Abstract
Earthworm egg capsules (cocoons) may acquire bacteria from the environment in which they are produced. We found that Ralstonia eutropha (pJP4) can be recovered from Eisenia fetida cocoons formed in soil inoculated with this bacterium. Plasmid pJP4 contains the genes necessary for 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4-dichlorophenol (2,4-DCP) degradation. In this study we determined that the presence of R. eutropha (pJP4) within the developing earthworm cocoon can influence the degradation and toxicity of 2,4-D and 2,4-DCP, respectively. The addition of cocoons containing R. eutropha (pJP4) at either low or high densities (102 or 105 CFU per cocoon, respectively) initiated degradation of 2,4-D in nonsterile soil microcosms. Loss of 2,4-D was observed within the first week of incubation, and respiking the soil with 2,4-D showed depletion within 24 h. Microbial analysis of the soil revealed the presence of approximately 104 CFU R. eutropha (pJP4) g−1 of soil. The toxicity of 2,4-DCP to developing earthworms was tested by using cocoons with or without R. eutropha (pJP4). Results showed that cocoons containing R. eutropha (pJP4) were able to tolerate higher levels of 2,4-DCP. Our results indicate that the biodegradation of 2,4-DCP by R. eutropha (pJP4) within the cocoons may be the mechanism contributing to toxicity reduction. These results suggest that the microbiota may influence the survival of developing earthworms exposed to toxic chemicals. In addition, cocoons can be used as inoculants for the introduction into the environment of beneficial bacteria, such as strains with biodegradative capabilities.
Earthworms are saprotrophic invertebrates which contribute to the overall structure, drainage, and aeration of soil ecosystems (11). In addition, earthworms directly and indirectly influence soil microbial communities, mainly through the processes of feeding, burrowing, and fecal (cast) deposition. Several studies have shown that earthworms can transport bacteria into the environment (6, 7, 9, 13, 16, 23, 27, 28, 29). In particular, previous studies have assessed the importance of earthworms for the transport of beneficial bacteria for use in biological control (27). More recently, earthworms have been implicated as vectors and contributors for bacterial gene transfer (6, 7). To date, the majority of these studies have focused on adult earthworms, with little attention to how developing cocoons or juvenile earthworms affect microbial communities.
Several studies have shown that microorganisms are associated with earthworm egg capsules (cocoons) (8, 18, 32). Morgan and Burrows (18) reported that the internal fluid of Eisenia fetida cocoons contained bacteria of the genera Nocardia, Pseudomonas, and Alcaligenes. Zachmann and Molina (32) were the first to quantify the bacteria present in egg capsules of E. fetida, with up to 108 CFU g−1 of cocoon. Attempts to identify several of the morphologically diverse cocoon isolates were inconclusive. In addition, Zachmann and Molina (32) determined that cocoons may acquire bacteria from the environment from which they are formed; however, the location and function of the bacteria within the cocoon were not addressed in that study. Recently, Daane et al. (8), using scanning electron microscopy, determined the location of bacteria within the developing earthworm cocoon. Examination of cocoon fragments containing well-developed juvenile earthworms revealed the presence of numerous rod-shaped microorganisms within the developing tissues. The microorganisms present did not appear to be detrimental to the developing earthworm tissue, which suggests a mutualistic association.
Earthworm cocoon fluid has been found to elicit an immune response against several bacterial species, which indicates that earthworms may be able to control the cocoon microbiota (30). This is also suggested by Daane et al. (7), who reported consistent recovery of Ralstonia eutropha (formerly Alcaligenes eutrophus) from Aporrectodea trapezoides cocoons formed in inoculated soil. In addition, Zachmann and Molina (32) reported that Escherichia coli could not be recovered from cocoons formed in microcosms inoculated with this bacterium.
Although cocoons have been found to contain and acquire microorganisms from the environment, few if any studies have been performed to assess how the earthworm egg capsule microbiota influences juvenile development, terrestrial microbial communities, or the fate of toxic chemicals in the environment. The latter is of particular interest because it would determine whether earthworm egg capsules could be used as biovectors for the introduction of beneficial bacteria, such as biological control, biodegradative, or plant-growth-promoting strains, into the environment.
The objectives of the present study were to use the 2,4-dichlorophenoxyacetic acid (2,4-D)-degrading bacterium, R. eutropha JMP222N (pJP4), and the compost earthworm, E. fetida as a model system to determine whether bacterial strains acquired by earthworm egg capsules can be released from the cocoons into the soil for degradation of 2,4-D. In addition, we assessed the ability of a degradative strain within the cocoon to influence the level of tolerance of the developing earthworm to 2,4-dichlorophenol (2,4-DCP).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
R. eutropha JMP222N is a nalidixic acid-resistant mutant of JMP222, a streptomycin-resistant, cured derivative of R. eutropha JMP134(pJP4) (21). R. eutropha JMP222N containing pJP4 was obtained by patch mating as previously described (7). Plasmid pJP4 is an IncP, broad-host-range plasmid containing genes for mercury resistance and 2,4-D and 2,4-DCP degradation (21). Strains were maintained on selective media at 4°C, and long-term storage was in 50% glycerol at −80°C. R. eutropha JMP222N(pJP4) was initially grown in minimal salts basal medium (26) containing 2,4-D and, thereafter, was routinely grown at 28°C on selective tryptone yeast (TY) agar medium (1) containing streptomycin, nalidixic acid, and HgCl2. R. eutropha JMP222N was routinely grown at 28°C on selective (streptomycin and nalidixic acid) TY agar medium. Filter-sterilized streptomycin, nalidixic acid, 2,4-D, and HgCl2 stock solutions were added to media after autoclaving to final concentrations of 500, 30, 400, and 10 μg ml−1, respectively.
For the inoculation of earthworm-mating microcosms, bacteria were grown in selective TY broth medium to mid- to late-exponential growth and harvested by centrifugation at 5,000 × g for 10 min. Cells were washed three times in a 0.1× mineral salts solution (5) (pH 7.0) and resuspended in the same solution to obtain 109 cells ml−1.
Chemicals.
2,4-DCP and 2,4-D (>98% purity) were purchased from Aldrich Chemical Co. (Milwaukee, Wis.). Streptomycin sulfate and nalidixic acid were obtained from Sigma Chemical Co. (St. Louis, Mo.). Mercuric chloride was obtained from EM Science (Gibbstown, N.J.).
Earthworms.
E. fetida (Savigny, 1826) earthworms were purchased from Carolina Biological Supply Co. (Burlington, N.C.). Laboratory stock cultures of E. fetida were propagated and maintained at room temperature in nonsterile Canadian sphagnum peat moss bedding (Magic Products, Amhurst Junction, Wis.). Ground oatmeal was supplied to the surface as a food source every 2 to 3 days.
Earthworm mating microcosms.
The microcosm design consisted of 1 liter Nalgene jars (Nalge Nunc International, Rochester, N.Y.) containing a sterile mixture of 128 g of bedding material, 32 g of microcrystalline cellulose (FMC Corp., Philadelphia, Pa.), and 280 ml of either 0.1× mineral salts solution (pH 7.0) or washed bacterial suspension at a final concentration of 108 cells g−1 of bedding (wet weight) as indicated below. The inoculated treatments received either R. eutropha JMP222N(pJP4) or R. eutropha JMP222N. All three mating microcosm treatments were established in three replications. Each microcosm received 40 adult (clitellate) earthworms (400 to 600 mg each) taken randomly from stock culture and was left undisturbed for 1-week intervals in continuous darkness at 24°C for the production of egg capsules.
Extraction and enumeration of bacteria from earthworm bedding.
All microcosms were analyzed for inoculated strains after 1 week of incubation. Microcosms inoculated with the degradative strain were also analyzed after 2 and 3 weeks of incubation. A 5-g subsample of bedding was aseptically taken, and bacterial cells were extracted from the bedding with 90 ml of sterile extractant solution containing 0.1% sodium pyrophosphate (pH 7.0) and 0.03% (vol/vol) Tween 80 (Sigma Chemical Co.). Bedding suspensions were shaken for 30 min at 250 rpm on a wrist-action shaker and allowed to settle for 10 min. A 10-ml portion of the upper, bedding-free phase was serially diluted in 0.01 M phosphate-buffered saline (pH 7.1) containing 0.03% Tween 80 (PBS-T) and spread plated, in duplicate, onto appropriate selective TY agar media. Bacteria were enumerated after 1 week of incubation at 28°C, and counts are reported as CFU per gram of bedding (wet weight).
Collection and bacterial analysis of earthworm egg capsules.
Cocoons were collected from earthworm mating microcosms at the end of a 1-week incubation period. Cocoons were also collected after the second and third weeks of incubation from microcosms inoculated with R. eutropha JMP222N(pJP4). The cocoons (age range, 0 to 7 days) were washed under running tap water, rinsed in distilled water, and incubated at room temperature for 12 days on water-saturated filter paper in petri dishes, yielding cocoons of ages ranging from 12 to 19 days. This age was chosen since viability can easily be assessed by observing either embryo movement or blood circulation. In addition, studies have shown bacterial numbers to increase with increasing cocoon age (8, 32). Therefore, an age range of 12 to 19 days provides for an accurate prediction of the bacterial numbers which will be released upon cocoon hatching, which usually begins around an age of 24 days but may take as long as 45 days for complete development. Prior to bacterial analyses, cocoons were examined for viability, weighed, and surface sterilized as previously described (6). A statistical analysis of the cocoon viability was performed by using a two-sample t test (Minitab release 11 computer package; Minitab, Inc., University Park, Pa.).
Five viable cocoons formed in each of the three earthworm mating microcosm treatments were analyzed for the presence of the inoculated bacterial strains. Each cocoon was homogenized by using a sterile tissue grinder in 2 ml of PBS-T and then analyzed as described for bedding analysis. Results are reported as CFU per cocoon.
2,4-D amended soil microcosms.
A Hubbard loamy sand (Udorthentic Haploboroll) was used for all degradation experiments and was described previously (6). The microcosms consisted of 3 g (dry weight) of nonsterile soil in 7-ml glass serum vials. A filter-sterilized solution of 2,4-D was added to each of the microcosms to give a final concentration of approximately 100 ppm and a moisture content of 16.5% (gravimetric determination), which is conducive to earthworm survival. The experimental design included treatments of nonsterile soil with or without added earthworm egg capsules. A sufficient number of microcosms for each treatment were made for duplicate analyses of 2,4-D at each time point. The cocoon-containing treatments included uninoculated cocoons (formed in uninoculated sterile bedding), JMP222N cocoons (formed in R. eutropha JMP222N inoculated bedding), and JMP222N(pJP4) cocoons [formed in R. eutropha JMP222N(pJP4) inoculated bedding]. For microcosms containing cocoons, two surface-sterilized viable cocoons, ranging in age from 12 to 19 days, were added to the soil.
Quantification of 2,4-D biodegradation.
The utilization of 2,4-D was determined by high-pressure liquid chromatography (HPLC). At each time point duplicate soil microcosms from each treatment were destructively sampled by vortexing for 2 min with 3 ml of distilled water. The soil slurry was transferred to a microcentrifuge tube and centrifuged at 16,000 × g for 5 min. The supernatant was filtered and analyzed by HPLC (Shimadzu model SCL-10A; Shimadzu, Columbia, Md.) equipped with a SphereClone ODS (2) column (250 by 4.6 mm; particle size, 5 μm; Phenomenex, Torrance, Calif.) with methanol-water-acetic acid (60:38:2, 1 ml min−1) as the eluant with detection at 280 nm by using a Shimadzu SPD-10A UV-visible variable-wavelength detector. The results are reported in parts per million as the mean from duplicate soil microcosms.
Toxicity of 2,4-DCP to earthworm egg capsules and adults.
Surface-sterilized cocoons containing viable embryos were exposed to various concentrations of 2,4-DCP. A filter-sterilized stock solution of 2,4-DCP in 0.1 N NaOH was added to molten 0.2% Noble agar (Difco, Detroit, Md.) and poured into sterile glass petri dishes and allowed to cool. The final concentrations of 2,4-DCP tested included 0.1, 0.5, 1, 3, 5, 10, 15, 20, 25, 30, 40, and 50 ppm. In addition, a control was included which did not contain 2,4-DCP. The same concentration of NaOH was present in each of the petri dishes independent of the 2,4-DCP concentration. Several cocoons from each of the three earthworm mating microcosm treatments were fully submersed into the various concentrations of semisolid 2,4-DCP agar and checked for viability over time at room temperature. Viability was assessed with a stereoscope and was based on observing either movement or circulating blood in the developing worms. The experiment was halted after the hatching of remaining viable cocoons.
Adult earthworms, taken randomly from stock cultures, were also tested by the above methodology. The adults were tested in 10, 15, 20, 25, and 30 ppm 2,4-DCP. Three adults were added for each concentration, except for the 30-ppm concentration, which had six adults. Earthworms were assessed for mortality over time and were considered dead when the worms no longer responded to physical stimuli.
RESULTS
Enumeration of inoculated strains in earthworm bedding.
Earthworm bedding was inoculated with either R. eutropha JMP222N(pJP4) or its plasmid-free derivative at an initial density of 108 cells g−1 (wet weight) of bedding. The bedding was analyzed for the inoculated strains after incubation for 1 week in the presence of 40 adult earthworms. Both inoculated strains decreased approximately 10-fold from the initial inoculation density to approximately 107 CFU g−1 of bedding at day 7. Neither of the R. eutropha strains could be isolated from the uninoculated control microcosms, with a level of detection of 9.0 × 101 CFU g−1 of soil.
To determine the survival of R. eutropha JMP222N(pJP4) over time, two earthworm mating microcosms were analyzed for 2 additional weeks. Each successive week revealed a decrease in the numbers of R. eutropha JMP222N(pJP4) recovered, with mean numbers of 1.4 × 107, 3.0 × 105, and 2.5 × 104 CFU g−1 for weeks 1, 2, and 3, respectively.
Egg capsule production.
Replicate earthworm mating microcosms were set up for each treatment in order to perform both degradation and toxicity studies. None of the earthworms from the earthworm mating microcosms died or showed signs of loss of secondary sexual characteristics (presence of clitellum) during the course of the experiments. The average numbers of cocoons produced per worm in the uninoculated and the R. eutropha JMP222N- and R. eutropha JMP222N(pJP4)-inoculated mating microcosms over a 7-day mating period were 1.37 ± 0.49, 1.43 ± 0.42, and 1.48 ± 0.40, respectively. The average total numbers of cocoons produced weekly from 40 adult earthworms over a 3-week period from two R. eutropha (pJP4)-inoculated mating microcosms were 51, 50, and 44 cocoons after weeks 1, 2, and 3, respectively. The greatest variability between the treatments was seen in the viability of the cocoons. The viabilities of cocoons collected from the uninoculated and the R. eutropha JMP222N(pJP4)-inoculated mating microcosms were 52 ± 8% and 92 ± 6%, respectively. The difference between the mean viabilities is significant (P = 0.0056).
Recovery of inoculated strains from earthworm egg capsules.
Viable cocoons were analyzed after 12 days of incubation at room temperature. The average cocoon weight was 13.1 ± 1.5 mg (mean ± standard deviation for 24 cocoons), and the selective agar plates used for the evaluation of surface sterilization revealed no growth.
A total of five cocoons, collected from bedding after 1 week of egg capsule production from each treatment, were analyzed for the inoculated strains. Neither of the R. eutropha strains could be recovered from cocoons collected from the uninoculated treatments, with the level of detection being 1.0 × 101 CFU cocoon−1. Cocoons formed in bedding inoculated with R. eutropha JMP222N had a mean number of 9.3 × 104 CFU cocoon−1. Similar results were found with the cocoons formed in bedding inoculated with R. eutropha JMP222N(pJP4), with a mean number of 1.0 × 105 CFU cocoon−1 of the degradative strain. In addition, several cocoons collected after the second and third weeks of egg capsule production from two microcosms inoculated with R. eutropha JMP222N(pJP4) were analyzed for the degradative strain. The cocoons had 1.1 × 104 and 1.2 × 102 CFU cocoon−1 of recoverable R. eutropha JMP222N(pJP4) within earthworm cocoons collected at weeks 2 and 3, respectively.
Degradation of 2,4-D in earthworm egg capsule containing soil microcosms.
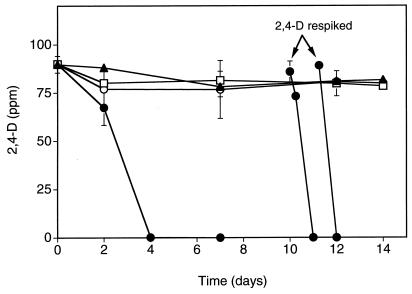
Degradation of 2,4-D by bacteria introduced with developing earthworm egg capsules was examined by placing two earthworm cocoons, collected from earthworm mating microcosms, in 3 g of nonsterile soil amended with 100 ppm of 2,4-D. The cocoons were approximately 1 week from hatching, and juvenile earthworms were observed in the soil microcosms as early as 5 days after the start of the experiment; however, complete hatching of all cocoons required up to 25 days from the onset of the experiment. Microcosms were analyzed over time for loss of 2,4-D by HPLC. Figure 1 shows the loss of 2,4-D only in soil microcosms containing cocoons which were formed in bedding inoculated with the degradative strain, R. eutropha JMP222N(pJP4). The concentration of 2,4-D fell to below the level of detection within 4 days in the R. eutropha JMP222N(pJP4) treatments. 2,4-D was utilized within 24 h after the remaining experimental microcosms were respiked at day 10 and day 11. In contrast, no appreciable loss of 2,4-D occurred in nonsterile microcosms or in microcosms with cocoons containing R. eutropha JMP222N or with cocoons formed in uninoculated bedding. Analysis of the soil after complete hatching, on day 11 from two of the microcosms to which R. eutropha JMP222N(pJP4) cocoons had been added, showed the presence of 1.5 × 104 CFU of R. eutropha JMP222N(pJP4) g−1 (wet weight) of soil.
FIG. 1.
Degradation of 2,4-D in nonsterile soil with or without added earthworm cocoons. Cocoons contained R. eutropha JMP222N(pJP4), R. eutropha JMP222N, or no degradative bacteria (nonsterile) and were about 1 week from hatching when added to the degradation microcosms at time zero. Juvenile worms containing microcosms were respiked with 100 ppm of 2,4-D on days 10 and 11. Data points represent the mean of two replicate samples. Error bars indicate the standard deviation, and where no standard bar is presented the value is less than the height of the symbol. Symbols: ▴, no cocoons; □, nonsterile cocoons; ○, JMP222N cocoons; ●, JMP222N(pJP4) cocoons.
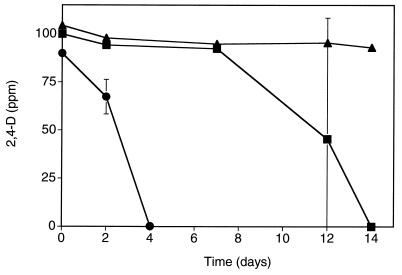
A comparative degradation experiment was set up with earthworm cocoons collected after 1 or 3 weeks from bedding initially inoculated with R. eutropha JMP222N(pJP4). The egg capsules collected after the first 7 days of cocoon production contained a mean density of 105 CFU of R. eutropha (pJP4) per cocoon and were considered high-inoculum cocoons. In contrast, cocoons collected after the third week of cocoon production contained a mean density of 102 CFU of R. eutropha (pJP4) per cocoon and were considered low-inoculum cocoons. Loss of 2,4-D was observed in both the high- and low-inoculum cocoon microcosms (Fig. 2). At the low-inoculum density of 102 CFU cocoon−1, 2,4-D degradation occurred after 7 days from the start of the experiment.
FIG. 2.
Comparison of degradation of 2,4-D in nonsterile soil with cocoons containing high (∼105 CFU cocoon−1; ●) or low (∼102 CFU cocoon−1; ■) densities of R. eutropha JMP222N(pJP4) or without cocoons (▴). Data points represent the mean of two replicate samples. Error bars indicate the standard deviation, and where no standard bar is presented the value is less than the height of the symbol.
Toxicity of 2,4-DCP to developing earthworms and adults.
Preliminary studies revealed that 2,4-D was not toxic to adults or developing earthworms at concentrations of up to 1,000 ppm (data not shown). However, the first intermediate product of 2,4-D degradation, 2,4-DCP, has been found to be extremely toxic to earthworms (10). Therefore, toxicity studies were undertaken to determine the ability of a degradative bacterial strain to influence the tolerance of the developing earthworm to 2,4-DCP.
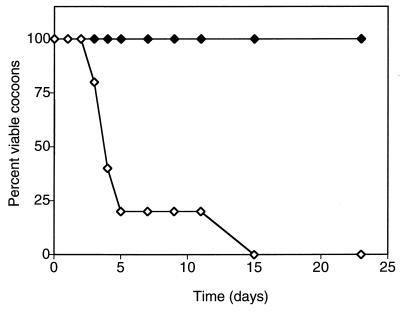
The toxicity of 2,4-DCP was assessed by embedding cocoons (12 to 19 days of age), with or without the degradative bacterial strain, into various concentrations of semisolid 2,4-DCP-containing agar. Cocoons which were formed in earthworm mating microcosms inoculated with R. eutropha JMP222N had results similar to those of cocoons formed in uninoculated bedding (data not shown). Table 1 shows the viability of cocoons after 23 days of continuous exposure to various concentrations of 2,4-DCP. Cocoons containing approximately 105 CFU of R. eutropha (pJP4) cocoon−1 showed a 33% mortality in 30 ppm of 2,4-DCP, with the remaining cocoons successfully hatching in this concentration. In contrast, cocoons formed in uninoculated bedding had a mortality of 40% at 15 ppm of 2,4-DCP. For cocoons with or without R. eutropha (pJP4), the mortality increased to 100% at 40 and 20 ppm of 2,4-DCP, respectively. Although the cocoons formed in uninoculated bedding were nonviable after long-term exposure to 20 ppm 2,4-DCP, viability was maintained for up to 15 days, with the greatest mortality (80%) occurring within the first 5 days (Fig. 3). The viability of cocoons containing the degradative strain R. eutropha (pJP4) exposed to 40 ppm of 2,4-DCP showed similar results in that the greatest mortality occurred within the first few days of incubation and 100% mortality occurring at 11 days (data not shown).
TABLE 1.
Viability of cocoons exposed to various concentrations of 2,4-DCP with or without R. eutropha JMP222N(pJP4)
| Concn (ppm)a | Viability (%) after 23 daysb in cocoons:
|
|
|---|---|---|
| Without R. eutropha (pJP4)c | Containing R. eutropha (pJP4)d | |
| 0 | 100 | 100 |
| 5 | 100 | 100 |
| 10 | 100 | 100 |
| 15 | 60 | 100 |
| 20 | 0 | 100 |
| 25 | 0 | 83 |
| 30 | 0 | 67 |
| 40 | 0 | 0 |
Exposure to 0.1, 0.5, 1, and 3 ppm of 2,4-DCP showed no mortality to developing earthworms with or without the degradative strain. Exposure to 50 ppm showed 100% mortality.
Five to six cocoons ranging in age of 12 to 19 days were tested for each concentration. The cocoons were embedded in 0.2% Noble agar containing 2,4-DCP in glass petri dishes.
Cocoons without R. eutropha (pJP4) were collected from nonsterile earthworm mating microcosms after 1 week of incubation.
R. eutropha (pJP4)-containing cocoons were collected from earthworm mating microcosms inoculated with R. eutropha JMP222N(pJP4) after 1 week of incubation.
FIG. 3.
Toxicity of 20 ppm of 2,4-DCP to cocoons formed in uninoculated or R. eutropha JMP222N(pJP4)-inoculated earthworm mating microcosms. Symbols: ◊, nonsterile cocoons; ⧫, R. eutropha (pJP4) cocoons.
Adult earthworms taken randomly from laboratory stock were exposed to 2,4-DCP in the same manner as cocoons to determine whether adults and developing worms have similar tolerance levels. Adult earthworms died within 48 h of exposure to 25 and 30 ppm of 2,4-DCP and survived up to 7 days in 20 ppm of 2,4-DCP. All of the adult earthworms survived long-term exposure (2 weeks) in 10 and 15 ppm of 2,4-DCP.
DISCUSSION
In the present study we determined that a degradative bacterial strain associated with earthworm cocoons can be released into the soil upon hatching and can initiate the biodegradation of the herbicide 2,4-D. This suggests that earthworm cocoons can be used as biovectors for the delivery of beneficial bacteria into the environment. Our study showed that utilization of 2,4-D was seen only in soil that contained cocoons which were formed in bedding inoculated with the degradative strain, R. eutropha JMP222N(pJP4). The number of days needed for complete loss of 2,4-D was significantly less when cocoons contained a higher density of R. eutropha (pJP4). Nevertheless, it should be noted that complete loss of 2,4-D was seen even when cocoons contained a mean density of ∼102 CFU of R. eutropha (pJP4) cocoon−1. Analysis of the soil, after complete hatching of the juvenile earthworms, revealed the presence of R. eutropha JMP222N(pJP4) at relatively high concentrations (∼104 CFU g−1 of soil). The use of surface-sterilized cocoons determined that the inoculation of the soil with the degradative strain was solely due to cocoon hatching and not to carryover from the inoculated mating-microcosm bedding material. Our data also suggest that the semipermeable nature of the earthworm cocoon shell may initiate the degradation of 2,4-D in vivo. To our knowledge, this represents the first study demonstrating the degradation of 2,4-D by R. eutropha (pJP4) with an earthworm egg capsule as a delivery vehicle.
The number of earthworm egg capsules produced per worm per week was not different between the uninoculated and inoculated mating-microcosm treatments. However, our results are higher than those previously published by Zachmann and Molina (32), who used similar mating conditions. In the present study, the decreasing number of egg capsules produced from the same earthworms over a 3-week period suggests that necessary resources may become depleted from the medium over time. Resource depletion was suggested in studies by van Gestal et al. (31) and Hartenstein et al. (12); however, more work in this area with the present system is needed to support this hypothesis.
The viability of cocoons formed in uninoculated sterile bedding was significantly lower than for cocoons collected from inoculated mating microcosms. However, no difference was seen in cocoon viability between uninoculated or inoculated microcosms after a second consecutive week of cocoon production (data not shown). A possible reason for the increase in viability of cocoons collected from the sterile microcosm is that the earthworms added to the mating microcosms were reinoculating the sterilized bedding with bacteria. This suggests that a certain density or population of bacteria may be required for proper earthworm development, but this hypothesis remains to be proven.
In the present study, we isolated an average of 105 CFU of either R. eutropha strain per cocoon from egg capsules formed in bedding inoculated with these bacteria. These results are similar to those reported by Daane et al. (7) from Aporrectodea trapezoides cocoons collected from soil columns inoculated with the same bacterial strain. In addition, in the present study cocoons collected weekly from a microcosm inoculated with R. eutropha JMP222N(pJP4) showed decreasing numbers of this strain from weeks 1 to 3, a finding which directly corresponds to the same decline seen in the bedding material.
The majority of toxicity studies have focused on adult earthworms (for a review, see reference 10). Several studies have assessed sublethal effects (juvenile growth, cocoon production or hatching, and immune system effects) (2, 3, 14, 15, 17, 19, 25, 31); however, none have examined the toxicity of chemicals to developing earthworms in cocoons directly. Several studies have shown that 2,4-D is not toxic to adult earthworms even at high concentrations; however, the first intermediate of its degradation, 2,4-DCP, has been found to be highly toxic (20, 22, 24). Therefore, we focused our attention on the toxicity of 2,4-DCP to developing earthworms. Our results show that the presence of the 2,4-D-degrading bacterium, R. eutropha (pJP4), within the developing cocoon greatly increases the tolerance to this highly toxic compound. Indeed, the exposure level allowing the successful hatching of juvenile earthworms was twice that of cocoons devoid of the degrading bacterium.
Studies have shown that juvenile earthworms are more sensitive to agrochemicals than mature worms (17, 25). Contrary to these results, we found no difference in the level of tolerance to 2,4-DCP between developing worms within the cocoon and adults. However, our results cannot be directly compared to previous studies because we used a continual exposure contact test rather than an artificial soil or filter paper test of limited exposure time. The present method of using a semisolid agar medium was appropriate because it allowed for total immersion of the cocoon and daily assessment of viability without disturbance. In addition, the concentration of agar used here immobilized the cocoon without interfering with normal development and hatching.
Taken together, our results show that the presence of a degradative strain within the developing earthworm cocoon can increase the tolerance to toxic compounds, which indicates that the bacteria were metabolically active in vivo. In addition, we have shown that earthworm cocoons can release beneficial strains of bacteria into the soil upon hatching and initiate biodegradation. The present work with 2,4-D as the model system is currently being expanded to include degradation of more recalcitrant compounds such as polyaromatic hydrocarbons and polychlorinated biphenyls. We are also interested in including beneficial microbes such as plant pathogen antagonists and rhizobia. In addition, it will be interesting to determine if this model system can be extended to include other agriculturally relevant species of earthworms, such as A. trapezoides, Lumbricus rubellus, and L. terrestris. Furthermore, the influence of soil texture, pH, moisture, and temperature on the rate and degree of degradation will be important to determine in future studies.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the New Jersey Commission on Science and Technology.
We thank Mervyn de Souza, Lisa Newman, and Michael Sadowsky for helpful discussions and suggestions regarding the manuscript. We also thank Gregory Flanagan and Quinn Im for technical assistance.
REFERENCES
- 1.Beringer J E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 2.Bouwman H, Reinecke A J. Effects of carbofuran on the earthworm, Eisenia fetida, using a defined medium. Bull Environ Contam Toxicol. 1987;38:171–178. doi: 10.1007/BF01606577. [DOI] [PubMed] [Google Scholar]
- 3.Bunn K E, Thompson H M, Tarrant K A. Effects of agrochemicals on the immune systems of earthworms. Bull Environ Contam Toxicol. 1996;57:632–639. doi: 10.1007/s001289900237. [DOI] [PubMed] [Google Scholar]
- 4.Clegg C D, Anderson J M, Lappin-Scott H M, van Elsas J D, Jolly J M. Interaction of a genetically modified Pseudomonas fluorescens with the soil-feeding earthworm Octolasion cyaneum (Lumbricidae) Soil Biol Biochem. 1995;27:1423–1429. [Google Scholar]
- 5.Cole M A, Elkan G H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973;4:248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daane L L, Molina J A E, Berry E C, Sadowsky M J. Influence of earthworm activity on gene transfer from Pseudomonas fluorescens to indigenous soil bacteria. Appl Environ Microbiol. 1996;62:515–521. doi: 10.1128/aem.62.2.515-521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daane L L, Molina J A E, Sadowsky M J. Plasmid transfer between spatially separated donor and recipient bacteria in earthworm-containing soil microcosms. Appl Environ Microbiol. 1997;63:679–686. doi: 10.1128/aem.63.2.679-686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daane L L, Molina J A E, Sadowsky M J. Scanning electron microscopy of the microflora in egg capsules of the earthworm Eisenia fetida. Pedobiologia. 1998;42:79–87. [Google Scholar]
- 9.Doube B M, Ryder M H, Davoren C W, Stephens P M. Enhanced root nodulation of subterranean clover (Trifolium subterraneum) by Rhizobium leguminosarum biovar trifolii in the presence of the earthworm Aporrectodea trapezoides (Lumbricidae) Biol Fertil Soils. 1994;18:169–174. [Google Scholar]
- 10.Edwards C A, Bohlen P J. The effects of toxic chemicals on earthworms. Rev Environ Contam Toxicol. 1992;125:23–99. [Google Scholar]
- 11.Edwards C A, Bohlen P J, Linden D R, Subler S. Earthworms in agroecosystems. In: Hendrix P F, editor. Earthworm ecology and biogeography in North America. Boca Raton, Fla: Lewis Publishers; 1995. pp. 185–213. [Google Scholar]
- 12.Hartenstein R, Neuhauser E F, Kaplan D L. Reproductive potential of the earthworm Eisenia foetida. Oecologia (Berlin) 1979;43:329–340. doi: 10.1007/BF00344959. [DOI] [PubMed] [Google Scholar]
- 13.Hendriksen N B. Effects of detritivore earthworms on dispersal and survival of the bacterium Aeromonas hydrophila. Acta Zool Fennica. 1995;196:115–119. [Google Scholar]
- 14.Lofs-Holmin A. Measuring growth of earthworms as a method of testing sublethal toxicity of pesticides. Swed J Agric Res. 1980;10:25–33. [Google Scholar]
- 15.Lofs-Holmin A. Measuring cocoon production of the earthworm Allolobophora caliginosa (Sav.) as a method of testing sublethal toxicity of pesticides. Swed J Agric Res. 1982;12:117–119. [Google Scholar]
- 16.Madsen E L, Alexander M. Transport of Rhizobium and Pseudomonas through soil. Soil Sci Soc Am J. 1982;46:557–560. [Google Scholar]
- 17.Martin N A. Toxicity of pesticides to Allolobophora caliginosa (Oligochaeta: Lumbricidae) N Z J Agric Res. 1986;29:699–706. [Google Scholar]
- 18.Morgan M, Burrows I. Earthworm/microorganism interactions. Rothamsted Exp Stn Rep. 1982;1982:104. [Google Scholar]
- 19.Neuhauser E F, Callahan C A. Growth and reproduction of the earthworm Eisenia fetida exposed to sublethal concentrations of organic chemicals. Soil Biol Biochem. 1990;22:175–179. [Google Scholar]
- 20.Neuhauser E F, Loehr R C, Malecki M R, Milligan D L, Durkin P R. The toxicity of selected organic chemicals to the earthworm Eisenia fetida. J Environ Qual. 1985;14:383–388. [Google Scholar]
- 21.Pemberton J M, Corney B, Don R H. Evolution and spread of pesticide degrading ability among soil micro-organisms. In: Timmis K N, Pühler A, editors. Plasmids of medical, environmental and commercial importance. Amsterdam, The Netherlands: Elsevier/North-Holland Biomedical Press; 1979. pp. 287–299. [Google Scholar]
- 22.Potter D A, Spicer P G, Redmond C T, Powell A J. Toxicity of pesticides to earthworms in Kentucky bluegrass turf. Bull Environ Contam Toxicol. 1994;52:176–181. doi: 10.1007/BF00198485. [DOI] [PubMed] [Google Scholar]
- 23.Reddell P, Spain A V. Earthworms as vectors of viable propagules of mycorrhizal fungi. Soil Biol Biochem. 1991;23:767–774. [Google Scholar]
- 24.Roberts B L, Dorough H W. Relative toxicities of chemicals to the earthworm Eisenia foetida. Environ Toxicol Chem. 1984;3:67–78. [Google Scholar]
- 25.Spurgeon D J, Hopkin S P. Effects of metal-contaminated soils on the growth, sexual development, and early cocoon production of the earthworm Eisenia fetida, with particular reference to zinc. Ecotox Environ Safety. 1996;35:86–95. doi: 10.1006/eesa.1996.0085. [DOI] [PubMed] [Google Scholar]
- 26.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 27.Stephens P M, Davoren C W, Ryder M H, Doube B M. Influence of the lumbricid earthworm Aporrectodea trapezoides on the colonization of wheat roots by Pseudomonas corrugata strain 2140R in soil. Soil Biol Biochem. 1993;25:1719–1724. [Google Scholar]
- 28.Stephens P M, Davoren C W, Ryder M H, Doube B M. Influence of the earthworm Aporrectodea trapezoides (Lumbricidae) on the colonization of alfalfa (Medicago sativa L.) roots by Rhizobium meliloti L5-30R and the survival of R. meliloti L5-30R in soil. Biol Fertil Soils. 1994;18:63–70. [Google Scholar]
- 29.Thorpe I S, Prosser J I, Glover L A, Killham K. The role of the earthworm Lumbricus terrestris in the transport of bacterial inocula through soil. Biol Fertil Soils. 1996;23:132–139. [Google Scholar]
- 30.Valembois P, Roch P, Lassegues M. Antibacterial molecules in annelids. In: Brehélin M, editor. Immunity in invertebrates. Berlin, Germany: Springer-Verlag; 1986. pp. 74–93. [Google Scholar]
- 31.van Gestel C A M, van Dis W A, van Breemen E M, Sparenburg P M. Development of a standardized reproduction toxicity test with the earthworm species Eisenia fetida andrei using copper, pentachlorophenol, and 2,4-dichloroaniline. Ecotox Environ Safety. 1989;18:305–312. doi: 10.1016/0147-6513(89)90024-9. [DOI] [PubMed] [Google Scholar]
- 32.Zachmann J E, Molina J A E. Presence of culturable bacteria in cocoons of the earthworm Eisenia fetida. Appl Environ Microbiol. 1993;59:1904–1910. doi: 10.1128/aem.59.6.1904-1910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]