Summary
Background
Incidence rates of SARS-CoV-2 infections in low-resource communities can inform vaccination strategies and non-pharmaceutical interventions (NPIs). Our objective was to estimate incidence over four epidemic waves in a slum in Rio de Janeiro, a proxy for economically deprived areas in the Global South.
Methods
Prospective cohort of children and household contacts screened for SARS-CoV-2 by PCR and serology (IgG). The incidence density of PCR positive infections estimated for each wave - the first wave, Zeta, Gamma and Delta - was compared to an index combining NPIs and vaccination coverage.
Findings
718 families and 2501 individuals were enrolled, from May 2020 to November 2021. The incidence density of SARS-CoV-2 infection due to the first wave was 2, 3 times that of the other waves. The incidence among children was lower than that of older participants, except in later waves, when vaccination of the elderly reached 90%. Household agglomeration was significantly associated with incidence only during the first wave.
Interpretation
The incidence of infection greatly exceeded rates reported in similar cohorts. The observed reduction in incidence in the elderly during the Delta variant wave, in spite of the rollback of NPIs, can be attributed to increased vaccine coverage. The high incidence in young people reinforces the importance of vaccination in this age group, a policy that has yet to receive the full support of some sectors of society.
Funding
UK Medical Research Council, Foundation for the Advancement of Science of the State of Rio de Janeiro, National Council for Scientific and Technological Development.
Keywords: Incidence density, COVID-19, Household transmission
Research in context.
Evidence before the study
We searched PubMed and Web of Science for publications on SARS-CoV-2 incidence density, published between 1 March 2020 and 26 February 2022. We used the keywords “incidence density” and “SARS-CoV-2” and searched for articles in English, French, Spanish and Portuguese. Cohort studies have estimated the incidence density of SARS-CoV-2 infection in families in the US and health care workers in India and Ethiopia, for the most part during the first wave of the COVID-19 pandemic. However, little is known about the incidence density of SARS-CoV-2 infection in families in low-resource, high household density communities, where compliance with non-pharmaceutical interventions (NPIs) such as lockdowns is unfeasible due to food insecurity.
Added value of this study
The objective of this study was to estimate the incidence of SARS-CoV-2 infection in a cohort of children and household contacts for each of the four waves of the pandemic between April 2020 and September 2021, accounting for age and household clustering. Our study participants reside in a community in Rio de Janeiro with high levels of poverty and violence. Ours is one of the few studies to characterize the incidence of SARS-CoV-2 in this setting. We evaluated incidence in relation to the stringency index — a scale for evaluating the adoption of NPIs in the city — and vaccination coverage by age group.
Implications of all the available evidence
The incidence of SARS-CoV-2 in families in a low-resource community in Rio de Janeiro was higher than in household cohorts in the US and among healthcare workers in India and Ethiopia. While the incidence of SARS-CoV-2 in children in our cohort was generally lower than that of older participants, the incidence in children increased later in the epidemic. In Brazil, immunization of children against SARS-CoV-2 continues to be opposed by some sectors of civil society and the government. Our findings highlight how important it is for the Brazilian government to stand behind the policy of vaccinating children despite political opposition.
Alt-text: Unlabelled box
Introduction
Epidemiological studies of SARS-CoV-2 incidence are valuable from a public health standpoint. Evaluating rates of new infections in a cohort can provide data to inform vaccination strategies and non-pharmaceutical interventions (NPIs). As discussed below, while many studies have estimated the incidence of SARS-CoV-2 in cohorts in Europe and North America1, 2, 3, 4 relatively little is known about incident cases of SARS-CoV-2 in the Global South.
An important development in the SARS-CoV-2 pandemic has been the emergence of variants of concern (VOCs) that may result in higher rates of transmission or reinfection than the first wave lineages.5,6 A number of studies have estimated SARS-CoV-2 incidence over one particular epidemic wave.1, 2, 3, 4 However, there is no data about rates of new SARS-CoV-2 cases in low-income, high population density areas during the period of circulation of different SARS-CoV-2 lineages. Little is known about the extent to which, if any, the incidence of SARS-CoV-2 infection changes from one SARS-CoV-2 epidemic wave to another in such areas, and if so, why.
The first SARS-CoV-2 lineage that became predominant in Rio de Janeiro in the austral summer of 2020 was B.1.1.33.7 Subsequently, the city experienced waves associated with the Variant of Interest (VOI) Zeta (P2)8; the VOCs Gamma (P.1/P.1.*), Delta (B.1.617.2/ AY.*) and Omicron (B.1.1.529) (genomic surveillance data available from: http://www.genomahcov.fiocruz.br/dashboard-en/; epidemiologic surveillance available from: https://doi.org/10.5281/zenodo.6335536). The present study examines from the first wave through the Delta wave, prior to the introduction of the Omicron VOC.
To control COVID-19, Rio de Janeiro implemented NPIs including the closure of schools, cancellation of public events during the New Year's Eve and Carnival seasons, and mandatory use of face masks, among others. Economic support for low-income families was limited to approximately US$100 per month from April 2020 to August 2020, and half this value for the next four months. This support was made available again beginning in April 2021 for the following five months. Schools for children ages 5–15 reopened partially and gradually beginning in August 2021 and fully in November 2021. Vaccination began on 20 January 2021 for the elderly and health professionals and gradually expanded to other age groups. Vaccination of adolescents 12–17 years old began in August 2021.
Like 60 other countries in the Global South, Brazil's economy is characterized by elevated indices of income inequality.9 Nevertheless, when formulating NPIs, governments often adopt a “one size fits all” approach without accounting for the effect of income on the capacity to comply with COVID-19 control measures.10 There may be far fewer resources available to sustain households in low-income communities, affecting the population's willingness to comply with NPIs. Understanding the main drivers of SARS-CoV-2 incidence in low-income communities and how they may have changed over the two years of the pandemic may provide valuable information to decision-makers for formulating future NPIs and immunization priorities. Such policies are likely to be necessary for the foreseeable future, as SARS-COV-2 may persist in human populations for a prolonged period.
The objective of this study was to estimate the incidence of SARS-CoV-2 infection in a cohort of children and household contacts in a slum in Rio de Janeiro. We calculated the incidence density for each of the four pandemic waves between April 2020 and September 2021, accounting for age and household clustering.
Methods
Recruitment and follow-up of participants
The present investigation, which was built upon our accompaniment of previous cohorts in this community,11, 12, 13, 14 is part of an ongoing prospective SARS-CoV-2 study.15 We offered study participation to all household contacts of children aged 13 and under who were seen at a primary health care centre for any reason including routine vaccinations and preventative care. The centre provides primary care to 4300 children in this age group per month. After enrolment, we collected samples in each household on study days 1 (first visit), 14 and 28, every three months during the first year, and twice in the second year. Enrolment took place from the beginning of the pandemic on May 15, 2020, to November 14, 2021.
Nasopharyngeal swabs and saliva were screened for SARS-CoV-2 by real-time RT-PCR to amplify the E gene and the RdRp region of the Orf1ab gene of SARS-CoV-2 using the SARS-CoV-2 E/RP molecular kit (Bio-Manguinhos, Rio de Janeiro, Brazil). Cycle thresholds (Ct) less than 40 were classified as positive. Serum tests were performed to screen for IgG antibodies by a Microparticle Chemiluminescent Immunoassay (CMIA), targeting the S gene (Abbott Laboratories, Abbott Park, IL, USA). SARS-CoV-2 RT-PCR and IgG serology assays were performed on all study participants at every visit according to the manufacturer's instructions.
The study was approved by institutional review boards at the Oswaldo Cruz Foundation and the London School of Tropical Medicine and Hygiene.
Case definition and covariates
A new SARS-CoV-2 infection was defined as a positive RT-PCR test. Seropositive results were not considered as new infections, as the precise date of seroconversion was not available. For the first three months of the study, however, as there was no previous contact with SARS-CoV-2, seropositive cases were also considered incident infections.
Incidence density was defined as the number of new SARS-CoV-2 cases per 1000 person weeks of exposure. In each period, the disease-free observation period for each participant was defined as the duration of observation during the period in question plus two additional weeks. The two extra weeks were included to account for the maximum interval for SARS-CoV-2 transmission, and the detection of infection by the study team. The at-risk time was truncated at the beginning and the end of each period if the follow-up spanned more than one period.
Households in which no individual was examined during an entire wave were considered lost to follow-up after eight unsuccessful contact attempts by telephone and smartphone messaging apps. Individuals in each household who missed one or more study visits were excluded only if the study team was unable to contact the participant during an entire wave. During the study period, young people and adults were eligible for vaccination, following a schedule defined by the municipal health department. The first age group eligible for vaccination was older adults. Children under 12 were not eligible to be vaccinated during the study period. Therefore, the at-risk time was truncated after the first dose.
To define the start and end of each epidemic wave, we determined the number of cases per week in wave t. The week with the lowest number of cases during a wave t was defined as the beginning of the next wave, (t+1). During each wave t, multiple lineages were detected by genomic surveillance. We named each wave t based on the predominant SARS-CoV-2 lineage during the wave.
Age was included in the model divided into categories based on the age groups targeted by vaccination campaigns in Rio de Janeiro: 0–4, 5–11, 12–17, 18–59, and sixty and older. In addition, we evaluated household clustering, defined as the ratio of the number of persons living in the household and the number of rooms, as the most relevant variable to estimate the incidence in the cohort.
Complementary indicators
Stringency index
The Stringency Index was developed to track and compare policy responses around the world, combining indicators in four domains: Containment and Closure, Economic Response, Health Systems, and Vaccine Policies.16 This indicator ranges from 0 representing no control measures to 100, which corresponds to complete lockdown. The index does not correlate specifically with incidence. Instead, it is an overall indicator of health authorities’ responses to factors including local trends in incidence, mortality, hospital capacity, and economic demands.
In Brazil, the variables were available for the capitals of each state. The data set is available from https://www.bsg.ox.ac.uk/research/research-projects/covid-19-government-response-tracker.17
Vaccination coverage
The Brazilian Health Ministry data on vaccination by age group was obtained by tabulating the open-source data from the COVID-19 vaccination information system (SI-PNI) available from https://opendatasus.saude.gov.br/dataset/covid-19-vacinacao as of February 2, 2022.
Genomic data
All genomic data from Rio de Janeiro evaluated in this study is available in the EpiCoV database at GISAID (www.gisaid.org) produced by the COVID-19 Fiocruz Genomic Surveillance Network and other genomic initiatives in Brazil. Details about genomic surveillance can be found at http://www.genomahcov.fiocruz.br/dashboard-en/.
Statistical models
As the response variable is infection vs non-infection, we used a logistic model to estimate the odds of each variable. In addition to age, which was included a priori, we also evaluated the significance of variables at the household level. Since the models were very similar according to the Watanabe–Akaike information criterion (WAIC),18 in addition to age, we chose a variable representing household clustering (persons/room), which we considered the most relevant indicator of housing conditions in the study site. The individuals who were included in the odds ratio calculation and who had sufficient follow-up time were retained in the incidence density calculation.
The probability of a participant being an incident infection is given by the expected value of the aforementioned logistic model. The duration of follow-up varies from participant to participant. Therefore, the estimated incidence density is the sum of the probability of being a SARS-CoV-2 positive or negative case, given the duration of follow-up of all the participants in each wave. The global incidence of infection in each wave is the expected number of infections including both SARS-CoV-2 detections by RT-PCR and seroconversion, divided by the number of people exposed in each wave. In addition, we tested the inclusion of two random effects to account for the expected dependence between individuals living in the same household and for the unstructured individual variability to account for unknown confounders (Supplemental Material).
Role of the funding source
The funders had no role in study design, data collection, data analysis, interpretation, writing of the report or decision to submit.
Results
Table 1 summarizes the sociodemographic profile of all participants according to the wave in which they were recruited. The interquartile range of age was similar across the epidemic waves. However, the median age was significantly lower in the Delta wave and higher in the Zeta wave (Supplemental Material). The age group that comprised the highest proportion of the cohort was working-age adults, which along with adolescents, represented 58% of those recruited. The majority of participants were women (60%). The total number of exams was greater during the first wave and similar for the following ones. Positive PCR ranged between 12 and 17% between the first three waves and decreased substantially in the Delta wave.
Table 1.
Sociodemographic and virologic characteristics of the study participants (N = 2501). IQR = interquartile range.
| Variable | Overall,N = 2501 | B.1.1.44,N = 884 | Zeta,N = 518 | Gamma,N = 644 | Delta,N = 455 |
|---|---|---|---|---|---|
| Median age in years (IQR) | 21 (8-37) | 19 (5-37) | 25 (8-37) | 22 (9-38) | 17 (8-34) |
| Age categories | N (%) | N (%) | N (%) | N (%) | N (%) |
| 0 to 4 years | 408 (16%) | 191 (22%) | 79 (15%) | 79 (12%) | 59 (13%) |
| 5 to 11 years | 503 (20%) | 167 (19%) | 96 (19%) | 126 (20%) | 114 (25%) |
| 12 to 17 years | 250 (10.0%) | 74 (8.4%) | 37 (7.1%) | 76 (12%) | 63 (14%) |
| 18 to 59 years | 1192 (48%) | 397 (45%) | 268 (52%) | 326 (51%) | 201 (44%) |
| 60 and older | 148 (5.9%) | 55 (6.2%) | 38 (7.3%) | 37 (5.7%) | 18 (4.0%) |
| Female | 1512 (60%) | 544 (62%) | 308 (59%) | 379 (59%) | 281 (62%) |
| SARS-COV-2 positive by RT-PCR | 744 (12%) | 139 (12%) | 200 (17%) | 287 (14%) | 118 (7%) |
| SARS-CoV-2 seropositive | 1479 (34%) | 339 (33%) | 199 (21%) | 318 (21%) | 623 (73%) |
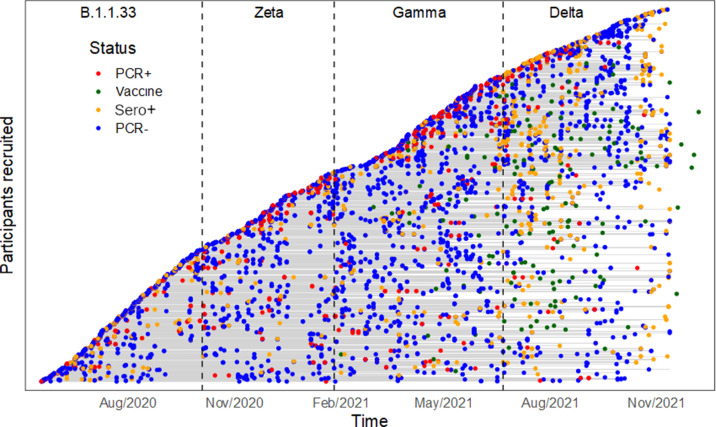
A total of 718 families were enrolled in the study; 40.5% were recruited in the first epidemic wave and approximately 20% in each of the subsequent ones. Concerning families recruited during the Zeta, Gamma and Delta waves, the proportion of families retained in subsequent waves was similar to the pattern observed among families recruited during the first wave. By the end of the study, 93.2% of the participants aged 12 and older who were eligible to be vaccinated had received at least one dose of a vaccine. The at-risk period and outcomes for each participant (RT-PCR and serology tests, and immunization) are summarized in Figure 1 and Supplementary Figure S3.
Figure 1.
Virologic and immunological outcomes for each cohort participant. The at-risk period for each individual in the cohort is represented by a horizontal grey line, beginning on the date of recruitment. The lines are stacked on top of each other to form the diagonal curve. The vertical dashed lines represent the end of one wave and the beginning of the next wave, as described in the Methods section. The dots represent each participant's RT-PCR results, positive serology results (colours described in the legend), and the date when each person received the first vaccine dose.
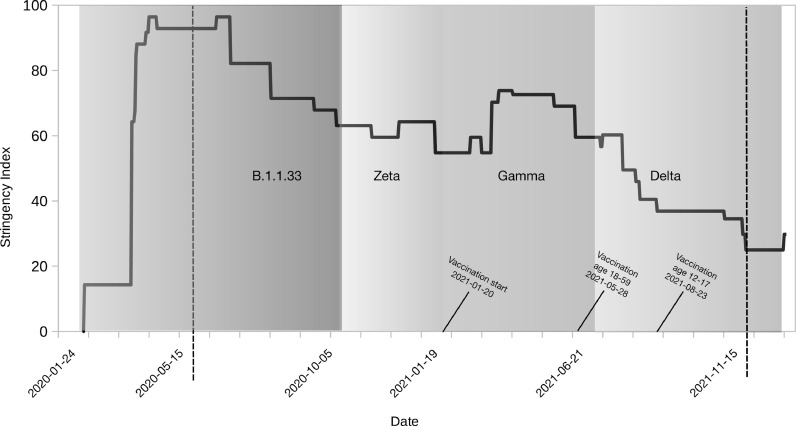
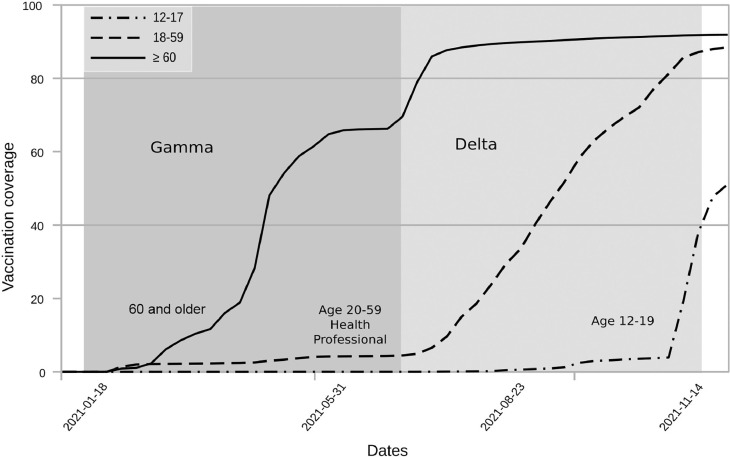
Figure 2 displays the four COVID-19 waves in Rio de Janeiro superimposed on a graph of the Stringency Index. During the initial three months, at the beginning of the epidemic, the value of the Stringency Index was indicative of lock-down. However, by the end of the first wave, these measures were relaxed, and the index oscillated between 60 and 70 points through the end of the Gamma wave. During the Delta wave, relatively few NPIs were implemented but the stringency index ticked up due to the rollout of vaccination. Four months after the start of vaccination, complete vaccination coverage among the elderly reached 60% (Figure 3). By the Delta wave, complete vaccination coverage reached 90% in this group. Vaccination of working-age adults coincided with the beginning of the Delta wave. By the end of the study period, complete vaccination coverage of working-age adults reached 90%. The vaccination of adolescents began in September 2021. Children 5–11 years of age were not vaccinated during the study period.
Figure 2.
Stringency of Non-pharmaceutical Interventions (NPIs) by SARS-CoV-2 waves. The black line shows the Stringency Index. The period of dominance of each lineage is depicted in different shades of grey. The dates marking the beginning and end of each wave are shown on the x-axis. The start of vaccination by age group is also indicated. The period analysed in this article is demarcated by dashed lines.
Figure 3.
Vaccine coverage by age group in the city of Rio de Janeiro during the study. The dates on the x-axis show the beginning of vaccination in each age group. The grey areas indicate the period of predominance of each Sars-CoV-2 lineage.
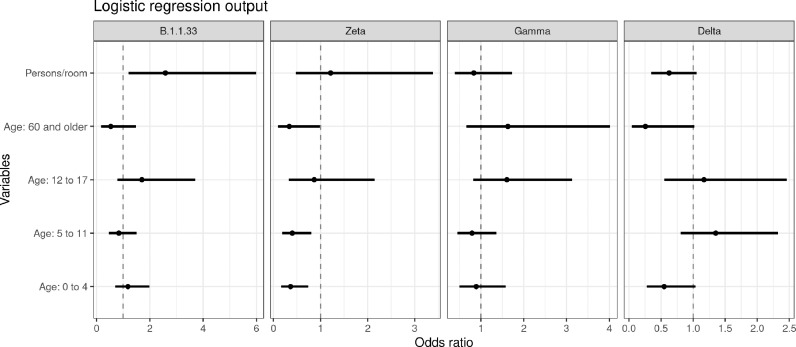
Sixteen models were evaluated (Supplementary Table S2). The model with the best WAIC score included just the family random effect. Figure 4 shows the odds ratio and credible intervals (0.025 and 0.975 quantiles) for the variables age group and household clustering.
Figure 4.
Association between age and the number of persons per room and the risk of being a SARS-CoV-2 case (odds ratios +/- credible intervals) by wave.
Compared to the working-age population (19–59 years of age), the odds ratio for children 0–4 years of age had a significant protective effect during the Zeta wave (odds ratio: 0.36) and there was a trend toward significance during the Delta wave (odds ratio: 0.5). A similar pattern was observed for people 60 and older. Being 60 or older had a significant protective effect during the Zeta wave (odds ratio: 0.34) and there was a trend toward a significant protective effect during the Delta wave (odds ratio: 0.25). In addition to these two age groups, being 5–11 years of age had a significant protective effect against being a SARS-CoV-2 case during the Zeta wave (odds ratio: 0.34). During the first wave and the Gamma waves, none of the age groups had a significantly increased or decreased risk of being a SARS-CoV-2 case.
During the first wave, the odds of being a SARS-CoV-2 case almost tripled with the number of persons per room in the household. For the subsequent waves, the effect of household clustering decreased log-linearly with the odds of being a SARS-CoV-2 case. During the Delta wave, there was a trend toward a significant effect of household clustering.
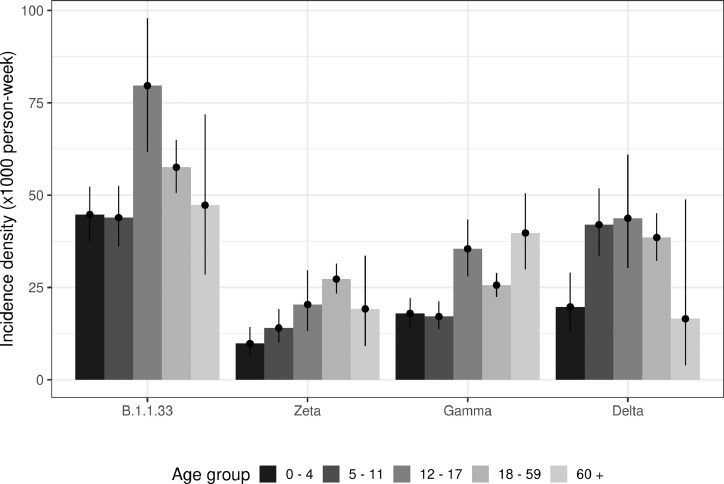
Figure 5 depicts the incidence density of SARS-CoV-2 infection adjusted by age and epidemic wave. The mean incidence density averaged across age groups during the first wave was 52.5 cases per 1000 person-weeks, which was 2.6 times greater than the incidence during the Zeta wave (19.7 per 1000 person-weeks) and 2.2 times that of the incidence during the Gamma wave (23.7 per 1000 person-weeks). The incidence density during the Delta wave was 35.6 per 1000 person-weeks. We carried out a sensitivity analysis to assess how different durations of follow-up influenced the ranking of the waves with respect to incidence. The results indicated that the aforementioned patterns - highest incidence during the first wave followed by the Delta, Gamma and Zeta waves - were robust across different durations of follow-up (Supplementary Material).
Figure 5.
Incidence density by age group in each epidemic wave.
Comparing age groups, the incidence density among children was generally lower than that of older participants throughout the study. For example, during the Delta wave, the incidence density among participants 60 and older was significantly lower than in younger age groups. Furthermore, during the Delta wave incidence among children 5–11 years old was similar to that of working-age adults.
Discussion
The incidence of SARS-CoV-2 infection in children and their household contacts in Rio de Janeiro greatly exceeded rates reported in similar cohorts in the Global North. The incidence of SARS-CoV-2 in our study population during the first wave was approximately eight times higher than the incidence reported for family-based cohorts in the US.1,2 Among the factors that may have contributed to the difference in incidence rates is the geographic scope of recruitment. Since the geographic areas from which families were recruited in the US were larger than that of our study, the cohorts followed in the US may have been more diverse than ours. On the other hand, few studies have investigated a severely economically deprived region like our study site, in which participants were at risk of food insecurity, and where violence affects their daily lives. It should be noted that fieldwork under these conditions was only possible because the interviewers were residents of the region hired during our cohort studies.11, 12, 13, 14, 15
Residents of low-resource communities may have higher exposure to SARS-CoV-2 than residents of high-resource communities because of household crowding and lower capacity to adhere to shelter-in-place orders due to food insecurity.19 Furthermore, due to COVID-19 denialism by prominent elected officials, there was contradictory messaging about NPIs and immunization, which further complicated compliance with COVID-19 control policies. With respect to other studies in developing countries, the incidence density in our cohort was similar to rates reported for healthcare workers in India and Ethiopia.20,21 These frontline workers likely had higher exposure to SARS-CoV-2 than our cohort, which would have tended to increase incidence.
In our cohort, the increase in the relative risk of SARS-CoV-2 infection during the first wave and Delta periods was two to three times that of the Zeta and Gamma periods. We can conjecture that relative risk was highest during the first wave because the population was completely non-adapted to the novel virus. The association between exposure to the Delta variant and higher relative transmission compared to other VOCs observed in our cohort is supported by experimental studies.22 The risk may have been higher during the Delta period because mutations in the SARS-CoV-2 Spike protein receptor-binding domain associated with the VOC Delta may have increased the transmissibility of the virus relative to previous lineages. The observed reduction in incidence in the elderly during the Delta variant wave, despite the rollback of NPIs, may have been attributable to increased vaccine coverage. A number NPIs were announced during this wave but not fully implemented; for instance, bars and restaurants were supposed to require proof of complete vaccination, but most did not. Masks were also not regularly required in stores and shopping centres.
In addition, we found that the risk of infection increased with household density. When we considered the number of persons per room in the household, the risk of being infected almost tripled in the first wave compared to the subsequent periods (Figure 3). One possible explanation for this difference is that residents were infected earlier in the epidemic in households with particularly high density. Subsequently, in the Zeta, Gamma, and Delta waves, the credible intervals of the variables persons/room overlapped with 1 indicating that the variable was neither associated with increased risk of being a SARS-CoV-2 case nor with decreased risk.
We evaluated two indicators estimated at the ecological level: The Stringency Index and vaccination coverage. The Stringency Index graph depicts how health authorities responded to factors such as SARS-CoV-2 incidence in the community. Since there may have been a delay between a change in incidence and the implementation of health authorities’ response, the index may not be perfectly synchronized with patterns of incidence, as is the case for the Gamma wave. However, the index reflects overall trends in incidence. It is well-established that compliance with NPIs and vaccination is protective against SARS-CoV-2 infection. Studies in other municipalities have demonstrated that the Stringency Index captures the population's response to NPIs at the city scale.23,24 The community we studied is located just 15 min from downtown by public transport and most residents commute to other neighbourhoods. Thus, the pattern we observe is very similar to other low-income communities in Rio de Janeiro. According to observational data on vaccination coverage on the scale of the city of Rio de Janeiro, there was a trend of increasing coverage during the study period. It is plausible to infer that this increase in vaccination was made possible because there was a growing acceptance of vaccination among individuals in the age groups that were eligible to be immunized. We can also reasonably surmise that with increasing vaccination coverage at the city scale, there was a decline in the risk that the virus would be introduced into any particular community or household.
In our study, the incidence of SARS-CoV-2 in children was generally lower than that of older participants, in accordance with a previous study of this population.15 However, the incidence in children increased later in the epidemic, underscoring the importance of making vaccines fully available to the young. The pattern of SARS-CoV-2 incidence by age group observed in this cohort can provide potentially valuable information to inform health policies. The World Health Organization endorsed the vaccination of children aged 5–11 with the BNT162b2 vaccine in November 2021.25 However, in Brazil, the vaccination of children in this age group continues to be opposed by certain groups in civil society and the government. The immunization of children aged 5–11 only began in Rio de Janeiro on 17 January 2022, after our study ended. It is reasonable to conclude that the high incidence of SARS-CoV-2 in children aged 5–11 in our cohort during the Delta wave (July to November 2021) was attributable at least in part to the fact that these children were not eligible for vaccination. Our findings highlight that it is crucial that the Brazilian government stand behind the policy of vaccinating children despite political opposition.
The main strengths of this study are the longitudinal design, which followed participants prospectively, and the eligibility criteria, which included participants regardless of symptoms, providing an accurate measure of incidence. These inclusion criteria and active follow up could provide an unbiased estimate of incidence in other similar populations in Latin America. Although communities with such high levels of poverty and violence are common in lower-income countries, the incidence of SARS-CoV-2 in this setting has seldom been studied due to the challenges of conducting fieldwork in this context. Ours is one of the few studies to characterize the incidence of SARS-CoV-2 in this setting. Among the limitations of the study is that households without children were not included.
During the final epidemic wave analysed, the Delta VOC, we identified a proportional increase in incidence density in the young. This finding strongly suggests that younger age groups need to be vaccinated, which runs counter to antivaccine messaging by some government officials. As Brazil has a long tradition of mass vaccination campaigns and Brazilians largely trust the public healthcare system,26 reducing SARS-CoV-2 incidence appears to be an attainable goal through increased access to vaccines and information campaigns publicizing the benefits of vaccinating children.
Contributors
M. S. C.: Study design, data interpretation, writing
L. S. B.: Study design, formal analysis, methodology, visualization
T. F.: Writing, data interpretation, study design, directly accessed and verified the underlying data reported in the manuscript
O. G. C.: Study design, formal analysis, software, visualization, directly accessed and verified the underlying data reported in the manuscript
L. D.: Data collection, Investigation, validation
G. C.: Writing - review & editing, software
P. C. R.: Methodology, Resources
C. S.: Writing - review & editing
J. W.: Writing - review & editing
M. S.: Methodology, Resources
P. B.: Study design, data interpretation, writing, funding acquisition
Data sharing statement
Interested researchers should contact the corresponding author. Requests will be assessed in accordance with best practices in cohort studies to ensure that data sharing would preserve confidentiality and be compatible with the terms of the Informed Consent Form used in this study.
Funding
UK Medical Research Council (MR/V033530/1), Foundation for the Advancement of Science of the State of Rio de Janeiro (E-26/202.862/2018, E-26/210.149/2020, and E-26/211.565/2019), National Council for Scientific and Technological Development (307282/2017-1).
Declaration of interests
None declared.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100283.
Appendix. Supplementary materials
References
- 1.Dawood F.S., Porucznik C.A., Veguilla V., et al. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in utah and New York City, New York. JAMA Pediatr. 2021;176:59–67. doi: 10.1001/jamapediatrics.2021.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawood F.S., Varner M., Tita A., et al. Incidence, clinical characteristics, and risk factors of SARS-CoV-2 infection among pregnant individuals in the United States. Clin Infect Dis. 2021:ciab713. doi: 10.1093/cid/ciab713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellingson K.D., Gerald J.K., Sun X., et al. Incidence of SARS-CoV-2 infection among health care personnel, first responders, and other essential workers during a prevaccination COVID-19 surge in Arizona. JAMA Health Forum. 2021;2(10) doi: 10.1001/jamahealthforum.2021.3318. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letizia A.G., Ge Y., Goforth C.W., et al. SARS-CoV-2 Seropositivity among US marine recruits attending basic training, United States, spring-fall 2020. Emerg Infect Dis. 2021;27(4):1188–1192. doi: 10.3201/eid2704.204732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konings F., Perkins M.D., Kuhn J.H., et al. SARS-CoV-2 variants of interest and concern naming scheme conducive for global discourse. Nat Microbiol. 2021;6(7):821–823. doi: 10.1038/s41564-021-00932-w. [DOI] [PubMed] [Google Scholar]
- 6.Koyama T., Platt D., Parida L. Variant analysis of SARS-CoV-2 genomes. Bull World Health Org. 2020;98:495–504. doi: 10.2471/BLT.20.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resende P.C., Delatorre E., Gräf T., et al. Evolutionary dynamics and dissemination pattern of the SARS-CoV-2 lineage B.1.1.33 during the early pandemic phase in Brazil. Front Microbiol. 2021;11:615280. doi: 10.3389/fmicb.2020.615280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voloch C.M., da Silva Francisco R., de Almeida L.G.P., et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J Virol. 2021;95(10):e00119–e00121. doi: 10.1128/JVI.00119-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Bank . Poverty and Shared Prosperity 2020: Reversals of Fortune. World Bank; Washington, D. C.: 2020. Poverty and Shared Prosperity 2020: Reversals of Fortune. [Google Scholar]
- 10.Vancini R.L., Camargo-Neto L., de Lira C.A.B., et al. Physical activity and sociodemographic profile of Brazilian people during COVID-19 outbreak: an online and cross-sectional survey. Int J Environ Res Public Health. 2020;17(21):7964. doi: 10.3390/ijerph17217964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedro R.S., Carvalho M.S., Girianelli V.R., et al. A populational-based birth cohort study in a low-income urban area in Rio de Janeiro, Brazil: implementation and description of the characteristics of the study. Cad Saúde Pública. 2019;35:e00023918. doi: 10.1590/0102-311X00023918. [DOI] [PubMed] [Google Scholar]
- 12.Brasil P., Pereira J.P., Moreira M.E., et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damasceno L., Terzian A.C.B., Fuller T., et al. Why did ZIKV perinatal outcomes differ in distinct regions of Brazil? An exploratory study of two cohorts. Viruses. 2021;13(5) doi: 10.3390/v13050736. Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Aguiar D.F., de Barros E.N.C., Ribeiro G.S., et al. A prospective, multicentre, cohort study to assess the incidence of dengue illness in households from selected communities in Brazil (2014–2018) Int J Infect Dis. 2021;108:443–453. doi: 10.1016/j.ijid.2021.04.062. [DOI] [PubMed] [Google Scholar]
- 15.Lugon P., Fuller T., Damasceno L., et al. SARS-CoV-2 infection dynamics in children and household contacts in a slum in Rio de Janeiro. Pediatrics. 2021;148(1) doi: 10.1542/peds.2021-050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster S., Anania J., Ellen L., Majumdar S., Goldszmidt R., Boby T. Blavatnik School of Government Working Paper; 2021. Variation in Government Responses to COVID-19 (Version 12.0) Internet[citado 10 de fevereiro de 2022]. Disponível em: www.bsg.ox.ac.uk/covidtracker. Oxford, UK: Oxford University; 2022. [Google Scholar]
- 17.Hale T., Angrist N., Hale A.J., et al. Government responses and COVID-19 deaths: global evidence across multiple pandemic waves. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0253116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe S. Asymptotic equivalence of bayes cross validation and widely applicable information criterion in singular learning theory. J Mach Learn Res. 2010;11:3571–3594. [Google Scholar]
- 19.Manfrinato C.V., Marino A., Condé V.F., Franco M., Stedefeldt E., Tomita LY. High prevalence of food insecurity, the adverse impact of COVID-19 in Brazilian favela. Public Health Nutr. 2021;24(6):1210–1215. doi: 10.1017/S1368980020005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhotra S., Kalaivani M., Lodha R., et al. Effectiveness of BBV152 Vaccine against SARS-CoV-2 Infections, hospitalizations and deaths among healthcare workers in the setting of high delta variant transmission in New Delhi, India. medRxiv. 2022 2022.01.22.22269701. [Google Scholar]
- 21.Gudina E.K., Ali S., Girma E., et al. Seroepidemiology and model-based prediction of SARS-CoV-2 in Ethiopia: longitudinal cohort study among front-line hospital workers and communities. Lancet Glob Health. 2021;9(11):E1517. doi: 10.1016/S2214-109X(21)00386-7. -E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mlcochova P., Kemp S.A., Dhar M.S., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorge D.C.P., Rodrigues M.S., Silva M.S., et al. Assessing the nationwide impact of COVID-19 mitigation policies on the transmission rate of SARS-CoV-2 in Brazil. Epidemics. 2021;35 doi: 10.1016/j.epidem.2021.100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vickers D.M., Baral S., Mishra S., et al. Stringency of containment and closures on the growth of SARS-CoV-2 in Canada prior to accelerated vaccine roll-out. Int J Infect Dis. 2022;118:73–82. doi: 10.1016/j.ijid.2022.02.030. IJID : official publication of the International Society for Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . World Health Organization; Geneva, Switzerland: 2021. Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing. [Google Scholar]
- 26.Paschoalotto M.A.C., Costa E., Almeida S.V., et al. Running away from the jab: factors associated with COVID-19 vaccine hesitancy in Brazil. Rev Saude Publica. 2021;55:97. doi: 10.11606/s1518-8787.2021055003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.