Abstract
Anaplastic large cell lymphoma (ALCL) is an aggressive type of non-Hodgkin’s lymphoma. More than three-fourths of anaplastic lymphoma kinase (ALK)-positive ALCL cases express the (nucleophosmin 1) NPM1-ALK fusion gene as a result of t(2;5) chromosomal translocation. The homodimerization of NPM1-ALK fusion protein mediates constitutive activation of the chimeric tyrosine kinase activity and downstream signaling pathways responsible for lymphoma cell proliferation and survival. Gilteritinib is a tyrosine kinase inhibitor recently FDA approved for the treatment of FMS-like tyrosine kinase mutation-positive acute myeloid leukemia. In this study, we demonstrate for the first time gilteritinib mediated growth inhibitory effects on NPM1-ALK driven ALCL cells. We utilized a total of five ALCL model cell lines, including both human and murine. Gilteritinib treatment inhibits NPM1-ALK fusion kinase phosphorylation and downstream signaling, resulting in induced apoptosis. Gilteritinib mediated apoptosis was associated with caspase 3/9, PARP cleavage, the increased expression of pro-apoptotic protein BAD, and decreased expression of anti-apoptotic proteins survivin and MCL-1. We also found downregulation of fusion kinase activity resulted in decreased c-Myc protein levels. Furthermore, cell cycle analysis indicates gilteritinib induced G0/G1 cell cycle arrest and reduced CD30 expression. In summary, our preclinical studies explored the novel therapeutic potential of gilteritinib in the treatment of ALCL cells expressing NPM1-ALK and potentially in other ALK or ALK-fusion driven hematologic or solid malignancies.
Keywords: NPM1-ALK, ALCL, Survival signaling, Gilteritinib, Cell cycle, Apoptosis
1. Introduction
Anaplastic large cell lymphoma (ALCL) is a clinically aggressive and pathologically distinct T-cell lymphoma with characteristic surface expression of CD30 (1). ALCL accounts for 5–10% of adult and 10–30% of pediatric and adolescent non-Hodgkin lymphoma (NHL) (2). The majority of systemic ALCLs express anaplastic lymphoma kinase (ALK), a gene encoding for a receptor tyrosine kinase, which is preferentially expressed in neural cells during the late embryonic stages (3). ALK chromosomal rearrangements are found in many different cancers. The most common ALK translocation, NPM1-ALK t(2;5)(p23;q35) is observed in more than 80% of ALK+ ALCL cases. The generated NPM1-ALK chimeric protein consists of the N-terminal oligomerization domain of NPM1 (1–117 amino acids) and the C-terminal kinase domain of ALK (1058–1620 amino acids) (4). In NPM1-ALK-positive ALCLs, the homodimerization of NPM1-ALK fusion protein catalyzes the autophosphorylation of its tyrosine kinase receptor constitutively in a ligand-independent manner (5). The NPM1-ALK fusion protein has been detected in abundance in the nucleus and cytoplasm, where it triggers downstream signaling pathways, such as STAT3, ERK, and AKT (6). Activation of these downstream effector molecules results in the inhibition of apoptosis, promotion of cell growth, and proliferation of ALCL cells.
The current standard therapy for ALK+ALCL is a combination chemotherapy using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (7). However, a significant number of patients develop refractory/relapsed disease after CHOP therapy. In the relapsed/refractory setting, patients are left with no effective treatment options, therefore an alternative therapeutic approach is warranted to better address the needs of the ALK+ ALCL population.
The recent expansion of knowledge on the understanding of molecular mechanisms of malignant neoplasms has aided in the development of effective drugs for the treatment of various cancers. One such example is the development of gilteritinib (Xospata), an oral, potent fms-like tyrosine kinase 3 (FLT3) inhibitor used to treat FLT3 mutation-positive relapsed/refractory acute myeloid leukemia (AML) (8,9). The results from recent clinical trial studies using gilteritinib as a single agent show improved survival of the patients with relapsed/refractory FLT3-mutated AML compared to standard therapy (10). In late 2018, the Food and Drug Administration (FDA) approved gilteritinib for the treatment of adult patients with relapsed or refractory FLT3-mutated AML (11). The kinase inhibitory activity assay results showed that gilteritinib also has binding activity with AXL and ALK (8).
In the present study, we report for the first time the growth inhibitory potential of gilteritinib in NPM1-ALK-positive ALCL preclinical models, including both human NPM1-ALK endogenously expressing cell lines (SR-786, DEL, SUP-M2, SU-DHL-1) and NPM1-ALK-transformed Ba/F3 murine cell line. We explored gilteritinib mediated inhibition of NPM1-ALK phosphorylation, downstream signaling, induction of apoptosis, cell cycle arrest, decreased cell proliferation, and reduced CD30 expression.
2. Methods
2.1. Cell culture:
SR-786, DEL, SUP-M2, and Ba/F3 cell lines were obtained from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany), and SU-DHL-1 cell line was obtained from ATCC (American Type Culture Collection, Manassas, VA, USA). All cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (Thermo Scientific, Waltham, MA, USA), and maintained at 37°C in a humidified incubator under 5% CO2. Cell lines were cultured up to 20 passages in a span of 8–12 weeks and regularly tested for mycoplasma contaminations using the Lonza MycoAlert kit.
2.2. Chemicals and Reagents:
Reagents and antibodies were purchased accordingly: Thermo Scientific, Waltham, MA, USA (RPMI-1640-SH30027), Corning Life Sciences, USA (Penicillin/Streptomycin-30–002-CI); InvivoGen, San Diego, CA, USA (Puromycin-58–58-2); STEMCELL Technologies, Vancouver, BC, Canada (MethoCult-H4100); Molecular Probes, Eugene, OR, USA (TO-PRO-3). MedKoo Biosciences, Morrisville, NC, USA (Gilteritinib-206139); Cell Signaling Technology, Beverly, MA, USA (NPM1-ALK-3333, phospho-NPM1-ALK-9687, phospho-STAT3–9145, phospho-AKT-4060, AKT-2920, ERK1/2–4695, Survivin-2802, c-Myc-5605); BD Biosciences San Jose, CA, USA (β-Actin-612656, STAT3–610189, phospho-ERK1/2–612358, PARP-556949, Caspase-9–551246, Caspase-3–610322, BAD-610391, FITC-CD30 (555829), FITC-IgG1κ isotype control-556649, FITC-Annexin V-556419); Sigma-Aldrich, St. Louis, MO, USA (FLAG-F3165)
2.3. Generation of transformed Ba/F3-FG-NPM1-ALK cell line:
As described in our previous study (12), full-length human NPM1-ALK cDNA (a gift from Dr. Toshiki Watanabe, The University of Tokyo, Japan) was amplified by PCR. The PCR product was then subcloned between XbaI and NheI restriction sites into the lentiviral plasmid pCDH-EF1-MCS-T2A-Puro vector (purchased from System Biosciences, Palo Alto, CA, USA). The clones were confirmed through Sanger DNA sequencing. The lentiviral plasmids, either empty vector pCDH-EF1-MCS-T2A-Puro or recombinant plasmid with FLAG (FG) tag, pCDH-EF1-FG-NPM1-ALK were transfected along with packaging and envelop plasmids psPAX2 and pMD2.G into HEK293T cells. The empty vector or pCDH-EF1-FG-NPM1-ALK lentiviral particles were transduced into Ba/F3 cells and stable clones were selected with puromycin. The Ba/F3-pCDH-vector cell growth was IL3-dependent while the transformed Ba/F3-FG-NPM1-ALK exhibited IL3-independent growth. The NPM1-ALK fusion gene mRNA expression levels were confirmed by RT-qPCR and protein levels were determined by Western blot using FLAG and NPM1-ALK antibodies.
2.4. Flow Cytometry:
Apoptosis was analyzed using FITC-Annexin V and TO-PRO-3 staining, followed by flow cytometry as described (13). Briefly, cell lines were treated with gilteritinib concentrations ranging from 5 – 20 nM. After a 48-hour incubation period, the control and treated cells were harvested, washed with PBS, and stained with both FITC-Annexin V and TO-PRO-3 in an Annexin V binding buffer solution for 15 minutes at room temperature. The percentage of apoptotic cells was measured by the BD Accuri C6 Plus flow cytometer (BD Biosciences San Jose, CA, USA).
2.5. Western blot analysis:
Control and treated cells were harvested after 24 hours of the incubation period. Cells were washed with PBS, lysed with lysis buffer (25mM Tris. HCl, 150mM NaCl, 25mM NaF, 0.5mM Na-orthovanadate, 1% Triton-X, 1mM Benzamidine) along with protease and phosphatase inhibitors. Each sample was incubated on ice for 20 minutes, followed by centrifugation at 15,000 RPM for 15 minutes at 4°C. Protein concentrations were estimated using the BCA protein assay kit (Pierce Biotechnology, Rockford, IL USA). Proteins were resolved using SDS-PAGE and then transferred onto a PVDF membrane. Immunoblotting was then carried out with respective antibodies, and blots were analyzed with the Odyssey IR scanner (LI-COR Biosciences, Lincoln, NE, USA).
2.6. Clonogenic cell survival assay:
DEL and SU-DHL-1 cell lines were used to investigate clonogenic survival assay. Cells were treated with 5 – 20 nM of gilteritinib for 24 hours, then each cell line was washed with PBS, diluted in MethoCult medium, and plated in triplicates. The cells were allowed to propagate into colonies. After eight days of incubation, the colonies were counted, and the viability was expressed as a percentage compared to the control.
2.7. Cell Cycle Analysis:
The effect of gilteritinib on the cell cycle was determined utilizing SU-DHL-1 and DEL cell lines. The cells were treated with gilteritinib concentrations ranging from 5 – 20 nM. After 24 hours of incubation, cells were washed with PBS and fixed with 70% ethanol. After fixation, the cells were washed with PBS, and the DNA was stained with propidium iodide for 15 minutes at room temperature in the dark. Cell-cycle analysis was performed using BD Accuri C6 Plus flow cytometer (BD Biosciences San Jose, CA, USA).
2.8. Analysis of CD30 expression:
SU-DHL-1 cells were treated with 5 – 20 nM of gilteritinib and incubated for 24 hours. The cells were harvested, washed with PBS, and incubated on ice for 1 hour in the dark with either FITC-conjugated CD30 or FITC-IgG1κ isotype control antibodies, diluted in 0.2% BSA/PBS. After the incubation period, cells were washed with 0.2% BSA/PBS, the pellet was resuspended in 200 μl of 0.2% BSA/PBS, and then analyzed using BD Accuri C6 Plus flow cytometer (BD Biosciences San Jose, CA, USA).
2.9. Statistical analyses:
Differences between control and drug-treated samples were assessed by paired two-tailed Students t-test using Microsoft Excel. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Gilteritinib inhibits activation of NPM1-ALK fusion kinase and downstream signaling
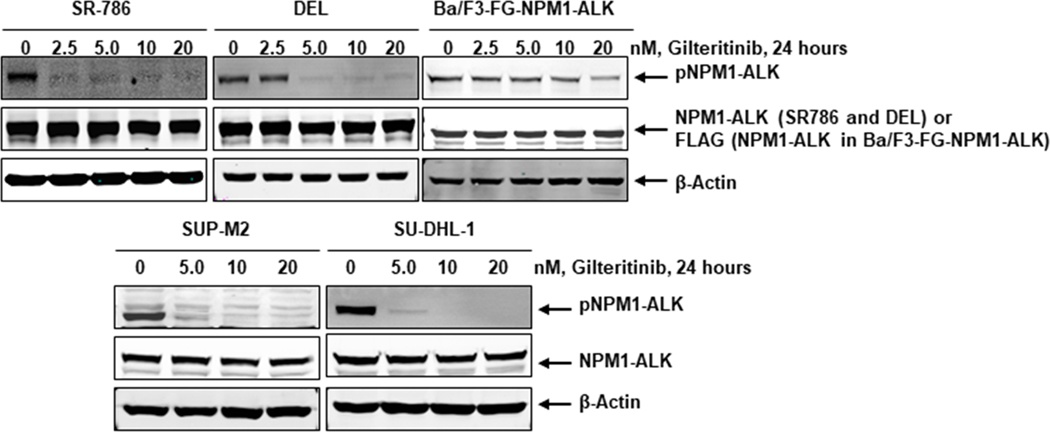
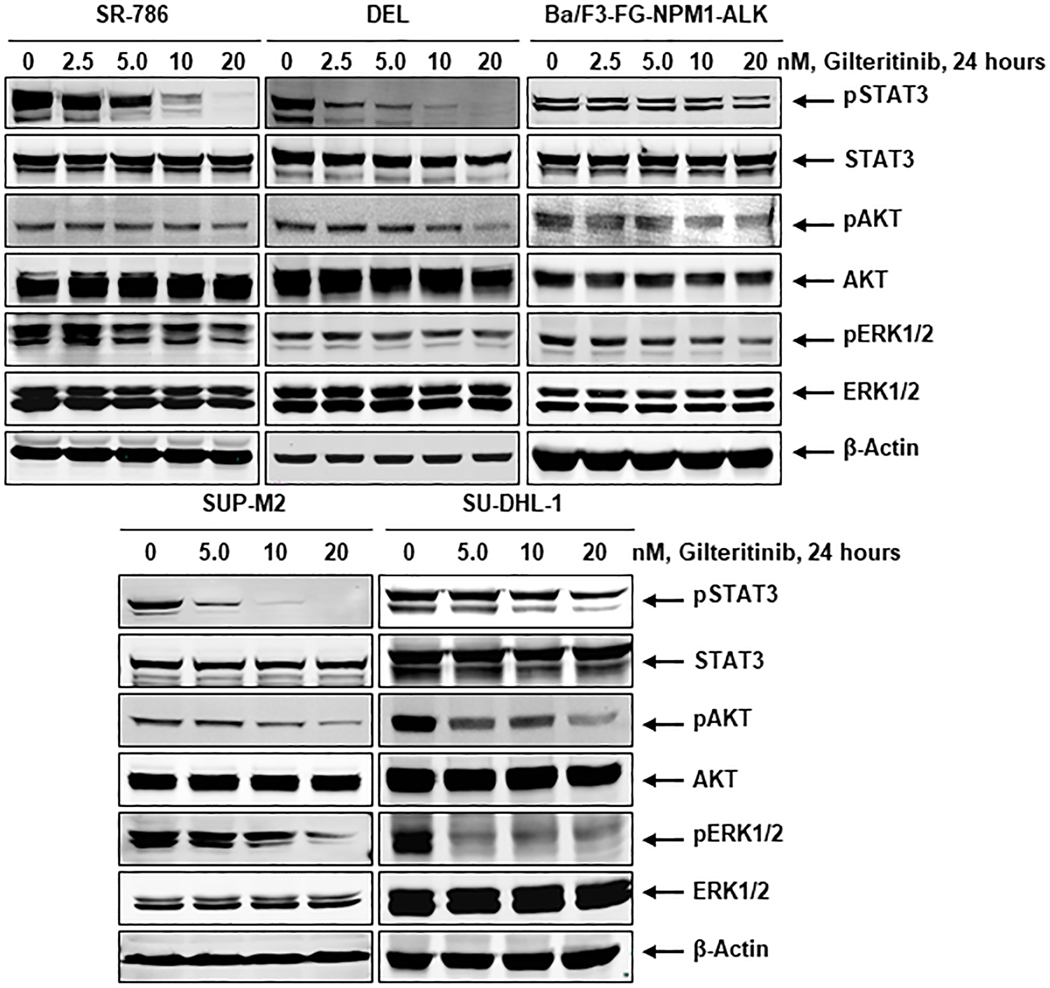
Chromosomal abnormalities, particularly chromosomal translocations, are strongly associated with several subtypes of leukemias and lymphomas (14). NPM1-ALK, a well-characterized fusion kinase, results in the development of the ALCL cells through the activation of a downstream survival signaling cascade (15). Constitutive activation of NPM1-ALK is vital for the survival of NPM1-ALK driven ALCL cells. The homodimerization of NPM1-ALK constantly phosphorylates the chimeric protein in a ligand-independent manner. The phosphorylation of NPM1-ALK results in continuous activation of the downstream pro-survival signaling cascade, including STAT3, AKT, and ERK1/2. Therefore, the inhibition of fusion kinase activity is a critical step for the induction of apoptosis in NPM1-ALK driven ALCL cells. To evaluate the effect of gilteritinib on fusion kinase activity, we used NPM1-ALK endogenously expressing human cell lines, SR-786, SU-DHL-1, DEL, SUP-M2, and genetically engineered Ba/F3-FG-NPM1-ALK murine cell line. All cell lines were treated with gilteritinib concentrations ranging from 2.5 – 20 nM for 24 hours. The Western blot analysis data after gilteritinib treatment clearly demonstrates that the fusion kinase NPM1-ALK phosphorylation was downregulated in a dose-dependent manner in both NPM1-ALK endogenously and ectopically expressing cell lines, as shown in Fig. 1. We hypothesized that the inhibition of NPM1-ALK phosphorylation by gilteritinib would presumably cause the downregulation of downstream signaling. Indeed, we observed that gilteritinib mediated downregulation of NPM1-ALK phosphorylation resulted in decreased levels of phosphorylated STAT3, AKT, and ERK1/2, while the total levels of these proteins remained unaffected (Fig. 2). Overall, these data suggest that gilteritinib is a potent inhibitor of the fusion kinase NPM1-ALK and its downstream signaling pathways.
Figure 1: Gilteritinib treatment inhibits activation of NPM1-ALK fusion kinase.
NPM1-ALK expressing SR-786, DEL, SUP-M2, SU-DHL-1, and Ba/F3-FG-NPM1-ALK cells were treated with indicated concentrations of gilteritinib for 24 hours. At the end of the treatment period, cell lysates were made, and Western blot analyses were performed for phospho-NPM1-ALK and total NPM1-ALK proteins. β-actin served as the loading control.
Figure 2: Inhibition of NPM1-ALK activation leads to inhibition of downstream survival signaling cascade.
NPM1-ALK expressing ALCL cell lines, SR-786, DEL, SUP-M2, SU-DHL-1, and Ba/F3-FG-NPM1-ALK were treated with the indicated concentrations of gilteritinib and Western blot analyses were performed for downstream effector molecules phospho-STAT3, phospho-AKT, phospho-ERK1/2 along with total proteins. β-actin served as the loading control.
3.2. Inhibition of NPM1-ALK mediated signaling by gilteritinib induces apoptosis
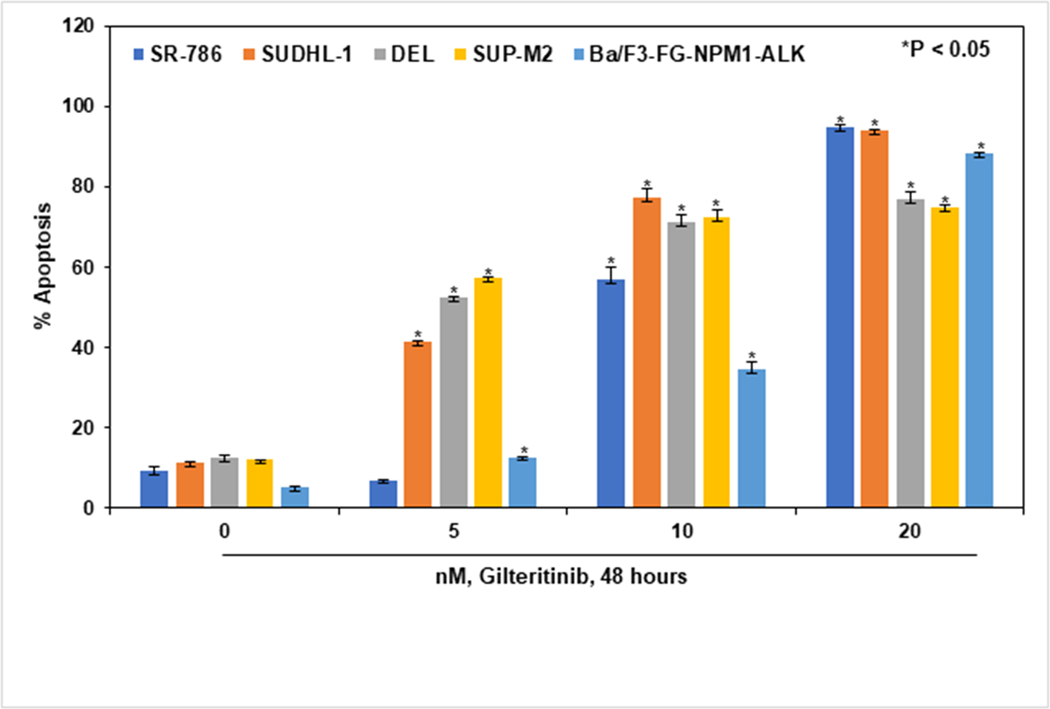
As a result of dysregulated signaling pathways, cancer cells are able to evade apoptosis via constitutively activated fusion kinases (16). We hypothesized that gilteritinib mediated inhibition of NPM1-ALK and its downstream signaling would result in the induction of apoptosis in these ALCL cells. We evaluated the effect of gilteritinib on ALCL cell lines, both NPM1-ALK endogenously expressing SR-786, SU-DHL-1, DEL, SUP-M2, and ectopically overexpressing Ba/F3-FG-NPM1-ALK. All ALCL cell lines were treated with gilteritinib concentrations ranging from 5 – 20 nM and incubated for 48 hours. The apoptosis in treated and untreated groups was measured using Annexin-V and TO-PRO-3 staining by flow cytometry. All tested NPM1-ALK fusion gene expressing ALCL cell lines were sensitive to gilteritinib and showed induction of apoptosis in a dose-dependent manner compared to controls (Fig. 3). Significant induction of apoptosis was observed at 5, 10, and 20 nM and 10, 20 nM concentrations of gilteritinib in SU-DHL-1, DEL, SUP-M2, and SR,786, Ba/F3-FG-NPM1-ALK, respectively. Our apoptosis data clearly demonstrate significant growth inhibitory effects of gilteritinib on both NPM1-ALK endogenously and ectopically overexpressed cell lines.
Figure 3: Gilteritinib induces apoptosis in ALCL cells.
NPM1-ALK expressing SR-786, SU-DHL-1, DEL, SUP-M2, and Ba/F3-FG-NPM1-ALK cell lines were treated with the indicated concentrations of gilteritinib for 48 hours. After treatment, cells were harvested and stained with FITC-annexin V and TO-PRO-3, and the percentages of apoptotic cells were determined by flow cytometry. Columns represent the mean of three independent experiments; Bars represent the standard error of the mean (SEM).
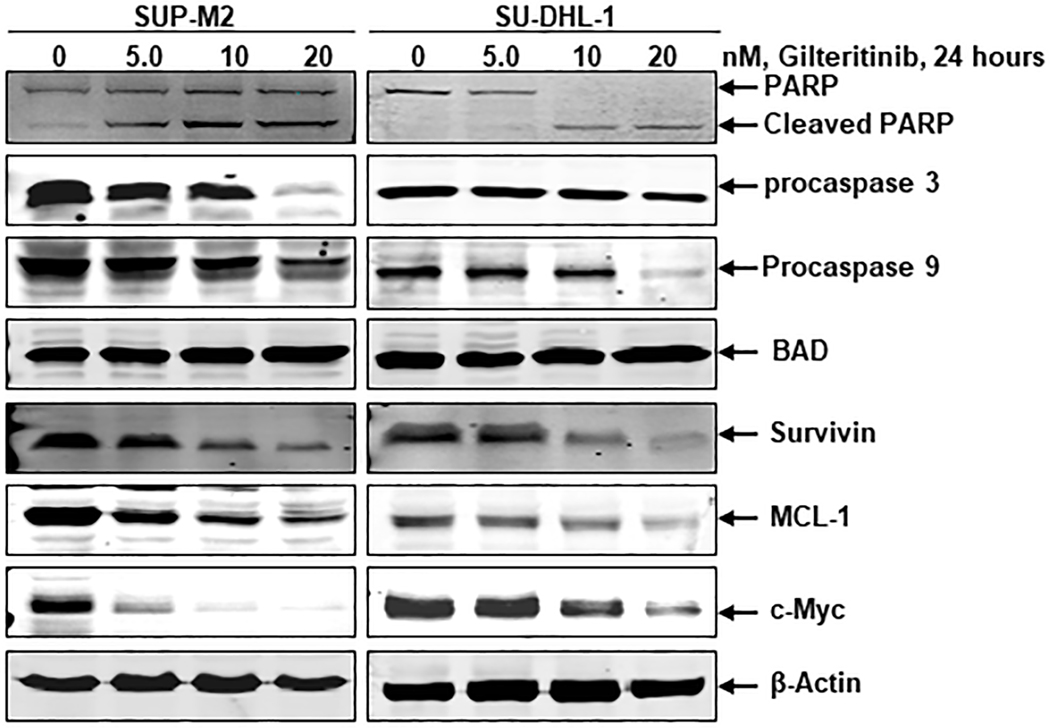
Next, we examined the effects of gilteritinib on the modulation of apoptotic regulatory proteins. Apoptosis is regulated by a balance of pro-apoptotic and anti-apoptotic proteins, while an imbalance between these two groups of BCL-2 family proteins results in tumor initiation (17). PARP is a nuclear poly (ADP-ribose) polymerase that catalyzes poly(ADP-ribosyl)ation of proteins involved in DNA repair. Caspase mediated proteolytic cleavage of PARP is a hallmark of apoptosis. To further support our data, the SUP-M2 and SU-DHL-1 cells were treated with gilteritinib concentrations ranging from 5 – 20 nM for a duration of 24 hours. Immunoblot analysis was performed to detect both pro- and anti-apoptotic proteins, activated caspases, and cleavage of PARP. Gilteritinib treatment in these ALCL cell lines induced PARP cleavage and downregulated procaspase levels of both apoptosis initiator caspase 9 and executioner caspase 3 (Fig. 4). Our results further indicate upregulation of pro-apoptotic protein BAD and downregulation of anti-apoptotic proteins survivin and MCL-1 in a dose-dependent manner (Fig. 4). In pediatric ALCL, activation of NPM1-ALK fusion protein induces c-Myc expression, which is involved in cell proliferation and transformation (18). Therefore, we also examined the effect of gilteritinib on c-Myc expression in these ALCL cells, which showed that the inhibition of NPM1-ALK fusion kinase activity by gilteritinib downregulated c-Myc expression dose-dependently (Fig. 4). All the evaluated apoptotic regulatory proteins were altered by inhibition of NPM1-ALK mediated survival signaling. This collection of data clearly indicate that gilteritinb induces apoptosis in NPM1-ALK driven ALCL cells.
Figure 4: Effect of gilteritinib on apoptotic-related proteins.
SUP-M2 and SU-DHL-1 cells were treated with the indicated concentrations of gilteritinib for 24 hours. At the end of the treatment, cell lysates were prepared, and Western blot analyses were performed for cleaved PARP, caspases 3, 9, BAD, Survivin, MCL1, and c-Myc. β-Actin served as the loading control.
3.3. Gilteritinib induces G0/G1 cell cycle arrest
The proportion of actively growing or dividing cells is higher in tumors than in normal tissue. This is often a result of dysfunction of cell cycle regulatory proteins, which in healthy tissues, play a vital role in preventing the uncontrolled division of cells. Proteins such as cyclins and cyclin-dependent kinases regulate the progress of a developing cell through the cell cycle. These regulatory proteins are often dysregulated in human cancers leading to neoplastic changes and terminal malignancy.
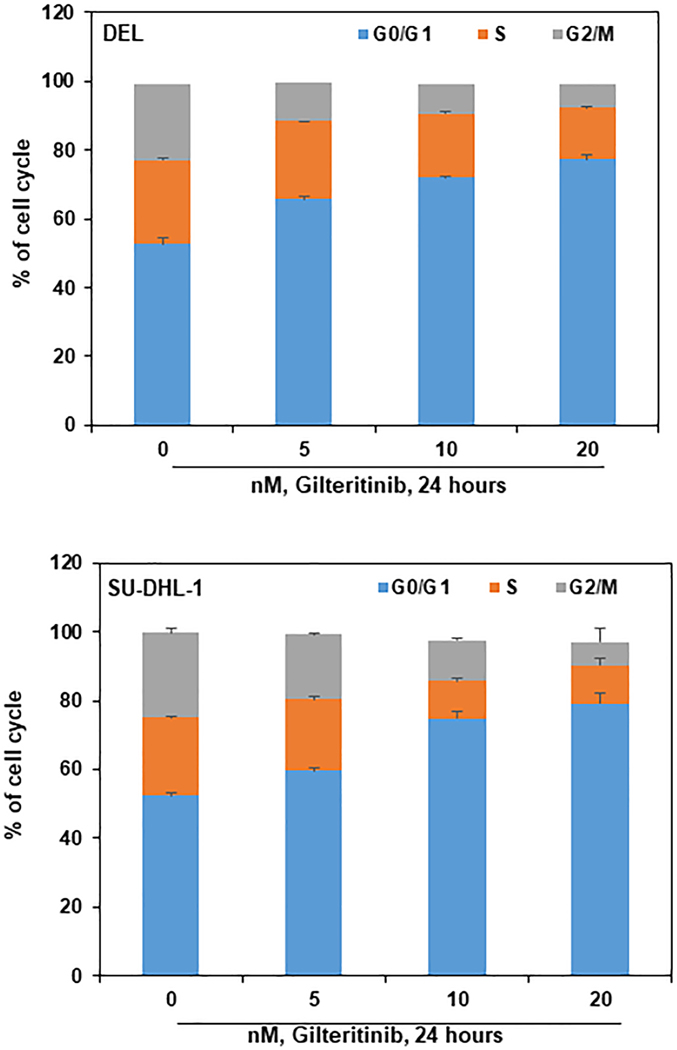
In order to understand the effect of gilteritinib treatment on cell cycle progression in ALCL cells, DEL and SU-DHL-1 were treated with gilteritinib concentrations ranging from 5 – 20 nM for 24 hours. The cells were harvested, fixed, and stained with propidium iodide, and the cell cycle status was analyzed by flow cytometry. Results showed that gilteritinib interferes at the G0/G1 phase of the cell cycle. As shown in Fig. 5, there is a significant increase in the percentage of cells in G0/G1 (24.6 and 26.8) phase, with a concomitant decrease in S (9.45 and 11.8), and G2/M (15.18 and 17.75) phases of the cell cycle compared to control of DEL and SU-DHL-1 cells, respectively. These results suggest that gilteritinib treatment inhibits cellular DNA synthesis leading to cell-cycle arrest at the G0/G1 phase with the reduction in S and G2/M phases.
Figure 5: Gilteritinib induces G0/G1 cell cycle arrest in ALCL cells:
DEL and SU-DHL-1 cells were treated with the indicated concentration of gilteritinib for 24 hours. Cells were fixed and stained with propidium iodide, and cell cycle status was determined by flow cytometry. Values represent the mean of three independent experiments. Bars represent the standard error of the mean (SEM).
3.4. Gilteritinib inhibits clonogenic survival of ALCL cells
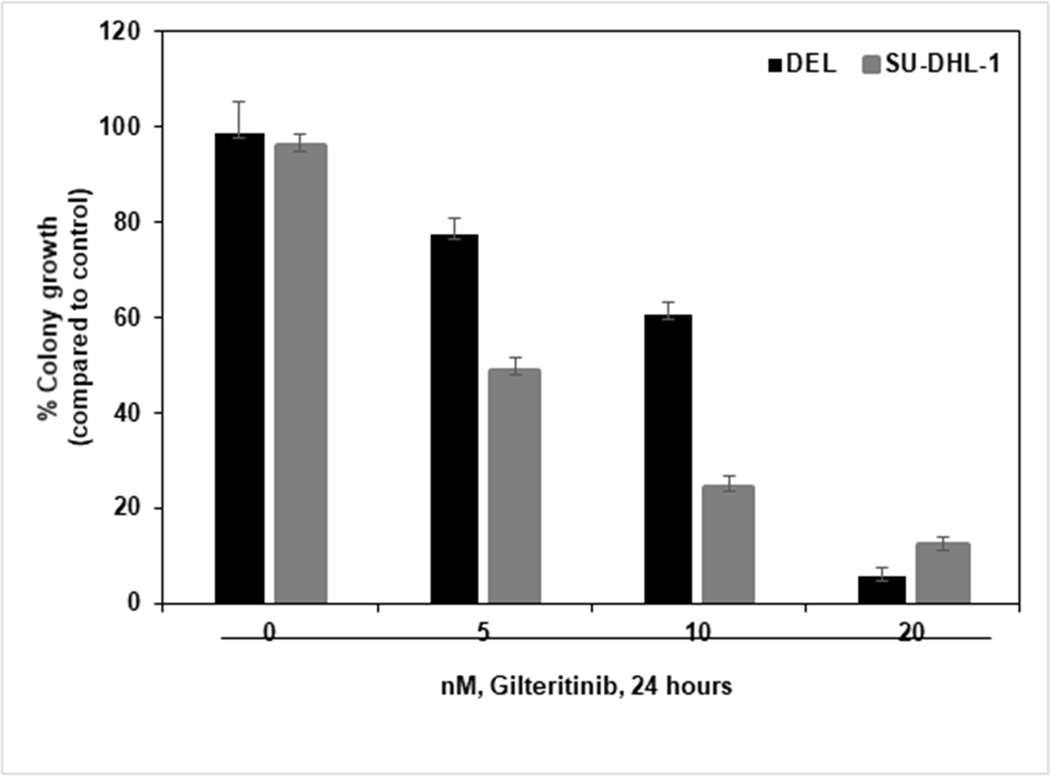
Malignant cells often demonstrate great clonogenic potential. The colonization of cells from a single cell involves uncontrolled cell division, which in turn is a characteristic feature of cancer cells. Clonogenic assays were used to evaluate the potential of the cells to form colonies after exposure to a drug. Here we examined gilteritinib induced growth inhibitory effects on the proliferation potential of ALCL cells. We treated DEL and SU-DHL-1 cells with various concentrations of gilteritinib for 24 hours. After treatment, cells were washed, resuspended in Methocult medium, and plated. The cells were then incubated for eight days, allowing them to grow and colonize. Colonies were counted in untreated and treated wells and expressed as a percentage compared to control cells. As shown in Fig. 6, gilteritinib treatment inhibited the colony growth of ALCL cells in a dose-dependent manner compared to controls. These results indicate that gilteritinib treatment inhibits the clonogenic potential of ALCL cells.
Figure 6: Gilteritinib inhibits the clonogenic potential of ALCL cells.
DEL and SU-DHL-1 cells were treated with indicated concentrations of gilteritinib for 24 hours, washed, mixed with MethoCult medium, and plated. After eight days of incubation, the number of colonies were counted. Columns represent the mean of three independent experiments; bars represent the SEM.
3.5. Gilteritinib inhibits CD30 expression in ALCL cells
CD30 overexpression is a characteristic of tumor cells in ALK+ALCL. The CD30 protein belongs to the TNF superfamily, and its expression plays an important role in the advancement of cell proliferation and survival. Constitutive activation of NPM1-ALK and downstream signaling proteins lead to the overexpression of the CD30 cell surface marker (19,20).
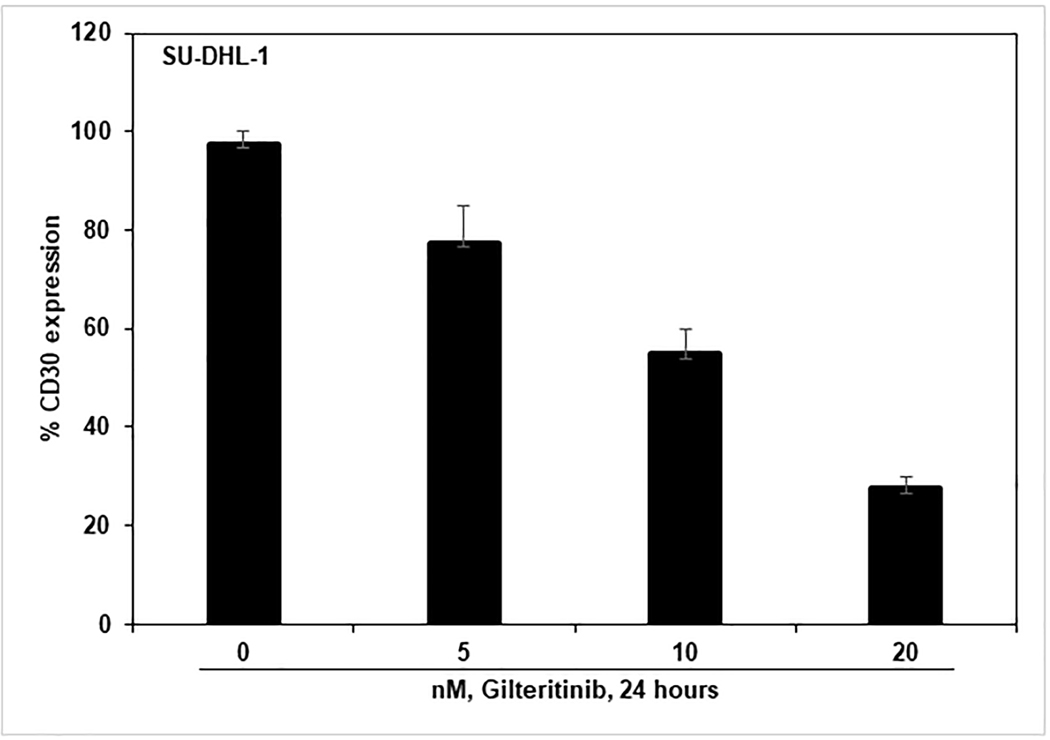
To understand the effect of gilteritinib on CD30 expression, we treated SU-DHL-1 cells with gilteritinib concentrations ranging from 5 – 20 nM and incubated for 24 hours. After the incubation period, cells were harvested, washed, and stained with FITC-conjugated CD30 antibody or FITC-IgG1κ isotype control diluted in 0.2% BSA/PBS on ice for 1 hour. The samples were then analyzed to determine expression levels of the CD30 marker using flow cytometry. The results demonstrated a significant decrease in the percentage of CD30 positive cells in treated cells compared to untreated cells (Fig. 7). This data indicates that gilteritinib treatment inhibits CD30 cell surface marker expression through the inhibition of NPM1-ALK kinase activity in ALCL cells.
Figure 7: Gilteritinib down-regulates CD30 surface expression in ALCL cells.
SU-DHL-1 cells were treated for 24 hours with indicated concentrations of gilteritinib and stained with anti-CD30-FITC along with respective isotype control antibodies. The CD30 positive cells were measured by flow cytometry. Columns represent the mean of three independent experiments; bars represent the SEM.
Discussion
The majority of systemic ALCL cases express ALK gene mutations with NPM1-ALK being the most common ALK-fusion in these lymphomas. Although most ALK-positive ALCL patients respond to anthracycline-based CHOP chemotherapy, failure of remission and resistance are often seen, indicating a need for an alternative therapeutic approach (7). Currently, there are a limited number of ALK inhibitors available to treat ALK+ patients. There is a clear need to either develop new ALK inhibitors or explore the efficacy of available receptor tyrosine kinase inhibitors and potentially repurposing them to target various types of ALK+ cancers. Crizotinib is one of the well-studied ALK inhibitors used in the treatment of echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion-positive non-small cell lung cancers (21). Crizotinib has also demonstrated an efficacious clinical response in ALK+ pediatric lymphomas. However, recent reports indicate crizotinib resistance in many ALK+ tumors, which is acquired through secondary kinase domain mutations or by other anti-apoptotic mechanisms (22–25).
Gilteritinib is a novel receptor tyrosine kinase inhibitor that has recently entered the drug discovery field and moved rapidly into the developmental process and subsequent clinical trials (26). In a recent phase III clinical trial, relapsed or refractory FLT3-mutated AML patients treated with gilteritinib had a long-term survival advantage and higher rates of remission compared to salvage chemotherapy (10). Another recent study demonstrated the feasibility and effectiveness of gilteritinib in pediatric FLT3-mutated AML (27). There are many ongoing clinical trials of gilteritinib in combination with various therapeutic agents. A phase I combination study of gilteritinib with induction and consolidation chemotherapy indicated a good safety profile, tolerability, and high response rates in FLT3-mutated AML treatment with either idarubicin or daunorubicin (28). Other combinational studies are exploring the efficacy of gilteritinib in combination with a BCL2 inhibitor or hypomethylating agents. A phase II clinical trial investigated the safety profile of combining gilteritinib with azacytidine in elderly AML patients unfit for standard induction therapy (29). In preclinical models, gilteritinib displayed growth inhibitory effects against mutations FLT3-ITD and/or FLT3/D835, which is the most acquired point mutation that confers resistance to other FLT3 inhibitors. In colorectal cancer cells expressing FLT3-ITD and FLT3/D835 gilteritinib showed induced cell death through PUMA upregulation (30). Studies also demonstrated gilteritinib activity against FLT3/F691, a mutation commonly found in AML cases after quizartinib resistance (31).
Based on previous in vitro kinase inhibitory activity assay profiling results on gilteritinib binding with ALK, and the positive impact of recent clinical trials, here we investigated the therapeutic value of gilteritinib repurposing in NPM1-ALK driven ALCL cells (9,10). In NPM1-ALK fusion driven ALCL cells, the fusion kinase is constitutively activated through the homodimerization of fusion partners through the N-terminal NPM1 oligomerization domain (32). Since fusion kinase activation is the first event to initiate lymphomagenesis, we examined the inhibitory effects of gilteritinib on the fusion kinase NPM1-ALK. We found the downregulation of NPM1-ALK kinase phosphorylation was evident in all utilized cell lines: four human (SR-786, SU-DHL-1, DEL, and SUP-M2) and one murine (Ba/F3-FG-NPM1-ALK), in a dose-dependent manner. These findings are consistent with other studies in which other ALK inhibitors showed similar results in ALCL cells (33,34). Activation of ALK triggers the upregulation of downstream survival signaling cascade, including the transcription factors STAT3, AKT, and ERK1/2, which are responsible for lymphoma cell proliferation and survival (35). We found inhibition of the fusion kinase by gilteritinib resulted in the downregulation of downstream signaling pathways. Since activated fusion kinase and downstream signaling are driving the survival of lymphoma cells, we further examined the effects of gilteritinib on apoptosis. Our findings showed that treatment with gilteritinib on NPM1-ALK endogenously expressing SR-786, SU-DHL-1, DEL, and SUP-M2 cells showed induced apoptosis in a concentration-dependent manner. Similar results were found in NPM1-ALK-ectopically expressing murine Ba/F3 cells. Apoptosis was associated with caspase activation and PARP cleavage. BCL2 family proteins are regulators of apoptosis, and an imbalance between anti- and pro-apoptotic proteins is observed in many cancers (36,37). Here, we identified gilteritinib treatment decreased the levels of anti-apoptotic proteins, survivin, and MCL-1, and increased the levels of pro-apoptotic protein, BAD. In ALCL cells with activated fusion kinase, NPM1-ALK induces c-Myc expression, which is involved in cell proliferation transformation (18). We investigated gilteritinib mediated inhibition of NPM1-ALK effects on c-Myc expression and found a significant decrease in c-Myc protein levels in gilteritinib treated ALCL cells. Next, we examined the cell cycle status to correlate with the gilteritinib mediated induction of apoptosis of ALCL cells. Our cell cycle analysis results showed that gilteritinib treatment induced cell cycle arrest at the G0/G1 phase with a concomitant decrease in S and G2/M phases in NPM1-ALK driven DEL and SU-DHL-1 cells. Similar effects on the cell cycle were observed with gilteritinib in FLT3-mutated AML cell lines (38). Activation of the NPM1-ALK fusion protein is vital for the proliferation of ALCL cells. Therefore, we investigated the effect of gilteritinib on the proliferation of NPM1-ALK driven ALCL cells using a standard methylcellulose clonogenic assay. The cells showed a remarkable decrease in colony growth after treatment with gilteritinib. DEL cells exhibited more response in the reduction of colony growth in comparison to SU-DHL-1, which could be due to variable expression of NPM1-ALK and downstream effector molecules in these cell lines. ALCL cells are characterized by a strong expression of the cytokine receptor CD30, a transmembrane glycoprotein member of the tumor necrosis factor (TNF) receptor family (39,40). In ALCL cells, CD30 surface marker is transcriptionally upregulated through NPM1-ALK activated ERK1/2 and STAT3 effector molecules, which contributes to lymphoma cell proliferation through activated NF-κB signaling (41). We analyzed gilteritinib mediated inhibition of NPM1-ALK on CD30 expression in SU-DHL-1 cells and found that the downregulation of the fusion kinase resulted in the decreased CD30 expression through inhibition of ERK1/2 and STAT3. In accordance, CD30 expression levels could potentially serve as a pharmacodynamic marker of therapeutic intervention of inhibiting NPM1-ALK signaling by gilteritinib in future clinical trials.
Our results comprehensively demonstrate gilteritinib inhibition on NPM1-ALK kinase activity and downstream survival signaling pathways, resulting in G0/G1 cell cycle arrest and apoptosis. Furthermore, our studies indicate gilteritinib treatment also decreased clonogenic potential and CD30 expression of lymphoma cells.
Altogether, our preclinical data support the rationale for in vivo testing of gilteritinib in NPM1-ALK+ ALCLs and also potentially in other ALK/ALK-fusion driven hematologic or solid malignancies.
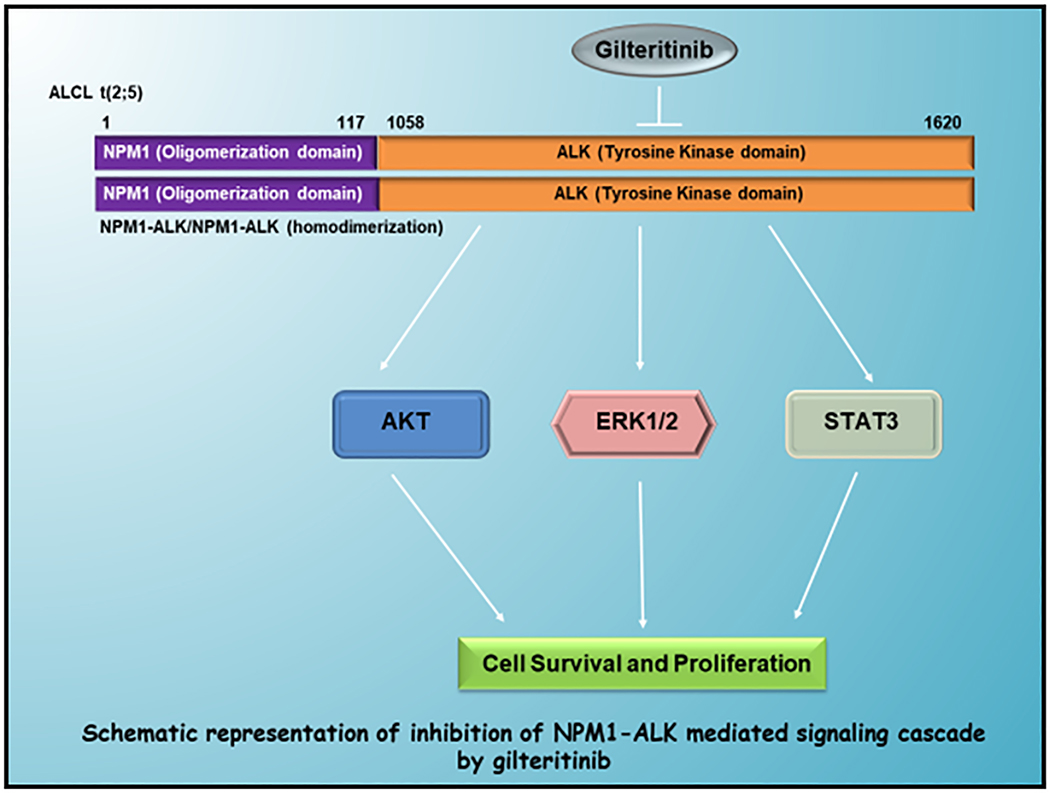
Figure 8: Visual Overview.
Implications:
Our preclinical results explore the use of gilteritinib for the treatment of NPM1-ALK driven anaplastic large cell lymphoma cells and pave a path for developing future clinical trials.
Acknowledgments
R. Balusu acknowledges the pilot award from American Cancer Society (ACS-IRG-16–194-07), Sosland Family Foundation Research Award, Hale Family Foundation, and Frontiers Clinical and Translational Pilot Award UL1T. R.A. Jensen is a recipient of P30-CA168524 from NCI. We are very grateful to Riley Baker (University of Kansas Medical Student) for thorough proofreading of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare no potential conflicts of interest
References
- 1.Damm-Welk C, Klapper W, Oschlies I, Gesk S, Rottgers S, Bradtke J, et al. Distribution of NPM1-ALK and X-ALK fusion transcripts in paediatric anaplastic large cell lymphoma: a molecular-histological correlation. Br J Haematol 2009;146(3):306–9. [DOI] [PubMed] [Google Scholar]
- 2.Montes-Mojarro IA, Steinhilber J, Bonzheim I, Quintanilla-Martinez L, Fend F. The Pathological Spectrum of Systemic Anaplastic Large Cell Lymphoma (ALCL). Cancers 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motegi A, Fujimoto J, Kotani M, Sakuraba H, Yamamoto T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. Journal of cell science 2004;117(Pt 15):3319–29. [DOI] [PubMed] [Google Scholar]
- 4.van der Krogt JA, Bempt MV, Ferreiro JF, Mentens N, Jacobs K, Pluys U, et al. Anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with the variant RNF213-, ATIC- and TPM3-ALK fusions is characterized by copy number gain of the rearranged ALK gene. Haematologica 2017;102(9):1605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falini B, Nicoletti I, Bolli N, Martelli MP, Liso A, Gorello P, et al. Translocations and mutations involving the nucleophosmin (NPM1) gene in lymphomas and leukemias. Haematologica 2007;92(4):519–32. [DOI] [PubMed] [Google Scholar]
- 6.Tabbo F, Barreca A, Piva R, Inghirami G, European TCLSG. ALK Signaling and Target Therapy in Anaplastic Large Cell Lymphoma. Front Oncol 2012;2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood 2015;126(1):17–25. [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Kaneko N, Ueno Y, Yamada M, Tanaka R, Saito R, et al. Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Investigational new drugs 2017;35(5):556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhillon S. Gilteritinib: First Global Approval. Drugs 2019;79(3):331–9. [DOI] [PubMed] [Google Scholar]
- 10.Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. The New England journal of medicine 2019;381(18):1728–40. [DOI] [PubMed] [Google Scholar]
- 11.Short NJ, Kantarjian H, Ravandi F, Daver N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther Adv Hematol 2019;10:2040620719827310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuravi S, Parrott E, Mudduluru G, Cheng J, Ganguly S, Saunthararajah Y, et al. CDC37 as a novel target for the treatment of NPM1-ALK expressing anaplastic large cell lymphomas. Blood cancer journal 2019;9(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balusu R, Fiskus W, Rao R, Chong DG, Nalluri S, Mudunuru U, et al. Targeting levels or oligomerization of nucleophosmin 1 induces differentiation and loss of survival of human AML cells with mutant NPM1. Blood 2011;118(11):3096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Gostissa M, Hildebrand DG, Becker MS, Boboila C, Chiarle R, et al. The role of mechanistic factors in promoting chromosomal translocations found in lymphoid and other cancers. Advances in immunology 2010;106:93–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov 2012;2(6):495–502. [DOI] [PubMed] [Google Scholar]
- 16.Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood 2007;110(7):2259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramesh P, Medema JP. BCL-2 family deregulation in colorectal cancer: potential for BH3 mimetics in therapy. Apoptosis 2020; 25(5): 305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raetz EA, Perkins SL, Carlson MA, Schooler KP, Carroll WL, Virshup DM. The nucleophosmin-anaplastic lymphoma kinase fusion protein induces c-Myc expression in pediatric anaplastic large cell lymphomas. The American journal of pathology 2002;161(3):875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Younes A. Role of CD30 targeting in malignant lymphoma. Current treatment options in oncology 2014;15(2):210–25. [DOI] [PubMed] [Google Scholar]
- 20.Al-Shamkhani A. The role of CD30 in the pathogenesis of haematopoietic malignancies. Current opinion in pharmacology 2004;4(4):355–9. [DOI] [PubMed] [Google Scholar]
- 21.Awad MM, Shaw AT. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol 2014;12(7):429–39. [PMC free article] [PubMed] [Google Scholar]
- 22.Torossian A, Broin N, Frentzel J, Daugrois C, Gandarillas S, Saati TA, et al. Blockade of crizotinib-induced BCL2 elevation in ALK-positive anaplastic large cell lymphoma triggers autophagy associated with cell death. Haematologica 2019;104(7):1428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4(120):120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Li Y, Zhang S, Gao C, Nie K, Ji Y. Primary resistance to crizotinib treatment in a non-small cell lung cancer patient with an EML4-ALK rearrangement: a case report. Cancer Biol Med 2018;15(2):178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prokoph N, Probst NA, Lee LC, Monahan JM, Matthews JD, Liang HC, et al. IL10RA Modulates Crizotinib Sensitivity in NPM1-ALK-positive Anaplastic Large Cell Lymphoma. Blood 2020. DOI: 10.1182/blood.2019003793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levis M, Perl AE. Gilteritinib: potent targeting of FLT3 mutations in AML. Blood Adv 2020;4(6):1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuoka K, Tsumura Y, Noguchi J, Sugawa M, Takaki T, Hiraki T, et al. Gilteritinib for pediatric FLT3 internal tandem duplication-positive recurrent acute myeloid leukemia. Rinsho Ketsueki 2020;61(4):322–6. [DOI] [PubMed] [Google Scholar]
- 28.Pratz KW, Cherry M, Altman JK, Cooper B, Cruz JC, Jurcic JG, et al. Updated Results from a Phase 1 Study of Gilteritinib in Combination with Induction and Consolidation Chemotherapy in Subjects with Newly Diagnosed Acute Myeloid Leukemia (AML). Blood 2018;132(Supplement 1):564. [Google Scholar]
- 29.Esteve J, Schots R, Bernal Del Castillo T, Lee J-H, Wang ES, Dinner S, et al. Multicenter, Open-Label, 3-Arm Study of Gilteritinib, Gilteritinib Plus Azacitidine, or Azacitidine Alone in Newly Diagnosed FLT3 Mutated (FLT3mut+) Acute Myeloid Leukemia (AML) Patients Ineligible for Intensive Induction Chemotherapy: Findings from the Safety Cohort. Blood 2018;132(Supplement 1):2736. [Google Scholar]
- 30.Li L, Lin L, Li M, Li W. Gilteritinib induces PUMA-dependent apoptotic cell death via AKT/GSK-3beta/NF-kappaB pathway in colorectal cancer cells. J Cell Mol Med 2020;24(3):2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chew S, Mackey MC, Jabbour E. Gilteritinib in the treatment of relapsed and refractory acute myeloid leukemia with a FLT3 mutation. Ther Adv Hematol 2020;11:2040620720930614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunchala P, Kuravi S, Jensen R, McGuirk J, Balusu R. When the good go bad: Mutant NPM1 in acute myeloid leukemia. Blood Rev 2018;32(3):167–83. [DOI] [PubMed] [Google Scholar]
- 33.Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L, et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci U S A 2007;104(1):270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George SK, Vishwamitra D, Manshouri R, Shi P, Amin HM. The ALK inhibitor ASP3026 eradicates NPM-ALK(+) T-cell anaplastic large-cell lymphoma in vitro and in a systemic xenograft lymphoma model. Oncotarget 2014;5(14):5750–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Hu J. Nucleophosmin1 (NPM1) abnormality in hematologic malignancies, and therapeutic targeting of mutant NPM1 in acute myeloid leukemia. Ther Adv Hematol 2020;11:2040620719899818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaattela M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene 2004;23(16):2746–56. [DOI] [PubMed] [Google Scholar]
- 37.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ 2018;25(1):65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno Y, Mori M, Kamiyama Y, Saito R, Kaneko N, Isshiki E, et al. Evaluation of gilteritinib in combination with chemotherapy in preclinical models of FLT3-ITD(+) acute myeloid leukemia. Oncotarget 2019;10(26):2530–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Leval L, Gaulard P. CD30+ lymphoproliferative disorders. Haematologica 2010;95(10):1627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Weyden CA, Pileri SA, Feldman AL, Whisstock J, Prince HM. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood cancer journal 2017;7(9):e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SY, Lee SY, Kandala G, Liou ML, Liou HC, Choi Y. CD30/TNF receptor-associated factor interaction: NF-kappa B activation and binding specificity. Proc Natl Acad Sci U S A 1996;93(18):9699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]