fewer main concepts and lower productivity scores when compared with healthy adults and adults with OIs.
Table 1.
Productivity measures as defined by MacWhinney (2017).
| Measure | Description |
|---|---|
| Duration | Total time of the sample in hours: minutes: seconds. |
| Total utterances | Includes all utterances used in computing MLU, plus unintelligible utterances (unintelligible); excludes non word utterances, for example a gesture only. |
| FREQ tokens aka Total Words | Total words tokens. Does not include repetitions and revisions. |
| Words per minute | FREQ tokens/duration converted to minutes |
| Propositional idea density | This measure was adapted from CPIDR3 (Computerized Propositional Idea Density Rater, third major version) CPIDR replicates Turner and Greene's rules (1977) for extracting propositions from text, based on the theory of Kintsch (1974) and the work of Snowdon et al. (1996) who showed that propositional idea density can be approximated by the number of verbs, adjectives, adverbs, prepositions, and conjunctions divided by the total number of words. |
| Retracing: retracing [//]: | Number of retracings (self-corrections or changes). |
| Disfluency index | Number of filled pauses, false starts revisions, repetitions divided by total no. of utterances. |
Note. MLU = mean length of utterance the number of morphemes (units of linguistic meaning) divided by number of utterances.
Method
Design
We used a cross-sectional observational study. All procedures were approved by the institutional review board.
Participants
Participants included in this study comprised four distinct groups: healthy adults, adults with nonsurgical orthopedic (OI) injuries, adults with mTBI, and adults with moderate to severe TBI. Adults in the mTBI and OI groups were recruited prospectively from an emergency medicine department (ED) in an academic medical center in the Midwestern United States. Eligible participants were those who presented to the ED and were diagnosed with an mTBI or a nonsurgical OI by a medical provider. After initial evaluation and care for their injuries, participants with mTBI or OI were discharged to home and participated in an assessment session 3–12 weeks after their injuries. This time period for inclusion in the study was chosen based on research stating typical physical recovery from TBI occurs after 2 weeks of injury (Carroll et al., 2004; Giza et al., 2018). The subacute period, cited in the literature as taking place between 3 weeks and 3 months postinjury (Bernstein, 2002; Iverson, 2005; Makdissi et al., 2015), is largely understudied and is of clinical importance because it is an important time period representing the appropriate time window for intervention; if symptoms are left untreated, individuals with mTBI are at risk for developing secondary symptoms such as anxiety or depression that can confound interpretation of cognitive symptoms (Bohnen & Jolles, 1992). Participants were included if they were (a) ages 18–55 years, (b) reported English as their primary language, and (c) were either diagnosed with TBI (International Classification of Diseases, Ninth Revision [ICD-9] Codes 850* and ICD-10 Codes S06.0*; World Health Organization, 1977, 2007), with diagnosis confirmed using a published definition of mTBI (Eisenberg et al., 2014) or were diagnosed with nonsurgical, traumatic OI as defined by ICD-9 Codes 800–829 and ICD-10 Codes S40-S49, S72, S82, and S92. Participants with OI were included in this study in order to distinguish between brain versus physical trauma on our variables of interest. Previous studies of discourse in adults with TBI have included OI control groups (Snow et al., 1997, 1999). In the current study, an OI comparison group was selected because it was believed that they shared demographic, pre-injury characteristics (e.g., risk-taking behaviors) with the participants with mTBI and had comparable levels of health care for their injuries. They also likely experienced similar levels of distress, which can potentially contribute to cognitive performance. Exclusion criteria for all participants were as follows: (a) a history of pre-injury medical or neurological disease affecting the brain (other than concussion), or language or learning disability; (b) indication of a health care surrogate on the medical record, which would indicate limited capacity to consent to research participation; or (c) failure of a pure-tone hearing screening using an air-conduction threshold of 30 dB. For more information regarding procedures for mTBI and OI groups, please see Norman et al. (2019a, 2019b).
Data for participants in the healthy and moderate to severe TBI group data were acquired from the shared online database, TalkBank (MacWhinney et al., 2011), specifically the Togher and Richardson databases. Healthy participants were selected from the Richardson database and are defined as “typically aging individuals with no history of neurological conditions who report English as their primary language” (Richardson & Dalton, 2016, p. 6). Participants in the moderate to severe group were selected from the Marshfield and Togher databases on TBIBank and were individuals diagnosed with a moderate to severe brain injury who also reported English as the primary language. Careful matching of groups by age and gender was employed prior to downloading of discourse data and analyses. Discourse data were collected using the TBI TalkBank Protocol (talkbank.org/AphasiaBank) for all groups. For the OI and mTBI groups, discourse samples were collected in person in a laboratory, recorded on audio, transcribed verbatim by a professional online transcription service (rev.com), and transferred to Codes for Human Analysis of Transcripts (CHAT; MacWhinney, 2000). For the healthy and moderate to severe TBI groups, transcripts were downloaded from TBIBank and transferred to CHAT (MacWhinney, 2000).
All participants completed the TBIBank protocol, and the Cinderella story retell was selected as the primary measure for transcription and analyses. The elicitation procedures are based on MacWhinney et al. (2011) protocol and can be found at: https://tbi.talkbank.org.1
Participant is presented with Cinderella book (pictures only).
Participant is informed that he or she will be telling a story and is asked whether Cinderella is a familiar story.
Participant is asked “Do you remember much about it? These pictures might remind you of how it goes. Take a look at the pictures and then I'll put the book away and ask you to tell the story in your own words.”
Participant is allowed to look through book (can be assisted with page turning if needed). If participant gives a response of fewer than three utterances, allow 10 s then prompt “What happened next” or “Go on.”
Continue until participant concludes story or it is clear he or she has finished. If no response, go to troubleshooting questions (https://aphasia.talkbank.org/protocol/).
For productivity measures, transcripts were entered into Computerized Language ANalysis (CLAN; MacWhinney, 2017) and time and linguistic coding was extracted using the MOR and EVAL programs (MacWhinney, 2017). Main concept analysis (MCA) content was scored by three independent raters who were master's-level speech-language pathology students. Procedures for MCA are detailed on Appendix A. CLAN transcripts were coded by one master's-level speech-language pathology student (P. H.), one trained research assistant, and by a licensed SLP (R. S. N.). Interrater reliability was established by each rater analyzing 20% of samples. Agreement was over 90% for all samples.
Discourse Measures
Because language performance by adults with TBI is well known to be influenced by cognitive function (Togher et al., 2014), our study team selected measures that would hypothetically be sensitive to cognitive changes after mTBI such as attention, working memory, and speed of information processing (Dean et al., 2012; Frencham et al., 2005; Miotto et al., 2010; Tombaugh et al., 2007; Walhovd et al., 2014). We hypothesized that deficits in these cognitive areas would significantly impact performance on discourse skills such as cohesion, lexical access, and productivity. Specific measures chosen were consistent with Coelho (2007)'s levels of discourse, and selected analyses were macrostructural (semantic content units) or microlinguistic (productivity, e.g., number of words, total words, words per minute).
Macrostructural Analysis: Linguistic Measures
MCA measures an individual's ability to express the general idea of a spoken narrative by considering the accuracy and completeness of their production on a scale from 1 to 3 (Richardson & Dalton, 2016). Appendix A demonstrates the MCA procedure as well as specific examples for scoring the Cinderella task, the discourse task selected for this study. We hypothesized that performance by participants in the TBI groups could be distinguished from the control (healthy and OI) groups using the criteria from the MCA method such as accuracy and completeness for the Cinderella Story Main Concepts (see Appendix A).
Microlinguistic Analyses: Productivity Measures
The productivity measures selected for analysis defined by MacWhinney (2017) were selected because of their sensitivity to TBI-related cognitive impairments (Galski et al., 1998; Togher et al., 2014) and are shown in Table 2.
Table 2.
Participant demographic characteristics.
| Demographic | Healthy (n = 19) | mTBI (n = 19) | OI (n = 15) | Mod–sev (n = 17) |
|---|---|---|---|---|
| Age (years), M (SD) | 27.97 (6.93) | 28.12 (7.78) | 28.22 (10.19) | 29.2 (8.19) |
| Age range | 21–43.90 | 18.5–48.40 | 19.8–52.10 | 20–46 |
| Female, n (%) | 12 (63.16) | 14 (73.68) | 10 (66.67) | 6 (35.29) |
| White, n (%) | 17 (89.47) | 17 (89.47) | 10 (66.67) | 17 (100.00) |
| African American, n (%) | 1 (5.26) | 0 | 3 (20) | 0 |
| Asian American, n (%) | 0 | 0 | 0 | 0 |
| Hispanic/Latino, n (%) | 1 (5.26) | 0 | 0 | 0 |
| Other, n (%) | 0 | 2 (10.53) | 2 (13.33) | 0 |
| Education (years), M (SD) | 15.76 (2.51) | 15.81 (0.45) | 15.87 (1.92) | 14.5 (1.87) |
Note. mTBI = mild traumatic brain injury; OI = orthopedic injury; Mod–sev = moderate to severe traumatic brain injury.
Statistical Analysis
We conducted a one-way analysis of variance (ANOVA) to compare mean total MCA and linguistic productivity scores across the four groups using SPSS Version 26.0 (p < .05). Games–Howell post hoc analyses were conducted to determine between-groups statistical differences. Outliers (n = 3) beyond the upper quartile and more than 2 SDs above/below the mean score of each variable were initially removed from analyses, but because their omission ultimately did not significantly alter results, the final analyses included the full sample.
Because data for four of our productivity variables (duration per second, total utterances, retracing, and dysfluency index) violated the normality assumption (Shapiro–Wilk tests < .05 threshold level), those variables were examined using the Kruskal–Wallis Test, a nonparametric one-way test of variance. The design and reporting were guided by relevant reporting frameworks (von Elm et al., 2008).
Results
We recruited a total of 70 participants (see Table 2). Groups did not differ significantly on key demographic variables (e.g., age, education). Of note, in three out of the four groups, female participants made up over 50% group. Table 3 lists descriptive statistics, including means and standard deviations for all the examined language variables.
Table 3.
Descriptive statistics for all language variables, arranged by group.
| Variable | N | M | SD | SE | 95% confidence interval for mean |
Minimum | Maximum | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||||
| Total MC | Healthy | 19 | 60.89 | 23.06 | 5.29 | 49.78 | 72.01 | 17 | 92 |
| OI | 15 | 55.40 | 16.26 | 4.19 | 46.40 | 64.40 | 28 | 81 | |
| mTBI | 19 | 54.89 | 19.22 | 4.41 | 45.63 | 64.16 | 23 | 82 | |
| Mod–sev | 17 | 31.41 | 15.55 | 3.77 | 23.42 | 39.41 | 11 | 59 | |
| Total | 70 | 50.93 | 21.79 | 2.60 | 45.73 | 56.12 | 11 | 92 | |
| Words/min | Healthy | 19 | 74.30 | 12.47 | 2.86 | 68.29 | 80.32 | 42.00 | 90.41 |
| OI | 15 | 143.81 | 34.48 | 8.90 | 124.71 | 162.90 | 92.37 | 221.70 | |
| mTBI | 19 | 138.37 | 31.71 | 7.27 | 123.09 | 153.65 | 85.98 | 195.81 | |
| Mod–sev | 16 | 64.32 | 37.50 | 9.38 | 44.34 | 84.30 | 24.34 | 150.33 | |
| Total | 69 | 104.74 | 46.46 | 5.59 | 93.58 | 115.90 | 24.34 | 221.70 | |
| Total words | Healthy | 19 | 142.95 | 60.50 | 13.88 | 113.79 | 172.11 | 60.00 | 274.00 |
| OI | 15 | 391.93 | 275.53 | 71.14 | 239.35 | 544.52 | 110.00 | 1292.00 | |
| mTBI | 19 | 358.68 | 209.95 | 48.17 | 257.49 | 459.88 | 90.00 | 698.00 | |
| Mod–sev | 17 | 283.94 | 215.06 | 52.16 | 173.37 | 394.52 | 59.00 | 786.00 | |
| Total | 70 | 289.10 | 219.32 | 26.21 | 236.80 | 341.40 | 59.00 | 1292.00 | |
| Density | Healthy | 19 | .50 | .03 | .01 | .49 | .51 | .45 | .54 |
| OI | 15 | .50 | .02 | .01 | .49 | .52 | .46 | .54 | |
| mTBI | 19 | .51 | .03 | .01 | .50 | .53 | .46 | .55 | |
| Mod–sev | 17 | .50 | .03 | .01 | .48 | .51 | .41 | .55 | |
| Total | 70 | .50 | .03 | .00 | .50 | .52 | .41 | .55 | |
| Total Utts | Healthy | 19 | 39.95 | 24.36 | 5.59 | 28.20 | 51.69 | 11.0 | 95.0 |
| OI | 15 | 38.27 | 32.56 | 8.41 | 20.23 | 56.30 | 12.0 | 147.0 | |
| mTBI | 19 | 36.11 | 20.42 | 4.68 | 26.26 | 45.95 | 8.0 | 75.0 | |
| Mod–sev | 17 | 25.59 | 13.65 | 3.31 | 18.57 | 32.61 | 9.0 | 59.0 | |
| Total | 70 | 35.06 | 23.52 | 2.81 | 29.45 | 40.67 | 8.0 | 147.0 | |
| Retracing | Healthy | 19 | 6.00 | 6.17 | 1.42 | 3.03 | 8.98 | .00 | 23.00 |
| OI | 15 | 3.80 | 2.68 | .69 | 2.32 | 5.28 | .00 | 9.00 | |
| mTBI | 19 | 4.53 | 4.07 | .94 | 2.56 | 6.49 | .00 | 15.00 | |
| Mod–sev | 17 | 3.24 | 2.14 | .52 | 2.14 | 4.33 | .00 | 7.00 | |
| Total | 70 | 4.46 | 4.23 | .52 | 3.45 | 5.47 | .00 | 23.00 | |
| Duration/s | Healthy | 19 | 340.00 | 211.54 | 48.53 | 238.04 | 441.96 | 79 | 890 |
| OI | 15 | 163.40 | 94.93 | 24.51 | 110.83 | 215.97 | 55 | 444 | |
| mTBI | 19 | 153.63 | 81.19 | 18.63 | 114.50 | 192.76 | 48 | 328 | |
| Mod–sev | 17 | 221.53 | 109.31 | 26.51 | 165.33 | 277.73 | 0 | 474 | |
| Total | 70 | 222.80 | 154.47 | 18.47 | 185.97 | 259.63 | 0 | 890 | |
| Disfluency index | Healthy | 19 | .19 | .09 | .02 | .15 | .24 | .06 | .36 |
| OI | 15 | .08 | .04 | .01 | .06 | .10 | .03 | .14 | |
| mTBI | 19 | .09 | .04 | .01 | .07 | .11 | .02 | .18 | |
| Mod–sev | 17 | .15 | .18 | .05 | .06 | .24 | .00 | .77 | |
| Total | 70 | .13 | .11 | .01 | .10 | .16 | .00 | .77 | |
Note. MC = main concept; OI = orthopedic injury; mTBI = mild traumatic brain injury; Mod–sev = moderate to severe traumatic brain injury; Utts = utterances; s = seconds.
MCAs
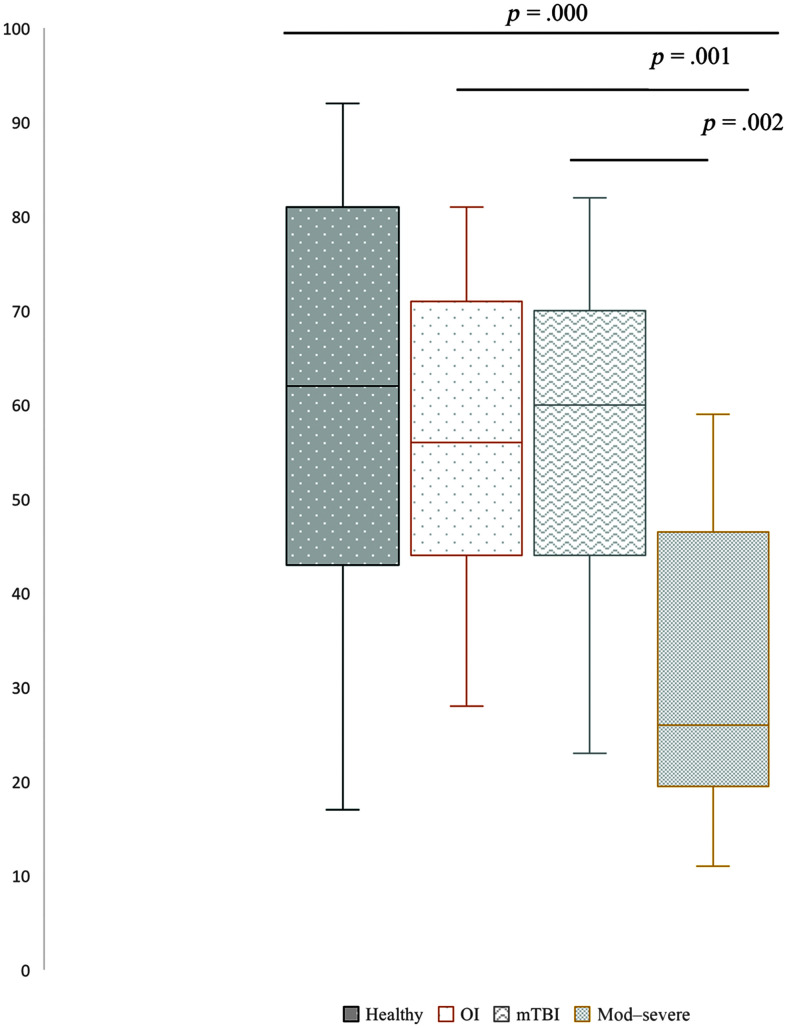
Table 4 shows the results of a one-way ANOVA for four language variables. Figure 1 illustrates a statistically significant difference in MCA scores among groups, F(3, 69) = 8.287, p = .000. A Games–Howell post hoc test (see Table 5) revealed that, in the production of total main concepts, individuals with moderate to severe TBI had significantly lower scores than healthy adults (p = .000), adults with OI (p = .001), and adults with mTBI (p = .002). There was a nonstatistically significant trend in MCA scores among the other three groups: Healthy adults had the highest scores (M = 60.89 SD = 23.06), followed by OI (M = 55.4, SD = 16.25), and adults with mTBI (M = 54.89, SD = 19.22), and those with moderate to severe TBI had the lowest MCA scores (M = 31.41, SD = 15.55).
Table 4.
Results of one-way analysis of variance for language variables.
| Variable | Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|---|
| Total Main concepts |
Between groups | 8961.346 | 3 | 2987.115 | 8.287 | .000 |
| Within groups | 23791.297 | 66 | 360.474 | |||
| Total | 32752.643 | 69 | ||||
| Words/min | Between groups | 88123.151 | 3 | 29374.384 | 32.566 | .000 |
| Within groups | 58629.730 | 65 | 901.996 | |||
| Total | 146752.881 | 68 | ||||
| Total words | Between groups | 656921.373 | 3 | 218973.791 | 5.429 | .002 |
| Within groups | 2662170.927 | 66 | 40335.923 | |||
| Total | 3319092.300 | 69 | ||||
| Density | Between groups | .003 | 3 | .001 | 1.210 | .313 |
| Within groups | .046 | 66 | .001 | |||
| Total | .048 | 69 | ||||
Note. Sig. = significance.
Figure 1.
Total main concepts by group. The mean difference is significant at the .05 level. mTBI = mild traumatic brain injury; OI = orthopedic injury; Mod–severe = moderate to severe traumatic brain injury.
Table 5.
Results of Games–Howell multiple comparisons post hoc test.
| Dependent variable | (I) Injury | (J) Injury | Mean difference (I−J) | SE | Sig. | 95% confidence interval |
|
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Total Main Concepts | Healthy | OI | 5.495 | 6.754 | .848 | −12.81 | 23.80 |
| mTBI | 6.000 | 6.887 | .820 | −12.58 | 24.58 | ||
| Mod–sev | 29.483* | 6.498 | .000 | 11.87 | 47.10 | ||
| OI | Healthy | −5.495 | 6.754 | .848 | −23.80 | 12.81 | |
| mTBI | .505 | 6.087 | 1.000 | −15.99 | 17.00 | ||
| Mod–sev | 23.988* | 5.643 | .001 | 8.62 | 39.36 | ||
| mTBI | Healthy | −6.000 | 6.887 | .820 | −24.58 | 12.58 | |
| OI | −.505 | 6.087 | 1.000 | −17.00 | 15.99 | ||
| Mod–sev | 23.483* | 5.802 | .002 | 7.80 | 39.16 | ||
| Mod–sev | Healthy | −29.483* | 6.498 | .000 | −47.10 | −11.87 | |
| OI | −23.988* | 5.643 | .001 | −39.36 | −8.62 | ||
| mTBI | −23.483* | 5.802 | .002 | −39.16 | −7.80 | ||
| Words/min | Healthy | OI | −69.502705* | 9.350335 | .000 | −96.09745 | −42.90796 |
| mTBI | −64.065474* | 7.816514 | .000 | −85.66542 | −42.46552 | ||
| Mod–sev | 9.984832 | 9.801973 | .741 | −17.74930 | 37.71897 | ||
| OI | Healthy | 69.502705* | 9.350335 | .000 | 42.90796 | 96.09745 | |
| mTBI | 5.437232 | 11.495666 | .964 | −25.88849 | 36.76295 | ||
| Mod–sev | 79.487538* | 12.927920 | .000 | 44.26467 | 114.71040 | ||
| mTBI | Healthy | 64.065474* | 7.816514 | .000 | 42.46552 | 85.66542 | |
| OI | −5.437232 | 11.495666 | .964 | −36.76295 | 25.88849 | ||
| Mod–sev | 74.050306* | 11.865928 | .000 | 41.75834 | 106.34227 | ||
| Mod–sev | Healthy | −9.984832 | 9.801973 | .741 | −37.71897 | 17.74930 | |
| OI | −79.487538* | 12.927920 | .000 | −114.71040 | −44.26467 | ||
| mTBI | −74.050306* | 11.865928 | .000 | −106.34227 | −41.75834 | ||
| Total words | Healthy | OI | −248.985965* | 72.483151 | .017 | −457.78008 | −40.19185 |
| mTBI | −215.736842* | 50.125604 | .002 | −355.47034 | −76.00335 | ||
| Mod–sev | −140.993808 | 53.975656 | .075 | −293.32924 | 11.34162 | ||
| OI | Healthy | 248.985965* | 72.483151 | .017 | 40.19185 | 457.78008 | |
| mTBI | 33.249123 | 85.913006 | .980 | −202.68721 | 269.18545 | ||
| Mod–sev | 107.992157 | 88.214737 | .617 | −133.75439 | 349.73870 | ||
| mTBI | Healthy | 215.736842* | 50.125604 | .002 | 76.00335 | 355.47034 | |
| OI | −33.249123 | 85.913006 | .980 | −269.18545 | 202.68721 | ||
| Mod–sev | 74.743034 | 70.997245 | .720 | −117.19292 | 266.67899 | ||
| Mod–sev | Healthy | 140.993808 | 53.975656 | .075 | −11.34162 | 293.32924 | |
| OI | −107.992157 | 88.214737 | .617 | −349.73870 | 133.75439 | ||
| mTBI | −74.743034 | 70.997245 | .720 | −266.67899 | 117.19292 | ||
| Density | Healthy | OI | .000705 | .007905 | 1.000 | −.02085 | .02226 |
| mTBI | −.010158 | .007859 | .574 | −.03137 | .01105 | ||
| Mod–sev | .006164 | .009315 | .910 | −.01930 | .03163 | ||
| OI | Healthy | −.000705 | .007905 | 1.000 | −.02226 | .02085 | |
| mTBI | −.010863 | .008657 | .598 | −.03434 | .01262 | ||
| Mod–sev | .005459 | .009997 | .947 | −.02177 | .03269 | ||
| mTBI | Healthy | .010158 | .007859 | .574 | −.01105 | .03137 | |
| OI | .010863 | .008657 | .598 | −.01262 | .03434 | ||
| Mod–sev | .016322 | .009961 | .373 | −.01071 | .04336 | ||
| Mod–sev | Healthy | −.006164 | .009315 | .910 | −.03163 | .01930 | |
| OI | −.005459 | .009997 | .947 | −.03269 | .02177 | ||
| mTBI | −.016322 | .009961 | .373 | −.04336 | .01071 | ||
Note. Sig. = significance; OI = orthopedic injury; mTBI = mild traumatic brain injury; Mod–sev = moderate to severe traumatic brain injury.
p < .05.
Productivity Measures
Table 4 shows statistically significant difference among all groups on words per minute and total words with nonsignificant differences for density. Table 5 shows results of Games–Howell post hoc analyses for the selected productivity measures. On the words per minute measure, those in the OI group produced more words per minute (M = 143.81, SD = 34.48) on average than any other group, followed by the mTBI group (M = 138.37, SD = 31.71; see Table 3). The healthy group produced, on average, about half of the words per minute (M = 74.30, SD = 12.47) than the OI group did, and the moderate to severe group, on average, produced the least amount per minute (M = 64.32, SD = 37.50). Table 5 demonstrates the significant differences on words per minute between the healthy and OI group, healthy and mTBI groups, OI and moderate to severe, and MTBI and moderate to severe. On the total words measure, the OI group was also the most talkative (M = 391.93, SD = 275.53), followed by mTBI (M = 358.68, SD = 209.95), and moderate to severe (M = 283.94, SD = 215.06), and lastly, the healthy participants produced the least number of words (M = 142.95, SD = 60.50). Significant differences were observed among healthy and all other groups (please see Appendix B for discourse for examples of discourse for each study group illustrating total word results). Statistical differences were not observed between any groups on density measures (see Table 5).
Nonparametric Results
A Kruskal–Wallis H test was performed to test differences in rank means among the four groups on measures of duration of Cinderella Story (in seconds), total utterances, retracing, and disfluency index. Significant differences were found between groups on measures of duration (in seconds; H = 14.664, p = .002; see Figure 2) and the dysfluency index (H = 18.651, p = 000). On the duration variable, the healthy group had the longest Cinderella stories (M = 340 s), followed by the moderate to severe group (M = 221.5 s), OI (M = 163 s), and lastly, mTBI (M = 153 s). Post hoc tests were then performed to test pairwise comparisons on these two variables. On the duration measure, significant differences were found between mTBI and moderate to severe TBI (p = .046), mTBI and healthy adults (p = .001), and OI and healthy adults (p = .003). On disfluency index scores, significant differences were found between the OI group and the healthy group (p = .000), between the mTBI and healthy group (p = .000), and the healthy and moderate to severe group (p = .006). There were no significant differences between the groups on total utterances (p = .194) and retracing (p = .798).
Figure 2.
Cinderella story duration (seconds). The mean difference is significant at the .05 level. mTBI = mild traumatic brain injury, OI = orthopedic injury; Mod–severe = moderate to severe traumatic brain injury.
Discussion
The aim of the current study was to characterize discourse-level language performance of adults with mTBI on a standardized elicitation task. To our knowledge, this is the first study to compare the discourse performance of adults with mTBI to demographically matched groups of healthy adults, orthopedic controls, and adults with moderate to severe TBI. The current study found significant differences in performance in both content and productivity measures among these groups. The discussion of our findings both reaffirms and expands the current understanding of language production of adults with mTBI and poses significant questions for continued research and clinical care.
Linguistic Content Measures
MCAs
An important finding from our study is the result of the MCA. These analyses demonstrated that individuals with moderate to severe TBI produced significantly fewer main concepts than all other groups in the sample. This result is consistent with previous studies of moderate to severe TBI (Kong et al., 2020; Power et al., 2020), but the current study expands the literature by placing the moderate to severe group performance within the context of the OI and mTBI group data. While there were no significant differences between the comparison groups, our data showed a subtle graded effect in performance. Although MCA is a robust measure for identifying language deficits in adults with moderate to severe TBI, the use of this method with the Cinderella discourse task was not sensitive to the subtle differences between mTBI and OI injuries; it did not adequately distinguish between these two different types of trauma in this sample of participants. This finding is potentially due to the linguistic demands of the Cinderella task, which may not necessarily reflect the cognitive-communication difficulties that are more consistent with a less severe form of TBI. Coelho (2002), in a study of discourse performance in adults with closed head injury, found that story retell tasks yielded better performance than story generation tasks. The study team attributed this finding to the fact that retell tasks, like the Cinderella task used in the current study, have comparatively lower demands on organization and cohesion and rather more linguistic demands, which are often spared in adults with head injury. Although the task employed in the current study did require participants to tell a story from memory, the use of a visual aid (i.e., the picture book) prior to beginning the task aided in story recall. Therefore, the utility of the MCA as a sensitive measure for mTBI-related communication deficits might potentially be best employed using an alternate discourse task that demands high-level cognitive-communication skills rather than purely linguistic skills.
Productivity Measures
Words per Minute and Total Words
In contrast to MCAs, examining the number of words per minute produced by the groups showed sensitivity to mTBI-related language performance when this measure was compared to the healthy and moderate to severe TBI groups; mTBI participants, on average, produced more words in comparison to both of these groups (see Appendix B for discourse samples). Notably, this measure did not distinguish between mTBI and OI groups, although the OI group, on average, produced more words per minute than mTBI and significantly more than healthy and moderate to severe groups. Because of the closely matched sampling of these two groups (OI and mTBI) and their similar initial and follow-up clinical care as well as contextual experiences (i.e., both groups lived in the same geographical region [Midwestern United States], received health care in the same hospital, discourse samples were collected by the same investigators in the same laboratory, etc.) in the time after their injuries, it is likely that these contextual and demographic factors may have played a role in their language performance, and thus, performance was very similar across many measures. One important issue to consider is whether or not these groups, particularly the mTBI group, had completed their recovery from their injuries or whether they might be experiencing “postconcussion syndrome.” These two groups of participants were part of a previous study examining language performance (Norman et al., 2019a, 2019b). Results from these studies indicated that while both groups did report symptomatology associated with their injuries, no significant differences were found between the groups on measures of cognition, sleep quality, or neurobehavioral symptoms as measured by the National Institutes of Health Toolbox Cognitive Battery (Carlozzi et al., 2014), the Pittsburgh Sleep Quality Index (Buysse et al., 1989), and the Neurobehavioral Symptom Inventory measure (Soble et al., 2014). Therefore, comparing the two groups on discourse measures was deemed appropriate.
Comparisons between healthy and moderate to severe TBI groups on words per minute yielded no significant differences between these two seemingly vastly different experimental groups, and, moreover, produced paradoxical outcomes. Both groups produced roughly half of the words the OI and mTBI groups produced in a minute. It is possible that this finding is a byproduct of the unique sampling employed in our study (use of database data for these two groups vs. collection of prospective data for mTBI and OI). For the groups on the extreme ends, healthy and moderate to severe TBI, it is difficult to ascertain whether these findings are a result of a potential cognitive-communication disorder in the moderate to severe group with comparatively higher storytelling efficiency (i.e., using fewer words to convey ideas) in the healthy group. For the prospectively recruited sample (mTBI and OI groups), it is possible that the mTBI and OI participants were more verbose during assessment because of the recency of their injuries, which may have caused them to (consciously or unconsciously) exert more effort and highlight their strengths during the experiment in order to appear recovered. This viewpoint is reinforced by results of the total words measure, with mTBI and OI outperforming participants in the healthy and moderate to severe TBI groups. One important phenomenon to consider is that individuals with subtle cognitive difficulties (as a result of brain or body trauma) may naturally use more words to communicate their ideas. This has been documented in other clinical populations with subtle, often subclinical characteristics such as adults with mild cognitive impairment (Dodge et al., 2015). One possibility is that groups that are using more total words are using fillers and pauses to appropriately and effectively communicate, while other groups are not using these socially acceptable conversational devices and thus the total word count is considerably lower in the latter group.
Density
The proposition density measure analysis was included in this study because it has been previously shown to be sensitive to TBI-related deficits (Coelho, Grela, et al., 2005) and populations with cognitive and language changes as a result of progressive disorders such as Alzheimer's disease dementia (Ivanova et al., 2014; Mueller et al., 2018). The proposition density measure, however, yielded no statistically significant differences among the groups. Propositional density has been hypothesized to be a measure of semantic complexity, heavily reliant upon nonlanguage processes such as attention and executive function. Our study results diverged from Coelho, Grela, et al. (2005) perhaps because of differences in how this measure was calculated; our study used the automated CHAT measure, adapted from Brown et al. (2007), calculated by “adding the number of verbs, adjectives, adverbs, prepositions, and conjunctions divided by the total number of words” (MacWhinney, 2017), while Coelho's study used the Kamhi and Johnson approach (Damico, 1990), which controls for grammaticality and sentence structure by calculating mean number of propositions divided by T-units, “a T-unit is an independent clause plus any subordinate clauses associated with it (p. 1140).”
In addition, it is quite possible that cognitive mechanisms underlying performance on this measure, including executive function and attentional processes (e.g., selection and sequencing of ideas), load on age-related deficits rather than a TBI-specific mechanism. Specifically, propositional density represents the efficiency with which ideas can be expressed, and is heavily reliant upon the inhibition of irrelevant and unwanted words, executive attention, and working memory. Indeed, evidence suggests that propositional density is lower in older versus younger adults (Kemper et al., 2001) and this finding is theorized to be due to declines in inhibition and executive functions rather than to reduced access to semantic storage (Soares et al., 2015), making propositional density insensitive to deficits in the current sample, which was relatively young and not yet at risk for age-related cognitive decline.
Duration
The total duration of the telling of the Cinderella story in seconds was another variable that distinguished the mTBI group from the healthy and moderate to severe TBI groups. The total time mTBI participants spent on telling the Cinderella story was significantly less than the healthy group and the moderate to severe group; contrasted with the words per minute measure for this group, this result provides evidence to an advantage in efficiency in the mTBI group over the healthy and moderate to severe TBI group. As the search for adequate measures for mTBI-related language measures continues, results of our study indicate that the use of this measure shows some promise. Individuals with mTBI often demonstrate working memory and attention deficits and the nature of the Cinderella task in which a visual cue is provide prior to data collection but not during might have contributed to a shorter narrative. Although on average, Cinderella stories told by the mTBI group were 10 s shorter than the OI group, significant differences between the two groups were not demonstrated so it is possible that OI participants might be also be vulnerable to working memory limitations. It is worthwhile to note that trauma of any type (whether it is to the body or the brain) has been demonstrated to evoke cognitive changes, so perhaps the same cognitive mechanism that is implicated during discourse performance in individuals with mTBI also contributes to shorter narratives in adults with OI (Landre et al., 2006; Troyanskaya et al., 2016). To our knowledge, discourse studies using OI controls have been limited but can shed light the current study results. OI controls in Snow et al. (1997) performed significantly different from participants with severe TBI on the following discourse criteria: providing insufficient information, being redundant, socially inappropriate, and difficulty with structuring discourse, with the participants with TBI demonstrating greater deficits on these criteria. In Snow et al. (1999), these results were replicated and statistically significant differences were again found between the groups on measures of errors, insufficient information, redundancy, and discourse structure. No significant differences were found between severe TBI and OI groups on measures of story grammar. In order to establish reliable tasks and norms pertaining to those tasks, using appropriate comparison groups is important. As research in mTBI discourse progresses, perhaps researchers should consider the use of both healthy community-based controls as well as orthopedic comparison groups.
For individuals at the ends of the severity spectrum, healthy and moderate to severe TBI, the findings for the duration variable are in stark contrast to those of the words per minute and total words variable discussed above; the healthy and moderate to severe group took far longer than the OI and mTBI groups to produce significantly less language output (e.g., the healthy group's narratives on average were twice as long as the mTBI group's). However, it is necessary to examine the duration of these samples in the context of their performance on both words per minute and the MCAs; for the moderate to severe TBI group, the verbal output during this duration time is likely not as meaningful and efficient compared to the healthy group, given that significantly more words per minute but fewer total main concepts were produced in the context of identical experimental conditions. We can conclude that the healthy group's output, in contrast, is far more meaningful and efficient than the moderate to severe TBI group's, given their superior performance on the MCA language measures.
Disfluency Index
Because of the well-established influence of cognition on communication performance as well as the risk of neurogenic fluency disorders in the mTBI population (Mattingly, 2015; Norman et al., 2013, 2018; Parrish et al., 2009), one measure of potential sensitivity to mTBI-related communication deficits was hypothesized to be the measure of a disfluency, which has been shown to be sensitive to moderate to severe TBI populations because of the association between sentence planning deficits, attentional control, and working memory deficits (Peach, 2013). In other cognitively impaired populations, such as adults with mild cognitive impairment, the dysfluency index has also been found to be a sensitive cognitive-linguistic marker (Mueller et al., 2018). Our use of the disfluency index, which encompasses speech behaviors that can potentially be attributed to TBI-related cognitive deficits (e.g., pauses, false starts, repetitions, and revisions), showed promise in distinguishing mTBI discourse performance from that of healthy controls. On this measure, the mTBI group and the two other injury groups (OI and moderate to severe TBI) showed statistically significant differences in performance in comparison to the healthy group. Pauses, false starts, repetitions, and revisions in all of the injury groups could potentially be attributed to cognitive deficits after injury such as processing speed difficulties, deficits in story organization, and recall. It is possible that the orthopedic group, while generally spared of neurological impairment, could potentially experience these cognitive symptoms as a result of distress in the early stages of their injuries. The use of the disfluency index in a story generation task such as the one used by Coelho, Grela, et al. (2005) holds promise for clinically distinguishing between mTBI and OI cognitive communication performance.
Differences on the magnitude of the disfluency index were unexpected; the healthy group performed worse (higher index score) on this measure than any injury group. Large standard deviations were observed in the healthy and moderate to severe groups, indicating that the high variability in scores potentially contributed to disfluency index findings in both groups. Because this is a relatively new area of research in mTBI, it is important to consider that other researchers have generally used this measure as a longitudinal measure to document decline over time and that the cross-sectional method in which it was analyzed for our study might present some limitations as it also removes the possible confound of social and personal style. Because the denominator of the disfluency index is the total number of words, it is also important to consider the differences on the total number of words and acknowledge that using fillers and pauses may be employed by some groups to check understanding, as social markers for conversation and the like (Dodge et al., 2015), thereby inflating the index score.
Total Utterances and Retracing
Measures of total utterances and retracing (self-corrections or changes) were found to, again, not statistically distinguish between mTBI and other groups, but these measures did show a graded effect in the expected direction (i.e., mTBI performance between OI and moderate to severe groups). Perhaps with a larger sample and more statistical power, these measures could potentially be utilized for diagnostic purposes; however, the clinical utility of these measures appears limited at this time.
Limitations
Limitations to our study include the overall culturally homogenous sample, both for the data collected prospectively and for the database data available online. Our sample was highly educated, primarily Caucasian, which makes generalization to other populations difficult. In addition, the study sample was disproportionately made up of female sex, with three of the study groups comprised predominantly by women. For the mTBI and OI groups, this sampling limitation was perhaps due to the nature of our recruitment process, in which participants had to have reported to the emergency room for their mTBI or OI in order to participate in the study. Studies have shown women, in general, seek medical care at higher rates (Bertakis et al., 2000), and this phenomenon has also been observed specifically for mTBI (Mollayeva et al., 2018). For the healthy group, the disproportion in the group was largely due to using a convenience sample from TBIBank with stringent inclusion/exclusion criteria. The impact of having predominantly female study samples should also be considered when interpreting our study findings. Although research has found a “female advantage” in certain domains post-TBI, including language, attention, and working memory (Ratcliff et al., 2007), the bulk of the research in this area has centered on social cognition outcomes after moderate to severe TBI, rather than language performance as the current study did. Because of the limited available literature on whether a female advantage exists in mTBI and OI and a sex-based comparison was outside of the scope of the current study, it is difficult to determine whether or not this phenomenon potentially impacted the results of our study. Future studies should explore the effect of sex on language-based outcomes in individuals with mTBI and OI.
The experimental task selected for this study was both a strength and a weakness. As a first step in this line of research, we selected a well-known discourse task to enable comparison to previous studies as well as the ability to use TalkBank data to increase the number of comparison groups. Ultimately, the task did not adequately distinguish between OI and mTBI groups, which indicated limited sensitivity to deficits in either population. The small sample size could have potentially also contributed to being unable to distinguish between OI and mTBI groups; however, moving forward, the results of this study could be used as a baseline for completing a power analysis that would be critical to designing a future study that is well powered statistically.
Conclusions
Understanding language function after mTBI is critical to SLPs practicing in the 21st century. In recent years, there has been an increase in clinical referrals of mTBI patients to SLP services, and SLPs have reported difficulty in addressing these individuals' therapeutic needs (Riedeman & Turkstra, 2018). Being able to efficiently treat cognitive-communication disorders starts with appropriate assessment measures. This study is a first step to understanding language performance in discourse for individuals with mTBI within the context of what we know about moderate to severe TBI discourse performance. Results of this study indicate that the use of narratives such as the Cinderella story have the potential to be used in this population. However, perhaps they need to be refined to capture the subtle cognitive-communication deficits that are characteristic to mTBI; functions such as working memory, processing speed, and executive function could be manipulated in future experiments to achieve this. The value of using the database control groups included in this study demonstrate the added utility of a living, active database system. A system such as this has the potential to continue to expand our knowledge base as the database itself expands with incremental contributions from the research and clinical communities. Because of the unique findings in our study regarding dysfluency index results compared to total words produced, a promising future direction for research is to analyze the types of fillers and fluency errors to determine if conversational fillers are indeed a device that individuals with mTBI use in order to navigate the cognitive and social aspects of conversation.
A unique clinical contribution from the current study and one which requires further refining is being able to distinguish mTBI-related communication deficits from deficits that arise from general physical trauma. Our study was unable to statistically distinguish between OI and mTBI on any measure, although graded effects were seen in the expected directions (i.e., worse performance in participants with mTBI). Understanding how brain trauma and body trauma are similar and different is a worthwhile endeavor in the study of mTBI. Comorbid conditions that occur as a result of body or brain trauma (e.g., depression, anxiety, intrusive thoughts, sleep disturbances) are potential mechanisms for the changes in cognitive communication that we observed in this study.
Future Directions
Cognitive and language performance of adults with mTBI is a pressing clinical issue, and there is growing interest in understanding the effects of trauma on long-term functional outcomes. While the research in mTBI and language is still at the early stages, results from our study, which employed carefully selected control groups and used validated and reliable task and analysis methods, can aid in the development of this knowledge. Further elucidating and characterizing language of adults with mTBI can influence the development of specific and sensitive clinical measures, which are critically needed. These measures can then be used to accurately identify individuals at risk of chronic symptoms known to negatively affect everyday interactions. Future research may consider an alternate analysis method or stimulus presentation that increases the cognitive demands of the elicitation task in order to increase sensitivity and specificity. Furthermore, as this area of research is explored, it will be necessary to address whether the role of SLPs in clinical care should be expanded to include the management of cognitive-communication disorders of individuals resulting from body or psychological trauma.
Acknowledgments
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR002646 to Rocio Norman and was also supported by National Institute of General Medical Sciences (R25GMO83252). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors wish to thank Aimee Balistreri and Kaytlin Hanson for their assistance with data collection. Many thanks to Mackenzie Cross, Sharon Chang, and Gabriel Fragozo for reliability and scoring.
Appendix A
Main Concept Analysis Procedure for Cinderella Discourse Task
|
Note. Based on Richardson and Dalton (2016).
Appendix B
Discourse Examples for Study Subgroups (Total Words in Parentheses)
| Healthy | mTBI | OI | Moderate–severe |
|---|---|---|---|
| Cinderella's father married a wealthy lady in the community. Eventually her father died, leaving Cinderella with her stepmother and stepsisters. They did not repeat her very well and used her pretty much as a servant in their household. She dreamed of being able to escape the situation and being able to be a part of the community. (57) | Okay, so this little girl named Cinderella lived with her dad, who was apparently a widower. And the dad met a woman who, by the way, her last name is the same as mine. Yeah. I don't get why, but they named her that, and, and um, she had three evil daughters. My dad had four evil daughters. But, um, he, he married her, and by some fault of nature that they don't disclose in the book, he dies. And she's left to the, uh, punishment of the evil stepmother, Tremaine, who didn't like her, and made her do all the nasty girl tasks that her three overprivileged daughters were not willing to do. And apparently, her daughters were jealous of Cinderella because she was pretty and they were really ugly, and they had great big noses. (137) | Okay so once upon a time um, there was a little girl named Cinderella. Uh her mother had passed away, but her father remarried and um, he remarried a woman with two daughters of her own. Um, but after some time he passed away uh, and Cinderella was left in the care of her stepmother. But she was quite evil and um, didn't treat Cinderella as well as her own daughters and she pretty much used her as a maid in the house. Um, Cinderella was friends with all of the animals of the house except for the cat which did not like her. It was a grumpy cat. Uh, so she cooked and cleaned and took care of everything in the house for her step mother and step sisters while you know sometimes looking out at the castle and dreaming about you know living there or at least getting out of her present situation. (155) | Once upon a time in a far away land. Pretty sure that's how all Disney movies start. There was a step daughter named Cinderella who lived with her stepmother and also her stepsisters in this giant big house and Cinderella hadta do absolutely everything from cleaning to mopping and taking care of her stepaunty and stepsisters. Sorry I might say that correctly, and constantly getting up very early in the morning to making dinners and all of their breakfasts including cleaning the house from the very bottom to the very top every single day. That right? (96) |
Funding Statement
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR002646 to Rocio Norman and was also supported by National Institute of General Medical Sciences (R25GMO83252).
Footnotes
TBIBank protocol procedures reprinted with permission from https://aphasia.talkbank.org/protocol/.
References
- American Speech-Language-Hearing Association. (1993). Definitions of communication disorders and variations [Relevant paper]. https://www.asha.org/policy/rp1993-00208/ [PubMed]
- American Speech-Language-Hearing Association. (2004). Knowledge and skills needed by speech-language pathologists providing services to individuals with cognitive-communication disorders. Retrieved May 1, from https://www.asha.org/policy/ks2004-00080/
- Barrow, I. M. , Hough, M. , Rastatter, M. P. , Walker, M. , Holbert, D. , & Rotondo, M. F. (2003). Can within-category naming identify subtle cognitive deficits in the mild traumatic brain-injured patient? Journal of Trauma and Acute Care Surgery, 54(5), 888–897. https://doi.org/10.1097/01.TA.0000057150.60668.7C0 [DOI] [PubMed] [Google Scholar]
- Barrow, I. M. , Hough, M. , Rastatter, M. P. , Walker, M. , Holbert, D. , & Rotondo, M. F. (2006). The effects of mild traumatic brain injury on confrontation naming in adults. Brain Injury, 20(8), 845–855. https://doi.org/10.1080/02699050600832445 [DOI] [PubMed] [Google Scholar]
- Bernstein, D. M. (2002). Information processing difficulty long after self-reported concussion. Journal of the International Neuropsychological Society, 8(5), 673–682. https://doi.org/10.1017/S1355617702801400 [DOI] [PubMed] [Google Scholar]
- Bertakis, K. D. , Azari, R. , Helms, L. J. , Callahan, E. J. , & Robbins, J. A. (2000). Gender differences in the utilization of health care services. Journal of Family Practice, 49(2), 147–147. [PubMed] [Google Scholar]
- Blyth, T. , Scott, A. , Bond, A. , & Paul, E. (2012). A comparison of two assessments of high level cognitive communication disorders in mild traumatic brain injury. Brain Injury, 26(3), 234–240. https://doi.org/10.3109/02699052.2012.654587 [DOI] [PubMed] [Google Scholar]
- Bogart, E. , Togher, L. , Power, E. , & Docking, K. (2012). Casual conversations between individuals with traumatic brain injury and their friends. Brain Injury, 26(3), 221–233. https://doi.org/10.3109/02699052.2011.648711 [DOI] [PubMed] [Google Scholar]
- Bohnen, N. , & Jolles, J. (1992). Neurobehavioral aspects of postconcussive symptoms after mild head injury. Journal of Nervous and Mental Disease, 8(11), 323–346. https://doi.org/10.1097/00005053-199211000-00002 [DOI] [PubMed] [Google Scholar]
- Brown, C. , Snodgrass, T. , & Covington, M. (2007). Computerized propositional idea density rater 3 (CPIDR 3) [Computer software]. Artificial Intelligence Center. [Google Scholar]
- Buysse, D. , Reynolds, C. , Monk, T. , Berman, S. , & Kupfer, D. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Byom, L. , & Turkstra, L. S. (2017). Cognitive task demands and discourse performance after traumatic brain injury. International Journal of Language & Communication Disorders, 52(4), 501–513. https://doi.org/10.1111/1460-6984.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi, N. E. , Tulsky, D. S. , Chiaravalloti, N. D. , Beaumont, J. L. , Weintraub, S. , Conway, K. , & Gershon, R. C. (2014). NIH Toolbox Cognitive Battery (NIHTB-CB): The NIHTB Pattern Comparison Processing Speed Test. Journal of the International Neuropsychological Society, 20(6), 630–641. https://doi.org/10.1017/S1355617714000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, L. , Cassidy, J. , Peloso, P. , Borg, J. , Von Holst, H. , Holm, L. , Paniak, C. , & Pepin, M. (2004). Prognosis for mild traumatic brain injury: Results of the WHO collaborating centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine, 43, 84–105. https://doi.org/10.1080/16501960410023859 [DOI] [PubMed] [Google Scholar]
- Chapman, S. B. , McKinnon, L. , Levin, H. S. , Song, J. , Meier, M. C. , & Chiu, S. (2001). Longitudinal outcome of verbal discourse in children with traumatic brain injury. The Journal of Head Trauma Rehabilitation, 16(5), 441–455. https://doi.org/10.1097/00001199-200110000-00004 [DOI] [PubMed] [Google Scholar]
- Coelho, C. (2007). Management of discourse deficits following traumatic brain injury: Progress, caveats, and needs. Seminars in Speech and Language, 28(2), 122–135. https://doi.org/10.1055/s-2007-970570 [DOI] [PubMed] [Google Scholar]
- Coelho, C. , Grela, B. , Corso, M. , Gamble, A. , & Feinn, R. (2005). Microlinguistic deficits in the narrative discourse of adults with traumatic brain injury. Brain Injury, 19(13), 1139–1145. https://doi.org/10.1080/02699050500110678 [DOI] [PubMed] [Google Scholar]
- Coelho, C. , Le, K. , Mozeiko, J. , Hamilton, M. , Tyler, E. , Krueger, F. , & Grafman, J. (2013). Characterizing discourse deficits following penetrating head injury: A preliminary model. American Journal of Speech Language Pathology, 22(2), S438–S448. https://doi.org/10.1044/1058-0360(2013/12-0076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, C. , Ylvisaker, M. , & Turkstra, L. S. (2005). Nonstandardized assessment approaches for individuals with traumatic brain injuries. Seminars in Speech and Language, 26(4), 223–241. https://doi.org/10.1055/s-2005-922102 [DOI] [PubMed] [Google Scholar]
- Coelho, C. A. (2002). Story narratives of adults with closed head injury and non-brain-injured adults. Journal of Speech, Language, and Hearing Research, 45(6), 1232–1248. https://doi.org/10.1044/1092-4388(2002/099) [DOI] [PubMed] [Google Scholar]
- Crawford, M. A. , Knight, R. G. , & Alsop, B. L. (2007). Speed of word retrieval in postconcussion syndrome. Journal of the International Neuropsychological Society, 13(1), 178–182. https://doi.org/10.1017/S135561770707021X [DOI] [PubMed] [Google Scholar]
- Damico, J. (1990). Best practices in school speech language pathology. The Psychological Corporation. [Google Scholar]
- Davis, G. A. , & Coelho, C. A. (2004). Referential cohesion and logical coherence of narration after closed head injury. Brain and Language, 89(3), 508–523. https://doi.org/10.1016/j.bandl.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Dean, P. J. , O'Neill, D. , & Sterr, A. (2012). Post-concussion syndrome: Prevalence after mild traumatic brain injury in comparison with a sample without head injury. Brain Injury, 26(1), 14–26. https://doi.org/10.3109/02699052.2011.635354 [DOI] [PubMed] [Google Scholar]
- Dewan, M. C. , Rattani, A. , Gupta, S. , Baticulon, R. E. , Hung, Y.-C. , Punchak, M. , Agrawal, A. , Adeleye, A. O. , Shrime, M. G. , & Rubiano, A. M. (2018). Estimating the global incidence of traumatic brain injury. Journal of Neurosurgery, 130(4), 1080–1097. https://doi.org/10.3171/2017.10.JNS17352 [DOI] [PubMed] [Google Scholar]
- Dodge, H. H. , Mattek, N. , Gregor, M. , Bowman, M. , Seelye, A. , Ybarra, O. , Asgari, M. , & Kaye, J. A. (2015). Social markers of mild cognitive impairment: Proportion of word counts in free conversational speech. Current Alzheimer Research, 12(6), 513–519. https://doi.org/10.2174/1567205012666150530201917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff, M. C. , Proctor, A. , & Haley, K. (2002). Mild traumatic brain injury (mTBI): Assessment and treatment procedures used by speech-language pathologists (SLPs). Brain Injury, 16(9), 773–787. https://doi.org/10.1080/02699050210128870 [DOI] [PubMed] [Google Scholar]
- Eisenberg, J. , Meehan, W. P. , & Mannix, R. (2014). Duration and course of post-concussive symptoms. Pediatrics, 133(6), 999–1006. https://doi.org/10.1542/peds.2014-0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, M. , Wald, M. M. , Xu, L. , & Coronado, V. G. (2010). Traumatic brain injury in the United States; emergency department visits, hospitalizations, and deaths, 2002–2006. Centers for Disease Control and Prevention.
- Frencham, K. A. , Fox, A. M. , & Maybery, M. T. (2005). Neuropsychological studies of mild traumatic brain injury: A meta-analytic review of research since 1995. Journal of Clinical and Experimental Neuropsychology, 27(3), 334–351. https://doi.org/10.1080/13803390490520328 [DOI] [PubMed] [Google Scholar]
- Galetto, V. , Andreetta, S. , Zettin, M. , & Marini, A. (2013). Patterns of impairment of narrative language in mild traumatic brain injury. Journal of Neurolinguistics, 26(6), 649–661. https://doi.org/10.1016/j.jneuroling.2013.05.004 [Google Scholar]
- Galski, T. , Tomkins, C. , & Johnston, M. V. (1998). Competence in discourse as a measure of social integration and quality of life in persons with traumatic brain injury. Brain Injury, 12(9), 769–782. https://doi.org/10.1080/026990598122160 [DOI] [PubMed] [Google Scholar]
- Ghayoumi, Z. , Yadegari, F. , Mahmoodi-Bakhtiari, B. , Fakharian, E. , Rahgozar, M. , & Rasouli, M. (2015). Persuasive discourse impairments in traumatic brain injury. Archives of Trauma Research, 4(1), Article e21473. https://doi.org/10.5812/atr.21473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza, C. , Greco, T. , & Prins, M. L. (2018). Concussion: Pathophysiology and clinical translation. Handbook of Clinical Neurology, 158, 51–61. https://doi.org/10.1016/b978-0-444-63954-7.00006-9 [DOI] [PubMed] [Google Scholar]
- Hardin, K. Y. , & Kelly, J. P. (2019). The role of speech-language pathology in an interdisciplinary care model for persistent symptomatology of mild traumatic brain injury. Seminars in Speech and Language, 40(1), 65–78. https://doi.org/10.1055/s-0038-1676452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova, I. , Salmon, D. P. , & Gollan, T. H. (2014). Which language declines more? Longitudinal versus cross-sectional decline of picture naming in bilinguals with Alzheimer's disease. Journal of the International Neuropsychological Society, 20(5), 534–546. https://doi.org/10.1017/S1355617714000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson, G. L. (2005). Outcome from mild traumatic brain injury. Current Opinion in Psychiatry, 18(3), 301–317. https://doi.org/10.1097/01.yco.0000165601.29047.ae [DOI] [PubMed] [Google Scholar]
- Karr, J. E. , Arenshenkoff, C. , & Garcia-Barrera, M. A. (2014). The neuropsychological outcomes of concussion: A systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology, 28(3), 321–336. https://doi.org/10.1037/neu0000037 [DOI] [PubMed] [Google Scholar]
- Kemper, S. , Thompson, M. , & Marquis, J. (2001). Longitudinal change in language production: Effects of aging and dementia on grammatical complexity and propositional content. Psychology and Aging, 16(4), 600–614. https://doi.org/10.1037/0882-7974.16.4.600 [DOI] [PubMed] [Google Scholar]
- King, K. A. , Hough, M. S. , Walker, M. M. , Rastatter, M. , & Holbert, D. (2006). Mild traumatic brain injury: Effects on naming in word retrieval and discourse. Brain Injury, 20(7), 725–732. https://doi.org/10.1080/02699050600743824 [DOI] [PubMed] [Google Scholar]
- Kintsch, W. (1974). The representation of meaning in memory. Erlbaum. [Google Scholar]
- Kong, A. P.-H. , Lau, D. K.-Y. , & Cheng, C. Y.-Y. (2020). Analysing coherence of oral discourse among Cantonese speakers in Mainland China with traumatic brain injury and cerebrovascular accident. International Journal of Speech-Language Pathology, 22(1), 37–47. https://doi.org/10.1080/17549507.2019.1581256 [DOI] [PubMed] [Google Scholar]
- Krug, H. , & Turkstra, L. S. (2015). Assessment of cognitive-communication disorders in adults with mild traumatic brain injury. SIG 2 Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 25(1), 17–35. https://doi.org/10.1044/nnsld25.1.17 [Google Scholar]
- Landre, N. , Poppe, C. J. , Davis, N. , Schmaus, B. , & Hobbs, S. E. (2006). Cognitive functioning and postconcussive symptoms in trauma patients with and without mild TBI. Archives of Clinical Neuropsychology, 21(4), 255–273. https://doi.org/10.1016/j.acn.2005.12.007 [DOI] [PubMed] [Google Scholar]
- Lê, K. , Coelho, C. , Mozeiko, J. , & Grafman, J. (2011). Measuring goodness of story narratives. Journal of Speech, Language, and Hearing Research, 54(1), 118–126. https://doi.org/10.1044/1092-4388(2010/09-0022) [DOI] [PubMed] [Google Scholar]
- LeBlanc, J. , de Guise, E. , Champoux, M. C. , Couturier, C. , Lamoureux, J. , Marcoux, J. , Maleki, M. , & Feyz, M. (2014, December). Acute evaluation of conversational discourse skills in traumatic brain injury. International Journal of Speech Language Pathology, 16(6), 582–593. https://doi.org/10.3109/17549507.2013.871335 [DOI] [PubMed] [Google Scholar]
- LeBlanc, J. , de Guise, E. , Feyz, M. , & Lamoureux, J. (2006, Dec). Early prediction of language impairment following traumatic brain injury. Brain Injury, 20(13–14), 1391–1401. https://doi.org/10.1080/02699050601081927 [DOI] [PubMed] [Google Scholar]
- LeBlanc, J. , Seresova, A. , Laberge-Poirier, A. , Tabet, S. , Correa, J. , Alturki, A. , Feyz, M. , & de Guise, E. (2020). Cognitive-communication skills and acute outcome following mild traumatic brain injury. Brain Injury, 34(11), 1472–1479. https://doi.org/10.1080/02699052.2020.1802669 [DOI] [PubMed] [Google Scholar]
- MacWhinney, B. (2000). The CHILDES Project: Tools for analyzing talk. Transcription format and programs. Psychology Press. [Google Scholar]
- MacWhinney, B. (2017). Tools for analyzing talk part 2: The CLAN program. Carnegie Mellon University. http://talkbank.org/manuals/CLAN. pdf [Google Scholar]
- MacWhinney, B. , Fromm, B. , Forbes, M. , & Holland, A. (2011). AphasiaBank: Methods for studying discourse. Aphasiology, 25(11), 1286–1307. https://doi.org/10.1080/02687038.2011.589893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makdissi, M. , Davis, G. , & McCrory, P. (2015). Clinical challenges in the diagnosis and assessment of sports-related concussion. Neurology, 5(1), 2–5. https://doi.org/10.1212/CPJ.0000000000000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini, A. , Zettin, M. , & Galetto, V. (2014). Cognitive correlates of narrative impairment in moderate traumatic brain injury. Neuropsychologia, 64, 282–288. https://doi.org/10.1016/j.neuropsychologia.2014.09.042 [DOI] [PubMed] [Google Scholar]
- Mattingly, E. O. (2015). Dysfluency in a service member with comorbid diagnoses: A case study. Military Medicine, 180(1), e157–e159. https://doi.org/10.7205/MILMED-D-14-00238 [DOI] [PubMed] [Google Scholar]
- Miotto, E. C. , Cinalli, F. Z. , Serrao, V. T. , Benute, G. G. , Lucia, M. C. , & Scaff, M. (2010). Cognitive deficits in patients with mild to moderate traumatic brain injury. Arquivos de Neuro-Psiquiatria, 68(6), 862–868. https://doi.org/10.1590/S0004-282X2010000600006 [DOI] [PubMed] [Google Scholar]
- Mollayeva, T. , El-Khechen-Richandi, G. , & Colantonio, A. (2018). Sex & gender considerations in concussion research. Concussion, 3(1), CNC51. https://doi.org/10.2217/cnc-2017-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozeiko, J. , Le, K. , Coelho, C. , Krueger, F. , & Grafman, J. (2011). The relationship of story grammar and executive function following TBI. Aphasiology, 25(6–7), 826–835. https://doi.org/10.1080/02687038.2010.543983 [Google Scholar]
- Mueller, K. D. , Koscik, R. L. , Hermann, B. P. , Johnson, S. C. , & Turkstra, L. S. (2018). Declines in connected language are associated with very early mild cognitive impairment: Results from the Wisconsin registry for Alzheimer's prevention. Frontiers in Aging Neuroscience, 9, 437. https://doi.org/10.3389/fnagi.2017.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, R. S. , Jaramillo, C. A. , Amuan, M. , Wells, M. A. , Eapen, B. C. , & Pugh, M. J. (2013). Traumatic brain injury in veterans of the wars in Iraq and Afghanistan: Communication disorders stratified by severity of brain injury. Brain Injury, 27(13–14), 1623–1630. https://doi.org/10.3109/02699052.2013.834380 [DOI] [PubMed] [Google Scholar]
- Norman, R. S. , Jaramillo, C. A. , Eapen, B. C. , Amuan, M. E. , & Pugh, M. J. (2018). Acquired stuttering in veterans of the wars in Iraq and Afghanistan: The role of traumatic brain injury, post-traumatic stress disorder and medications. Military Medicine, 183(11–12), 526–534. https://doi.org/10.1093/milmed/usy067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, R. S. , Shah, M. N. , & Turkstra, L. S. (2019a). Language comprehension after mild traumatic brain injury: The role of speed. American Journal of Speech-Language Pathology, 28(4), 1479–1490. https://doi.org/10.1044/2019_AJSLP-18-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, R. S. , Shah, M. N. , & Turkstra, L. S. (2019b). Reaction time and cognitive-linguistic performance in adults with mild traumatic brain injury. Brain Injury, 33(9), 1173–1183. https://doi.org/10.1080/02699052.2019.1632487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, R. S. , Swan, A. A. , Jenkins, A. , Ballard, M. , Amuan, M. , & Pugh, M. J. (2020). Updating and refining prevalence rates of traumatic brain injury–related communication disorders among post-9/11 veterans: A chronic effects of neurotrauma consortium study. Perspectives of the ASHA Special Interest Groups. Advance online publication. https://doi.org/10.1044/2020_PERSP-20-00011 [Google Scholar]
- Parrish, C. , Roth, C. , Roberts, B. , & Davie, G. (2009). Assessment of cognitive-communication disorders of mild traumatic brain injury sustained in combat. SIG 2 Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 19(2), 47–57. https://doi.org/10.1044/nnsld19.2.47 [Google Scholar]
- Peach, R. K. (2013). The cognitive basis for sentence planning difficulties in discourse after traumatic brain injury. American Journal of Speech Language Pathology, 22(2), S285–S297. https://doi.org/10.1044/1058-0360(2013/12-0081) [DOI] [PubMed] [Google Scholar]
- Power, E. , Weir, S. , Richardson, J. , Fromm, D. , Forbes, M. , MacWhinney, B. , & Togher, L. (2020). Patterns of narrative discourse in early recovery following severe traumatic brain injury. Brain Injury, 34(1), 98–109. https://doi.org/10.1080/02699052.2019.1682192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin, S. , Mateer, C. , & Tweeten, R. (1998). Neuropsychological assessment of individuals with mild traumatic brain injury. The Clinical Neuropsychologist, 12(1), 21–30. https://doi.org/10.1076/clin.12.1.21.1724 [Google Scholar]
- Ratcliff, J. J. , Greenspan, A. I. , Goldstein, F. C. , Stringer, A. Y. , Bushnik, T. , Hammond, F. M. , Novack, T. A. , Whyte, J. , & Wright, D. W. (2007). Gender and traumatic brain injury: Do the sexes fare differently? Brain Injury, 21(10), 1023–1030. https://doi.org/10.1080/02699050701633072 [DOI] [PubMed] [Google Scholar]
- Richardson, J. D. , & Dalton, S. G. (2016). Main concepts for three different discourse tasks in a large non-clinical sample. Aphasiology, 30(1), 45–73. https://doi.org/10.1080/02687038.2015.1057891 [Google Scholar]
- Riedeman, S. , & Turkstra, L. (2018). Knowledge, confidence, and practice patterns of speech-language pathologists working with adults with traumatic brain injury. American Journal of Speech-Language Pathology, 27(1), 181–191. https://doi.org/10.1044/2017_AJSLP-17-0011 [DOI] [PubMed] [Google Scholar]
- Ruben, R. J. (2000). Redefining the survival of the fittest: Communication disorders in the 21st century. The Laryngoscope, 110(2), 241–245. https://doi.org/10.1097/00005537-200002010-00010 [DOI] [PubMed] [Google Scholar]
- Snow, P. , Douglas, J. , & Ponsford, J. (1997). Conversational assessment following traumatic brain injury: A comparison across two control groups. Brain Injury, 11(6), 409–429. https://doi.org/10.1080/026990597123403 [DOI] [PubMed] [Google Scholar]
- Snow, P. , Douglas, J. , & Ponsford, J. (1999). Conversational discourse abilities following severe traumatic brain injury: A follow-up study. Brain Injury, 12(11), 911–935. https://doi.org/10.1080/026990598121981 [DOI] [PubMed] [Google Scholar]
- Snowdon, D. A. , Kemper, S. J. , Mortimer, J. A. , Greiner, L. H. , Wekstein, D. R. , & Markesbery, W. R. (1996). Linguistic ability in early life and cognitive function and Alzheimer's disease in late life: Findings from the Nun Study. JAMA, 275(7), 528–532. https://doi.org/10.1001/jama.1996.03530310034029 [PubMed] [Google Scholar]
- Soares, F. C. , de Oliveira, T. C. , de Macedo, L. D. , Tomás, A. M. , Picanço-Diniz, D. L. , Bento-Torres, J. , Bento-Torres, N. V. , & Picanço-Diniz, C. W. (2015). CANTAB object recognition and language tests to detect aging cognitive decline: An exploratory comparative study. Clinical Interventions in Aging, 10, 37–48. https://doi.org/10.2147/cia.S68186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soble, J. , Silva, M. , Vandeploeg, R. , Curtiss, G. , Belanger, H. , Donnell, A. , & Scott, S. (2014). Normative data for the Neurobehavioral Symptom Inventory (NSI) and post-concussion symptom profiles among TBI, PTSD and nonclinical samples. The Clinical Neuropsychologist, 28(14), 614–632. https://doi.org/10.1080/13854046.2014.894576 [DOI] [PubMed] [Google Scholar]
- Stubbs, E. , Togher, L. , Kenny, B. , Fromm, D. , Forbes, M. , MacWhinney, B. , McDonald, S. , Tate, R. , Turkstra, L. , & Power, E. (2018). Procedural discourse performance in adults with severe traumatic brain injury at 3 and 6 months post injury. Brain Injury, 32(2), 167–181. https://doi.org/10.1080/02699052.2017.1291989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togher, L. , McDonald, S. , Coelho, C. A. , & Byom, L. (2014). Cognitive communication disability following TBI. In McDonald S., Togher L., & Code C. (Eds.), Social and communication disorders following traumatic brain injury (pp. 89–118). Routledge. [Google Scholar]
- Tombaugh, T. N. , Rees, L. , Stormer, P. , Harrison, A. G. , & Smith, A. (2007). The effects of mild and severe traumatic brain injury on speed of information processing as measured by the computerized tests of information processing (CTIP). Archives of Clinical Neuropsychology, 22(1), 25–36. https://doi.org/10.1016/j.acn.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Troyanskaya, M. , Pastorek, N. , Scheibel, R. , Petersen, N. , Walder, A. , Henson, H. , & Levin, H. S. (2016). Choosing appropriate comparison group participants in studies of veterans: Characteristics of orthopedically injured and uninjured operation enduring freedom/operation Iraqi freedom/operation new dawn veterans. Journal of Clinical and Experimental Neuropsychology, 38(7), 811–819. https://doi.org/10.1080/13803395.2016.1167172 [DOI] [PubMed] [Google Scholar]
- Tucker, F. M. , & Hanlon, R. E. (1998). Effects of mild traumatic brain injury on narrative discourse production. Brain Injury, 12(9), 783–792. https://doi.org/10.1080/026990598122179 [DOI] [PubMed] [Google Scholar]
- Turner, A. & Greene, E. (1977). The construction and use of a propositional text base (Technical Report No. 63, pp. 77-63). Institute for the Study of Intellectual Behavior, University of Colorado .
- von Elm, E. , Altman, D. G. , Egger, M. , Pocock, S. J. , Gotzsche, P. C. , & Vandenbroucke, J. P. (2008). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Journal of Clinical Epidemiology, 61(4), 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Voss, J. D. , Connolly, J. , Schwab, K. A. , & Scher, A. I. (2015). Update on the epidemiology of concussion/mild traumatic brain injury. Current Pain and Headache Reports, 19(7), 32. https://doi.org/10.1007/s11916-015-0506-z [DOI] [PubMed] [Google Scholar]
- Walhovd, K. B. , Johansen-Berg, H. , & Káradóttir, R. T. (2014). Unraveling the secrets of white matter—Bridging the gap between cellular, animal and human imaging studies. Neuroscience, 276, 2–13. https://doi.org/10.1016/j.neuroscience.2014.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman-Hakes, C. , Murray, B. , Moineddin, R. , Ronchon, E. , Cullen, N. , Gargaro, J. , & Colantino, A. (2013). Evaluating the impact of treatment for sleep/wake disorders on recovery of cognition and communication in adults with chronic TBI. Brain Injury, 27(12), 1364–1376. https://doi.org/10.3109/02699052.2013.823663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (1977). International classification of diseases and related health problems, 9th edition.
- World Health Organization. (2007). International classification of diseases and related health problems, 10th revision.