Abstract
BACKGROUND
Although ischemic coronary artery disease (CAD) is the most common etiology of heart failure (HF), the extent to which patients with new-onset HF actually undergo an ischemic work-up and/or revascularization is not well defined.
OBJECTIVES
This study sought to analyze the patterns of testing for ischemic CAD and revascularization in patients with new-onset HF.
METHODS
This was a retrospective cohort study using Truven Health MarketScan Commercial and Medicare databases from 2010 to 2013. The occurrence of noninvasive and invasive ischemic CAD testing and revascularization procedures were examined among patients with new inpatient HF diagnoses during the index hospitalization and within 90 days of admission.
RESULTS
Among 67,161 patients identified with new-onset HF during an inpatient hospitalization, only 17.5% underwent testing for ischemic CAD during the index hospitalization, increasing to 27.4% at 90 days. Among patients with new-onset HF, only 2.1% underwent revascularization during the index hospitalization for HF; by 90 days, the revascularization rate had increased to 4.3%. Of the tests performed for ischemic CAD, stress testing (nuclear stress testing or stress echocardiography) was performed in 7.9% of new-onset HF patients during the index hospitalization (14.6% within 90 days), whereas coronary angiography was performed in 11.1% of patients during the index hospitalization (16.5% within 90 days). In adjusted analyses, HF patients carrying a baseline diagnosis of CAD had greater odds of noninvasive ischemic testing (odds ratio: 1.25; 95% confidence interval: 1.17 to 1.33; p < 0.0001), as well as invasive ischemic testing (odds ratio: 1.93; 95% confidence interval: 1.83 to 2.05; p < 0.0001), at the index hospitalization than those without baseline CAD.
CONCLUSIONS
The majority of patients hospitalized for new-onset HF did not receive testing for ischemic CAD either during hospitalization or within 90 days, which suggests significant underutilization of ischemic CAD assessment in new-onset HF patients.
Keywords: angiography, invasive, ischemic CAD, noninvasive, revascularization, stress testing
Heart failure (HF) is a major cause of morbidity and mortality in an increasingly aging population, resulting in a high burden to patients and to the health care system (1,2). In fact, HF is one of the only cardiovascular diseases for which the rates of hospitalization and mortality have progressively worsened over the past 25 years (3,4). More than 915,000 new cases of HF are diagnosed in the United States each year (1,5), and at age 40, there is a 20% overall lifetime risk of developing HF (6).
Many patients with HF also have concomitant coronary artery disease (CAD) (7,8). The estimated prevalence of CAD in patients with HF ranges from 50% to 65% (9,10). The presence of CAD is common, not only in patients with HF with reduced ejection fraction (HFrEF), but also in patients with HF with preserved ejection fraction (11,12). Epidemiological data further demonstrate that the most common cause of HF is no longer hypertension or valvular heart disease, but rather ischemic CAD (6). This is relevant, not only because ischemic CAD represents a potentially treatable (or reversible) cause of HF, but also because the presence of CAD can be synergistically and independently associated with worsened long-term outcomes. The recently reported 10-year outcomes of the STICH (Surgical Treatment for Ischemic Heart Failure) trial demonstrated a mortality benefit of revascularization with coronary artery bypass graft (CABG) surgery over optimal medical therapy for patients with ischemic cardiomyopathy (13). Thus, identification of an underlying ischemic etiology of HF is integral to clinical management strategies for HF. The 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for the management of HF currently designate a Class IIa indication to both noninvasive and invasive assessment of ischemic CAD in HF patients (14). However, there are limited data available on how many patients with new-onset HF actually undergo an ischemic work-up and/or revascularization following an HF diagnosis, particularly in a contemporary real-world setting.
Therefore, we sought to perform an analysis of a large commercial administrative claims database in order to assess the patterns of ischemic testing and revascularization for patients hospitalized with new-onset HF.
METHODS
Adult inpatients admitted with a principal diagnosis of HF between 2011 and 2013 were identified in the Truven Health MarketScan Commercial and Medicare Supplemental databases (Truven Health Analytics, Ann Arbor, Michigan), which include health plan enrollment data and detailed patient-level administrative claims for inpatient, outpatient, and pharmacy services. The MarketScan research databases consist of fully adjudicated and paid claims, including both the patient and health plan payments, for approximately 35 million commercially insured patients annually. All data are linked by unique encrypted identifiers and are compliant with the Health Insurance Portability and Accountability Act privacy standards. Institutional review board approval was not required for this retrospective analysis of de-identified data.
Heart failure was identified as the presence of one International Classification of Diseases-Ninth Revision-Clinical Modification (ICD-9-CM) code 428.xx as a principal (i.e., admitting) diagnosis on an inpatient hospitalization. Patients with a principal diagnosis of HF on an inpatient admission in the preceding 12 months were excluded. Baseline clinical characteristics were assessed in the 6 months before and including the date of the index HF hospitalization, with details for CAD, hypertension, hyperlipidemia, diabetes, smoking (assessed using claims for smoking cessation or counseling), prior stroke, arrhythmia, renal disease, chronic obstructive pulmonary disease (COPD), malignancy, and dementia. Performance of an echocardiogram, stress echocardiogram, nuclear stress test (single-photon emission computed tomography or thallium), right heart catheterization, and diagnostic coronary angiography at the index HF hospitalization and during the first 90 days post-index were evaluated, as was revascularization with percutaneous coronary intervention (PCI) or CABG surgery. A noninvasive assessment for ischemic CAD was defined as either stress echocardiography or nuclear stress testing; invasive assessment for ischemic CAD was defined as diagnostic coronary angiography.
Procedures were identified by the presence of either a relevant Medicare Severity Diagnosis Related Group or an ICD-9-CM code, Current Procedure Terminology, or Health Care Common Procedure Coding System procedure code. Billing codes for these relevant procedures were reviewed by 2 independent reviewers (D.D., A.J.K.) before data queries.
STATISTICAL ANALYSES.
Standard statistical tests were used for descriptive comparisons, including the Fisher exact test for dichotomous outcomes and Student t test for continuous outcomes. Logistic regression was used to identify demographic and clinical predictors of receiving noninvasive testing in patients with new-onset HF. All analyses were conducted using Stata version 12 (StataCorp LP, College Station, Texas).
RESULTS
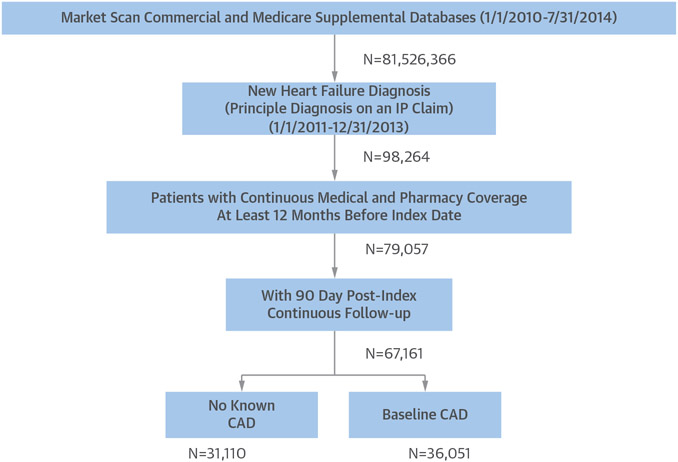
Of the 81,526,366 patient cases available in the MarketScan Commercial and Medicare Supplemental databases from 2010 to 2014, 98,264 patients with a diagnosis of new-onset HF as the primary diagnosis on an inpatient claim were identified (Figure 1). Among these patients, 79,057 patients had contin-ous insurance coverage at least 12 months before the index date (ensuring that they did not have an HF diagnosis that was missed), and 67,161 patients had at least 90-day post-index continuous follow-up. A total of 42,479 of these patients had a baseline diagnosis of CAD as specified with a CAD ICD-9-CM code in their chart preceding or on the same day as their hospitalization; 36,578 patients did not.
FIGURE 1. Patient Flow.
From databases containing >81 million patient records, a total of 67,161 heart failure patients were included in this analysis. CAD = coronary artery disease; IP = inpatient.
The mean age of the study population was 73.7 years with a slight male predominance (52.4%) (Table 1). With regard to cardiovascular comorbidities, 83.3% of all patients had hypertension, whereas around one-half had hyperlipidemia, diabetes mellitus, or baseline CAD. The most common noncardiac comorbidity was renal disease of any kind, which affected 49.5% of patients. Approximately one-third of patients had either HFrEF, HF with preserved ejection fraction, or mixed or unspecified HF.
TABLE 1.
Patient Characteristics (N = 67,161)
| Age, yrs | 73.68 ± 13.7 |
| Sex | |
| Female | 31,959 (47.6) |
| Male | 35,202 (52.4) |
| Index year | |
| 2011 | 24,412 (36.4) |
| 2012 | 23,485 (35.0) |
| 2013 | 19,264 (28.7) |
| Heart failure type | |
| With preserved ejection fraction | 21,163 (31.5) |
| With reduced ejection fraction | 23,134 (34.5) |
| Mixed/unspecified | 22,864 (34.0) |
| Cardiovascular characteristics | |
| CAD | 36,051 (53.7) |
| Hypertension | 55,912 (83.3) |
| Hyperlipidemia | 31,412 (46.8) |
| Diabetes | 31,268 (46.6) |
| Smoking | 7,332 (10.9) |
| Prior stroke | 6,952 (10.4) |
| PAD | 15,201 (22.6) |
| Major arrhythmia | 8,360 (12.5) |
| Nonmajor arrhythmia | 40,082 (59.7) |
| Prior noninvasive ischemic work-up | 11,842 (17.6) |
| Prior invasive ischemic work-up | 7,885 (11.7) |
| Comorbid conditions | |
| Renal disease | 33,211 (49.5) |
| Chronic obstructive pulmonary disease | 28,464 (42.4) |
| Malignancy | 12,487 (18.6) |
| Dementia | 3,737 (5.6) |
Values are mean ± SD or n (%).
CAD = coronary artery disease; PAD = peripheral arterial disease.
TESTING FOR ISCHEMIC CAD.
Overall, a minority of patients received any testing for ischemic CAD either during the index hospitalization or within the subsequent 90 days (Table 2). Patients with no history of CAD at baseline were less likely to undergo testing for ischemic CAD (rate of testing during index hospitalization: 16.5% in those without CAD vs. 18.3% with known CAD; p < 0.001; rate of testing within 90 days: 26.9% in those without CAD vs. 27.8% with known CAD; p = 0.009).
TABLE 2.
Ischemic CAD Testing and Revascularization
| No CAD (n = 31,110) |
CAD (n = 35,051) |
All Patients (N = 67,161) |
p Value | |
|---|---|---|---|---|
| Noninvasive assessment for ischemia | ||||
| During index hospitalization | 2,472 (8.0) | 2,851 (7.9) | 5,323 (7.9) | 0.86 |
| Within 90 days post-index | 4,576 (14.7) | 5,203 (14.4) | 9,779 (14.6) | 0.31 |
| Invasive assessment for ischemia | ||||
| During index hospitalization | 3,002 (9.7) | 4,424 (12.3) | 7,426 (11.1) | <0.0001 |
| Within 90 days post-index | 4,775 (15.4) | 6,293 (17.5) | 11,068 (16.5) | <0.0001 |
| Invasive or noninvasive assessment for ischemia | ||||
| During index hospitalization | 5,146 (16.5) | 6,601 (18.3) | 11,747 (17.5) | <0.0001 |
| Within 90 days post-index | 8,364 (26.9) | 10,018 (27.8) | 18,382 (27.4) | 0.009 |
| Neither invasive nor noninvasive assessment for ischemia | ||||
| During index hospitalization | 25,964 (83.5) | 29,450 (81.7) | 55,414 (82.5) | <0.0001 |
| Within 90 days post-index | 22,746 (73.1) | 26,033 (72.2) | 48,779 (72.6) | 0.009 |
| Noninvasive assessment for ischemia during baseline | 2,809 (9.0) | 9,033 (25.1) | 11,842 (17.6) | <0.0001 |
| Invasive assessment for ischemia during baseline | 854 (2.8) | 7,031 (19.5) | 7,885 (11.7) | <0.0001 |
| Invasive or noninvasive assessment for ischemia during baseline | 3,399 (10.9) | 13,025 (36.1) | 16,424 (24.5) | <0.0001 |
| With revascularization during index hospitalization | 146 (0.5) | 1,230 (3.4) | 1,376 (2.1) | <0.0001 |
| PCI only | 116 (0.4) | 901 (2.5) | 1,017 (1.5) | |
| CABG only | 30 (0.1) | 317 (0.9) | 347 (0.5) | |
| Hybrid revascularization | 0 (0.0) | 12 (0.0) | 12 (0.0) | |
| With revascularization within 90 days post-index | 635 (2.0) | 2,260 (6.3) | 2,895 (4.3) | <0.0001 |
| PCI only | 406 (1.3) | 1,578 (4.4) | 1,984 (3.0) | |
| CABG only | 225 (0.7) | 646 (1.8) | 871 (1.3) | |
| Hybrid revascularization | 4 (0.0) | 36 (0.1) | 40 (0.1) |
Values are n (%).
CABG = coronary artery bypass grafting; CAD = coronary artery disease; PCI = percutaneous coronary intervention.
During the index HF hospitalization, fewer than 1 in 10 patients (7.9%) received noninvasive ischemic testing for CAD, which was defined as exercise or pharmacological testing with or without an imaging modality such as myocardial perfusion imaging or echocardiography. This rate increased to 14.6% at 90 days following the index admission (Table 2). Notably, there were no differences in the unadjusted rates of noninvasive testing if a patient had a previously known diagnosis of CAD or not. By contrast, patients with baseline CAD were more likely to undergo an invasive CAD assessment (defined as coronary angiography) compared with those without baseline CAD during the index hospitalization (9.7% in those without CAD vs. 12.3% with known CAD; p < 0.001) and within 90 days (15.4% vs. 17.5%; p < 0.001).
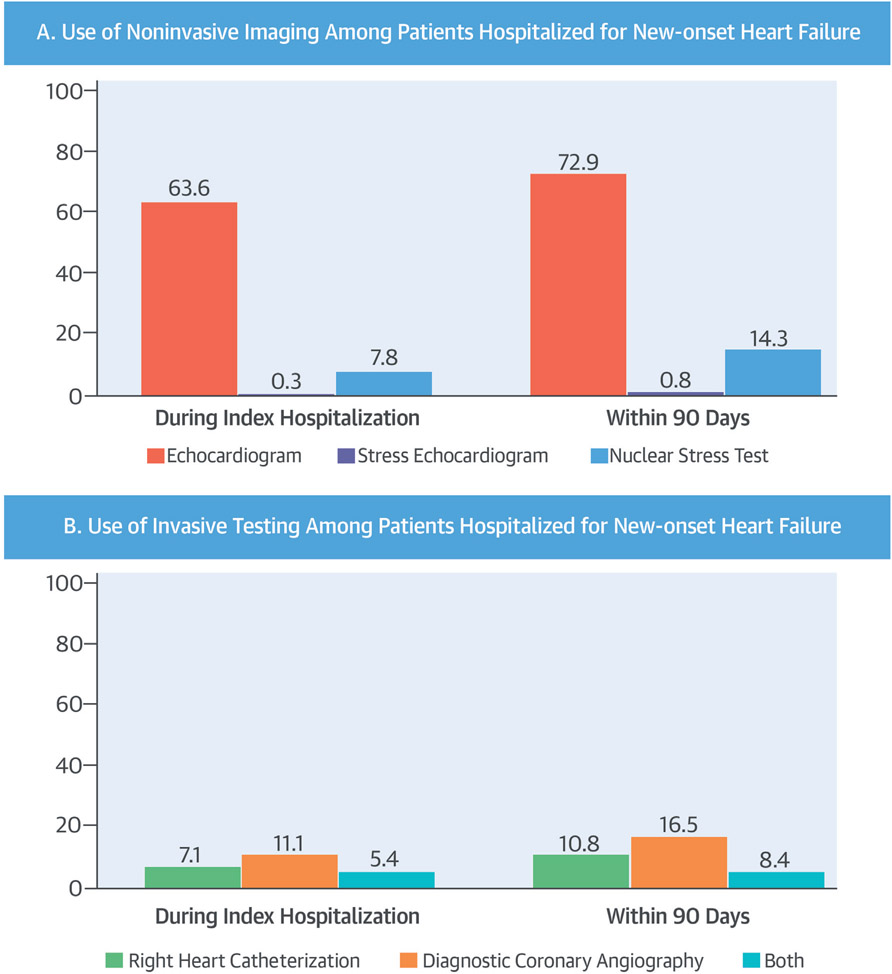
When examining various noninvasive testing modalities utilized for the work-up of new-onset HF, a standard 2-dimensional echocardiogram was the most commonly performed test, occurring in 63.6% of patients during the index hospitalization and 72.9% of patients within 90 days (Central Illustration). Performance of a nuclear stress test came in a distant second at both time periods, whereas stress echocardiography utilization barely registered, reaching a high of only 0.8% of patients within 90 days. In regard to invasive testing modalities, coronary angiography alone was utilized more frequently than right heart catheterization alone or the combination of both coronary angiography and right heart catheterization Central Illustration).
CENTRAL ILLUSTRATION. Ischemic Work-Up in HF.
In this retrospective cohort study, ischemic coronary artery disease (CAD) testing and revascularization procedures were examined among 67,161 patients with new inpatient heart failure (HF) diagnoses during the index hospitalization and within 90 days of admission. Overall, only a minority of patients received any testing for ischemic CAD and those without a history of CAD at baseline received even less. Of the noninvasive (A) and invasive (B) imaging modalities used during index hospitalization and through 90-day follow-up, a standard 2-dimensional echocardiogram was the most commonly performed test.
MULTIVARIABLE ANALYSES.
In multivariable analysis, baseline CAD (odds ratio [OR]: 1.25; 95% confidence interval [CI]: 1.17 to 1.33; p < 0.001) was associated with greater odds of noninvasive testing in patients with new-onset HF. Hypertension, hyperlipidemia, HFrEF, and mixed or unspecified HF were also associated with greater odds of noninvasive testing, whereas elderly age (>70 years), prior stroke, peripheral arterial disease (PAD), prior arrhythmias, renal disease, COPD, dementia, and prior noninvasive and invasive work-up for ischemia were associated with lesser odds of undergoing noninvasive testing (Table 3). With regard to invasive testing, multivariable analysis demonstrated that baseline CAD (OR: 1.93; 95% CI: 1.83 to 2.05; p < 0.0001) was associated with greater odds of invasive ischemic CAD testing. Smoking and HFrEF were also associated with greater odds of invasive ischemic CAD, whereas elderly age, earlier year of enrollment, hypertension, diabetes, prior stroke, PAD, nonmajor arrhythmia, renal disease, COPD, malignancy, dementia, and prior invasive work-up for ischemia were associated with lesser odds (Table 4).
TABLE 3.
Multivariable Analysis for Noninvasive Testing
| Noninvasive Testing |
||||
|---|---|---|---|---|
| No (n = 61,838) |
Yes (n = 5,323) |
Adjusted OR (95% CI) |
p Value | |
| Age group | ||||
| 18–70 yrs | 22,700 (36.71) | 2,538 (47.68) | Reference group | |
| 71–85 yrs | 25,098 (40.59) | 2,224 (41.78) | 0.85 (0.793–0.901) | <0.0001 |
| >85 yrs | 14,040 (22.70) | 561 (10.54) | 0.36 (0.327–0.399) | <0.0001 |
| Sex | ||||
| Female | 29,580 (47.83) | 2,379 (44.69) | Reference group | |
| Male | 32,258 (52.17) | 2,944 (55.31) | 1.05 (0.986–1.111) | 0.13 |
| Index year | ||||
| 2011 | 22,501 (36.39) | 1,911 (35.90) | Reference group | |
| 2012 | 21,578 (34.89) | 1,907 (35.83) | 1.04 (0.973–1.112) | 0.25 |
| 2013 | 17,759 (28.72) | 1,505 (28.27) | 0.99 (0.925–1.068) | 0.87 |
| Heart failure type | ||||
| Preserved ejection fraction | 19,514 (31.56) | 1,649 (30.98) | Reference group | |
| Reduced ejection fraction | 21,118 (34.15) | 2,016 (37.87) | 1.38 (1.282–1.491) | <0.0001 |
| Mixed/unspecified | 21,206 (34.29) | 1,658 (31.15) | 1.31 (1.208–1.422) | <0.0001 |
| Cardiovascular characteristics | ||||
| CAD | 33,200 (53.69) | 2,851 (53.56) | 1.25 (1.172–1.329) | <0.0001 |
| Hypertension | 51,484 (83.26) | 4,428 (83.19) | 1.11 (1.024–1.202) | 0.01 |
| Hyperlipidemia | 28,866 (46.68) | 2,546 (47.83) | 1.09 (1.026–1.160) | 0.005 |
| Diabetes | 28,608 (46.26) | 2,660 (49.97) | 1.06 (0.994–1.120) | 0.08 |
| Smoking | 6,690 (10.82) | 642 (12.06) | 1.05 (0.956–1.146) | 0.3200 |
| Prior stroke | 6,532 (10.56) | 420 (7.89) | 0.82 (0.741–0.914) | <0.0001 |
| PAD | 14,159 (22.90) | 1,042 (19.58) | 0.95 (0.879–1.019) | 0.15 |
| Major arrhythmia | 7,769 (12.56) | 591 (11.10) | 0.86 (0.781–0.939) | 0.001 |
| Nonmajor arrhythmia | 37,377 (60.44) | 2,705 (50.82) | 0.79 (0.742–0.835) | <0.0001 |
| Prior noninvasive ischemic work-up | 11,248 (18.19) | 594 (11.16) | 0.56 (0.509–0.610) | <0.0001 |
| Prior invasive ischemic work-up | 7,529 (12.18) | 356 (6.69) | 0.49 (0.437–0.550) | <0.0001 |
| Comorbid conditions | ||||
| Renal disease | 30,754 (49.73) | 2,457 (46.16) | 0.88 (0.826–0.930) | <0.0001 |
| CABG | 26,509 (42.87) | 1,955 (36.73) | 0.77 (0.728–0.821) | <0.0001 |
| Malignancy | 11,593 (18.75) | 894 (16.80) | 0.96 (0.891–1.038) | 0.31 |
| Dementia | 3,586 (5.80) | 151 (2.84) | 0.62 (0.521–0.731) | <0.0001 |
TABLE 4.
Multivariable Analysis for Invasive Testing
| Invasive Testing |
||||
|---|---|---|---|---|
| No (n = 59,735) |
Yes (n = 7,426) |
Adjusted OR (95% CI) |
p Value | |
| Age group | ||||
| 18–70 yrs | 20,555 (34.41) | 4,683 (63.06) | Reference group | |
| 71–85 yrs | 24,908 (41.70) | 2,414 (32.51) | 0.45 (0.428–0.480) | <0.0001 |
| >85 yrs | 14,272 (23.89) | 329 (4.43) | 0.11 (0.094–0.119) | <0.0001 |
| Sex | ||||
| Female | 29,011 (48.57) | 2,948 (39.70) | Reference group | |
| Male | 30,724 (51.43) | 4,478 (60.30) | 1.04 (0.987–1.098) | 0.14 |
| Index year | ||||
| 2011 | 21,809 (36.51) | 2,603 (35.05) | Reference group | |
| 2012 | 20,913 (35.01) | 2,572 (34.64) | 1.04 (0.983–1.109) | 0.16 |
| 2013 | 17,013 (28.48) | 2,251 (30.31) | 1.18 (1.105–1.255) | <0.0001 |
| Heart failure type | ||||
| Preserved ejection fraction | 19,912 (33.33) | 1,251 (16.85) | Reference group | |
| Reduced ejection fraction | 19,354 (32.40) | 3,780 (50.90) | 1.82 (1.705–1.940) | <0.0001 |
| Mixed/unspecified | 20,469 (34.27) | 2,395 (32.25) | 0.75 (0.693–0.815) | <0.0001 |
| Cardiovascular characteristics | ||||
| CAD | 31,627 (52.95) | 4,424 (59.57) | 1.93 (1.826–2.049) | <0.0001 |
| Hypertension | 50,052 (83.79) | 5,860 (78.91) | 0.93 (0.874–1.000) | 0.049 |
| Hyperlipidemia | 28,031 (46.93) | 3,381 (45.53) | 0.98 (0.930–1.038) | 0.53 |
| Diabetes | 27,966 (46.82) | 3,302 (44.47) | 0.80 (0.759–0.845) | <0.0001 |
| Smoking | 6,218 (10.41) | 1,114 (15.00) | 1.15 (1.066–1.239) | <0.0001 |
| Prior stroke | 6,461 (10.82) | 491 (6.61) | 0.76 (0.684–0.835) | <0.0001 |
| PAD | 13,934 (23.33) | 1,267 (17.06) | 0.90 (0.840–0.964) | 0.003 |
| Major arrhythmia | 7,205 (12.06) | 1,155 (15.55) | 1.04 (0.963–1.115) | 0.34 |
| Nonmajor arrhythmia | 36,474 (61.06) | 3,608 (48.59) | 0.76 (0.719–0.799) | <0.0001 |
| Prior noninvasive ischemic work-up | 10,517 (17.61) | 1,325 (17.84) | 1.02 (0.953–1.093) | 0.57 |
| Prior invasive ischemic work-up | 7,412 (12.41) | 473 (6.37) | 0.29 (0.263–0.322) | <0.0001 |
| Comorbid conditions | ||||
| Renal disease | 30,460 (50.99) | 2,751 (37.05) | 0.61 (0.576–0.642) | <0.0001 |
| CABG | 25,752 (43.11) | 2,712 (36.52) | 0.79 (0.749–0.834) | <0.0001 |
| Malignancy | 11,405 (19.09) | 1,082 (14.57) | 0.89 (0.828–0.955) | 0.0001 |
| Dementia | 3,638 (6.09) | 99 (1.33) | 0.41 (0.335–0.506) | <0.0001 |
A total of 2.1% of all patients underwent coronary revascularization during the index HF hospitalization, whereas 4.3% experienced revascularization within 90 days (Table 2). Patients more frequently underwent revascularization if they had a prior known diagnosis of CAD than if they did not. PCI was more commonly used as a revascularization modality than CABG. In multivariable analysis, baseline CAD (OR: 9.27; 95% CI: 7.74 to 11.10; p < 0.0001), male sex, diabetes, smoking, and PAD were associated with greater odds for revascularization in patients with new-onset HF, whereas elderly age, COPD, malignancy, and dementia were associated with lesser odds of revascularization (Table 5).
TABLE 5.
Multivariable Analysis for Revascularization
| Revascularization |
||||
|---|---|---|---|---|
| No (n = 65,785) |
Yes (n = 1,376) |
Adjusted OR (95% CI) |
p Value | |
| Age group | ||||
| 18–70 yrs | 24,500 (37.24) | 738 (53.6) | Reference group | |
| 71–85 yrs | 26,761 (40.68) | 561 (40.8) | 0.67 (0.597–0.757) | <0.0001 |
| >85 yrs | 14,524 (22.08) | 77 (5.6) | 0.20 (0.155–0.253) | <0.0001 |
| Sex | ||||
| Female | 31,484 (47.86) | 475 (34.5) | Reference group | |
| Male | 34,301 (52.14) | 901 (65.5) | 1.16 (1.030–1.302) | 0.01 |
| Index year | ||||
| 2011 | 23,913 (36.35) | 499 (36.3) | Reference group | |
| 2012 | 23,024 (35.00) | 461 (33.5) | 0.97 (0.855–1.109) | 0.69 |
| 2013 | 18,848 (28.65) | 416 (30.2) | 1.12 (0.983–1.287) | 0.08 |
| Heart failure type | ||||
| Preserved ejection fraction | 20,921 (31.80) | 242 (17.6) | Reference group | |
| Reduced ejection fraction | 22,480 (34.17) | 654 (47.5) | 1.40 (1.223–1.607) | <0.0001 |
| Mixed/unspecified | 22,384 (34.03) | 480 (34.9) | 0.72 (0.607–0.86 0) | <0.0001 |
| Cardiovascular characteristics | ||||
| CAD | 34,821 (52.93) | 1,230 (89.4) | 9.27 (7.743–11.098) | <0.0001 |
| Hypertension | 54,782 (83.27) | 1,130 (82.1) | 0.85 (0.728–0.986) | 0.03 |
| Hyperlipidemia | 30,707 (46.68) | 705 (51.2) | 0.88 (0.779–0.983) | 0.02 |
| Diabetes | 30,475 (46.33) | 793 (57.6) | 1.14 (1.012–1.275) | 0.03 |
| Smoking | 7,120 (10.82) | 212 (15.4) | 1.19 (1.017–1.393) | 0.03 |
| Prior stroke | 6,831 (10.38) | 121 (8.8) | 0.86 (0.708–1.041) | 0.12 |
| PAD | 14,830 (22.54) | 371 (27.0) | 1.17 (1.032–1.330) | 0.01 |
| Major arrhythmia | 8,179 (12.43) | 181 (13.2) | 0.73 (0.617–0.856) | <0.0001 |
| Nonmajor arrhythmia | 39,394 (59.88) | 688 (50.0) | 0.71 (0.636–0.797) | <0.0001 |
| Prior noninvasive ischemic work-up | 11,543 (17.55) | 299 (21.7) | 0.94 (0.818–1.070) | 0.33 |
| Prior invasive ischemic work-up | 7,722 (11.74) | 163 (11.9) | 0.51 (0.430–0.606) | <0.0001 |
| Comorbid conditions | ||||
| Renal disease | 32,528 (49.45) | 683 (49.6) | 0.90 (0.805–1.010) | 0.07 |
| CABG | 27,944 (42.48) | 520 (37.8) | 0.74 (0.662–0.833) | <0.0001 |
| Malignancy | 12,306 (18.71) | 181 (13.2) | 0.71 (0.602–0.831) | <0.0001 |
| Dementia | 3,715 (5.65) | 22 (1.6) | 0.41 (0.270–0.636) | <0.0001 |
SENSITIVITY ANALYSES.
In order to mitigate the effects of any ischemic evaluation performed before the diagnosis of new onset HF, we repeated the aforementioned analyses but excluded any patient who had any previously coded ischemic evaluation. The total population for these sensitivity analyses consisted of 50,737 patients, of whom 27,711 (54.6%) did not carry a diagnosis of CAD.
Patients without any prior ischemic evaluation who developed new onset HF had comparable rates of noninvasive or invasive testing during the index hospitalization (18.7%) and at 90 days (28.6%) when compared with the entire study population (Online Table 1). Patients with known CAD were once again more likely to undergo both noninvasive and invasive ischemic testing than those without known CAD. Among patients without any form of prior ischemic evaluation with new-onset HF, only 1.9% underwent revascularization during the index hospitalization for HF and only 4.1% within 90 days.
Of the tests performed for ischemic CAD in patients without any prior ischemic evaluation, nuclear stress testing was performed in 8.8% of patients during their index hospitalization (15.5% within 90 days) and stress echocardiogram in 0.4% during their index hospitalization (0.8% within 90 days), whereas coronary angiography was performed in 11.5% during their index hospitalization and 16.8% within 90 days (Online Table 2). Once again, in multivariable analyses, HF patients carrying a baseline diagnosis of CAD had greater odds of noninvasive ischemic testing (OR: 1.24; 95% CI: 1.16 to 1.32; p < 0.001), as well as invasive ischemic testing (OR: 2.06; 95% CI: 1.93 to 2.19; p < 0.0001) than those without baseline CAD (Online Table 3).
DISCUSSION
The current study represented the largest and most contemporary analysis of the patterns of ischemic testing and revascularization in patients with new-onset HF. The principal finding of this analysis was that almost three-quarters of patients with new-onset HF did not receive any ischemic CAD testing within 90 days of index admission. This finding was particularly striking both given how prevalent CAD is among patients with HF, as well as in light of the adverse outcomes associated with concomitant CAD and HF. Notably, the rates of revascularization procedures following an index HF admission were even lower (occurring in <5% of patients within 90 days of an index HF admission), which in part may reflect the low rates of upstream ischemic testing. Both of these findings, based upon analyses from an administrative claims database, suggested a significant diagnostic testing and treatment gap for a high-risk patient population with one of the highest prevalence rates of CAD.
The present findings were in agreement with a comparable, albeit smaller, observational study reporting patterns of diagnostic testing among patients with new-onset HF (15). In the aforementioned study, Farmer et al. (15) examined cardiovascular imaging utilization patterns in 37,099 patients in the Cardiovascular Research Network Heart Failure study who presented with de novo HF and showed that only 36.9% of patients had a CAD assessment. These findings, as well as those in the current analysis, are sobering, given the Class IIa indication afforded to ischemic testing in both the 2013 ACC/AHA guidelines for the management of HF (14) as well as other consensus documents (16,17). In these guidelines, stress nuclear imaging or stress echocardiography are acceptable options for assessing ischemic CAD in patients presenting with HF who have known CAD and no angina unless they are ineligible for revascularization. Despite these recommendations, most patients with new-onset HF—even those with a known diagnosis of CAD—did not receive a work-up for ischemic CAD. Even assuming incomplete reporting of testing within the current database, when combining the present findings with the prevalence of CAD in patients with HF and the reported annual incidence of 915,000 patients with new-onset HF (18), it can be estimated that every year more than 325,000 patients with new-onset HF and CAD might not be adequately assessed for ischemic CAD.
The potential underuse of ischemic CAD testing in patients with new-onset HF has several potential implications beyond the empiric desire to establish a definitive etiology for HF. The omission of ischemic CAD testing may prevent patients from being treated with aggressive guideline-directed medical therapies for CAD, which can both alleviate symptoms and reduce hard cardiovascular events (19). In the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) registry, performance of coronary angiography during the index hospitalization for acute HF was associated with an increased utilization of aspirin, statins, and myocardial revascularization as well as a reduced risk of death 60 to 90 days after discharge (20). Additionally, upfront ischemic testing can lead to lower resource utilization in terms of emergency department visits and rehospitalization for complications of CAD and HF (20,21). Lastly, a significant proportion of patients with HF and left ventricular dysfunction have the potential for clinically important improvements in left ventricular function after the appropriate use of coronary revascularization (22-24). A number of observational studies have demonstrated both improved survival and left ventricular function with revascularization compared with medical therapy alone (25-27). The recently reported 10-year outcomes of the STICH trial demonstrated an all-cause mortality benefit to surgical revascularization compared with optimal medical therapy among patients with depressed ventricular function (13,28,29). Further, quality of life was improved through surgical revascularization (30). The undertesting identified in the present analysis was not just limited to ischemic CAD testing: more than one-quarter of patients with newly diagnosed HF did not even undergo a 2-dimensional transthoracic echocardiogram, despite the fact that the ACC/AHA guidelines establish echocardiography as a Class I recommendation in the work-up for new-onset HF (14). This speaks to a larger issue at hand, namely that patients being hospitalized for new-onset HF may not be receiving an appropriate HF work-up in general. In an increasingly cost-conscious era where there is concern for excessive testing in lower-risk patients, there certainly appears to be concomitant underutilization of appropriate testing in higher-risk patients as well, consistent with a treatment-risk paradox.
STUDY LIMITATIONS.
The analysis relied exclusively upon claims data to track diagnoses, testing, and procedures. As such, errors in coding or non-submission of claims may have resulted in the omission of diagnostic tests and procedures. Nevertheless, given how closely physician and hospital reimbursements within the United States are tied to testing and procedural coding, the effect of undercoding/miscoding was likely somewhat mitigated within this analysis. Additionally, administrative codes have been shown to be fairly accurate for cardiovascular diagnoses, perhaps due to their relationship with levels of reimbursement (31,32). Another potential limitation related to an inability to separate routine exercise treadmill testing (without adjunctive imaging) from other forms of stress testing due to overlapping Current Procedure Terminology codes. Claims data are limited in that they lack granular information surrounding each patient’s HF admission, such as the severity of HF symptoms, ventricular function, other comorbidities, and medication usage, which could have helped to further explain differences in ischemic CAD testing and revascularization. Furthermore, outcomes data were incompletely captured by these databases, particularly if adverse outcomes occurred outside of a hospital setting. This information would have been useful to determine whether patients who did or did not undergo ischemic CAD testing and/or revascularization had disparate outcomes. Lastly, the rates of more specialized imaging modalities, such as cardiac computed tomography, magnetic resonance imaging, and positron emission tomography were not assessed; however, the fact that these imaging modalities are not as commonly available as the imaging modalities assessed in our study suggests that including these modalities would not have appreciably altered our results.
CONCLUSIONS
Within the context of a large administrative claims database, the great majority of patients hospitalized for new-onset HF did not receive any testing for ischemic CAD, either during the index hospitalization or within the subsequent 90 days. These data suggested significant underutilization of guideline-recommended assessments for ischemic CAD in new-onset HF patients.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PRACTICE-BASED LEARNING AND IMPROVEMENT:
Despite the potential reversibility of heart failure in patients with ischemic heart disease, most are not evaluated for coronary disease during the 90 days after hospitalization for new-onset HF.
TRANSLATIONAL OUTLOOK:
Additional research is needed to explore the factors responsible for the limited implementation of guideline-recommended diagnostic evaluation of patients with new-onset heart failure and to develop measures that enhance the detection of potentially reversible causes.
Acknowledgments
This study was supported by an unrestricted educational grant from Abiomed Inc. Dr. Doshi has received an educational grant from Abiomed. Dr. Ben-Yehuda has received institutional research grants from Abiomed and Thoratec. Dr. Bonafede is an employee of Truven Health, which was awarded a contract to conduct this study in collaboration with Abiomed. Dr. Josephy is an employee of Abiomed. Dr. Karmpaliotis is on the speakers bureaus of Abbott Vascular, Boston Scientific, Medtronic, and Asahi. Dr. Parikh is on the speakers bureaus of Abbott Vascular, Medtronic, CSI, St. Jude Medical, and Boston Scientific; and is on the advisory boards of Abbott Vascular, Medtronic, and Philips. Dr. Moses is a consultant for Boston Scientific and Abiomed. Dr. Kirtane has received institutional research grants to Columbia University from Boston Scientific, Medtronic, Abbott Vascular, Abiomed, St. Jude Medical, Vascular Dynamics, and Eli Lilly. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ACC
American College of Cardiology
- AHA
American Heart Association
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- ICD-9-CM
International Classification of Diseases-Ninth Revision-Clinical Modification
- OR
odds ratio
- PAD
peripheral arterial disease
- PCI
percutaneous coronary intervention
Footnotes
APPENDIX For supplemental tables, please see the online version of this article.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 2.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol 2008;52:428–34. [DOI] [PubMed] [Google Scholar]
- 4.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–22. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002;106:3068–72. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation 2006;114:1202–13. [DOI] [PubMed] [Google Scholar]
- 8.Velazquez EJ, Pfeffer MA. Acute heart failure complicating acute coronary syndromes: a deadly intersection. Circulation 2004;109:440–2. [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–402. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–57. [DOI] [PubMed] [Google Scholar]
- 11.Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation 2009;119:3070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 13.Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 15.Farmer SA, Lenzo J, Magid DJ, et al. Hospital-level variation in use of cardiovascular testing for adults with incident heart failure: findings from the cardiovascular research network heart failure study. J Am Coll Cardiol Img 2014;7:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherty JD, Bax JJ, De Luca L, et al. Acute heart failure syndromes in patients with coronary artery disease early assessment and treatment. J Am Coll Cardiol 2009;53:254–63. [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 19.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- 20.Flaherty JD, Rossi JS, Fonarow GC, et al. Influence of coronary angiography on the utilization of therapies in patients with acute heart failure syndromes: findings from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2009;157:1018–25. [DOI] [PubMed] [Google Scholar]
- 21.Mallidi J, Penumetsa S, Friderici JL, Saab F, Rothberg MB. The effect of inpatient stress testing on subsequent emergency department visits, readmissions, and costs. J Hosp Med 2013;8:564–8. [DOI] [PubMed] [Google Scholar]
- 22.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol 2002;39:1151–8. [DOI] [PubMed] [Google Scholar]
- 23.Auerbach MA, Schoder H, Hoh C, et al. Prevalence of myocardial viability as detected by positron emission tomography in patients with ischemic cardiomyopathy. Circulation 1999;99:2921–6. [DOI] [PubMed] [Google Scholar]
- 24.Bonow RO. The hibernating myocardium: implications for management of congestive heart failure. Am J Cardiol 1995;75:17A–25A. [DOI] [PubMed] [Google Scholar]
- 25.O’Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intraaortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation 2012;126:1717–27. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez EJ, Williams JB, Yow E, et al. Long-term survival of patients with ischemic cardiomyopathy treated by coronary artery bypass grafting versus medical therapy. Ann Thorac Surg 2012;93:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maini B, Naidu SS, Mulukutla S, et al. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: the USpella Registry. Catheter Cardiovasc Interv 2012;80:717–25. [DOI] [PubMed] [Google Scholar]
- 28.Mark DB, Knight JD, Velazquez EJ, et al. Quality-of-life outcomes with coronary artery bypass graft surgery in ischemic left ventricular dysfunction: a randomized trial. Ann Intern Med 2014;161:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doenst T, Cleland JG, Rouleau JL, et al. Influence of crossover on mortality in a randomized study of revascularization in patients with systolic heart failure and coronary artery disease. Circ Heart Fail 2013;6:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005;43:480–5. [DOI] [PubMed] [Google Scholar]
- 32.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004;148:99–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.