Abstract
It has been reported that the COVID-19 pandemic has predisposed adolescents to risky behaviors such as substance use and subsequent substance use disorder (SUD). However, it is unknown how the pandemic has changed the prevalence of SUD among adolescents in Uganda. We aimed to determine the prevalence of SUD and associated factors among adolescents in southwestern Uganda. Retrospectively, psychiatry ward records from November 2018 to July 2021 were collected from the largest tertiary hospital in southwestern Uganda. A total of 441 adolescent records were included in the analysis, with a mean age was 17±1.88 years, and the majority were males (50.34%). The overall prevalence of SUD was 7.26% (5.90% and 9.80% before and during the pandemic). Despite a little rise in SUD (3.9% increment) during the COVID-19 pandemic, there was no statistical difference compared to before the pandemic. The likelihood of being diagnosed with SUD was more among older adolescents at any period. In addition, having a diagnosis of bipolar mood disorder reduced the likelihood of SUD during the pandemic. This study indicated no statistical change in the diagnosis of SUD among adolescents before and during the COVID-19 pandemic. As older-male adolescents (17 to 19 years) were at higher risk of SUD, there is a need for early intervention for this group.
Introduction
Adolescence (10–19 years) is characterized by a series of developmental changes, which are highly impacted by social, cultural, and nutritional influences [1]. A vast array of neurodevelopmental changes occur during this time, including cortical thinning, gray matter volume reductions, increases in white matter volume, synaptic pruning, and reorganization within cortical and limbic regions [2–5]. These neurodevelopmental changes give rise to characteristic behaviors during adolescence, such as improvements in cognition and executive functions, increases in reward sensitivity, novelty-seeking, risk-taking behavior, and a tendency to spend more time with peers [6–8]. Some of these behavioral characteristics, in turn, contribute to a greater likelihood of initiating substance use [9, 10].
Substance use by adolescents remains a significant public health concern [1]. More than 50% of substance use initiation cases occur during adolescence [11, 12]. The common substance used among adolescents is alcohol, followed by marijuana and cigarette smoking [13, 14]. In the USA, cigarette smoking trends have been increasing due to the introduction of e-cigarettes, although cannabis is the most common substance used by adolescents [14]. In sub-Sahara Africa–a region with the highest population of adolescents, the overall prevalence of adolescent substance use was estimated at 41.6% [15]. A study in Kenya among pregnant adolescents (aged 14–18 years) found alcohol use disorder at 13.2% [16]. In Uganda, 60–71% of school-going children (12 to 24 years) use addictive substances, especially alcohol (19.3%) and Kuber (smokeless tobacco, used sublingually) at 4.4% [13]. In a study among adolescents attending the Makerere/Mulago Columbia Adolescent Health Clinic in Mulago, 15.6% used at least an addictive substance, with alcohol being dominant at 15.2% of the total population [17]. Moreover, an earlier age of onset of substance use is significantly associated with the risk of developing a substance use disorder (SUD) later in life [1, 18, 19]. Despite studies showing a prevalence of substance use among adolescents in Uganda, no particular study has been carried out to investigate the prevalence of SUD in the country.
Prior Ugandan studies reported that factors associated with substance use (as opposed to substance use disorder) among adolescents include the death of a mother, suffering from chronic illness, depression, and having friends or family members with substance use problems [16, 17]. However, following the COVID-19 pandemic, substance use and substance use disorders have been reported to have increased along with changes in associated factors [20, 21]. The factors leading to the aforementioned increase in substance use are due to the drastic change in the lifestyle of most individuals due to lockdown, social isolation, restriction of movement, and economic shutdown [20, 21]. The COVID-19 associated social and economic restrictions led to decreased opportunities for regulation of stress, anxiety, existing mental health conditions, domestic violence, child maltreatment, and traumatic experiences, hence paving the way for substance use among adolescent victims as a mode of coping [20–23]. Eventually, suicide occurrences were caused by the inaccessibility of alcohol due to lockdown, and alcohol withdrawal symptoms were reported during the pandemic [24, 25].
In Uganda, there is limited evidence on the impact of the COVID-19 pandemic on substance use disorder among the vast young population. Yet, the country had its schools closed for almost two years with no online studies or other means [26], thus removing this known protective factor (staying in school and school connectedness) against substance use among adolescents [27]. Furthermore, previous literature reports that adolescents out of school are at a higher risk of using addictive substances [28], thus putting them at risk of being diagnosed with SUD. Therefore, in this study, a retrospective record review was performed to compare the prevalence and associated factors of SUD based on two-time points, that is, before and during the COVID-19 pandemic among adolescents with mental health problems attending a psychiatric ward in Uganda.
Methods
Study area and design
This study was based on a retrospective review of the outpatient registers (Health Management Information Systems [HMIS] form 031) of the Mbarara Regional Referral Hospital (MRRH) psychiatry ward from November 2018 to July 2021. The psychiatry unit manages patients with mental illnesses confirmed by mental health professionals only and those with medical conditions that have mental illness presentations such as HIV. MRRH psychiatry ward is the largest psychiatric facility in southwestern Uganda, with a stationed child and adolescent psychiatrist and a functional specialist addiction clinic. Therefore, the facility handles and manages many adolescents especially referrals from all over southwestern Uganda, with mental illnesses, including SUD. HMIS form 031 captures the following information; patient’s number, name, address, age, anthropometric measurements, gender, next of kin, substances of addiction used, investigations, physical symptoms, diagnosis, and treatment given. At the beginning of the COVID-19 pandemic in Uganda (March 2020), 16 months data of before the COVID-19 pandemic and 17 months during the pandemic, were collected for this study.
Inclusion and exclusion criteria
Using the HMIS form 031 register, information of all adolescents aged 10 to 19 years [29] was extracted. After retrieving all the eligible registers from the psychiatry department records office, a total of 720 records were retrieved. Due to reattendances to the clinic, records with the same patient number, age, gender, patients’ names initials, year of attendance, and diagnosis were considered duplicates, and only one record was included in this study. A total of 279 records were reattendances in the same calendar year for the same diagnosis, and so were excluded from the final analysis as duplicates.
Data collection, management and quality control
The following information was extracted from the HMIS form 031: (i) patient’s number, (ii) patient name initials, (iii) age, (iv) gender (male, female), (v) substances of addiction used (alcohol; cannabis; cigarettes (smoked tobacco), Kuber (smokeless tobacco, commonly used sublingually) [30]; miraa/khat [Catha Edulis Forsk]–plant leaves that contain stimulant similar to Amphetamine [31]; others such as Akandi [32], and glue), and (vi) diagnosis. Age was categorized into three groups based on the stage of adolescence [33]; early adolescents (10–14 years), middle adolescence (15–17 years), and late adolescence (18 to 19 years). Data were entered parallel by two pairs of individuals (JA and EA) and (SMN and CK), and in case of any discrepancies, MMK resolved them. Data were cleaned in Microsoft Excel.
Substance use disorder and mental health diagnosis
The mental health diagnoses at the MRRH psychiatry ward are based on the ICD-11. Patients with physical diagnoses are referred to the department for management of their psychiatric manifestations or comorbidities. For this study, a diagnosis of SUD was considered for an individual who had been diagnosed with any type of SUD, such as alcohol use disorder, cannabis use disorder, among others [34]. An individual can be diagnosed with more than one type of substance use disorder. In addition, individuals with SUD can have both mental comorbidities such as depression, anxiety, or physical comorbidities such as HIV.
Ethics
The present study was conducted in accordance with the Declaration of Helsinki 2013 [35] and was approved by the Mbarara University of Science and Technology research ethics committee (MUST-2021-229). However, the formal consent was waivered since the data was retrospective, and it was not possible to track the involved participants. All of the information is anonymously presented in this study and there are no ethical concerns.
Data analysis
Data were analyzed using STATA version 12.0. Categorical variables were presented with frequencies and percentages. Age was presented in terms of mean and standard deviation. The Gaussian assumption was used to assess for normality based on the Shapiro-Wilks test and histograms. Chi-square tests for categorical variables or student t-tests for continuous variables were performed to determine significant differences between individuals with a diagnosis of SUD and those without. Inferential analysis of two groups, before and during the pandemic, was performed with the included study variables. Binary logistic analysis for factors associated with SUD was performed. Factors significant at bivariate logistic analysis were tested for collinearity using variance inflation factors (VIF), and those with a VIF below three were included in the final model at multiple logistic regression. The significant level was at less than 5% for a 95% confidence interval.
Results
A total of 441 records were finally included in the present analysis, with 65.31% (n = 288) being recorded before the COVID-19 pandemic. The average age of the participants included was 17±1.88 years, with almost half (48.75%) being in their late adolescence (18–19 years). About half were males (50.34%), and the commonest psychiatric diagnosis made was bipolar disorder (40.59%), followed by schizophrenia and other primary psychosis (27.66%). Detailed information on adolescent data use in this study can be found in Table 1.
Table 1. Distribution of the socio-demographics and comorbid mental and physical illnesses with substance abuse disorder.
| Variable | Total; n (%) | Substance use disorder | X2 (p-value) | |
|---|---|---|---|---|
| No; 409 (92.74%) | Yes; 32 (7.26%) | |||
| Socio-demographic information | ||||
| Admission time | ||||
| Before pandemic | 288 (65.31) | 271 (94.10) | 17 (5.90) | 2.26 (0.133) |
| During pandemic | 153 (34.69) | 138 (90.20) | 15 (9.80) | |
| Age (mean, SD) | 17.00,1.88 | 16.91,1.90 | 18.16,1.02 | -3.66 (<0.001) |
| Stage of adolescence | ||||
| Early | 43 (9.75) | 43 (100) | 0 | 17.91 (<0.001) |
| Middle | 183 (41.50) | 178 (97.27) | 5 (2.73) | |
| Late | 215 (48.75) | 188 (87.44) | 27 (12.56) | |
| Gender | ||||
| Female | 217 (49.66) | 217 (100) | 0 | 34.06 (<0.001) |
| Male | 220 (50.34) | 188 (85.45) | 32 (14.55) | |
| Comorbid mental illnesses and physical illnesses | ||||
| 1. Schizophrenia and other primary psychosis (6A2) | ||||
| No | 319 (72.34) | 288 (90.28) | 31 (9.72) | 10.38 (<0.001) |
| Yes | 122 (27.66) | 121 (99.18) | 1 (0.82) | |
| 2. Bipolar mood disorders (6A8) | ||||
| No | 262 (59.41) | 232 (88.55) | 30 (11.45) | 16.87 (<0.001) |
| Yes | 179 (40.59) | 177 (98.88) | 2 (1.12) | |
| 3. Depression | ||||
| No | 394 (89.34) | 363 (92.13) | 31 (7.87) | 0.36 (0.548) |
| Yes | 47 (10.66) | 46 (97.87) | 1 (2.13) | |
| 4. Anxiety and fear-related disorders (6B0) | ||||
| No | 426 (96.60) | 394 (92.49) | 32 (7.51) | 1.21 (0.270) |
| Yes | 15 (3.40) | (100) | 0 | |
| 5. Neurodevelopmental disorders (6A0) | ||||
| No | 431 (97.73) | 399 (92.58) | 32 (7.42) | 0.80 (0.371) |
| Yes | 10 (2.27) | 10 (100) | 0 | |
| 6. Factitious disorders (6D5) | ||||
| No | 435 (98.64) | 403 (92.64) | 32 (7.36) | 0.48 (0.490) |
| Yes | 6 (1.36) | 6 (100) | 0 | |
| 7. Elimination disorders (6C0) | ||||
| No | 440 | 408 (92.73) | 32 (7.27) | 0.08 (0.779) |
| Yes | 1 | 1 (100) | 0 | |
| 8. Personality disorder (8D1) | ||||
| No | 439 (99.55) | 407 (92.71) | 32 (7.29) | 0.16 (0.692) |
| Yes | 2 (0.45) | 2 (100) | 0 | |
| 9. Dissociative disorders (6B6) | ||||
| No | 440 (99.77) | 408 (92.73) | 32 (7.27) | 0.08 (0.779) |
| Yes | 1 (0.23) | 1 (100) | 0 | |
| 10. Disruptive behavior and dissocial disorders (6C9) | ||||
| No | 439 (99.55) | 407 (92.71) | 32 (7.29) | 0.16 (0.692) |
| Yes | 2 (0.45) | 2 (100) | 0 | |
| 11. Catatonia | ||||
| No | 440 (99.77) | 408 (92.73) | 32 (7.27) | 0.08 (0.779) |
| Yes | 1 (0.23) | 1 (100) | 0 | |
| 12. Disorders specifically associated with stress disorders (6B4) | ||||
| No | 431 (97.73) | 400 (92.81) | 31 (7.19) | 0.11 (0.735) |
| Yes | 10 (2.27) | 9 (90.00) | 1 (10.00) | |
| 13. Mental and behavioral disorders associated with pregnancy, childbirth and the puerperium, (6E2) | ||||
| No | 440 (99.77) | 408 (92.73) | 32 (7.27) | 0.08 (0.779) |
| Yes | 1 (0.23) | 1 (100) | 0 | |
| 14. Disorders of bodily distress and bodily experience (6C2) | ||||
| No | 432 (97.96) | 400 (92.59) | 32 (7.41) | 0.72 (0.397) |
| Yes | 9 (2.04) | 9 (100) | 0 | |
| 15. Impulsive control disorders (6C7) | ||||
| No | 438 (99.32) | 406 (92.69) | 32 (7.31) | 0.24 (0.627) |
| Yes | 3 (0.68) | 3 (100) | 0 | |
| 16. HIV | ||||
| No | 428 (97.05) | 396 (92.52) | 32 (7.48) | 1.05 (0.306) |
| Yes | 13 (2.95) | 13 (100) | 0 | |
Prevalence of substance abuse disorder and substance use types
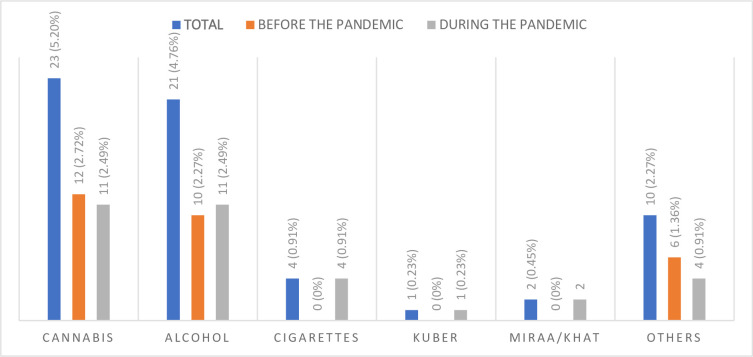
The overall prevalence of SUD was 7.26% (n = 32), where 5.90% (n = 17) adolescents had SUD before the pandemic and 9.80% (n = 15) during the pandemic. Although a 3.9% increment of SUD during the COVID-19 pandemic was observed, the difference was not statistically significant (χ2 = 2.26, p = 0.133). The most used substance was cannabis (5.21%, n = 23/441), followed by alcohol, 4.76%, (n = 21/441), and the least used was Kuber 0.23% (n = 1/441). There was a statistically significant difference between cigarette use and the pandemic period (p = 0.023), although other substances were not significantly associated (Fig 1).
Fig 1. Substances used before and during the COVID-19 pandemic.
Distribution of study variables with substance use disorder
The mean age of adolescents with SUD was higher compared to those without SUD (18.16 vs. 16.91 years; t = -3.66, p<0.001), that is, 12.56% of late adolescents had been diagnosed with SUD, whereas it was 2.73% for middle-aged adolescents and there was no SUD case in early adolescents, and this association of age group with SUD was statistically significant χ2 = 17.91, p<0.001). Furthermore, all adolescents with SUD were males (14.55% vs. 0%), and the association between gender and SUD was statistically significant (χ2 = 34.06, p<0.001). In addition, adolescents with schizophrenia and bipolar mood disorders were less likely to be diagnosed with SUD. For details about the relationship between the study variables and SUD, please refer to Table 1.
There was a statistical difference between the stage of adolescents and SUD before the COVID-19 pandemic (χ2 = 14.77, p = 0.001), whereas no difference was observed during the pandemic. More males were statistically more diagnosed with SUD during the two periods (p<0.001); however, they were more prevalent during the pandemic than before the pandemic (18.29% vs. 12.32%). Before the pandemic, adolescents without comorbidity of schizophrenia and other primary psychosis (6A2) were less prone to SUD (8.13% vs. 0%, χ2 = 6.83, p = 0.009); but such association was not statistically significant during the pandemic. Similarly, adolescents without comorbid bipolar disorder were more likely to be diagnosed with SUD in both periods (Table 2).
Table 2. Distribution of the socio-demographics and comorbid mental and physical illnesses with substance abuse disorder based on before and during the pandemic.
| Variables | Before COVID-19 pandemic | During the COVID-19 pandemic | ||||
|---|---|---|---|---|---|---|
| No | Yes | χ2 (p-value) | No | Yes | χ2 (p-value) | |
| (271, 94.10%) | (17, 5.90%) | (138, 90.20%) | (15, 9.80%) | |||
| Stage of adolescence | ||||||
| Early | 29 (100) | 0 | 14.77 (0.001) | 14 (100) | 0 | 4.64 (0.098) |
| Middle | 117 (99.15) | 1 (0.85) | 61 (93.85) | 4 (6.15) | ||
| Late | 125 (88.65) | 16 (11.35) | 63 (85.14) | 11 (14.86) | ||
| Gender | ||||||
| Female | 148 (100) | 0 | 19.38 (<0.001) | 69 (100) | 0 | 14.01 (<0.001) |
| Male | 121 (87.68) | 17 (12.32) | 67 (81.71) | 15 (18.29) | ||
| Comorbid mental illnesses and physical illnesses | ||||||
| 1. Schizophrenia and other primary psychosis (6A2) | ||||||
| No | 192 (91.87) | 17 (8.13) | 6.83 (0.009) | 96 (87.27) | 14 (12.73) | 3.78 (0.052) |
| Yes | 79 (100) | 0 | 42 (97.67) | 1 (2.33) | ||
| 2. Bipolar mood disorders (6A8) | ||||||
| No | 156 (90.17) | 17 (9.83) | 12.01 (0.001) | 76 (85.39) | 13 (14.61) | 5.55 (0.018) |
| Yes | 115 (100) | 0 | 62 (96.88) | 2 (3.13) | ||
| 3. Depression | ||||||
| No | 239 (93.36) | 17 (6.64) | 2.26 (0.133) | 124 (89.86) | 14 (10.14) | |
| Yes | 32 (100) | 0 | 14 (93.33) | 1 (6.67) | 0.18 (0.667) | |
| 4. Anxiety and fear-related disorders (6B0) | ||||||
| No | 262 (93.91) | 17 (6.09) | 0.58 (0.445) | 132 (89.80) | 15 (10.20) | 0.68 (0.410) |
| Yes | 9 (100) | 0 | 6 (100) | 0 | ||
| 5. Neurodevelopmental disorders (6A0) | ||||||
| No | 264 (93.95) | 17 (6.05) | 0.45 (0.502) | 135 (90.00) | 15 (10.00) | 0.33 (0.564) |
| Yes | 7 (100) | 0 | 3 (100) | 0 | ||
| 6. Factitious disorders (6D5) | ||||||
| No | 266 (93.99) | 17 (6.01) | 0.32 (0.572) | 137 (90.13) | 15 (9.87) | 0.11 (0.741) |
| Yes | 5 (100) | 0 | 1 (100) | 0 | ||
| 7. Elimination disorders (6C0) | ||||||
| No | 270 (94.08) | 17 (5.92) | 0.06 (0.802) | 138 (90.20) | 15 (9.80) | NA |
| Yes | 1 (100) | 0 | 0 | 0 | ||
| 8. Personality disorder (8D1) | ||||||
| No | 269 (94.06) | 17 (5.94) | 0.13 (0.722) | 138 (90.20) | 15 (9.80) | NA |
| Yes | 2 (100) | 0 | 0 | 0 | ||
| 9. Dissociative disorders (6B6) | ||||||
| No | 270 (94.08) | 17 (5.92) | 0.06 (0.802) | 138 (90.20) | 15 (9.80) | NA |
| Yes | 1 (100) | 0 | 0 | 0 | ||
| 10. Disruptive behavior and dissocial disorders (6C9) | ||||||
| No | 270 (94.08) | 17 (5.92) | 0.06 (0.802) | 137 (90.13) | 15 (9.87) | 0.11 (0.741) |
| Yes | 1 (100) | 0 | 1 (100) | 0 | ||
| 11. Catatonia | ||||||
| No | 271 (94.10) | 17 (5.90) | NA | 137 (90.13) | 15 (9.87) | 0.11 (0.741) |
| Yes | 0 | 0 | 1 (100) | 0 | ||
| 12. Disorders specifically associated with stress disorders (6B4) | ||||||
| No | 267 (94.01) | 17 (5.99) | 0.25 (0.614) | 133 (90.48) | 14 (9.52) | 0.33 (0.564) |
| Yes | 4 (100) | 0 | 5 (83.33) | 1 (16.67) | ||
| 13. Mental and behavioral disorders associated with pregnancy, childbirth and the puerperium, (6E2) | ||||||
| No | 271 (94.10) | 17 (5.90) | NA | 137 (90.13) | 15 (9.87) | 0.11 (0.741) |
| Yes | 0 | 0 | 1 (100) | 0 | ||
| 14. Disorders of bodily distress and bodily experience (6C2) | ||||||
| No | 264 (93.95) | 17 (6.05) | 0.45 (0.502) | 136 (90.07) | 15 (9.93) | 0.22 (0.639) |
| Yes | 7 (100) | 0 | 2 (100) | 0 | ||
| 15. Impulsive control disorders (6C7) | ||||||
| No | 269 (94.06) | 17 (5.94) | 0.13 (0.722) | 136 (90.07) | 15 (9.93) | 0.22 (0.639) |
| Yes | 2 (100) | 0 | 2 (100) | 0 | ||
| 16. HIV | ||||||
| No | 261 (93.88) | 17 (6.12) | 0.65 (0.420) | 135 (90.00) | 15 (10.00) | 0.33 (0.564) |
| Yes | 10 (100) | 0 | 3 (100) | 0 | ||
Factors associated with having a substance use disorder diagnosis
The variables that were significant (p<0.05) at inferential statistics (i.e., chi-square and t-test), were included in logistic analyses and those significant at bivariate logistic regressions were considered for model building. For both periods combined, all the included variables; age, comorbidity of schizophrenia and other primary psychosis (6A2), and bipolar disorder had a VIF below 3 and mean VIF of 1.21, indicating good collinearity. The final model had a sensitivity of 0%, specificity of 100%, a negative predictive value of 92.74%, and would correctly classify 92.74% of the diagnosis of SUD in both periods. The model had good goodness of fit for all the included variables, with a p-value of 0.590. The likelihood of being diagnosed with SUD increased with age [adjusted odds ratio (aOR) = 1.81, 95% confidence interval (CI) = 1.31–2.52, p<0.001]. However, having a diagnosis of bipolar mood disorder (aOR = 0.04, CI = 0.01–0.22, p = 0.001) and schizophrenia and other primary psychosis (aOR = 0.03, CI = 0.01–0.13, p<0.001) was protective against SUD.
Before the pandemic, only an increase in age was associated with SUD; whereas an increase in age and having comorbidity of bipolar disorder were associated with SUD during the pandemic. These were tested for collinearity and all had a VIF of 1. Therefore, they were included in the final model, which had a sensitivity of 0%, specificity of 100%, a negative predictive value of 90.20%, and would correctly classify 90.20% of the diagnosis of SUD during the pandemic. The likelihood of being diagnosed with SUD increased with an increase in age during the pandemic (aOR = 1.72, CI = 1.06–2.80, p = 0.028). However, having a diagnosis of bipolar disorder reduced the likelihood of being diagnosed with SUD (aOR = 0.18, CI = 0.04–0.87, p = 0.032) (Table 3).
Table 3. Logistic analysis for factors associated with substance use disorder.
| Variable | Total sample | Before the pandemic | During the pandemic | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bivariable analysis | Multivariable analysis | Bivariable analysis | Bivariable analysis | Multivariable analysis | ||||||
| Crude Odds ratio (95% confidence interval) | p-value | Crude Odds ratio (95% confidence interval) | p-value | Crude Odds ratio (95% confidence interval) | p-value | Crude Odds ratio (95% confidence interval) | p-value | Adjusted odds ratio (95% confidence interval) | P-value | |
| Age | 1.86 (1.31–2.62) | <0.001 | 1.81 (1.31–2.52) | <0.001 | 1.98 (1.20–3.24) | 0.007 | 1.76 (1.08–2.86) | 0.023 | 1.72 (1.06–2.80) | 0.028 |
| Gender | ||||||||||
| Female | 1 | 1 | 1 | |||||||
| Male | Omitted | Omitted | Omitted | |||||||
| Schizophrenia and other primary psychosis (6A2) | ||||||||||
| No | 1 | 1 | 1 | 1 | ||||||
| Yes | 0.77 (0.01–0.57) | 0.012 | 0.03 (0.01–0.22) | 0.001 | Omitted | 0.16 (0.02–1.28) | 0.085 | |||
| Bipolar mood disorders | ||||||||||
| No | 1 | 1 | 1 | 1 | 1 | |||||
| Yes | 0.09 (0.02–0.37) | 0.001 | 0.04 (0.01–0.17) | <0.001 | Omitted | 0.19 (0.04–0.87) | 0.032 | 0.18 (0.04–0.87) | 0.032 | |
Discussion
This study aimed to investigate substance use disorder (SUD) among adolescents before and during the COVID-19 pandemic using a retrospective record review of the outpatient register (HMIS form 031 at the Mbarara Regional Referral psychiatry ward, the largest tertiary hospital in southwestern Uganda). The overall prevalence of SUD was 7.26% considering all the adolescent records over two periods (5.90% and 9.80%, before and after the pandemic, respectively). There was no statistically significant difference in SUD during the two periods despite a slightly higher rate reported during the pandemic. In addition, the use of cigarettes was statistically higher during the COVID-19 pandemic. The likelihood of being diagnosed with SUD was more among older adolescents at any period. However, having a diagnosis of bipolar mood disorder reduced the likelihood of being diagnosed with SUD during the pandemic.
Despite a little rising in SUD (3.9% increase) during the COVID-19 pandemic, there was no statistically significant change compared to before the pandemic; a finding similar to other studies that found that cannabis and alcohol use did not change during the pandemic [36]. Lockdowns during the pandemic presented an opportunity to reduce the availability of substances due to the disruption in the distribution chain, as seen in other countries. For example, a 17% and 24% decline in marijuana and alcohol availability in the USA was reported during the pandemic; given the situation of substance unavailability in the USA, the substance use rates had not significantly decreased [36]. Despite these findings, other studies reported increased substance use among adolescents during the pandemic of 25.33% and 9.33% increment in alcohol and other substance use, respectively [37].
The use of substances in our study was higher than that reported in Indonesia at 5.3% for the commonly used substance—alcohol [38]. Cigarette use was statistically significantly more during the COVID-19 pandemic, which is contradictory to other studies that found no significant comparison [36]. These findings indicate that adolescents procured and accessed substances from their own homes since schools were closed during the pandemic [39]. Vaping materials and cigarettes are generally obtained through peer networks [40], and their availability kept increasing due to increased interaction during school closure making cigarettes more accessible during the lockdown.
This study found that an increase in age was associated with an increased likelihood of being diagnosed with SUD, a factor consistently associated with substance use among adolescents, as reported by many researchers [38, 40]. An increase in age leads to increased curiosity, bandwagon effect, and impulsivity towards substance use [41]. The use of addictive substances, particularly alcohol, is restricted under 18 in Uganda [42]. Therefore, adolescents who tend towards the accepted age (late adolescence, 18 to 19 years in this study; for example) were more likely to be diagnosed with SUD.
Males are more likely than females to take alcohol and drugs. The more exposure to a substance, the higher the risk of being diagnosed with SUD. Male’s predominance in substance use may be associated with the cultural acceptance of substance use among the male gender, especially in this patriarchal society [43, 44]. Also, the use of substances shows the masculinity in an individual man since weak men are believed to be unable to handle alcohol or other substances [44, 45]. Moreover, adolescents are always watching television and on the internet, which has adverts promoting male use of substances. For example, the use of alcohol by most celebrities in songs and most sports are sponsored by alcohol-producing companies [28]. The increased likelihood of being diagnosed with SUD among males could also be an effect of the increased tolerance towards alcohol that puts them at a higher risk for SUD [45, 46].
Individuals with primary psychotic or mood disorders usually use addictive substances to cope with their symptoms [47, 48]. Although teenagers with primary psychosis or mood symptoms mostly experience their first episodes of the primary disorder, most of them have not yet developed coping mechanisms, including substance use, unlike their counterparts who have experienced several episodes. This may be the case supporting the present finding that having a diagnosis of a primary psychotic or mood disorder was less associated with SUD.
The interpretation of this study’s findings should be made with caution in view of the following limitations. First of all, it was a retrospective cross-sectional study whose data depended on the efficiency of record reporting by the department staff, an aspect that has caused inconsistencies with HMIS reporting [49]. In addition, many variables that could have influenced the findings, such as stressors and family environment, were not retrieved from the registry and hence not included in the analysis. Finally, the data included in the analysis were from one facility, limiting the generalization of the findings throughout the country.
Conclusions
The present study is one of the first approaches to investigating the change of SUD after the COVID-19 pandemic inception. The findings reported herein indicate a slight increment of SUD among Ugandan adolescents during the pandemic. Therefore, there is a need to screen and treat adolescents for SUD as the pandemic progresses, especially among male-older adolescents. Some strategies, for example, reopening schools, and increasing support in schools and families for managing and identifying SUD to contribute towards early intervention for preventing the hazardous consequences of SUD, such as an increase in crime, are highly suggested.
Acknowledgments
The authors acknowledge their affiliation, the African Center for Suicide and Research. Also, aknowledge the support of the Department of Psychiatry Mbarara Regional Referral Hospital.
Data Availability
All the data used is available from: https://doi.org/10.6084/m9.figshare.19411322.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Hamidullah S, Thorpe HHA, Frie JA, Mccurdy RD, Khokhar JY. Adolescent substance use and the brain: behavioral, cognitive and neuroimaging correlates. Frontiers in Human Neuroscience. 2020;14(298). doi: 10.3389/fnhum.2020.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider M. Adolescence as a vulnerable period to alter rodent behavior. Cell and Tissue Research. 2013;354(1):99–106. Epub 2013/02/23. doi: 10.1007/s00441-013-1581-2 [DOI] [PubMed] [Google Scholar]
- 3.Jaworska N, MacQueen G. Adolescence as a unique developmental period. Journal of Psychiatry & Neuroscience: JPN. 2015;40(5):291–3. Epub 2015/08/21. doi: 10.1503/jpn.150268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumontheil I. Adolescent brain development. Current Opinion in Behavioral Sciences. 2016;10:39–44. doi: 10.1016/j.cobeha.2016.04.012 [DOI] [Google Scholar]
- 5.Thorpe HHA, Hamidullah S, Jenkins BW, Khokhar JY. Adolescent neurodevelopment and substance use: receptor expression and behavioral consequences. Pharmacology & Therapeutics. 2020;206:107431. Epub 2019/11/11. doi: 10.1016/j.pharmthera.2019.107431 [DOI] [PubMed] [Google Scholar]
- 6.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–63. Epub 2000/05/19. doi: 10.1016/s0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- 7.Choudhury S, Blakemore SJ, Charman T. Social cognitive development during adolescence. Social Cognitive and Affective Neuroscience. 2006;1(3):165–74. Epub 2008/11/06. doi: 10.1093/scan/nsl024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romer D. Adolescent risk taking, impulsivity, and brain development: implications for prevention. Developmental Psychobiology. 2010;52(3):263–76. Epub 2010/02/23. doi: 10.1002/dev.20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisdahl KM, Sher KJ, Conway KP, Gonzalez R, Feldstein Ewing SW, Nixon SJ, et al. Adolescent brain cognitive development (ABCD) study: overview of substance use assessment methods. Developmental Cognitive Neuroscience. 2018;32:80–96. Epub 2018/03/22. doi: 10.1016/j.dcn.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garofoli M. Adolescent substance abuse. Primary Care. 2020;47(2):383–94. Epub 2020/05/20. doi: 10.1016/j.pop.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Blanco C, Flórez-Salamanca L, Secades-Villa R, Wang S, Hasin DS. Predictors of initiation of nicotine, alcohol, cannabis, and cocaine use: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). The American Journal on Addictions. 2018;27(6):477–84. Epub 2018/08/09. doi: 10.1111/ajad.12764 [DOI] [PubMed] [Google Scholar]
- 12.Gray KM, Squeglia LM. Research Review: What have we learned about adolescent substance use? Journal of Child Psychology and Psychiatry. 2018;59(6):618–27. Epub 2017/07/17. doi: 10.1111/jcpp.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbo C, Okello ES, Muhwezi W, Akello G, Ovuga E. Alcohol, substance use and psychosocial competence of adolescents in selected secondary schools in Uganda: a cross sectional survey. International Neuropsychiatric Disease Journal. 2016;7(2):25387. doi: 10.9734/INDJ/2016/25387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future National Survey Results on Drug Use, 1975–2017: Overview, key findings on adolescent drug use. Institute for Social Research, University of Michigan, 2018.Available from: http://files.eric.ed.gov/fulltext/ED594190.pdf. [Google Scholar]
- 15.Olawole-Isaac A, Ogundipe O, Amoo EO, Adeloye D. Substance use among adolescents in sub-Saharan Africa: a systematic review and meta-analysis. South African Journal of Child Health. 2018;2018(1). Available from: https://hdl.handle.net/10520/EJC-10deb396ce [Google Scholar]
- 16.Kimbui E, Kuria M, Yator O, Kumar M. A cross-sectional study of depression with comorbid substance use dependency in pregnant adolescents from an informal settlement of Nairobi: drawing implications for treatment and prevention work. Annals of General Psychiatry. 2018;17(1):53. doi: 10.1186/s12991-018-0222-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry MB, Bakeera-Kitaka S, Lubega K, Snyder SA, LaRussa P, Pfeffer B. Depressive symptoms, sexual activity, and substance use among adolescents in Kampala, Uganda. African Health Sciences. 2019;19(2):1888–96. doi: 10.4314/ahs.v19i2.12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taioli E, Wynder EL. Effect of the age at which smoking begins on frequency of smoking in adulthood. The New England Journal of Medicine. 1991;325(13):968–9. Epub 1991/09/26. doi: 10.1056/NEJM199109263251318 [DOI] [PubMed] [Google Scholar]
- 19.Viner RM, Taylor B. Adult outcomes of binge drinking in adolescence: findings from a UK national birth cohort. Journal of Epidemiology and Community Health. 2007;61(10):902–7. Epub 2007/09/18. doi: 10.1136/jech.2005.038117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enns A, Pinto A, Venugopal J, Grywacheski V, Gheorghe M, Kakkar T, et al. Substance use and related harms in the context of COVID-19: a conceptual model. Health Promotion and Chronic Disease Prevention in Canada: Research, Policy and Practice. 2020;40(11–12):342–9. Epub 2020/09/17. doi: 10.24095/hpcdp.40.11/12.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Molecular Psychiatry. 2021;26(1):30–9. Epub 2020/09/16. doi: 10.1038/s41380-020-00880-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fegert JM, Vitiello B, Plener PL, Clemens V. Challenges and burden of the Coronavirus 2019 (COVID-19) pandemic for child and adolescent mental health: a narrative review to highlight clinical and research needs in the acute phase and the long return to normality. Child and Adolescent Psychiatry and Mental Health. 2020;14(1):20. doi: 10.1186/s13034-020-00329-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardell JD, Kempe T, Rapinda KK, Single A, Bilevicius E, Frohlich JR, et al. Drinking to cope during COVID-19 pandemic: the role of external and internal factors in coping motive pathways to alcohol use, solitary drinking, and alcohol problems. Alcoholism, Clinical and Experimental Research. 2020;44(10):2073–83. Epub 2020/09/02. doi: 10.1111/acer.14425 [DOI] [PubMed] [Google Scholar]
- 24.Dsouza DD, Quadros S, Hyderabadwala ZJ, Mamun MA. Aggregated COVID-19 suicide incidences in India: Fear of COVID-19 infection is the prominent causative factor. Psychiatry Research. 2020;290:113145. Epub 2020/06/17. doi: 10.1016/j.psychres.2020.113145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syed NK, Griffiths MD. Nationwide suicides due to alcohol withdrawal symptoms during COVID-19 pandemic: a review of cases from media reports. Journal of Psychiatry Research. 2020;130:289–91. Epub 2020/09/01. doi: 10.1016/j.jpsychires.2020.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Africanews. Schools remain closed in Uganda for over 77 weeks due to covid 19 2021. Available from: https://www.africanews.com/2021/10/28/schools-remain-closed-in-uganda-for-over-77-weeks-due-to-covid-19//. [Google Scholar]
- 27.Weatherson KA, O’Neill M, Lau EY, Qian W, Leatherdale ST, Faulkner GEJ. The protective effects of school connectedness on substance use and physical activity. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine. 2018;63(6):724–31. Epub 2018/10/03. doi: 10.1016/j.jadohealth.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 28.Kabwama SN, Matovu JKB, Ssenkusu JM, Ssekamatte T, Wanyenze RK. Alcohol use and associated factors among adolescent boys and young men in Kampala, Uganda. Substance Abuse Treatment, Prevention, and Policy. 2021;16(1):49. doi: 10.1186/s13011-021-00385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organisation. Adolescent health 2021. Available from: https://www.who.int/health-topics/adolescent-health#tab=tab_1. [Google Scholar]
- 30.The School Series. Kuber 2022. Available from: http://school-series.com/drugs/67-kuber#:~:text=Kuber%20is%20smokeless%20tobacco%20that,because%20it%20has%20no%20smell. [Google Scholar]
- 31.Wabe NT. Chemistry, pharmacology, and toxicology of khat (catha edulis forsk): a review. Addiction & health. 2011;3(3–4):137–49. Epub 2011/07/01. [PMC free article] [PubMed] [Google Scholar]
- 32.Kaggwa MM, Nkola R, Najjuka SM, Bongomin F, Ashaba S, Mamun MA. Extrapyramidal side effects in a patient with alcohol withdrawal symptoms: a reflection of quality of the mental health care system. Risk Management and Healthcare Policy. 2021;14:2789–95. doi: 10.2147/RMHP.S314451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Association of Maternal & Child Health Programs. Adolescent development 2021. Available from: http://www.amchp.org/programsandtopics/AdolescentHealth/projects/Pages/AdolescentDevelopment.aspx#:~:text=Researchers%20suggest%20adolescence%20undergo%20three,and%20late%20adolescence%2Fyoung%20adulthood.&text=Early%20Adolescence%20occurs%20between%20ages%2010%2D14. [Google Scholar]
- 34.Basu D, Ghosh A. Substance use and other addictive disorders in international classification of Diseases-11, and their relationship with diagnostic and statistical Manual-5 and international classification of Diseases-10. Indiaan Journal of Social Psychiatry. 2018;34(5):54–62. doi: 10.4103/ijsp.ijsp_83_17 [DOI] [Google Scholar]
- 35.World Medical Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 36.Miech R, Patrick ME, Keyes K, O’Malley PM, Johnston L. Adolescent drug use before and during U.S. national COVID-19 social distancing policies. Drug and Alcohol Dependence. 2021;226:108822. doi: 10.1016/j.drugalcdep.2021.108822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodge KA, Skinner AT, Godwin J, Bai Y, Lansford JE, Copeland WE, et al. Impact of the COVID-19 pandemic on substance use among adults without children, parents, and adolescents. Addictive Behaviors Reports. 2021;14:100388. doi: 10.1016/j.abrep.2021.100388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen LT, Siste K, Hanafi E, Murtani BJ, Christian H, Limawan AP, et al. Insights into adolescents’ substance use in a low–middle-income country during the COVID-19 pandemic. Frontiers in Psychiatry. 2021;12:1743. doi: 10.3389/fpsyt.2021.739698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hearst MO, Fulkerson JA, Maldonado-Molina MM, Perry CL, Komro KA. Who needs liquor stores when parents will do? The importance of social sources of alcohol among young urban teens. Preventive Medicine. 2007;44(6):471–6. doi: 10.1016/j.ypmed.2007.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenk KM, Toomey TL, Shi Q, Erickson DJ, Forster JL. Do sources of cigarettes among adolescents vary by age over time? Journal of Child & Adolescent Substance Abuse. 2014;23(2):137–43. Epub 2014/02/25. doi: 10.1080/1067828X.2012.750972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Argyriou E, Um M, Carron C, Cyders MA. Age and impulsive behavior in drug addiction: A review of past research and future directions. Pharmacology, Biochemistry, and Behavior. 2018;164:106–17. Epub 2017/08/06. doi: 10.1016/j.pbb.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasirye R. Uganda and the new alcohol control bill 2016 Sweden: Movendi International; 2016. [cited 2021]. Available from: https://movendi.ngo/blog/2016/09/26/uganda-new-alcohol-control-bill-2016/#:~:text=No%20person%20under%20the%20age,sale%20and%20consumption%20of%20alcohol. [Google Scholar]
- 43.Dumbili EW. ’What a man can do, a woman can do better’: gendered alcohol consumption and (de)construction of social identity among young Nigerians. BMC Public Health. 2015;15:167. Epub 2015/04/18. doi: 10.1186/s12889-015-1499-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwamoto DK, Cheng A, Lee CS, Takamatsu S, Gordon D. "Man-ing" up and getting drunk: the role of masculine norms, alcohol intoxication and alcohol-related problems among college men. Addictive Behavoir 2011;36(9):906–11. Epub 2011/05/31. doi: 10.1016/j.addbeh.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR, Ahlström S, Bondy S, et al. Gender differences in alcohol consumption and adverse drinking consequences: cross-cultural patterns. Addiction (Abingdon, England). 2000;95(2):251–65. Epub 2000/03/21. doi: 10.1046/j.1360-0443.2000.95225112.x [DOI] [PubMed] [Google Scholar]
- 46.Moonajilin MS, Kamal MKI, Mamun FA, Safiq MB, Hosen I, Manzar MD, et al. Substance use behavior and its lifestyle-related risk factors in Bangladeshi high school-going adolescents: An exploratory study. PLoS One. 2021;16(7):e0254926. Epub 2021/07/22. doi: 10.1371/journal.pone.0254926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer TD, McDonald JL, Douglas JL, Scott J. Do patients with bipolar disorder drink alcohol for different reasons when depressed, manic or euthymic? Journal of Affective Disorders. 2012;136(3):926–32. Epub 2011/10/05. doi: 10.1016/j.jad.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 48.Cetty L, Shahwan S, Satghare P, Devi F, Chua BY, Verma S, et al. Hazardous alcohol use in a sample of first episode psychosis patients in Singapore. BMC Psychiatry. 2019;19(1):91. doi: 10.1186/s12888-019-2073-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muhindo R, Joloba E. Health Management Information System (HMIS); Whose Data is it Anyway? Contextual Challenges. Review of Public Administration and Management. 2016;4. doi: 10.4172/2315-7844.1000190 [DOI] [Google Scholar]