Abstract
The sexually dimorphic trait of height is one aspect of the experience of transgender and gender‐diverse (TGD) individuals that may influence their gender dysphoria and satisfaction with their transition. In this article, we have reviewed the current knowledge of the factors that contribute to one's final adult height and how it might be affected in TGD youth who have not experienced their gonadal puberty in the setting of receiving gonadotropin‐releasing hormone analog (GnRHa) and gender‐affirming hormonal treatment. Additional research is needed to characterize the influence of growth and final adult height on the lived experience of TGD youth and adults and how to best assess their growth, predict their final adult height, and how medical transition can be potentially modified to help them meet their goals.
Keywords: blockers, gender diverse, growth, height, hormones, transgender
1. INTRODUCTION
The attainment of one's final adult height is a complex interplay of environmental, genetic, familial, nutritional, and hormonal factors. The sexually dimorphic trait of height is one aspect of the experience of transgender and gender‐diverse (TGD) individuals that may influence their gender dysphoria and satisfaction with their transition, as cis‐gender men are on average 11–13 cm taller than cis‐gender women. 1 In the initial report of a transmasculine adolescent treated in the Netherlands with GnRH analog (GnRHa) at 13 years of age, exogeneous testosterone at age 17 years, and gender‐affirming surgery as a young adult, he attained an adult height of 169.5 cm, which is −2.0 standard deviations by normative data for Dutch men and −0.16 standard deviations for Dutch women. 2 The authors noted that the patient “would have liked to be taller.” 2
Additional research is needed to characterize the influence of growth and final adult height on the lived experience of TGD youth and adults and how to best assess their growth, predict their final adult height, and how medical transition can be potentially modified to help them meet their goals. In this article, we have reviewed the current knowledge of the factors that contribute to one's final adult height and how it might be affected in TGD youth who have not experienced their gonadal puberty in the setting of receiving gonadotropin‐releasing hormone analog (GnRHa) and gender‐affirming hormonal treatment.
2. FACTORS INFLUENCING LINEAR GROWTH
Prepubertal growth in TGD youth does not differ from their cis‐gender peers, as medical intervention, which would alter the sex steroid milieu, is not indicated until the onset of puberty. 3 Prior to pubertal onset, both birth‐assigned sexes have similar heights during childhood. Linear growth in childhood and adolescence occurs via the process of endochondral ossification at the cartilaginous epiphyseal plate (“growth plate”), located near the distal ends of the long bones. 4 , 5 , 6 Regulation of growth is complex and occurs by both paracrine and endocrine mechanisms including involvement of growth hormone (GH), which stimulates production primarily of insulin‐like growth factor‐I (IGF‐1) from the liver, as well as contributions from thyroid hormone, glucocorticoids, androgens, and sex steroids. 7 , 8 , 9 Additionally, paracrine effects from fibroblast growth factors, bone morphogenic proteins, Indian hedgehog, and parathyroid hormone have also been shown to be involved in disorders of short stature and may be influenced by sex steroids. 5 , 10 , 11 , 12 , 13 Other non‐endocrine factors such as genetic disorders, renal disease, pre‐natal factors, and malnutrition may also negatively impact growth. 5 Additionally, height is influenced by sex chromosomes with genes involved in height on both the X and Y chromosome, as supported by sex chromosome aneuploidies, in which an increase in the number of chromosomes, especially the Y chromosome, leads to tall stature. 14 , 15
3. PUBERTAL GROWTH PATTERNS
Puberty initiation is marked by the secretion of gonadotropin‐releasing hormone (GnRH) from the hypothalamus in a pulsatile fashion. 16 This secretory pattern is also the target of GnRHa in the management of pediatric gender dysphoria. 17 Pulsatile GnRH stimulates pituitary‐luteinizing hormone (LH) and follicle‐stimulating hormone section and this in turn causes downstream activation of the gonads. There is a predominance of production of testosterone by the testes and estrogen from the ovary, which leads to secondary sexual characteristics and the pubertal growth spurt. 16 Testosterone also increases GH secretion in birth‐assigned boys and estrogen increases GH secretion in both assigned sexes. 18 Birth‐assigned boys demonstrate increased GH pulse amplitude and higher cumulative GH concentrations compared with birth‐assigned girls. 18 , 19 , 20 Patient with coexisting hypogonadism and GH deficiency will not have a pubertal growth spurt when GH alone is replaced, as sex steroids stimulate GH as well as IGF‐1 and have direct effects on the epiphyseal plate. 18 Thus, GH and sex steroids are necessary for a normal pubertal growth spurt. 18
Clinicians caring for TGD youth may note that the pubertal growth begins with the onset of breast budding (e.g., Tanner stage 2 breast) in birth‐assigned females, while in birth‐assigned boys, this occurs in the later stages of puberty (e.g., Tanner stage 4 genitalia). 16 , 21 , 22 There is sexual dimorphism in the increase in GH secretion that accounts for at least some of the height differentials between adult birth‐assigned males and females associated with these pubertal stages. 19 , 23 The pubertal growth spurt contributes to approximately 17% of final adult height, equating to 27.5–29 cm in birth‐assigned girls and 30–31 cm in birth‐assigned boys. 1 In the absence of receiving GnRHa for gender dysphoria, birth‐assigned males are on average 13 centimeters taller compared with birth‐assigned females. 1 It is thought that this height differential is from a combination of factors (Figure 1). The first is timing; birth‐assigned‐females reach the peak height velocity on average two years earlier than birth‐assigned males (11.5 and 13.5 years, respectively). This allows for more time for gain in height before puberty. Second, birth‐assigned males reach a greater peak height velocity of approximately 9.5 cm/year, compared with 8.3 cm/year in birth‐assigned girls. 1 , 24 , 25
FIGURE 1.
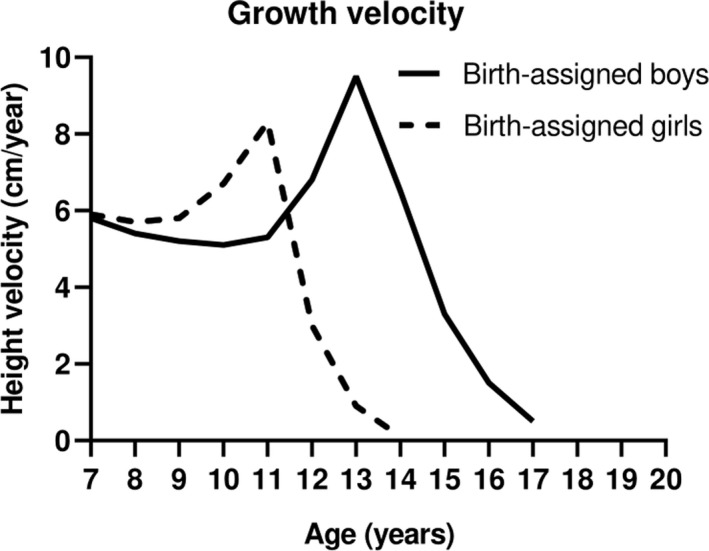
Pubertal growth velocities by birth‐assigned sex. Birth‐assigned men achieve a taller adult height in the setting of a testosterone‐associated puberty in part because of more time for prepubertal growth and an increased peak and longer duration of the pubertal growth spurt compared with birth‐assigned females who have undergone an estrogen‐associated puberty. Adapted from Ref. 1, 24
Bone growth continues to occur while the epiphyseal plate remains cartilaginous; linear growth ends when the entire bone has ossified as a result of the process of senescence of the chondrocytes. 18 , 26 This is thought to occur when the chondrocytes in the proliferative zone experience an accumulation of estrogen exposure and eventually become replaced with osteocytes. 27 Clinically, observations from case reports of individuals with estrogen receptor defects or aromatase deficiency demonstrate very delayed epiphyseal closure and associated tall stature. 28 No published studies have fully characterized the impact of final adult height or current height in an actively growing TGD youth and its impact on their gender dysphoria or lived experience.
4. ASSESSMENT OF ANTHROPOMETRIC MEASUREMENTS IN TGD YOUTH
Assessment of a TGD youth's anthropometric parameters is a component of the physical examination and should be approached in a sensitive, gender‐affirmative manner. Methods to achieve accuracy and precision of growth measurements may be undervalued by clinicians, yet are of paramount importance. Assessment of height is best carried out by the use of a wall‐mounted stadiometer when feasible. 29 Currently, there is no research or clinical practice guidelines to support whether the birth‐assigned or affirmed gender's growth charts should be used for the assessment of growth and development. 30 Additionally, baseline anthropometric parameters appear to be similar to population‐based normative data, while some gender‐diverse cohorts have reported increased rates of obesity. 31 , 32 For children over the age of 2 years, it is currently recommended that children are evaluated using the Center for Disease Control charts for weight, height, and body mass index (BMI) from the age of 2–20 years. 33 The lack of research to guide the utilization of the birth‐assigned vs. affirmed sex growth charts, which may alter the assessment of changes in anthropometric parameters, is challenging. 30 This may also be complicated by interpretation of any changes in weight or BMI percentile in the setting of a concomitant eating disorder or disordered eating behavior, which is an associated comorbidity of gender dysphoria. 34 Kidd et al. have proposed the interpretation of growth within both gendered growth charts and consideration for the prior patient history, pubertal stage, and any gender‐affirming pharmacological interventions that may alter weight and height. The authors suggest continuing to use the birth‐assigned growth chart during GnRHa therapy until gender‐affirming hormones are implemented based on the underlying physiology. Specifically, TGD youth can be regarded as hypogonadal youth who would be expected to display a growth pattern similar to cis‐gender peers with constitutional delay of growth and development until they begin gender‐affirming hormones, at which time it may be most appropriate to use the affirmed‐sex growth chart or use of both gendered growth charts.
5. METHODS TO PREDICT FINAL ADULT HEIGHT IN TGD YOUTH
No published literature provides guidance on how to best predict the final adult height for TGD youth receiving GnRHa and gender‐affirming hormonal treatment. Without medical intervention, adult height may be estimated using the corrected mid‐parental target height, which is commonly determined by the Tanner method, derived by taking an average of the parents’ heights (after adjusting for a 6.5 cm addition for birth‐assigned males or reduction for birth‐assigned females). 35 For example, a mother who is 160 cm and a father who is 180 cm would be expected to have birth‐assigned female child who attained an average adult height of 163.5 cm or a birth‐assigned male child who attained an average adult height of 176.5 cm. As visible by a comparison of siblings of the same birth‐assigned sex, variations in height are common, and these predictions fall within ±10 cm (approximately 1.5 standard deviations) of the predicted height. In TGD youth, the effects of the intersection of puberty‐suppressing medications and gender‐affirming hormones in influencing a final adult height closer to the birth‐assigned or affirmed gender have not been fully studied. In our experience, we have found that the height of transgender female adolescents who received GnRHa in early puberty followed by exogenous estradiol therapy attained a final adult height shorter than their birth‐assigned (e.g., male) prediction, but taller than their affirmed sex (e.g., female) prediction.
Another tool commonly used for height prediction by pediatric endocrinologists is the determination of skeletal, or bone, age from a left‐hand radiograph. 36 The determination of skeletal age is performed by comparing with age‐matched and sex‐matched standards, most commonly using the Greulich and Pyle atlas. 37 There are several methods to predict adult height, such as the Bayley‐Pinneau method, based on this skeletal age, but in general, this lacks applicability to many people, as the standards are based on Caucasian children. 36 , 38 , 39 , 40 Applying these prediction methods to an adolescent who is in the process of medical transition is not studied, especially in light that pubertal suppression leads to delays in skeletal maturation, gender‐affirming hormones are being implemented in age ranges (e.g., 14–16 years) that would be consistent with delayed puberty in cis‐gender peers and the unknown influence of gender‐affirming hormones on final height predictions. 3 , 41
6. USE OF GnRHa IN FOR PUBERTAL SUPPRESSION IN GENDER DYSPHORIA
GnRH analogues (GnRHa) are class of medications that lead to reversible pubertal suppression and are standard of care in TGD youth who meet strict diagnostic criteria. 3 GnRHa are synthetic derivatives of the native GnRH decapeptide, which differ predominantly via substitution of the 6th amino acid (glycine), which leads to greater potency and prolonged half‐life compared with native GnRH. 42 GnRHa initially acts as a “super‐agonist” causing a transient “flare‐up” phase during which time, the gonadotropins, LH and FSH, and downstream sex steroids increase. 42 This initial phase is followed by subsequent reduction of gonadotropins and therefore, sex steroids, through desensitization of gonadotropes in the pituitary gland (Figure 2). 42 , 43 In pediatrics, there is extensive clinical experience by pediatric endocrinologists in the implementation of GnRHa since the 1980s for central precocious puberty (CPP). 44
FIGURE 2.
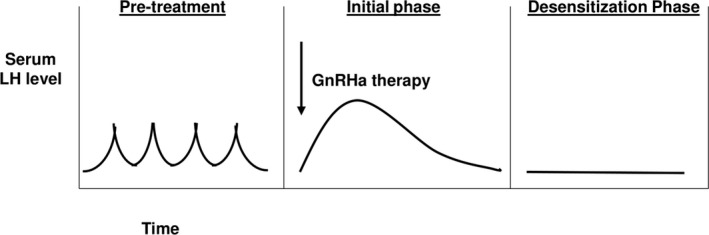
Serum LH levels in response to GnRHa therapy. Prior to medical transition, LH exhibits pulsatile secretion in response to its upstream stimulator, GnRH. With treatment with GnRHa, LH levels initially increase during a “flare‐up” followed by a “desensitization” phase leading to gonadotrope suppression and loss of LH pulsatility. Adapted from Ref. 42. LH, Luteinizing Hormone; GnRH, Gonadotropin‐releasing hormone; GnRHa, Gonadotropin‐releasing hormone Analog
When TGD youth meet eligibility criteria for GnRHa as established by current clinical practice guidelines, implementation of pubertal suppression can be considered when youth develop Tanner stage 2 (e.g., initial breast budding in birth‐assigned females, testicular volume ≥4 ml in birth‐assigned males) of puberty or later. 3 However, the utilization of GnRHa remains an off‐label use of medication, as currently there is no FDA‐approved indication for gender dysphoria. 3 , 45 , 46 Use of GnRHa agonist in youth with gender diversity, previously classified as gender identity disorder, was first implemented in the Netherlands in one of the oldest and largest pediatric transgender health programs. 47 , 48 Known informally as “the Dutch protocol,” this involved the use of GnRHa at Tanner stage 2 of puberty followed by subsequent consideration for gender‐affirming hormones (e.g., exogenous estradiol or testosterone) at 16 years of age and gender‐affirming surgeries after 18 years of age if desired by the patient. 47 This was then adopted by pediatric transgender health programs in the United States. 49 Psychological outcome data when this initial cohort was analyzed between the ages of 20 and 22 years demonstrated that transgender young adults who had been treated with GnRHa at the onset of pubertal development, as well as gender‐affirming hormones and gender‐affirming surgery, had similar psychological outcomes compared with population‐based normative data. 50
GnRHa formulations include intranasal, intramuscular, and subcutaneous injectables of various duration, as well as subcutaneous implants. 44 , 51 In gender dysphoric youth, most clinical experience includes injectable and subcutaneous implants. Injectable forms of GnRHa (e.g., leuprolide acetate and triptorelin) are also available as 1‐, 3‐, and 6‐month formulations, which require careful timing of follow‐up injections to maintain pubertal suppression and monitoring for development of the rare side effect of sterile abscesses. 44 Histrelin acetate implants (e.g., Supprelin LA) are also commonly prescribed for their long duration of action, as well as for their efficacy; however, they require that a small incision be made in the subcutaneous tissue, typically of the upper arm, and may also be cost‐prohibitive. 44 More cost‐effective histrelin acetate 50 mg subcutaneous implants, such as Vantus, which deliver 50 mcg/day, may be alternatives to Supprelin LA, which delivers 65 mcg/day, and are likely to be equally effective in TGD youth. 52 , 53 Of note, the brand SupprelinLA has a pediatric indication for precocious puberty, while Vantas does not have; however, in the United States, Supprelin LA currently costs approximately $42,873, while Vantas costs significantly less at approximately $5,098. 54 , 55 The use of a histrelin acetate implant is approved for one year; however, clinical experience from CPP demonstrates a longer duration of action of two years. 45 , 56 , 57 These authors also have clinical experience with TGD patients lost to follow‐up with histrelin implants still effective at 3–4 years.
Longitudinal studies of efficacy and safety in TGD youth are needed; however, initial follow‐up data in gender dysphoric youth support its safety. 58 Breakage of the implant has been reported in CPP with surgical explantation after one year, typically in the setting of subcutaneous encapsulation or scar tissue; however, this surgical complication upon removal has not been characterized in gender‐diverse children and it is unknown how duration of placement greater than one or two years influences this outcome. 56 , 59 In addition to these considerations, the effects of cognitive, physical (e.g., bone health) and surgical implications of pubertal suppression also should be weighed. 60
7. IMPACT OF GnRHa ON LINEAR GROWTH AND FINAL ADULT HEIGHT IN TGD YOUTH
A reduction in height velocity during GnRHa therapy in an actively growing child to a prepubertal growth velocity is an expected side effect in medical transition and has been shown to also lead to a reduction in height standard deviation score (SDS). 47 , 58 , 61 GnRHa has been used in some instances as a treatment for short stature, when used in conjunction with GH therapy, in cis‐gender children to lead to a taller adult height. 62
As birth‐assigned males are taller than birth‐assigned females, current or final height could impact the experience of the gender diverse population to live in their affirmed gender, for example, a transmasculine adult with a low‐normal height of cis‐gender females has a very low height for cis‐gender men. Within the gender‐diverse community, some individuals describe themselves as having “height dysphoria” and in some instances could be severe enough to meet criteria within the diagnosis of body dysmorphia, although this has not been formally described in the research literature. Additionally, how this correlates with actual height has not been described. No studies have evaluated the impact of current or final adult height on gender dysphoria, nor characterized the heights of gender‐diverse adults compared with cis‐gender identified peers.
Several measures have been created to assess body image in the TGD population. 63 , 64 Height and stature are included as a component to the Body Image Scale (BIS) created by Lindgren and Pauly in the as part of a 30‐element scale of body dissatisfaction. 64 Sub‐analysis typically includes height as a “neutral” characteristic that is described by the authors as “hormonally unresponsive” compared with traits like genitalia or analyzed by body image subscale for muscularity and posture, and height is not commonly reported as an individual variable. 64 , 65 , 66 , 67 Transgender men have been shown to have statistically higher rates of body dissatisfaction with their height compared with transgender women; however, transgender men have also been reported to have satisfaction with their height. 66 , 68
BIS data from the Netherlands cohort showed that in 70 TGD youth aged 12–16 years, GnRHa therapy alone did not impact body dissatisfaction; however, this was assessed after only one‐year duration. 69 A follow‐up study by the same authors of 55 young TGD adults who had been treated using GnRHa followed by gender‐affirming hormones and gender‐affirming surgery showed that transgender women had improved body dissatisfaction, but transgender men failed to show a statistically significant difference. 50 A more recent study of 95 American TGD youth aged 8–16 years demonstrated that transfeminine youth had significantly higher rates of body dissatisfaction compared with transmasculine youth prior to receiving GnRHa. 70 None of these studies have reported specifically the ratings of body dissatisfaction of height in TGD youth.
8. IMPACT OF GnRHa ON WEIGHT AND METABOLIC PROFILE OF TGD YOUTH
Counseling related to changes in weight and need for lifestyle modification should be approached in a thoughtful manner, especially as TGD youth are at an increased risk for disordered eating and body dissatisfaction. 34 Weight gain is listed as a common side effect of GnRH agonist, including leuprolide and histrelin acetate. 45 , 46 Assessment of weight in children with CPP treated with GnRH agonist has demonstrated mixed results and in some groups, weight gain appears to be transient. 57 , 71 , 72 , 73 , 74 Yang et al. found a differential response in changes in BMI in CPP, with the baseline normal‐weight birth‐assigned females showing a significant increase in BMI‐standard deviation‐score (SDS) in comparison with the group that was overweight at baseline that did not; however, this has been supported other studies. 72 , 73
Anthropometric response to GnRHa treatment in gender dysphoria warrants specific scientific investigation, as intervening in the underlying biology of CPP may not mimic the biology of classically timed puberty treated in gender dysphoria. A small study of 11 TGD youth followed on GnRHa for two years or longer demonstrated an increase in fat mass percentage and decrease in lean body mass percentage. 47 Klink et al. reported no change in BMI SDS between GnRHa and beginning gender‐affirming hormones in 34 TGD youth aged 11–18 years. 41 Similarly, Schagen et al.’s cohort of 128 TGD adolescent reported no change in the BMI SDS one year after receiving GnRHa therapy in birth‐assigned males, but birth‐assigned females did demonstrate a statistically significant increase in BMI SDS during this time. 58 Both of these studies included patients who were predominantly late to post‐pubertal with a median Tanner stage 4 breast development in birth‐assigned females and median Tanner stage 4–5 genitalia in birth‐assigned males. Additionally, a recent study of 71 adolescent transgender females and 121 transgender males found that absolute BMI significantly increased during treatment with GnRHa alone; BMI SDS was not reported. 75 Research studies are needed to understand if any changes in weight gain are transient or of longer duration, especially as it may impact metabolic health. Lower HDL‐C levels as compared with NHANES controls have been observed and decreased insulin sensitivity has been reported as well in TGD youth. 31 , 76
9. SPECIAL CONSIDERATION FOR PROLONGED GnRHa USE IN NON‐BINARY YOUTH
Future research is also needed to evaluate the outcomes specifically of non‐binary identified youth who may desire GnRHa without desire to also progress to gender‐affirming hormones. Newer publications have begun characterizing the mental health and medical outcomes of non‐binary adults, but even fewer studies have described non‐binary youth. 77 Non‐binary youth experience less access to health care than binary transgender youth. 78 Outcome data on non‐binary youth treated with prolonged pubertal suppression are needed. Current clinical practice guidelines do not provide guidance for medical providers and should be discussed within the ethical framework of balancing autonomy, benefits, and risks as well as exploring alternative medical options. 78 , 79 From a growth perspective, prolonged suppression by GnRHa has the potential result in a taller‐than‐expected adult height, extrapolated from growth trends from adults with untreated hypogonadism that begins before epiphyseal closure. 80
10. ALTERNATIVES TO GNRHA FOR PUBERTAL SUPPRESSION
Administration of GnRHa for TGD with gender dysphoria may vary based on local availability, comfort level of the medical provider, cost and/or insurance coverage. 53 , 81 , 82 Before the availability of GnRHa, progestins such as medroxyprogesterone or cyproterone acetate, depending on the geographical location of clinical practice, were the mainstay of therapy for children with CPP. 83 , 84 , 85 The use of depot or oral medroxyprogesterone can be considered, especially in settings where GnRH agonist is too costly or not available; however, GnRH agonist should be utilized as first‐line therapy when possible due to better efficacy and an improved side effect profile. 3 , 86 The anti‐androgen, cyproterone acetate, available outside of the United States, has been widely used in adult transwoman to suppress endogenous androgens and there is some experience reported in adolescents, although effects on growth velocity were not studied. 3 , 87 , 88 A small but statistically significant reduction in height SDS has been reported in 21 transgender adolescent girls treated with cyproterone acetate, while no significant reduction was noted in 44 transgender adolescent boys treated with the progestin, lynestrenol, for pubertal suppression. However, this is most likely related to mean age at progestin initiation of 17.1 years in transgender girls and 16.1 years in transgender boys in this study, when most linear growth has already been attained. 88 Therefore, the effects of progestins on growth velocity are not well‐characterized. Combined oral contraceptives, which contain supraphysiologic dose of ethinyl estradiol, should not be used for HPG suppression in actively growing child, as estradiol fuses the epiphyseal cartilage. 89
11. INFLUENCE OF GENDER‐AFFIRMING HORMONES ON GROWTH
Current dosing protocols for pubertal induction for gender‐affirming estradiol and testosterone administration are predominantly derived from experience with cis‐gender hypogonadal patients and initial studies in adolescents support its safety. 3 , 90 , 91 , 92 , 93 , 94 Pediatric endocrinologists previously manipulated height in cis‐gender girls through administration of high‐dose estrogen to attenuate adult height by accelerating epiphyseal fusion. 95 , 96 , 97 While this practice has fallen out of favor, this approach may still be practiced by some pediatric endocrinologists when desired by parents of children with severe intellectual disability. 98 One case series of 11 transgender girls described an average adult height reduction of 4.3 cm in the setting of receiving a maximum dose of 6 mg of oral 17‐beta estradiol or 200 mcg of oral ethinyl estradiol, but adult height was still above average for Dutch females. The authors concluded that if height attenuation is desired, exogenous estradiol should be used at higher doses and prior to the age of 16 years. 99 Another case series of transgender girls using similar doses of exogenous estradiol noted that only one of six subjects had a perceived adult height reduction; however, the authors acknowledge a variation in dosage paradigms and bone age at initiation of estradiol therapy, which may have impacted their results. 94 While 17‐beta estradiol is now preferred to ethinyl estradiol for reasons of more favorable thromboembolic profile, transdermal 17‐beta estradiol has been associated with an increase in IGF‐1 levels in some studies compared with oral 17‐beta estradiol; however, no randomized control studies have compared the adult height of transgender girls based on route of administration. 100 , 101
Finally, short stature has been treated by some pediatric endocrinologists using aromatase inhibitors that prevent the conversion of testosterone to estradiol and therefore should prolong the growth potential of the chondrocytes in the epiphyseal plate. 102 , 103 , 104 , 105 , 106 This method has been an approach to optimize height in cis‐gender boys. The use of non‐aromatizable androgens, such as the use of the use of dihydrotestosterone in cis‐gender boys and oxandrolone in girls with Turner syndrome, has been found to increase height without advancing the bone age. 107 , 108 Oxandrolone has been previously suggested by current clinical practice guidelines to be a therapeutic option in short transmasculine youth. 3 A recent publication suggests that oxandrolone use in transmasculine adolescents may lead to small gains in adult height compared with mid‐parental target height, However, this study was confounded by some patients within subgroups also receiving GnRH agonist therapy. 109 Nonetheless, oxandrolone may be a potential future therapy in youth 10 years and older to minimize the risk of dose‐dependent virilization and potential risk for bone age advancement in younger children. 110 , 111
12. FUTURE DIRECTIONS
Many questions remain unanswered in understanding how best to assess growth and use of height prediction tools in TGD youth, as well as the influences of timing, route of administration, and duration of puberty‐suppressing medications and gender‐affirming hormones on final adult height. In gender‐diverse youth, the combination of some endogenous hormone exposure before GnRH agonist therapy with delayed implementation of gender‐affirming hormones, compared with continuous endogenous hormone exposure in cis‐gender peers, may potentially result in sub‐optimally timed sex steroid exposure, potentially affecting chondrocyte senescence and negatively impacting adult height. Future therapeutic tools that could be innovative in TGD care include the potential use of other alternatives to GnRHa such as GnRH antagonists or other progestins, such as depot medroxyprogesterone, the synthetic steroid danazol or the anti‐androgen biclutamide. 16 , 86 , 112 Additionally, growth attenuation or promotion strategies through manipulation of gender‐affirming hormone dosing or through the use of adjunctive medications such as aromatase inhibitors, the non‐aromatizable androgens, or growth hormone therapy may prove in the future to have benefit, although these options are highly speculative currently, as their potential benefits have not been studied in TGD youth. 106 , 108 Regardless, as clinicians continue to provide patient‐centered care, a discussion of the youth's growth, including adult height prediction, and exploration of any family‐based values around height and how this may influence their gender dysphoria should be included as part of their comprehensive care.
DISCLOSURES
SAR has no disclosures. JMC served on an advisory board for Endo Pharmaceuticals in June 2018.
AUTHORS’ CONTRIBUTIONS
Drs. Roberts and Carswell contributed equally as they conceptualized the content of this review article, drafted the manuscript, and edited and reviewed the final manuscript.
ACKNOWLEDGEMENTS
The authors thank their transgender and gender‐diverse youth and families for the privilege of being their medical providers.
Funding information
Funding includes National Institutes of Health K12HD051959 (PI: Goldstein) and Society for Pediatric Research Bridging to Success Award, both supporting SAR.
REFERENCES
- 1. Abbassi V. Growth and normal puberty. Pediatrics. 1998;102(2 Pt 3):507‐511. [PubMed] [Google Scholar]
- 2. Cohen‐Kettenis PT, Schagen SEE, Steensma TD, de Vries ALC, Delemarre‐van de Waal HA. Puberty suppression in a gender‐dysphoric adolescent: a 22‐year follow‐up. Arch Sex Behav. 2011;40(4):843‐847. 10.1007/s10508-011-9758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hembree WC, Cohen‐Kettenis PT, Gooren L, et al. Endocrine treatment of gender‐dysphoric/gender‐incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869‐3903. 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 4. Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech. 1994;28(6):505‐519. 10.1002/jemt.1070280606. [DOI] [PubMed] [Google Scholar]
- 5. van der Eerden BCJ, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev. 2003;24(6):782‐801. 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- 6. Nilsson O, Marino R, Luca FD, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res Paediatr. 2005;64(4):157‐165. 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 7. Lui JC, Nilsson O, Baron J. Recent insights into the regulation of the growth plate. J Mol Endocrinol. 2014;53(1):T1‐T9. 10.1530/JME-14-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray PG, Clayton PE. Endocrine control of growth. Am J Med Genet C Semin Med Genet. 2013;163(2):76‐85. 10.1002/ajmg.c.31357. [DOI] [PubMed] [Google Scholar]
- 9. Roselló‐Díez A, Joyner AL. Regulation of long bone growth in vertebrates; it is time to catch up. Endocr Rev. 2015;36(6):646‐680. 10.1210/er.2015-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karp SJ, Schipani E, St‐Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related‐protein‐dependent and ‐independent pathways. Development. 2000;127(3):543‐548. [DOI] [PubMed] [Google Scholar]
- 11. Kindblom JM, Nilsson O, Hurme T, Ohlsson C, Savendahl L. Expression and localization of Indian hedgehog (Ihh) and parathyroid hormone related protein (PTHrP) in the human growth plate during pubertal development. J Endocrinol. 2002;174(2):R1‐R6. 10.1677/joe.0.174r001. [DOI] [PubMed] [Google Scholar]
- 12. Hirai T, Chagin AS, Kobayashi T, Mackem S, Kronenberg HM, Scott MP. Parathyroid hormone/parathyroid hormone‐related protein receptor signaling is required for maintenance of the growth plate in postnatal life. Proc Natl Acad Sci 2011;108(1):191‐196. 10.1073/pnas.1005011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emons J, Chagin AS, Sävendahl L, Karperien M, Wit JM. Mechanisms of Growth Plate Maturation and Epiphyseal Fusion. Horm Res Paediatr. 2011;75(6):383‐391. 10.1159/000327788. [DOI] [PubMed] [Google Scholar]
- 14. Ottesen AM, Aksglaede L, Garn I, et al. Increased number of sex chromosomes affects height in a nonlinear fashion: A study of 305 patients with sex chromosome aneuploidy. Am J Med Genet A. 2010;152A(5):1206‐1212. 10.1002/ajmg.a.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis JA, Stebbing M, Harrap SB. Significant population variation in adult male height associated with the Y chromosome and the aromatase gene. J Clin Endocrinol Metab. 2001;86(9):4147‐4150. 10.1210/jcem.86.9.7875. [DOI] [PubMed] [Google Scholar]
- 16. Roberts SA, Kaiser UB. GENETICS IN ENDOCRINOLOGY: Genetic etiologies of central precocious puberty and the role of imprinted genes. Eur J Endocrinol. 2020;183(4):R107‐R117. 10.1530/EJE-20-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boepple PA, Mansfield MJ, Wierman ME, et al. Use of a potent, long acting agonist of gonadotropin‐releasing hormone in the treatment of precocious puberty. Endocr Rev. 1986;7(1):24‐33. 10.1210/edrv-7-1-24. [DOI] [PubMed] [Google Scholar]
- 18. Leung K‐C, Johannsson G, Leong GM, Ho KKY. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25(5):693‐721. 10.1210/er.2003-0035. [DOI] [PubMed] [Google Scholar]
- 19. Perry RJ, Farquharson C, Ahmed SF. The role of sex steroids in controlling pubertal growth. Clin Endocrinol (Oxf). 2008;68(1):4‐15. 10.1111/j.1365-2265.2007.02960.x. [DOI] [PubMed] [Google Scholar]
- 20. Rose SR, Municchi G, Barnes KM, et al. Spontaneous growth hormone secretion increases during puberty in normal girls and boys. J Clin Endocrinol Metab. 1991;73(2):428‐435. 10.1210/jcem-73-2-428. [DOI] [PubMed] [Google Scholar]
- 21. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13‐23. 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albertsson‐Wikland K, Rosberg S, Karlberg J, Groth T. Analysis of 24‐hour growth hormone profiles in healthy boys and girls of normal stature: relation to puberty. J Clin Endocrinol Metab. 1994;78(5):1195‐1201. 10.1210/jcem.78.5.8175978. [DOI] [PubMed] [Google Scholar]
- 24. Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966;41(220):613‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317‐329. 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 26. Meinhardt UJ, Ho KKY. Modulation of growth hormone action by sex steroids. Clin Endocrinol (Oxf). 2006;65(4):413‐422. 10.1111/j.1365-2265.2006.02676.x. [DOI] [PubMed] [Google Scholar]
- 27. Weise M, De‐Levi S, Barnes KM, Gafni RI, Abad V, Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci USA. 2001;98(12):6871‐6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bulun SE. Aromatase and estrogen receptor α deficiency. Fertil Steril. 2014;101(2):323‐329. 10.1016/j.fertnstert.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Louer AL, Simon DN, Switkowski KM, Rifas‐Shiman SL, Gillman MW, Oken E. Assessment of child anthropometry in a large epidemiologic study. J Vis Exp JoVE. 2017;(120). 10.3791/54895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kidd KM, Sequeira GM, Dhar CP, Montano GT, Witchel SF, Rofey D. Gendered body mass index percentile charts and transgender youth: making the case to change charts. Transgender Health. 2019;4(1):297‐299. 10.1089/trgh.2019.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Millington K, Schulmeister C, Finlayson C, et al. Physiological and metabolic characteristics of a cohort of transgender and gender‐diverse youth in the United States. J Adolesc Health. 2020;67(3):376‐383. 10.1016/j.jadohealth.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brocksmith VM, Alradadi RS, Chen M, Eugster EA. Baseline characteristics of gender dysphoric youth. J Pediatr Endocrinol Metab. 2018;31(12):1367‐1369. 10.1515/jpem-2018-0250. [DOI] [PubMed] [Google Scholar]
- 33. Use and Interpretation of the WHO and CDC Growth Charts for Children from Birth to 20 Years in the United States: 4. https://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/growthchart.pdf. Accessed December 30, 2020. [Google Scholar]
- 34. Nagata JM, Ganson KT, Austin SB. Emerging trends in eating disorders among sexual and gender minorities. Curr Opin Psychiatry. 2020;33(6):562‐567. 10.1097/YCO.0000000000000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanner JM, Goldstein H, Whitehouse RH. Standards for children’s height at ages 2–9 years allowing for heights of parents. Arch Dis Child. 1970;45(244):755‐762. 10.1136/adc.45.244.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin DD, Wit JM, Hochberg Z, et al. The use of bone age in clinical practice – part 1. Horm Res Paediatr. 2011;76(1):1‐9. 10.1159/000329372. [DOI] [PubMed] [Google Scholar]
- 37. Alshamrani K, Messina F, Offiah AC. Is the Greulich and Pyle atlas applicable to all ethnicities? A systematic review and meta‐analysis. Eur Radiol. 2019;29(6):2910‐2923. 10.1007/s00330-018-5792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Satoh M. Bone age: assessment methods and clinical applications. Clin Pediatr Endocrinol Case Rep Clin Investig. 2015;24(4):143‐152. 10.1297/cpe.24.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roemmich JN, Blizzard RM, Peddada SD, et al. Longitudinal assessment of hormonal and physical alterations during normal puberty in boys. IV: Predictions of adult height by the Bayley‐Pinneau, Roche‐Wainer‐Thissen, and Tanner‐Whitehouse methods compared. Am J Hum Biol. 1997;9(3):371‐380. [DOI] [PubMed] [Google Scholar]
- 40. Ostojic SM. Prediction of adult height by Tanner‐Whitehouse method in young Caucasian male athletes. QJM Mon J Assoc Physicians. 2013;106(4):341‐345. 10.1093/qjmed/hcs230. [DOI] [PubMed] [Google Scholar]
- 41. Klink D, Caris M, Heijboer A, van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin‐releasing hormone analog treatment and cross‐sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab. 2015;100(2):E270‐275. 10.1210/jc.2014-2439. [DOI] [PubMed] [Google Scholar]
- 42. Millar RP, Lu Z‐L, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin‐releasing hormone receptors. Endocr Rev. 2004;25(2):235‐275. 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- 43. Conn PM, Crowley WF. Gonadotropin‐releasing hormone and its analogues. N Engl J Med. 1991;324(2):93‐103. 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 44. Eugster EA. Treatment of Central Precocious Puberty. J Endocr Soc. 2019;3(5):965‐972. 10.1210/js.2019-00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Supprelin LA (histrelin acetate) label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022058s006lbl.pdf. Accessed December 22, 2020.
- 46. Lupron Depot‐Ped (leuprolide acetate) label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020263s036lbl.pdf. Accessed December 22, 2020.
- 47. de Waal D‐V, Cohen‐Kettenis PT. Clinical management of gender identity disorder in adolescents: a protocol on psychological and paediatric endocrinology aspects. Eur J Endocrinol. 2006;155(suppl_1):S131–S137. 10.1530/eje.1.02231. [DOI] [Google Scholar]
- 48. Cohen‐Kettenis PT, van Goozen SH. Pubertal delay as an aid in diagnosis and treatment of a transsexual adolescent. Eur Child Adolesc Psychiatry. 1998;7(4):246‐248. 10.1007/s007870050073. [DOI] [PubMed] [Google Scholar]
- 49. Spack NP, Edwards‐Leeper L, Feldman HA, et al. Children and adolescents with gender identity disorder referred to a pediatric medical center. Pediatrics. 2012;129(3):418–425. 10.1542/peds.2011-0907 [DOI] [PubMed] [Google Scholar]
- 50. de Vries ALC, McGuire JK, Steensma TD, Wagenaar ECF, Doreleijers TAH, Cohen‐Kettenis PT. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics. 2014;134(4):696–704. 10.1542/peds.2013-2958 [DOI] [PubMed] [Google Scholar]
- 51. Carel J‐C, Eugster EA, Rogol A, et al. Consensus statement on the use of gonadotropin‐releasing hormone analogs in children. Pediatrics. 2009;123(4):e752‐762. 10.1542/peds.2008-1783. [DOI] [PubMed] [Google Scholar]
- 52. Olson‐Kennedy J, Streeter LH, Garofalo R, Chan Y‐M, Rosenthal SM. Histrelin implants for suppression of puberty in youth with gender dysphoria: a comparison of 50 mcg/Day (Vantas) and 65 mcg/Day (SupprelinLA Transgender Health. 2021;6(1):36–42. 10.1089/trgh.2020.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stevens J, Gomez‐Lobo V, Pine‐Twaddell E. Insurance coverage of puberty blocker therapies for transgender youth. Pediatrics. 2015;136(6):1029‐1031. 10.1542/peds.2015-2849. [DOI] [PubMed] [Google Scholar]
- 54. Supprelin LA Prices, Coupons & Patient Assistance Programs. Drugs.com. https://www.drugs.com/price‐guide/supprelin‐la. Accessed December 29, 2020.
- 55. Vantas Prices, Coupons & Patient Assistance Programs. Drugs.com. https://www.drugs.com/price‐guide/vantas. Accessed December 29, 2020
- 56. Lewis KA, Goldyn AK, West KW, Eugster EA. A single histrelin implant is effective for 2 years for treatment of central precocious puberty. J Pediatr. 2013;163(4):1214‐1216. 10.1016/j.jpeds.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rahhal S, Clarke WL, Kletter GB, et al. Results of a second year of therapy with the 12‐month histrelin implant for the treatment of central precocious puberty. Int J Pediatr Endocrinol. 2009;2009:812517. 10.1155/2009/812517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schagen SEE, Cohen‐Kettenis PT, Delemarre‐van de Waal HA, Hannema SE. Efficacy and safety of gonadotropin‐releasing hormone agonist treatment to suppress puberty in gender dysphoric adolescents. J Sex Med. 2016;13(7):1125‐1132. 10.1016/j.jsxm.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 59. Silverman LA, Neely EK, Kletter GB, et al. Long‐term continuous suppression with once‐yearly histrelin subcutaneous implants for the treatment of central precocious puberty: a final report of a phase 3 multicenter trial. J Clin Endocrinol Metab. 2015;100(6):2354‐2363. 10.1210/jc.2014-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mahfouda S, Moore JK, Siafarikas A, Zepf FD, Lin A. Puberty suppression in transgender children and adolescents. Lancet Diabetes Endocrinol. 2017;5(10):816‐826. 10.1016/S2213-8587(17)30099-2. [DOI] [PubMed] [Google Scholar]
- 61. Attie KM, Ramirez NR, Conte FA, Kaplan SL, Grumbach MM. The pubertal growth spurt in eight patients with true precocious puberty and growth hormone deficiency: evidence for a direct role of sex steroids. J Clin Endocrinol Metab. 1990;71(4):975‐983. 10.1210/jcem-71-4-975. [DOI] [PubMed] [Google Scholar]
- 62. Ranke MB. Treatment of children and adolescents with idiopathic short stature. Nat Rev Endocrinol. 2013;9(6):325‐334. 10.1038/nrendo.2013.71. [DOI] [PubMed] [Google Scholar]
- 63. Becker I, Nieder TO, Cerwenka S, et al. Body Image in Young Gender Dysphoric Adults: A European Multi‐Center Study. Arch Sex Behav. 2016;45(3):559‐574. 10.1007/s10508-015-0527-z. [DOI] [PubMed] [Google Scholar]
- 64. Lindgren TW, Pauly IB. A body image scale for evaluating transsexuals. Arch Sex Behav. 1975;4(6):639‐656. 10.1007/BF01544272. [DOI] [PubMed] [Google Scholar]
- 65. van de Grift TC, Cohen‐Kettenis PT, Steensma TD, et al. Body satisfaction and physical appearance in gender dysphoria. Arch Sex Behav. 2016;45:575‐585. 10.1007/s10508-015-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van de Grift TC, Elaut E, Cerwenka SC, et al. Effects of medical interventions on gender dysphoria and body image: a follow‐up study. Psychosom Med. 2017;79(7):815‐823. 10.1097/PSY.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shirdel‐Havar E, Steensma TD, Cohen‐Kettenis PT, Kreukels BPC. Psychological symptoms and body image in individuals with gender dysphoria: A comparison between Iranian and Dutch clinics. Int J Transgenderism. 2018;20(1):108‐117. 10.1080/15532739.2018.1444529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bozkurt A, Isikli H, Demir F, et al. Body image and personality traits of male‐to‐female transsexuals and homosexuals. Soc Behav Personal. 2006;34(8):927. 10.2224/sbp.2006.34.8.927. [DOI] [Google Scholar]
- 69. de Vries ALC, Steensma TD, Doreleijers TAH, Cohen‐Kettenis PT. Puberty suppression in adolescents with gender identity disorder: a prospective follow‐up study. J Sex Med. 2011;8(8):2276‐2283. 10.1111/j.1743-6109.2010.01943.x. [DOI] [PubMed] [Google Scholar]
- 70. Chen D, Abrams M, Clark L, et al. Psychosocial characteristics of transgender youth seeking gender‐affirming medical treatment: baseline findings from the trans youth care study. J Adolescent Health. 2020. 10.1016/j.jadohealth.2020.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yoon JW, Park HA, Lee J, Kim JH. The influence of gonadotropin‐releasing hormone agonists on anthropometric change in girls with central precocious puberty. Korean J Pediatr. 2017;60(12):395‐402. 10.3345/kjp.2017.60.12.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim SW, Kim YB, Lee JE, et al. The influence of gonadotropin releasing hormone agonist treatment on the body weight and body mass index in girls with idiopathic precocious puberty and early puberty. Ann Pediatr Endocrinol Metab. 2017;22(2):95‐101. 10.6065/apem.2017.22.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang WJ, Ko KH, Lee KH, Hwang IT, Oh YJ. The different effects of gonadotropin‐releasing hormone agonist therapy on body mass index and growth between normal‐weight and overweight girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2017;22(1):49‐54. 10.6065/apem.2017.22.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vuralli D, Ozon ZA, Gonc EN, Alikasifoglu A, Kandemir N. Long‐term effects of GnRH agonist treatment on body mass index in girls with idiopathic central precocious puberty. J Pediatr Endocrinol Metab JPEM. 2020;33(1):99‐105. 10.1515/jpem-2019-0214. [DOI] [PubMed] [Google Scholar]
- 75. Klaver M, Mutsert R, van der Loos MATC, et al. Hormonal treatment and cardiovascular risk profile in transgender adolescents. Pediatrics. 2020;145(3): 10.1542/peds.2019-0741. [DOI] [PubMed] [Google Scholar]
- 76. Nokoff NJ, Scarbro SL, Moreau KL, et al. Body composition and markers of cardiometabolic health in transgender youth on gonadotropin‐releasing hormone agonists. Transgender Health. 2021;6(2):111–119. 10.1089/trgh.2020.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reisner SL, Hughto JMW. Comparing the health of non‐binary and binary transgender adults in a statewide non‐probability sample. PLoS One. 2019;14(8):e0221583. 10.1371/journal.pone.0221583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chew D, Tollit MA, Poulakis Z, Zwickl S, Cheung AS, Pang KC. Youths with a non‐binary gender identity: a review of their sociodemographic and clinical profile. Lancet Child Adolesc Health. 2020;4(4):322‐330. 10.1016/S2352-4642(19)30403-1. [DOI] [PubMed] [Google Scholar]
- 79. Pang KC, Notini L, McDougall R, et al. Long‐term puberty suppression for a nonbinary teenager. Pediatrics. 2020;145(2): 10.1542/peds.2019-1606. [DOI] [PubMed] [Google Scholar]
- 80. Uriarte MM, Baron J, Garcia HB, Barnes KM, Loriaux DL, Cutler GB. The effect of pubertal delay on adult height in men with isolated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1992;74(2):436‐440. 10.1210/jcem.74.2.1449545. [DOI] [PubMed] [Google Scholar]
- 81. Nahata L, Quinn GP, Caltabellotta NM, Tishelman AC. Mental health concerns and insurance denials among transgender adolescents. LGBT Health. 2017;4(3):188‐193. 10.1089/lgbt.2016.0151. [DOI] [PubMed] [Google Scholar]
- 82. Dowshen NL, Christensen J, Gruschow SM. Health insurance coverage of recommended gender‐affirming health care services for transgender youth: shopping online for coverage information. Transgender Health. 2019;4(1):131‐135. 10.1089/trgh.2018.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kauli R, Pertzelan A, Prager‐Lewin R, Grünebaum M, Laron Z. Cyproterone acetate in treatment of precocious puberty. Arch Dis Child. 1976;51(3):202‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Warren MP, Mathews JH, Morishima A, Vande WR. The effect of medroxyprogesterone acetate on gonadotropin secretion in girls with precocious puberty. Am J Med Sci. 1975;269(3):375‐381. 10.1097/00000441-197505000-00010. [DOI] [PubMed] [Google Scholar]
- 85. Lee PA. Medroxyprogesterone therapy for sexual precocity in girls. Am J Dis Child 1960. 1981;135(5):443‐445. 10.1001/archpedi.1981.02130290041015. [DOI] [PubMed] [Google Scholar]
- 86. Jain J, Kwan D, Forcier M. Medroxyprogesterone acetate in gender‐affirming therapy for transwomen: results from a retrospective study. J Clin Endocrinol Metab. 2019;104(11):5148‐5156. 10.1210/jc.2018-02253. [DOI] [PubMed] [Google Scholar]
- 87. Tack LJW, Heyse R, Craen M, et al. Consecutive cyproterone acetate and estradiol treatment in late‐pubertal transgender female adolescents. J Sex Med. 2017;14(5):747‐757. 10.1016/j.jsxm.2017.03.251. [DOI] [PubMed] [Google Scholar]
- 88. Tack LJW, Craen M, Lapauw B, et al. Proandrogenic and antiandrogenic progestins in transgender youth: differential effects on body composition and bone metabolism. J Clin Endocrinol Metab. 2018;103(6):2147‐2156. 10.1210/jc.2017-02316. [DOI] [PubMed] [Google Scholar]
- 89. Cutler GB. The role of estrogen in bone growth and maturation during childhood and adolescence. J Steroid Biochem Mol Biol. 1997;61(3–6):141‐144. [PubMed] [Google Scholar]
- 90. Vogiatzi M, Tursi JP, Jaffe JS, Hobson S, Rogol AD. Testosterone use in adolescent males: current practice and unmet needs. J Endocr Soc. 2020;5(1): 10.1210/jendso/bvaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Klein KO, Rosenfield RL, Santen RJ, et al. Estrogen replacement in turner syndrome: literature review and practical considerations. J Clin Endocrinol Metab. 2018;103(5):1790‐1803. 10.1210/jc.2017-02183. [DOI] [PubMed] [Google Scholar]
- 92. Olson‐Kennedy J, Okonta V, Clark LF, Belzer M. Physiologic response to gender‐affirming hormones among transgender youth. J Adolesc Health. 2018;62(4):397‐401. 10.1016/j.jadohealth.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jarin J, Pine‐Twaddell E, Trotman G, et al. Cross‐sex hormones and metabolic parameters in adolescents with gender dysphoria. Pediatrics. 2017;139(5):e20163173. 10.1542/peds.2016-3173. [DOI] [PubMed] [Google Scholar]
- 94. Hannema SE, Schagen SEE, Cohen‐Kettenis PT, Delemarre‐van de Waal HA. Efficacy and safety of pubertal induction using 17β‐estradiol in transgirls. J Clin Endocrinol Metab. 2017;102(7):2356‐2363. 10.1210/jc.2017-00373. [DOI] [PubMed] [Google Scholar]
- 95. Crawford JD. Treatment of tall girls with estrogen. Pediatrics. 1978;62(6 Pt 2):1189‐1195. [PubMed] [Google Scholar]
- 96. Goldzieher MA. Treatment of excessive growth in the adolescent female. J Clin Endocrinol Metab. 1956;16(2):249‐252. 10.1210/jcem-16-2-249. [DOI] [PubMed] [Google Scholar]
- 97. Freed SC. Suppression of growth in excessively tall girls. J Am Med Assoc. 1958;166(11):1322. 10.1001/jama.1958.62990110001011. [DOI] [PubMed] [Google Scholar]
- 98. Gunther D, Diekema D. Attenuating growth in children with profound developmental disability. Arch Pediatr Adolesc Med. 2006;160:1013‐1017. 10.1001/archpedi.160.10.1013. [DOI] [PubMed] [Google Scholar]
- 99. Hellinga I, Wiepjes C, Vink D, Rotteveel J, Klink D. Final Height Reduction in Transgender Adolescent Girls: A Case Series. In: ESPE Abstracts. Vol 92. Bioscientifica; 2019. https://abstracts.eurospe.org/hrp/0092/hrp0092rfc11.6. Accessed April 27, 2021 [Google Scholar]
- 100. Isotton AL, Wender MCO, Casagrande A, Rollin G, Czepielewski MA. Effects of oral and transdermal estrogen on IGF1, IGFBP3, IGFBP1, serum lipids, and glucose in patients with hypopituitarism during GH treatment: a randomized study. Eur J Endocrinol. 2012;166(2):207‐213. 10.1530/EJE-11-0560. [DOI] [PubMed] [Google Scholar]
- 101. Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(10):2430‐2438. 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90(12):6396‐6402. 10.1210/jc.2005-1392. [DOI] [PubMed] [Google Scholar]
- 103. Hero M, Wickman S, Dunkel L. Treatment with the aromatase inhibitor letrozole during adolescence increases near‐final height in boys with constitutional delay of puberty. Clin Endocrinol Oxf. 2006;64(5):510‐513. 10.1111/j.1365-2265.2006.02499.x. [DOI] [PubMed] [Google Scholar]
- 104. Pedrosa LF, de Oliveira JM, Thomé PRV, Kochi C, Damiani D, Longui CA. Height increment and laboratory profile of boys treated with aromatase inhibitors with or without growth hormone. Horm Metab Res. 2017;49(10):778‐785. 10.1055/s-0043-116944. [DOI] [PubMed] [Google Scholar]
- 105. Wickman S, Sipilä I, Ankarberg‐Lindgren C, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: a randomised controlled trial. Lancet Br Ed. 2001;357(9270):1743‐1748. 10.1016/s0140-6736(00)04895-9. [DOI] [PubMed] [Google Scholar]
- 106. Mauras N, Ross JL, Gagliardi P, et al. Randomized trial of aromatase inhibitors, growth hormone, or combination in pubertal boys with idiopathic, short stature. J Clin Endocrinol Metab. 2016;101(12):4984‐4993. 10.1210/jc.2016-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Keenan BS, Richards GE, Ponder SW, Dallas JS, Nagamani M, Smith ER. Androgen‐stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin‐like growth factor‐I in the treatment of short stature and delayed puberty. J Clin Endocrinol Metab. 1993;76(4):996‐1001. 10.1210/jcem.76.4.8473416. [DOI] [PubMed] [Google Scholar]
- 108. Wit JM, Oostdijk W. Novel approaches to short stature therapy. Best Pract Res Clin Endocrinol Metab. 2015;29(3):353‐366. 10.1016/j.beem.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 109. Grimstad FW, Knoll MM, Jacobson JD. Oxandrolone use in trans‐masculine youth appears to increase adult height: preliminary evidence [published online ahead of print April 5, 2021]. LGBT Health. 2021. 10.1089/lgbt.2020.0355 [DOI] [PubMed] [Google Scholar]
- 110. Gravholt CH, Andersen NH, Conway GS, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177(3):G1‐G70. 10.1530/EJE-17-0430. [DOI] [PubMed] [Google Scholar]
- 111. Jackson ST, Rallison ML, Buntin WH, Johnson SB, Flynn RR. Use of oxandrolone for growth stimulation in children. Am J Dis Child 1960. 1973;126(4):481‐484. 10.1001/archpedi.1973.02110190399007. [DOI] [PubMed] [Google Scholar]
- 112. Ferrero S, Barra F, Maggiore LR. Current and emerging therapeutics for the management of endometriosis. Drugs. 2018;78(10):995‐1012. 10.1007/s40265-018-0928-0. [DOI] [PubMed] [Google Scholar]


