Abstract
The production of Alternaria alternata f. sp. lycopersici host-specific toxins (AAL toxins) and epoxide hydrolase (EH) activity were studied during the growth of this plant-pathogenic fungus in stationary liquid cultures. Media containing pectin as the primary carbon source displayed peaks of EH activity at day 4 and at day 12. When pectin was replaced by glucose, there was a single peak of EH activity at day 6. Partial characterization of the EH activities suggests the presence of three biochemically distinguishable EH activities. Two of them have a molecular mass of 25 kDa and a pI of 4.9, while the other has a molecular mass of 20 kDa and a pI of 4.7. Each of the EH activities can be distinguished by substrate preference and sensitivity to inhibitors. The EH activities present at day 6 (glucose) or day 12 (pectin) are concomitant with AAL toxin production.
Alternaria alternata f. sp. lycopersici is a fungal pathogen that causes the Alternaria stem canker disease of tomatoes (17). During disease development and in liquid culture, the pathogen secretes host-specific toxins (AAL toxins) which, in purified form, elicit cell death patterns characteristic of the stem canker (36). The ability of the pathogen to infect the leaves, stems, and green fruit of tomatoes is limited to genotypes that are homozygous for the recessive allele (asc/asc) of the Asc gene (11). The Asc gene also regulates toxin sensitivity; thus, the toxins function as chemical determinants of the stem canker disease (11). Moreover, AAL toxins, which are members of the same class of sphinganine analog mycotoxins as fumonisins, inhibit ceramide synthase in rat hepatocytes (28) and induce apoptosis in monkey kidney cells (41). Unlike the case with fumonisins (24), the effects of chronic exposure to AAL toxins on animal health are still unresolved. The first of the AAL toxins (toxin A [TA]) was characterized in 1981 (7), and more recently (8), new isomeric toxins were purified and characterized (Fig. 1). The presence of one pair of vicinal diols, free or esterified, in the structure of each of the AAL toxins (Fig. 1) suggests the possible involvement of an epoxide hydrolase (EH) in their synthesis. This hypothetical mechanism is supported by the fact that one of the oxygen atoms of the diol came from direct incorporation of atmospheric oxygen and the other came from water (10).
FIG. 1.
Structures and proposed metabolic pathway for AAL toxins from A. alternata f. sp. lycopersici.
EHs (EC 3.3.2.3) catalyze the hydrolysis of epoxides or arene oxides to their corresponding diols by the addition of water (34). Several members of this ubiquitous enzyme subfamily have been described in organisms as diverse as mammals (21), plants (37), insects (13), and microorganisms (16). Because of their involvement in the metabolism of various xenobiotics (27, 42), many of which are suspected to be carcinogenic, and natural oxylipins (29, 45), mammalian enzymes have been extensively studied (see reference 21 for a review). Based on sequence similarity, most EHs are members of the α/β hydrolase fold family (1). This family of enzymes hydrolyzes their substrates in a two-step mechanism involving the formation and hydrolysis of a covalent alkyl-enzyme intermediate formed with a nucleophilic aspartic acid (4, 26, 40). In comparison with mammalian EHs, little is known about EHs from other species, especially from filamentous fungi. An EH induced by a cutin extract of plants has been partially characterized from Fusarium solani pisi (25). This enzyme apparently is related to the ability of the mycelium to infect some plants (44). More recently, fungal EHs have attracted attention for their potential in asymmetric organic synthesis (1). However, little is known of the physiological significance of these enzymes. In the case of dematiaceous fungi, EH activities are constitutively expressed coincident with secondary metabolite pigment production in stationary phase or idiophase (19).
In a preliminary study (35), AAL toxin production by A. alternata f. sp. lycopersici was shown to occur concomitant with the expression of an EH activity. Moreover, both AAL toxin production and EH activity were enhanced by clofibrate, which is well known to induce EH in mammals (19). However, some questions have not been answered. Is there a direct link between the enzyme and production of AAL toxins, i.e., is the EH involved in the toxin metabolism? Is the increase in EH activity that is measured following the administration of clofibrate due to increased production of the same enzyme or production of a new form? To answer these questions, we first investigated the effects of the pH, the carbon source, the time of fermentation, and the presence of clofibrate on the production of EH activity and of toxin. Second, we characterized the EH activities obtained under different culture conditions.
MATERIALS AND METHODS
Microorganisms and chemicals.
The single-conidium isolate (12) of A. alternata f. sp. lycopersici (AS27-3) used herein was originally isolated from a field-infected tomato plant (17) and maintained in the laboratory on cornmeal agar. [14C]cis-9-10-epoxystearic acid (ESA), [3H]trans-stilbene oxide (t-SO), [3H]cis-stilbene oxide (c-SO), [3H]trans-1,3-diphenylpropene oxide (t-DPPO), and [3H]cis-1,3-diphenylpropene oxide (c-DPPO) were previously synthesized in this laboratory (1, 18). [3H]labeled-juvenile hormone III (JH-III) was purchased from Amersham Life Science (Arlington Heights, Ill.). Unlabeled t-DPPO was synthesized as described previously (1). Chalcone oxide inhibitors were synthesized in the laboratory (32). Other chemicals were purchased from Aldrich Chemicals (Milwaukee, Wis.) and used without any further purification. The liquid scintillation cocktail CytoScint was purchased from Fisher Scientific (Fairlawn, N.J.). The bicinchoninic acid (BCA) reagent for protein concentration determination was obtained from Pierce, Inc. (Rockford, Ill.).
Media and culture conditions.
A. alternata f. sp. lycopersici and A. alternata (black mold) were grown on liquid media containing (in grams per liter): glycine, 0.75; NaCl, 0.1; K2HPO4 · 3H2O, 1.31; MgSO4 · 7H2O, 0.5; CaCl2 · 2H2O, 0.13; yeast extract, 0.5; malic acid 0.69; and pectin (P9135; Sigma), 22.3, or glucose, 20.7. Both media were adjusted to a final pH of 3.7 and inoculated at a final concentration of 3.3 × 103 conidia/ml of medium, and 30-ml portions were dispensed into plastic petri dishes (three replicates) and grown at room temperature (20 to 25°C) under cool-white fluorescent lighting (12 h/day). For the pH study, the above glucose medium was adjusted to the desired pH between 2.1 and 6.0 with 10 N NaOH or 5 N HCl, brought to volume, and inoculated, and 30-ml portions were dispensed into plastic petri dishes (four replicates). Cell culture filtrate and mycelium material were prepared by vacuum filtration (Whatman no. 1) at 2 to 15 days after inoculation, according to each experiment. The dry mass of mycelium was measured after drying at 80°C under a vacuum to a constant weight (usually for 24 h).
Subcellular extract preparation.
The harvested mycelium was resuspended in 100 mM sodium phosphate buffer (pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride (PMSF), EDTA, and dithiothreitol (DTT) (buffer A) and was disrupted with a Polytron homogenizer (9,000 rpm for 2 min). The homogenate was centrifuged at 10,000 × g for 20 min at 4°C. The protein concentration of the supernatant (crude extract) was estimated by a BCA assay using bovine serum albumin (BSA) as a standard.
Enzyme assays.
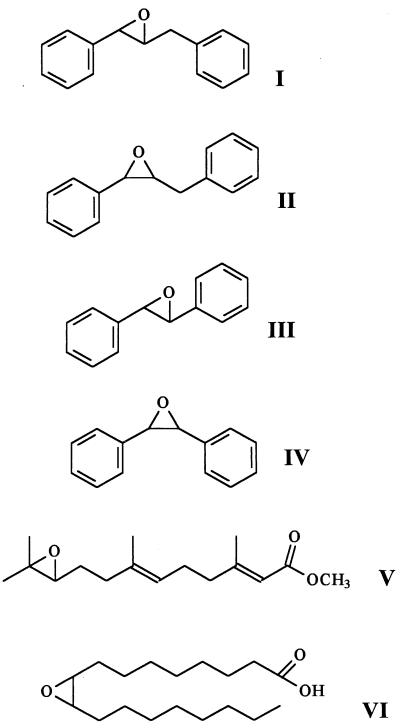
The EH activities of the crude extracts were measured routinely by using t-DPPO (compound I) as described previously (5). Briefly, 100 μl of cell extracts diluted in 100 mM sodium phosphate buffer (pH 7.4) containing 0.1 mg of BSA/ml was incubated at 30°C for 2 min. t-DPPO (1 μl of 5 mM solution in dimethyl formamide [DMF]) was added (final concentration, 50 μM) with a Hamilton repeating dispenser syringe; a standard deviation of less than 5% in the amount added was observed. The mixture was incubated at 30°C for 10 min. The reaction was quenched by the addition of 60 μl of methanol. Iso-octane (200 μl) permitted the extraction of the remaining epoxide (99%), while 91% of the diol formed stayed in the aqueous phase (5). The quantity of diol formed was determined by using a liquid scintillation counter (model 1409; Wallac, Gaithersburg, Md.) to quantify the radioactivity contained in the aqueous phase. Assays were performed in triplicate. One unit of EH corresponds to the amount of enzyme that catalyzed the formation of 1 μmol of diol per min under the above conditions. The linearity of the assay was verified versus the enzyme concentration (0 to 2 mU/ml) and the incubation time (0 to 30 min). The EH activities of crude extracts were also measured by using c-DPPO (compound II), t-SO (compound III), c-SO (compound IV), JH-III (compound V), and ESA (compound VI) as described previously (1, 33, 42).
Inhibition studies.
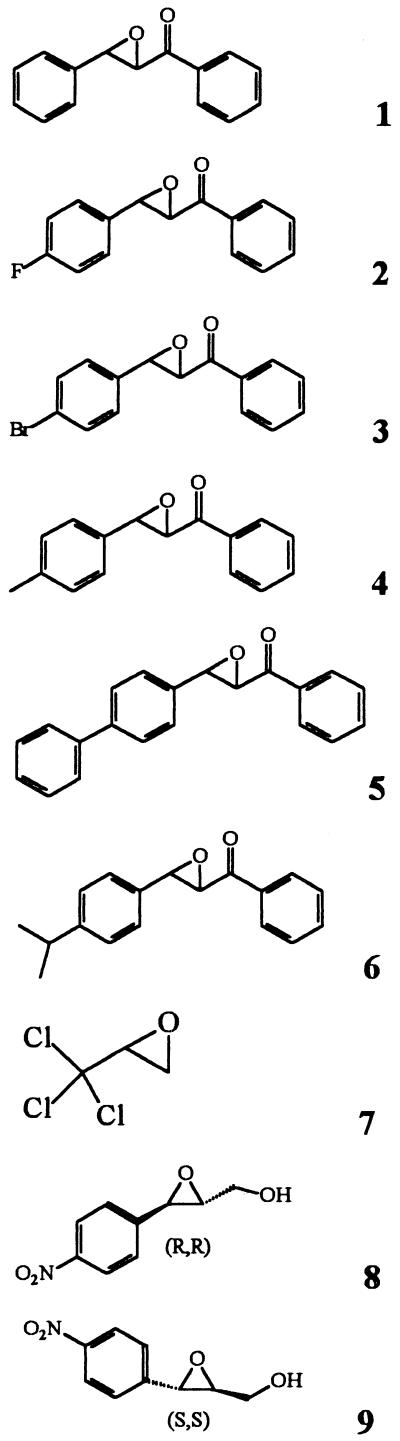
To measure the effect of group-selective reagents, 100 μl of enzyme extracts in phosphate buffer (pH 7.4; 0.5 mU/ml) was incubated at 30°C for 15 min with 1 μl of the inhibitor solution in DMF or water (final concentration of inhibitor, 1 mM). The remaining activity was then determined by using t-DPPO (compound I) as described above. Results are generated from at least three separate runs, each in triplicate. For the determination of the concentration of inhibitor that reduces enzyme activity by 50% (IC50), 100 μl of enzyme extracts in phosphate buffer (pH 7.4; 0.5 mU/ml) was incubated at 30°C for 15 min with 1 μl of the inhibitor solution in DMF (final concentration of inhibitor, 0.05 to 250 μM). The remaining activity was then determined by using t-DPPO (compound I) as described above. IC50s were determined by regression of at least five datum points, with a minimum of two points in the linear region of the curve on either side of the IC50. The curve was generated from at least three separate runs, each in triplicate, to obtain the standard deviation in Table 4. In at least one run, compounds of similar potency were included to ensure the rank order of inhibitors.
TABLE 4.
Effect of competitive inhibitors on the EH activity of extracts of A. alternata f. sp. lycopersici with t-DPPO as a substrate a
| Inhibitor | IC50 (μM)b
|
|||||
|---|---|---|---|---|---|---|
| Pectin medium
|
Glucose medium (day 6)
|
|||||
| Day 6
|
Day 12
|
|||||
| Control | With clofibrate | Control | With clofibrate | pH 4 | pH 6 | |
 |
32 ± 4 | 41 ± 4 | >250 | 21 ± 3 | 12 ± 3 | 18 ± 2 |
| 21 ± 3 | 16 ± 2 | >250 | 15 ± 3 | 8 ± 2 | 16 ± 3 | |
| 100 ± 8 | 92 ± 7 | >250 | 10 ± 2 | 10 ± 1 | 7 ± 1 | |
| 160 ± 10 | 230 ± 10 | 182 ± 13 | 2 ± 1 | 4 ± 1 | 2 ± 1 | |
| >250 | >250 | >250 | >250 | >250 | >250 | |
| 56 ± 6 | 60 ± 7 | 8 ± 1 | 68 ± 7 | 135 ± 7 | 40 ± 8 | |
| >250 | >250 | >250 | >250 | >250 | >250 | |
| >250 | >250 | >250 | >250 | >250 | >250 | |
| 36 ± 3 | 32 ± 2 | 158 ± 9 | 43 ± 4 | 32 ± 3 | 36 ± 2 | |
Enzyme extracts (0.5 mU/ml) were incubated with inhibitors for 15 min in phosphate buffer (pH 7.4) at 30°C prior to substrate introduction.
Mean ± standard deviation (n = 3).
Determination of the molecular weight and pI.
The molecular weight associated with EH activity in the crude extract was estimated from the elution profile on a gel filtration column. The enzyme extract (0.5 ml) was applied to a Sephacryl-S100 (Pharmacia, Uppsala, Sweden) column (1.5 by 100 cm), equilibrated with buffer A (flow rate, 10 ml/h; fraction volume, 1 ml). The molecular weight was calculated by comparing the elution constant (Kav) of the EH activity with that of the following standard proteins: alcohol dehydrogenase (150 kDa), BSA (66 kDa), ovalbumin (43 kDa), chymotrypsinogen A (25 kDa), and RNase A (13.7 kDa). The void and exclusion volumes were determined by using dextran blue and vitamin B12. Electrofocusing was performed with pH 4.0 to 6.5 gradient gels by using the Pharmacia LKB Multiphor system and standard Pharmacia procedures. After migration the gel was cut in 5-mm slices from the anode to the cathode. The gel pieces were incubated in 0.5 ml of buffer A during 1 h at 4°C. The EH activity in the buffer was then measured.
Measurement of toxin production.
The quantity of AAL toxins present in the cell culture filtrate was determined by using an indirect immunoassay method with sera from mouse 57 and coating antigen-C-BSA (38). TA purified in our laboratory and shown by 1H NMR to be at least 95% pure (9) was used as an analytical standard.
RESULTS
Effect of pH.
Cells of A. alternata f. sp. lycopersici were grown on glucose media that had a range of pH values between 2.1 and 6.0. Based on results obtained in a preliminary study to determine the kinetics of toxin production (data not shown), cells were harvested after 6 days of culture. Mycelial growth, EH activities, and AAL toxin production are dependent on the pH of the medium (Table 1). Fungal growth is not observed at day 6 in the lowest-pH medium; however, growth is apparent in pH 2.6 medium and increases to a maximum (average, 4.3 g · liter−1) in media at pH 3.6 through pH 6.0. A fivefold increase in both EH activity and AAL toxin production is observed for cultures grown at pH 3.1 compared to pH 2.6. EH activity increases another 2-fold, but AAL toxin levels decrease 20-fold, when the pH of the medium is increased from 3.1 to 3.6. For higher pH values, the EH activity and the AAL toxin production level off at averages of 1.8 mU · mg−1 and 13 mg · liter−1, respectively. Our standard pectin medium (35) has a final pH of 3.7, and no attempt was made to study the effect of pH on pectin media for the following reasons: (i) sterilization by autoclaving alters the initial pH of pectin media, (ii) pectin strongly buffers the standard medium at pH 3.7, and (iii) filter sterilization after autoclaving is impractical due to the viscosity of pectin in the media. For the study of factors other than pH, cultures were conducted at pH 3.7 for both glucose and pectin media.
TABLE 1.
Effect of the initial pH on the growth, EH activity, and AAL toxin production of A. alternata f. sp. lycopersicia
| pH | Dry mass (g · liter−1) | EH activity (mU · mg−1)b | Concn of toxinc (mg · liter−1) |
|---|---|---|---|
| 2.1 | NGd | ||
| 2.6 | 0.9 ± 0.2 | 0.6 ± 0.1 | 123 ± 24 |
| 3.1 | 2.6 ± 0.3 | 3.0 ± 0.4 | 562 ± 101 |
| 3.6 | 4.7 ± 0.3 | 6.0 ± 0.5 | 21 ± 10 |
| 4.1 | 4.5 ± 0.6 | 2.6 ± 0.3 | 18 ± 8 |
| 4.6 | 4.5 ± 0.6 | 1.8 ± 0.1 | 13 ± 5 |
| 5.1 | 4.2 ± 0.7 | 1.5 ± 0.1 | 14 ± 2 |
| 5.6 | 4.0 ± 0.2 | 1.6 ± 0.2 | 14 ± 3 |
| 6.0 | 4.1 ± 0.4 | 1.7 ± 0.1 | 7 ± 1 |
The fungus was grown in petri dishes containing 30 ml of medium with glucose as the carbon source. The mycelium was harvested on the 6th day of culture. Results shown are means ± standard deviations for four culture replicates.
One unit is equivalent to 1 μmol of diol formed/min.
Total immunoreactive material expressed as TA equivalents.
NG, no growth.
Effect of the carbon source and of the presence of clofibrate.
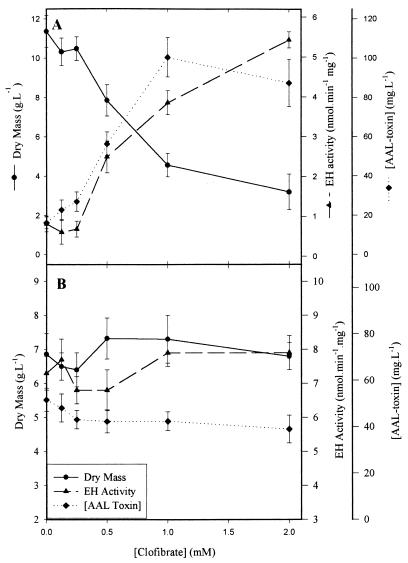
In the absence of clofibrate, twofold-greater growth of the mycelium is observed on pectin (11 g · liter−1 [Fig. 2A]) compared to glucose medium (7 g · liter−1 [Fig. 2B]) after 6 days of culture, while nine- and four-fold-higher EH activity and AAL toxin production, respectively, are observed on glucose medium. Interestingly, the addition of clofibrate to the culture has different effects on fungal growth in media with different carbon sources. On pectin media, at a concentration of clofibrate higher than 0.25 mM, a significant decrease in biomass production is observed, while significant increases in both EH activity and toxin production are observed. For 1 mM and higher clofibrate concentrations, production of the AAL toxins levels off at around 90 mg · liter−1, while the EH activity rises more slowly to reach a value of 5.5 mU · mg−1 at 2 mM. These results are consistent with earlier observations (35) and are close to those obtained on glucose media without clofibrate (Fig. 2B). When A. alternata f. sp. lycopersici is grown on the glucose media, the addition of clofibrate has no significant (α = 0.05) effect on mycelium growth, EH activity, or AAL toxin production.
FIG. 2.
Effect of clofibrate concentration on the growth, EH activity, and toxin production of A. alternata f. sp. lycopersici grown in petri dishes containing 30 ml of medium. Clofibrate was added in ethanol (0.5% of the total volume of culture) 2 days after culture inoculation; the mycelium and cell culture filtrate were obtained on the 6th day of culture. (A) Pectin as the carbon source; (B) glucose as the carbon source.
Kinetics of growth, EH activity, and toxin production.
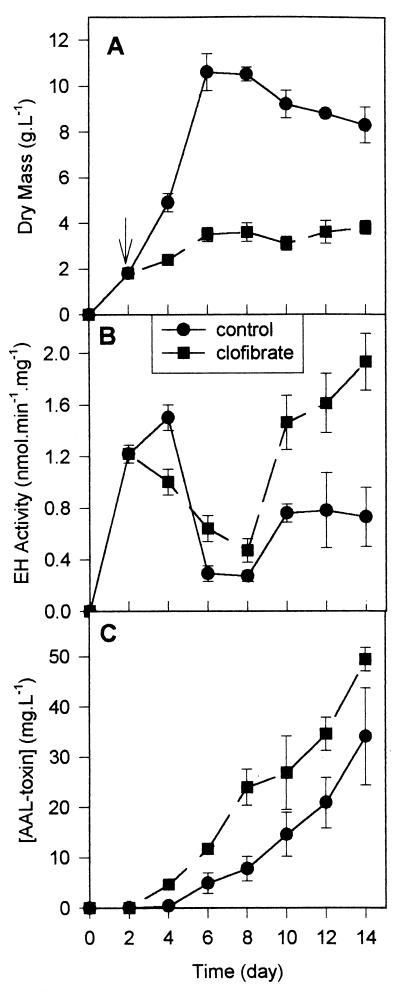
Culture of A. alternata f. sp. lycopersici in pectin medium was monitored over a 2-week period (Fig. 3). In the absence of clofibrate, an exponential phase is observed between days 2 and 6, and cells reach a biomass value of 11 g · liter−1. The addition of 1 mM clofibrate on the 2nd day of incubation (Fig. 3A) stops the growth of the mycelium at around 3.5 g of dry biomass · liter−1. In the absence or presence of clofibrate, two peaks of EH activities are observed (Fig. 3B): the first peak at around 3 days of culture (beginning of the exponential phase) and the second peak at around 12 days (during the stationary phase). The clofibrate decreases the first peak but increases the second peak twofold. A similar pattern is observed for the total EH activity, expressed as enzymatic units per gram of mycelium (data not shown). For both culture conditions, AAL toxin production starts on day 4 and continues over the period sampled (Fig. 3C). However, in the presence of clofibrate, two to three times more AAL toxins are synthesized.
FIG. 3.
Kinetics of growth, EH activities, and AAL toxin production by A. alternata f. sp. lycopersici grown in petri dishes containing 30 ml of medium with pectin as the carbon source and with (■) or without (●) 1 mM clofibrate. Clofibrate was added on the 2nd day of culture (arrow). (A) Fungal biomass; (B) EH specific activity with t-DPPO as the substrate; (C) concentration of AAL toxin expressed as TA equivalents.
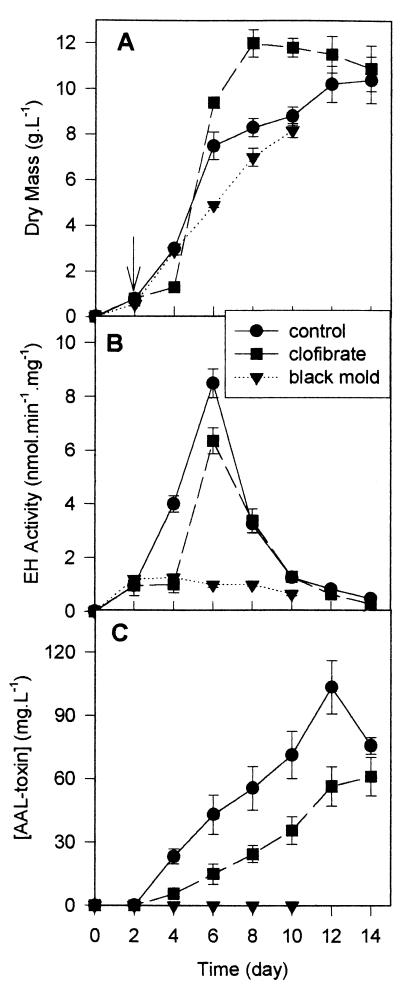
On a glucose medium (Fig. 4), an exponential phase of growth is observed between days 2 and 6, and clofibrate, at 1 mM, has little effect on the growth of A. alternata f. sp. lycopersici (Fig. 4A). Compared to the pectin medium, most EH activity is associated with the exponential-growth phase of the glucose culture and not with the stationary phase (Fig. 4B). AAL toxin (Fig. 4C) appears in the medium on the 2nd day of culture (the same time as the EH), and its concentration increases almost linearly over the 15 days of the study (during the exponential and stationary phases). Clofibrate slightly decreases EH activity and AAL toxin production (Fig. 4B and C). A similar pattern is observed for the total EH activity, expressed as enzymatic units per gram of mycelium (data not shown), indicating a decrease in EH production induced by the peroxisomal proliferator. AAL toxins appear in the medium at the same time that EH activity is first detected (on day 2) and continue to rise over the test period. As found in previous work using pectin media (35), the non-toxin-producing isolate, A. alternata (black mold), has only very low EH activities (10-fold less than A. alternata f. sp. lycopersici) (Fig. 4B), and the EH activity remains at this level over the sampling period.
FIG. 4.
Kinetics of growth, EH activities, and AAL toxin production by A. alternata f. sp. lycopersici grown in petri dishes containing 30 ml of medium with glucose as the carbon source and with (■) or without (●) 1 mM clofibrate. Clofibrate was added on the 2nd day of culture (arrow). A. alternata (black mold) (▾) was grown under the same conditions without any clofibrate added. (A) Fungal biomass; (B) EH specific activity with t-DPPO as a substrate; (C) concentration of AAL toxin expressed as TA equivalents.
Molecular mass and pI determination.
Crude cell extracts were prepared from A. alternata f. sp. lycopersici grown on pectin medium at days 6 and 12 of culture in the absence or presence of 1 mM clofibrate and on glucose media at pH 4.0 and 6.0 at day 6.
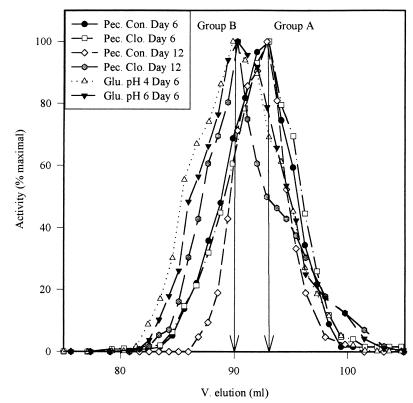
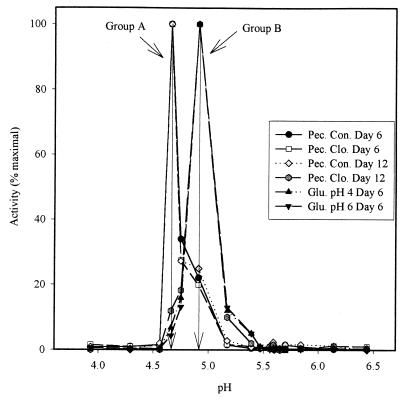
The native molecular weights of the EH activities were determined by gel filtration (Fig. 5). Only one peak of activity was observed for each extract. The elution profiles indicate an approximate molecular weight of the major form of EH activity present in the cell extracts (Table 2). Based on these molecular weights, the cell extracts could be separated in two groups of EH activity: the first (group A) at 20 kDa (pectin control medium on days 6 and 12 and pectin medium with clofibrate on day 6) and the second (group B) at 25 kDa (glucose media at pH 4 and 6 and pectin medium with clofibrate on day 12). However, the elution profiles (Fig. 5) lack the resolution to exclude the presence of a minor component, and their shapes suggest the presence of at least two different molecular weight forms in all the extracts.
FIG. 5.
Elution profiles from a Sephacryl-S100 column of EH activities in cell extracts of A. alternata f. sp. lycopersici grown in liquid culture. Pec., pectin medium; Con., control; Clo., with clofibrate; Glu., glucose medium.
TABLE 2.
Estimation of the major native molecular weights and pIs of major EH activities from A. alternata f. sp. lycopersici a
| Medium | Enzyme extract characteristic
|
||
|---|---|---|---|
| Molecular massb (Da) | pIbc | Ratio of 4.7 to 4.9 pI | |
| Pectin | |||
| Day 6 | |||
| Control | 20 ± 1 | 4.7 ± 1 | 4.7 |
| With clofibrated | 20 ± 1 | 4.7 ± 1 | 5.0 |
| Day 12 | |||
| Control | 20 ± 1 | 4.7 ± 1 | 3.9 |
| With clofibrated | 25 ± 1 | 4.9 ± 1 | 0.12 |
| Glucose, day 6 | |||
| pH 4 | 25 ± 1 | 4.9 ± 1 | 0.07 |
| pH 6 | 25 ± 1 | 4.9 ± 1 | 0.05 |
Only the characteristics of the major activity are given. Both the 20,000 and the 25,000 Da and the 4.7 and 4.9 pIs appear to be present in all the samples assayed.
Results are means ± standard deviations (n = 3).
Yields in activity between 30 and 40% were obtained.
Added at 1 mM.
The pIs of the EH activities were determined by isoelectric focusing (Fig. 6). Only one peak of activity was observed for each extract. The elution profiles indicate an approximate pI of the major form of EH activity present in the cell extracts (Table 2). Based on these values, the cell extracts could be separated in the same two groups of EH activity found with the molecular weights: group A at pI 4.7 and group B at pI 4.9. Moreover, the 20-kDa enzyme from gel permeation (group A) gave predominantly pI of 4.7, while the 25-kDa enzyme (group B) gave predominantly a pI of 4.9. The shapes of the activity profiles (Fig. 6) suggest the presence of the two different EH activity forms in all the extracts. The ratio of the 4.7 pI to the 4.9 pI (Table 2) indicates that the minor EH activity represents 5 to 20% of the total activity.
FIG. 6.
Profiles of EH activity of A. alternata f. sp. lycopersici cell extracts from a 4.0 to 6.5 pH gradient electrofocusing gel (Pharmacia LKB Multiphor System). Pec., pectin medium; Con., control; Clo., with clofibrate; Glu., glucose medium.
Substrate selectivity.
The EH activities of the six extracts were measured for six substrates available in the laboratory: four benzyl- or phenyl-substituted epoxides (compounds I to IV) and two natural lipid epoxides (compounds V and VI). The relative rates of hydration of the different substrates are shown in Table 3. The two groups (A and B) of EH activities are significantly different (α = 0.05) from each other for the six substrates. However, the six epoxides tested allow the segregation of group A into two subgroups of similar activity: A1 (pectin control medium on day 6 and pectin medium with clofibrate on day 6) and A2 pectin control medium on day 12). Groups A1 and A2 have relatively more activity for the JH-III and ESA substrates than group B. Group B is more active on t-DPPO and c-DPPO. For all three groups, only low activity is found on t-SO and c-SO. The high specific activity obtained for the three groups with the terpenoid epoxide is surprising. For most of the EHs studied, the turnover of such trisubstituted epoxides is much lower than that of less-hindered compounds (31, 42).
TABLE 3.
Substrate selectivity of EH activities of A. alternata f. sp. lycopersici extracts
| Substrate | Relative activitya (mean ± SD) (n = 3)
|
|||||
|---|---|---|---|---|---|---|
| Pectin medium
|
Glucose medium (day 6)
|
|||||
| Day 6
|
Day 12
|
|||||
| Control | With clofibrate | Control | With clofibrate | pH 4 | pH 6 | |
 |
100 ± 4 | 100 ± 4 | 100 ± 14 | 100 ± 14 | 100 ± 7 | 100 ± 11 |
| 23 ± 2 | 23 ± 2 | 16 ± 1 | 34 ± 4 | 49 ± 5 | 48 ± 5 | |
| 7.3 ± 0.4 | 7.1 ± 0.4 | 8.4 ± 0.9 | 4.4 ± 0.4 | 9.2 ± 0.2 | 5.3 ± 0.5 | |
| 0.5 ± 0.1 | 0.6 ± 0.1 | 11 ± 1 | 1.4 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | |
| 257 ± 18 | 231 ± 25 | 371 ± 37 | 128 ± 5 | 123 ± 1 | 145 ± 21 | |
| 139 ± 18 | 128 ± 7 | 200 ± 24 | 61 ± 14 | 73 ± 10 | 76 ± 11 | |
Relative to activity with t-DPPO, taken as 100%. Specific activities for t-DPPO, in nanomoles per minute per milligram, are as follows: 1.13 for pectin control medium on day 6, 1.21 for pectin medium with clofibrate on day 6, 0.7 for pectin control medium on day 12, 1.4 for pectin medium with clofibrate on day 12, 8.3 for glucose medium at pH 4.0, and 1.9 for glucose medium at pH 6.0.
Group-selective reagents and inhibitor specificity.
In a second comparison, the effects of different inhibitors on the activities of extracts were tested. At first, the effects of reagents selective for functional groups on the protein were tested. Metal chelators (EDTA and o-phenanthroline) as well as an inhibitor of serine esterases and proteases (PMSF) showed no inhibition of the six enzymatic extracts. Reducing reagents (DTT and cysteine) did not have significant effects on the activities, while in contrast, oxidants (m-chloroperbenzoic acid and H2O2) were potent inhibitors. Similar results were obtained with other EHs (14, 30, 42). Strong sulfhydryl-modifying reagents (HgCl2 and 4-hydroxymercuribenzoate sodium) totally inhibited all the extracts, while the three groups of enzyme give different results with weaker sulfhyhydryl reagents (dithiodinitrobenzoic acid [DTNB] and iodoacetamide). In the conditions tested, group A1 is slightly inhibited by DTNB (33 ± 3%) and iodoacetamide (18 ± 5%), while group A2 is not inhibited by DTNB and is totally inhibited by iodoacetamide. Group B is strongly inhibited by DTNB (75 ± 5%) and slightly inhibited by iodoacetamide (33 ± 5%). These results suggest the presence of an exposed sulfhydryl group only on the B enzyme, while the A2 enzyme should have an accessible disulfide bond. An exposed sulfhydryl group was demonstrated on the mammalian soluble EH (sEH) but not on the microsomal EH (mEH) (42). Under the conditions tested, the histidine reagent (ω-bromonitroacetophenone [ω-Br-NPA]) gave significantly different levels of inhibition with the three groups of enzyme: 63 ± 3, 41 ± 4, and 81 ± 5% inhibitions for A1, A2, and B enzymes, respectively. The three fungal enzymes are only partially inhibited by ω-Br-NPA, while under similar conditions mammalian mEH and sEH are totally inhibited. Moreover, for these mammalian enzymes, ω-Br-NPA was shown to bind covalently a histidine residue of the active site (14, 15). The lower inhibition of the three fungal enzymes by ω-Br-NPA may simply reflect a difficulty for the compound to reach the catalytic histidine, its reaction with other nucleophiles in the extract, or the turnover of the alkylated enzyme.
In a separate study, the IC50s of known EH inhibitors (21) were measured (Table 4). Based on the distribution of the results obtained, the six extracts could be divided into the same three groups of activities that were found above with the substrate selectivity study. Compounds 1, 2, and 9 are good inhibitors of groups A1 and B but not of group A2. Compound 4 is a good inhibitor of the B enzyme but not of the A1 and A2 enzymes. Only group A2 is well inhibited by compound 6, which is not a very good inhibitor of the two other groups. Compounds 5, 7, and 8 are not inhibitors of the three fungal enzymes. Interestingly, compound 5 is the best inhibitor known for the mammalian sEH (32), and compound 7 is the best inhibitor known for the mEH (21). Moreover, like the mammalian sEHs (21), all the extracts are more sensitive to the (2S,3S)-para-nitrophenyl-glycidol (compound 9) than to the (2R,3R) enantiomer (compound 8).
DISCUSSION
This report describes the production and characterization of EH activities from A. alternata f. sp. lycopersici in relation to the production of AAL toxins. The results obtained clearly show that the pH (Table 1) and the medium composition (Fig. 2) markedly influence AAL toxin production and EH activities by A. alternata f. sp. lycopersici. They are enhanced at acidic pHs and by the use of glucose. The fungus displays very different characteristics when the major carbon source of the medium is changed from pectin to glucose (Fig. 3 and 4). The use of glucose as a carbon source resulted in a 10-fold increase in EH production in comparison to that with pectin, a “natural” substrate. This was surprising given that EH activities for F. solani pisi (25) and Aspergillus niger (31) were shown to be repressed in the presence of glucose. On the glucose medium, the EH activity seems related to increases in fungal biomass. A similar effect was found for A. niger (31). However, it has been reported recently (20) that in the case of Beauveria densa, a dematiaceous fungus, EH production is coincident with a secondary pigment produced in stationary phase or idiophase. We have not observed such a phenomenon, but the EH activity might be, in vivo, related to the metabolism of some secondary fungal chemical. On the pectin medium, two peaks of EH activities are produced at different periods during mycelium growth. This result was quite surprising given that only one peak of EH activity was demonstrated for several other fungi (20, 25, 31). With pectin as a substrate, the AAL toxins appear in the medium when the first peak of EH activity disappears. This result suggests that this peak of EH activity may not be directly related to AAL toxin production. Moreover, AAL toxin production occurred in parallel with the appearance of the second peak of EH activity (Fig. 3). With glucose as the carbon source, the fact that EH activity and the production of AAL toxins appear at the same time (2nd day [Fig. 4]) is consistent with the hypothesis that there is an epoxide hydration step in the biosynthetic pathway of AAL toxins. However, the fact that EH activity and AAL toxin productions are not parallel throughout the growth period could be explained in several ways which are consistent with this hypothesis. First, it is possible that the step catalyzed by EH is not rate limiting, and a perfect correlation of activity and AAL toxin production is not critical. A second possibility and caution is that this study uses surrogate substrates and not the suspected precursors to the AAL toxins. Thus, the assay could detect several EH activities, while only one of the apparent minority activities could be more closely associated with the toxin production. Moreover, the proposed hypothesis is supported by the observation that the non-toxin producer, A. alternata (black mold), has only very low EH activities (10-fold less than the strain producing toxin) (Fig. 4B), and these EH activities remain at this level over the sampling period.
All of these results obtained under different culture conditions suggest the presence of more than one EH. To test the hypothesis of the presence of more than one EH, EH activities obtained in different culture conditions were characterized for molecular and enzymatic properties. Results obtained for the molecular properties (Table 2) suggest the presence of at least two different EH activities. One (group A) displays a molecular mass of 20 kDa and a pI of 4.7, while the other (group B) weighs approximately 25 kDa and has a pI of 4.9. The mass values found are smaller than the masses for most of the EHs previously described: monomeric masses between 30 and 70 kDa are generally found (31), but a 17-kDa bacterial EH has been described recently (39). For all the EH activities described, pI values between 4.5 and 6.0 have been found (31). Based on substrate and inhibitor spectra (Tables 3 and 4), group A could be subdivided in two distinguishable groups of enzymatic activities (A1 and A2). However, one of course cannot absolutely distinguish classes of enzymes based on substrate selectivity and differential inhibition. The observed differences could be due to the fact that in crude extracts, other proteins present can affect substrate activities and inhibitor sensitivity. Based on subcellular location and substrate selectivity, several EH activities have been described in mammals (21), insects (23), and plants (3). To our knowledge, it is the first time that more than one EH activity has been reported in a microorganism.
The A1 enzyme activity, the first peak of activity produced on the pectin medium, is probably not directly related to toxin production because toxin production occurred only as this peak was decreasing. On this medium, the second peak of activity produced is different in the absence (group A2) and presence (group B) of clofibrate. The fact that in the presence of clofibrate, twice as much of the AAL toxins is produced suggests that the group B enzyme is related to AAL toxin biosynthesis. This trend is consistent with the high level of toxin production observed on the glucose medium, where the group B enzyme is produced at much higher levels. Moreover, pI determinations (Fig. 6 and Table 2) show that on pectin media with or without clofibrate, at least 20% of the EH activity characterized at day 6 is from the group B enzyme. This result indicates that, as observed on the glucose medium, on pectin media the group B enzyme is produced at the same time the toxin is produced, strongly suggesting that the group B enzyme is associated with the production of AAL toxins. Recently, EH activities in yeast and bacteria have been described in relation to the presence of carotenoid pigments as secondary metabolites (6) or related to the metabolism of limonene (39). In A. alternata f. sp. lycopersici, all the EH fractions show very high catalytic activity on JH-III, especially the A2 enzyme. Such a result is consistent with the possible involvement of the fungal EH activities in the metabolism of terpenes. In mammals, the sEH hydrolyzed juvenile hormone and other terpenoic epoxides (22). The competitive inhibitors tested herein (Table 4) are substrates of the mammalian sEH with low turnover (21). If a similar mode of action occurs with the fungal EHs, such compounds would be of limited utility in evaluating these enzymes in vivo. However, the determination of the inhibitory potency of different chalcone oxides will allow us to develop new affinity matrices for the purification of the fungal enzymes, as was done for the mammalian EHs (43), and to develop new, potent inhibitors. These new tools will allow us to better understand the role of these enzymes in the metabolism of the AAL toxins and possibly, in the future, to block AAL toxin production in infected plants.
ACKNOWLEDGMENTS
This work was supported in part by NIEHS grant R01-ES02710, NIEHS Superfund Basic Research Program ES04699, NIEHS Center for Environmental Health Sciences grant 1P30-ES05707, and UC Davis EPA Center for Ecological Health Research grant CR819658.
REFERENCES
- 1.Archelas A, Furstoss R. Epoxide hydrolases: new tools for the synthesis of fine organic chemicals. Trends Biotechnol. 1998;16:108–116. doi: 10.1016/S0167-7799(97)01161-X. [DOI] [PubMed] [Google Scholar]
- 2.Beetham J K, Grant D, Arand M, Garbarino J, Kiyosue T, Pinot F, Oesch F, Belknap W R, Shinozaki K, Hammock B D. Gene evolution of epoxide hydrolases and recommended nomenclature. DNA Cell Biol. 1995;14:61–71. doi: 10.1089/dna.1995.14.61. [DOI] [PubMed] [Google Scholar]
- 3.Blée E, Shuber F. Occurrence of fatty acid epoxide hydrolases in soybean (Glycine max): purification and characterization of the soluble form. Biochem J. 1992;282:711–714. doi: 10.1042/bj2820711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borhan B, Jones A D, Pinot F, Grant D F, Kurth M J, Hammock B D. Mechanism of soluble epoxide hydrolase: formation of an α-hydroxy ester-enzyme intermediate through Asp-333. J Biol Chem. 1995;270:26923–26930. doi: 10.1074/jbc.270.45.26923. [DOI] [PubMed] [Google Scholar]
- 5.Borhan B, Mebrahtu T, Nazarian S, Kurth M J, Hammock B D. Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem. 1995;231:188–200. doi: 10.1006/abio.1995.1520. [DOI] [PubMed] [Google Scholar]
- 6.Botes A L, Steenkamp J A, Letloenyane M Z, Van Dyk M S. Epoxide hydrolase activity of Chryseomonas luteola for the asymmetric hydrolysis of aliphatic mono-substituted epoxides. Biotechnol Lett. 1998;20:427–430. [Google Scholar]
- 7.Bottini A T, Bowen J R, Gilchrist D G. Phytotoxins. II. Characterization of a phytotoxic fraction from Alternaria alternata f. sp. lycopersici. Tetrahedron Lett. 1981;22:2723–2726. [Google Scholar]
- 8.Caldas E D, Jones A D, Ward B, Winter C K, Gilchrist D G. Structural characterization of three new AAL toxins produced by Alternaria alternata f. sp. lycopersici. J Agric Food Chem. 1994;42:327–333. [Google Scholar]
- 9.Caldas E D, Jones A D, Winter C K, Ward B, Gilchrist D G. Analysis of sphinganine analog mycotoxins by electrospray ionization mass spectrometry. Anal Chem. 1995;67:196–207. [Google Scholar]
- 10.Caldas E D, Sadilkova K, Ward B, Jones A D, Winter C K, Gilchrist D G. Biosynthetic studies of fumonisin B1 and AAL toxins. J Agric Food Chem. 1998;46:4734–4743. [Google Scholar]
- 11.Clouse S D, Gilchrist D G. Interaction of the asc locus in F8 paired lines of tomato with Alternaria alternata f. sp. lycopersici and AAL-toxin. Phytopathology. 1986;77:80–82. [Google Scholar]
- 12.Clouse S D, Martensen A N, Gilchrist D G. Rapid purification of host-specific pathotoxins from Alternaria alternata f. sp. lycopersici by solid-phase adsorption on octadecylsilane. J Chromatogr. 1985;350:255–263. doi: 10.1016/s0021-9673(01)93524-1. [DOI] [PubMed] [Google Scholar]
- 13.Debernard S, Morisseau C, Severson T F, Feng L, Wojtasek H, Prestwich G D, Hammock B D. Expression and characterization of the recombinant juvenile hormone epoxide hydrolase (JHEH) from Manduca sexta. Insect Biochem Mol Biol. 1998;28:409–419. doi: 10.1016/s0965-1748(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 14.Diezte E C, Stephens J, Magdalou J, Bender D M, Moyer M, Fowler B, Hammock B D. Inhibition of human and murine cytosolic epoxide hydrolase by group-selective reagents. Comp Biochem Physiol. 1993;104B:299–308. doi: 10.1016/0305-0491(93)90372-c. [DOI] [PubMed] [Google Scholar]
- 15.Dubois G C, Appella E, Levin W, Lu A Y H, Jerina D M. Hepatic microsomal epoxide hydrase: involvement of a histidine at the active site suggests a nucleophilic mechanism. J Biol Chem. 1978;253:2932–2939. [PubMed] [Google Scholar]
- 16.Faber K, Mischtiz M, Kroutil W. Microbial epoxide hydrolases. Acta Chem Scand. 1996;50:249–258. [Google Scholar]
- 17.Gilchrist D G, Grogan R G. Production and nature of a host-specific toxin from Alternaria alternata f. sp. lycopersici. Phytopathology. 1976;66:880–886. [Google Scholar]
- 18.Gill S S, Ota K, Hammock B D. Radiometric assays for mammalian epoxide hydrolases and glutathione-S-transferase. Anal Biochem. 1983;131:273–282. doi: 10.1016/0003-2697(83)90166-5. [DOI] [PubMed] [Google Scholar]
- 19.Grant D F, Moody D E, Beetham J, Storms D H, Moghadam M F, Bohran B, Pinot F, Winder B, Hammock B D. The response of soluble epoxide hydrolase and other hydrolytic enzymes to peroxisome proliferators. In: Moody D E, editor. Peroxisome proliferators: unique inducers of drug-metabolizing enzymes. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 97–112. [Google Scholar]
- 20.Grogan G, Roberts S M, Willetts A J. Novel aliphatic epoxide hydrolase activities from dematiaceous fungi. FEMS Microbiol Lett. 1996;141:239–243. doi: 10.1111/j.1574-6968.1996.tb08391.x. [DOI] [PubMed] [Google Scholar]
- 21.Hammock B D, Grant D, Storm D. Epoxide hydrolases. In: Sipes I, McQueen C, Gandolfi A, editors. Comprehensive toxicology. Oxford, United Kingdom: Pergamon Press; 1997. pp. 283–306. [Google Scholar]
- 22.Hammock B D, Gill S S, Stamoudis V, Gilbert L I. Soluble mammalian epoxide hydratase: action on juvenile hormone and other terpenoic epoxides. Comp Biochem Physiol. 1976;53B:263–265. doi: 10.1016/0305-0491(76)90045-6. [DOI] [PubMed] [Google Scholar]
- 23.Harshman L G, Casas J, Dietze E C, Hammock B D. Epoxide hydrolase activities in Drosophila melanogaster. Insect Biochem. 1991;21:887–894. [Google Scholar]
- 24.Javed T, Bennett G A, Richard J L, Dombrink-Kurtzman M A, Cote L M, Buck W B. Mortality in broiler chicks on feed amended with Fusarium proliferatum culture material or with purified fumonisin B1 and moniliformin. Mycopathologia. 1993;123:171–184. doi: 10.1007/BF01111269. [DOI] [PubMed] [Google Scholar]
- 25.Kolattukudy P E, Brown L. Fate of naturally occurring epoxy acids: a soluble hydrase, which catalyses cis hydration, from Fusarium solani pisi. Arch Biochem Biophys. 1975;166:599–607. doi: 10.1016/0003-9861(75)90425-7. [DOI] [PubMed] [Google Scholar]
- 26.Lacourciere G M, Armstrong R N. The catalytic mechanism of microsomal epoxide hydrolase involves an ester intermediate. J Am Chem Soc. 1993;115:10466–10467. [Google Scholar]
- 27.Meijer J, DePierre J W. Cytosolic epoxide hydrolase. Chem Biol Int. 1988;64:207–249. doi: 10.1016/0009-2797(88)90100-7. [DOI] [PubMed] [Google Scholar]
- 28.Merril A H, Jr, Wang E, Gilchrist D G, Riley R T. Fumonisins and other inhibitors of de novo sphingolipid biosynthesis. Adv Lipid Res. 1993;26:215–234. [PubMed] [Google Scholar]
- 29.Moghaddam M, Grant D, Cheek J, Greene J F, Hammock B D. Bioactivation of leukotoxin and isoleukotoxin to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morisseau C. L’époxyde hydrolase d’Aspergillus niger: purification, caracterisation et utilisation pour la preparation d’époxydes et de diols optiquement purs. Thèse de docteur en science de l’Université de la Méditterranée, Marseille, France. 1995. [Google Scholar]
- 31.Morisseau C, Venturi G, Moussou P, Baratti J C. Effect of carbon and nitrogen sources on the production of epoxide hydrolase from Aspergillus niger. Biotechnol Tech. 1998;12:805–809. [Google Scholar]
- 32.Morisseau C, Du G, Newman J W, Hammock B D. Mechanism of mammalian soluble epoxide hydrolase inhibition by chalcone oxide derivatives. Arch Biochem Biophys. 1998;356:214–228. doi: 10.1006/abbi.1998.0756. [DOI] [PubMed] [Google Scholar]
- 33.Mumby S M, Hammock B D. A partition assay for epoxide hydrases acting on insect juvenile hormone and an epoxide-containing juvenoid. Anal Biochem. 1979;92:16–21. doi: 10.1016/0003-2697(79)90619-5. [DOI] [PubMed] [Google Scholar]
- 34.Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and oleofinic compounds. Xenobiotica. 1972;3:305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- 35.Pinot F, Caldas E D, Schmidt C, Gilchrist D G, Jones A D, Winter C K, Hammock B D. Characterization of epoxide hydrolase activity in Alternaria alternata f. sp. Lycopersici. Possible involvement in toxin production. Mycopathologia. 1998;140:51–58. doi: 10.1023/a:1006829330490. [DOI] [PubMed] [Google Scholar]
- 36.Siler D J, Gilchrist D G. Properties of host-specific toxins produced by Alternaria alternata f. sp. lycopersici in culture and in tomato plants. Physiol Mol Plant Pathol. 1983;23:265–274. [Google Scholar]
- 37.Stapleton A, Beetham J K, Pinot F, Gabarino J E, Rockhold D R, Friedman M, Hammock B D, Belknap W R. Cloning and expression of soluble epoxide hydrolase from potato. Plant J. 1994;6:251–258. doi: 10.1046/j.1365-313x.1994.6020251.x. [DOI] [PubMed] [Google Scholar]
- 38.Szurdoki F, Troudale E, Ward B, Gee S J, Hammock B D, Gilchrist D G. Synthesis of protein conjugates and development of immunoassays for AAL toxins. J Agric Food Sci. 1996;44:1796–1803. [Google Scholar]
- 39.van der Werf M J, Overkamp K M, de Bont J A M. Limonene-1,2-epoxide hydrolase from Rhodococcus erythropolis DCL14 belongs to a novel class of epoxide hydrolases. J Bacteriol. 1998;180:5052–5057. doi: 10.1128/jb.180.19.5052-5057.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verschueren K H G, Seljée F, Rozeboom H J, Kalk K H, Dijkstra B W. Crystallographic analysis of the catalytic mechanism of haloalkane dehalogenase. Nature. 1993;363:693–698. doi: 10.1038/363693a0. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Jones C, Ciacci-Zanella J, Holt T, Gilchrist D G, Dickman M B. Fumonisins and Alternaria alternata lycopersici toxins: sphingaline analog mycotoxins induce apoptosis in monkey kidney cells. Proc Natl Acad Sci USA. 1996;93:3461–3465. doi: 10.1073/pnas.93.8.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wixtrom R N, Hammock B D. Membrane-bound and soluble fraction epoxide hydrolases: methodological aspects. In: Zakim D, Vessey D A, editors. Biochemical pharmacology and toxicology. New York, N.Y: John Wiley & Sons; 1985. pp. 1–93. [Google Scholar]
- 43.Wixtrom R N, Silva M H, Hammock B D. Affinity purification of cytosolic epoxide hydrolase using derivatized epoxy-activated Sepharose gels. Anal Biochem. 1988;169:71–80. doi: 10.1016/0003-2697(88)90256-4. [DOI] [PubMed] [Google Scholar]
- 44.Woloshuk C P, Kolattukudy P E. Mechanism by which contact with plant cuticle triggers cutinase gene expression in the spores of Fusarium solani f. sp. pisi. Proc Natl Acad Sci USA. 1986;83:1704–1708. doi: 10.1073/pnas.83.6.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeldin D C, Wei S, Falck J R, Hammock B D, Snapper J R, Capdevila J H. Metabolism of epoxyeicosatrienoic acids by cytosolic epoxide hydrolase: substrate structural determinants of asymmetric catalysis. Arch Biochem Biophys. 1995;316:443–451. doi: 10.1006/abbi.1995.1059. [DOI] [PubMed] [Google Scholar]