Abstract
Background
Evidence and guidelines increasingly support an individualised approach to care for people with type 2 diabetes and individualisation of glycaemic targets in response to patient factors.
Methods
We undertook a scoping review of the literature for evidence of factors impacting upon glycated haemoglobin target individualisation in adults with type 2 diabetes. Data were analysed thematically with the themes inductively derived from article review.
Findings
Evidence suggests that presence of cardiovascular disease, hypoglycaemia unawareness, severe hypoglycaemia, limited life expectancy, advanced age, long diabetes duration, frailty, cognitive impairment, disability, extensive comorbidity, diabetes distress and patient preference should inform the setting of glycaemic targets.
Conclusion
The management of people with diabetes is complex. In clinical practice, many patients will have a variety of factors that should be considered when personalising their care. Approaches to personalised care and glycaemic treatment targets should be undertaken as part of a shared decision-making process between physician and patient. Use of electronic records might enable greater efficiency and more widespread use of personalised care plans for people with diabetes.
KEYWORDS: individualisation, glycated haemoglobin, patient factors, type 2 diabetes
Background
As highlighted by the National Institute for Health and Care Excellence type 2 diabetes guidelines, the need for individualised care in diabetes is increasingly important.1 Diabetes prevalence in the UK is such that many patients are unlikely to receive regular specialist input for their diabetes care. Conversely, people with diabetes encounter non-specialists with greater frequency. It is important to consider individual characteristics of people with diabetes before agreeing on appropriate glycaemic targets. Discussing and agreeing individualised glycated haemoglobin (HbA1c) targets as part of care plans are paramount to improving patient experience and care.
The objective of this scoping review was to collect and discuss the evidence on the use of individualised HbA1c targets in people with type 2 diabetes and to identify any existing gaps in knowledge as areas of potential future research.
Methods
This review was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews (PRISMA-ScR) statement and registered in the online Open Science Framework database (https://osf.io/snjpr) prior to data extraction.2
Search strategy
MEDLINE, AMED, PsycINFO and Embase were searched from inception to 01 June 2021. We completed a comprehensive search using free text and Medical Subject Headings (MeSH) for various forms of the following terms (in titles and abstracts): individualisation, glycaemic and target. The terms and truncated variants of the terms were combined for study retrieval. Additional articles were identified through backward and forward searching. The final search strategy can be found in supplementary material S1, Table S1.
Study selection
Publications were included if they were in the English language, included adult people with type 2 diabetes and were full text. Studies of any design were included to encompass the variety of factors that impact glycaemic target individualisation. Articles were excluded if they were not reporting an original study; had a small sample size (n<15) or did not cover themes relating to the individualisation of HbA1c targets in diabetes.
Study quality and data extraction
Two authors reviewed study quality of the included quantitative studies using the National Institutes of Health Quality Assessment Tools (QATs).3 The QATs used were study-design specific. For the included qualitative study, the Critical Skills Appraisal Programme qualitative checklist was used.4 Studies were rated as good, fair or poor depending on risk of bias. Data were extracted and tabulated on article characteristics (publication year, country of origin, number of participants and study type) and contextual factors (diabetes type and theme). Data were thematically analysed, with no formal quantitative synthesis taking place due to significant methodological heterogeneity in the included studies.
Results
Fig 1 details the records obtained from the search. After screening, 59 full-text articles were evaluated, of which, 11 were excluded for the reasons documented in Fig 1. Forty-eight studies were included following screening and review against eligibility criteria. Overall, risk of bias was rated low in 26 of the included studies, moderate in 16 and high in five (supplementary material S1, Table S2). The included qualitative study was rated good (supplementary material S1, Table S3).
Fig 1.
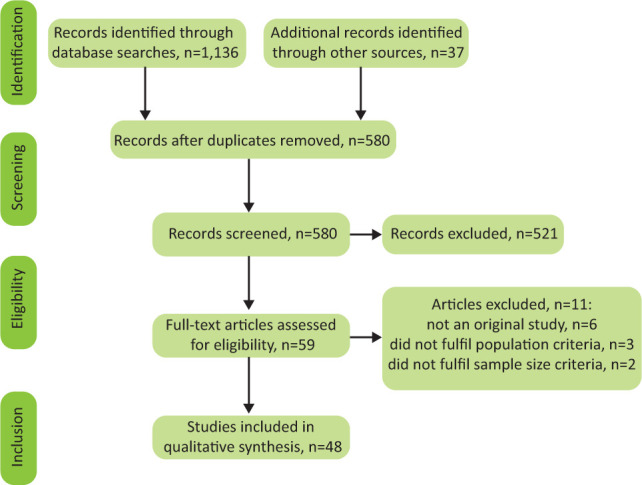
Process of inclusion of studies and stages.
Study characteristics
The study characteristics are shown in Table 1.5–52 Sample sizes ranged from 28 to 264,687 (median 3,572; interquartile range 533–11,140.
Table 1.
Overview of included studies
| Author, publication year | Themes | Country | Number | Study type | Population |
|---|---|---|---|---|---|
| Deusenberry et al, 201221 | Age | USA | 692 | Case-control | T2DM |
| Glynn et al, 199922 | Age | USA | 161,700 | Case-control | T1DM and T2DM |
| Ha et al, 201223 | Age | South Korea | 320 | Case series | T1DM and T2DM |
| Lipska et al, 201524 | Age | USA | 1,288 | Cross-sectional | T1DM and T2DM |
| Monami et al, 201325 | Age | Italy | 854 | Case-control | T2DM |
| Shorr et al, 199716 | Age | USA | 19,932 | Cohort study | T2DM |
| Strain et al, 201717 | Age | Europe | 278 | RCT | T2DM |
| Zhong et al, 201719 | Age | UK | 264,687 | Cohort study | T1DM and T2DM |
| The ACCORD Study Group, 200811 | Age, comorbidity, complications, frailty | USA, Canada | 10,251 | RCT | T2DM |
| The ADVANCE Collaborative Group, 200812 | Age, comorbidity, complications, duration, frailty | Asia, Australia, Europe, North America | 11,140 | RCT | T2DM |
| Duckworth et al, 20096 | Age, comorbidity, complications, frailty | USA | 1,791 | RCT | T2DM |
| UK Prospective Diabetes Study Group, 199814 | Age, complications | UK | 3,867 | RCT | T2DM |
| Lipska et al, 201343 | Age, duration, hypoglycaemia | USA | 9,094 | Cross-sectional | T2DM |
| Yi et al, 201818 | Age, hypoglycaemia | China | 23,680 | Cohort study | T2DM |
| Ben-Ami et al, 199920 | Age, hypoglycaemia | Israel | 102 | Case series | T1DM and T2DM |
| O'Connor et al, 200315 | Age, psychosocioeconomic | USA | 1,109 | Cohort study | T1DM and T2DM |
| Blaum et al, 200329 | Comorbidity, frailty | USA | 7,447 | Cross-sectional | T1DM and T2DM |
| Adler et al, 19995 | Complications | UK | 5,063 | RCT | T2DM |
| Holman et al, 20087 | Complications | UK | 3,277 | Cohort study | T2DM |
| Mellbin et al, 20138 | Complications | Many | 12,537 | RCT | T2DM |
| Mukamal et al, 20019 | Complications | USA | 1,935 | Cohort study | T1DM and T2DM |
| Nathan, 201410 | Complications | USA | 1,441 | Cohort study | T1DM |
| The ORIGIN Trial Investigators, 201213 | Complications | Many | 12,537 | RCT | T2DM |
| McCoy et al, 201244 | Duration, hypoglycaemia | USA | 1,020 | Case-control | T1DM and T2DM |
| Kalyani et al, 201031 | Frailty | USA | 6,097 | Cross-sectional | T1DM and T2DM |
| Bruce et al, 201832 | Frailty | Australia | 367 | Cohort study | T2DM |
| Currie et al, 201033 | Frailty | UK | 27,965 | Cohort study | T2DM |
| de Galan et al, 200934 | Frailty | Asia, Australia, Europe, North America | 11,140 | RCT | T2DM |
| Huang et al, 201135 | Frailty | USA | 71,092 | Cohort study | T2DM |
| Liccini et al, 201636 | Frailty | USA | 198 | Cohort study | T1DM and T2DM |
| Twito et al, 201326 | Frailty | Israel | 2,994 | Cohort study | T1DM and T2DM |
| Van Hateren et al, 201127 | Frailty | Holland | 374 | Cohort study | T2DM |
| Yanagita et al, 201828 | Frailty | Japan | 132 | Cohort study | T2DM |
| Punthakee et al, 201230 | Frailty | USA, Canada | 2,956 | Cohort study | T2DM |
| Bonds et al, 201038 | Hypoglycaemia | USA, Canada | 10,194 | Cohort study | T2DM |
| Chen et al, 201739 | Hypoglycaemia | China | 90 | RCT | T2DM |
| Hsu et al, 201340 | Hypoglycaemia | Taiwan | 9,220 | Cohort study | T2DM |
| Huang et al, 201441 | Hypoglycaemia | USA | 72,310 | Cohort study | T2DM |
| Kong et al, 201442 | Hypoglycaemia | Hong Kong | 8767 | Cohort study | T2DM |
| Whitmer et al, 200945 | Hypoglycaemia | USA | 16,667 | Cohort study | T2DM |
| Zoungas et al, 201037 | Hypoglycaemia | Asia, Australia, Europe, North America | 11,140 | Cohort study | T2DM |
| Brown et al, 200846 | Psychosocioeconomic | USA | 332 | Cross-sectional | T2DM |
| Chin et al, 200847 | Psychosocioeconomic | USA | 537 | Cross-sectional | T1DM and T2DM |
| Ciechanowski et al, 200048 | Psychosocioeconomic | USA | 367 | Cross-sectional | T1DM and T2DM |
| Egede et al, 200549 | Psychosocioeconomic | USA | 10,025 | Cohort study | T1DM and T2DM |
| Finkelstein et al, 200350 | Psychosocioeconomic | USA | 242,067 | Case-control | T1DM and T2DM |
| Huang et al, 200551 | Psychosocioeconomic | USA | 28 | Qualitative | T2DM |
| Huang et al, 200652 | Psychosocioeconomic | USA | 519 | Cross-sectional | T2DM |
RCT = randomised controlled trial; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
Individualising glycaemic targets in response to patient factors
Full-text review of the articles revealed several emergent themes on the use of individualised HbA1c targets. Articles were coded according to theme. Concurrence of themes between articles resulted in the determination of key patient factors where individualised HbA1c targets were beneficial. These factors were:
-
bull
presence of established cardiovascular disease5–14
- bull
-
bull
presence of frailty, disability, cognitive impairment or comorbidity6,11,12,14,26–36
- bull
-
bull
presence of psychosocial, social or economic issues.15,46–52
Individualised HbA1c targets and established cardiovascular disease
Evidence from the UK Prospective Diabetes Study (UKPDS) in people with type 2 diabetes showed that, in their patient population, early intensive glycaemic control resulted in improved microvascular and macrovascular outcomes.5,7,14
The Action to Control Cardiovascular Risk in Diabetes (ACCORD), Action in Diabetes Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) and Veterans Affairs Diabetes Trial (VADT) trials were subsequently undertaken to further evaluate the effects of intensive glycaemic control on outcomes in people with pre-existing type 2 diabetes.6,11,12 Three and a half years in, the ACCORD trial was halted due to increased all-cause mortality seen in the intensive control (HbA1c 47 mmol/mol (6.4%)) group. Despite having similar objectives, ADVANCE and VADT trials showed no difference in macrovascular outcomes. Explanations for the differences in outcomes seen in these trials vary and uncertainty remains.
In the ACCORD trial, patients experiencing severe hypoglycaemia, whether in the intensive or standard glycaemic control arms, were noted to have increased mortality rates. These data have been echoed in the Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial that showed severe hypoglycaemia was associated with an increased risk of a composite major adverse cardiac event (MACE; cardiovascular (CV) death, non-fatal myocardial infarction (MI) or stroke), all-cause mortality, CV death (separate from the composite event) and arrhythmic death in people with CV risk factors and type 2 diabetes.8,13
Since the results of these trials, pharmaceutical treatment options have advanced dramatically. Newer agents such as glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose co-transporter-2 inhibitors (SGLT2is) demonstrate cardiovascular and mortality benefits over older treatment options studied in ACCORD, ADVANCE and VADT trials with comparable efficacy on HbA1c levels.53 Despite this, the legacy of the ACCORD trial has meant that clinicians must practise caution in applying intensive HbA1c targets to those at risk of CV disease due to the additional risk of severe hypoglycaemia associated with achieving intensive glycaemic control.10 Alongside the use of individualised HbA1c targets, aggressive modification of all CV risk factors (such as blood pressure and lipid modification) is crucial in reducing long-term CV mortality in people with diabetes. In those with risk factors for CV disease, or with pre-existing CV disease, adjustment of HbA1c treatment targets to avoid severe hypoglycaemia should be considered alongside consideration of switching to GLP-1RAs or SGLT2is in those with increased CV risk, heart failure or chronic kidney disease (CKD).9
Individualised HbA1c targets and advancing age and diabetes duration
The care of older adults presents unique challenges. There is conflicting evidence on whether clinicians over- or under-treat diabetes in the elderly.22,24 Those with advanced age are more likely to have a longer duration of diabetes, higher risk of hypoglycaemia, higher levels of CV comorbidity, higher levels of inpatient and outpatient service utilisation, and inappropriately intensive treatment for their diabetes.14–16,19–21,23,24
Follow-up data from the ACCORD, ADVANCE and VADT trials suggest elderly patients with a longer duration of diabetes are unlikely to gain macrovascular benefits from intensive glycaemic control and may be exposed to excess risk of severe hypoglycaemia, increased morbidity and mortality.6,11,12,18
These findings are expanded upon in a study by Monami et al: follow-up over 6 years in those with a longer diabetes duration (10 years and over) showed that mortality only increases when HbA1c levels are greater than 68 mmol/mol (>8.4%).25 Similarly, over the same follow-up when considering those aged 71 years and over, mortality was shown to increase only when HbA1c levels rose above 68 mmol/mol (>8.4%).
Despite guidance on glycaemic target individualisation and lack of macrovascular benefits, a European study by Strain et al on factors affecting physician glycaemic target-setting behaviours for elderly patients (aged 70 years and over) showed that rigid, particularly aggressive, uniform glycaemic targets are still commonly used in line with national performance indicators.17,54 It is likely that a historical lack of consensus among guidelines and difficulty in accounting for the possible risks and benefits of adjusting glycaemic targets are contributory factors.47
Bearing in mind the diminishing returns of improving glycaemic control, safe, effective treatment of elderly patients with a longer duration of diabetes requires constant re-evaluation of the expected gains in macrovascular risk reduction versus the expected risks of intensive glycaemic control. A relaxed glycaemic target between 58 and 69 mmol/mol (7.5%–8.5%) aimed at avoiding hypoglycaemia and uncontrolled hyperglycaemia with an individualised care plan should be considered in older patients with established diabetes of long duration.17 Excess mortality would be better addressed through modification of other reversible CV risks, such as lipid and blood pressure control in these patients.55,56 There is limited follow-up data in younger people (aged under 60 years) with type 2 diabetes of long duration. Further work is needed in determining appropriate HbA1c targets in this group.
Individualised HbA1c targets and frailty, disability, cognitive impairment and comorbidity
Frailty, disability, cognitive impairment and comorbidity are often seen to be interrelated, with a degree of overlap and are increasingly prevalent in the western world as the population ages.57 Lower HbA1c values are both a risk factor for developing frailty and in those with frailty, and are associated with an increased risk of stroke, dementia and mortality.36,28 In patients aged 60–64 years with disability and newly diagnosed type 2 diabetes, the benefits of intensive glycaemic control in those with only low levels of functional impairment are marginal at best (106 quality-adjusted days).58 People with diabetes have a two-to-three-fold increased odds of disability irrespective of glycaemic control, cardiovascular disease and obesity are seen as the main contributors.29,31 Sub-optimal glycaemic control alone is not a significant predictor for disability and should not be the main consideration when agreeing appropriate HbA1c targets with patients.34 Alongside frailty and disability, presence of cognitive impairment in people with type 2 diabetes significantly increases the risk of severe hypoglycaemia, major CV events, CV death and all-cause death.30,34 Comorbid patients with diabetes would be expected to have a shorter length of life with a subsequent reduction in time for the development of diabetes complications.59 In studies evaluating older people with diabetes with cardiovascular comorbidities, intensive glycaemic control has shown no mortality benefit and has not resulted in a reduction of further CV endpoints.6,11,12,59
The complexity of accounting for these variables has resulted in a conflicting evidence base on the association between HbA1c and mortality.26,27,32,33,35 As such, agreeing appropriate HbA1c targets with patients is highly nuanced. In general, for otherwise healthy older adults, a target of <58 mmol/mol (<7.5%) probably reflects the best compromise between risk and benefit. An individualised glycaemic target between 58 and 69 mmol/mol (7.5%–8.5%) to avoid hypoglycaemia, symptomatic hyperglycaemia and medication burden should be considered in adults with co-existing frailty, disability, comorbidity or cognitive impairment.59,60
The use of individualised HbA1c targets and problematic hypoglycaemia
Severe hypoglycaemia has been suggested as one of the reasons why the ‘intensive glycaemic control’ arm of the ACCORD trial was noted to have excess mortality, though no direct causal relationship has been established.59 Retrospective analysis of the ADVANCE dataset by Zoungas et al showed that severe hypoglycaemia in patients with type 2 diabetes was associated with a statistically significant increase in the risk of major macrovascular events (hazard ratio (HR) 2.88; 95% confidence interval (CI) 2.01–4.12), major microvascular events (HR 1.81; 95% CI 1.19–2.74), CV death (HR 2.68; 95% CI 1.72–4.19) and death from any cause (HR 2.69; 95% CI 1.97–3.67; all p<0.001).37 It is possible that severe hypoglycaemia is contributory to these outcomes but it is more likely that severe hypoglycaemia is a general marker of clinical vulnerability in these individuals.
The risk of hypoglycaemia increases independently with advancing age, duration of diabetes and presence of CKD.41,42 Symptomatic hypoglycaemia in diabetes, whether mild or severe, is a significant source of hospitalisation (HR 2.09; 95% CI 1.63–2.67) and death (HR 2.48; 95% CI 1.41–4.38) and is associated with increased morbidity, all-site cancer, disability, medical visits, diabetes-related medical costs, medication costs, healthcare resource utilisation, and reduced quality of life, well-being and self-management.18,20,38,40,42,44,61 Independent of glycaemic control, comorbid status and diabetes treatment, hypoglycaemia is associated with a greater risk of dementia (2.39% per year; 95% CI 1.72–3.01).45 In older adults with Alzheimer's dementia, Chen et al showed a reduced progression of dementia, reduced rate of hypoglycaemia, reduced medication burden and reduced rate of diabetes complications over a 3-year follow-up period in patients following a moderate rather than intensive glucose control strategy.39 Risk of severe hypoglycaemia in type 2 diabetes is highest in those achieving near-normal glycaemia (HbA1c <42 mmol/mol (<6.0%)) or with very poor glycaemic control (HbA1c ≥75 mmol/mol (≥9.0%)).43
Since the data presented by ACCORD and ORIGIN trials, it is recognised that glycaemic targets for patients with hypoglycaemia unawareness or preceding severe hypoglycaemia should be individualised to avoid hypoglycaemia at the expense of a relaxed HbA1c target. Special care should be taken in the management of comorbid patients and patients with longer diabetes durations, such as the demographics of the patients in the ACCORD study. The unique clinical course of each patient reinforces the need to individualise glycaemic targets in response to hypoglycaemia risk.41 A reasonable suggestion is to assign an individualised glycaemic target that avoids severe hypoglycaemia and preserves hypoglycaemia awareness. This may mean that in younger, healthier patients whose diabetes is controlled with dietary and lifestyle interventions alone, a non-diabetic glycaemic target (<48 mmol/mol; <6.5%) may be appropriate but in patients with limited life expectancy, a higher HbA1c target (<69 mmol/mol; <8.5%) sufficient to prevent the symptoms of hyperglycaemia would be acceptable. Those in the intermediary zone who would not necessarily be in ‘good’ health, but whose life expectancy is not limited may thus benefit from a glycaemic target between 58 and 69 mmol/mol (7.5%–8.5%), depending on individual circumstances.
The use of individualised HbA1c targets and psychosocioeconomic concerns
As a chronic disease process, diabetes is increasingly recognised to have a significant impact on psychological outcomes and mental health. Studies show that more than one-third of people with diabetes have depression at a level that impairs functioning, quality of life, adherence to medical treatment, glycaemic control, and increases healthcare utilisation, healthcare cost and the risk of diabetic complications.48,49,62 Coexistent depression and diabetes increases the risk of death from all causes in excess of the summative effects of having either condition in isolation.48,49,62
Studies in older people with diabetes (aged 65 years and over) show that vulnerable adults have even greater levels of depression, have increased concern related to medication side effects, have trouble remembering to take medications, have required increased assistance with medication taking, feel overwhelmed following visits to clinicians, find taking their diabetes medications unpleasant and are less willing to take insulin.46,50 Depending on how burdensome an individual views their treatment, improvements in glycaemic control can result in net harm and reduced quality of life (despite improved HbA1c) in older adults.56 A cross-sectional study by Chin et al evaluated the preferences of older adults (aged 65 years and over) with diabetes regarding the quality of life trade-offs between aggressive glycaemic control and the avoidance of diabetes complications.47 The study found that standard glycaemic targets were acceptable for most patients but that, where treatment negatively impacted upon quality of life or where the gains in quality of life were neutral, standard glycaemic targets were problematic. Reinforcement of the importance of a dialogue between patients and physicians in a shared decision-making process to include consideration of overall lifegoals, patient preferences towards different treatment approaches and diabetic complications is paramount.
Quality of life in people with diabetes is impacted by the adverse effects of diabetes treatments as well as the route of treatment delivery (injected or oral).47 Reductions in quality of life due to diabetes treatments can be large, with wide inter-person variation.46,52 Modelling studies of the NHANES diabetes population (2011–2012, aged 25–75 years) shows that the individualisation of glycaemic targets according to risk of future complications and patient age is cost saving (mainly due to reduced medication usage) and results in gains in quality-adjusted life-years (mainly due to reduced medication burden in the over-treated) over the course of a lifetime without substantially impacting patient outcomes.63
Exploratory studies into diabetes healthcare goals that are most important for patients describe social and functional goals rather than biochemical goals targeting risks and complications.51 A shared decision-making process that takes social and functional goals into account may be an approach that is key to ensuring the successful implementation of individualised diabetes care.15
Discussion
Current diabetes literature and up-to-date evidence-based guidelines report on the importance of using individualised HbA1c targets for people with diabetes. Our review discusses the evidence on individualised HbA1c targets in response to established cardiovascular disease, advanced age, long diabetes duration, frailty, disability, cognitive impairment, presence of comorbidity, problematic hypoglycaemia and psychosocioeconomic considerations. We believe a considered approach should be taken before agreeing an individualised HbA1c target, taking into account an informed opinion from patients on the respective risks and benefits of higher and lower HbA1c targets, alongside review of the presence of relevant characteristics that may influence decision making (Fig 2). Recurrent themes in the reviewed literature demonstrated the importance of a multifactorial approach to micro- and macrovascular risk management, ensuring lipid modification and blood pressure management are optimised alongside using individualised HbA1c targets.
Fig 2.
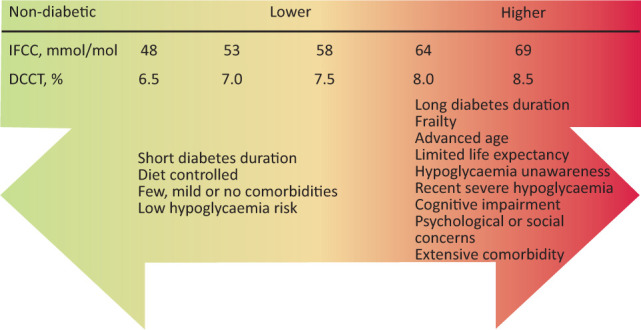
Decision aid in patient–physician encounters when mutually agreeing an individualised glycated haemoglobin target. People with diabetes should be fully informed wherever possible to reach a shared decision with their physician on a target appropriate for them based on their characteristics. DCCT = Diabetes Control and Complications Trial; HbA1c = glycated haemoglobin; IFCC = International Federation of Clinical Chemistry.
In the UK, national diabetes quality performance indicators target a specific HbA1c at a population level.64 While this may be useful at reducing population-level risk, population-level HbA1c performance indicators do not adequately consider the additional costs and adverse effects of a uniform glycaemic target to a heterogeneous diabetes population.43 Furthermore, these indicators are not consistent with widely published evidence-based guidelines encouraging individualised approaches.54 We suggest implementation of additional quality indicators; for example, flagging low HbA1c values in patients with frailty as a marker of over-treatment to encourage appropriate glycaemic target individualisation.
Strengths and weaknesses
The majority of the studies (26) included in this review were rated as having a low risk of bias. Due to the diverse nature of the topics included in this review, often including patient populations that are difficult to recruit, we decided against excluding studies where the risk of bias was moderate or high, instead accepting this as a limitation.
Individualised care and glycaemic targets are equally as important in type 1 diabetes but can often differ with care plans and targets used in people with type 2 diabetes. We excluded studies referring to type 1 diabetes only as this area of diabetes care remains largely in the realm of specialists.
Gaps in the literature remain on the evaluation of the impact of using individualised glycaemic targets on healthcare outcomes for people with type 2 diabetes. Few studies between 2012 and 2018 have evaluated this, with some useful insights: individualised glycaemic targets are cost-effective; improve quality-adjusted life-years; reduce rates of severe hypoglycaemia, medication burden and healthcare utilisation; and increase glycaemic target-achievement.17,63,65–68
Conclusion
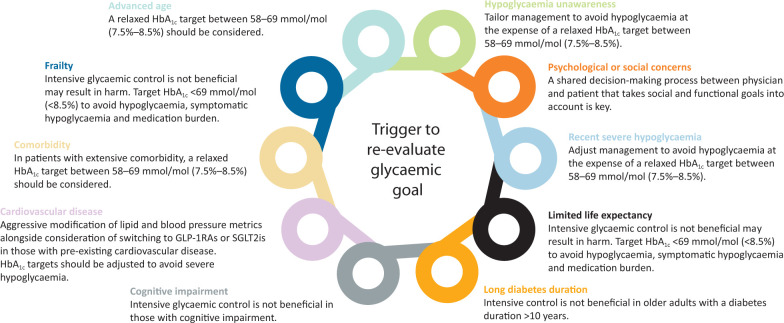
The management of people with diabetes is complex. In clinical practice, many patients will have a variety of factors (Fig 3) that should be considered when personalising their care and assigning individualised glycaemic targets. Our findings suggest that a significant body of evidence exists for adjusting glycaemic targets in response to individual patient factors. Approaches to personalised care and glycaemic treatment target setting need to be undertaken as part of a shared decision-making process between physician and patient. Further efforts are needed to improve practice and to adjust national performance measures that incentivise the pursuit of uniform tight glycaemic targets. Future work evaluating the impact of using individualised glycaemic targets in people with diabetes and on the use of electronic records as a tool to aid this process could enable increased efficiency and more widespread use of personalised care plans in diabetes.67
Fig 3.
Patient factors that should prompt a re-evaluation of glycaemic goals in people with diabetes. HbA1c = glycated haemoglobin; GLP-1RA = glucagon-like peptide-1 receptor agonist; SGLT2i = sodium-glucose co-transporter-2 inhibitor.
Key points
Use of individualised glycaemic targets in people with type 2 diabetes is endorsed by national guidelines.
Current guidelines are non-specific regarding the decision-making process for adjusting glycaemic targets.
Individualising glycaemic targets should be considered as part of a shared decision-making process between physician and patient.
A variety of patient characteristics should prompt a re-evaluation of appropriate glycated haemoglobin targets by physicians.
Agreeing on glycated haemoglobin targets with patients is highly nuanced. Factors such as established cardiovascular disease, diabetes duration, life expectancy, episodes of severe hypoglycaemia, hypoglycaemia unawareness, presence of significant comorbidity, and presence of psychological or social concerns should be considered.
Supplementary material
Additional supplementary material may be found in the online version of this article at www.rcpjournals.org/clinmedicine:
S1 – Search strategy and study quality.
References
- 1.National Institute for Health and Care Excellence . Type 2 diabetes in adults: management: NICE Guideline [NG28]. NICE, 2021. www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-pdf-1837338615493 [Google Scholar]
- 2.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health . Study quality assessment tools. NIH, 2014. www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Google Scholar]
- 4.Critical Appraisal Skills Programme . CASP checklist: 10 questions to help you make sense of a qualitative research. CASP, 2018. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf [Google Scholar]
- 5.Adler AI, Neil HAW, Manley SE, Holman RR, Turner RC. Hyperglycemia and hyperinsulinemia at diagnosis of diabetes and their association with subsequent cardiovascular disease in the United Kingdom Prospective Diabetes Study (UKPDS 47). Am Heart J 1999;138:S353–9. [DOI] [PubMed] [Google Scholar]
- 6.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39. [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. [DOI] [PubMed] [Google Scholar]
- 8.Mellbin LG, Rydén L, Riddle MC, et al. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J 2013;34:3137–44. [DOI] [PubMed] [Google Scholar]
- 9.Mukamal KJ, Nesto RW, Cohen MC, et al. Impact of diabetes on long-term survival after acute myocardial infarction: Comparability of risk with prior myocardial infarction. Diabetes Care 2001;24:1422–7. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014;37:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The ACCORD Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72. [DOI] [PubMed] [Google Scholar]
- 13.The ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–28. [DOI] [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- 15.O'Connor PJ, Desai JR, Solberg LI, Rush WA, Bishop DB. Variation in diabetes care by age: Opportunities for customization of care. BMC Fam Pract 2003;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shorr RI. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med 1997;157:1681. [PubMed] [Google Scholar]
- 17.Strain WD, Agarwal AS, Paldánius PM. Individualizing treatment targets for elderly patients with type 2 diabetes: factors influencing clinical decision making in the 24-week, randomized INTERVAL study. Aging (Albany NY) 2017;9:769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi Y, Li Y, Hou A, et al. A retrospective cohort study of patients with type 2 diabetes in China: associations of hypoglycemia with health care resource utilization and associated costs. Diabetes Ther 2018;9:1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong VW, Juhaeri J, Cole SR, et al. Incidence and trends in hypoglycemia hospitalization in adults with type 1 and type 2 diabetes in England, 1998-2013: A retrospective cohort study. Diabetes Care 2017;40:1651–60. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999;159:281–4. [DOI] [PubMed] [Google Scholar]
- 21.Deusenberry CM, Coley KC, Korytkowski MT, Donihi AC. Hypoglycemia in hospitalized patients treated with sulfonylureas. Pharmacotherapy 2012;32:613–7. [DOI] [PubMed] [Google Scholar]
- 22.Glynn RJ, Monane M, Gurwitz JH, Choodnovskiy I, Avorn J. Aging, comorbidity, and reduced rates of drug treatment for diabetes mellitus. J Clin Epidemiol 1999;52:781–90. [DOI] [PubMed] [Google Scholar]
- 23.Ha WC, Oh SJ, Kim JH, et al. Severe hypoglycemia is a serious complication and becoming an economic burden in diabetes. Diabetes Metab J 2012;36:280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monami M, Vitale V, Lamanna C, et al. HbA1c levels and all-cause mortality in type 2 diabetic patients: Epidemiological evidence of the need for personalised therapeutic targets. Nutr Metab Cardiovasc Dis 2013;23:300–6. [DOI] [PubMed] [Google Scholar]
- 26.Twito O, Ahron E, Jaffe A, et al. New-onset diabetes in elderly subjects: association between HbA1c levels, mortality, and coronary revascularization. Diabetes Care 2013;36:3425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Hateren KJJ, Landman GWD, Kleefstra N, et al. Glycaemic control and the risk of mortality in elderly type 2 diabetic patients (ZODIAC-20). Int J Clin Pract 2011;65:415–9. [DOI] [PubMed] [Google Scholar]
- 28.Yanagita I, Fujihara Y, Eda T, et al. Low glycated hemoglobin level is associated with severity of frailty in Japanese elderly diabetes patients. J Diabetes Investig 2018;9:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blaum CS, Ofstedal MB, Langa KM, Wray LA. Functional status and health outcomes in older Americans with diabetes mellitus. J Am Geriatr Soc 2003;51:745–53. [DOI] [PubMed] [Google Scholar]
- 30.Punthakee Z, Miller ME, Launer LJ, et al. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: Post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: Results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care 2010;33:1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruce DG, Davis WA, Davis TME. Glycaemic control and mortality in older people with type 2 diabetes: The Fremantle Diabetes Study Phase II. Diabetes Obes Metab 2018;20:2852–9. [DOI] [PubMed] [Google Scholar]
- 33.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. Lancet 2010;375:481–9. [DOI] [PubMed] [Google Scholar]
- 34.de Galan BE, Zoungas S, Chalmers J, et al. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: The action in diabetes and vascular disease: Preterax and diamicron modified release controlled evaluation (ADVANCE) trial. Diabetologia 2009;52:2328–36. [DOI] [PubMed] [Google Scholar]
- 35.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: The diabetes and aging study. Diabetes Care 2011;34:1329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liccini AP, Malmstrom TK. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J Am Med Dir Assoc 2016;17:846–51. [DOI] [PubMed] [Google Scholar]
- 37.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–8. [DOI] [PubMed] [Google Scholar]
- 38.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Wang J, Wang LJ, Lin H, Huang PJ. Blood glucose intervention plans and elderly diabetes mellitus with dementia. Eur Rev Med Pharmacol Sci 2017;21:2702–7. [PubMed] [Google Scholar]
- 40.Hsu PF, Sung SH, Cheng HM, et al. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: A nationwide population-based study. Diabetes Care 2013;36:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus. JAMA Intern Med 2014;174:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong APS, Yang X, Luk A, et al. Severe hypoglycemia identifies vulnerable patients with type 2 diabetes at risk for premature death and all-site cancer: The Hong Kong Diabetes Registry. Diabetes Care 2014;37:1024–31. [DOI] [PubMed] [Google Scholar]
- 43.Lipska KJ, Warton EM, Huang ES, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes: the diabetes and aging study. Diabetes Care 2013;36:3535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCoy RG, Shah ND, Van Houten HK, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitmer RA. Hypoglycemic Episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown SES, Meltzer DO, Chin MH, Huang ES. Perceptions of quality-of-life effects of treatments for diabetes mellitus in vulnerable and nonvulnerable older patients. J Am Geriatr Soc 2008;56:1183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chin MH, Drum ML, Jin L, et al. Variation in treatment preferences and care goals among older patients with diabetes and their physicians. Med Care 2008;46:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciechanowski P, Katon WJ, Russo J. Depression and diabetes. Arch Intern Med 2000;160:3278–85. [DOI] [PubMed] [Google Scholar]
- 49.Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 2005;28:1339–45. [DOI] [PubMed] [Google Scholar]
- 50.Finkelstein EA, Bray JW, Chen H, et al. Prevalence and costs of major depression among elderly claimants with diabetes. Diabetes Care 2003;26:415–20. [DOI] [PubMed] [Google Scholar]
- 51.Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc 2005;53:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang ES, Shook M, Jin L, Chin MH, Meltzer DO. The impact of patient preferences on the cost-effectiveness of intensive glucose control in older patients with new-onset diabetes. Diabetes Care 2006;29:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021;372:m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461–98. [DOI] [PubMed] [Google Scholar]
- 55.Gæde P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93. [DOI] [PubMed] [Google Scholar]
- 56.Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients' risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med 2014;174:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc 2012;60:1487–92. [DOI] [PubMed] [Google Scholar]
- 58.Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: A decision analysis. Ann Intern Med 2008;149:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ismail-Beigi F, Moghissi E, Tiktin M, et al. Individualizing glycemic targets in type 2 diabetes mellitus: Implications of recent clinical trials. Ann Intern Med 2011;154:554–9. [DOI] [PubMed] [Google Scholar]
- 60.Sinclair AJ, Paolisso G, Castro M, et al. European diabetes working party for older people 2011 clinical guidelines for type 2 diabetes mellitus: executive summary. Diabetes Metab 2011;37:S27–38. [DOI] [PubMed] [Google Scholar]
- 61.Cryer PE. Minimizing hypoglycemia in diabetes. Diabetes Care 2015;38:1583–91. [DOI] [PubMed] [Google Scholar]
- 62.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care 2001;24:1069–78. [DOI] [PubMed] [Google Scholar]
- 63.Laiteerapong N, Cooper JM, Skandari MR, et al. Individualized glycemic control for U.S. Adults with type 2 diabetes a cost-effectiveness analysis. Ann Intern Med 2018;168:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Institute for Health and Care Excellence . NICE Quality and Outcomes Framework indicator: The percentage of patients on the CKD register in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less. NICE, 2015. www.nice.org.uk/standards-and-indicators/qofindicators/the-percentage-of-patients-on-the-ckd-register-in-whom-the-last-blood-pressure-reading-measured-in-the-preceding-12-months-is-140-90-mmhg-or-less-nm117 [Google Scholar]
- 65.Bieszk N, Grabner M, Wei W, Bonine N, Stephenson J. The SWEET SPOTS study: a real-world interpretation of the 2012 American Diabetes Association Position Statement regarding individualized A1C targets. Risk Manag Healthc Policy 2016;9:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmieder RE, Gitt AK, Koch C, et al. Achievement of individualized treatment targets in patients with comorbid type-2 diabetes and hypertension: 6 months results of the DIALOGUE registry. BMC Endocr Disord 2015;15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berkowitz SA, Atlas SJ, Grant RW, Wexler DJ. Individualizing HbA1c targets for patients with diabetes: Impact of an automated algorithm within a primary care network. Diabet Med 2014;31:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miñambres I, Mediavilla JJ, Sarroca J, Pérez A. Meeting individualized glycemic targets in primary care patients with type 2 diabetes in Spain. BMC Endocr Disord 2016;16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]