Abstract
Listeria monocytogenes is a foodborne pathogen and the causative agent of listeriosis, a disease associated with high fatality (20–30%) and hospitalization rates (>95%). ATP-Binding Cassette (ABC) transporters have been demonstrated to be involved in the general stress response. In previous studies, in-frame deletion mutants of the ABC transporter genes, LMOf2365_1875 and LMOf2365_1877, were constructed and analyzed; however, additional work is needed to investigate the virulence potential of these deletion mutants. In this study, two in vitro methods and one in vivo model were used to investigate the virulence potential of in-frame deletion mutants of ABC transporter genes. First, the invasion efficiency in host cells was measured using the HT-29 human cell line. Second, cell-to-cell spread activity was measured using a plaque forming assay. Lastly, virulence potential of the mutants was tested in the Galleria mellonella wax moth model. Our results demonstrated that the deletion mutant, ⊿LMOf2365_1875, displayed decreased invasion and cell-to-cell spread efficiency in comparison to the wild-type, LMOf2365, indicating that LMOf2365_1875 may be required for virulence. Furthermore, the reduced virulence of these mutants was confirmed using the Galleria mellonella wax moth model. In addition, the expression levels of 15 virulence and stress-related genes were analyzed by RT-PCR assays using stationary phase cells. Our results showed that virulence-related gene expression levels from the deletion mutants were elevated (15/15 genes from ⊿LMOf2365_1877 and 7/15 genes from ⊿LMOf2365_1875) compared to the wild type LMOf2365, suggesting that ABC transporters may negatively regulate virulence gene expression under specific conditions. The expression level of the stress-related gene, clpE, also was increased in both deletion mutants, indicating the involvement of ABC transporters in the stress response. Taken together, our findings suggest that ABC transporters may be used as potential targets to develop new therapeutic strategies to control L. monocytogenes.
Introduction
Listeria monocytogenes, a Gram-positive foodborne pathogen, is an important public health concern since it can cause listeriosis associated with a mortality rate of approximately 20 to 30% in animals and humans [1]. Listeriosis outbreaks have been associated with the consumption of contaminated food products, which include ready-to-eat (RTE) meats and dairy and more recently fresh produce [2–4]. L. monocytogenes is also commonly found in the environment, and it is difficult to eliminate this pathogen from food processing facilities since it is able to survive under harsh conditions such as low pH and high salt [5].
Listeriosis occurs primarily in immunocompromised individuals, including pregnant women, the newborn, and the elderly [1]. L. monocytogenes virulence involves adhesion and invasion to host cells, escape from vacuoles, intracellular growth, and cell-to-cell spread [6]. The activity of well-characterized virulence factors is involved in each stage of the process [7], and many genes linked to each stage have been identified [8,9]. The prfA gene encodes a transcriptional regulator that turns on transcription of several virulence genes, including hly, plcA, plcB, and inlA [10,11]. A transcriptional regulator, encoded by the sigB gene positively regulates transcription of stress-related genes, including clpC and clpE [12]. Several genes involved in adhesion are actA, ami, fbpA, and flaA, and internalin A and B (inlA and inlB) that facilitate invasion into mammalian cells [13]. The hly gene encodes for listeriolysin O, a pore-forming toxin, which in combination with the action of two phospholipases, plcA and plcB, is responsible for escape of L. monocytogenes from vacuoles. Intracellular motility and cell-to-cell spread involve the action of the actA and iap genes [6].
In recent years, an infection model using larvae of the greater wax moth, Galleria mellonella, has been shown to be a promising model to assess virulence of numerous human pathogens, including L. monocytogenes [14–16]. Advantages of this model are its low cost, easy manipulation, ethical acceptability, and the capability to incubate larvae at 37°C, which is human body temperature and is a prerequisite for the optimal expression of various key virulence factors in L. monocytogenes [17]. Most importantly, the innate immune system of G. mellonella resembles that of mammals, with enzymes, reactive oxygen species, and antimicrobial peptides necessary against protection from bacterial infection [14]. In addition, the G. mellonella model has also been successfully utilized to explore cadmium resistance in L. monocytogenes [18], as well as comparison of the transcriptomes from different isolates [19].
ATP-Binding Cassette (ABC) transporters serve as major transport systems in bacteria [20]. More than 30 copies of different ABC transporters are found in the L. monocytogenes genome [21]. Typically, an ABC transporter consists of several subunits, including a nucleotide-binding domain, a transmembrane domain, and/or a solute-binding domain [22]. ABC transporters can be used as targets in the development of antibacterial vaccines and therapies [23]. In addition to transport, some ABC transporters have been demonstrated to be involved in virulence. For example, an ABC transporter that is associated with resistance to antimicrobial peptides contributes to the virulence of Salmonella [24].
Liu and Ream [25] showed that LMOf2365_1875 (ABC transporter, manganese-binding protein), LMOf2365_1876 (manganese ABC transporter; permease protein), and LMOf2365_1877 (manganese ABC transporter; ATP-binding protein) were highly induced in milk at 4°C; however, this ABC transporter operon was inhibited in RTE meats [26]. Magnesium is the potential substrate for this transporter, and it is also present in other L. monocytogenes strains [27,28]. To our knowledge, it is not under control of key L. monocytogenes transcriptional regulators such as SigB. Previous studies have shown that the in-frame deletion mutants ⊿LMOf2365_1875 and ⊿LMOf2365_1877 had no overall growth defects in Brain Heart Infusion (BHI) medium, but were sensitive to salt, acid, and nisin, indicating that LMOf2365_1875 and LMOf2365_1877 may be involved in the general stress response [21]. However, there have been no studies on the virulence potential of these deletion mutants. In this paper, we tested the virulence potential of the two in-frame deletion mutants of ⊿LMOf2365_1875 and ⊿LMOf2365_1877 to gain insight into the possible role of the ABC transporter during infection of the human host.
Manganese is involved in bacterial virulence [22,29]. For example, acquisition of Mn (II) is required for intracellular survival and replication of Salmonella enterica serovar Typhimurium in macrophages and for virulence in vivo [30]. Since LMOf2365_1875 encodes for a putative ABC transporter, manganese-binding protein, we hypothesized that manganese transport may be blocked in ⊿LMOf2365_1875; therefore, virulence was reduced in L. monocytogenes. While this hypothesis needs further testing, it is supported by the following lines of evidence. Manganese also plays an important role in streptococcal virulence [31]. An ABC transporter named MtsA that is involved in manganese transport in Streptococcus pyogenes was related to virulence since a deletion mutant resulted in attenuated virulence [32]. MtsA shares 98% similarity with LMOf2365_1877(AAT04647.1) and 72% similarity with LMOf2365_1875 (AAT04645.1). In addition, an Agrobacterium tumefaciens mutant with a manganese transport deficiency had attenuated virulence in plants [33]. Similarly, iron acquisition is also required for virulence in L. monocytogenes since an ABC transporter mutant impaired in heme uptake displayed decreased virulence [34].
Materials and methods
Bacterial strains and cell line culture conditions
L. monocytogenes strain F2365 (isolated from Mexican-style soft cheese) [35] was used in the current study since its genome is fully sequenced and annotated [28]. L. monocytogenes F2365, L. monocytogenes Scott A, L. innocua, and two isogenic deletion mutants (⊿LMOf2365_1875 and ⊿LMOf2365_1877) of the parent strain LMOf2365 (Table 1) stored at -80°C as glycerol stocks were streaked onto BHI agar (Sigma-Aldrich St. Louis, MO) and incubated at 37°C for overnight prior to performing each experiment. The human colon adenocarcinoma cell line HT-29 (ATCC, Manassas, VA, USA) was maintained as described previously [36].
Table 1. Listeria strains used in this study.
| Bacterial strains | Reference/source |
|---|---|
| L. monocytogenes F2365 wild-type serotype 4b strain, genome sequenced | [28] |
| L. monocytogenes Scott A | Gift from R. D. Joerger (University of Delaware) |
| L. innocua | aERRC collection |
| ΔLMOf2365_1875, 1875deletion | [21] |
| Δ LMOf2365_1877, 1877deletion | [21] |
aEastern Regional Research Center.
Cell invasion assays
HT-29 cells (ATCC HTN-38) were used to determine the virulence of the Listeria strains [37]. L. monocytogenes strains (L. monocytogenes Scott A, isogenic deletion mutants ΔLMOf2365_1875 and ΔLMOf2365_1877, and the parental LMOf2365) and L. innocua were used for the invasion assays performed as described previously [36]. In brief, HT-29 cells were grown in 24-well tissue culture plates for 5 days to obtain almost confluent monolayers. Strains of L. monocytogenes (L. monocytogenes Scott A, isogenic deletion mutants ΔLMOf2365_1875 and ΔLMOf2365_1877, and the parental LMOf2365) and L. innocua were grown to log-phase (OD600nm ~0.3) at 37°C. HT-29 cell monolayers incubated in medium without antibiotics for 24 h were infected for 1 h at 37°C with 107 CFU bacterial cells in 300 μl BHI medium (Becton Dickinson and Co., Sparks, MD). The cell monolayers were washed with DMEM and incubated in DMEM containing gentamicin (100 μg/ml) for 1.5 h at 37°C. HT-29 cell monolayers were gently washed three times with phosphate buffered saline (pH 7.4) and then disrupted with 1 ml cold sterile water (4°C). Viable bacteria were counted after plating serial dilutions onto TSA. The results were expressed as the percentage of CFU recovered after 2 h relative to the number of bacterial cells deposited per well. Three independent experiments were performed for each strain.
Plaque forming assays (PFAs)
Strains of L. monocytogenes (L. monocytogenes Scott A, isogenic deletion mutants ΔLMOf2365_1875 and ΔLMOf2365_1877, and the parental LMOf2365) and L. innocua were used for PFA assays, which were performed using HT-29 cells as described previously [36,37]. In brief, confluent HT-29 cell monolayers were incubated in medium without antibiotics for 24 h. The log-phase Listeria cells (described above) were used to infect HT-29 cell monolayers with a dilution series of 102 to 107 CFU/ml cells per well, and then incubated for 2 h at 37°C. After removing the bacterial suspensions, cell monolayers were washed with DMEM and incubated in DMEM containing 100 μg/ml of gentamicin for 1.5 h. Each well was then covered with DMEM with 0.5% agarose containing 10 μg/ml gentamicin. After solidification, 400 μl of the same liquid medium were added to the top of the agar to prevent starvation. Tissue culture plates were incubated for 3 days at 37°C under 5% CO2 (v/v). Enumeration of formed plaques was performed using an inverted microscope. The results were expressed as log numbers of plaques per 107 CFU/ml deposited per well. Experiments were performed in duplicate and repeated twice for each strain.
G. mellonella injection and mortality assay
The assessment of virulence of L. monocytogenes strains in this study was conducted using the G. mellonella larvae model, described in our previous work [16]. L. monocytogenes strains were grown overnight at 37˚C in BHI broth and on BHI agar plates. The overnight liquid cultures in BHI broth were washed twice and serially diluted with phosphate buffered saline (PBS). Appropriate dilutions were plated onto BHI agar and incubated for 24 h at 37˚C to obtain the CFU count. Colony counts were used to calculate the bacterial inoculum for Galleria infection. A set of 20 Galleria larvae of the similar size (approximately 200–300 mg), light-colored, with a good motility, were inoculated with appropriate dilutions of L. monocytogenes in the PBS (Fisher BioReagents), for final concentrations of 106 and 105CFU/larva. Inoculated larvae were incubated at 37°C and monitored for mortality and phenotypic changes, including changes in color, motility, dryness, and pupation for a period of seven days. For each treatment, the number of dead larvae was recorded daily for up to seven days. From these data, percent mortality was calculated. Each trial included one set of ten uninoculated larvae and one set of ten larvae inoculated with sterile 0.85% saline solution. One group of uninoculated larvae served as a control for adaptation of Galleria larvae to 37°C, while a second group served as a “manipulation” control. Experiments were conducted with three independent trials.
RT-PCR analysis of virulence and stress-related genes
Strains of L. monocytogenes (deletion mutants ΔLMOf2365_1875 and ΔLMOf2365_1877, as well as the wild type LMOf2365 parent strain) (Table 1) were inoculated in 5 ml of BHI and grown with agitation (200 rpm) for 12 h at 37°C. Total RNA was isolated from the above strains of L. monocytogenes as previously described [25]. Primers targeting 15 genes related to virulence and stress response (Table 2) were designed as previously described [36]. The spoG housekeeping gene was used as an internal control (Table 2). cDNA synthesis and real-time PCR analysis conducted in this study were described in our previous work [25]. Reactions without reverse transcriptase were used as negative controls. RT-PCR assays were performed three times for each strain.
Table 2. Oligonucleotides used for real-time PCR to evaluate the virulence and stress related genes in L. monocytogenes.
| GENE | Forward primer sequences (5’-3’) | Role in virulence | Product size (bp) |
|---|---|---|---|
| actA | F: AAGAGTTGAACGGAGAGGT R: TCAGCTAGGCGATCAATTTC |
Adhesion, invasion, vaculole lysis, and intracellular motility [6] | 121 |
| ami | F: GTAACCATTCGCGATGACTC R: CTTGAATAGCGAACCCTTGA |
Adhesion [6] | 100 |
| clpC | F: GTAACCATTCGCGATGACTC R: CTTGAATAGCGAACCCTTGA |
stress response [38] | 100 |
| clpE | F: CAGAAGCACTAACAGCAGCA R: TCACCGTATTTTCGTCCAGT |
stress response [39] | 141 |
| fbpA | F: GCGGTCGAAGTAGTGAAAGA R: AGCTAGTTCTTGGCGGATTT |
adhesion [6] | 126 |
| flaA | F: CGCAAGAACGTTTAGCATCT R: ATGGATGAGTTTTTGCTTGC |
adhesion [40] | 127 |
| hly | F: GAATGCAATTTCGAGCCTAA R: AGTCATTCCTGGCAAATCAA |
Lysis of vacuoles [41] | 133 |
| iap | F: GAAAAACAAGCTGCACCAGT R: CTGTTGGTGCTTTAGGTGCT |
Invasion and actin-based activity [42] | 109 |
| inlA | F: ATGGGATTTTGCGACAGATA R: CGGAAGGTGGTGTAGTGTTC |
Invasion [6] | 143 |
| inlB | F: ACCTAAACCTCCGACCAAAC R: TCGTTTCCGCTTTAAACATC |
Invasion [6] | 140 |
| lap | F: ATCCCTTCCCTAACACTTGG R: GTGGAAGTTTGAACCATTGC |
Adhesion [43] | 133 |
| plcA | F: AAGACGAGCAAAACAGCAAC R: CTCGTGTCAGTTCTGGGAGT |
Vacuole lysis [6] | 100 |
| plcB | F: ATCCTATCCACCAGGCTACC R: TCTTTCACGTCATTTGAGCA |
Vacuole lysis [6] | 117 |
| pfrA | F: CGCAAGAACGTTTAGCATCT R: ATGGATGAGTTTTTGCTTGC |
Transcriptional regulator, virulence [12] |
127 |
| sigB | F: TCGCAAATATTCCCAAGGTA R: TGACGGTGAATTCCGTGATA |
Transcription factor, stress response [12] | 127 |
| spoG | F: TGACGGTGAATTCCGTGATA R: TCAGCAGAAACGGATTCAGA |
Internal control gene [25] | 147 |
Statistical analysis
Data collected from this study were analyzed using the Student’s t test of the Statistical Analysis Software (SAS Institute Inc., Cary, NC) for paired comparison with P < 0.05 considered significant.
Results and discussion
Deletion mutant ⊿LMOf2365_1875 displayed reduced invasion and cell-to-cell spread activities in the HT-29 cell line
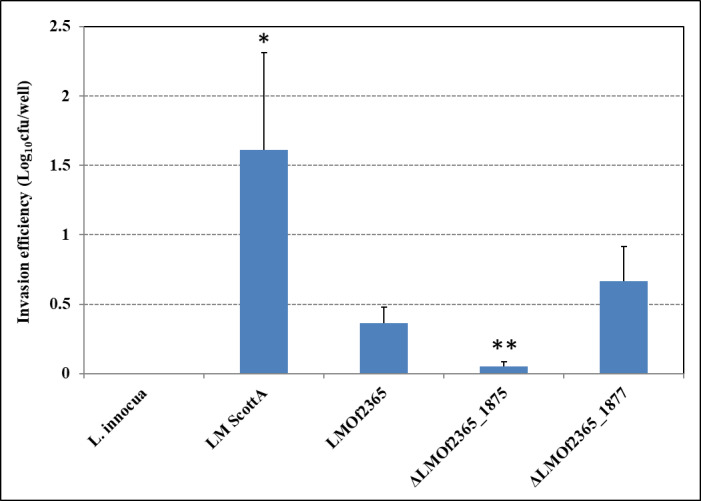
Previous studies identified mutants of an ABC transporter responsible for oligopeptide transport in L. monocytogenes that were defective in host infection [44]. Since ABC transporters are membrane proteins, they may be involved in the adhesion of L. monocytogenes to human host cells. To understand if LMOf2365_1875 and LMOf2365_1877 are involved in causing host infection, cell invasion and plaque forming in vitro assays using HT-29 cell monolayers were employed to test the virulence potential of each deletion mutant. As shown in Fig 1, L. monocytogenes Scott A expressed the highest invasion (1.6 log10 CFU/well), and L. monocytogenes F2365 (LMOf2365) also had a high invasion efficiency (0.4 log10 CFU/well). Both L. monocytogenes F2365 and L. monocytogenes Scott A belong to serotype 4b strains, which is the serotype most often associated with outbreaks of listeriosis. The adhesion and invasion efficiency of LMOf2365 was lower compared to the LM Scott A strain (with a p value of 0.03), which is consistent with the fact that LMOf2365 has a truncated inlB gene that is involved in adhesion and invasion [45]. Non-pathogenic L. innocua, used as a negative control, showed no invasion. The deletion mutant strain ⊿LMOf2365_1875 showed a deficiency in invasion (0.1 log10 CFU/well) (with a p-value of 0.003), whereas ⊿LMOf2365_1877 had a slightly higher invasion efficiency (0.7 log10 CFU/well) (with a p-value of 0.12) compared to the wild type strain (LMOf2365).
Fig 1. Invasion of Listeria strains in HT-29 cells.
HT-29 cell monolayers were incubated with Listeria strains grown to stationary phase for invasion assays. Viable intracellular bacteria were counted after plating serial dilutions on BHI agar plates. The results were expressed as log numbers of CFU recovered relative to the number of bacterial cells (107) deposited per well. Each experiment was conducted in duplicate and repeated three times. Significant differences from parental wild type LMOf2365 strain are shown (*, p-value < 0.05; **, p-value < 0.01).
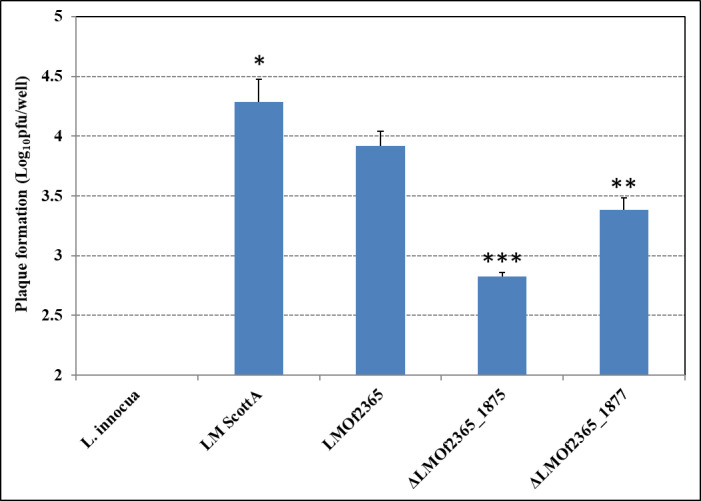
The other in vitro assay for Listeria virulence was based on the ability of strains to form plaques on HT-29 monolayers. L. monocytogenes F2365 formed a higher number of plaques (approximately 3.9 log10 pfu/well) in comparison to the two mutants but a lower number compared to Scott A (with a p-value of 0.05) (Fig 2). In contrast, no plaques were visible with the non-pathogenic L. innocua strain. ⊿LMOf2365_1875 formed 71% lower number of plaques (2.8 log10 pfu/well) compared to the wild type (with a p-value of 0.0002) whereas there was a smaller difference in plaque forming ability between ⊿LMOf2365_1877 (3.4 log10 pfu/well) and the wild type LMOf2365 (3.9 log10 pfu/well) (with a p-value of 0.002). Examining all of the data, results from the invasion and plaque forming virulence assays demonstrated that the deletion mutant ⊿LMOf2365_1875 displayed some weakness in invasion and intracellular cell-to-cell spread in HT-29 monolayers, suggesting that LMOf2365_1875 may be required for L. monocytogenes virulence.
Fig 2. Plaque formation of Listeria strains in HT-29 cells.
Results are presented as the log10 numbers of plaque-forming units (pfu) per well ± standard deviation of triplicate. Significant differences from parental wild type LMOf2365 strain are shown (*, p-value < 0.05; **, p-value < 0.01; ***, p-value < 0.001).
The ⊿LMOf2365_1875 and ⊿LMOf_1877 showed reduced virulence in the G. mellonella model
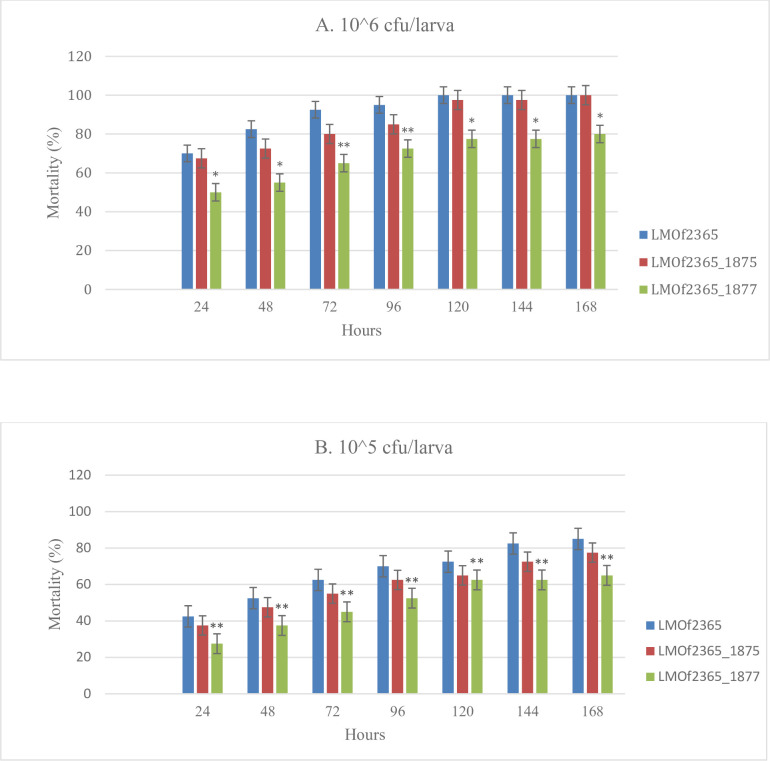
The Galleria mellonella insect larvae model has been successfully utilized to assess virulence properties of various L. monocytogenes isogeneic mutants [16]. In this work we studied two isogenic deletion mutants, ⊿LMOf2365_1875 and ⊿LMOf2365_1877 and compared them with the parent strain LMOf2365. Our results (Fig 3A and 3B) showed that both mutant strains, regardless of the inoculated dose (106 or 105 CFU/larva), exhibited reduced mortality, hence lower virulence potential, compared to the parent strain LMOf2365. At the dose of 106 CFU/larva (Fig 3A), virulence of the deletion mutant⊿LMOf2365_1875 was lower than that of the LMOf2365 parental strain over the first 96 h of the monitoring period, after which the virulence potential of both strains appeared to be similar. The difference in the first 96 h was not statistically significant. On the other hand, the deletion mutant ⊿LMOf_1877, expressed a significantly lower mortality rate/virulence potential compared to both LMOf2365 and ⊿LMOf_1875 throughout the monitoring period of infected larvae. We have observed a similar trend at the dose of 105 CFU/larva (Fig 3B). Although the difference in mortality rates between the parental strain and ⊿LMOf2365_1875, as well as the mutants themselves, was not significant, the difference between the parent strain LMOf2365 and LMOf2365_1877 was significant (Fig 3B). It appears that the mutant LMOf2365_1877 had an increased effect on virulence compared to ⊿LMOf2365_1875 as evidenced by lower mortality. This effect is especially expressed at the higher inoculum dose. Previous work [16] showed that L. monocytogenes isogenic mutants of prfA, hlyA, virR and virS had a significant effect on mortality in the Galleria model while inlA and inlB had marginal effects, which correlates with the known effects of these genes on Listeria virulence.
Fig 3.
Comparison of mortality rates of deletion mutants ⊿LMOf2365_1875 and ⊿LMOf2365_1875 with wild type LMOf2365 in G. mellonella using 106 CFU/larva (A) and 105 CFU/larva (B). *Significant difference from both parental strain and ΔLMO2365_1875. ** Significant difference from parental strain. Data presented are the averages of three independent experiments.
The expression levels of virulence and stress-related genes were elevated in ⊿LMOf2365_1875 and ⊿LMOf2365_1877 under stationary phase
To determine the gene expression levels in ⊿LMOf2365_1875 and ⊿LMOf2365_1877 under stationary-phase conditions, 15 genes related to virulence and stress response [46] were chosen for real-time PCR assays. All of the virulence-related genes were up-regulated in ⊿LMOf2365_1877 in comparison to the wild type parental LMOf2365 strain (Table 3), indicating that LMOf2365_1877 negatively regulated virulence and stress gene expression under stationary phase growth conditions. Although the expression level of pfrA, the major virulence regulator in L. monocytogenes, was relatively higher (21.4-fold) in ⊿LMOf2365_1875 compared to the wild type parental strain LMOf2365, expression levels of the genes (actA, plcA, plcB and hly) regulated by pfrA were not up-regulated in ⊿LMOf2365_1875. On the other hand, the expression levels of other virulence-related genes (ami, inlA, inlB and fbpA) were up-regulated. Our previous studies indicated that stationary phase cells of the deletion mutants (⊿LMOf2365_1875 and ⊿LMOf2365_1875) were more resistant to multiple stress conditions [21], indicating that they may contribute to the general stress response. The gene expression levels of three stress-related genes (sigB, clpC, and clpE) were tested using RT-PCR assays. As shown in Table 3, the expression levels of clpC were moderately elevated (6.8 and 10.1-fold) in ⊿LMOf2365_1875 and ⊿LMOf2365_1877, respectively. The increased levels of stress-related gene, clpC, expression confirmed our previous observation that these deletion mutants may contribute to general stress. In addition, the expression levels of clpE and sigB were also elevated (6.8 and 4.0-fold, respectively) in ⊿LMOf2365_1877.
Table 3. Relative expression levels of virulence and stress-related genes in wild-type and deletion mutants of L. monocytogenes F2365.
| actA | ami | iap | inlA | inlB | lap | fbpA | plcA | hly | pfrA | clpC | clpE | sigB flaA plcB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMOf2365a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 1 1 |
| ΔLMOf2365_1875 | -1.4b | 19.6 | 1.2 | 18.3 | 11.2 | -1.0 | 5.6 | 1.1 | -3.2 | 21.4 | 6.9 | 1.1 | -1.4 63.3 –1.4 |
| Δ LMOf2365_1877 | 8 | 25.6 | 10.6 | 30.7 | 11.8 | 4.0 | 12.3 | 8.0 | 7.7 | 1.9 | 10.1 | 6.8 | 4.0 3.5 12.7 |
aThe expression levels of the genes in the mutant strains were normalized to that in wild-type LMOf2365 strains.
b Numbers are average values from three independent experiments.
In this study, the deletion mutants showed reduced virulence in terms of invasion and cell-to-cell spreading ability; however, a number of virulence genes showed increase expression under stationary-phase growth. This seems to be contradictory, but the gene expression experiments were not performed under conditions that would occur during infection. It is likely that the virulence gene expression levels were repressed due to catabolite repression under stationary-phase growth conditions; however, these genes were de-repressed in ⊿LMOf2365_1875 and ⊿LMOf2365_1877.
We did not perform the complementation experiments for the deleted genes because these deletions are in frame (21), and which by design assures non-interference of other genes at the transcription level. A complementation experiment may not provide any additional information. In addition, gene complementation in Listeria, whether by plasmid or by an integration vector, do not exactly mimic the wild type situation because of the difference in genetic machinery involved in complementation and the topology of the complemented gene.
Conclusions
The virulence potential of the deletion mutants, ΔLMOf2365_1875 and ΔLMOf2365_1877, was assessed using both in vitro (invasion and plaque forming ability) and in vivo (G. mellonella insect model) assays. Our study showed for the first time that LMOf2365_1875 encoding for a manganese-binding protein of an ABC transporter might be required for virulence. In the G. mellonella model, decreased mortality of the deletion mutant ⊿LMOf2365_1877 also indicates a possible role in the virulence potential of L. monocytogenes. In addition, the gene expression levels of L. monocytogenes virulence and stress-related genes were elevated in ΔLMOf2365_1877 under normal laboratory growth conditions. Targeting virulence factors could be a promising approach to develop new strategies against resistant microorganisms.
Acknowledgments
We would like to thank Amy Ream for RT-PCR analysis, and Dr. James Smith for critical reading of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Norton DM, Braden CR. Foodborne listeriosis. In: Ryser ET, Marth E, editors. Listeria, Listeriosis and Food Safety. CRC Press, Boca Raton, FL; 2007. pp. 305–356. [Google Scholar]
- 2.Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55: 476–511. doi: 10.1128/mr.55.3.476-511.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming DW, Cochi SL, MacDonald KL, Brondum J, Hayes PS, et al. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985; 312: 404–407. doi: 10.1056/NEJM198502143120704 [DOI] [PubMed] [Google Scholar]
- 4.Garner D, Kathariou S. Fresh produce-associated listeriosis outbreaks, sources of concern, teachable moments, and insights. J Food Prot. 2016;79:337–44. doi: 10.4315/0362-028X.JFP-15-387 [DOI] [PubMed] [Google Scholar]
- 5.Kathariou S. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J Food Prot. 2002; 65:1811–1829. doi: 10.4315/0362-028x-65.11.1811 [DOI] [PubMed] [Google Scholar]
- 6.Camejo A, Carvalho F, Reis O, Leitão E, Sousa S, Cabanes D. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence. 2011;2(5):379–94. doi: 10.4161/viru.2.5.17703 [DOI] [PubMed] [Google Scholar]
- 7.Pizarro-Cerdá J, Cossart P. Subversion of cellular functions by Listeria monocytogenes. J Pathol. 2006; 208: 215–223. doi: 10.1002/path.1888 [DOI] [PubMed] [Google Scholar]
- 8.Portnoy DA, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992; 60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VáZquez-Boland JA, Kuhn M, Berche P, Chakraborty T, DomíNguez-Bernal G, Goebel W, et al. , 2001. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2010; 14:584–640. doi: 10.1128/CMR.14.3.584-640.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty R, Kidd KK. Response. Science. 1992. Feb 28; 255(5048):1052–1053. doi: 10.1126/science.255.5048.1052-b [DOI] [PubMed] [Google Scholar]
- 11.Ireton K., Payrastre B., Cossart P. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J Biol Chem. 1999; 274:17025–17032. doi: 10.1074/jbc.274.24.17025 [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Oliver HF, Raengpradub S, Palmer ME, Orsi RH, Wiedmann M, et al. Transcriptomic and phenotypic analyses suggest a network between the transcriptional regulators HrcA and sigmaB in L. monocytogenes. Appl Environ Microbiol. 2007; 73:7981–7991. doi: 10.1128/AEM.01281-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho F, Sousa S, Cabanes D. How Listeria monocytogenes organizes its surface for virulence. Front Cell Infect Microbiol. 2014; 4:48. doi: 10.3389/fcimb.2014.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramarao N, Leroux C, Lereclus D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J Vis Exp. 2012; 70:4392. doi: 10.3791/4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, et al. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol. 2010; 76:310–317. doi: 10.1128/AEM.01301-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rakic Martinez M, Wiedmann M, Ferguson M, Datta AR. Assessment of Listeria monocytogenes virulence in the Galleria mellonella insect larvae model. PLoS One. 2017. Sep 12;12(9): e0184557. doi: 10.1371/journal.pone.0184557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson J, Mandin P, Renzoni A, Chiaruttini C, Springer M, Cossart P. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell. 2002; 110:551–561. doi: 10.1016/s0092-8674(02)00905-4 [DOI] [PubMed] [Google Scholar]
- 18.Parsons C, Lee S, Jayeola V, Kathariou S. Novel cadmium resistance determinant in Listeria monocytogenes. Appl Environ Microbiol. 2017; Feb 15;83(5): e02580–16. doi: 10.1128/AEM.02580-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BH, Garmyn D, Gal L, Guérin C, Guillier L, Rico A, et al. Exploring Listeria monocytogenes transcriptomes in correlation with divergence of lineages and virulence as measured in Galleria mellonella. Appl Environ Microbiol. 2019;85(21), e01370–19. doi: 10.1128/AEM.01370-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka KJ, Song S, Mason K, Pinkett HW. Selective substrate uptake: the role of ATP-binding cassette (ABC) importers in pathogenesis. Biochim Biophys Acta Biomembr. 2018. Apr;1860(4):868–877. doi: 10.1016/j.bbamem.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Ceruso M, Gunther IV NW, Pepe T, Cortesi ML, Fratamico P. Construction of Listeria monocytogenes mutants with in-frame deletions in putative ATP-Binding Cassette (ABC) transporters and analysis of their growth under stress conditions. J Microb Biochem Technol. 2012. a; 4:141–146. doi: 10.4172/1948-5948.1000085 [DOI] [Google Scholar]
- 22.Lewis VG, Ween MP, McDevitt CA. The role of ATP-binding cassette transporters in bacterial pathogenicity. Protoplasma. 2012; 249:919–942. doi: 10.1007/s00709-011-0360-8 [DOI] [PubMed] [Google Scholar]
- 23.Garmory HS, Titball RW. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect Immun. 2004; 72:6757–6763. doi: 10.1128/IAI.72.12.6757-6763.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eswarappa SM, Panguluri KK, Hensel M, Chakravortty D. The yejABEF operon of Salmonella confers resistance to antimicrobial peptides and contributes to its virulence. Microbiology. 2008;154(Pt 2):666–678. doi: 10.1099/mic.0.2007/011114-0 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Ream A. Gene expression profiling of Listeria monocytogenes strain F2365 during growth in ultrahigh-temperature-processed skim milk. Appl Environ Microbiol. 2008; 74:6859–6866. doi: 10.1128/AEM.00356-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae D, Crowley MR, Wang C. Transcriptome analysis of Listeria monocytogenes grown on a ready-to-eat meat matrix. J Food Prot. 2011; 74:1104–1111. doi: 10.4315/0362-028X.JFP-10-508 [DOI] [PubMed] [Google Scholar]
- 27.Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, et al. Comparative genomics of Listeria species. Science. 2001. Oct 26;294(5543):849–52. doi: 10.1126/science.1063447 [DOI] [PubMed] [Google Scholar]
- 28.Nelson KE, Fouts DE, Mongodin EF, Ravel J, DeBoy RT, Kolonay JF, et al. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 2004; 32:2386–2395. doi: 10.1093/nar/gkh562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006; 60:187–209. doi: 10.1146/annurev.micro.60.080805.142149 [DOI] [PubMed] [Google Scholar]
- 30.Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn (II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002; 70(11):6032–42. doi: 10.1128/IAI.70.11.6032-6042.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eijkelkamp BA, McDevitt CA, Kitten T. Manganese uptake and streptococcal virulence. Biometals. 2015; 28:491–508. doi: 10.1007/s10534-015-9826-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janulczyk R, Ricci S, Björck L. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect Immun. 2003; 71:2656–2664. doi: 10.1128/IAI.71.5.2656-2664.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heindl JE, Hibbing ME, Xu J, Natarajan R, Buechlein AM, Fuqua C. Discrete responses to limitation for iron and manganese in Agrobacterium tumefaciens: influence on attachment and biofilm formation. J Bacteriol. 2015; 198(5):816–829. doi: 10.1128/JB.00668-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin B, Newton SM, Shao Y, Jiang X, Charbit A, Klebba PE. Iron acquisition systems for ferric hydroxamates, haemin and haemoglobin in Listeria monocytogenes. Mol Microbiol. 2006; 59:1185–1198. doi: 10.1111/j.1365-2958.2005.05015.x [DOI] [PubMed] [Google Scholar]
- 35.Linnan MJ, Mascola L, Lou XD, Goulet V, May S, Salminen C, et al. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med. 1988; 319: 823–828. doi: 10.1056/NEJM198809293191303 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Yoo BB, Hwang CA, Suo Y, Sheen S, Khosravi P, Huang L. LMOf2365_0442 encoding for a fructose specific PTS permease IIA may be required for virulence in L. monocytogenes strain F2365. Front Microbiol. 2017. a; 8:1611. doi: 10.3389/fmicb.2017.01611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roche SM, Gracieux P, Milohanic E, Albert I, Virlogeux-Payant I, Témoin S., et al. Investigation of specific substitutions in virulence genes characterizing phenotypic groups of low-virulence field strains of Listeria monocytogenes. Appl Environ Microbiol. 2005; 71:6039–6048. doi: 10.1128/AEM.71.10.6039-6048.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair S, Milohanic E, Berche P. ClpC ATPase is required for cell adhesion and invasion of Listeria monocytogenes. Infect Immun. 2000.68:7061–7068. doi: 10.1128/IAI.68.12.7061-7068.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair S, Frehel C, Nguyen L, Escuyer V, Berche P. ClpE, a novel member of the HSP100 family, is involved in cell division and virulence of Listeria monocytogenes. Mol Microbiol. 1999; 31:185–196. doi: 10.1046/j.1365-2958.1999.01159.x [DOI] [PubMed] [Google Scholar]
- 40.Dons L, Eriksson E, Jin Y, Rottenberg ME, Kristensson K, Larsen CN, Bresciani J, Olsen JE et al. Role of flagellin and the two component CheA/CheY system of Listeria monocytogenes in host cell invasion and virulence. Infect Immun. 2004; 72:3237–3244. doi: 10.1128/IAI.72.6.3237-3244.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988; 167:1459–1471. doi: 10.1084/jem.167.4.1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilgrim S, Kolb-Mäurer A, Gentschev I, Goebel W, Kuhn M. Deletion of the gene encoding p60 in Listeria monocytogenes leads to abnormal cell division and loss of actin-based motility. Infect Immun. 2003; 71:3473–3484. doi: 10.1128/IAI.71.6.3473-3484.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandiripally VK, Westbrook DG, Sunki GR, Bhunia AK. Surface protein p104 is involved in adhesion of Listeria monocytogenes to human intestinal cell line, Caco-2. J Med Microbiol. 1999; 48: 117–124. doi: 10.1099/00222615-48-2-117 [DOI] [PubMed] [Google Scholar]
- 44.Schauer K, Geginat G, Liang C, Goebel W, Dandekar T, Fuchs TM. Deciphering the intracellular metabolism of Listeria monocytogenes by mutant screening and modelling. BMC Genomics. 2010; 11:573. doi: 10.1186/1471-2164-11-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nightingale KK, Milillo SR, Ivy RA, Ho AJ, Oliver HF, Wiedmann M. Listeria monocytogenes F2365 carries several authentic mutations potentially leading to truncated gene products, including inlB, and demonstrates atypical phenotypic characteristics. J Food Prot. 2007; 70: 482–488. doi: 10.4315/0362-028x-70.2.482 [DOI] [PubMed] [Google Scholar]
- 46.Olesen I, Vogensen FK, Jespersen L. Gene transcription and virulence potential of Listeria monocytogenes strains after exposure to acidic and NaCl stress. Foodborne Pathog Dis. 2009; 6:669–680. doi: 10.1089/fpd.2008.0243 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.