Abstract
Appropriate synchronization of the timing of behaviors with the circadian clock and adequate sleep are both important for almost every physiological process. The timing of the circadian clock relative to social (ie, local) clock time and the timing of sleep can vary greatly among individuals. Whether the timing of these processes is stable within an individual is not well-understood. We examined the stability of circadian-controlled melatonin timing, sleep timing, and their interaction across ~ 100 days in 15 students at a single university. At three time points ~ 35-days apart, circadian timing was determined from the dim-light melatonin onset (DLMO). Sleep behaviors (timing and duration) and chronotype (ie, mid-sleep time on free days corrected for sleep loss on school/work days) were determined via actigraphy and analyzed in ~ 1-month bins. Melatonin timing was stable, with an almost perfect relationship strength as determined via intraclass correlation coefficients ([ICC]=0.85); average DLMO timing across all participants only changed from the first month by 21 minutes in month 2 and 5 minutes in month 3. Sleep behaviors also demonstrated high stability, with ICC relationship strengths ranging from substantial to almost perfect (ICCs = 0.65–0.85). Average DLMO was significantly associated with average chronotype (r2 = 0.53, P <.01), with chronotype displaying substantial stability across months (ICC = 0.61). These findings of a robust stability in melatonin timing and sleep behaviors in young adults living in real-world settings holds promise for a better understanding of the reliability of previous cross-sectional reports and for the future individualized strategies to combat circadian-associated disease and impaired safety (ie, “chronomedicine”).
Keywords: Circadian rhythm / physiology, Human, Melatonin / biosynthesis, Melatonin / metabolism, Saliva / metabolism, Sleep / physiology, Time factors
1 |. INTRODUCTION
Alignment between the internal circadian clock with behaviors is fundamental to the functioning of almost every physiological process, cognition, and overall health.1 Despite knowledge that humans have a relatively stable circadian period when they are studied in highly controlled environments (eg, multi-day tightly-controlled laboratory protocols in dim-light settings),2,3 the invention of electrical lighting has allowed individuals to express dramatic differences in circadian phase, in timing (ie, chronotype) and/or in preference for morning or evening activities.4,5 In fact, similarly aged individuals living in the same city can exhibit a range up to 11-hour difference in the timing of dim-light melatonin onset (DLMO), the established circadian phase marker.6,7 Thus, alignment between the internal circadian clock and the social clock (ie, on a watch or device) varies greatly on an individual-by-individual basis. However, whether circadian phase is stable across multiple months for an individual living in real-world settings is unknown. In a study that included two separate in-laboratory visits with fixed sleep/wake times preceding each visit, Kantermann and Eastman found that (i) individuals did not have significantly different circadian period or phase timing between visits, and (ii) individuals that had large (~3 hour) changes in their fixed sleep/wake schedules between the two visits had earlier circadian timing at the second visit.8 Understanding the stability of circadian phase across multiple visits in individuals not living on a fixed schedule is necessary in order to establish future circadian-based therapeutic (ie, “chronotherapy”) recommendations.
The timing and duration of sleep and their relationships with endogenous circadian timing are also vital components of overall health and cognitive processing.9 In cross-sectional analyses, epidemiological studies that only assessed behavior using a single-time questionnaire or across short intervals have found associations between shorter sleep durations and increased risk for cardiovascular disease,10 obesity,11 diabetes12 and workplace accidents or injury.13 Although cross-sectional study design is an effective way to obtain an estimate of daily behaviors, it assumes that a single measurement is representative, and without bias (eg, changing behavior because of data collection on that day). Such a design may be limited in its ability to accurately document the time-varying dynamics of sleep timing and duration and may inaccurately conclude the presence or absence of a scientific relationship. For example, a single question about self-reported sleep duration is not associated with coronary artery calcification, but objective sleep duration measured over 6 days is associated with incidence of coronary calcification.14 It is not clear, however, if 6 days of measurement is an accurate depiction of habitual sleep duration. Workday vs. free-day changes in sleep timing and duration are well known. Variation of sleep metrics is high when examining individual variability across 3 days of objective sleep, including one weekend day, but are more consistent when measuring sleep metrics between years.15 Finally, given that measuring DLMO in the laboratory is expensive and burdensome to both participants and investigators, understanding the stability of more easily attainable objective chronotype markers from sleep/wake patterns (ie, mid-sleep time on free days corrected for sleep loss on work days [MSFsc]),16 which are highly correlated with DLMO,4,17 may be helpful for making circadian-based assessments to be used for recommendations at the individual and population level.
Identifying the stability of objective measurements of sleep and circadian outcomes longitudinally, including both weekdays/workdays and weekend/free days, is imperative to understand and target modifiable behaviors to improve health and wellbeing. Moreover, examination of how the stability of these repeated measures may appear at both the group level, where the noise from large inter-individual differences in circadian and sleep outcomes could make these measures appear less stable, and at the individual level is necessary for accurate understanding of stability. Lastly, recognizing the stability of these independent variables that predict poor health and safety outcomes is vital in understanding profiles of risk and potential future treatment options (ie, “chronotherapy” or “chronomedicine”).
2 |. MATERIALS AND METHODS
2.1 |. Participants
Participants (n = 15, 10 male; aged 19.1 years [± standard error of mean (SEM)] 0.3, range 18–21) were recruited at one university. They were eligible to participate if they were not currently employed in night shift work and had not traveled more than one time zone in the 3 months prior to and during the protocol. All participants provided written informed consent, and approval of study procedures was obtained from the Partner’s Healthcare Institutional Review Board. This study was part of a larger investigation that was registered at clinicaltrials.gov as NCT02846077. Note, only a subset of participants from the overall trial partook in this semester-long study, and no participants or their data from previous publications18–20 were used in the current study.
2.2 |. Study procedures
Immediately after obtaining consent, participants were provided a wrist actigraphy monitor (MotionLogger; Ambulatory Monitoring, Ardsley, NY) to continuously wear on their nondominant arm and were sent twice-daily electronic sleep diaries to complete once in the morning upon awakening and once in the evening immediately prior to sleep. Participants were instructed to wear the monitor at all times, with the exception of when the device might get wet or damaged. The study was conducted over ~ 100 days during one spring semester.
At three time points spaced ~ 35-days apart (ie, beginning, middle, and end of the spring semester), participants were admitted to the Brigham and Women’s Hospital Center for Clinical Investigation and Intensive Physiologic Monitoring Unit to assess DLMO timing as a marker of circadian timing. The first DLMO assessment occurred in mid-February and the last in mid-May. As data were collected across the Standard Time-to-Daylight Saving Time (DST) transition in month 2, all DLMO assessments were completed ≥ 5 days after spring social time change to DST. Upon admittance to the laboratory (~15:30), participants were not allowed to use any personal light-emitting electronic devices and ambient lighting was dimmed to ~ 4 lux. Saliva samples were collected hourly starting at ~ 16:00 and ending at ~ 07:00 (16 samples per participant). In the 20 minutes immediately prior to each saliva sample, participants maintained a constant seated posture and were instructed to refrain from eating or drinking to minimize exogenous influences on melatonin concentrations. At all other times during the ~ 16-hour overnight stay, participants were allowed to move within the study room, remain seated, sleep in a seated position in their chair, and/or eat a small provided snack. The saliva was later assayed for melatonin.
2.3 |. Analysis
Melatonin circadian phase (ie, DLMO) was calculated as the linear interpolated point in time at which melatonin concentrations crossed and remained above a 5 pg/ml threshold.7 Actigraphic sleep onset, offset, and duration data were manually scored using the electronic sleep-wake diaries data to set rest episode start and end times, which were used to calculate sleep episode duration.18,21 These data were binned into three approximately 1-month bins including days preceding and between in-laboratory visits (~35 days). Daytime naps and “all-nighters” were not included in the present analysis (average of 11.4 sleep episodes removed per participant). Sleep metrics in the 3 days after the DST transition (March 12–15th, 2017) were removed to reduce the potential for artificial changes in sleep timing due to the 1-hour advance in clock time. Sleep timing data from days −8 to + 8 relative to this transition are shown in Figure Phase angle of entrainment was calculated as the difference in timing of DLMO for each month’s in-laboratory visit and the preceding month’s average sleep onset. Chronotype was calculated using actigraphy-based weekday (ie, assumed work/school day) and weekend (ie, assumed free day) sleep onset and offset times and the MSFsc formula from the Munich Chronotype Questionnaire (MCTQ).16 Sunset, sunrise, and solar midpoints were determined for each day during the study using weather.gov.
Linear mixed-effects models were used to determine group-level differences in average values of each variable across months of study, with month as the fixed effect and participant as the random effect to account for individual differences. If the model fit was significant, dependent t tests were performed to identify specific differences between individual months with a Bonferroni correction to correct for multiple comparisons (P <0.017 for statistical significance). Intraclass correlation coefficients (ICC) were calculated to examine individual consistency across months for a single score using a two-way mixed-effects model.22 The strength of ICC scores was defined using the following criteria: slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and almost perfect (0.81–1.00).23 Standard deviations were computed within an individual across the three months and then averaged across the group. Associations between the three-month averages of melatonin circadian phase and chronotype were determined using a Pearson correlation. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC). We did not have statistical power to analyze by sex.
3 |. RESULTS
The average intra-individual differences in DLMO timing were 00:16 (± standard error of mean (SEM) 00:18, range −2:09 to 1:52) between month 1 and month 2; 0:02 (00:22, −01:16 to 03:16) for month 1 and month 3; and −00:17 (00:19, −02:14–01:41) for month 2 and 3, with no significant differences across months (F2,23 = 0.57, P = 0.57; Figures 1A and 2). When examining individual stability, we found that the timing of DLMO displayed an almost perfect relationship strength across the three months of examination (ICC = 0.85), with only two participants having > 2h difference between months (Figure 3A). Average standard deviation in DLMO timing across the three months was 0.6h (0.1, 0.1 – 1.6h).
FIGURE 1.
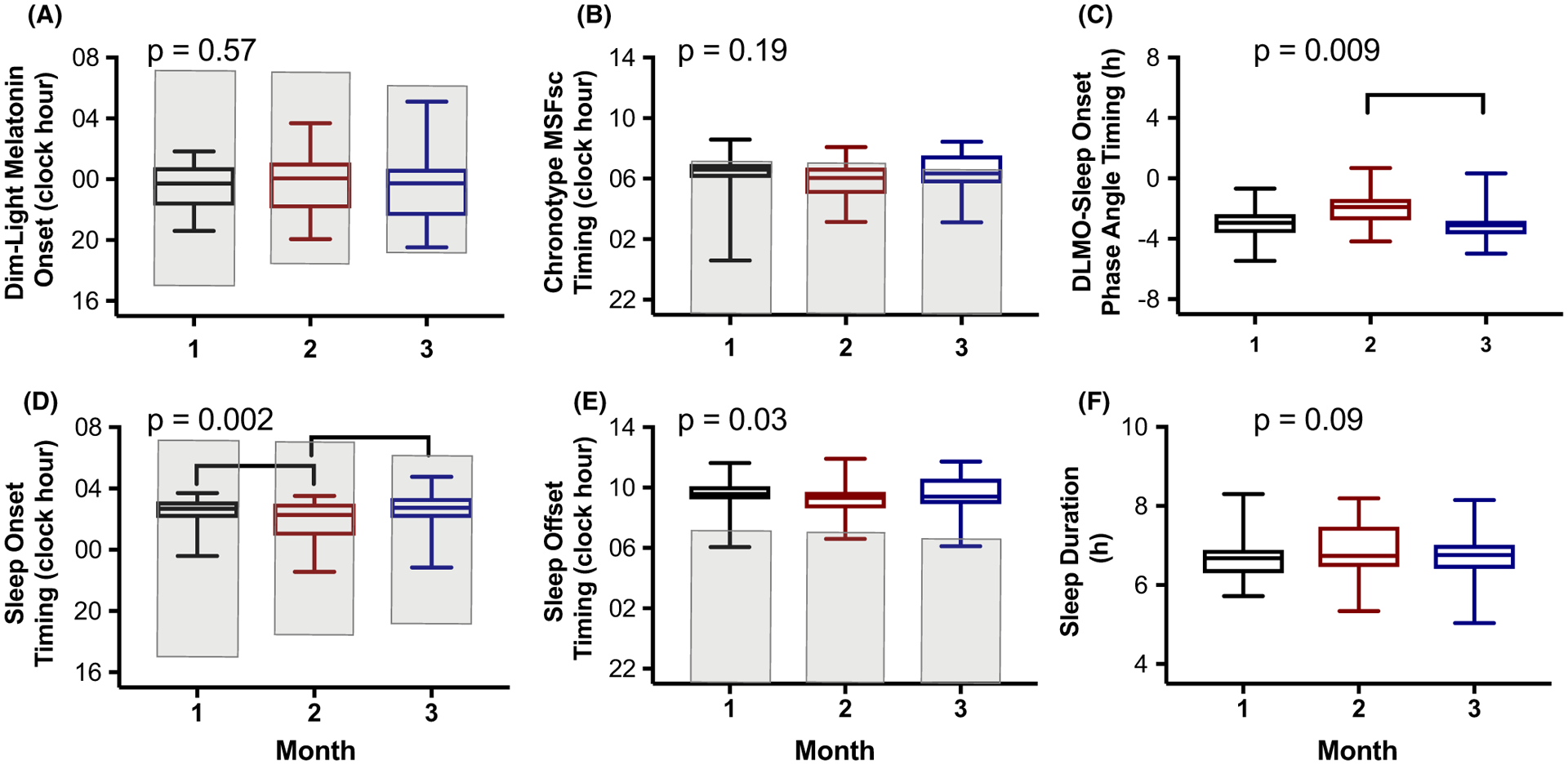
Circadian and sleep metrics across three separate months. Summary statistics of the average-within-an-individual data are presented as box plots with the center line denoting the median, the lower and upper lines of the box representing the 25th to 75th percentiles, respectively, and the whiskers representing the minimum and maximum of the group. The black box plot is for month 1 data, red for month 2 data, and blue for month 3 data. Shaded areas (a,b,d,e) represent the median timing of the solar night during each month (ie, sunset to sunrise duration). P values are derived from mixed-effects models with month as the fixed effect and participant as the random effect and brackets denote significant differences at the end of each line after correcting for multiple comparisons (P < 0.017 considered significant).
FIGURE 2.
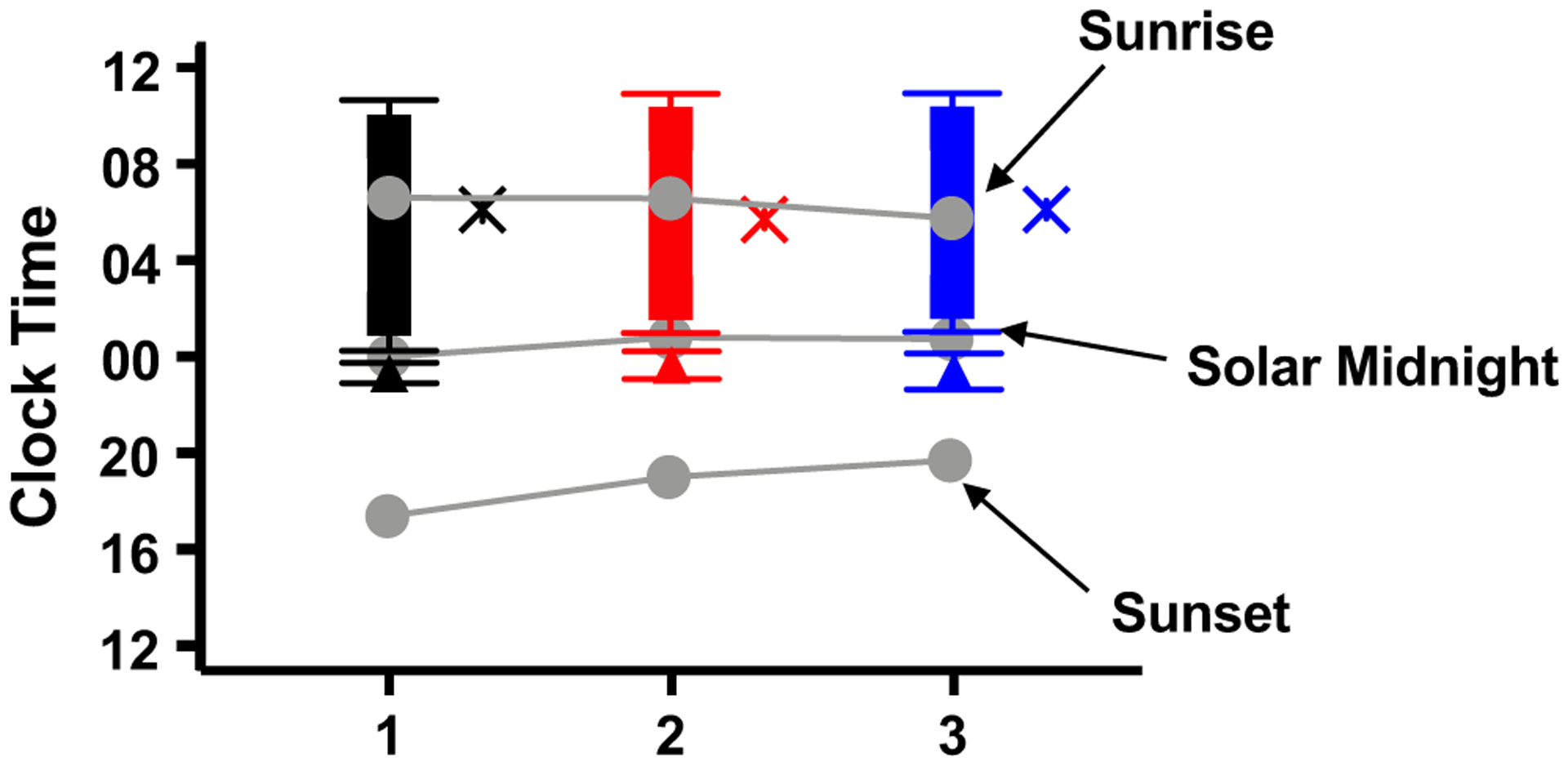
Average timing of dim-light melatonin onset (DLMO), sleep onset and offset, chronotype, and solar timing. Black symbols denote month 1, red month 2, and blue month 3. Triangles denote average DLMO, solid bars denote sleep, with the ends of each bar denoting average sleep onset and offset timing, and the X symbols denote chronotype (MSFsc). Gray circles denote sunset, solar midnight, and sunrise. Error bars are standard error of the mean.
FIGURE 3.
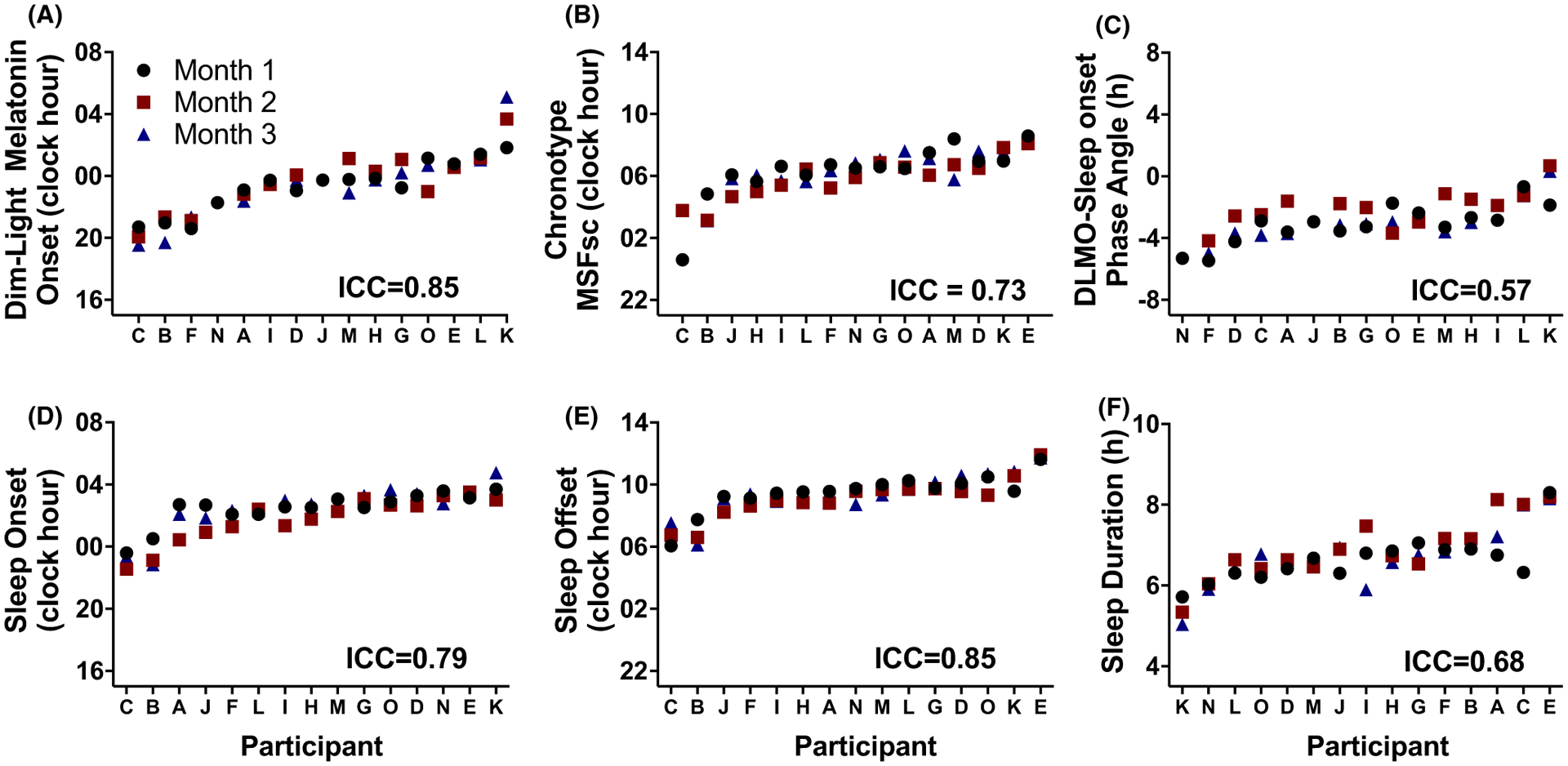
Individual differences in circadian and sleep metrics across three separate months. Participants are ordered from earlier to later timing. Black circles denote month 1, red squares month 2 and blue triangles month 3.
There was a significant positive association between DLMO and actigraphy-derived MSFsc, such that a later timing of DLMO was associated with a later timing of MSFsc (r2 = 0.54, P = 0.002) (Figure S2). MSFsc average intra-individual differences were −00:25 (00:20, −01:43 −03:11) for between month 1 and month 2; −00:01 (00:20, −02:38 −03:10) for month 1 and month 3; and 00:25 (00:11, −00:58 −01:10) for month 2 and 3, with no significant differences across months (F2,28 = 1.79, P = 0.19; Figures 1B and 2). Moreover, MSFsc timing across months displayed a substantial relationship strength (Figure 3B), with an average standard deviation of MSFsc timing between months of 0.7h (0.1, 0.2 −2.2h).
The average intra-individual differences of phase angle of entrainment significantly differed across months, with differences of 0.9h (0.4, −1.9 −2.6) between month 1 and month 2; −0.002h (0.3, −1.2 −2.2) for month 1 and month 3; and −0.9h (0.3, −2.5 −0.7) for month 2 and 3 (F2,23 = 5.78, P = 0.009; Figures 1C and 2). Dependent t tests revealed that month 2 had a significantly larger phase angle as compared to month 3 (t(11)=3.5, P = 0.005). The ICC for phase angle of entrainment was of moderate strength (ICC = 0.57, Figure 3C), and the standard deviation of phase angle of entrainment for the group was 0.8h (0.09, 0.3 −1.4h).
When examining sleep metrics, we found that sleep onset and offset timing significantly differed across months for our population, with average intra-individual differences in sleep onset timing of −00:42 (00:12, −02:16 −00:34) for between month 1 and month 2; −00:03 (00:11, −01:40 −01:04) for month 1 and month 3; and 00:39 (00:11, −00:32 −01:46) for month 2 and 3 (F2,28 = 8.23, P = 0.002; Figures 1D and 2). Dependent t tests revealed that month 2 had significantly earlier sleep onset timing as compared to both month 1 (t(14)=3.5, P = 0.004) and month 3 (t(14)=3.5, P = 0.004). Average intra-individual differences in sleep offset timing between those 3 months was −00:36 (00:09, −01:10 −01:00); −00:02 (00:12, −01:38 −01:28); and 00:20 (00:10, −00:51 −01:23), respectively (F2,28 = 3.81, P = 0.03; Figures 1E and 2), with no significant differences between particular months (all P > 0.04). Taken together, these onset and offset timings resulted in average intra-individual differences in sleep duration of 0.3h (0.2, −0.5 −1.7) for between month 1 and month 2; 0.1h (0.2, −0.9 −1.7) for month 1 and month 3; and −0.2h (0.1, −1.6 −0.4) for month 2 and 3, which did not significantly differ across months (F2,28 = 2.61, P = 0.09; Figures 1F and 2).
The ICC for sleep outcomes ranged from substantial in relationship strength (sleep onset and sleep duration) to almost perfect (sleep offset) in relationship strength (Figure 3D–F). Average standard deviation for sleep onset was 0.6h (0.07, 0.2 −1.2h), 0.5h (0.05, 0.1 −0.8h) for sleep offset, and 0.3h (0.1, 0.08 −1.0h) sleep duration.
When the data were analyzed relative to solar (instead of social) time, because the timing of the DLMO and sleep variables predominately followed social timing, all outcomes significantly changed across months with the transition to DST (Figure S3).
4 |. DISCUSSION
Documenting the reproducibility and stability of melatonin circadian phase, sleep behaviors, and the relationship between these two physiological processes is paramount to considering the reliability of previous cross-sectional reports on the impact of proper circadian alignment and the consequences of circadian misalignment. The current study revealed that across three months of measurement, the timing of circadian phase and sleep metrics in college students living in real-world settings has high stability at the individual level, yet differences appear in sleep onset, offset, and phase angle of entrainment at the group level due to inter-individual noise. Taken together, these data demonstrate the robust nature of internal circadian phase and sleep timing in real-world settings at the individual level and suggest that cross-sectional studies regarding circadian timing and sleep behaviors may reflect habitual physiology and behaviors in individuals. These findings are of critical importance as they further describe the stability of many processes (ie, circadian and sleep timing and events in relation to timing of those processes) that have come to the forefront as potential mechanisms for poor health and cognitive function, and thus, countermeasures targeting these behaviors may increase their confidence in data provided by cross-sectional observations.
Our findings that circadian phase and sleep metrics had high individual stability across multiple measurements in real-world sleep settings agrees with previous reports of the consistency of these metrics in structured and laboratory-based sleep environments. Using multiple in-laboratory visits spanning across 9 months to a year, Kantermann and Eastman found that DLMO was reproducible within ~ 2h, dependent on the controlled sleep/wakefulness schedules preceding each laboratory visit.8 Similarly, Benloucif and colleagues found stable melatonin onset metrics (within ~ 45 minutes) in participants maintaining controlled sleep/wake schedules up to 9 months apart24 and Revell and colleagues found that DLMO was within ~ 30 minutes when measured 1-week apart in individuals maintaining a tightly-controlled schedule on weekdays, but allowed to sleep 1h later on the weekend.25 In our sample of participants that entered the laboratory after living with uncontrolled real-world sleep/wake schedules, the average differences in DLMO timing between months 1, 2, and 3 were 21 and 5 minutes, respectively. Our smaller range of DLMO findings may be due to closer measurement timeframes (all within ~ 100 days as compared to 9 months) or potential smaller changes in sleep/wake timing preceding the measurements due to unchanged class schedules across the study. It should also be noted that we see this consistency despite changes in seasons and other life events and school activities across this interval. Importantly, if the data are examined while also considering the solar seasonal changes, particularly with the hour change in sunset, sunrise, and solar midpoints due to DST, it is clear that the participants in our study predominately followed social clock timing rather than solar timing. This observation is consistent with previous reports of no difference in melatonin circadian phase when studying individuals with access to electrical lighting near the summer and winter solstice5 and limited changes in free-day sleep timing relative to solar time across DST.26 Our findings have significant implications in regard to timing daily events with an individual’s internal circadian phase (ie, “chronotherapy” or “chronomedicine”), as it would suggest that a measurement of circadian phase is stable over time in young adults. This may be beneficial in identifying therapies for individuals with a circadian sleep/wake disorder27 and could also have implications for targeting the circadian timing of eating18 or the timing of drug distribution as a majority of widely sold drugs on the market have circadian gene targets28 and dosage of medications could potentially be reduced if the circadian phase of optimal drug effectiveness is known.29 However, further research is needed to assess whether this stability is evident in other clinical populations.
In regard to sleep timing, duration, and their relationship to internal circadian timing, despite several significant differences in the group-level averages across months, primarily driven by changes in sleep timing during month 2, monthly intra-individual stability was high. Other observations examining sleep in college students across a semester have found similar group-level differences in sleep outcomes30,31 and studies of older individuals are also in agreement with our findings of consistent actigraphic sleep outcomes within individuals.15 Although we cannot account for the causes as to why sleep timing changed during the second month in our cohort, the fact that individual stability was still robust in the measured sleep metrics highlights the need to examine these outcomes at the individual level. Our data add to this body of literature, particularly in relation to group and individual consistency and the relationship with circadian timing. Moreover, the almost perfect and substantial individual consistencies of monthly averages of sleep onset, offset, and duration could be of particular importance for cognitive performance, mood, and health, as more irregular sleep timing and duration on a daily timescale have recently been shown to play a role in poorer academic performance,7,19 mood/wellbeing,29 and cardiometabolic health.32 It is interesting to note, however, that sleep onset timing was slightly less stable than offset timing, likely due to external factors that govern the need to awaken at a particular time with the use of an alarm clock (eg, school, sports, work), whereas sleep onset timing can be more flexible. Thus, later sleep onset timing is likely to have a greater influence on shorter sleep durations observed in society.33 In fact, 73% of our participants slept < 7h on average across the entirety of the study, an amount less than recommended by the National Sleep Foundation.33 Individuals obtaining chronic insufficient sleep have previously been shown to have poorer performance,34,35 and— particularly concerning for safety outcomes—a disassociation between objective performance and subjective alertness.35,36 In our population, the relationship between objective chronic sleep durations and the stability of these metrics was previously unknown in real-world settings and our findings may have implications for the accuracy of single-time administered performance and mood testing reflecting habitual levels.
Lastly, our findings that DLMO was associated with chronotype is in agreement with previous reports,4,17 along with our findings of a high repeatability of chronotype metrics.8 Importantly, our metric of mid-sleep time on free days corrected was obtained using objective sleep timing data rather than a single questionnaire, potentially strengthening the physiological implications of this relationship. These findings suggest that chronotype is moderately stable across months, which could be beneficial when considering the use of chronotype for work-scheduling purposes across a monthly timeframe37 or for therapeutic strategies.38 Future work is needed to track the stability of chronotype in different age groups, as chronotype changes with aging,16 and across the year to test for seasonal or light/dark cycle effects.4,5
As our study was conducted in a small number of college students, additional studies are needed in other populations including different age groups, family or work situations, and those with any type of disorder that affects sleep or circadian rhythms. For example, patients with delayed sleep-wake phase disorder have been found to have less stable sleep and DLMO timing when measured either 5 days39 or two weeks40 apart as compared to healthy controls. The variability of sleep or circadian timing over months may be important in the disease process and/or for diagnosing or monitoring some health metrics; cross-sectional studies have already demonstrated the importance of day-to-day variability in sleep in multiple outcomes.7,41,42 By utilizing the repeated measure design of 3 measures in real-world settings, however, we increase generalizability of our study findings. Moreover, our low sample may have also allowed for individuals with higher variability in certain metrics to have a greater influence in the strength of our ICC analysis or group differences. We hypothesize that a larger sample would strengthen the observed ICC values. Lastly, our participant’s knowledge of partaking in a study testing sleep behaviors may have influenced their sleep/wake habits and artificially increased their sleep/wake stability. Nevertheless, the duration of our study’s data collection interval (>100 days of measurement) likely lessened any study-specific effects. We did not observe any systematic changes across the protocol, as might be expected if the novelty of the study diminished and participants returned to more habitual schedules.
In summary, our findings of the nearly perfect stability of circadian phase and sleep metrics in young adults in real-world settings have significant implications not just for better understanding of the reliability of previous cross-sectional reports, but also for the future of individualized strategies and treatments to combat disease and improve safety. Importantly, our findings suggest that future work taking into account an individual’s circadian timing can have confidence that this timing may remain stable across several months, and that other markers of sleep/wake timing (ie, chronotype) could be considered a potential proxy for this timing.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the participants and Center for Clinical Investigation staff for their support in conducting these studies. This work was funded by the National Institutes of Health (Dr McHill was supported in part by K01HL146992, F32DK107146, and T32HL007901; Drs. Barger and Czeisler were supported in part by R01OH011773 and Dr Barger by R01AG044416; Dr Klerman was supported in part by K24HL105664, R01HL114088, R01GM105018, R01HL128538, and P01AG009975; Drs. Sano and Picard were supported in part by R01GM105018; NIH grant 1UL1 TR001102-01, 8UL1TR000170-05, UL1 RR 025758, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science).
Funding information
National Institutes of Health, Grant/Award Number: K01HL146992, F32DK107146, T32HL007901, R01OH011773, R01AG044416, K24HL105664, R01HL114088, R01GM105018, R01HL128538, P01AG009975 and R01GM105018
CONFLICTS OF INTEREST
AWM reports speaker honorarium or travel reimbursement fees from the Utah Sleep Research Society and the California Precast Concrete Association; AS has received travel reimbursement or honorarium payments from Gordon Research Conferences, Pola Chemical Industries, Leuven Mindgate, American Epilepsy Society, and IEEE. She has received research support from Microsoft, Sony Corporation, NEC Corporation, and POLA chemicals, and consulting fees from Gideon Health and Suntory Global Innovation Center; CJH has received travel reimbursement from Integrated Safety Support and Deliberate Innovation; LKB is on the scientific advisory board for CurAegis Technologies. She has received consulting fees from University of Pittsburgh, Sygma, Insight and Puget Sound Pilots; CAC reports grants to BWH from FAA, NHLBI, NIA, NIOSH, NASA, and DOD; is/was a paid consultant to AARP (2018), American Academy of Dental Sleep Medicine (2017, 2018), Eisenhower Medical Center (2018), Emory University (2019), Ganésco, Inc (2017), Inselspital Bern (2019), Institute of Digital Media and Child Development (2017, 2018, 2019), Klarman Family Foundation (2017, 2018, 2019), M. Davis and Co (2018), Physician’s Seal (2019), Samsung (2016), Sleep Research Society Foundation (2019), State of Washington Board of Pilotage Commissioners (2018), Tencent Holdings Ltd (2019), Teva Pharma Australia (2019, 2020), UC San Diego (2018), University of Michigan (2017), University of Washington (2017, 2018), and Vanda Pharmaceuticals Inc (2017, 2018, 2019, 2020), in which Dr Czeisler also holds an equity interest; received travel support from Annenberg Center for Health Sciences at Eisenhower (2018), Aspen Brain Institute (2018), Bloomage International Investment Group, Inc (2018, 2019), UK Biotechnology and Biological Sciences Research Council (2019), Bouley Botanical (2017, 2018, 2019), Dr Stanley Ho Medical Development Foundation (2019), European Biological Rhythms Society (2017, 2019), German National Academy of Sciences (Leopoldina) (2019), Illuminating Engineering Society (2018), National Safey Council (2017, 2018, 2019), National Sleep Foundation (2017, 2018, 2019), Society for Research on Biological Rhythms (2018), Sleep Research Society Foundation (2018), Stanford Medical School Alumni Association (2019), Tencent Holdings Ltd (2019), University of Zurich (2018), and Vanda Pharmaceuticals Inc (2017, 2018, 2019), Ludwig-Maximilians-Universität München (2018), National Highway Transportation Safety Administration (2018), Office of Naval Research (2018), Salk Institute for Biological Studies/Fondation Ipsen (2018), The National Academy of Sciences, Engineering, and Medicine (2017), The Wonderful Company (2017), Department of Defense (2017); receives research/education support through BWH from Cephalon, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Harmony Biosciences LLC (2019), Jazz Pharmaceuticals PLC Inc (2017, 2018, 2019, 2020), Johnson & Johnson (2019), NeuroCare, Inc (2019), Philips Respironics Inc/Philips Homecare Solutions (2017, 2018, 2019, 2020), Regeneron Pharmaceuticals (2018, 2019, 2020), Regional Home Care (2019), Teva Pharmaceuticals Industries Ltd, Sanofi SA, Optum, ResMed, San Francisco Bar Pilots, Sanofi, Schneider, Simmons, Sysco, Philips, Vanda Pharmaceuticals; is/was an expert witness in legal cases, including those involving Advanced Power Technologies, Aegis Chemical Solutions LLC (2019), Amtrak (2019); Casper Sleep Inc (2019), C&J Energy Services (2019), Complete General Construction Co (2017), Dallas Police Association (2019), Enterprise Rent-A-Car (2019), Espinal Trucking/Eagle Transport Group LLC/Steel Warehouse Inc (2017, 2018, 2019), FedEx, Greyhound Lines Inc/Motor Coach Industries/FirstGroup America (2017, 2018, 2019), Pomerado Hospital/Palomar Health District (2017, 2018), PAR Electrical Contractors Inc (2019), Product & Logistics Services LLC/Schlumberger Technology Corp/Gelco Fleet Trust (2019), Puckett Emergency Medical Services LLC (2019), South Carolina Central Railroad Company LLC (2017, 2018), Union Pacific Railroad (2019), United Parcel Service/UPS Ground Freight Inc (2017, 2018), and Vanda Pharmaceuticals (2019, 2020); serves as the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc; and receives royalties from McGraw Hill, and Philips Respironics (2017, 2018, 2019) for the Actiwatch-2 and Actiwatch Spectrum devices. Dr Czeisler’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict of interest policies; RP receives consulting fees from and owns shares in Empatica, Inc, which sells two wearable sensors that are used in clinical trials for collecting autonomic and physical activity data during sleep/wake. She receives speaker fees from Stern Strategy; EBK has received travel reimbursement from the Sleep Research Society, the National Sleep Foundation, the Santa Fe Institute, the World Conference of Chronobiology, the Gordon Research Conference, and the German Sleep Society (DGSM); she was paid by the Puerto Rico Trust for a grant review, and has consulted for the National Sleep Foundation.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this manuscript will be made available by the authors, upon request, to any qualified researcher.
REFERENCES
- 1.Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol. 2016;26(10):R432–443. [DOI] [PubMed] [Google Scholar]
- 2.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. [DOI] [PubMed] [Google Scholar]
- 3.Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 2011;108(Suppl 3):15602–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright Kenneth P Jr, McHill Andrew W, Birks Brian R, Griffin Brandon R, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23(16):1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stothard ER, McHill AW, Depner CM, et al. Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr Biol. 2017;27(4):508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McHill AW, Czeisler CA, Phillips AJK, et al. Caloric and macro-nutrient intake differ with circadian phase and between lean and overweight young adults. Nutrients. 2019;11(3), 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips AJK, Clerx WM, O’Brien CS, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep. 2017;7(1):3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantermann T, Eastman CI. Circadian phase, circadian period and chronotype are reproducible over months. Chronobiol Int. 2018;35(2):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czeisler CA. Perspective: casting light on sleep deficiency. Nature. 2013;497(7450):S13. [DOI] [PubMed] [Google Scholar]
- 10.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. [DOI] [PubMed] [Google Scholar]
- 11.Bjorvatn B, Sagen IM, Oyane N, et al. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16(1):66–76. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–867. [DOI] [PubMed] [Google Scholar]
- 13.Nakata A Effects of long work hours and poor sleep characteristics on workplace injury among full-time male employees of small-and medium-scale businesses. J Sleep Res. 2011;20(4):576–584. [DOI] [PubMed] [Google Scholar]
- 14.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30(6):793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–1039. [DOI] [PubMed] [Google Scholar]
- 17.Kantermann T, Sung H, Burgess HJ. Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. J Biol Rhythms. 2015;30(5):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHill AW, Phillips AJ, Czeisler CA, et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106(5):1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer D, McHill AW, Sano A, et al. Irregular sleep and event schedules are associated with poorer self-reported well-being in US college students. Sleep. 2020;43(6):zsz300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sano A, Taylor S, McHill AW, et al. Identifying objective physiological markers and modifiable behaviors for self-reported stress and mental health status using wearable sensors and mobile phones: observational study. J Med Internet Res. 2018;20(6):e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barger LK, Flynn-Evans EE, Kubey A, et al. Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. Lancet Neurol. 2014;13(9):904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1(1):30. [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 24.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20(2):178–188. [DOI] [PubMed] [Google Scholar]
- 25.Revell VL, Kim H, Tseng CY, Crowley SJ, Eastman CI. Circadian phase determined from melatonin profiles is reproducible after 1 wk in subjects who sleep later on weekends. J Pineal Res. 2005;39(2):195–200. [DOI] [PubMed] [Google Scholar]
- 26.Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr Biol. 2007;17(22):1996–2000. [DOI] [PubMed] [Google Scholar]
- 27.Keijzer H, Smits MG, Duffy JF, Curfs LM. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev. 2014;18(4):333–339. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB. Dosing time matters. Science. 2019;365(6453):547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkins J, Shaw P. Self-reported sleep quality in college students: a repeated measures approach. Sleep. 1992;15(6):545–549. [DOI] [PubMed] [Google Scholar]
- 31.Pilcher JJ, Ott ES. The relationships between sleep and measures of health and weil-being in college students: a repeated measures approach. Behav Med. 1998;23(4):170–178. [DOI] [PubMed] [Google Scholar]
- 32.Zuraikat FM, Makarem N, Redline S, Aggarwal B, Jelic S, St-Onge M-P. Sleep regularity and cardiometabolic heath: is variability in sleep patterns a risk factor for excess adiposity and glycemic dys-regulation? Curr Diab Rep. 2020;20(8):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foundation NS. Executive summary of the 2005 “Sleep in America” poll. 2005.
- 34.Cohen DA, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2(14):14ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHill AW, Hull JT, Wang W, Czeisler CA, Klerman EB. Chronic sleep curtailment, even without extended (>16-h) wakefulness, degrades human vigilance performance. Proc Natl Acad Sci USA. 2018;115(23):6070–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bermudez EB, Klerman EB, Czeisler CA, Cohen DA, Wyatt JK, Phillips AJ. Prediction of vigilant attention and cognitive performance using self-reported alertness, circadian phase, hours since awakening, and accumulated sleep loss. PLoS One. 2016;11(3):e0151770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vetter C, Fischer D, Matera JL, Roenneberg T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol. 2015;25(7):907–911. [DOI] [PubMed] [Google Scholar]
- 38.Almoosawi S, Vingeliene S, Gachon F, et al. Chronotype: implications for epidemiologic studies on chrono-nutrition and cardiometabolic health. Adv Nutr. 2019;10(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess HJ, Park M, Wyatt JK, Rizvydeen M, Fogg LF. Sleep and circadian variability in people with delayed sleep-wake phase disorder versus healthy controls. Sleep Med. 2017;34:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson LA, McGlashan EM, Hosken IT, Anderson C, Phillips AJK, Cain SW. Sleep and circadian instability in delayed sleep-wake phase disorder. J Clin Sleep Med. 2020;16(9):1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Sci Rep. 2018;8(1):14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2020;75(9):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, upon request, to any qualified researcher.


