ABSTRACT
Despite a short history since its first isolation, Akkermansia muciniphila has been extensively studied in relation to its effects on human metabolism. A recent human intervention study also demonstrated that the bacterium is safe to use for therapeutic purposes. The best-known effects of A. muciniphila in human health and disease relate to its ability to strengthen gut integrity, modulate insulin resistance, and protect the host from metabolic inflammation. A further molecular mechanism, induction of GLP-1 secretion through ICAM-2 receptor, was recently discovered with the identification of a new bacterial protein produced by A. muciniphila. However, other studies have suggested a detrimental role for A. muciniphila in specific host immune settings. Here, we evaluate the molecular, mechanistic effects of A. muciniphila in host health and suggest some of the missing links to be connected before the organism should be considered as a next-generation biotherapeutic agent.
KEYWORDS: Akkermansia muciniphila, molecular mechanisms, biotherapeutics, metabolic inflammation, effective compounds
1. Introduction
Advances in large-scale sequencing methods have enabled the investigation of whole microbial communities and has stimulated research on the human gut microbiome. In particular, the gut microbiome has been extensively investigated in relation to its association with clinical aspects of metabolic disorders.1,2 Obesity was one of the first host pathologies demonstrated to be clearly associated with particular gut microbial ecologies3,4 and was the metabolic disease for which A. muciniphila was first reported to exhibit a beneficial effect on host physiology.5
Akkermansia is a Gram-negative, oval-shaped, non-motile, and oxygen-tolerant anaerobic bacterium with only a short history of investigation due to its relatively recent isolation.6,7 Derrien et al. (2004) first isolated Akkermansia muciniphila (type strain MucT; ATCC BAA-835), a member of the Verrucomicrobia phylum in the gut of humans and animals.6,8,9 It was found to account for 1–3% of the total gut bacteria and persists throughout the life of the colonized host.10,11 A. muciniphila is mainly localized to the distal parts of the small and large intestines, where it utilizes mucin as its main energy source to provide the amino acids and sugar groups required for bacterial growth.6,12–14 It is known that the bacterium has the enzymatic capability to degrade the complex mucin structure.15,16 When A. muciniphila degrades mucin, it releases less complex carbohydrates from the mucin layer and produces organic acids such as acetate and propionate.6
During searches for bacteria beneficial to human health, A. muciniphila has emerged as one of the most promising biotherapeutic agents, since it has been shown to confer multiple beneficial effects in relation to certain metabolic diseases. These effects include the improvement of metabolic parameters, the strengthening of gut integrity, the stimulation of gut peptide hormone secretion, and the amelioration of metabolic inflammation.5,17,18 Such clinical benefits have resulted in the bacterium being tested for safety in human intervention studies.19,20 It was presumed to be safe after live or pasteurized A. muciniphila at 1010 cells were supplied daily for 3 months in overweight/obese individuals, without adverse effects.19 The safety of the bacterium was also confirmed in patients with type 2 diabetes (T2D).20 Here, we briefly review the current molecular mechanisms attributed to A. muciniphila and revisit its role in metabolic diseases. We also explore missing links to be filled before this organism could be considered as a biotherapeutic agent.
2. Molecular mechanistic function of A. muciniphila in metabolic diseases
A number of studies have provided evidence that A. muciniphila plays an important role in the regulation of host metabolic processes associated with metabolic disorders.5,21–23 In particular, the ability of A. muciniphila to reduce gut permeability has been identified as a major mechanism by which it regulates host metabolism.5,24,25 While gut mucus captures bacteria and prevents epithelial abrasion as a first line of host defense, the tight junction barrier of the gut inhibits the leakage of bacterial antigens from the lumen into epithelial tissues.26,27 Although A. muciniphila is renowned for mucin degradation, it is also able to increase the production of mucin by increasing both the number and density of goblet cells in high fat diet (HFD)-induced mice, thus restoring the thickness of the mucus layer and thereby strengthening the intestinal barrier.5,15,18 Amuc 1100, an outer membrane protein of A. muciniphila, was reported to decrease body weight and fat mass while improving the epithelial tight junction in HFD-induced obese mice.28 A number of molecular mechanisms by A. muciniphilia contributes to the amelioration of metabolic diseases have been identified17,18,29 and further examples are discussed below.
2-1. Communication through gut peptide hormone secretion
The role of gut microbiota in modulating the release of intestinal peptide hormones that affect appetite and energy homeostasis has been highlighted by a number of previous studies.30–32 L cells, which are intestinal enteroendocrine cells, play a crucial role in the gut-brain axis. They detect the presence of nutrients, microbiota, and metabolites via a G protein-coupled receptor (GPCR) and respond by secreting gut peptide hormones in association with elevated intracellular calcium (Ca2+) concentration, which signals to the brain to regulate satiety.33–35 Specifically, glucagon-like peptide-1 (GLP-1), a hormone induced by L cells, has been shown to suppress appetite.36 GLP-1 is also categorized as an incretin hormone due to its role in postprandial glucose clearance, by facilitating insulin secretion from pancreatic beta cells.37 In addition, GLP-1 stimulates brown adipose tissue (BAT) thermogenesis via activation of the AMPK pathway, thereby increasing energy expenditure.38 Not surprisingly, due to its proximity to L cells, the gut microbiota plays a crucial role in enteroendocrine cell differentiation and gut hormone secretion, particularly GLP-1.39 For example, A. muciniphila treatment increased the level of 2-oleoylglycerol, an endogenous GPR119 agonist, in the mice ileum (Figure 1).5 Additionally, it has recently been shown that A. muciniphila supports GLP-1 release via a newly discovered protein, P917. The molecular basis of the action of this novel protein will be further discussed later in this review.
Figure 1.
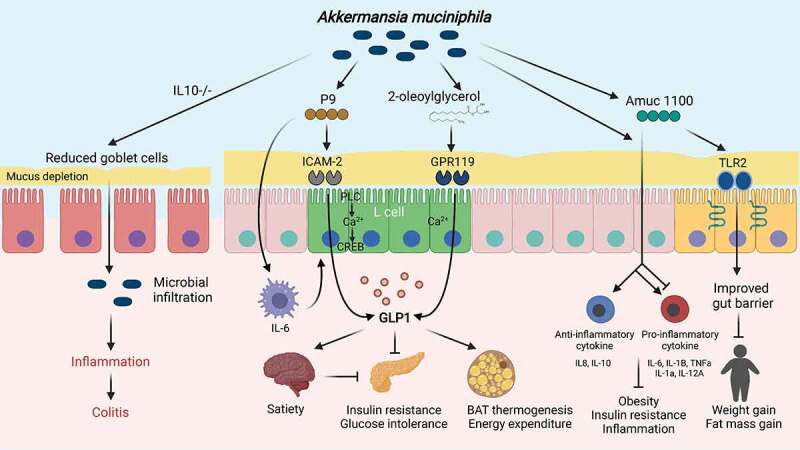
Akkermansia regulation of host metabolism. An overview of the molecular mechanisms for its action in the gut. Activation of TLR2 by Amuc 1100, an outer membrane protein of A. muciniphila, improves the epithelial tight junction and decreases body weight and fat mass. The protein can also trigger a range of anti- and pro-inflammatory cytokines, preventing obesity, insulin resistance, and inflammation in the visceral adipose tissue. A hormone induced by L cells, glucagon like peptide-1 (GLP-1), is implicated in satiety and glucose homeostasis. A. muciniphila regulates GLP-1 by increasing an agonist (2-oleoylglycerol) of the GPR119 endocannabinoid receptor. P9 protein produced by A. muciniphila binds to intercellular adhesion molecule-2 (ICAM-2) and activates phospholipase C (PLC), intracellular Ca2+ signaling, and CREB. P9-stimulated IL-6 expression in macrophages is involved in GLP-1 production. Repeated oral gavage of A. muciniphila promotes inflammation in IL10-/- mice as a result of increased microbial infiltration due to reduced numbers of mucin-filled goblet cells. The figure was created using BioRender.com.
2-2. Anti-inflammatory effects mediated by A. muciniphila
Previous research has hinted at an immunological relationship between A. muciniphila and host metabolic inflammation (Figure 1).18,29 In mice, daily oral administration of A. muciniphila after 4 weeks on a HFD, reduced visceral adipose tissue inflammation by increasing the fraction of regulatory T cells (Tregs) within the total CD4 T cell population and significantly reduced IL-6 and IL-1β expression in the visceral adipose tissue.18 Given that IL-6 and IL-1β secretion are known to promote adipose tissue inflammation, and are linked to obesity and insulin resistance, it was proposed that this anti-inflammatory activity of A. muciniphila could be the mechanism by which it ameliorates these metabolic disorders.40,41 Since Tregs are known to be key players in the regulation of adaptive immune responses associated with metabolic disorder-induced chronic inflammation,42,43 A. muciniphila-induced increases in Treg populations could be another of the organism’s mechanisms for modulating such inflammation. Dysregulation of the adaptive immune system was also associated with A. muciniphila colonization. The organism was found to be prevalent in immunodeficient Rag1−/− mice, lacking T and B cells, but bone marrow transfer from Rag+/+ to Rag1−/− mice reduced the levels of A. muciniphila in their guts.29 The crosstalk between immune system and A. muciniphila is also a representative characteristic of its anti-inflammatory effects in the intestinal mucosal barrier.44–46 The abundance of A. muciniphila has been found to be markedly reduced in patients with inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis (UC), implicating the organism in protection against inflammatory intestinal injury.44,45 The effect of A. muciniphila in systemic and intestinal anti-inflammation was demonstrated in an in vivo study using a dextran sulfate sodium (DSS)-induced colitis mouse model.46 Daily oral gavage of A. muciniphila for 14 days improved colitis by significantly downregulating pro-inflammatory cytokines such as TNF-α, IL-6, IL-1α, and IL-12A in mouse serum and colon tissue. In addition, the level of the immunomodulatory cytokine IL-10 was significantly enhanced by A. muciniphila administration. As effective compounds, both the A. muciniphila proteins, Amuc 1100 and P9, were also found to have immunomodulatory and immunometabolic functions. A. muciniphila and Amuc 1100 induced a range of anti- and pro-inflammatory cytokines (i.e., IL-8, IL-6, IL-1β, IL-10 and TNF-α).47 P9 was demonstrated to have IL-6 dependent anti-obesogenic effects.17 It is intriguing that these two studies found the elevated level of IL-6 by A. muciniphila unlike other HFD or inflammation models. IL-6 is a pleiotropic cytokine that can be involved in both pro- and anti-inflammation through trans- and classic signaling, respectively.48,49 Although it is not yet investigated if the reduction/enhancement of IL-6 observed with Akkermasia is linked to these signaling pathways, the cytokine has been implicated in metabolic processes such as hepatic inflammation and glucose homeostasis via BAT.50,51 In human subjects, it was also recently shown to delay gastric emptying and, consequently, postprandial insulin secretion.52 As a potent known GLP-1 stimulator,53 IL-6 induced by A. muciniphila may follow additional mechanisms than being a direct anti-/pro-inflammatory cytokine in the host metabolic homeostasis, which warrants further studies.
3. Potential therapeutic role of A. muciniphila in neurological disorders
In addition to its favorable effects in the gut, recent studies have provided a link between A. muciniphila and several neurological disorders such as autism spectrum disorder (ASD), psychological disorders associated with IBD, refractory epilepsy, Alzheimer’s disease (AD), and amyotrophic lateral sclerosis (ALS).54–58 According to a meta-analysis based on five clinical studies, children with ASD have a lower abundance of Akkermansia in their gut than control groups.54 Although relevant mechanistic studies have yet to be undertaken, it is possible that Akkermansia may ameliorate the increased gut permeability seen in ASD patients.59 A. muciniphila was associated with a therapeutic effect on depression associated with IBD when administered in a colitis mouse model under chronic restraint stress.55 The authors also discovered that the fecal samples of UC patients with depression had a lower abundance of A. muciniphila than those from control UC patients, demonstrating the link between the intestinal bacteria and psychological disorders via the gut-brain axis. Co-administration of A. muciniphila and Parabacteroides merdae to ketogenic diet-fed mice resulted in protective effects from seizure via increases in the ratio of hippocampal GABA to glutamate.56 More specifically, changes in the two bacteria, promoted by the ketogenic diet, reduced gamma-glutamyltranspeptidase activity and colonic and serum ketogenic gamma-glutamylated amino acids, changing the metabolism of brain GABA/glutamate. Daily administration of A. muciniphila in an AD mouse model (i.e., APP/PS1), for 6 months, not only improved metabolic disorders including glucose homeostasis and lipid metabolism, but also AD-related symptoms such as amyloid β-protein deposition in the brain and cognitive impairment.57 However, the interaction of the brain with serum metabolites derived from A. muciniphila was involved in ALS, a neurodegenerative disease marked by the death of motor neurons.58 Notably, A. muciniphila supplementation in Sod1-Tg mice improved motor function and brain atrophy through the accumulation of A. muciniphila-associated nicotinamide in the central nervous system. These results suggest that A. muciniphila has a broad mechanistic potential to exert influence on both its residential and remote sites, thus highlighting the need to be cautious prior to using the bacteria in clinical settings.
4. Controversial points relating to immunometabolic functions of A. muciniphila
Although dozens of beneficial effects of A. muciniphila on human and mouse health have been reported, a few studies have challenged this positive role by showing possible adverse effects of the bacterium. A pilot study of pediatric type 1 diabetes mellitus (T1DM) patients revealed that the genus Akkermansia was significantly more abundant in patients with poor glycemic control (HbA1c >7.5%).60 In a genetically susceptible T1D mouse model, it was shown that early-life antibiotic treatment accelerated T1D development and changed taxonomic composition of the gut microbiome including increases in the abundance of Akkermansia.61,62 This effect was seen even with a single course of pulsed antibiotic therapy, underscoring the significance of dysregulated immunological- and microbial gut environment in immunometabolic disorders.
Despite its known anti-inflammatory properties, several studies have claimed that A. muciniphila can promote colitis, depending on the immunological setting.63–65 Observations of enriched A. muciniphila on the colonic mucosa and in feces of mice treated with DSS questioned whether the organism plays a beneficial role in the gut.64,65 Another study demonstrated that repeated oral gavage of A. muciniphila led to the induction of colitis and intestinal inflammation in specific pathogen-free and germ-free IL10−/− mice. By contrast, the non-mucin-degrading bacterium, Bacteroides acidifaciens, did not induce the same pathologies.63 When IL10−/− mice were compared with wild-type mice, thinner mucus layers were observed in the IL10−/− mice, leading the authors to hypothesize that the mucin-degrading capacity of A. muciniphila facilitates microbial infiltration to the intestinal mucosa and stimulates bacterial-driven inflammation (Figure 1). In their early study of IL-10-/- mice treated with Enterococcus faecium, Ganesh et al. reported an adverse role for A. muciniphila, since a decrease in A. muciniphila was associated with lower caecal inflammation.66 In a subsequent study, using a simplified human intestinal microbiota (SIHUMI) mouse model, it was demonstrated that the co-presence of A. muciniphila with Salmonella enterica Serovar Typhimurium (SIHUMI-AS) induced more severe inflammation than was seen in mice with S. Typhimurium only (SIHUMI-S).67 Reduced numbers of mucin-filled goblet cells and thinner mucus layers were found in SIHUMI-AS mice indicating that mucin degradation by A. muciniphila can exacerbate pathogen exposure in the host.
A beneficial role for Akkermansia is also disputed in multiple sclerosis and Parkinson’s disease, as patients with these diseases consistently exhibit higher levels of the bacterium than control groups.68–72 Exposure of peripheral blood mononuclear cells of healthy subjects to A. muciniphila induced Th1 lymphocytes differentiation, implying pro-inflammatory effects of the bacteria in the host.69 These findings emphasize the need to further elucidate the specific mechanisms and molecular targets through which A. muciniphila modulates host immunity in the gut environment where countless interactions between microbes exist. Thus, the effects of A. muciniphila, in a range of immunological settings should be extensively investigated before the organism is used for therapeutic purposes.
5. A. muciniphila as a biotherapeutic agent: the missing links for clinical translation
The beneficial effects of A. muciniphila, observed in numerous human cohort studies as well as animal models, led researchers to evaluate the organism in clinical trials. A translational clinical study that involved washed microbiota transplantation (WMT), an alternative method of fecal microbiota transplantation (FMT), demonstrated that 53.7% of Chinese IBD patients showed improvement in clinical parameters (e.g., stool frequency, rectal bleeding, and physician’s assessment) with significantly increased abundance of A. muciniphila.73 When the response group and non-response group were compared, the non-response group did not exhibit significant changes in the A. muciniphila levels, implying a prognostic role for this bacterium. A study group in Belgium carried out a human clinical trial to address the association between A. muciniphila and metabolic disorders.19 Although oral administration of A. muciniphila did not result in reductions in body weight, fat mass, or hip circumference, it did improve insulin sensitivity, reduce insulinemia, and reduce plasma cholesterol in overweight/obese insulin-resistant participants. Furthermore, pasteurized A. muciniphila showed slightly superior results than viable A. muciniphila. This human intervention study has significance as it paved the way for the organism to be used as a safe biotherapeutic agent. Another clinical intervention study in T2D patients in the United States also confirmed the safe use of a probiotic cocktail containing A. muciniphila for the improvement of postprandial glucose control.20 The findings of that study led to the development of market-ready Akkermansia products either as a sole bacterium or as part of a probiotic cocktail.
While most studies aiming to verify a biotherapeutic role for A. muciniphila have focused on its exogenous administration, they have overlooked the inherent abundance of the bacteria in the host. Indeed, it was recently reported that the abundance of Akkermansia varies with ethnicity.73,74 East Asians, for example, harbored lower levels of Akkermansia compared to Whites, although this was not necessarily linked to unfavorable metabolic features in a FMT experiment.74 With regard to the therapeutic administration of Akkermansia, it is important to establish whether endogenous Akkermansia abundance or the nature of Akkermansia strains is most critical for inducing metabolic changes in the host. To fully comprehend the role of Akkermansia in the prevention and treatment of immunometabolic diseases, below we suggest areas for further investigation.
5-1. Effective compounds in functionality of A. muciniphila
Since it was discovered that A. muciniphila was favorable to host health, researchers have investigated the bacterial components responsible for the benefits afforded by this organism. Extracellular vesicles (EVs), also known as outer membrane vesicles (OMVs), were the first A. muciniphila components to be evaluated.75 The protective effects of EVs, derived from A. muciniphila, were confirmed by multiple animal models and human cell lines.24,75–78 When a colon epithelial cell line (CT26) was pretreated with A. muciniphila-derived EVs (AmEVs), it reduced the pro-inflammatory cytokine production induced by E. coli-derived EVs.75 The authors additionally verified the anti-inflammatory role of AmEVs using a colitis mouse model. AmEVs also replicated the anti-obesogenic functions of A. muciniphila itself.24,76 In addition to beneficial effects linked to the gut, AmEVs were also reported to elicit potent anti-cancer activity via their immunomodulatory actions and effects on the gut-brain axis through serotonin production.77,78
Consistent effects on lipid disorders and insulin resistance, seen with pasteurized bacteria, led to the discovery of the A. muciniphila outer membrane protein, Amuc 1100.28 The mechanism of action of this protein was explained by its ability to activate TLR2 (Figure 1), in human peripheral blood mononuclear cells, and to increase the development of transepithelial electrical resistance in Caco-2 cells.28,47 Since the discovery of Amuc 1100, many favorable effects of A. muciniphila on host health have been attributed to the protein. These include anti-inflammatory and anti-tumorigenesis activity in DSS/azoxymethane treated mice,79 restoration of aberrant tryptophan levels in colitis mice,80 up-regulation of serotonin (i.e., 5-HT) metabolism in RIN-14B and Caco-2 cells,81 and improvement of depression-like behavior in chronic stress-induced mice.82 The beneficial health effects observed with a single, purified, A. muciniphila protein have expanded prospects for the commercialization of A. muciniphila, particularly in light of the difficulty encountered in culturing live, whole bacteria in bulk.
In an effort to find new, A. muciniphila targets with therapeutic potential, we recently identified the P9 protein and subsequently investigated its mechanism of action.17 We first confirmed its beneficial effects on body weight reduction and glucose tolerance and further revealed that it could promote thermogenesis in BAT and induce GLP-1 secretion. By contrast, Amuc 1100-induced GLP-1 secretion was not observed in this study. The mechanism of action of P9 related to its ability to bind to the intercellular adhesion molecule-2 (ICAM-2) and activate phospholipase C (PLC), intracellular Ca2+ signaling, and the cAMP response element-binding protein (CREB), rather than it acting as an agonist of the GLP receptor (Figure 1). This was the first demonstration of a link between ICAM-2 and GLP-1 secretion, as well as its activation by a bacterial protein. Additionally, it was observed that IL-6 was related to the GLP-1 secretion pathway induced by P9 treatment. P9 will need to be tested in additional disease models to see if it elicits the same effects via the same mechanisms, given that the protein has only been examined in a diet-induced obese mouse model. Further advances in our knowledge of the mechanisms by which P9 acts will help provide a fuller explanation of how A. muciniphila benefits host health.
5-2. Different functionality at the subspecies level
Several studies have used genome sequencing to investigate the genetic diversity of A. muciniphila isolates.83–85 In a study of human gastrointestinal tract metagenomic libraries, three out of 23 metagenomes were found to include distinct Akkermansia species, based on average nucleotide identity (ANI).83 The authors further used a validation twin cohort to suggest that different species of the genus Akkermansia can co-colonize the same host. Phylogenetic analysis based on 16S rRNA sequences and single nucleotide polymorphisms (SNPs) found in A. muciniphila genomes has identified a number of A. muciniphila clades.84,86 The three clades discovered, based on SNPs in different mammalian hosts (e.g., humans, mice, and pigs), were shown to have distinct functionalities such as phosphatidylinositol signaling system and amino sugar and nucleotide sugar metabolism. A recent genomic study based on metagenome assembled genomes (MAGs) discovered four clades of A. muciniphila (AmI, AmII, AmIII, and AmIV), with AmII synthesizing vitamin B12, which is required for succinate to propionate conversion.87 Another study investigated the phenotypes and functionalities of these clades, for example, growth rate, oxygen sensitivity, cell aggregation, and mucin fermentation.88 Interestingly, a large genomic analysis of Akkermansia (> 2,000 MAGs) identified five species-level clades, on the basis of their associations with different proportions of CRISPR spacers and matching bacteriophages, and revealed further information about the distinct functions of Akkermansia clades.85 The different functionalities exhibited by unique A. muciniphila clades, offers the opportunity to develop only those clades with the most desirable therapeutic benefits for clinical purposes. Analysis of MAGs reported sub-clades of A. muciniphila and clade-specific functioning without the need to cultivate these bacteria. However, it will be necessary to investigate whether or not there is an association between specific clades and beneficial effects in relation to immunometabolic diseases.
5-3. Crosstalk with other bacteria in the gut
On its own, A. muciniphila appears to be a potent host health modulator. However, in the complex gut environment, with trillions of residents, the organism is very likely to interact with other gut residents to elicit beneficial effects on the host. No changes in microbiota composition were observed in an overweight/obese human intervention study with live and pasteurized A. muciniphila treatments.19 However, factors known to affect the abundance of A. muciniphila in the gut, such as a HFD, fiber intake, medicine administration, antibiotics treatment, and immune dysregulation, all involve significant alterations in community diversity and composition. Moreover, several studies on the health effects of colonization by other probiotic bacteria, in both mice and human subjects, reported concomitant increases in the prevalence of A. muciniphila.89–91 In particular, Alard et al. found that a probiotic mixture containing Lactobacillus rhamnosus and Bifidobacterium animalis restored the levels of A. muciniphila, which was decreased in HFD-fed-mice while L. rhamnosus alone rather decreased the abundance of the organism.89 In various disease cases, an increase in A. muciniphila abundance was connected to the presence of other gut bacteria.56,92–94 Treatment of C. difficile-infected mice with Bacteroides fragilis resulted in concomitant increases in the levels of A. muciniphila, as well as higher mouse survival rates and increased gut barrier integrity.92 In IBD patients and allergic asthmatic children, the levels of both A. muciniphila and Faecalibacterium prausnitzii were shown to be lower than in non-disease control groups.93,94 Both of these organisms reside close to mucus layer in the intestine, and it has been suggested that oligosaccharides, vitamins, and acetate released from mucus, by A. muciniphila, support the growth of F. prausnitzii.93 Furthermore, co-administration of A. muciniphila and Parabacteroides in mice fed a ketogenic diet was shown to promote seizure protection, while the same protection was not observed with A. muciniphila alone.56 A co-culturing experiment demonstrated that sugars released by A. muciniphila indirectly increased butyrate producing bacteria (e.g., Anaerostipes caccae, Eubacterium hallii, and F. prausnitzii), and thus intestinal butyrate levels95 . Furthermore, the authors investigated the trophic interaction between A. muciniphila and A. caccae using a co-culture experiment followed by transcriptome analysis.96 In the presence of A. caccae, A. muciniphila upregulated its mucin-degrading genes. To date, only a few bacterial species have been experimentally examined for their cross-feeding effects with A. muciniphila. With increasing evidence of the ability of A. muciniphila to interact with a variety of other gut microbes, such co-culture experiments need to be expanded to include multiple species, to elucidate the extent of the metabolic interactions occurring in the gut that involve this organism.
6. Conclusions and outlook
Being one of the most abundant bacteria in the gut, along with the demonstration of its favorable effects on human health, A. muciniphila has sparked excitement as a promising biotherapeutic agent. However, an incomplete picture of the mechanisms by which it affects host metabolism, as well as contradictory data relating to its beneficial effects, cautions the need for further investigation before the bacterium can be considered as a safe, valuable and efficacious therapeutic agent for the treatment of immunometabolic disorders. The most well-studied effect of A. muciniphila, in the amelioration of metabolic diseases, is enhancement of the integrity of the intestinal barrier. More complex molecular mechanisms, such as GLP-1 induction, have only recently been discovered and one such mechanism is associated with the novel P9 protein produced by this bacterium. Until now, the significance of A. muciniphila in human health and disease had been limited to aspects of metabolic disease control. However, further investigation of the interactions of A. muciniphila with other gut bacteria may reveal metabolic crosstalk that impacts other diseases and host immunological conditions.
A. muciniphila has been extensively studied over a relatively short period of time. This has led to the discovery of its roles in metabolic disease amelioration, the identification of (as yet) incomplete mechanisms by which it achieves these beneficial effects, the evaluation of its efficacy in human intervention trials, and the identification of potential market/therapeutic products produced by the organism. With the safety of the organism proven in human subjects, the next objectives are to determine the appropriate treatment doses with whole bacterial cells or purified therapeutic components, and to confirm whether the molecular mechanisms of immunometabolism regulation observed in mice are also seen in humans. This is especially the case for those components not yet tested in humans, and for disease models where live/pasteurized bacteria did not achieve the desired external phenotypic modifications. With the promising outlook for A. muciniphila, as a therapeutic agent, we are set to identify the next generation of probiotics.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1C1C2004318, No. 2021R1A2B5B03086637, and No. 2015M3C9A4053391).
Disclosure statement
GK is a founder of KoBioLabs, Inc., a company that aims to characterize the role of host–microbiome interactions in chronic diseases. The other authors declare no competing interests.
References
- 1.Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K.. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health—pathophysiology and therapeutic strategies. Gastroenterology. 2021;160(2):573–13. doi: 10.1053/j.gastro.2020.10.057. [DOI] [PubMed] [Google Scholar]
- 2.Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AAM, Jonkers DMAE, Oosting M, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51(4):600–605. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley RE, Turnbaugh PJ, Klein S, Gordon JI.. Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. P Natl Acad Sci USA. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Micr. 2004;54(5):1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 7.Ouwerkerk JP, van der Ark KCH, Davids M, Claassens NJ, Finestra TR, de Vos WM, Belzer C. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer. Appl Environ Microb. 2016;82(23):6983–6993. doi: 10.1128/AEM.01641-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin D, Raab N, Pinto Y, Rothschild D, Zanir G, Godneva A, Mellul N, Futorian D, Gal D, Leviatan S. Diversity and functional landscapes in the microbiota of animals in the wild. Science. 2021;372(6539):eabb5352. doi: 10.1126/science.abb5352. [DOI] [PubMed] [Google Scholar]
- 9.Geerlings SY, Ouwerkerk JP, Koehorst JJ, Ritari J, Aalvink S, Stecher B, Schaap PJ, Paulin L, de Vos WM, Belzer C. Genomic convergence between Akkermansia muciniphila in different mammalian hosts. Bmc Microbiol. 2021;21(1):298. doi: 10.1186/s12866-021-02360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microb. 2008;74(5):1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microb. 2007;73(23):7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Herreweghen F, Van den Abbeele P, De Mulder T, De Weirdt R, Geirnaert A, Hernandez-Sanabria E, Vilchez-Vargas R, Jauregui R, Pieper DH, Belzer C. In vitro colonisation of the distal colon by Akkermansia muciniphila is largely mucin and pH dependent. Benef Microbes. 2017;8(1):81–96. doi: 10.3920/BM2016.0013. [DOI] [PubMed] [Google Scholar]
- 13.van der Ark KCH, Aalvink S, Suarez-Diez M, Schaap PJ, de Vos WM, Belzer C. Model-driven design of a minimal medium for Akkermansia muciniphila confirms mucus adaptation. Microb Biotechnol. 2018;11(3):476–485. doi: 10.1111/1751-7915.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye F, Shen H, Li Z, Meng F, Li L, Yang J, Chen Y, Bo X, Zhang X, Ni M. Influence of the biliary system on biliary bacteria revealed by bacterial communities of the human biliary and upper digestive tracts. Plos One. 2016;11(3):e0150519. doi: 10.1371/journal.pone.0150519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trastoy B, Naegeli A, Anso I, Sjogren J, Guerin ME. Structural basis of mammalian mucin processing by the human gut O-glycopeptidase OgpA from Akkermansia muciniphila. Nat Commun. 2020;11(1):4844. doi: 10.1038/s41467-020-18696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Zhang XY, Guo RR, Cai ZP, Hu XC, Chen H, Wei S, Voglmeir J, Liu L. Cloning, purification and biochemical characterization of two beta-N-acetylhexosaminidases from the mucin-degrading gut bacterium Akkermansia muciniphila. Carbohyd Res. 2018;457:1–7. doi: 10.1016/j.carres.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim JH, Han D, Cha KH, Moon SH, Lee K. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. 2021;6(5):563–+. doi: 10.1038/s41564-021-00880-5. [DOI] [PubMed] [Google Scholar]
- 18.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae J-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 19.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–+. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, Eid J, Gines J, Iyer M, Justice N. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care. 2020;8(1):e001319. doi: 10.1136/bmjdrc-2020-001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katiraei S, de Vries MR, Costain AH, Thiem K, Hoving LR, van Diepen JA, Smits HH, Bouter KE, Rensen PCN, Quax PHA. Akkermansia muciniphila exerts lipid-lowering and immunomodulatory effects without affecting neointima formation in hyperlipidemic APOE*3-Leiden.CETP mice. Mol Nutr Food Res. 2020;64(15):e1900732–e. doi: 10.1002/mnfr.201900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YJ, Zhong ZQ, Wang BJ, Xia XW, Yao WY, Huang L, Wang Y, Ding W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacol. 2019;44(12):2054–2064. doi: 10.1038/s41386-019-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu F, Guo X, Zhang M, Ou Z, Wu D, Deng L, Lu Z, Zhang J, Deng G, Chen S. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe. 2020;61:102138. doi: 10.1016/j.anaerobe.2019.102138. [DOI] [PubMed] [Google Scholar]
- 24.Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med.2018;50(2)e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao Y, Kuang Z, Li C, Guo S, Xu Y, Zhao D, Hu Y, Song B, Jiang Z, Ge Z. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes. 2021;13(1):1927633. doi: 10.1080/19490976.2021.1927633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ermund A, Schütte A, Johansson MEV, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol - Gastrointest Liver Physiol. 2013;305(5):G341–G7. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Guo C, Zhang D, Zhang J, Wang X, Geng C. The altered tight junctions: an important gateway of bacterial translocation in cachexia patients with advanced gastric cancer. J Interferon Cytokine Res. 2014;34(7):518–525. doi: 10.1089/jir.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Sparks JB, Karyala SV, Settlage R, Luo XM. Host adaptive immunity alters gut microbiota. ISME J. 2015;9(3):770–781. doi: 10.1038/ismej.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breton J, Tennoune N, Lucas N, Francois M, Legrand R, Jacquemot J, Goichon A, Guérin C, Peltier J, Pestel-Caron M. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab. 2016;23(2):324–334. doi: 10.1016/j.cmet.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Panaro BL, Tough IR, Engelstoft MS, Matthews RT, Digby GJ, Møller CL, Svendsen B, Gribble F, Reimann F, Holst J. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab. 2014;20(6):1018–1029. doi: 10.1016/j.cmet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schéle E, Grahnemo L, Anesten F, Hallén A, Bäckhed F, Jansson JO. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology. 2013;154(10):3643–3651. doi: 10.1210/en.2012-2151. [DOI] [PubMed] [Google Scholar]
- 33.McCauley HA, Matthis AL, Enriquez JR, Nichol JT, Sanchez JG, Stone WJ, Sundaram N, Helmrath MA, Montrose MH, Aihara E. Enteroendocrine cells couple nutrient sensing to nutrient absorption by regulating ion transport. Nat Commun. 2020;11(1):4791. doi: 10.1038/s41467-020-18536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye L, Bae M, Cassilly CD, Jabba SV, Thorpe DW, Martin AM, Lu H-Y, Wang J, Thompson JD, Lickwar CR. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29(2):179–96 e9. doi: 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Symonds EL, Peiris M, Page AJ, Chia B, Dogra H, Masding A, Galanakis V, Atiba M, Bulmer D, Young RL. Mechanisms of activation of mouse and human enteroendocrine cells by nutrients. Gut. 2015;64(4):618–626. doi: 10.1136/gutjnl-2014-306834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7—36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol Regul Integr Comp Physiol. 1998;274(1):R23–R9. doi: 10.1152/ajpregu.1998.274.1.R23. [DOI] [PubMed] [Google Scholar]
- 37.Shigeto M, Ramracheya R, Tarasov AI, Cha CY, Chibalina MV, Hastoy B, Philippaert K, Reinbothe T, Rorsman N, Salehi A. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J Clin Invest. 2015;125(12):4714–4728. doi: 10.1172/JCI81975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 39.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han MS, White A, Perry RJ, Camporez JP, Hidalgo J, Shulman GI, Davis RJ. Regulation of adipose tissue inflammation by interleukin 6. Proc Natl Acad Sci U S A. 2020;117(6):2751–2760. doi: 10.1073/pnas.1920004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nov O, Shapiro H, Ovadia H, Tarnovscki T, Dvir I, Shemesh E, Kovsan J, Shelef I, Carmi Y, Voronov E. Interleukin-1beta regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. Plos One. 2013;8(1):e53626. doi: 10.1371/journal.pone.0053626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. Plos One. 2011;6(1):e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin THJ. Mucolytic bacteria with increased prevalence in IBD mucosa augmentin vitroutilization of mucin by other bacteria. Am J Gastroenterol | ACG. 2010;105(11):2420–8. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 45.Rajilić-Stojanović M, Shanahan F, Guarner F, de Vos WM. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis. 2013;19(3):481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 46.Bian XY, Wu WR, Yang LY, Lv LX, Wang Q, Li YT, Fung K, Ip M. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2019;10:10. doi: 10.3389/fmicb.2019.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ottman N, Reunanen J, Meijerink M, Pietila TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M, Boeren S. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. Plos One. 2017;12(3):e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6(5):583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 49.Malchow S, Thaiss W, Jänner N, Waetzig GH, Gewiese-Rabsch J, Garbers C, Yamamoto K, Rose-John S, Scheller J. Essential role of neutrophil mobilization in concanavalin A-induced hepatitis is based on classic IL-6 signaling but not on IL-6 trans-signaling. Biochim Biophys Acta. 2011;1812(3):290–301. doi: 10.1016/j.bbadis.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Wunderlich FT, Ströhle P, Könner AC, Gruber S, Tovar S, Brönneke HS, Juntti-Berggren L, Li L-S, van Rooijen N, Libert C. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010;12(3):237–249. doi: 10.1016/j.cmet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng Y-H. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lang Lehrskov L, Lyngbaek MP, Soederlund L, Legaard GE, Ehses JA, Heywood SE, Wewer Albrechtsen NJ, Holst JJ, Karstoft K, Pedersen BK. Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. 2018;27(6):1201–11.e3. doi: 10.1016/j.cmet.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu M, Xu X, Li J, Li F. Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front Psychiatry. 2019;10:473. doi: 10.3389/fpsyt.2019.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen T, Wang R, Duan Z, Yuan X, Ding Y, Feng Z, Bu F, Liu S, Wang Q, Zhou J. Akkermansia muciniphila protects against psychological disorder-induced gut microbiota-mediated colonic mucosal barrier damage and aggravation of colitis. Front Cell Infect Mi. 2021;11:723856. doi: 10.3389/fcimb.2021.723856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–41.e13. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ou ZH, Deng LL, Lu Z, Wu FF, Liu WT, Huang DQ, Szendroedi J, Roden M, Müssig K. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr Diabetes. 2020;10(1):10. doi: 10.1038/s41387-020-0111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–480. [DOI] [PubMed] [Google Scholar]
- 59.de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 60.Lakshmanan AP, Kohil A, El Assadi F, Al Zaidan S, Al Abduljabbar S, Bangarusamy DK, Jia X, Li L, Bai J, Zhang B, et al. Akkermansia, a possible microbial marker for poor glycemic control in qataris children consuming arabic diet-A pilot study on pediatric T1DM in Qatar. Nutrients. 2021;14(1):13. doi: 10.3390/nu14010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livanos AE, Greiner TU, Vangay P, Pathmasiri W, Stewart D, McRitchie S, Li H, Chung J, Sohn J, Kim S. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol. 2016;1(11):16140. doi: 10.1038/nmicrobiol.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X-S, Li J, Krautkramer KA, Badri M, Battaglia T, Borbet TC, Koh H, Ng S, Sibley RA, Li Y. Antibiotic-induced acceleration of type 1 diabetes alters maturation of innate intestinal immunity. eLife. 2018;7:e37816. doi: 10.7554/eLife.37816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seregin SS, Golovchenko N, Schaf B, Chen JC, Pudlo NA, Mitchell J, Baxter NT, Zhao LL, Schloss PD, Martens EC. NLRP6 protects II10-/- Mice from colitis by limiting colonization of akkermansia muciniphila. Cell Rep. 2017;19(4):733–745. doi: 10.1016/j.celrep.2017.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Håkansson Å, Tormo-Badia N, Baridi A, Xu J, Molin G, Hagslätt ML, Karlsson C, Jeppsson B, Cilio CM, Ahrné S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med. 2015;15(1):107–120. doi: 10.1007/s10238-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwab C, Berry D, Rauch I, Rennisch I, Ramesmayer J, Hainzl E, Heider S, Decker T, Kenner L, Müller M. Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. ISME J. 2014;8(5):1101–1114. doi: 10.1038/ismej.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ganesh BP, Richter JF, Blaut M, Loh G. Enterococcus faecium NCIMB 10415 does not protect interleukin-10 knock-out mice from chronic gut inflammation. Benef Microbes. 2012;3(1):43–50. doi: 10.3920/BM2011.0050. [DOI] [PubMed] [Google Scholar]
- 67.Ganesh BP, Klopfleisch R, Loh G, Blaut M, Ryffel B. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. Plos One. 2013;8(9):e74963. doi: 10.1371/journal.pone.0074963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proceedings of the National Academy of Sciences 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proceedings of the National Academy of Sciences 2017; 114(40):10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vallino A, Dos Santos A, Mathé CV, Garcia A, Morille J, Dugast E, Shah SP, Héry-Arnaud G, Guilloux C-A, Gleeson PJ, et al. Gut bacteria Akkermansia elicit a specific IgG response in CSF of patients with MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e688. doi: 10.1212/NXI.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, Bork P, Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9(1):39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disorders. 2017;32(5):739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang T, Li P, Wu X, Lu GC, Marcella C, Ji XH, Ji G, Zhang F. Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biot. 2020;104(23):10203–10215. doi: 10.1007/s00253-020-10948-7. [DOI] [PubMed] [Google Scholar]
- 74.Ang QY, Alba DL, Upadhyay V, Bisanz JE, Cai J, Lee HL, Barajas E, Wei G, Noecker C, Patterson AD. The East Asian gut microbiome is distinct from colocalized white subjects and connected to metabolic health. eLife. 2021;10:e70349. doi: 10.7554/eLife.70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SGRoh TY, Myung SJ, Extracellular vesicles derived from gut microbiota, especially akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. Plos One. 2013;8(10):e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y, Luo M, Zhou H, Li C, Luk SKA, Zhao G, Fung K, Ip M. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front Microbiol. 2019;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaghoubfar R, Behrouzi A, Ashrafian F, Shahryari A, Moradi HR, Choopani S, Hadifar S, Vaziri F, Nojoumi SA, Fateh A. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci Rep-Uk. 2020;10(1):22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo ZW, Xia K, Liu YW, Liu JH, Rao SS, Hu XK, Chen C-Y, Xu R, Wang Z-X, Xie H. Extracellular vesicles from akkermansia muciniphila elicit antitumor immunity against prostate cancer via modulation of CD8(+) T cells and macrophages. Int J Nanomed. 2021;16:2949–2963. doi: 10.2147/IJN.S304515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang LJ, Tang L, Feng YM, Zhao SY, Han M, Zhang C, Yuan G, Zhu J, Cao S, Wu Q. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8 + T cells in mice. Gut. 2020;69(11):1988–1997. doi: 10.1136/gutjnl-2019-320105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gu Z, Pei W, Shen Y, Wang L, Zhu J, Zhang Y, Fan S, Wu Q, Li L, Zhang Z. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 2021;12(20):10184–10195. doi: 10.1039/D1FO02172A. [DOI] [PubMed] [Google Scholar]
- 81.Wang J, Xu W, Wang R, Cheng R, Tang Z, Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021;12(8):3597–3610. doi: 10.1039/D1FO00115A. [DOI] [PubMed] [Google Scholar]
- 82.Cheng RR, Xu WJ, Wang JC, Tang ZQ, Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila alleviates the depression-like behavior of depressed mice induced by chronic stress. Biochem Bioph Res Co. 2021;566:170–176. doi: 10.1016/j.bbrc.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 83.van Passel MWJ, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, Chain PSG, Woyke T, Palva A, de Vos WM. The genome of akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. Plos One.2011;6(3):e16876. doi: 10.1371/journal.pone.0016876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo XF, Li SH, Zhang JC, Wu FF, Li XC, Wu D, Zhang M, Ou ZH, Jie ZY, Yan QL. Genome sequencing of 39 Akkermansia muciniphila isolates reveals its population structure, genomic and functional diverisity, and global distribution in mammalian gut microbiotas. Bmc Genomics. 2017;18:18. doi: 10.1186/s12864-016-3397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karcher N, Nigro E, Puncochar M, Blanco-Miguez A, Ciciani M, Manghi P, Zolfo M, Cumbo F, Manara S, Golzato D. Genomic diversity and ecology of human-associated Akkermansia species in the gut microbiome revealed by extensive metagenomic assembly. Genome Biol. 2021;22:22. doi: 10.1186/s13059-020-02241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Belzer C, de Vos WM. Microbes inside—from diversity to function: the case of Akkermansia. ISME J. 2012;6(8):1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirmiz N, Galindo K, Cross KL, Luna E, Rhoades N, Podar M, Flores GE. Comparative genomics guides elucidation of vitamin B-12 biosynthesis in novel human-associated akkermansia strains. Appl Environ Microb. 2020;86(3):e02117–19. doi: 10.1128/AEM.02117-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC, Dallow E, Remick B, Barton GM, David LA. Genotypic and phenotypic diversity among human isolates of akkermansia muciniphila. Mbio. 2021;12(3):e00478–21. doi: 10.1128/mBio.00478-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alard J, Lehrter V, Rhimi M, Mangin I, Peucelle V, Abraham A-L, Mariadassou M, Maguin E, Waligora-Dupriet A-J, Pot B. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ Microbiol. 2016;18(5):1484–1497. doi: 10.1111/1462-2920.13181. [DOI] [PubMed] [Google Scholar]
- 90.Toscano M, De Grandi R, Stronati L, De Vecchi E, Drago L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: a preliminary study. World J Gastroenterol. 2017;23(15):2696–2704. doi: 10.3748/wjg.v23.i15.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dizman N, Hsu J, Bergerot PG, Gillece JD, Folkerts M, Reining L, Trent J, Highlander SK, Pal SK. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med. 2021;10(1):79–86. doi: 10.1002/cam4.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng H, Yang S, Zhang Y, Qian K, Zhang Z, Liu Y, Wang Y, Bai Y, Fan H, Zhao X. Bacteroides fragilis prevents clostridium difficile infection in a mouse model by restoring gut barrier and microbiome regulation. Front Microbiol. 2018;9. doi: 10.3389/fmicb.2018.02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopez-Siles M, Enrich-Capo N, Aldeguer X, Sabat-Mir M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M. Alterations in the abundance and co-occurrence of akkermansia muciniphila and faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Mi. 2018;8:281. doi: 10.3389/fcimb.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Demirci M, Tokman HB, Uysal HK, Demiryas S, Karakullukcu A, Saribas S, Cokugras H, Kocazeybek BS. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopath. 2019;47(4):365–371. doi: 10.1016/j.aller.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 95.Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol J, de Vos WM. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B 12 Production by intestinal symbionts. Mbio. 2017;8(5):e00770–17. doi: 10.1128/mBio.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chia LW, Hornung BVH, Aalvink S, Schaap PJ, de Vos WM, Knol J. Deciphering the trophic interaction between Akkermansia muciniphila and the butyrogenic gut commensal Anaerostipes caccae using a metatranscriptomic approach. Anton Leeuw Int J G. 2018;111(6):859–873. doi: 10.1007/s10482-018-1040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


