Summary
Relapsed/refractory multiple myeloma (RRMM) is known to have a high burden of disease and complications associated with refractoriness to prior lines of therapy. Severe pain and fatigue symptoms and impairments in physical and emotional functioning have been strongly linked to reduced health‐related quality of life (HRQoL) in patients with RRMM. Assessment of patient reported‐outcome measures from the pivotal, Phase II HORIZON study (OP‐106; NCT02963493) in patients with RRMM (n = 64) demonstrated that melphalan flufenamide (melflufen) plus dexamethasone treatment preserved HRQoL. Patients had clinically meaningful improvements, even after eight treatment cycles, in relevant scales such as global health status/QoL, physical functioning, emotional functioning, pain, and fatigue. Patients with triple‐class–refractory disease (n = 50) displayed similar improvements. Patient‐reported outcome deterioration was delayed for a substantial amount of time in patients who experienced a response to melflufen plus dexamethasone treatment relative to patients who did not experience a response. These findings support the notion that treatment with melflufen plus dexamethasone may sustain or improve HRQoL over time in patients with RRMM, including in patients with triple‐class–refractory disease for whom outcomes are generally worse. The clinical benefits observed in patients from the HORIZON trial are encouraging and supportive of translation into real‐world practice.
Keywords: patient‐reported outcomes, melflufen, melphalan flufenamide, health‐related quality of life, relapsed/refractory multiple myeloma
Introduction
Survival in multiple myeloma (MM) has improved substantially in recent years due to advances in therapeutic agents and approaches. Despite this, patients continue to relapse and survival after exposure to the three main classes of drugs [proteasome inhibitors (PIs), immunomodulatory drugs, and anti‐cluster of differentiation 38 (CD38) monoclonal antibodies (mAbs)] results in triple‐class–exposed or –refractory disease, with suboptimal outcomes. 1 , 2 , 3 Moreover, this improvement in survival has contributed to an increased proportion of patients with relapsed/refractory MM (RRMM) living with the burden of symptoms and complications associated with the disease and prior lines of therapy. 3 , 4 RRMM is associated with severe symptoms, of which pain, fatigue, physical functioning, and emotional functioning have been strongly linked to impairments in health‐related quality of life (HRQoL) of patients. 5 , 6 Treatment goals for late‐stage patients with RRMM should therefore not only include extending survival but also managing disease and treatment‐related symptoms and preserving, or potentially improving, HRQoL. 4 , 7
Melphalan flufenamide (melflufen) is a first‐in‐class peptide‐drug conjugate (PDC) that targets aminopeptidases and thereby rapidly releases alkylating agents inside tumour cells, and has been approved by the United States Food and Drug Administration (FDA) for use in patients with RRMM. 8 , 9 , 10 , 11 , 12 , 13 In the pivotal, Phase II, HORIZON study (OP‐106; ClinicalTrials.gov identifier: NCT02963493), melflufen plus dexamethasone showed clinically meaningful efficacy with an overall response rate (ORR) of 29%, median progression‐free survival of 4·2 months, median overall survival of 11·6 months, and a manageable safety profile in patients with advanced RRMM. 14 The present analysis focusses on patient‐reported outcomes (PROs) collected in a subset of patients in the HORIZON study.
Methods
Study design and participants
The HORIZON study was a pivotal, single‐arm, multicentre, Phase II study of melflufen plus dexamethasone in patients with RRMM who received at least two prior lines of therapy, including an immunomodulatory agent and PI, and who were refractory to pomalidomide and/or an anti‐CD38 mAb. Additional eligibility criteria have been previously described. 14
In each 28‐day cycle, patients received a 30‐min central intravenous infusion of melflufen 40 mg (starting dose) on day 1 in combination with oral dexamethasone 40 mg (20 mg if aged ≥75 years) on days 1, 8, 15, and 22 until disease progression, unacceptable toxicity, or withdrawal. Dose reductions for melflufen‐related toxicities were allowed after the first cycle in 10‐mg increments down to the lowest dose of 20 mg, as previously described. 14 Multiple dose reduction steps for dexamethasone were permitted based on starting dose. The 40 mg starting dose could be reduced to 20 mg in step 1, then 12 mg in step 2, and the 20 mg starting dose could be reduced to 12 mg in step 1, then 8 mg in step 2. The primary end‐point was ORR [partial response or better (≥PR)] assessed by the investigator and confirmed by independent review.
The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation guidelines for Good Clinical Practice. The protocol was reviewed and approved by national regulatory authorities and an independent ethics committee or institutional review board at each study centre. Each patient provided written informed consent.
Following protocol amendment 5, PRO assessments were added to capture functional status and well‐being. Analyses included patients with valid baseline and at least one post‐baseline assessment. Subgroup analyses were performed for patients with triple‐class–refractory MM (MM that is refractory to or intolerant of one or more immunomodulatory drug, one or more PI, and one or more anti‐CD38 mAb).
Outcome measures
Two PRO measures were used to assess functional status and well‐being, as a prespecified secondary end‐point in the HORIZON study: the EuroQol five Dimensions‐three Levels (EQ‐5D‐3L) questionnaire 15 and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30 version 3 (EORTC QLQ‐C30). 16 The EQ‐5D‐3L is a generic measure of health status. The EQ visual analogue scale (VAS) is intended to rate ‘health today’ with anchors ranging from 0 (worst imaginable health state) to 100 (best imaginable health state). The EORTC QLQ‐C30 is used to assess HRQoL of patients with cancer participating in international clinical trials, with a 1‐week recall period, with raw scores transformed to a range of 0–100 (higher scores represent better functioning for functional status/QoL and functional scales, and an increase in symptoms for the symptom scales), and calculated according to the QLQ‐C30 scoring manual. 16 For the purpose of these analyses, fatigue and pain were identified as the most relevant symptom scales for patients with RRMM from the EORTC QLQ‐C30, in addition to global health status/QoL and physical and emotional functioning. 5 , 6 , 17
Questionnaires were administered at baseline, before dosing at day 1 of cycles two, four, six and eight, at the end of treatment, and at follow‐up visits for progression‐free and overall survival. Completion rates were calculated as the proportion of patients who completed each relevant PRO scale, out of the total number who were dosed (from the same protocol amendment) at each cycle. Cycle four was considered a relevant time interval for capturing important PRO changes and was of primary interest for the change from baseline analyses.
Statistical analyses
To understand whether PRO results were stable over time, the mean changes from baseline were estimated and 95% confidence intervals (CIs) were calculated. The EORTC QLQ‐C30 symptom scales were defined as stable, based on available minimal important difference thresholds, if the 95% CI were within −18 to 13 units for global health status/QoL, within −18 to 10 for physical functioning, within −24 to 24 units for pain, and within −20 to 15 units for fatigue. 17 A lower bound of the 95% CI for global health status/QoL scale and physical functioning scale falling below −18 units, and an upper bound of the 95% CI for pain and fatigue scales above 24 and 15 units, respectively, were seen as deterioration. 17 An upper bound for the 95% CI for global health status/QoL and physical functioning scale above 13 and 10 units, respectively, and the lower bound of the 95% CI for the pain and fatigue scales falling below −24 and −20 units, respectively, were seen as improvement. 17 Outputs were shown using descriptive statistics and graphical representations for the proportion of patients with impairments or meaningful improvements.
Analyses were performed based on data in all cycles, as the change from baseline using mixed models for repeated measures (MMRM). The models included baseline score as a covariate and visit (cycle). Least‐square (LS) means for change from baseline at the different cycles and two‐sided 95% CIs were calculated. No imputation of missing values was performed. Univariate analyses for the mean changes from baseline to cycle four, using 95% CI based on the t‐distribution, were also performed.
The proportion of patients with improvement from baseline in EORTC QLQ‐C30 scores over time was assessed. On individual scores, improvement cut‐offs of 5–10 points (indicative of small improvements) and ≥10 points (indicative of medium to large improvements) from baseline were defined using previously established minimal important differences for MM. 18 , 19 , 20 , 21 , 22 The relevant scales were examined at both cut‐offs as the number of improved patients per cycle and the percentage of improved patients of the total number per cycle.
Time until definite deterioration was assessed, defined as the time interval between the baseline measurement and the first ≥10 point deterioration from baseline (decrease for functional scales and global health status/QoL, increase for symptom scales) in EORTC‐QLQ‐C30 score with no further improvement during the remaining assessments. 23 A change of ≥10 points from baseline was selected as the threshold based on the minimal important difference established in Osoba et al. 20 Death was considered as an event when it occurred within a period of time defined by 1·5‐times the period between two assessments (three treatment cycles or 12 weeks) as planned in the study protocol, and as previously established. 23 , 24 Patients were considered lost to follow‐up for HRQoL and were censored at the last assessment if a ≥10 point deterioration from baseline was not observed, or if a ≥10 point deterioration was followed by an improvement at a subsequent cycle. 25 All patients with baseline and one or more post‐baseline PRO assessment were included in time until definite deterioration analyses. Patients were stratified as responders [achieving ≥PR or a minimal response or better (≥MR)], or as non‐responders [patients with stable disease (SD), progressive disease (PD), or not evaluable]. Time until definite deterioration was calculated using the Kaplan–Meier method and described using medians and 95% CIs using a log(−log(survival)) distribution.
Results
Of the 72 patients who enrolled after the protocol amendment to allow PRO assessment, 64 had valid baseline and post‐baseline PRO assessments. At the data cut‐off date of 25 August 2020 used for the analyses, 11 patients remained on therapy. Baseline characteristics were generally comparable between patients from whom PROs were collected and those in the overall population (Table I). The median (range) age was 67 (46–84) years), 19% had International Staging System Stage III disease, 36% had high‐risk cytogenetics at study entry, 30% had extramedullary disease, and patients had received a median of five prior lines of therapy (range, two to 10). In all, 50 patients had triple‐class–refractory disease. At cycles two, four, six, and eight, 91%, 95%, 96%, and 100% of patients with ongoing treatment completed EQ VAS PRO assessments, and 96%, 95%, 96%, and 100% completed global health status/QoL PRO assessments, respectively (Table SI). Completion rates were similar for other scales assessed from the EORTC‐QLQ‐C30.
Table I.
Baseline characteristics of the patient reported outcome group and overall HORIZON patient population.
| Characteristics |
Patient‐reported outcome group (n = 64) |
Overall (N = 157) |
|---|---|---|
| Age, years, median (range) | 67 (46–84) | 65 (35–86) |
| Male sex, n (%) | 33 (52) | 89 (57) |
| Time since diagnosis at study entry, years, median (range)* | 7·0 (0·7–17·1) | 6·5 (0·7–24·6) |
| International Staging System Stage I/II/III, %† | 48/32/19 | 40/31/25 |
| ECOG PS 0/1/2, % | 27/63/11 | 25/59/16 |
| Baseline albumin, median (range), g/l‡ | 39 (19–47) | 38 (19–52) |
| <35 g/l, n (%) | 11 (17) | 47 (30) |
| ≥35 g/l, n (%) | 53 (83) | 110 (70) |
| High LDH (≥1·5 × ULN) at study entry, n (%) | 6 (10) | 24 (15) |
| High risk cytogenetics at study entry, n (%) | 23 (36) | 59 (38) |
| Extramedullary disease at study entry, n (%) | 19 (30) | 55 (35) |
| No. prior lines of therapy, median (range) | 5 (2–10) | 5 (2–12) |
| Triple‐class–refractory, n (%)§ | 50 (78) | 119 (76) |
| Refractory to prior alkylators, n (%) | 34 (53) | 92 (59) |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Time since initial diagnosis is calculated relative to first dose of study drug.
In the patient reported outcome group and the overall population, two patients and six patients had unknown/missing International Staging System stage, respectively.
Baseline laboratory values are defined as the most recent assessment prior to administration of the first dose of study drug.
Triple‐class–refractory is defined as refractory or intolerant to one or more proteasome inhibitor, one or more immunomodulatory drug, and one or more anti‐CD38 monoclonal antibody.
Patient‐reported outcomes and treatment with melflufen plus dexamethasone
The mean baseline EQ VAS score was 61·4 in the overall group and was slightly lower in the triple‐class–refractory subgroup at 59·8. The EQ VAS scores were generally consistent throughout the course of treatment with melflufen plus dexamethasone, with an LS mean change from baseline at treatment cycle six of –0·4 in the overall group and 1·1 in the triple‐class–refractory subgroup (Fig 1A). The proportion of patients in the overall group with impairments across EQ‐5D‐3L dimensions of anxiety/depression, mobility, pain/discomfort, self‐care, and usual activities remained relatively stable over time from baseline through treatment cycle eight (Fig 1B). Worsening pain/discomfort and impairment with usual activities were the most frequent across all treatment cycles examined.
Fig 1.
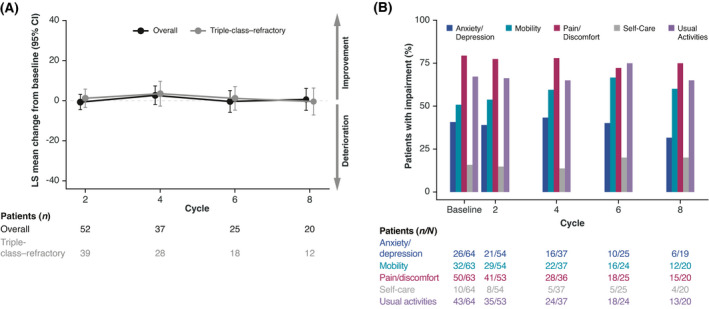
EQ‐5D‐3L change from baseline and proportion of patients with impairments as assessed by EQ‐5D‐3L domains over treatment cycles. (A) LS mean change from baseline in EQ visual analogue scale over time for the overall group (black) and triple‐class–refractory subgroup (grey). Numbers underneath (A) and denominators underneath (B) represent non‐missing patients who completed the patient‐reported outcome measure in each respective cycle. (B) Proportion of total patients with detectable impairments in EQ‐5D‐3L scales per treatment cycle. CI, confidence interval; EQ‐5D‐3L, EuroQol five Dimensions‐three Levels. [Colour figure can be viewed at wileyonlinelibrary.com]
The baseline EORTC QLQ‐C30 global health status/QoL score in the triple‐class–refractory subgroup was 56·7, slightly lower than the overall group score of 58·6. The overall LS mean changes from baseline in EORTC QLQ‐C30 global health status/QoL, emotional functioning, physical functioning, and fatigue scores were relatively stable in the overall group and in the triple‐class–refractory subgroup (Fig 2A). Pain was also relatively stable across treatment in the overall group and the triple‐class–refractory subgroup. The MMRM LS mean change from baseline results was comparable to the univariate analyses by cycle. Overall, these data indicate that PRO measures were stable over time in patients treated with melflufen plus dexamethasone.
Fig 2.
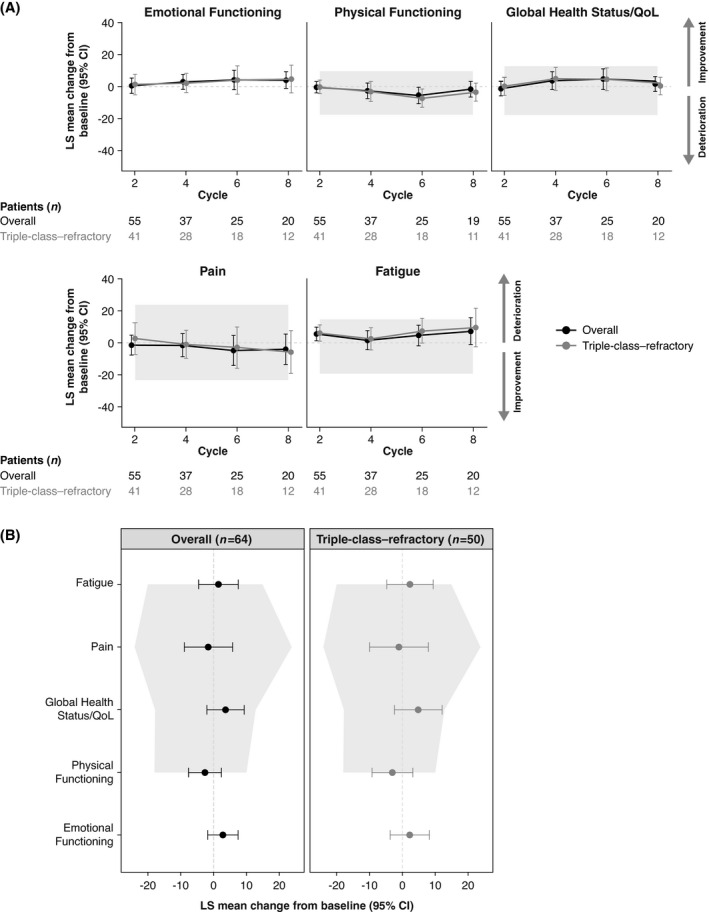
The LS mean change from baseline in EORTC QLQ‐C30. (A) LS mean change from baseline in EORTC QLQ‐C30 over treatment cycles in the overall group (black) and the triple‐class–refractory subgroup (grey). (B) Stability of scales assessed by change from baseline at cycle four. Higher scores represent improvement for global health status/QoL, emotional functioning, and physical functioning scales, and lower scores represent improvement for pain and fatigue symptom scales. Numbers underneath each plot represent non‐missing patients who completed the patient‐reported outcome measure in each respective cycle. Shaded area represents 95% confidence interval where no minimal important difference is observed. 17 CI, confidence interval; EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30; LS, least squares; QoL, quality of life.
A closer examination of LS mean change from baseline of the EORTC QLQ‐C30 scales at cycle four was performed to investigate the stability of PROs after patients had completed several cycles of treatment. In the overall group and the triple‐class–refractory subgroup, LS mean change from baseline scores across all scales were stable, and 95% CIs did not exceed previously established minimal important difference limits for MM (Fig 2B). 17 The univariate analysis for change from baseline at cycle four for patients who completed and tolerated three treatment cycles revealed similar results.
The proportion of patients with meaningful improvements in EORTC QLQ‐C30 was assessed using two relevant minimal important difference cut‐offs of 5–10 points for a small improvement 22 , 26 and ≥10 points for a medium‐to‐large improvement 19 , 21 from baseline through cycle eight. In the overall group, the total proportion of patients with improvements, as assessed by both cut‐offs, was relatively stable across treatment cycles for each scale (Fig 3A). Among those patients in the overall group who showed improvement, a majority improved by ≥10 points in the global health status/QoL scale in all treatment cycles, and by ≥10 points in the emotional functioning scale in cycles four to eight. The opposite trend was observed in the physical functioning scale, with a greater proportion of patients improving by a measure of 5–10 points in cycles four to eight (Fig 3A). The trend in the magnitude of improvements in global health status/QoL and emotional functioning scales in the triple‐class–refractory subgroup was similar to that observed in the overall population (Fig 3B).
Fig 3.
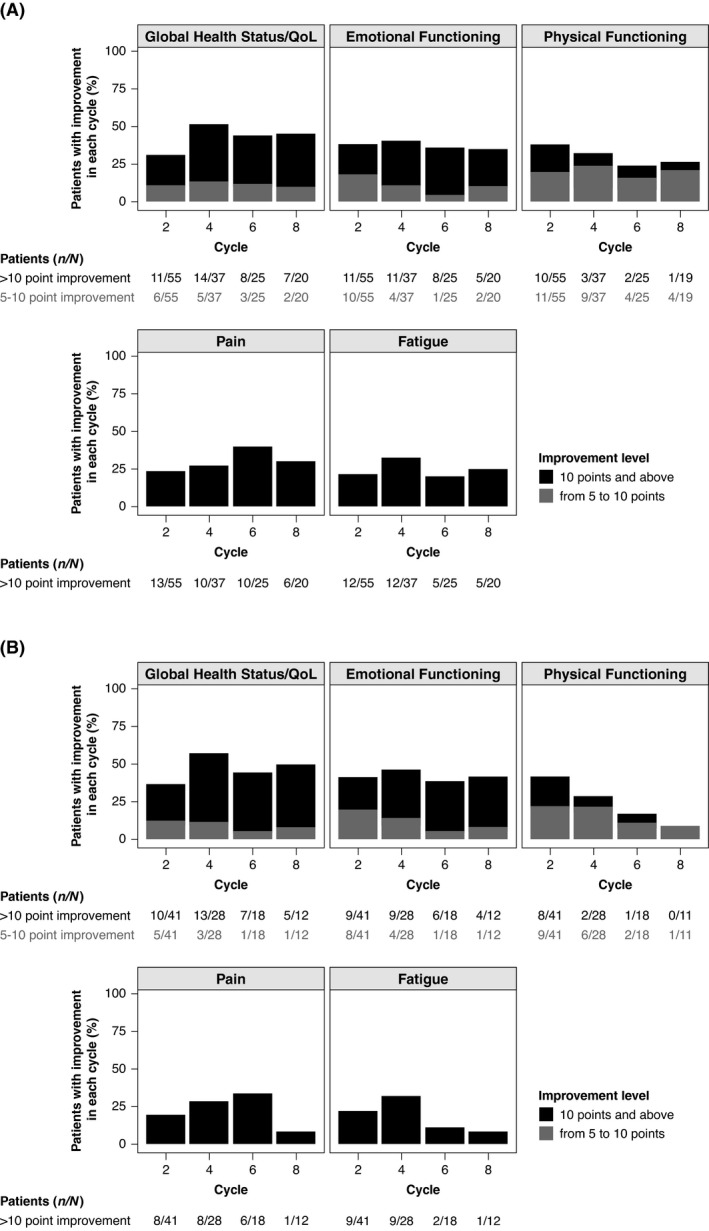
Proportion of patients with meaningful improvements from baseline in EORTC QLQ‐C30 scales across treatment cycles. Proportion of patients with improvements in EORTC QLQ‐C30 scales, assessed by minimal clinically important differences of 5–10 points (grey) or ≥10 points (black) in (A) the overall group and (B) the triple‐class–refractory subgroup. EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30; QoL, quality of life.
Improvements in pain and fatigue across both the overall group and the triple‐class–refractory subgroup were only detected at the medium to large cut‐off of ≥10 points. Improvements in pain and fatigue were observed across nearly all treatment cycles; however, there were few patients with these improvements in the triple‐class–refractory subgroup at cycle eight (Fig 3B). Together, these findings indicate that in patients who experienced improvements in PROs, these improvements were clinically meaningful over multiple cycles of treatment.
Time until definite deterioration analysis for EORTC QLQ‐C30 scales of focus
A time until definite deterioration analysis was performed to assess the time until definite deterioration in score using EORTC global health status/QoL and pain scales as measures (Fig 4). For global health status/QoL, patients who achieved a ≥MR had a median time until definite deterioration of 13·8 months compared with 2·9 months in patients who did not achieve an MR (Fig 4A). A similar trend was observed for the pain scale, with patients achieving a ≥MR having a median time until definite deterioration of 13·8 months compared with 3·1 months for patients who did not achieve an MR (Fig 4B). Similar results were observed for other EORTC QLQ‐C30 scales of focus and when evaluating patients who achieved a ≥PR compared with those who did not (Figure S1A, B; Table SII). When patients who did not achieve a ≥MR were separated into those with PD or SD/not evaluable, the median time until definite deterioration for global health status/QoL and pain were similar, but slightly worse for those with PD compared with SD/not evaluable (Figure S1C, D).
Fig 4.
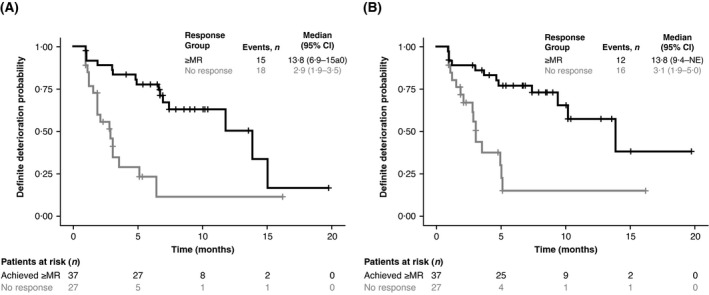
Time until definite deterioration analysis for EORTC QLQ‐C30 global health status/QoL and pain scales. Time until definite deterioration for patients who achieved an ≥MR versus non‐responders (i.e. patients with SD, not evaluable, or PD) for the EORTC QLQ‐C30 (A) global health status scale and (B) pain scale. Black, patients who achieved a ≥MR; grey, non‐responders (patients with SD, not evaluable, or PD). CI, confidence interval; EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30; MR, minimal response; NE, not evaluable; PD, progressive disease; SD, stable disease.
Discussion
Relapsed/refractory multiple myeloma is a late‐stage disease often associated with advanced patient age, frailty and an increased likelihood of comorbidities. 27 Patients with RRMM in HORIZON had similar baseline HRQoL as in other studies in RRMM, 18 , 26 , 28 , 29 , 30 , 31 , 32 with a mean baseline EQ VAS score of 61·4 in HORIZON, compared with 63·1 and 57·6 reported in USA 28 and European Union + Russia 29 daratumumab early access programme analyses, respectively. Patients with RRMM in HORIZON had poor baseline HRQoL relative to other advanced cancers, including cancers of the bladder, brain, breast, colon/rectum, head/neck, hepatobiliary tract/pancreas, kidney, lung, lymphoma, ovary or prostate, for which mean EQ VAS scores ranged from 61·8 to 72·0. 33 In addition to having the highest percentage of patients (79%) reporting pain/discomfort compared with other cancer types, 51% of patients in HORIZON reported mobility impairment; second only to those with prostate cancer (58%). 33 The finding that patients in HORIZON are representative of broader RRMM populations is supportive of the utility of the analyses in the present study, and the finding that RRMM has similar, and potentially higher, disease burden relative to other advanced cancers further supports the unmet needs for HRQoL and symptom management in patients with RRMM.
In HORIZON, the proportion of patients in the overall group with EQ‐5D‐3L impairments, the change from baseline in EORTC QLQ‐C30 function and symptom scales, and the percentage of patients with some improvement in EORTC QLQ‐C30 function and symptom scales were generally stable across treatment cycles. Previous examinations of improvements in EORTC QLQ‐C30 function and symptom scales have typically used one of two accepted cut‐offs, at ≥5 or ≥10 points of improvement from baseline. 20 , 21 , 22 , 26 Our analyses reveal that although patient improvements in later cycles may be smaller in magnitude (5–10 points), these improvements are still clinically meaningful. In addition, time until definite deterioration analyses indicate that PRO deterioration is delayed for a substantial amount of time in patients with RRMM who experience a response to melflufen plus dexamethasone treatment.
Patient‐reported outcome data in the literature are limited for patients with triple‐class–refractory disease in RRMM. Our present findings that patients with RRMM and triple‐class–refractory disease have slightly worse baseline PRO measures than the overall group are not surprising, given that this population has particularly poor prognoses among patients with RRMM, 34 , 35 representing an area of high unmet therapeutic need. Despite these prognoses, patients with triple‐class–refractory disease in HORIZON displayed relatively stable PRO measures over time. Our present finding that melflufen plus dexamethasone treatment can sustain HRQoL in this subgroup is encouraging and warrants further investigation in a larger population.
The present study has several limitations. Because PRO assessments were added as an addendum to protocol version 5, the populations in these analyses were relatively small. Given that 85% of all patients in HORIZON received study medication at cycle two, but 48% received study medication at cycle four, the subset included in the PRO analyses is inherently biased towards patients with better outcomes. Lastly, only patients with a baseline and a post‐baseline PRO assessment were included, given that analyses focussed on changes in PRO scores from baseline.
Collectively, these findings support the notion that treatment with melflufen plus dexamethasone may sustain or improve HRQoL over time in patients with RRMM, despite the high disease burden of this patient population as well as the associated morbidity and should help augment the successful translation of the clinical benefit seen in those patients participating in the HORIZON trial across to real‐world practice. 36
Author contributions
Alessandra Larocca, Xavier Leleu, Cyrille Touzeau, Joan Bladé, Agne Paner, María‐Victoria Mateos, Michele Cavo, Christopher Maisel, Adrían Alegre, Albert Oriol, Anastasios Raptis, Paula Rodriguez‐Otero, Amitabha Mazumder, Jacob Laubach, Omar Nadeem and Paul G. Richardson performed the research. María‐Victoria Mateos and Paul G. Richardson designed the research study; and Anna Sandberg, Marie Orre, Anna Torrång and Nicolaas A. Bakker performed PRO analyses and analysed the data. All authors had access to the data and all authors contributed to the writing and editing of the paper.
Conflict of interest
Alessandra Larocca reports honoraria and personal fees from Bristol Myers Squibb, Celgene, Janssen, and Takeda and personal fees from Amgen and GlaxoSmithKline. Cyrille Touzeau reports consultancy and honoraria from AbbVie, Amgen, Celgene, GlaxoSmithKline, Jannsen, Novartis, and Takeda, honoraria from Sanofi, and research support from AbbVie. Albert Oriol reports consultancy for Amgen, Celgene/Bristol Myers Squibb, Sanofi, GlaxoSmithKline and Janssen. Joan Bladé reports honoraria from Amgen, Celgene, Janssen, Oncopeptides, and Takeda. Agne Paner reports honoraria from Amgen and Celgene and consultancy/advisory fees from AbbVie, Cellectar, Janssen, Karyopharm, and Takeda. María‐Victoria Mateos received personal fees from AbbVie, Adaptive, Amgen, Celgene, GlaxoSmithKline, Janssen, Roche, Seattle Genetics, and Takeda. Michele Cavo received personal fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Sanofi, and Takeda. Christopher Maisel received honoraria from Amgen, Celgene, Incyte, Janssen, Karyopharm, Kite, Takeda, and Verastem and consulting fees from Bayer. Adrían Alegre received honoraria from Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Oncopeptides, Sanofi, and Takeda. Albert Oriol reports consultancy or advisory role and speakers’ bureau for Bristol Myers Squibb, Sanofi, and Amgen. Paula Rodriguez‐Otero reports honoraria from AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen, Kite, Oncopeptides, and Sanofi. Omar Nadeem reports personal fees from Amgen, Celgene, Janssen, Sanofi, and Takeda. Xavier Leleu, Anastasios Raptis, Amitabha Mazumder, and Jacob Laubach have nothing to disclose. Anna Sandberg, Marie Orre, and Nicolaas A. Bakker are employees of and own stock and stock options in Oncopeptides. Anna Torrång is a consultant at Oncopeptides. Paul G. Richardson received grant support from Bristol Myers Squibb, grant support and honoraria from Celgene, Karyopharm, Oncopeptides, and Takeda, and honoraria from Janssen, Sanofi, and Secura Bio.
Funding
This study was sponsored by Oncopeptides AB, which also provided support for manuscript editorial assistance.
Supporting information
Table SI. Completion rates per cycle for patient‐reported outcome measures.
Table SII. Median and confidence limits for time until definite deterioration analysis of scores of focus.
Fig S1. Time until definite deterioration analysis of patients who achieved a response versus those who did not achieve an MR or PR. Time until definite deterioration for patients who achieved a ≥PR (black) versus non‐responders (i.e. patients with SD, PD, or not evaluable; grey) for the EORTC‐QLQ‐C30 (A) global health status/QoL scale and (B) pain scale. Time until definite deterioration for patients who achieved an ≥MR (black) versus non‐responders, with patients with SD or not evaluable grouped (grey) separately from patients with PD (teal) for the EORTC QLQ‐C30 (C) global health status scale and (D) pain scale.
Acknowledgements
This work is supported by Oncopeptides AB. Medical writing support was provided Elizabeth G. Wheatley, PhD, and Katherine Mills‐Lujan, PhD, CMPP, of Team 9 Science, with funding from Oncopeptides AB.
References
- 1. Laubach J, Richardson P, Anderson K. Multiple myeloma. Annu Rev Med. 2011;62:249–64. [DOI] [PubMed] [Google Scholar]
- 2. Thorsteinsdottir S, Dickman PW, Landgren O, Blimark C, Hultcrantz M, Turesson I, et al. Dramatically improved survival in multiple myeloma patients in the recent decade: results from a Swedish population‐based study. Haematologica. 2018;103: e412–e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to CD38‐targeted monoclonal antibody therapy. Leukemia. 2019;33:2266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vogl DT, Delforge M, Song K, Guo S, Gibson CJ, Ervin‐Haynes A, et al. Long‐term health‐related quality of life in transplant‐ineligible patients with newly diagnosed multiple myeloma receiving lenalidomide and dexamethasone. Leuk Lymphoma. 2018;59:398–405. [DOI] [PubMed] [Google Scholar]
- 5. LeBlanc MR, Hirschey R, Leak Bryant A, LeBlanc TW, Smith SK. How are patient‐reported outcomes and symptoms being measured in adults with relapsed/refractory multiple myeloma? A systematic review. Qual Life Res. 2020;29:1419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosinol L, Ocio EM, Grande M, Fernandez‐Nistal A, Ruiz‐Zorrilla A, Montoto C. Impact of patients characteristics on health‐related quality of life in patients with relapsed or refractory multiple myeloma: results from the Charisma study. Poster presented at the 24th European Hematology Association (EHA) Annual Congress; June 13–16, 2019; Amsterdam, The Netherlands; Poster PF615.
- 7. Jordan K, Proskorovsky I, Lewis P, Ishak J, Payne K, Lordan N, et al. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health‐related quality of life in patients with multiple myeloma: results of a European, multicenter cohort study. Support Care Cancer. 2014;22:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chauhan D, Ray A, Viktorsson K, Spira J, Paba‐Prada C, Munshi N, et al. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan‐flufenamide, against multiple myeloma cells. Clin Cancer Res. 2013;19:3019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ray A, Ravillah D, Das DS, Song Y, Nordström E, Gullbo J, et al. A novel alkylating agent melflufen induces irreversible DNA damage and cytotoxicity in multiple myeloma cells. Br J Haematol. 2016;174:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gullbo J, Wickstrom M, Tullberg M, Ehrsson H, Lewensohn R, Nygren P, et al. Activity of hydrolytic enzymes in tumour cells is a determinant for anti‐tumour efficacy of the melphalan containing prodrug J1. J Drug Target. 2003;11:355–63. [DOI] [PubMed] [Google Scholar]
- 11. Wickström M, Haglund C, Lindman H, Nygren P, Larsson R, Gullbo J. The novel alkylating prodrug J1: diagnosis directed activity profile ex vivo and combination analyses in vitro. Invest New Drugs. 2008;26:195–204. [DOI] [PubMed] [Google Scholar]
- 12. Wickström M, Nygren P, Larsson R, Harmenberg J, Lindberg J, Sjöberg P, et al. Melflufen ‐ a peptidase‐potentiated alkylating agent in clinical trials. Oncotarget. 2017;8:66641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. PEPAXTO® (melphalan flufenamide) [package insert]. Waltham, MA: Oncopeptides Inc; 2021. [Google Scholar]
- 14. Richardson PG, Oriol A, Larocca A, Blade J, Cavo M, Rodriguez‐Otero P, et al. Melflufen and dexamethasone in heavily pretreated relapsed and refractory multiple myeloma. J Clin Oncol. 2021;39:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Reenen M, Oppe M, Secnik Boye K, Herdman M, Kennedy‐Martin M, Kennedy‐Martin T, et al. EuroQol Research Foundation. EQ‐5D‐3L User Guide; 2018. Available from: https://euroqol.org/publications/user‐guides.
- 16. Fayers P, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. On behalf of the EORTC Quality of Life Group. EORTC QLQ‐C30 scoring manual. 3rd edn. Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 17. Kvam AK, Fayers P, Wisloff F. What changes in health‐related quality of life matter to multiple myeloma patients? A prospective study. Eur J Haematol. 2010;84:345–53. [DOI] [PubMed] [Google Scholar]
- 18. Stewart AK, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, et al. Health‐related quality‐of‐life results from the open‐label, randomized, phase III ASPIRE trial evaluating carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in patients with relapsed multiple myeloma. J Clin Oncol. 2016;34:3921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Royle K‐L, Gregory WM, Cairns DA, Bell SE, Cook G, Owen RG, et al. Quality of life during and following sequential treatment of previously untreated patients with multiple myeloma: findings of the Medical Research Council Myeloma IX randomised study. Br J Haematol. 2018;182:816–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. J Clin Oncol. 1998;16:139–44. [DOI] [PubMed] [Google Scholar]
- 21. Ludwig H, Pönisch W, Knop S, Egle A, Hinke A, Schreder M, et al. Quality of life in patients with relapsed/refractory multiple myeloma during ixazomib‐thalidomide‐dexamethasone induction and ixazomib maintenance therapy and comparison to the general population. Leuk Lymphoma. 2020;61:377–86. [DOI] [PubMed] [Google Scholar]
- 22. Ludwig H, Moreau P, Dimopoulos MA, Mateos M‐V, Kaiser M, Hajek R, et al. Health‐related quality of life in the ENDEAVOR study: carfilzomib‐dexamethasone vs bortezomib‐dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J. 2019;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balakrishnan N, Rao CR. Advances in survival analysis, Volume 23, 1st ed. (Handbook of Statistics series). Amsterdam, The Netherlands: Elsevier; 2004. [Google Scholar]
- 24. Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V‐325 Study Group. J Clin Oncol. 2007;25:3210–6. [DOI] [PubMed] [Google Scholar]
- 25. Anota A, Hamidou Z, Paget‐Bailly S, Chibaudel B, Bascoul‐Mollevi C, Auquier P, et al. Time to health‐related quality of life score deterioration as a modality of longitudinal analysis for health‐related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52. [DOI] [PubMed] [Google Scholar]
- 27. Hari P, Romanus D, Luptakova K, Blazer M, Yong C, Raju A, et al. The impact of age and comorbidities on practice patterns and outcomes in patients with relapsed/refractory multiple myeloma in the era of novel therapies. J Geriatr Oncol. 2018;9:138–44. [DOI] [PubMed] [Google Scholar]
- 28. Chari A, Mark TM, Krishnan A, Stockerl‐Goldstein K, Usmani SZ, Londhe A, et al. Results of an early access treatment protocol (EAP) of daratumumab in United States patients with relapsed or refractory multiple myeloma. Blood. 2016;128:Abstract 2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cook G, Corso A, Streetly M, Mendeleeva LP, Ptushkin V, Couturier C, et al. Results of the daratumumab monotherapy early access treatment protocol (EAP) in patients from Europe and Russia with relapsed or refractory multiple myeloma. 24th EHA Abstract Book. 2019:PS1425.
- 30. Leleu X, Kyriakou C, Vande Broek I, Murphy P, Bacon P, Lewis P, et al. Prospective longitudinal study on quality of life in relapsed/refractory multiple myeloma patients receiving second‐ or third‐line lenalidomide or bortezomib treatment. Blood Cancer J. 2017;7:e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richardson PG, Schlossman RL, Roy AN, Panneerselvam A, Acharyya S, Sopala M, et al. Patient‐reported outcomes of multiple myeloma patients treated with panobinostat after ≥2 lines of therapy based on the international phase 3, randomized, double‐blind, placebo‐controlled PANORAMA‐1 trial. Br J Haematol. 2018;181:628–36. [DOI] [PubMed] [Google Scholar]
- 32. Robinson D Jr, Esseltine DL, Regnault A, Meunier J, Liu K, van de Velde H. The influence of baseline characteristics and disease stage on health‐related quality of life in multiple myeloma: findings from six randomized controlled trials. Br J Haematol. 2016;174:368–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pickard AS, Jiang R, Lin HW, Rosenbloom S, Cella D. Using patient‐reported outcomes to compare relative burden of cancer: EQ‐5D and functional assessment of cancer therapy‐general in eleven types of cancer. Clin Ther. 2016;38:769–77. [DOI] [PubMed] [Google Scholar]
- 34. Usmani S, Ahmadi T, Ng Y, Lam A, Desai A, Potluri R, et al. Analysis of real‐world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016;21:1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar SK, Dimopoulos MA, Kastritis E, Terpos E, Nahi H, Goldschmidt H, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31:2443–8. [DOI] [PubMed] [Google Scholar]
- 36. Richardson PG, San Miguel JF, Moreau P, Hajek R, Dimopoulos MA, Laubach JP, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real‐world setting. Blood Cancer J. 2018;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Completion rates per cycle for patient‐reported outcome measures.
Table SII. Median and confidence limits for time until definite deterioration analysis of scores of focus.
Fig S1. Time until definite deterioration analysis of patients who achieved a response versus those who did not achieve an MR or PR. Time until definite deterioration for patients who achieved a ≥PR (black) versus non‐responders (i.e. patients with SD, PD, or not evaluable; grey) for the EORTC‐QLQ‐C30 (A) global health status/QoL scale and (B) pain scale. Time until definite deterioration for patients who achieved an ≥MR (black) versus non‐responders, with patients with SD or not evaluable grouped (grey) separately from patients with PD (teal) for the EORTC QLQ‐C30 (C) global health status scale and (D) pain scale.


