Abstract
Viral respiratory tract infections are the main causative agents of the onset of infection-induced asthma and asthma exacerbations that remain mechanistically unexplained. Here we found that deficiency in signaling via type I interferon receptor led to deregulated activation of group 2 innate lymphoid cells (ILC2 cells) and infection-associated type 2 immunopathology. Type I interferons directly and negatively regulated mouse and human ILC2 cells in a manner dependent on the transcriptional activator ISGF3 that led to altered cytokine production, cell proliferation and increased cell death. In addition, interferon-γ (IFN-γ) and interleukin 27 (IL-27) altered ILC2 function dependent on the transcription factor STAT1. These results demonstrate that type I and type II interferons, together with IL-27, regulate ILC2 cells to restrict type 2 immunopathology.
Type 2 immune responses evolved to respond to parasitic helminth infections but can also be triggered by a wide variety of other stimuli ranging from bacterial and viral infections to venoms, endogenous host ligands and vaccine adjuvants. They are characterized by the expression of specific signature cytokines, including interleukin 4 (IL-4), IL-5 and IL-13, that in concert ‘instruct’ effector mechanisms that are critical not only for defending the host against metazoan parasites but also for maintaining and restoring tissue integrity and homeostasis after infectious and non-infectious challenge1. It has been widely accepted that the exclusive source of type 2 signature cytokines are TH2 cells, a subpopulation of CD4+ helper T cells of the adaptive immune system. However, studies have now identified previously unknown innate lymphoid cell (ILC) populations in mice and humans that, like TH2 cells, require the transcription factor GATA-3 for their development2, can produce large quantities of type 2 signature cytokines and are now collectively referred to as ‘group 2 innate lymphoid cells’ (ILC2 cells)3.
ILC2 cells are activated by cytokines, including TSLP, IL-25 and IL-33 (ref. 4). IL-25 (IL-17E), a member of the IL-17 family, induces type 2 responses through the IL-25 receptor (IL-17RB), whereas IL-33 utilizes the IL-33 receptor that consists of ST2 (IL-1RL1) and IL-1RAcP. ILC2 cells serve as central regulators of type 2 immune responses through the ‘instruction’ of diverse effector pathways5. The secretion of IL-5 and IL-9 by ILC2 cells leads to the recruitment and activation of eosinophils and mast cells. Through the production of IL-13, ILC2 cells induce the proliferation and activation of goblet cells and mucus production. Moreover, IL-13 promotes the migration of activated dendritic cells (DCs) to draining lymph nodes for TH2 priming6. Moreover, the release of IL-4 induces class-switch recombination to immunoglobulin E (IgE), and the production of amphiregulin by ILC2 cells contributes to epithelial integrity and tissue repair7.
Clinical observations and rodent models of human disease have revealed that ILC2 cells serve critical roles in allergic inflammatory responses such as asthma, atopic dermatitis and allergic lung inflammation induced by pathogens. Notably, mouse models of allergic inflammation induced by ragweed protein, ovalbumin and papain, as well as fungal and viral lung infections, have demonstrated that ILC2 cells are sufficient to drive allergic lung responses, which suggests that ILC2 cells need to be tightly regulated for the control of pulmonary inflammatory pathologies8. However, the mechanisms that negatively regulate the proliferation and cytokine production of ILC2 cells remain largely unknown.
Asthma is a hallmark type 2 immune response–mediated disease characterized by the presence of chronic inflammation in the lower airways, which results in airflow obstruction and airway hyper-responsiveness (AHR)9. Paradoxically, viral respiratory tract infections, archetypal triggers of potent innate and adaptive type 1 immune responses, which are characterized by the production of type I interferons and interferon-γ (IFN-γ)-producing lymphoid cells10, frequently give rise to pulmonary type 2 immunopathology11. Viral respiratory infection is the dominant cause of type 2 immunity–mediated asthma and asthma exacerbations and is associated with ~85% of pediatric disease and ~75% of adult disease that remains mechanistically unexplained12. As the induction of innate type I interferons is impaired in asthmatic patients13,14 and many viruses exert strategies to dampen their induction15–17, we hypothesized that type I interferons might have a central role in the regulation of type 2 immunity and its associated pathologies.
Here we report that deficiency in signaling via type I interferon receptor led to deregulated activation of ILC2 cells and infection-associated type 2 immunopathology. Type I interferons directly regulated mouse and human ILC2 cells in a manner dependent on the transcriptional activator ISGF3 that led to diminished cytokine production and cell proliferation and increased cell death. This demonstrated that type I interferons regulated ILC2 cells to restrict type 2 immunopathologies and fibrotic disease.
RESULTS
IFNAR deficiency leads to elevated activation of ILC2 cells
As an elevated type 2 immunological profile was observed in hospitalized patients during the 2009 H1N1 influenza A virus (IAV) pandemic18,19, we first chose an established animal model of IAV infection to verify our hypothesis. As reported20, mice deficient in the type I interferon receptor IFNAR1 (Ifnar1−/− mice) were more susceptible to intranasal infection with IAV (strain A/Puerto Rico/8/1934 H1N1) than were their C57BL/6 wild-type counterparts (Supplementary Fig. 1a). However, after challenge with a non-lethal dose of IAV, no substantial difference between these mice was observable in their expression of the gene encoding the IAV non-structural protein NS1 and, subsequently, wild-type and Ifnar1−/− mice recovered comparably well (Supplementary Fig. 1b,c). Despite those findings, immuno-histochemical analysis of infected lungs revealed more inflammatory cells, goblet cells, epithelial hyperplasia, alveolar wall thickening and pulmonary fibrosis in the Ifnar1−/− mice than in the wild-type mice (Fig. 1a–c). In addition, we observed a significantly greater abundance of pulmonary neutrophils and eosinophils (Fig. 1d) and a higher concentration of serum IgE (Fig. 1e) in the Ifnar1−/− mice than in the wild-type mice, which suggested that signaling via type I interferon receptor was needed to restrain innate and adaptive type 2 immunity during pulmonary viral infection. Expression of the pro-inflammatory mediators IL-1β and TNF was lower in the lungs of IAV-infected Ifnar1−/− mice than in those of their IAV-infected wild-type counterparts (Supplementary Fig. 1d,e). Moreover, in agreement with a published study20, expression of the chemokine CCL2 was lower in Ifnar1−/− mice than in wild-type mice, as were counts of inflammatory monocytes (Supplementary Fig. 1f,g), which suggested that type 2 immunological parameters were selectively enhanced in the IFNAR-deficient mice after infection with a low dose of IAV.
Figure 1.
Deficiency in signaling via type Is interferon receptor results in increased ILC2 cells and type 2 immunopathology. (a,b) Pathology scores, as density of inflammatory infiltration (a), and hyperplasia of alveolar type 2 pneumocytes (left), bronchial and bronchiolar epithelial hyperplasia (middle), and alveolar wall thickening and fibrosis in affected parenchyma (right) (b), in lungs obtained from wild-type (WT) C57BL/6 and Ifnar1−/− mice (age and sex matched; n = 3–7 per group) treated with PBS (Mock) or infected intranasally with 20 plaque-forming units of IAV, assessed by microcopy as in c. (c) Microscopy of sections of lungs from mice as in a,b, stained with hematoxylin and eosin (H&E) 5 d after infection, with periodic acid Schiff (PAS) 10 d after infection, or with Masson’s trichrome (MT) 15 d after infection. Scale bars, 200 μm. (d) Quantification of neutrophils and eosinophils from lungs as in a,b, assessed by flow cytometry. (e) Enzyme-linked immunosorbent assay (ELISA) of IgE in serum from mice as in a,b. (f,g) Quantitative RT-PCR analysis of the pulmonary expression of Il33 and Ifnb in mice as in a,b; results were calculated by the change-in-cycling-threshold (Ct) method and were normalized to those of the control gene Gapdh and are presented relative to those of mice treated with PBS (Mock). (h) Quantification of ILC2 cells in PBS-perfused lungs from mice as in a,b, assessed by flow cytometry. ND, not detectable. Each symbol (a,b,d,h) represents an individual mouse; small horizontal lines indicate the mean (± s.d. of triplicates). *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 (Mann-Whitney test). Data are representative of three independent experiments (mean and s.d. of triplicates in e–g).
The release of IL-33 during infection with IAV leads to the activation of ILC2 cells, which causes AHR in the absence of a functional T cell and B cell compartment21. Such findings demonstrate that activation of ILC2 cells during infection is critical for IAV-associated pulmonary type 2 immunopathology21. Consistent with those results, obtained with an H3N1 strain of IAV21, we found that intranasal infection of wild-type mice with a sub-lethal dose of H1N1 IAV led to an increase in IL-33 in the lungs of infected mice as early as day 5 after infection (Fig. 1f). The highest levels of Ifnb mRNA were also observed 5 d after IAV challenge (Fig. 1g), which suggested cross-regulation of IL-33 and type I interferons. Because activation of ILC2 cells is a central regulator of innate and adaptive type 2 immunity and its associated pathologies8, we investigated whether signaling via type I interferons affected ILC2 cells during pulmonary infection with IAV. Intranasal infection of wild-type mice with a low dose of IAV led to an increase in the number of ILC2 cells in the lungs of the challenged mice (Fig. 1h). Ifnar1−/− mice exhibited significantly more lung ILC2 cells after IAV challenge than did their wild-type counterparts (Fig. 1h), which demonstrated that type I interferons limited the activation of the pulmonary ILC2 cells during this IAV infection. Pulmonary concentrations of IL-33 were not significantly different in wild-type mice relative to their concentrations in Ifnar1−/− mice throughout the course of infection (Supplementary Fig. 1h), which indicated that type I interferons did not regulate IL-33 during this IAV challenge. We obtained similar results by infecting wild-type and Ifnar1−/− mice with the gastrointestinal parasitic nematode Heligmosomoides polygyrus, which requires the induction of innate and adaptive type 2 immunity for efficient host protection22. IFNAR1 deficiency resulted in more intestinal neutrophils, eosinophils and ILC2 cells and higher concentrations of serum IgG1 and IgE than that of wild-type mice (Supplementary Fig. 2a–d). These observations collectively demonstrated that deficiency in signaling via type I interferon receptor resulted in deregulated activation of ILC2 cells and infection-associated type 2 immunopathology.
Type I interferons directly regulate mouse and human ILC2 cells
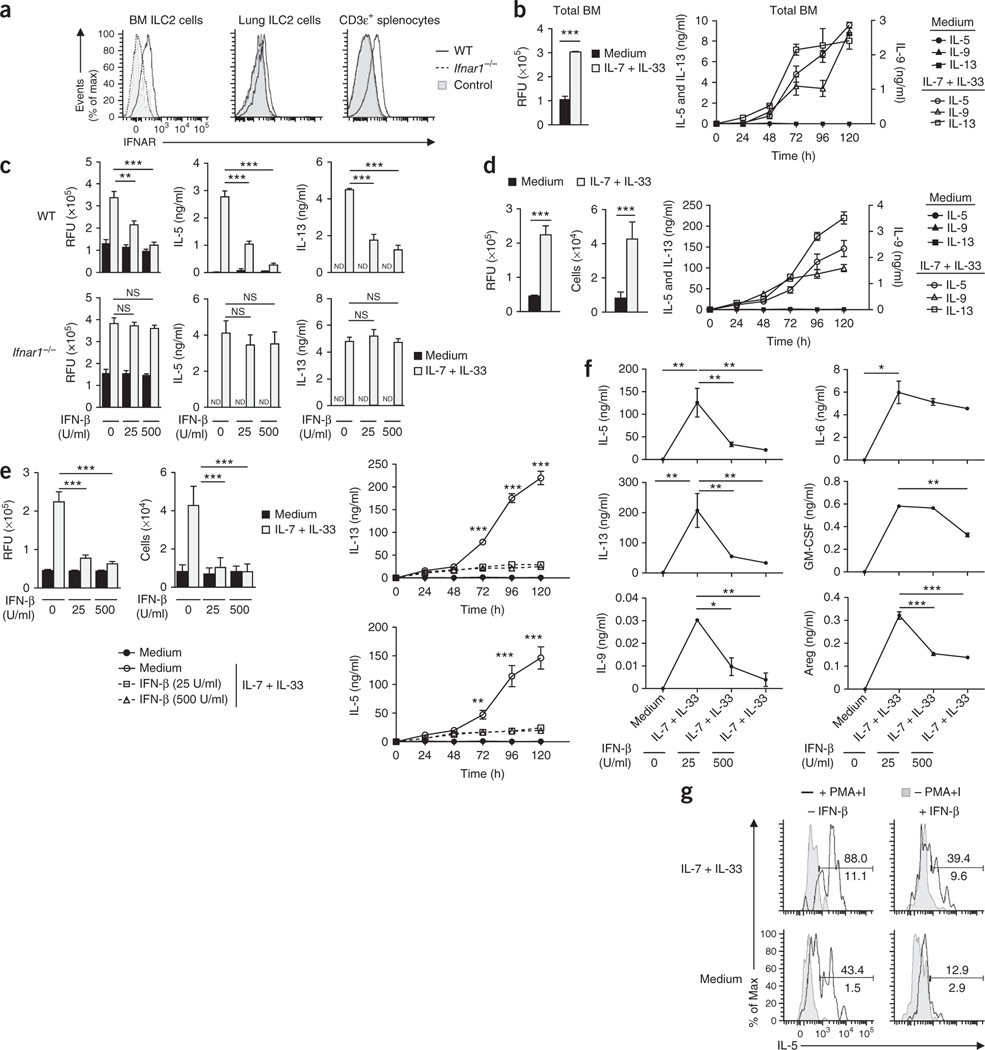
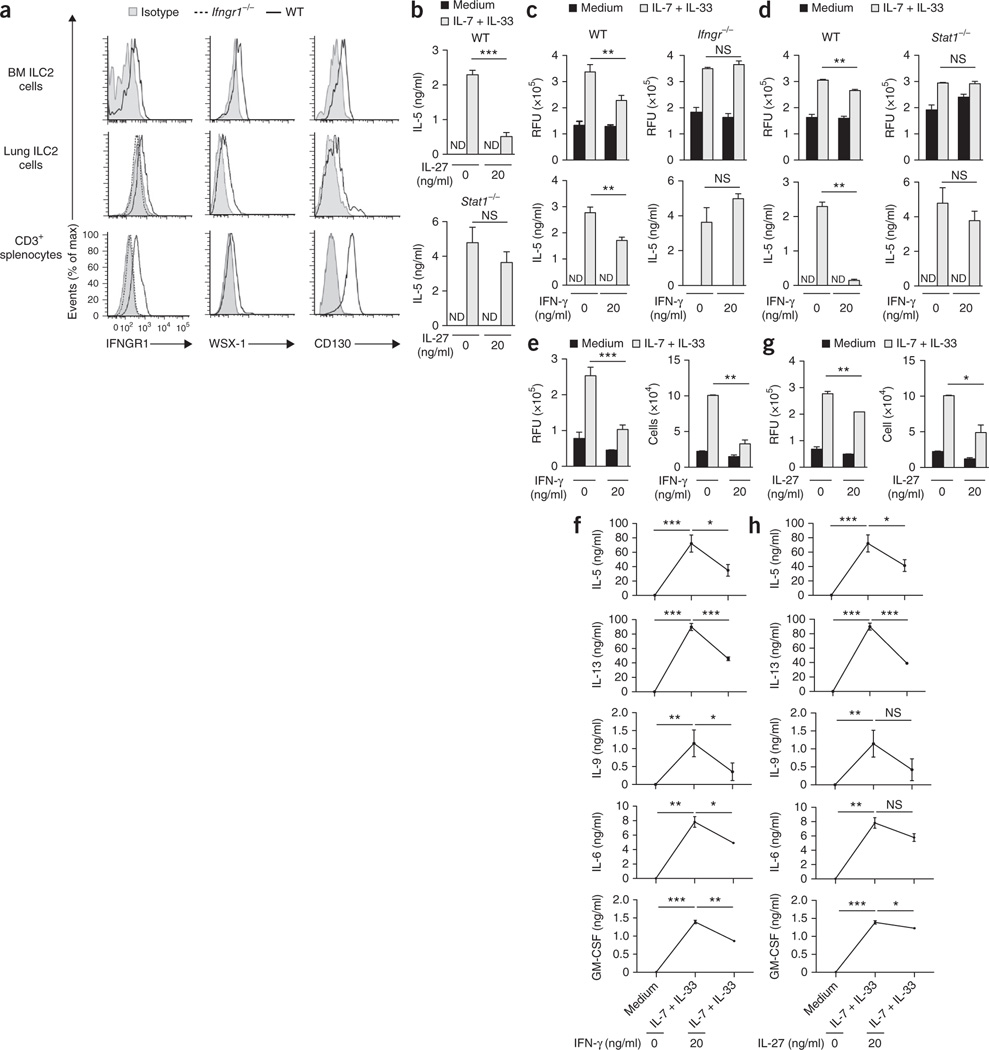
Since bone marrow (BM) ILC2 cells as well as lung ILC2 cells expressed type I interferon receptor at their surface (Fig. 2a), we next investigated whether type I interferons directly regulated the activation and cytokine production of ILC2 cells. Stimulation of total mouse BM cells with IL-7 plus IL-33 induces the production of IL-5 and IL-13, with BM ILC2 cells being the dominant source of these cytokines23. We therefore used total mouse BM cell cultures to first assess the role of type I interferons in the function of ILC2 cells. When stimulated for 5 d with IL-7 plus IL-33, BM cells proliferated and produced IL-5, IL-9 and IL-13 (Fig. 2b). The addition of IFN-β blunted the IL-33-mediated proliferation and cytokine release of BM cells in a dose- and IFNAR1-dependent manner (Fig. 2c). To exclude the possibility of secondary regulatory mechanisms involving non-ILC2 cells in culture conditions and to add further evidence that type I interferons directly regulated ILC2 function, we established a method for the isolation and ex vivo expansion of mouse BM-derived ILC2 cells that enabled us to gain access to sufficient numbers of purified ILC2 cells (Supplementary Fig. 3a–d). Pure BM-derived ILC2 cells stimulated with IL-7 plus IL-33 produced large quantities of IL-5 and IL-13, as well as readily detectable levels of the cytokines IL-6, IL-9, GM-CSF and amphiregulin (Fig. 2d–f). However, we did not observe the release of IL-4 above the limit of detection when we stimulated ILC2 cells with a combination of IL-7 plus IL-33 (data not shown). Type I interferons altered the proliferation and cytokine release of pure ILC2 cells in a dose-dependent manner (Fig. 2e,f), which demonstrated that type I interferons directly regulated the function of ILC2 cells. Furthermore, type I interferons reduced the capacity of ILC2 cells stimulated with IL-7 plus IL-33 to release the cytokines IL-5, IL-6, IL-9, IL-13, GM-CSF and amphiregulin (Fig. 2f). To further assess the effect of type I interferons on lung ILC2 cells, we enriched pulmonary ILC2 cells from naive wild-type mice and cultured them with IL-7 plus IL-33 in the presence or absence of type I interferons. Consistent with a published study2, GATA-3+ ILC2 cells expressed IL-5 protein upon stimulation with the phorbol ester PMA plus ionomycin (Fig. 2g). The addition of IL-7 plus IL-33 further boosted the ability of pulmonary GATA-3+ ILC2 cells to produce IL-5 and yielded about 90% cytokine-producing cells (Fig. 2g). The addition of type I interferons massively reduced the capacity of lung ILC2 cells to produce IL-5 (Fig. 2g), which demonstrated that type I interferons were key regulators of these ILC2 cells.
Figure 2.
Type I interferons restrain the proliferation and cytokine production of mouse ILC2 cells. (a) Cell surface expression of IFNAR on BM ILC2 cells, lung ILC2 cells and CD3ε+ splenocytes from wild-type and Ifnar1−/−mice. Control, fluorescence minus one. (b) Viability of wild-type BM cells stimulated for 5 d in medium alone (control) or with IL-7 plus IL-33 (each at 10 ng/ml), presented as relative fluorescent units (RFU) (left), and ELISA of IL-5 and IL-13 (left vertical axis) and of IL-9 (right vertical axis) in supernatants of those cells (right). (c) Proliferation of wild-type or Ifnar1−/− BM cells stimulated for 5 d as in b in the presence (25 or 500) or absence (0) of IFN-β (25 or 500 U/ml), presented as in b (left), and ELISA of IL-5 and IL-13 in supernatants of those cells (right). (d) Proliferation (left) and quantification (middle) of BM-derived wild-type ILC2 cells stimulated for 5 d as in b, and ELISA and multiplex assay of IL-5, IL-13 and IL-9 in supernatants of those cells (right), presented as in b. (e) Proliferation, presented as in b (top left), and quantification (top middle), of BM-derived wild-type ILC2 cells stimulated for 5 d as in c, and ELISA and multiplex assay of IL-5, IL-13 and IL-9 in supernatants of those cells (right), presented as in b. (f) ELISA and multiplex assay of cytokines in supernatants of cells as in e, at day 5. (g) Intracellular IL-5 in GATA-3+ ILC2 cells among wild-type lung lymphoid cells subjected to enrichment by Percoll gradient and stimulated for 4 h as in b (left margin), alone (− PMA+I) or in combination with PMA and ionomycin (+ PMA+I) (key), with (+ IFN-β) or without (− IFN-β) the addition of IFN-β (500 U/ml) (above plots), analyzed by intracellular staining and flow cytometry. Numbers above (+ PMA+I) and below (− PMA+I) bracketed lines indicate percent IL-5+ ILC2 cells among GATA-3+ ILC2 cells. NS, not significant (P > 0.05); *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 (Mann-Whitney test). Data are representative of two (a,g) or three (b–f) independent experiments (mean and s.d. of triplicates in b–f).
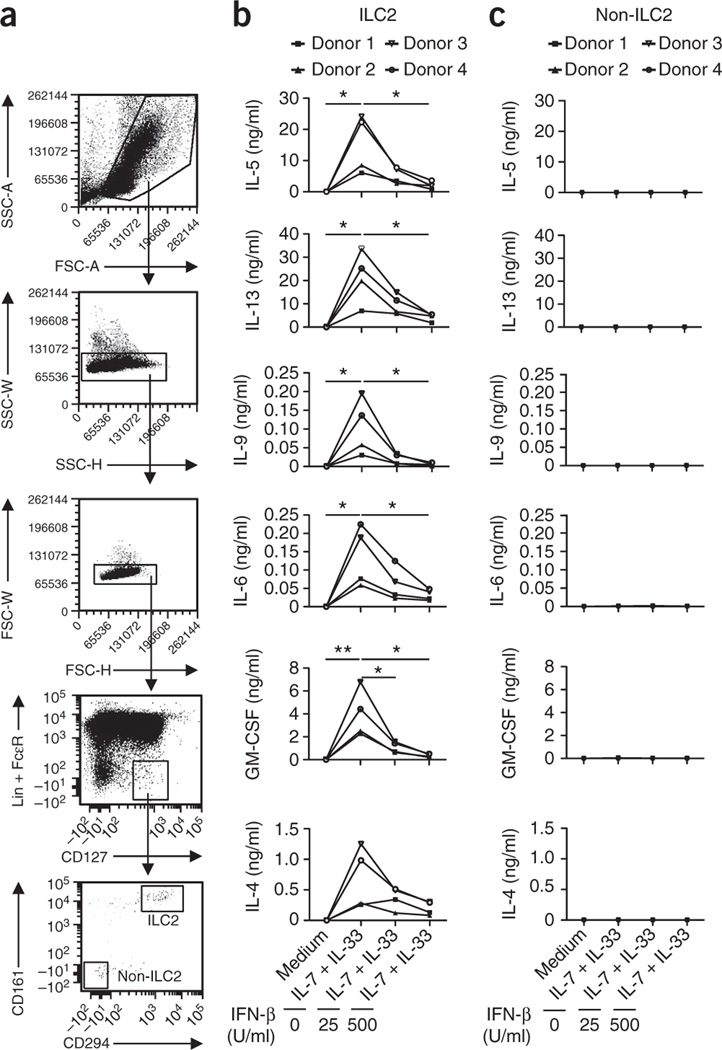
To investigate whether type I interferons also regulate the function of human ILC2 cells, we purified human cord blood–derived, lineage marker–negative (Lin−) FcεRI−CD127+CD161+CD294+ ILC2 cells and stimulated them with IL-7 plus IL-33 only or in combination with IFN-β. We used a cord blood–derived Lin−FcεRI−CD127+CD161−CD294− cell purity of the ILC2 population obtained (Fig. 3a). Similar to mouse ILC2 cells, purified human ILC2 cells produced large quantities of IL-5 and IL-13, as well as detectable amounts of IL-6, IL-9 and GM-CSF (Fig. 3b). In agreement with published reports24, human ILC2 cells also secreted IL-4 (Fig. 3b). Cytokine secretion by human ILC2 cells was significantly decreased by the addition of IFN-β, in a dose-dependent manner (Fig. 3b). In contrast, barely detectable amounts of cytokines were released by non-ILC2 cells under the same culture conditions (Fig. 3c), which highlighted the purity of the obtained ILC2 populations.
Figure 3.
Human ILC2 cells are regulated by type I interferons. (a) Gating strategy for ILC2 cells isolated from human cord blood, after setting of lymphocyte and singlet gates, human ILC2 cells were defined as Lin−FcεRI−CD127+CD161+CD294+ and sorted. The Lin−FcεRI−CD127+CD161−CD294− cell population (non-ILC2 cells) served as a negative control. SSC, side scatter; FSC, forward scatter. (b) Multiplex assay of cytokines in supernatants of cells sorted (from n = 4 donors) as in a as ILC2 cells, and non-ILC2 cells (c), then stimulated for 5 d with medium or IL-7 plus IL-33 in the presence or absence of IFN-β (as in Fig. 2f). *P ≤ 0.05 and **P ≤ 0.01 (Mann-Whitney test). Data are representative of four experiments.
As type I interferons have been shown to regulate the plasticity of lymphoid cells25, we investigated whether the stimulation of ILC2 cells with type I interferons would alter their ability to produce signature cytokines of ILC1 and ILC3 cells. The secretion of IL-22 by mouse ILC2 cells was below the limit of detection (data not shown). Moreover, the secretion of IL-17A and IFN-γ by mouse and human ILC2 cells was just slightly above the limit of detection (data not shown), which suggested that in the settings tested, type I interferons did not induce substantial release of ILC1 or ILC3 signature cytokines from mouse or human ILC2 cells. Collectively, these observations identified type I interferons as a potent direct regulator of ILC2 function in mice and humans.
Mechanisms of type I interferon–mediated regulation of ILC2 cells
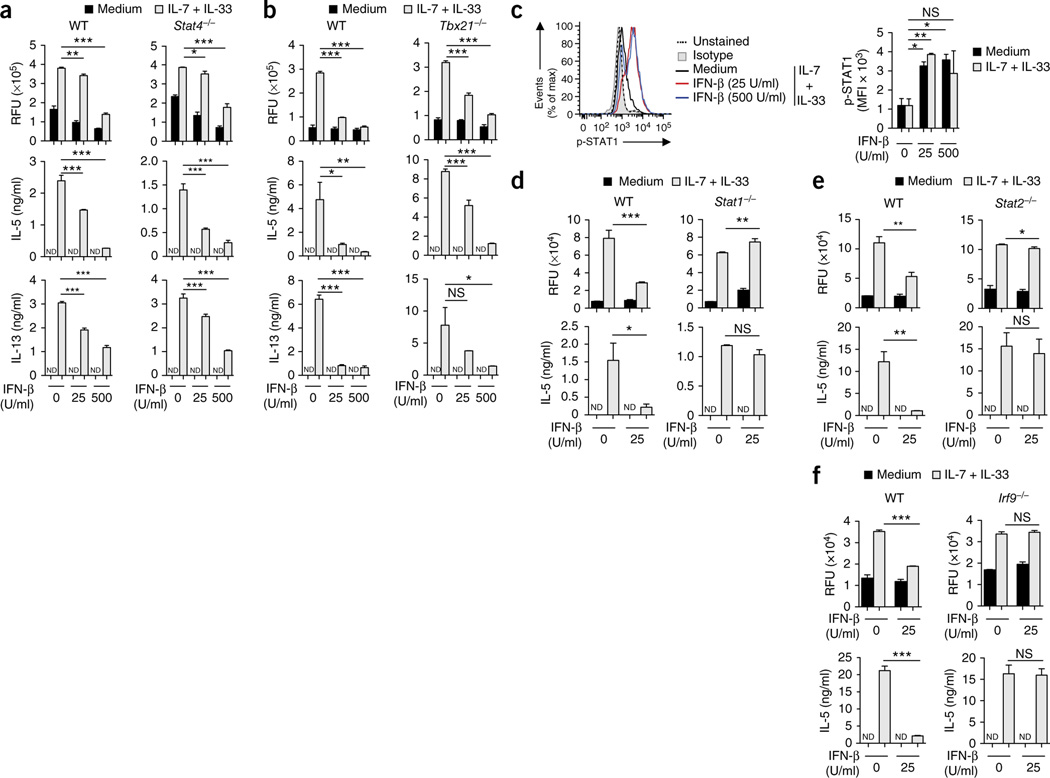
To delineate the mechanism of type I interferon–mediated regulation of ILC2 function, we first investigated the signaling pathway engaged downstream of IFNAR. All type I interferons share a common receptor, composed of IFNAR1 and IFNAR2, that signals through the formation of heterodimers of the signal transducers STAT1 and STAT2, which further associate with the transcription factor IRF9 to form the heterotrimeric ISGF3 complex. However, depending on the cell type and biological context, IFNAR can also induce the activation and nuclear translocation of STAT1-STAT1 homodimers that bind to IFN-γ-activated sequences in the promoters of IFN-γ-induced genes25. In addition, IFNAR has been shown to signal through STAT4 (ref. 25), STAT2-STAT6 heterodimers26 or the transcription factor T-bet27, or to activate inducible nitric oxide synthase (iNOS)28, to regulate gene transcription. We observed that type I interferon–mediated inhibition of the proliferation and cytokine release of ILC2 cells occurred independently of STAT4 and T-bet (Fig. 4a,b), as well as of iNOS and STAT6 (Supplementary Fig. 4), but required STAT1, STAT2 and IRF9 (Fig. 4c–f), which demonstrated an essential role for ISGF3-mediated gene expression in this.
Figure 4.
Mechanisms of type I interferon–mediated regulation of ILC2 cells. (a,b) Proliferation of BM cells obtained from wild-type and Stat4−/− mice (a) or wild-type and T-bet-deficient (Tbx21−/−) mice (b) and stimulated for 5 d with medium or IL-7 plus IL-33 in the presence or absence of IFN-β (as in Fig. 2c) (top row), and ELISA of IL-5 and IL-13 in supernatants of those cells (below). (c) Intracellular staining and flow cytometry analyzing phosphorylated (p-) STAT1 in sorted and expanded BM-derived wild-type ILC2 cells stimulated for 15 min as in a,b (left), and mean fluorescence intensity (MFI) of phosphorylated STAT1 in those cells (right). Isotype, isotype-matched control antibody. (d–f) Proliferation of BM cells obtained from wild-type and Stat1−/− mice (d), wild-type and Stat2−/− mice (e) or wild-type and Irf9−/− mice (f) and stimulated for 5 d with medium or IL-7 plus IL-33 (as in Fig. 2c) in the presence or absence of IFN-β (25 U/ml) (top row), and ELISA of IL-5 in supernatants of those cells (bottom row). *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 (Mann-Whitney test). Data are representative of three (a,b) or two (c,d–f) independent experiments (mean and s.d. (c) or mean and s.d. of triplicates (a,b,d–f)).
The stimulation of pure BM-derived ILC2 cells with IL-7 plus IL-33 induced cell division (Fig. 5a) and enhanced viability (Fig. 5b). The addition of IFN-β resulted in reduced capacity of the cells to proliferate (Fig. 5a) and led to increased cell death over time (Fig. 5b). The stimulation of ILC2 cells with IFN-β resulted in reduced numbers of cytokine-producing ILC2 cells with lower intracellular levels of IL-5 and IL-13 (Fig. 5c,d). The addition of IFN-β to ILC2 cells that had already been stimulated still significantly reduced their proliferation and cytokine production (Supplementary Fig. 5a; P values in legend). Moreover, removal of IFN-β from the cultures had little influence on its suppressive effect on ILC2 cells (Supplementary Fig. 5b). As GATA-3 is essential for the development and maintenance of ILC2 cells2, we further investigated the effect of type I interferons on GATA-3 expression. Stimulation of ILC2 cells with IFN-β for several days led to reduced expression of GATA-3 (Supplementary Fig. 5c). However, despite its suppression of proliferation and cytokine production, the removal of IFN-β restored GATA-3 expression (Supplementary Fig. 5d), which suggested a contributory but non-essential role for the regulation of GATA-3 by type I interferons. Collectively these observations showed that type I interferons directly regulated ILC2 cells in an ISGF3-dependent manner that led to reduced production of cytokines and cell proliferation, as well as increased cell death.
Figure 5.
Stimulation of ILC2 cells with type I interferons reduces proliferation, viability and cytokine production. (a) Proliferation of sorted and expanded BM-derived wild-type ILC2 cells labeled with the division-tracking dye CFSE and then stimulated for 3 or 5 d (above plots) with medium or IL-7 plus IL-33 in the presence or absence of IFN-β (as in Fig. 2d), analyzed by flow cytometry. (b) Proliferation of sorted and expanded BM-derived wild-type ILC2 cells stimulated for 2 or 3 d (left margin) as in a, assessed by staining with annexin V and viability dye (LIVE/DEAD), followed by flow cytometry. (c) Expression of IL-5 and IL-13 by sorted and expanded BM-derived wild-type ILC2 cells stimulated for 3 d as in a, assessed by intracellular flow cytometry. (d) Quantification of IL-5+ and IL-13+ cells and intracellular staining of IL-5 and IL-13 (presented as mean fluorescence intensity relative to that of isotype-matched control antibody), for cells as in c. Numbers in quadrants (b,c) indicate percent cells in each. *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 (Mann-Whitney test). Data are representative of two independent experiments (mean and s.d. in d).
Type I interferons regulate ILC2 cells in vivo
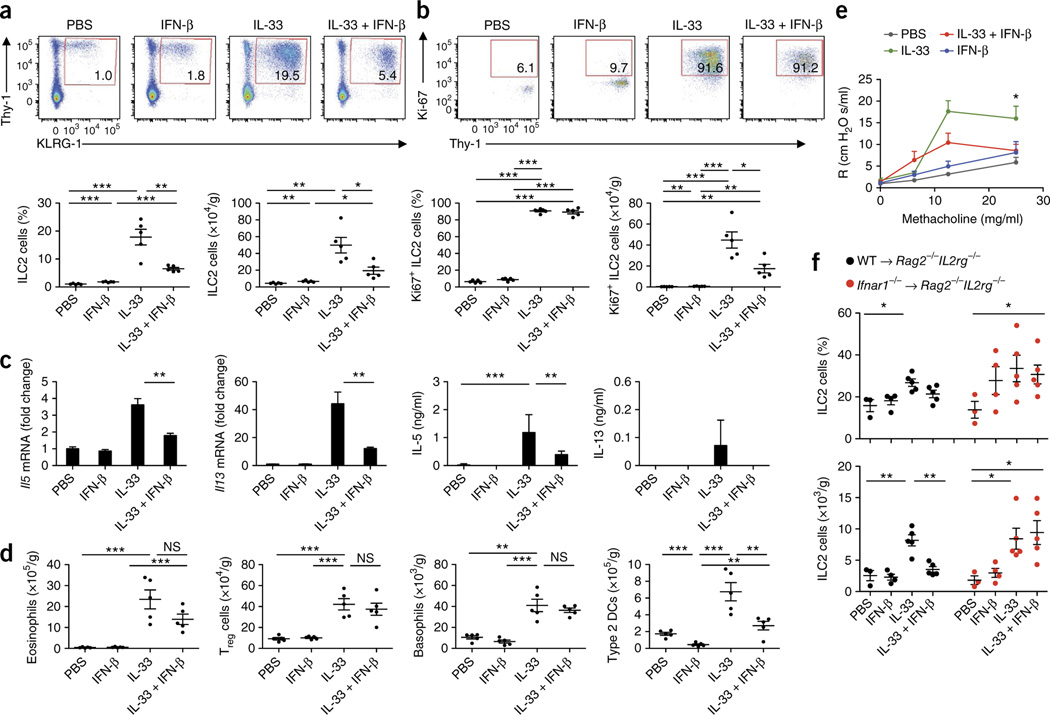
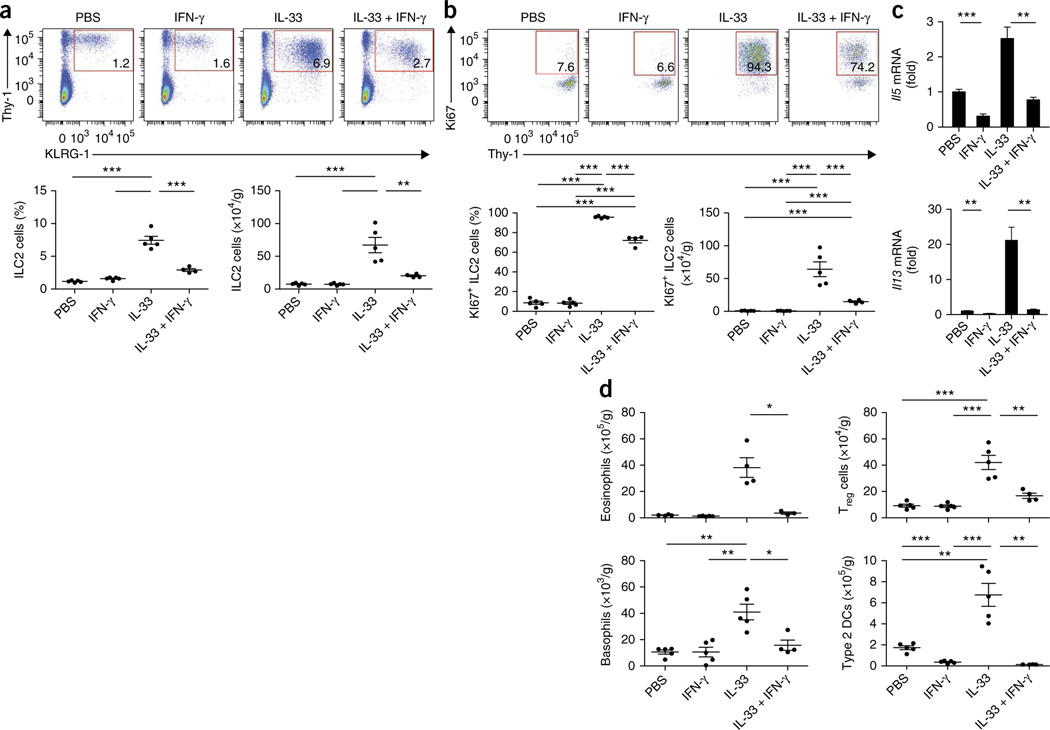
To confirm our finding that ILC2 cells were regulated by type I interferons and to further investigate the cellular immunological cascade in vivo, we administrated IL-33 intranasally to mice alone and in combination with IFN-β. In accordance with published studies29, the IL-33 challenge led to a substantial increase in the frequency and absolute number of ILC2 cells in the lungs (Fig. 6a). However, the frequency and total number of pulmonary ILC2 cells were significantly lower after administration of IL-33 in conjunction with IFN-β than after challenge with IL-33 alone (Fig. 6a). Staining of pulmonary ILC2 cells with the proliferation marker Ki67 demonstrated that IL-33 challenge substantially induced the proliferation of pulmonary ILC2 cells (Fig. 6b). Challenge with IL-33 in combination with IFN-β significantly reduced the number of Ki67+ lung ILC2 cells (Fig. 6b), which highlighted the finding that type I interferons potently regulated the proliferative capacity of ILC2 cells in vivo. Consistent with the changes in the pulmonary ILC2 population, the expression of Il5 and Il13 mRNA, as well as that of IL-5 and IL-13 protein (Fig. 6c) and Il6 mRNA (Supplementary Fig. 6b), was significantly lower in the lungs of mice that received IL-33 in combination with IFN-β than in those of mice that received IL-33 only. Moreover, IFN-β significantly reduced the IL-33-mediated expression of genes encoding the type 2 immunity markers CCL17, CCL22 and CHI3L3 (ref. 30), as well as fibronectin 1 (ref. 31), a marker of fibrosis (Supplementary Fig. 6c–f; P values in legend). IL-33 challenge further led to an increase in basophils, eosinophils, type 2 DCs32,33 and regulatory T cells (Treg cells) (Fig. 6d). Whereas co-administration of IFN-β did not alter the IL-33-mediated increase in pulmonary basophils and Treg cells, we observed significantly lower numbers of type 2 DCs after co-administration of IFN-β (Fig. 6d). Similarly, IFN-β restrained the IL-33-mediated increase in the abundance of pulmonary eosinophils (Fig. 6d) and expression of mRNA encoding the eosinophil chemotactic factor CCL24 (Supplementary Fig. 6g). Furthermore, the increase in IL-33-mediated expression of CD11c by eosinophils, indicative of cell activation34, was reduced by co-injection of type I interferons (Supplementary Fig. 6h,i). IL-33-induced AHR was significantly suppressed by IFN-β (Fig. 6e).
Figure 6.
Type I interferons restrict ILC2 cells and type 2 immunopathology in vivo. (a) Flow cytometry (top) of lung cells from wild-type mice challenged intranasally for 3 consecutive days with PBS, IFN-β, IL-33, or IL-33 plus IFN-β (above plots), assessed 24 h after the final treatment. Numbers in outlined areas indicate percent Lin−KLRG1+Thy-1+ ILC2 cells. Below, frequency (left) and total number (right) of ILC2 cells as above (n = 5 mice per group; age and sex matched). (b) Flow cytometry (top) of lung cells from mice as in a. Numbers in outlined areas indicate percent pulmonary Ki67+ ILC2 cells. Below frequency (left) and total number (right) of Ki67+ ILC2 cells as above. (c) Quantitative RT-PCR analysis of Il5 and Il13 mRNA in lung tissue (left) and ELISA of IL-5 and IL-13 in bronchoalveolar lavage fluid (right) of mice as in a (mRNA results presented as in Fig. 1f,g). (d) Total eosinophils, basophils, Treg cells and type 2 DCs in mice as in a, assessed by flow cytometry. (e) Lung AHR in mice as in a, assessed as pulmonary resistance (R) after delivery of various concentrations of aerosolized methacholine (horizontal axis). (f) Frequency (top) and total number (bottom) of pulmonary ILC2 cells from Rag2−/−Il2rg−/− host mice given wild-type donor ILC2 cells (WT→Rag2−/−Il2rg−/−) or Ifnar1−/− donor ILC2 cells (Ifnar1−/−→ Rag2−/−Il2rg−/−), then challenged as in a, assessed by flow cytometry. Each symbol (a,b,d,f) represents an individual mouse; small horizontal lines indicate the mean (± s.d.). *P ≤ 0.05; **P ≤ 0.01 and ***P ≤ 0.001 (Mann-Whitney test (a–d,f) or repeated-measures ANOVA, followed by Dunnett’s multiple-comparison test (e)). Data are representative of two experiments (a–d; mean and s.d.) or one experiment (e,f; mean and s.d.).
To confirm that IFN-β acted directly on ILC2 cells in vivo, we performed adoptive-transfer experiments. We isolated lung ILC2 cells from wild-type or Ifnar1−/− mice and expanded these in vitro, then transferred the cells into ILC2-deficient Rag2−/−Il2rg−/− host mice. Challenge of the host mice with IL-33 led to a substantial increase in pulmonary ILC2 cells in Rag2−/−Il2rg−/− host mice that received wildtype ILC2 cells, as well as in Rag2−/−Il2rg−/− host mice that received Ifnar1−/− ILC2 cells (Fig. 6f). However, after the administration of IL-33 in conjunction with IFN-β, the frequency and number of pulmonary ILC2 cells were significantly lower only in Rag2−/−Il2rg−/− host mice that received wild-type ILC2 cells, not in Rag2−/−Il2rg−/− host mice that received Ifnar1−/− ILC2 cells (Fig. 6f). This demonstrated that direct IFNAR signaling on ILC2 cells was needed to restrain the IL-33-mediated activation of ILC2 cells.
Type II interferon and IL-27 negatively regulate ILC2 cells
Not only type I interferons but also the sole type II interferon (IFN-γ) are often produced in the course of the same immune response, and published reports have suggested that type I interferons induce the production of IFN-γ in innate and adaptive lymphoid cells25. Moreover, IL-27 has emerged as an interferon-mediated regulator of innate and adaptive immune responses35. As BM and lung ILC2 cells expressed receptors for IFN-γ (IFNGR1) and IL-27 (WSX-1 and CD130) (Fig. 7a), we investigated whether IL-27 and IFN-γ were able to regulate ILC2 function and subsequent induction of type 2 immunity. In vitro analysis revealed that IL-27 altered the IL-33-mediated release of IL-5 by BM cells in a STAT1-dependent manner (Fig. 7b). Moreover, we observed that IFN-γ altered the IL-33-mediated proliferation of BM cells and their release of IL-5 in a manner dependent on the IFN-γ receptor IFNGR1 and STAT1 (Fig. 7c,d) but independent of STAT2, IRF9, IFNAR, STAT4, T-bet, iNOS and STAT6 (Supplementary Fig. 7a–f). Those observations were substantiated by experiments with pure ILC2 cells, which demonstrated that IFN-γ as well as IL-27 altered the proliferation and cytokine release of pure BM ILC2 cells in a dose-dependent manner (Fig. 7e–h). Furthermore, IFN-γ as well as IL-27 reduced the capacity of IL-7- and IL-33-stimulated ILC2 cells to release cytokines IL-5, IL-6, IL-9, IL-13 and GM-CSF (Fig. 7f,h). As noted after stimulation with type I interferons, IFN-γ as well as IL-27 induced only undetectable to low levels of ILC1 or ILC3 signature cytokines (data not shown).
Figure 7.
IL-27 and IFN-γ regulate ILC2 function. (a) Cell surface expression of IFNGR1, WSX-1 and CD130 on BM and lung ILC2 cells, as well as splenocytes. (b) ELISA of IL-5 in supernatants of wild-type and Stat1−/− BM cells stimulated for 5 d with medium or with IL-7 plus IL-33 (each at 10 ng/ml) in the presence (20) or absence (0) of IL-27 (20 ng/ml). (c,d) Proliferation of wild-type and Ifngr1−/− BM cells (c) or wild-type and Stat1−/− BM cells (d) stimulated for 5 d in as in b (top row), and ELISA of IL-5 in supernatants of those cells (bottom row). (e,f) Proliferation (e, left) and quantification (e, right) of sorted and expanded BM-derived wild-type ILC2 cells stimulated for 5 d with medium or with IL-7 plus IL-33 (each at 10 ng/ml) in the presence or absence of IFN-γ (20 ng/ml), and ELISA and multiplex assay of cytokines in supernatants of cells as in e (f). (g,h) Proliferation (g, left) and quantification (g, right) of cells stimulated as in e in the presence or absence of IL-27 (20 ng/ml), and ELISA and multiplex assay of cytokines in supernatants of cells as in g (h). *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 (Mann-Whitney test). Data are representative of two (a,b) or three (c–h) independent experiments (mean and s.d. of triplicates in b–e,g).
To further confirm the observations obtained for IFN-γ-mediated ILC2 regulation and to delineate the cellular immunological cascade in vivo, we administered IL-33 intranasally, alone and in combination with IFN-γ, to wild-type mice. The frequency and total number of pulmonary ILC2 cells (Fig. 8a), as well as of Ki67+ ILC2 cells (Fig. 8b), were markedly lower in mice given IL-33 in combination with IFN-γ than in mice that received IL-33 only. Moreover, we observed reduced expression of Il5 and Il13 mRNA (Fig. 8c), as well as of Il6, Ccl17, Ccl22, Chi3l3, Fn1 and Ccl24 mRNA (Supplementary Fig. 8a–f), in mice given IL-33 in combination with IFN-γ than in mice that received IL-33 only. Furthermore, co-administration of IFN-γ potently reduced the IL-33-mediated increase in pulmonary eosinophils and type 2 DCs and, in contrast to type I interferon, also altered the induction of basophils and Treg cells (Fig. 8d); this demonstrated that IFN-γ was a potent regulator of this ILC2 function and pulmonary type 2 immunity in vivo. Thus, type I interferons, IFN-γ and IL-27 synergistically restricted type 2 immunopathology and fibrosis through the regulation of ILC2 cells.
Figure 8.
IFN-γ restricts ILC2 cells and type 2 immunopathology in vivo. (a) Flow cytometry (top) of lung cells from wild-type mice challenged intranasally for three consecutive days with PBS, IFN-γ, IL-33, or IL-33 plus IFN-γ (above plots), assessed 24 h after the final treatment. Numbers in outlined areas indicate percent Lin−KLRG1+Thy-1+ ILC2 cells. Below, frequency (left) and total number (right) of ILC2 cells as above (n = 4–5 mice per group; age and sex matched). (b) Flow cytometry (top) of lung cells from mice as in a. Numbers in outlined areas indicate percent Ki67+ ILC2 cells. Below, frequency (left) and total number (right) of Ki67+ ILC2 cells as above. (c) Quantitative RT-PCR analysis of Il5 and Il13 mRNA in lung cells from mice as in a (presented as in Fig. 1f,g). (d) Total eosinophils, basophils, type 2 DCs and Treg cells in mice as in a, assessed by flow cytometry. Each symbol (a,b,d) represents an individual mouse; small horizontal lines indicate the mean (± s.d.). *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 (Mann-Whitney test). Data are representative of two independent experiments (mean and s.d. in c).
DISCUSSION
ILC2 cells are characterized by their ability to produce immense amounts of type 2 cytokines early in an immunological challenge without any antigen-specific activation and are sufficient to induce type 2 immunopathologies, such as allergies and virus-induced asthma in mice and humans21. We found here that type I interferons acted as a master regulator of ILC2 function and thereby controlled innate and adaptive type 2 immunity and its associated pathologies.
Type I interferons reduced the capacity of IL-33-stimulated mouse and human ILC2 cells to proliferate and to release the type 2 signature cytokines IL-4, IL-5, IL-9 and IL-13, as well as IL-6 and GM-CSF. This was correlated with lower numbers of pulmonary eosinophils and type 2 DCs and reduced expression of markers of the type 2 immune response and fibrosis. We observed increased ILC2 cells and more deregulated innate and adaptive type 2 immunity in type I interferon receptor–deficient mice infected with IAV or H. polygyrus than in infected wild-type mice, which demonstrated that type I interferons were central regulators of ILC2-mediated immunity.
Results of studies of the susceptibility of IFNAR-deficient mice to infection with influenza virus and their viral loads of influenza virus have been controversial20,36,37. Differences in mouse strain backgrounds and variations in viral doses tested have been suggested to account for the diverse results20,38. Beyond its ‘instruction’ of direct antiviral activity, type I interferons’ regulation of monocytes and neutrophils has been shown to contribute to resistance to infection with influenza virus20. Although deficiency in IFNAR signaling resulted in increased susceptibility to infection with IAV, we did not observe significant differences between wild-type and Ifnar1−/− mice in their viral loads after infection with a sub-lethal dose of IAV. Our observations therefore suggested that the enhanced type 2 inflammation observed in Ifnar1−/− mice after infection with a sub-lethal dose was not due to increased viral replication–induced tissue damage and that viral load was uncoupled from type 2 immunopathology. Interestingly, we observed serum IgE concentrations were already higher in uninfected Ifnar1−/− mice, which further highlighted the proposal that lack of sensing of type I interferon pre-disposed the mice to elevated type 2 immune responses. A report has demonstrated that germ-free mice, which lack bacterial intestinal flora as well as viral intestinal flora, have more gut-resident ILC2 cells than do standard pathogen-free mice39. This effect is reversed upon infection with murine norovirus39. Although the possibility remains to be tested, it is likely that type I interferons induced by murine norovirus contribute to the restraint of intestinal ILC2 cells.
In agreement with published reports21, we observed that expression of Il13 mRNA was significantly elevated in lungs of mice infected with IAV, which correlated with an increase in IL-33 protein and the number of ILC2 cells. However, despite equal induction of IL-33 protein in lungs of wild-type mice and those of Ifnar1−/− mice, the number of ILC2 cells was significantly higher in Ifnar1−/− mice, which suggested that the ILC2 cells were directly regulated by type I interferons. Indeed, after the administration of IL-33 in conjunction with IFN-β, the frequency and number of pulmonary ILC2 cells were significantly lower only in Rag2−/−IL2rg−/−mice that had received wild-type ILC2 cells, not in Rag2−/−IL2rg−/−mice that had received Ifnar1−/− ILC2 cells; this demonstrated that direct IFNAR signaling by ILC2 cells was needed to restrain the IL-33-mediated activation of ILC2 cells. However, as type I interferons acts on many cell types, we cannot exclude the possibility that type I interferon–mediated regulation of cells other than ILC2 cells contributed to the restraint type 2 immunity during infection with IAV.
Depending on the cell type and biological context, the type I interferon receptor can activate different downstream signaling pathways to regulate cell differentiation and function. The ability of type I interferons to regulate ILC2 function required the heterotrimeric ISGF3 complex consisting of STAT1, STAT2 and IRF9, while STAT4, STAT6 and iNOS were dispensable for this. The ligation of type I interferon receptor activates T-bet-mediated signaling27, and T-bet is required for the fate and function of Nkp46-expressing ILC3 cells40–42. However, type I interferon–mediated regulation of ILC2 function occurs independently of T-bet. STAT1 exerts critical roles in receptor-mediated signaling of IFN-γ25 and IL-27 (ref. 43), and we observed here that STAT1 was essential for the ability of both IFN-γ and IL-27 to regulate ILC2 function. Similar observations are reported in the accompanying paper demonstrating that IFN-γ and IL-27 are potent regulators of ILC2 cells44.
Type I interferons altered the cytokine production and proliferation of and enhanced the death of ILC2 cells. In addition, we observed that treatment with type I interferons reduced GATA-3 expression in ILC2 cells. However, we observed that despite its suppression of proliferation and cytokine production, removal of IFN-β restored GATA-3 expression, which suggested a contributing but non-essential role for the regulation of GATA-3 by type I interferons.
Our results are supported by clinical findings demonstrating that polymorphisms in human genes encoding components of the interferon pathway are associated with type 2 immunopathologies. For example, single-nucleotide polymorphisms in introns of IFNG are associated with asthma45. In addition, polymorphisms in the genes encoding the two IFN-γ receptor chains, IFN-γR1 and IFN-γR2, as well in genes encoding the central downstream signaling molecules STAT1, STAT2 and IRF1, are associated with asthma46,47, which further highlights the role of the interferon pathway in restraining type 2 pathologies.
Pulmonary viral infection frequently causes AHR, and lower interferon expression has been reported in asthmatic patients. Both mouse ILC2 cells and human ILC2 cells are induced after respiratory viral infection21, which is paradoxical, given the usually typical type 1 cytokine–based immune response that follows viral challenge. Upon infection of the airways, ILC2 cells contribute to type 2 immunopathology and loss of airway function21. Our data therefore provide a mechanistic explanation for the clinical findings and suggest that polymorphisms in genes encoding components of the interferon pathways, as well as pathogen-mediated interference of interferon expression and signaling, alter the ability of the immune system to restrain ILC2 cells and predispose the host to disease. Notably, treatment with interferon has been reported to have a positive effect in patients suffering severe asthma48. Increasing interferon signaling and lowering the interferon signaling threshold through blockade of negative feedback mechanisms are therefore promising strategies for the development of treatments for type 2 immunopathologies and fibrotic disease.
METHODS
Methods and any associated references are available in the online version of the paper.
ONLINE METHODS
Mice.
C57BL/6 wild-type mice (000664; Jax), Stat6−/− mice (005977; Jax), Tbx21−/− mice (004648; Jax) and Ifngr1−/− mice (003288; Jax) were purchased from the Jackson Laboratory. Inos−/− mice (4236) and Rag2−/−IL2rg−/− mice (4111) mice were obtained from Taconic. Ifnar1−/− (Ifnar1tm1Agt) were a gift from J.L. Gommerman (University of Toronto). Stat4Ity14/Ity14 mice were generated and contributed by M.M.E. and D.M.; these mice carry a loss-of-function mutation in Stat4 and called ‘Stat4−/− mice’ here to simplify the nomenclature in this article. Adult age- and sex-matched mice were used. Animals were bred at McGill University in an specific pathogen–free environment, and all experiments were in accordance with legislation outlined in the regulations and standard guidelines of McGill University. A.M.G. provided bones from Stat1−/− and Stat2−/− mice, and K.L.M. provided bones from Irf9−/− mice.
Reagents.
IL-2, IL-7, IL-25, IL-27 and TSLP were purchased from R&D Systems. IL-33 was obtained from R&D systems or BioLegend. IFN-β and IFN-γ (mouse and human) were purchased from PBL and Peprotech, respectively. Red blood cells were lysed with red blood cell lysis buffer (Sigma).
IAV.
The mouse-adapted IAV H1N1 strain A/Puerto Rico/8/34 was propagated and titrated by plaque assay on Madin-Darby canine kidney cells as described49. As quantitative RT-PCR analysis of viral load is highly sensitive and specific50,51, we analyzed expression of the influenza virus gene encoding the nonstructural protein NS1 (Ns1) to compare the viral loads of wild-type mice and Ifnar1−/− mice after infection with a sublethal dose of IAV.
Anesthesia and IAV infection.
Mice were anesthetized by intramuscular injection of a mixture of ketamine and xylazine in PBS. For sublethal infection, 5 or 20 plaque-forming units of IAV per 20 g body weight were administered intranasally. Mice were monitored daily and were killed at the appropriate time point.
Lung histopathological analysis.
After perfusion, lungs were inflated with 10% buffered formalin acetate, then were fixed at room temperature and embedded in paraffin. Sections 5 μm in thickness were stained with hematoxylin and eosin, periodic acid–Schiff or Masson’s trichrome. Scores were assigned for inflammatory infiltration density, alveolar type 2 pneumocyte hyperplasia, bronchial-bronchiolar epithelial hyperplasia and alveolar wall thickening and fibrosis in affected parenchyma. The grading scheme was as follows: 1, minimal (<25% of total section surface); 2, mild (25–50% of total section surface); 3, moderate (>50–75% of total section surface); and 4, marked (<75% of total section surface).
Bronchoalveolar lavage.
Upon exposure of the trachea, bronchoalveolar lavage fluid was obtained by slow injection of 0.5 ml PBS into the lung, which was subsequently recovered again in the syringe. The fluid was centrifuged at 582g at 4 °C and the supernatant was stored at −80 °C for further analysis.
In vitro BM assay.
BM was isolated from femur and tibia of mice. After lysis of red blood cells, 1 × 106 cells were cultured for 5 d in RPMI-1640 medium supplemented with 10% FBS, 2 mM glutamine, 100 U/ml Penicillin and 100 μg/ml streptomycin, in a flat-bottom 96-well plate. IL-7 and IL-33 (both at 10 ng/ml) were added where appropriate.
Expansion of BM ILC2 cells.
BM cells were isolated and then were stained with antibodies to lineage markers (TCRβ, TCRγδ, CD3ε, CD11b, CD11c, Ter119, Gr1 (Ly6G), CD45R (B220), NK1.1 and CD5), as well as antibodies to CD25, c-Kit and Sca-1 (all in Supplementary Table 1). BM ILC2 cells were sorted as Lin−Sca-1+c-Kit−CD25+ cells as described23. Sorted cells were expanded in complete medium (RPMI-1640 medium containing 10% FBS, 2 mM glutamine, 100 U/ml penicillin/100 μg/ml streptomycin, 50 μg/ml gentamicin and 80 μM 2-mercaptoethanol), and IL-2, IL-7, IL-25 and IL-33 (each at 50 ng/ml) and TSLP (20 ng/ml). After 15 d of expansion, cells were allowed to ‘rest’ for 2 d in complete medium containing only IL-2 and IL-7 (10 ng/ml) and then were used in different experimental approaches.
Viability assay.
AlamarBlue (Invitrogen) is a redox indicator that yields in colorimetric changes in response to metabolic activity. At the appropriate time points, alamarBlue was added to the cell culture in accordance to the manufacturer’s recommendations (Invitrogen).
Protein quantification in serum, lungs and cell culture supernatants.
Cytokines (IL-5, IL-9, IL-13, IL-17A, IL-22, IFN-γ and amphiregulin) in supernatants of ILC2 cell cultures were analyzed with ELISA kits from R&D Systems. Serum IgG1 was assessed by sandwich ELISA with antibodies from Southern Biotechnologies (coating antibody, goat antibody to mouse immunoglobulin, heavy and light chains (1010–01); standard, mouse IgG1 (15H6; 0102–01); detection antibody, biotin-conjugated goat antibody to mouse IgG1 (1070–08)) and serum IgE was analyzed with a mouse IgE ELISA MAX kit (BioLegend). In some experiments, cytokines in supernatants of ILC2 cell cultures were analyzed by multiplex assay (Eve Technologies). For analysis of IL-33 protein in whole lungs, tissues were homogenized in PBS containing protease inhibitors and, after centrifugation, supernatants were stored at −80 °C for further analysis.
Isolation of ILC2 cells from human cord blood.
Peripheral blood mononuclear cells from human cord blood underwent enrichment by lymphocyte separation medium (Wisent). Cells were washed with ice-cold FACS buffer (2% FBS in PBS) and were subsequently stained and sorted for the purification of human ILC2 cells (Lin−CD127+CRTH2+CD161+). The human lineage ‘cocktail’ and antibodies (Supplementary Table 1) were obtained from BioLegend. This protocol has been approved by the ethics committee of Centre de Recherche du Centre Hospitalier de l’Université de Montréal (The Research Center of the Centre Hospitalier de L’Université de Montréal).
Staining of cell suspensions for flow cytometry.
Fc receptors were blocked with 2.4G2 hybridoma supernatant (HB-197; American Type Culture Collection) and subsequently surface staining of cells in FACS buffer (2% FBS in PBS) was performed for 30 min on ice. Antibodies used for flow cytometry staining are in Supplementary Table 1. Dead cells were identified by staining with a LIVE/DEAD Fixable Aqua Dead Cell Stain Kit or LIVE/DEAD Fixable bright violet Dead Cell Stain Kit according to the manufacturer’s instructions (Invitrogen). For the detection of early-stage apoptotic cells, Annexin V staining was performed according to the manufacturer’s recommendations (eBioscience). For intracellular staining, a Foxp3 staining buffer set was used according to the manufacturer’s recommendations (eBioscience). Data were acquired on a FACSCanto II (BD Biosciences) and were analyzed with FlowJo software (Tree Star). After setting of singlet gate, viable cell populations were further characterized by staining for CD11b+Ly6C+Ly6G+ polymorphonuclear neutrophils, CD11b+Ly6C+Ly6G− inflammatory monocytes, and CD45+SiglecF+ CD11c− eosinophils. Lung ILC2 cells were characterized as follows: pulmonary cells were isolated and stained for KLRG1, Thy-1, ICOS, T1/ST2 and CD127 and for lineage markers (TCRβ, TCRγδ, CD3ε, Gr-1, CD11b, TER-119, B220, NK1.1, CD5 and CD11c). After setting of singlet and lymphocyte gates, viable Lin− ILC2 cells were defined as T1-ST2+Thy-1+ cells that expressed KLRG1, CD127 and ICOS. After setting of singlet gate and exclusion of Siglec-F+CD11c− cells, viable pulmonary type 2 cells (DCs) were characterized as B220−MHCIIhiCD11c+CD301b+ cells. Viable pulmonary basophils were defined as SSCloCD49b+FcεRI+ cells, while viable pulmonary Treg cells were defined as CD4+Foxp3+ cells.
Administration of IL-33 and interferon and lung preparation.
Mice were anesthetized before administration of cytokines by intramuscular injection of a mixture of ketamine and xylazine in PBS. IL-33 (500 ng per mouse) was applied intranasally together with IFN-β (5,000 U per mouse) or IFN-γ (1 μg per mouse) for 3 consecutive days. 24 h after the final treatment, mice were killed and lungs were perfused with 10 ml ice-cold PBS. The left lung lobe was separated and stored in RNAlater (Ambion) or frozen in liquid nitrogen for RNA preparation. The remaining lung was digested as described52 with minor modifications. Lungs were cut into small pieces that were digested in RPMI-1640 medium containing 5% FBS and 0.5 mg/l collagenase type IV (Sigma) and 0.02 mg/ml DNase I (Roche). After 1 h of digestion, lung pieces were homogenized with a 10-ml syringe with an 18G1½ needle and were filtered through a 100-μm cell strainer. Cell suspensions were washed once with flow cytometry buffer (2% FBS in PBS) and were subsequently stained for analysis by flow cytometry.
Quantitative RT-PCR.
Lung tissues were homogenized with a MagNA Lyser (Roche). Total RNA was extracted with TRIzol reagent (Life Technologies) according to the manufacturer’s instructions. After treatment with DNase I, cDNA was synthesized with Oligo(dT)12–18 Primer (Life Technologies) and SuperScript III Reverse Transcriptase (Life Technologies). Primers for quantitative RT-PCR are in Supplementary Table 2. Quantitative RT-PCR was carried out with Power SYBR Green (Life Technologies) in an Applied Biosystems StepOnePlus Real-Time PCR System. mRNA expression was calculated by the change-in-cycling-threshold (Ct) method, and results were normalized to those of the control gene Gapdh.
CFSE staining.
Pure BM-derived ILC2 cells were stained with 1 μM CFSE (carboxyfluorescein diacetate succinimidyl ester; Life Technologies) according to the manufacturer’s recommendations.
Detection of phosphorylated STAT1 in ILC2 cells.
Upon stimulation, cells were immediately fixed in 1.6% PFA and then were incubated for 10 min at room temperature. Cells were washed with PBS and were permeabilized by the addition of 100% ice-cold methanol. After an incubation period of at least 10 min at room temperature, cells were washed once with PBS and once with flow cytometry buffer and then were further incubated for 30 min at room temperature with the appropriate antibodies (Supplementary Table 1). After staining, cells were washed and resuspended in flow cytometry buffer and were further analyzed by flow cytometry.
Ex vivo stimulation of pulmonary ILC2 cells.
Cell suspensions of lung preparation underwent enrichment via a 40%−70% Percoll gradient. Cells (1 × 106 in a 96-well plate) were cultured in RPMI-1640 medium supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamycin and 55 μM β-mercapthoethanol, together with IL-7 and IL-33 (50 ng/ml), with or without IFN-β 500 U/ml, and in combination with PMA (phorbol 12-myristate 13-acetate) and ionomycin. Cells were stimulated in the presence of BD GolgiPlug (BD Biosicences).
Pulmonary AHR measurement.
Lung AHR was assessed by analysis of pulmonary resistance after delivery of aerosolized methacholine to mice treated with PBS, IFN-β or IL-33 alone, or IL-33 plus IFN-β, through use of the FlexiVent system (SCIREQ)53.
Transfer of pulmonary ILC2 cells.
Pulmonary ILC2 cells isolated from wild-type and Ifnar1−/− mice were stained and sorted by flow cytometry as Lin−CD45+ST2+ cells as described54. ILC2 cells then underwent expansion for 5–10 d in IL-2 and IL-7 (all at 50 ng/ml) and then were injected intravenously into Rag2−/−IL2rg−/− host mice (42,000 cells per mouse). At 3 h after cell transfer, host mice were challenged.
Infection with H. polygyrus.
BALB/c mice were used for passage of H. polygyrus. Mice were infected by gavage with 200 stage 3 larvae of H. polygyrus.
Statistical analysis.
Data were analyzed with GraphPad Prism 6 software (GraphPad Software). The Mann-Whitney test was used for determination of the significance of differences between two groups. One-way ANOVA, including the Bonferroni’s multiple-comparison test, was used for analysis of IL-33 in IAV-infected lung tissues. The significance of AHR measurements was evaluated by repeated-measures ANOVA, followed by Dunnett’s multiple-comparison test.
Supplementary Material
ACKNOWLEDGMENTS
We thank J.L. Gommerman (University of Toronto) for Ifnar1−/− (Ifnar1tm1Agt) mice; D. Xue and G.A. Kaufman for help with AHR measurements; and B. Charbonneau for technical support. Supported by the Canadian Institutes of Health Research (J.H.F. and C.U.D.; MOP-114972 for the J.H.F. laboratory; postdoctoral fellowship for C.U.D.; MOP-89821 operating funds for S.M.V.), the Canadian Foundation of Innovation (Leaders Opportunity Fund infrastructure grant for the J.H.F. laboratory), the German National Academy of Sciences Leopoldina (C.U.D.), The American Association of Immunologists Careers in Immunology Fellowship Program (J.H.F. and C.U.D.) and Canada Research Chair in Host Responses to Virus Infections (S.M.V.).
Footnotes
Any Supplementary Information and Source Data files are available in the online version of the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Gause WC, Wynn TA & Allen JE Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat. Rev. Immunol. 13, 607–614 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyler T. et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37, 634–648 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spits H. et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Diefenbach A, Colonna M. & Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 41, 354–365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenzie AN, Spits H. & Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Halim TY et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monticelli LA et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12, 1045–1054 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlow JL & McKenzie AN Type-2 innate lymphoid cells in human allergic disease. Curr. Opin. Allergy Clin. Immunol. 14, 397–403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambrecht BN & Hammad H. The immunology of asthma. Nat. Immunol. 16, 45–56 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Yoo JK, Kim TS, Hufford MM & Braciale TJ Viral infection of the lung: host response and sequelae. J. Allergy Clin. Immunol. 132, 1263–1276, quiz 1277 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansel TT, Johnston SL & Openshaw PJ Microbes and mucosal immune responses in asthma. Lancet 381, 861–873 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Edwards MR, Bartlett NW, Hussell T, Openshaw P. & Johnston SL The microbiology of asthma. Nat. Rev. Microbiol. 10, 459–471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wark PA et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201, 937–947 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards MR et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 6, 797–806 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Sastre A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 162, 12–18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barik S. Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr. Top. Microbiol. Immunol. 372, 173–191 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Feng Q, Langereis MA & van Kuppeveld FJ Induction and suppression of innate antiviral responses by picornaviruses. Cytokine Growth Factor Rev. 25, 577–585 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S. et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N. Engl. J. Med. 361, 1935–1944 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Terai M. et al. Early induction of interleukin-5 and peripheral eosinophilia in acute pneumonia in Japanese children infected by pandemic 2009 influenza A in the Tokyo area. Microbiol. Immunol. 55, 341–346 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Seo SU et al. Type I interferon signaling regulates Ly6Chi monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 7, e1001304 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YJ et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 12, 631–638 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds LA, Filbey KJ & Maizels RM Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin. Immunopathol. 34, 829–846 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brickshawana A, Shapiro VS, Kita H. & Pease LR Lineage−Sca1+c-Kit−CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J. Immunol. 187, 5795–5804 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mjösberg J. et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 37, 649–659 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G. Type I interferon: friend or foe? J. Exp. Med. 207, 2053–2063 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Jiang M. & Pernis AB IFN-α activates Stat6 and leads to the formation of Stat2:Stat6 complexes in B cells. J. Immunol. 163, 3834–3841 (1999). [PubMed] [Google Scholar]
- 27.Huber JP & Farrar JD Regulation of effector and memory T-cell functions by type I interferon. Immunology 132, 466–474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diefenbach A. et al. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 8, 77–87 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Barlow JL et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 132, 933–941 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Murray PJ et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffield JS, Lupher M, Thannickal VJ & Wynn TA Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 8, 241–276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumamoto Y. et al. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 39, 733–743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami R. et al. A unique dermal dendritic cell subset that skews the immune response toward Th2. PLoS ONE 8, e73270 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motomura Y. et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 40, 758–771 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Sweeney CM et al. IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav. Immun. 25, 1170–1181 (2011). [DOI] [PubMed] [Google Scholar]
- 36.García-Sastre A. et al. The role of interferon in influenza virus tissue tropism. Virol. 72, 8550–8558 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price GE, Gaszewska-Mastarlarz A. & Moskophidis D. The role of α/β and γ interferons in development of immunity to influenza A virus in mice. J. Virol. 74, 3996–4003 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blazejewska P. et al. Pathogenicity of different PR8 influenza A virus variants in mice is determined by both viral and host factors. Virology 412, 36–45 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Kernbauer E, Ding Y. & Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rankin LC et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat. Immunol. 14, 389–395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sciumé G. et al. Distinct requirements for T-bet in gut innate lymphoid cells. Exp. Med. 209, 2331–2338 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klose CS et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Hunter CA. & Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity 37, 960–969 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moro K. et al. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat. Immunol. doi: 10.1038/ni.3309 (23 November 2015). [DOI] [PubMed] [Google Scholar]
- 45.Kumar A. & Ghosh B. A single nucleotide polymorphism (A → G) in intron 3 of IFNγ gene is associated with asthma. Genes Immun. 9, 294–301 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Pinto LA et al. STAT1 gene variations, IgE regulation and atopy. Allergy 62, 1456–1461 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Nakao F. et al. Association of IFN-γ and IFN regulatory factor 1 polymorphisms with childhood atopic asthma. J. Allergy Clin. Immunol. 107, 499–504 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Kroegel C. et al. Interferon-alphacon-1 treatment of three patients with severe glucocorticoid-dependent asthma. Effect on disease control and systemic glucocorticosteroid dose. Respiration 73, 566–570 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Gaush CR & Smith TF Replication and plaque assay of influenza virus in an established line of canine kidney cells. Appl. Microbiol. 16, 588–594 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruest A, Michaud S, Deslandes S. & Frost EH Comparison of the Directigen flu A+B test, the QuickVue influenza test, and clinical case definition to viral culture and reverse transcription-PCR for rapid diagnosis of influenza virus infection. J. Clin. Microbiol. 41, 3487–3493 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward CL et al. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J. Clin. Virol. 29, 179–188 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flaczyk A. et al. IL-33 signaling regulates innate and adaptive immunity to Cryptococcus neoformans. J. Immunol. 191, 2503–2513 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Kaufman GN et al. Intravenous immunoglobulin attenuates airway hyperresponsiveness in a murine model of allergic asthma. Clin. Exp. Allergy 41, 718–728 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Maazi H. et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 42, 538–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.