Abstract
Background
Existing clinical studies supported the potential efficacy of mesenchymal stromal cells as well as derived exosomes in the treatment of COVID-19. We aimed to explore the safety and efficiency of aerosol inhalation of the exosomes derived from human adipose-derived MSCs (haMSC-Exos) in patients with COVID-19.
Methods
The MEXCOVID trial is a phase 2a single-arm, open-labelled, interventional trial and patients were enrolled in Jinyintan Hospital, Wuhan, China. Eligible 7 patients were assigned to receive the daily dose of haMSCs-Exos (2.0 × 108 nano vesicles) for consecutively 5 days. The primary outcomes included the incidence of prespecified inhalation-associated events and serious adverse events. We also observed the demographic data, clinical characteristics, laboratory results including lymphocyte count, levels of D-dimer and IL-6 as well as chest imaging.
Results
Seven severe COVID-19 related pneumonia patients (4 males and 3 females) were enrolled and received nebulized haMSC-Exos. The median age was 57 year (interquartile range (IQR), 43 year to 70 year). The median time from onset of symptoms to hospital admission and administration of nebulized haMSC-Exos was 30 days (IQR, 15 days to 40 days) and 54 d (IQR, 34 d to 69 d), respectively. All COVID-19 patients tolerated the haMSC-Exos nebulization well, with no evidence of prespecified adverse events or clinical instability during the nebulization or during the immediate post-nebulization period. All patients presented a slight increase of serum lymphocyte counts (median as 1.61 × 109/L vs. 1.78 × 109/L). Different degrees of resolution of pulmonary lesions after aerosol inhalation of haMSC-Exos were observed among all patients, more obviously in 4 of 7 patients.
Conclusions
Our trial shows that a consecutive 5 days inhalation dose of clinical grade haMSC-Exos up to a total amount of 2.0 × 109 nano vesicles was feasible and well tolerated in seven COVID-19 patients, with no evidence of prespecified adverse events, immediate clinical instability, or dose-relevant toxicity at any of the doses tested. This safety profile is seemingly followed by CT imaging improvement within 7 days. Further trials will have to confirm the long-term safety or efficacy in larger population.
Trial Registration: MEXCOVID, NCT04276987.
Keywords: COVID-19, Mesenchymal stromal cell, Exosomes, Inhalation, Extracellular vesicles
Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been rampant across the globe for more than two years and so far killing over five million people. Mesenchymal stromal cells (MSCs) and their derived extracellular vesicles (EVs) are a potential treatment for COVID-19 due to their capability to modulate the immune response, promote pathogen clearance and mitigate the severity of organ injuries.
Many clinical studies have demonstrated that mesenchymal stromal cells (MSCs) and their derived exosomes (MSCs-Exo) significantly reduced lung inflammation resulting from different types of lung injury. A study of 11 patients with COVID-19-associated ARDS showed that intravenous infusion (a total of 60 × 107 cells) of human umbilical cord MSCs (UC-MSCs) or placental MSCs (PL-MSCs) rapidly improved respiratory distress along with reducing the excessive inflammatory response [1]. Several randomized, double-blind, placebo-controlled trials suggested that UC-MSC (a total of 10–12 × 107 cells) promoted recovery of lung lesion in COVID-19 patients without safety risk [2–5]. However, the small sample-size of patients included in the trials, the important heterogeneity in the severity of the included patients, the tissue source, the therapeutic doses, the timing of the cells administration, the percentage of viability, the bioactivity and the inter-batch variability of the MSCs, are all methodological limitations that preclude drawing any definitive conclusions about the efficacy of MSCs in this indication. Furthermore, intravenous MSCs-based therapy-related issues include the potential risk of mutagenicity and oncogenicity, the uncertainty about the wide range of MSCs viability after preparation for infusion and the optimized methods for cryopreservation, thawing, and production of MSCs [6, 7].
MSCs-Exo, on the other hand, own similar therapeutic properties to MSCs in lung injury models, with more accessibility to be prepared, stored, and delivered to the bedside while circumventing certain limitations and caveats inherent to using parent cells. So far, most published clinical trials about MSCs as well as MSCs-Exo focusing on COVID-19 associated ARDS were administered intravenously. In these studies, although the safety profile of MSCs and MSC-EVs treatment was suggested to be correct, the nonsignificant therapeutic effect might lie in the route of administration. The nebulized route constitutes a particularly interesting route of administration in the context of lung damage, given its excellent performance in terms of the bioavailability of the drug delivered to the targeted pulmonary site [8]. To date, studies evaluating the efficacy of clinical-grade MSCs-derived exosomes remain sparse, and the feasibility of nebulized route of administration has never been investigated, even though attractive in the context of severe SARS-CoV-2-induced pneumonia.
Given the severe situation of COVID-19 worldwide, we aimed to assess the safety of aerosol inhalation of the exosomes derived from human adipose-derived MSCs (haMSCs-Exo) in the treatment of patients with severe COVID-19 related pneumonia, to explore the optimum dosage as well as delivery route of MSCs-based therapy for acute respiratory diseases.
Methods
Study design and participants
The phase 2a single-arm, open labelled, interventional clinical trial MEXCOVID study (NCT04276987) was approved by the Ethics Commission of Jinyintan hospital as well as Rui-jin Hospital, Shanghai Jiao-tong University School of Medicine, Shanghai, China, and conducted at Jinyintan hospital, Wuhan, China, starting enrollment from March 16th, 2020. The inclusion criteria included 1) ages are differing from 18 years old to 75 years old, 2) confirmed infection with SARS-CoV-2 with PCR, 3) according to the fifth version of the guidelines on the Diagnosis and Treatment of COVID-19 by the National Health Commission, COVID-19 severity was classified as severe or critical type. The full inclusion and exclusion criteria are shown in Table 1. Due to the restricted accessibility during the epidemic period, all the candidates had already been admitted to the ICU and received antiviral therapy and other supportive care, while some patients received antibiotic treatment, antifungal treatment, glucocorticoid, and oxygen support at the appropriate situation. All the eligible patients met the criteria by the day of enrollment, one day before haMSC-Exos administration. Written Informed consent was obtained after discussion with patient or an appropriate surrogate. The process was carried out within the isolation units at Jinyintan hospital. Senior doctors (XD, HS and DCC) were responsible for introducing the protocol to the candidates and all the participants were voluntary to sign the informed consent with the presence of DCC and HS with their signatures simultaneously. All the documents were recorded by taking photographs. A total of seven patients received the initial dose of haMSC-Exos (2.0 × 108 particles per day) for five consecutive days (total cumulative therapeutic dose of 1.0 × 109 haMSC-Exos per patient), based on the well tolerated dose of haMSCs-Exo from MEXVT study (NCT04313647). Data from the first patient were reviewed for safety before proceeding with an enrollment of next patients.
Table 1.
The eligibility criteria of the MEXCOVID-19 study
| Inclusion criteria |
|
1. The subjects or their family members voluntarily participated in the study and signed the informed consent 2. Ages are differing from 18 years old to 75 years old 3. Confirmed infection with SARS-CoV-2 4. According to the fifth version of the guidelines on the Diagnosis and Treatment of COVID-19 by the National Health Commission, COVID-19 severity is classified as severe or critical type: Severe type: (1) Respiratory, distress, respiratory rate 30 per minute (2) Oxygen saturation on ambient air at rest ≤ 93% (3) Partial pressure of oxygen in arterial blood/ fraction of inspired oxygen ≤ 300 mmHg Critical type: (1) Respiratory failure occurs, and mechanical ventilation is required (2) Shock occurs (3) Patients with other organ dysfunction needing intensive care unit monitoring treatment |
| Exclusion criteria |
|
1. Patients with severe allergy history 2. Pneumonia caused by bacteria, mycoplasma, chlamydia, Legionella, fungi, parasites, or other viruses 3. HAP/VAP (hospital-acquired pneumonia/ventilator-acquired pneumonia) caused by lung cancer or other known reasons 4. Suffering from carcinoid tumor or carcinoid syndrome 5. Recent use of immunosuppressive drugs 6. History of epilepsy, needing continuous anticonvulsant therapy, or having received anticonvulsant therapy in the past three years 7. History of severe chronic lung diseases or requiring long-term home oxygen therapy 8. Undergoing hemodialysis or peritoneal dialysis 9. According to the local laboratory values, the creatinine clearance rate less than 15 ml/min 10. Moderate or severe hepatic failure (child Pugh score > 12) 11. Expecting to receive any of the following drugs during the study period: valproic acid or sodium dipropionate used within 2 weeks before screening; 5-tryptamine reuptake inhibitors, tricyclic antidepressants, 5-HT1 receptor agonists (triptans), or monoamine oxidase inhibitors (or MAOIs used within 2 weeks before screening) 12. Cannot understand and implement the investigation plan 13. Suffering from lower extremities deep venous thrombosis or pulmonary embolism in the past 3 months 14. Undergoing ECMO or high-frequency oscillatory ventilation 15. People with HIV, hepatitis virus, or syphilis 16. Pregnant or nursing females 17. According to the judgment of the researcher, the one who has a low probability of being included in the group (such as frailty, etc.) |
Clinical-grade human adipose-derived MSCs-Exosomes (haMSC-Exos) were prepared from Cellular Biomedicine Group, Inc. (CBMG, Shanghai, China, https://www.cellbiomedgroup.com) and the detailed process of manufacture and quality control of haMSC-Exos were presented in our previous articles [9]. haMSC-Exos were prepared from CBMG and shipped frozen, from Shanghai to Wuhan, directly to the clinical site in a validated dry ice shipper with a continuous temperature monitoring device. Upon receipt, the Exos solution was inspected and stored in a controlled, continuously monitored in − 20 °C storage tank within the isolation units. All the handovers as well as signature documents were recorded by taking photographs. Prior to administration, the solutions were thawed, reconstituted at the clinical site.
Procedures
The Data Safety Monitoring Group (DSMG) was comprised of critical care physicians with MEXVT trial experience and was responsible for reviewing data for each patient and making recommendations regarding continuing, stopping, or altering the trial. The skin test of haMSC-Exos was performed before the first inhalation as described [9]. Starting from the morning of Day 1, inhalation of haMSC-Exos was administered using a mesh nebulizer set (Aerogen Solo system, Ireland) at 9am each day with a total volume of 6 ml diluted with normal saline for 30 min for five consecutive days. All patients were monitored closely for any changes in a prescribed list of temperature, respiratory or cardiovascular parameters. Follow-up laboratory tests such as white blood cell count, lymphocyte count, chemistry panels assessing liver and kidney function, C-reactive protein (CRP), lactate dehydrogenase (LDH), interleukin 6 (IL-6), and CT scan were collected at baseline and after the cumulative dose of inhalation treatment. The incidence and nature of all serious adverse events were reviewed and independently evaluated by the DSMG to determine whether they were thought to be related to haMSC-Exos inhalation, with a particular focus on events that would be unexpected in COVID-19.
Clinical outcomes
The primary objectives were to assess the safety and tolerability. We recorded the vital signs of all the participants at the different periods before and after inhalation. Meanwhile, we reported the incidence of all serious adverse events, including death, and the incidence of prespecified inhalation-associated events, such as fever, shortness of breath, diarrhea and epilepsy, etc., and non-serious adverse events thought to be related to the nebulization process. Clinical information for the patients before and after 5-day inhalation treatment was obtained from a review of the hospital computer medical system and included the following: 1) demographic data, principal symptoms, days of admission from symptom onset, and comorbidity; 2) various therapeutic data, including mechanical ventilation, antiviral therapies, antiviral or antifungal therapies, steroids, and convalescent plasma (CP) therapy. CP transfusion defined as one dose of 200 mL of inactivated CP derived from recently recovered donors with the neutralizing antibody titers above 1:640 was transfused to the patients within 4 h as an addition to maximal supportive care and antiviral agents [10]. All the candidates were enrolled in CP transfusion unless they had previous allergic history to plasma or ingredients (sodium citrate), or severe organ dysfunction, who were not suitable for CP transfusion; 3) laboratory data, including white blood cell count, lymphocyte count, chemistry panels assessing liver and kidney function, CRP, LDH, IL-6 and; 4) chest imaging scoring data.
Regarding the CT score, all CT images were reviewed by two independent radiologists using a viewing console. Decisions were reached by consensus. Each segment of the lung was reviewed for opacification, and the lesion size was described as small (diameter < 1 cm), medium (diameter, 1 to < 3 cm), large (diameter, 3 cm to < 50% of the segment), or segmental (> 50% of the segment), scored as 1 to 4 point, respectively [11]. The lesion was assessed segment by segment, and the total score ranged up to 72 points. The form of the lesion (mainly ground-glass opacity and consolidation) was classified as patchy or oval according to its shape on serial images.
Statistical analysis
Continuous variables were presented as median and 25–75th interquartile range (IQR). CT score before and after inhalation treatment were compared using Wilcoxon test (nonparametric equivalent of the paired t test). Systemic clinical outcomes and biomarker values were compared using Kruskal–Wallis tests. All statistical analysis was performed using GraphPad Prism software (La Jolla, California, USA). Remaining analyses are descriptive.
Results
Clinical characteristics of participants
From March 16th, 2020 to March 25th, 2020, seven severe COVID-19 patients (4 males and 3 females) were enrolled and received aerosol inhalation of haMSC-Exos. The median age was 57 y (IQR, 43 y to 70 y) (Table 2). The median time from onset of symptoms to hospital admission and aerosol inhalation of haMSC-Exos was 30 days (IQR, 15 days to 40 days) and 54 days (IQR, 34 days to 69 days), respectively. All these seven patients had a fever at disease onset. The second common symptoms on the onset day of haMSC-Exos administration were shortness of breath (5 of 7) and cough (5 of 7). Malaise (3 patients), expectoration (1 patient), sore throat (1 patient), headache (1 patient), and diarrhea (1 patient) were less common. Five patients had underlying chronic diseases, including hypertension, diabetes, chronic obstructive pulmonary disease (COPD), and hyperthyroidism. All of them were given antiviral and antibiotic or antifungal treatment (Table 3). Besides, three patients received corticosteroid therapy, while four patients received convalescent plasma transfusion (Table 3). As of April 5th, 2020, all seven patients were discharged from the hospital.
Table 2.
Demographic information and baseline characteristics of patients in MEXCOVID study
| Age (years) | Sex | Occupation | Allergic history | Smoking history (pack years) | Clinical classification | Days of admission from symptom onset (days) | Days of haMSCs-Exos nebulization from symptom onset (days) | Principal symptoms | Comorbidity | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 34 | Male | Unemployed | Denied | 400 | Severe | 15 | 32 | Fever, shortness of breath | Hypertension |
| Patient 2 | 51 | Female | Self-employed | Denied | None | Severe | 15 | 59 | Fever, sore throat, shortness of breath, cough, expectoration, malaise | None |
| Patient 3 | 43 | Male | Employee | Denied | None | Severe | 30 | 34 | Fever, cough | Diabetes, Hypertension |
| Patient 4 | 60 | Male | Employee | Denied | 800 | Severe | 40 | 85 | Fever, cough, malaise, shortness of breath | Chronic obstructive pulmonary disease, hypertension |
| Patient 5 | 57 | Female | Unemployed | Denied | None | Severe | 11 | 69 | Fever, headache, diarrhea | Hyperthyroidism |
| Patient 6 | 75 | Male | Unemployed | Denied | None | Severe | 44 | 54 | Fever, cough, shortness of breath, malaise | None |
| Patient 7 | 70 | Female | Unemployed | Penicillin | None | Severe | 38 | 47 | Fever, cough, shortness of breath | Diabetes |
Table 3.
Other treatments of patients in MEXCOVID study
| Treatment received | Oxygen support | |||||
|---|---|---|---|---|---|---|
| Antiviral treatment | Antibiotic or antifungal treatment | Corticosteroids treatment | Convalescent plasma transfusion | Before haMSCs-Exo nebulization | After haMSCs-Exo nebulization | |
| Patient 1 | Arbidol, ribavirin, IFN-α | Cefoperagone sodium and tazobactam sodium | None | Yes | Nasal cannula | Nasal cannula |
| Patient 2 | Arbidol, ribavirin, IFN-α, lopinavir-ritonavir | Cefoperagone sodium and tazobactam sodium, meropenem | Methylprednisolone | Yes | Nasal cannula | Nasal cannula |
| Patient 3 | Arbidol, IFN-alpha | None | None | None | Nasal cannula | Nasal cannula |
| Patient 4 | Arbidol, oseltamivir | Cefoperagone sodium and tazobactam sodium, meropenem, moxifloxacin | Methylprednisolone | Yes | High-flow nasal cannula | Nasal cannula |
| Patient 5 | IFN-α | Cefoperagone sodium and tazobactam sodium, meropenem | Methylprednisolone | Yes | Nasal cannula | Nasal cannula |
| Patient 6 | Arbidol, IFN-α | Cefoperagone sodium and tazobactam sodium, | None | None | High-flow nasal cannula | Nasal cannula |
| Patient 7 | Ganciclovir | Moxifloxacin, meropenem, fluconazole | None | None | Nasal cannula | Nasal cannula |
Clinical manifestations, laboratory and radiological findings
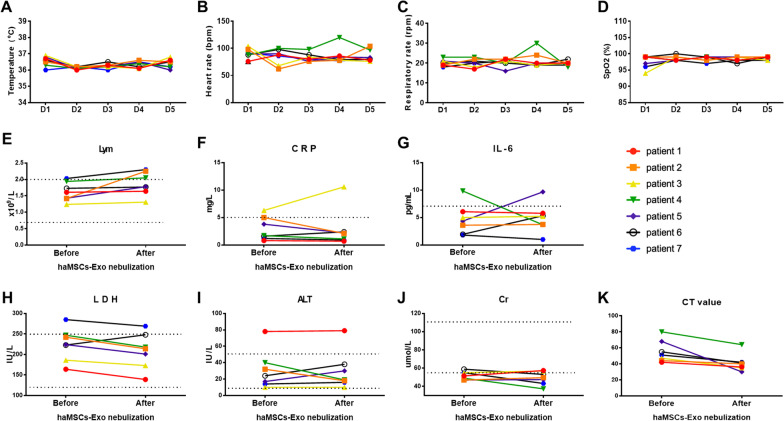
All of 7 COVID-19 patients tolerated the haMSC-Exos nebulization well, with no evidence of prespecified adverse events or clinical instability, aggravation of existing symptoms, during the nebulization or in the immediate post-nebulization period. The vital signs (in temperature, heart rate, respiratory rate, and saturation oxygen) of the seven patients stayed stable during the five-day aerosol inhalation course (Fig. 1A–D).
Fig. 1.
Clinical characters of patients in the MEXCOVID study. A Temperature B heart rate C respiratory rate D oxyhemoglobin saturation before and after hMSC-Exos nebulization in COVID-19 patients. E–J Dynamic changes of laboratory parameters before and after haMSC-Exos nebulization in COVID-19 patients. K Chest CT score before and after haMSC-Exos nebulization in COVID-19 patients. The dotted horizontal line represents the reference value range. haMSC-Exos human adipose-derived MSCs-Exosomes; COVID-19 coronavirus disease 2019; Lym lymphocyte; CRP C reactive protein; IL-6 Interleukin-6; LDH Lactate dehydrogenase; ALT alanine aminotransferase; Cr: creatinine
As a critical prognostic indicator of COVID-19, lymphocytopenia has been on an improving trend after aerosol inhalation of haMSC-Exos (median as 1.61 × 109/L vs. 1.78 × 109/L) in MEXCOVID, all seven patients showing an increase of lymphocyte counts (Fig. 1E). In terms of inflammation biomarkers, a trend towards a decrease was observed, including C-reactive protein (CRP) (a decrease found in 6 out of 7) (Fig. 1F), interleukin-6 (IL-6) (a decrease found in 5 out of 7) (Fig. 1G), lactate dehydrogenase (LDH) (a decrease found in 6 out of 7) (Fig. 1H). The alanine aminotransferase (ALT) (median as 78 IU/L vs. 79 IU/L) remained within normal range before and after aerosol treatment in patients except for patient 1 (Fig. 1I). The stable ALT and creatinine (Cr) (median as 51 μmol/L vs. 40 μmol/L) level indicated that aerosol inhalation of haMSC-Exos had no hepatotoxicity or nephrotoxicity (Fig. 1I–J). The CT score value (median as 51 points before treatment vs. 40 points after treatment, p = 0.0559) of these seven patients dropped after aerosol therapy (Fig. 1K).
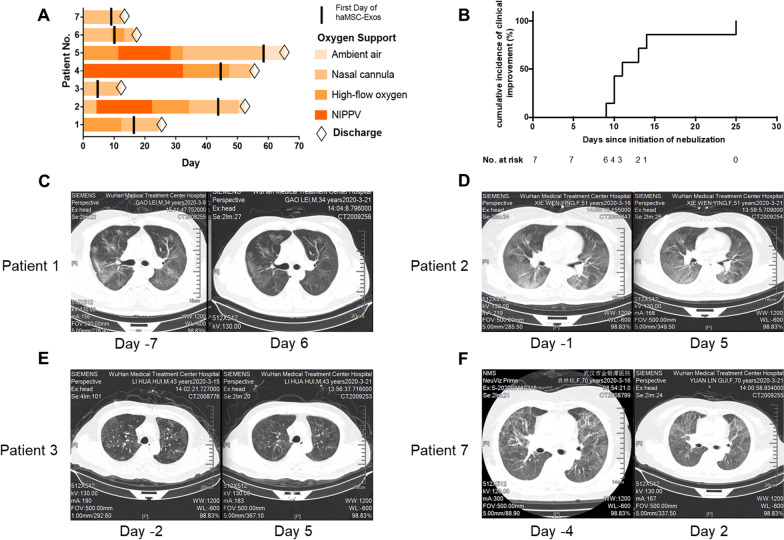
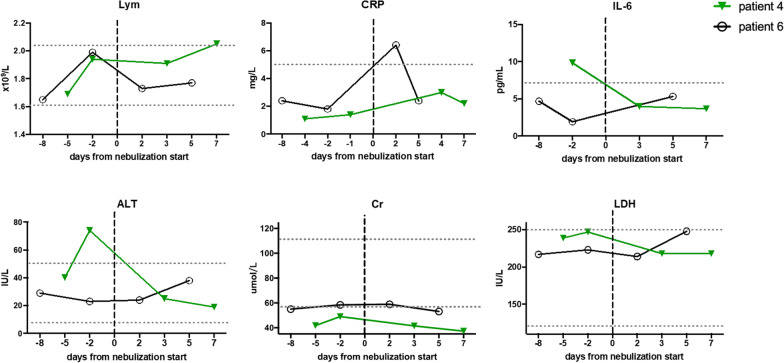
Of all the seven patients, patients 4 and 6 were receiving high-flow oxygen at the beginning of aerosol inhalation of haMSC-Exos, and switched to nasal cannula at the 3rd and 4th day of nebulization (Fig. 2A). Despite the change of oxygen support method in patient 4 and patient 6 before and after aerosol therapy, no striking amelioration in laboratory parameters was observed (Fig. 3).
Fig. 2.
Clinical improvement from baseline, the onset day of inhalation treatment, in individual patients in the MEXCOVID study. A Changes in oxygen-support status from baseline in individual patients. B Cumulative incidence of clinical improvement from the onset of neublization. C–F Changes of chest CT scan before and after haMSC-Exos inhalation in COVID-19 patients
Fig. 3.
Dynamic changes of laboratory parameters in patient 4 and patient 6 before and after haMSC-Exos nebulization. The dotted horizontal line represents the reference value range. Lym lymphocyte; CRP C reactive protein; IL-6 interleukin-6; ALT alanine aminotransferase; Cr creatinine; LDH lactate dehydrogenase
Different degrees of resolution of pulmonary lesions after aerosol inhalation of haMSC-Exos were observed in all patients. Representative chest CT images before and after aerosol inhalation of haMSC-Exos of patient 1, patient 2, patient 3, and patient 7 are shown in Fig. 2B–E. Patient 7, a 70-year-old female hospitalized 38 days from symptom onset (dso) who received aerosol therapy since 47 dso, showed the most obvious pulmonary image improvement (Fig. 2E). Compared with the result at 43 dso, massive infiltration and ground-glass opacity disappeared on the CT image performed at 48 dso. The feedbacks from all the accessible follow-up visits are shown in Table 4.
Table 4.
Follow-up feedbacks of patients after nebulization treatment
| Days from the onset of nebulization treatment | Clinical Symptoms | COVID-19 antibody test | Lymphocyte count | Alanine aminotransferase (ALT) (U/L) | Creatinine (Cr) (μmol/L) | Chest CT images | |
|---|---|---|---|---|---|---|---|
| Patient 1 | Day 20 | Denied | IgM ( −), IgG ( −) | 2.29 × 109/L | N/A | N/A | Obvious absorption of infiltration at both lobes compared with the day of symptom onset |
| Day 36 | Denied | N/A | N/A | N/A | N/A | Slight absorption compared with Day 20 | |
| Patient 2 | Day 41 | Denied | IgM ( −), IgG ( +) | 2.14 × 109/L | 45 | 53 | Partial absorption of infiltration compared with the day of symptom onset |
| Patient 3 | Day 22 | Denied | N/A | N/A | N/A | N/A | Normal |
| Day 59 | Denied | N/A | 3.72 × 109/L | 9 | 63 | Normal | |
| Patient 4 | Day 17 | Mild cough | N/A | N/A | N/A | N/A | Infiltration at both lobes, similar to the day of symptom onset |
| Day 43 | Denied | N/A | N/A | N/A | N/A | Infiltration at both lobes, similar to Day 17 | |
| Patient 5 | Day 36 | Denied | IgM ( −), IgG ( +) | 2.52 × 109/L | 20 | 55 | Slight absorption compared with the day of symptom onset |
| Patient 6 | Day 24 | Mild cough | N/A | N/A | N/A | 49 | N/A |
| Patient 7 | Day 28 | Denied | IgM ( −), IgG ( +) | 1.82 × 109/L | 10 | 50 | Obvious absorption of infiltration at both lobes compared with Day 2 |
| Day 52 | Denied | N/A | 1.97 × 109/L | N/A | N/A | Slight absorption compared with Day 28 |
Discussion
Given the emerging crisis of COVID-19 pandemic, it would be of great value to explore a new initiative inhalation route of haMSC-Exos-based therapy on this viral respiratory infection mainly involving terminal bronchioles in its most severe form: the ARDS. We used vibrating mesh nebulizers in all our trials because there is a body of evidence supporting the use of mesh rather than jet nebulizers [12–14]. Mass median aerodynamic diameters were slightly smaller with mesh nebulizers compared to jet nebulizers. Thus, the particle size of haMSC-Exos around 100 nm meets the nebulization requirement to reach the distal lung theoretically. Our preliminary preclinical data have determined the tolerance and efficacy by implementing a mesh nebulization system in pneumonia rodent model, showing the relative improvement in survival rate [9].
Previous findings show that inhalation administration of haMSC-Exos was well tolerated in healthy volunteers in MEXVT trial [9], with no evidence of prespecified adverse events, immediate clinical instability, or dose-limiting toxicity at any of the doses tested. The human immune system and its response to external stimuli were more complex, the mass dosage equivalence did not necessarily apply when transferring from rodents to humans. For maximum safety, we started the testing dose from a tenfold reduction (1 × 109 particles per patient). Also, in our previous fluorescence uptake experimental set [9], the strongest fluorescence intensity was found at 24 h post-nebulization and then gradually decreased afterwards. We therefore determined the treatment interval starting from once per day at the fixed time of each day. The primary outcomes in this study suggested that haMSC-Exos inhalation was safe among severe COVID-19 patients. Based on continuous reviews by DSMG, none of the severe adverse events reported in COVID-19 patients in MEXCOVID trial were related to multiple administrations of haMSCs-Exos inhalation.
In the present study, most of investigated patients (5 of 7) were achieved by an improvement of clinical symptoms as well as CT image scores. Although no significant differences in biomarkers and respiratory and cardiovascular parameters were found, it remained possible that differences in baseline severity of illness confounded the secondary outcomes we recorded. For example, 2 of 7 patients have improved their respiratory status by switching from high flow nasal cannula (HFNC) to standard oxygen canula after nebulization. No statistical differences were seen in laboratory results except for a favorable shift of lymphocytes and IL-6 levels. Notably, the favorable changes observed in CT imaging even within 7 days. One possible reason would be the benefit of haMSC-Exos nebulization for COVID-19 patients, especially in those with lung infiltrates. Due to the delay of enrollment, we would not be able to exclude the potential bias that it might be the normal and spontaneous course of the disease. Although conclusions about efficacy and biomarker response are unwarranted, the consistency in the results in terms of tolerability and short-term safety is still encouraging for future clinical application.
Our trial has some limitations. First, with only seven patients, we can neither generalize our phase 2 experience, nor draw conclusions about the efficacy of haMSCs-Exo for COVID-19. Since the vibrating mesh nebulized route constituted a particularly interesting route of administration in the context of lung injury, it remained to be of great value to identify how the inhalation of Exos diffused into the airway tree with the advanced real-time tracing technologies. Second, because of several procedures such as safety and tolerance test in healthy volunteers, ethics approval and quality control of exosomes product, we have to enroll our first patient in mid-March 2020 when the epidemic of COVID-19 in Wuhan has been under control. Most of surviving severe patients have been in recovery phase. Whether a different timing of administration would have been associated with different outcomes cannot be determined. Third, all patients were treated with multiple other agents (including antiviral medications), and it is not possible to determine whether the improvement observed could have been related to therapies other than haMSC-Exos inhalation. Last, since daily SARS-CoV-2 nucleic acid tests were not available in all patients, the dynamics of the viremia of SARS-CoV-2 remained unclear. The optimal timing for nebulized administration of haMSCs-Exo, therefore, needs to be determined in the future.
Conclusions
Our trial shows that a consecutive 5 days inhalation dose of clinical grade haMSC-Exos up to a total amount of 2.0 × 109 nano vesicles was feasible and well tolerated in seven COVID-19 patients, with no evidence of prespecified adverse events, immediate clinical instability, or dose-relevant toxicity at any of the doses tested. This safety profile is seemingly followed by CT imaging improvement within 7 days. Further trials will have to confirm the long-term safety or efficacy in larger population.
Acknowledgements
We thank all the healthy volunteers and patients who participated in the clinical trials. We express our sincere appreciation to Cellular Biomedicine Group for sponsoring this study and the following individuals and institutions for their support and assistance for the research, Tony Liu, Li Zhang, Jia-qiang Ren, Ying-lu Chen, Xiao-le Song, Ji-gang Lei, Meng Li and Dong Xu from the Cellular Biomedicine Group.
Abbreviations
- haMSC-Exos
The exosomes derived from human adipose-derived MSCs
- IQR
Interquartile range
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- MSCs
Mesenchymal stromal cells
- EVs
Extracellular vesicles
- MSCs-Exo
Mesenchymal stromal cells derived exosomes
- UC-MSCs
Human umbilical cord MSCs
- PL-MSCs
Placental MSCs
- DSMG
Data Safety Monitoring Group
- CRP
C-reactive protein
- LDH
Lactate dehydrogenase
- IL-6
Interleukin 6
- CP therapy
Convalescent plasma therapy
- COPD
Chronic obstructive pulmonary disease
- ALT
Alanine aminotransferase
- Cr
Creatinine
- dso
Days from symptom onset
- HFNC
High flow nasal cannula
Author contributions
YGZ, JMQ designed the trial. MMS, DCC, XD, HS collected data. MMS, YGZ, AM analyzed data. CXD, DCC, MMS, AM, YGZ, JMQ interpreted data. MMS, YGZ, AM, JMQ provided the plan and wrote the report, while DCC, JMQ provided editorial overview and modified the report. MMS, YGZ prepared the figures. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81630001), National Innovative Research Team of High-level Local Universities in Shanghai, National Key Research & Development Program of China (2018YFE0102400), Shanghai Shenkang Hospital Development Center Clinical Science and Technology Innovation Project (SHDC12018102, SHDC2020CR1002A) and Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases, Shanghai (20dz2261100).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The MEXCOVID study was approved by the Ethics Commission of Jinyintan hospital, Wuhan, China as well as Rui-jin Hospital, Shanghai Jiao-tong University School of Medicine, Shanghai, China (KY2020-32). Written Informed consent was obtained after discussion with patient or an appropriate surrogate.
Consent for publication
Consent for publication involving individual details, images and lab results have been obtained from all the patients.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying-Gang Zhu, Meng-meng Shi, Antoine Monsel and Cheng-xiang Dai have contributed equally
Contributor Information
Ying-Gang Zhu, Email: robinzyg@gmail.com.
Jie-Ming Qu, Email: jmqu0906@163.com.
References
- 1.Hashemian S-MR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini S-E, Hossieni H, Keshel SH, Naderpour Z, Hajizadeh-Saffar E, Shajareh E, Jamaati H, Soufi-Zomorrod M, Khavandgar N, Alemi H, Karimi A, Pak N, Rouzbahani NH, Nouri M, Sorouri M, Kashani L, Madani H, Aghdami N, Vasei M, Baharvand H. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther 2021;12:91. [DOI] [PMC free article] [PubMed]
- 2.Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, Hirani K, Bell CA, Kusack H, Rafkin L, Baidal D, Pastewski A, Gawri K, Leñero C, Mantero AMA, Metalonis SW, Wang X, Roque L, Masters B, Kenyon NS, Ginzburg E, Xu X, Tan J, Caplan AI, Glassberg MK, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, Wang S, Zhang C, Yuan X, Xu Z, Huang L, Fu JL, Li Y, Zhang Y, Yao WQ, Liu T, Song J, Sun L, Yang F, Zhang X, Zhang B, Shi M, Meng F, Song Y, Yu Y, Wen J, Li Q, Mao Q, Maeurer M, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther 2021;6:. [DOI] [PMC free article] [PubMed]
- 4.Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, Mariana N, Antarianto RD, Liem IK, Kispa T, Mujadid F, Novialdi N, Luviah E, Kurniawati T, Lubis AMT, Rahmatika D. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10:1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, Diehl JL, Demoule A, Annane D, Marois C, Demeret S, Weiss E, Voiriot G, Fartoukh M, Constantin JM, Mégarbane B, Plantefève G, Malard-Castagnet S, Burrel S, Rosenzwajg M, Tchitchek N, Boucher-Pillet H, Churlaud G, Cras A, Maheux C, Pezzana C, Diallo MH, Ropers J, Menasché P, Larghero J; APHP STROMA–CoV-2 Collaborative Research Group. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care. 2022;26(1):48. [DOI] [PMC free article] [PubMed]
- 6.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. biology and potential therapeutic value. Am J Respir Crit Care Med 2017;196:266–273. [DOI] [PMC free article] [PubMed]
- 8.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi MM, Yang QY, Monsel A, Yan JY, Dai CX, Zhao JY, Shi GC, Zhou M, Zhu XM, Li SK, Li P, Wang J, Li M, Lei JG, Xu D, Zhu YG, Qu JM. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J Extracell Vesicles. 2021;10(10):e12134. doi: 10.1002/jev2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong KT, Antonio GE, Hui DSC, Lee N, Yuen EHY, Wu A, Leung CB, Rainer TH, Cameron P, Chung SSC, Sung JJY, Ahuja AT. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228:395–400. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 12.Zhou QT, Tang P, Leung SSY, Chan JGY, Chan H-K. Emerging inhalation aerosol devices and strategies: Where are we headed? Adv Drug Deliv Rev. 2014;75:3–17. doi: 10.1016/j.addr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Galindo-Filho VC, Alcoforado L, Rattes C, Paiva DN, Brandão SCS, Fink JB, Dornelas de Andrade A. A mesh nebulizer is more effective than jet nebulizer to nebulize bronchodilators during non-invasive ventilation of subjects with COPD: A randomized controlled trial with radiolabeled aerosols. Respir Med 2019;153:60–67. [DOI] [PubMed]
- 14.Rouby JJ, Sole-Lleonart C, Rello J. Ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: understanding nebulization of aminoglycosides and colistin. Intensive Care Med. 2020;46:766–770. doi: 10.1007/s00134-019-05890-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.