Abstract
In one of the largest, most comprehensive studies on borderline personality disorder (BPD) to date, this article places into context associations between this diagnosis and (1) 16 different psychiatric disorders, (2) eight somatic illnesses, and (3) six trauma and adverse behaviors, e.g., violent crime victimization and self-harm. Second, it examines the sex differences in individuals with BPD and their siblings. A total of 1,969,839 Swedish individuals were identified from national registers. Cumulative incidence with 95% confidence intervals (CI) was evaluated after 5 years of follow-up from BPD diagnosis and compared with a matched cohort. Associations were estimated as hazard ratios (HR) with 95% CIs from Cox regression. 12,175 individuals were diagnosed with BPD (85.3% female). Individuals diagnosed with BPD had higher cumulative incidences and HRs for nearly all analyzed indicators, especially psychiatric disorders. Anxiety disorders were most common (cumulative incidence 95% CI 33.13% [31.48–34.73]). Other notable findings from Cox regressions include psychotic disorders (HR 95% CI 24.48 [23.14–25.90]), epilepsy (3.38 [3.08–3.70]), violent crime victimization (7.65 [7.25–8.06]), and self-harm (17.72 [17.27–18.19]). HRs in males and females with BPD had overlapping CIs for nearly all indicators. This indicates that a BPD diagnosis is a marker of vulnerability for negative events and poor physical and mental health similarly for both males and females. Having a sibling with BPD was associated with an increased risk for psychiatric disorders, trauma, and adverse behaviors but not somatic disorders. Clinical implications include the need for increased support for patients with BPD navigating the health care system.
Subject terms: Psychiatric disorders, Depression, Addiction, Bipolar disorder, Schizophrenia
Introduction
Borderline Personality Disorder (BPD; Diagnostic and Statistical Manual of Mental Disorders [DSM] terminology), or Emotionally Unstable Personality Disorder (International Classification of Diseases [ICD] terminology), is a serious psychiatric disorder estimated to affect 1.7% of the worldwide population [1]. The core features of this diagnosis include: unstable interpersonal relationships, recurring self-harm, and emotional dysregulation [2].
Receiving a BPD diagnosis has been repeatedly associated with a high degree of psychiatric and somatic comorbidities, traumatic events, and criminal behavior [3–5]. These results have shaped the clinical perception and research directions of BPD, however specific estimates across psychiatric disorders, somatic illnesses, trauma, and adverse behavior have never been comprehensively presented in one study. Moreover, the majority of past studies were limited to smaller clinical samples or cross-sectional community samples, which did not allow for unselected samples nor longitudinal data. Additional longitudinal trait-based epidemiological studies have examined BPD symptoms in twins, however, few participants reached the full diagnostic criteria and thus may not be reflective of individuals with severe BPD symptoms [6, 7]. Therefore, it is of interest to examine these estimates in a representative and well-powered population-based study in order to provide the context for a BPD diagnosis and reframe misconceptions.
Psychiatric disorders
Psychiatric comorbidities are the rule rather than the exception in patients with BPD. A Swedish national study reported that 95.7% of individuals with a BPD diagnosis had a comorbid psychiatric diagnosis [8]. Mood disorders, post-traumatic stress disorder (PTSD), impulsive disorders, and bipolar disorders are commonly associated with BPD symptoms and diagnosis [9–11]. Precise estimates for these comorbidities are lacking, especially for rare and serious disorders, e.g., psychotic disorders [12].
Somatic illnesses
Individuals with BPD use health care services at a higher rate than those with other personality disorders, and even elevated BPD symptoms are associated with receiving disability [13, 14]. BPD has been linked to poor somatic health, such as obesity, diabetes, gastrointestinal disease, cardiovascular disease, hypertension, chronic pain, and sexually transmitted infections [3, 15]. It is likely that somatic illnesses associated with BPD have been overlooked in smaller studies, for example, infertility [16, 17]. Moreover, patients often perceive the severity of their illness worse than reports based on medical records, highlighting the importance of objective measurement [18].
Trauma and adverse behaviors
Up to 90% of patients with BPD are estimated to have a history of childhood trauma [1]. BPD has been linked to an increased risk for sexual abuse victimization in adulthood [19] and physical trauma resulting from accidents [3]. However, the association between BPD and other trauma types, e.g., death of a family member, is unclear. The relationship between trauma and BPD is theorized to be bidirectional, although the evidence for this is conflicting [20]. Similar to somatic reports, capturing objective measures of trauma would prevent biases that may arise from self-reports [21].
The association between violent crime and a BPD diagnosis is well documented, however less literature exists on nonviolent offenses [22]. It is unclear which nonviolent offenses are predominant in patients with BPD, although it is likely borne from impulsive actions. As recurrent self-harm is a core feature of BPD [2], we expect the rate of self-harm requiring medical attention to be higher than the general population. However, a precise estimate from a population sample is unknown.
Sex differences
BPD is predominately diagnosed in females, although evidence suggests that this is largely the result of a diagnostic bias [23]. Moreover, gender differences have been found for symptom expression and comorbidities [22]. Males have a higher prevalence of substance use disorders and antisocial personality disorder and exhibit symptoms related to aggression; while females show increased rates of risky behavior and an increased prevalence of comorbid mood disorders, eating disorders, and PTSD [22, 24]. Males are typically underrepresented in BPD studies, thus potential differences in outcomes and precursors of BPD is of particular importance. Given a diagnostic bias, males would need greater symptom severity in order to be diagnosed, leading to a difference in symptom severity between the sexes. With this, we hypothesize elevated rates across most categories for males with BPD compared to their female counterparts.
Further, this raises etiological questions about the differences between the sexes. With our hypothesis that males with BPD will have more severe symptomatology, we postulate that individuals with a brother diagnosed with BPD will have higher rates of diagnoses and adverse outcomes across all domains compared to those with a sister with a BPD diagnosis, similar to the so-called female protective effect in autism, with the sexes reversed [25].
Present study
The primary aim of this study was to describe the extent of the association for individuals with BPD and (1) psychiatric disorders, (2) somatic illnesses, and (3) trauma and adverse behaviors in a Swedish nationwide sample. As sensitivity analyses, we examined the temporal order of these associations, sex-specific differences for individuals, and those with siblings diagnosed with BPD.
Materials and methods
Study population
The study population included individuals born in Sweden between January 1st, 1973 and December 31st, 1993 with a personal identity number and a biological mother identifiable in the register (2,177,075). We excluded individuals with a congenital malformation (113,566), those who died before age 18 (6972), and/or emigrated before age 18 (62,664). Thus, 1,969,839 individuals were included in our study.
Data sources
Swedish personal identity numbers were used to link multiple Swedish registers in order to identify the cohort and create the analyzed variables, termed here as “indicators” (Table 1) [26–30]. The National Patient Register (NPR) contains administrative data from in-patient and specialist outpatient care (but not primary care) with diagnosis made by licensed medical doctors. The diagnoses have been externally validated by reviewing a random subset of patient’s medical records with comparison to the received diagnostic code. The positive predictive value of the psychiatric and somatic diagnoses in the register is high; out of the investigated medical records 80% or more retained the stated diagnostic code upon review [29]. Physical trauma requiring medical attention was found to have an acceptable positive predictive value of 74% [30, 31].
Table 1.
Utilized Swedish national registers.
| Source | Contained information | Use in study |
|---|---|---|
| National Patient Register | ICD diagnoses from all inpatient and outpatient specialist care after 2001; while only information on inpatient is available before this date. During our follow up period, two ICD revisions were used: the ICD-9 from 1987 until 1996 and the ICD-10 from January 1, 1997 and onwards | Determine all psychiatric and somatic illnesses |
| National Crime Register | All criminal convictions in the Swedish general court | Determine criminal convictions |
| Longitudinal Integration Database for Health Insurance and Labor Market Studies | Income, use of social services and benefits, and neighborhood quality | Determine poverty and neighborhood quality |
| Medical Birth Register | Information on birth, birthdate, and maternal prenatal period. | Identify cohort |
| Migration Register | Immigration and emigration to and from Sweden | Identify cohort and for censoring |
| Cause of Death Register | Date and cause of death | Identify cohort and for censoring |
BPD diagnosis was defined by receiving an Emotionally Unstable Personality Disorder diagnosis in the NPR (ICD 10th revision: F60.3) by psychiatrists in in- or outpatient psychiatric clinics. In one validation study, structured interviews had been used in 36% of examined personality disorders (including other personality disorders than BPD), as identified from medical charts. However, the positive predictive value, i.e., proportion of diagnoses validated upon review, for the 26 BPD-diagnoses investigated was high regardless if structured interviews had been used or not, between 77% (based on DSM-criteria) and 100% (based on ICD-criteria); inter-rater agreement was between 85% (ICD-criteria) and 100% (DSM-criteria) [32]. Another validation study based on 70 medical charts with a BPD-diagnosis, and reported a positive predictive value of 81%, with an inter-rater agreement of 93% [33].
The indicators in our study were selected based on an existing data linkage, which contains a subset of all ICD-codes (Supplementary Table 1). Indicators were placed into three groups: psychiatric disorders, somatic illnesses, and trauma and adverse behaviors (Supplementary Tables 2–4). As a sensitivity analysis, we analyzed the three most common subcategories for umbrella indicators with many subtypes, e.g., autoimmune disorders. Adverse behaviors included violent/nonviolent crime and self-harm. Trauma included accidents requiring medical attention, violent crime victimization requiring medical attention, death of a close family member, and childhood neighborhood quality.
We only considered time of the first observed event for all indicators, except for childhood poverty and neighborhood quality.
The study was approved by the Regional Ethics Committee in Stockholm, Sweden (Dnr 2013/862 31/5). As our study participants were non-identifiable, no informed consent was needed by Swedish law.
Statistical analysis
Cumulative incidence
First, we estimated cumulative incidence for those with and without a BPD diagnosis in order to quantify the associations between BPD and the indicators on an absolute scale. Each patient with BPD was matched with ten individuals not diagnosed with BPD on birth year and sex. Follow up for individuals with BPD began at the date of the first observed BPD diagnosis and continued until the end of follow-up, December 31, 2013, this date was also used for their matched non-exposed individuals. We calculated 5-year cumulative incidence, interpreted as the probability of the event occurring within 5 years, while accounting for censoring. We used Kaplan-Meier estimation to estimate the cumulative incidence as 1 minus the survival function.
Associations between BPD diagnosis and the indicators
To quantify the association between BPD and our indicators, hazard ratios (HR) with 95% confidence intervals (CI) were obtained using sex-stratified Cox regression. Age was used as an underlying time score and we adjusted for birth cohort (1973–1977, 1978–1982, 1983–1987, and 1988–1993). BPD diagnosis was treated as a binary, time-constant, exposure regardless of when the diagnosis occurred. We followed each individual from birth or the start of ICD-9, January 1, 1987, until the first instance of either death, emigration, indicator occurrence, or end of follow-up. We did not account for competing risks, since standard methods introduce changes in the association dependent on whether the exposure is associated with the competing outcome [34].
We adjusted the analysis for multiple testing according to Benjamini–Hochberg method, with an alpha of 0.05, we obtained a false discovery rate p value threshold of 8.34 · 10−58 for the main analysis and 0.04 for secondary analyses [35].
Secondary analyses
Sex-separated and sibling analysis
To evaluate potential differences between males and females diagnosed with BPD, we repeated the cumulative incidence-, association-, and time-varying analyses by analyzing males and females separately. To investigate potential etiological differences, we repeated the association analyses among men and women separately, split by exposure being having a full sister or having a full brother with a BPD diagnosis.
Time-varying sensitivity analyses
By not considering timing of exposure in our main analysis, we assumed that individuals were exposed since birth although the BPD diagnosis occurred at a later age. This means that we were “borrowing information from the future”, an approach that may introduce bias. Therefore, we repeated all Cox regression analyses comparing the indicators before or after a BPD diagnosis to treat exposures as time-varying. Risk factor analyses considered the indicators to be exposures prior to a BPD diagnosis, while outcome analyses considered indicators as an outcome following a BPD diagnosis.
SAS was used for data management and all subsequent analysis was done in R using the survival package [36].
Results
Descriptive statistics
The cohort consisted of 1,969,839 individuals (48.8% female) with 12,175 individuals with BPD (85.3% female; 0.6% of sample) (Table 2; absolute values Supplementary Tables 5–7). Total follow-up time was 32,637,932 person-years, calculated from the first possible time of BPD diagnosis, i.e., from its introduction in 1997, and onward. The diagnosis was evenly distributed between birth year cohorts, and the total cohort had a mean age of 29.69 years at the end of follow-up.
Table 2.
Descriptive information.
| Total cohort No. % | With BPD diagnosis No. % | Without BPD diagnosis No. % | ||||
|---|---|---|---|---|---|---|
| Individuals | 1,969,839 | 100% | 12,175 | 0.6% | 1,957,664 | 99.4% |
| Follow-up time, person-years | 32,637,932 | 202,513 | 32,435,419 | |||
| Males | 1,007,774 | 51.2% | 1,785 | 14.7% | 1,005,989 | 51.4% |
| Females | 962,065 | 48.8% | 10,390 | 85.3% | 951,675 | 48.6% |
| Birth year | ||||||
| 1973–1977 | 461,587 | 23.4% | 2,356 | 19.4% | 459,231 | 23.4% |
| 1978–1982 | 421,516 | 21.4% | 2,978 | 24.5% | 418,538 | 21.3% |
| 1983–1987 | 439,982 | 22.3% | 3,487 | 28.6% | 436,495 | 22.2% |
| 1988–1993 | 646,754 | 32.8% | 3,354 | 27.5% | 643,400 | 32.9% |
Cumulative incidences
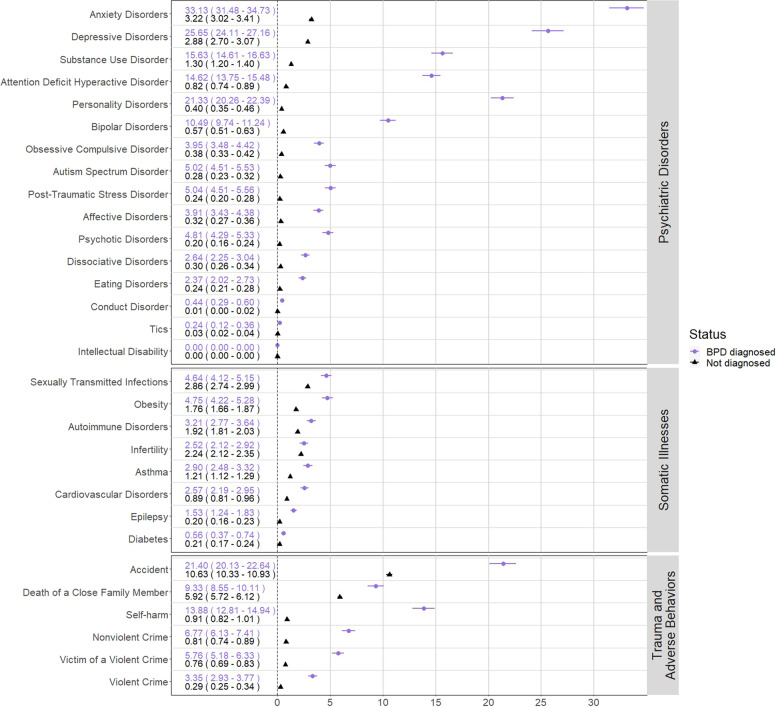
The 5-year cumulative incidence showed increased incidences in individuals with BPD compared to the matched control sample for all indicators, except intellectual disability (Fig. 1; Supplementary Figs. 1–7). The largest cumulative incidences were for anxiety disorders (Cumulative incidence [95% CI]; BPD 33.13% [31.48–34.73%]; not BPD (NBPD) 3.17% [2.98–3.79%]), major depressive disorder (BPD 25.65% [24.11–27.16%]; NBPD 3.04% [2.85–3.24%]) personality disorders (BPD 21.33% [20.26–22.39%]; NBPD 0.36% [0.31–0.41%]), accidents requiring medical attention (BPD 21.50% [20.13–22.64%]; NBPD 10.91% [10.60–11.21%]), and attention-deficit hyperactive disorder (BPD 14.62% [13.75–15.48%]; NBPD 0.81% [0.74–0.88%]). Intellectual disability had zero first event occurrences after date of BPD-diagnosis for both exposed and unexposed.
Fig. 1. Cumulative incidence by 5 years after Borderline Personality Disorder diagnosis, estimate in percent and (95% confidence interval).
The cumulative incidence of each of the main indicators broken down by subgroups.
Associations between BPD diagnosis and indicators
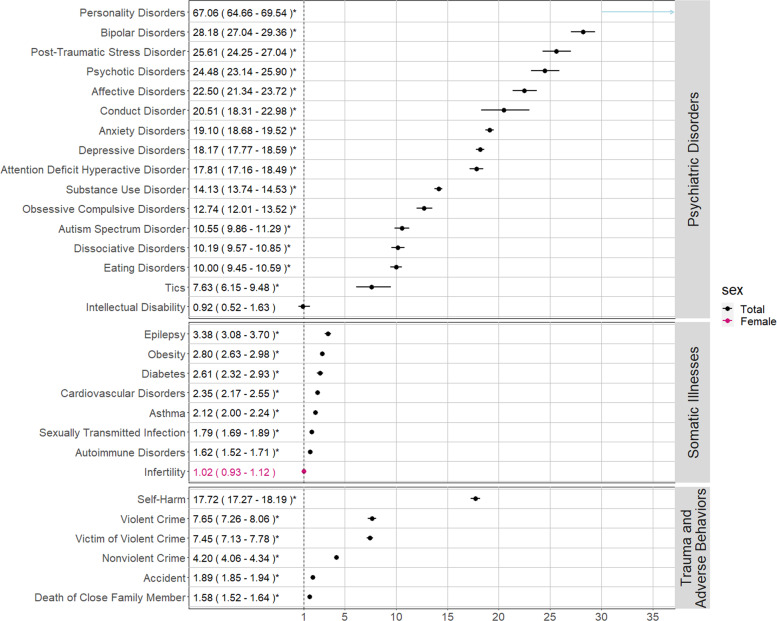
All HRs for BPD and the indicators included in our main analysis were statistically significant, i.e., larger than 1, after correcting for multiple testing, except for intellectual disability and infertility in females (Fig. 2). The majority of the indicators under the umbrella categories were also statistically significantly larger than 1 (Supplementary Figs. 8 and 9).
Fig. 2. Associations with Borderline Personality Disorder diagnosis, hazard ratio and (95% confidence interval).
*Statistically significant after correcting for multiple testing using the Benjamini-Hochberg method, resulting in a p value threshold of 8.34 · 10−58.
Psychiatric disorders
Psychiatric disorders had the highest HRs across all analyses. The highest HRs were personality disorders not including BPD (HR [95% CI] 67.06 [64.66–69.54]), bipolar disorders (28.18 [27.04–29.36]), and PTSD (25.61 [24.25–27.04]). Psychotic disorders were also elevated (24.48 [23.14–25.90]).
Somatic illnesses
The largest HRs for the association between BPD and somatic illnesses were for epilepsy (3.38 [3.08–3.70]), obesity (2.8 [2.63–2.98]), and diabetes (2.61 [2.32–2.93]).
Traumatic events and adverse behaviors
The strongest association regarding traumatic events was violent crime victimization (7.45 [7.13–7.78]). Death of a close family member also had a positive association (1.58 [1.52–1.64]). Self-harm had the highest hazard ratios of adverse behaviors (17.72 [17.27–18.19]). Committing a violent crime (7.65 [7.26–8.06]) had a stronger association than nonviolent crimes (4.20 [4.06–4.34]). Impulsive nonviolent crimes, i.e., property damage (6.66 [6.05–7.33]), had a stronger association than planned crime, possessing fake identification (1.94 [1.19–3.17]). Although they were analyzed as risk factors by definition, the HRs for poverty in childhood (1.93 [1.86–2.00]) and childhood neighborhood quality (1.52 [1.47–1.59]) were positively associated with BPD diagnosis (Supplementary Fig. 10 and Supplementary Table 5).
Secondary analyses
Sex-separated and sibling analysis
Females with BPD had higher cumulative incidences compared to males with BPD in nearly all somatic disorders, e.g., obesity (male 1.80 [0.97–2.62]; female 5.29 [4.68–5.90]); while the inverse was true for adverse behaviors and traumas, e.g., committing a violent crime (male 10.27 [8.14–12.35]; female 2.44 [2.06–2.83]). However, the CIs frequently overlapped between the sexes (Supplementary Figs. 11–23). HRs were largely uniform (Supplementary Fig. 24). Males had higher HRs for bipolar disorder (male 36.31 [32.62–40.41]; female 27.11 [25.93–28.33]), PTSD (male 34.99 [29.54–41.44], female 24.72 [23.35–26.18]) and affective disorders (male 31.42 [27.63–35.72]; female 21.32 [20.13–22.59]). Somatic disorders were mostly uniform across sex. Committing a violent crime was relatively more elevated in females (male 6.90 [6.38–7.46]; female 8.25 [7.68–8.86]). Additionally, being a victim of a violent crime requiring medical attention had a higher HR in females (male 5.05 [4.56–5.58]; female 8.58 [8.17–9.01]).
Individuals with siblings diagnosed with BPD had increased rates of indicators, however, many CIs contained 1, especially within somatic disorders (Supplementary Tables 9–11). Individuals with brothers diagnosed with BPD had higher HRs than those with sisters who were diagnosed in 87 out of 123 indicators. Psychiatric disorders had the strongest association, e.g., PTSD (males with brothers diagnosed with BPD (BBPD) 6.76 [3.74–12.24], males with sisters diagnosed with BPD (SBPD) 2.62 [1.78–3.87], females with BBPD 4.09 [2.72–6.16], females with SBPD 3.62 [3.02–4.35]).
Time-varying sensitivity analyses
Broadly, the magnitude of HRs for each indicator stayed relatively consistent when treating indicators as a risk factor prior to, or as an outcome following, a BPD diagnosis (Supplementary Tables 8, 12–13 and Supplementary Figs. 10, 25, and 26. Some notable exceptions were personality disorders (HR [95% CI] risk factor for subsequent BPD diagnosis 71.50 [68.36–74.78]; outcome following a BPD diagnosis 43.77 [41.40–46.28]), bipolar disorders (risk 35.94 [34.16–37.82]; outcome 17.27 [16.12–18.51), and psychotic disorders (risk 25.82 [24.19–27.56]; outcome 17.96 [16.23–19.87]) which had higher HRs leading up to a BPD diagnosis, and epilepsy (risk 2.89 [2.59–3.22]; outcome 5.36 [4.53–6.34]) that had a higher HR following a BPD diagnosis.
Discussion
In this population-based study, which included 12,175 individuals with BPD in a total sample of 1,969,839 Swedes, we found that BPD was associated with an increased risk for psychiatric comorbidities, somatic illnesses, traumatic events, and adverse behaviors. Our findings replicate previously known associations and identify unknown or understudied associations, e.g., epilepsy, infertility, and death of close family members. Moreover, this work extends findings to include both sexes and their siblings.
Psychiatric disorders
BPD was strongly associated with all psychiatric disorders except intellectual disability. We found a robust association between BPD and all other personality disorders, mood disorders, eating disorders, PTSD, and substance use disorders. Certain findings need additional vigilance from clinicians and researchers, e.g., the strong association with psychotic disorders. This finding follows the historical implication of the term borderline, coined to indicate that the patients were on the borderline between psychosis and neurosis [37]. Psychotic experiences are often treated as transient symptoms according to DSM guidelines, although symptoms are often perpetual [12]. Our finding highlights the importance of carefully assessing psychotic symptoms in BPD, and indicates that psychotic disorders are indeed overrepresented in individuals with BPD [38].
Somatic illnesses
Individuals with BPD had a higher risk of almost all somatic comorbidities in the main analysis except for female infertility. A Danish study also found a positive association between somatic disorders and combined personality disorders, however, their estimates were smaller than our results [39]. This could suggest that a BPD diagnosis has a worse prognosis than other personality disorders. In line with the literature, epilepsy and metabolic-related comorbidities, such as type 2 diabetes and obesity, had the strongest associations [10]. Previous studies have indicated a relationship between epilepsy and BPD, and here we show a clear association [40].
Trauma and adverse behaviors
Individuals with BPD were at a higher risk of all traumatic events both before and after receiving their diagnosis. Namely, individuals with BPD were at a higher risk of seeking medical care due to violent crime victimization, especially sexual assault, which had the strongest association. This supports the expansive literature linking sexual assault and a BPD diagnosis [19]. Second, there was a positive association between BPD and the death of a close family member, which has only been reported in studies involving the death of a parent in childhood [41]. Third, our study identified an underreported positive link between childhood poverty and poor neighborhood quality and subsequent BPD diagnosis [42]. Although the CIs overlapped, sex-separated analysis found that males had higher rates of traumatic events, except for sexual assault, fitting within previous literature on BPD symptoms and trauma [43].
The consistent time-varying results provide evidence for the theory of a close, cyclical relationship between trauma and BPD [1, 20]. Our sibling analysis found evidence to support an overlap in genetic and/or environmental etiology between traumatic events and BPD diagnosis, previously theorized to be present [44].
As expected, BPD individuals had higher instances of adverse behaviors: self-harm, violent and nonviolent crime. In line with previous findings, the most common criminal behaviors were aggressive in nature, such as making violent threats and assault [2, 4]. Additionally, our study identified that impulsive nonviolent crimes such as property damage or petty theft are more common than planned crimes, e.g., having fake identification.
Sex differences and time-varying findings
The HRs were largely uniform across sexes, even though the absolute proportions, i.e., cumulative incidences, sometimes differed substantially. This suggests that this disorder confers similar increase in rates of comorbidities between males and females. A diagnostic bias between the sexes could result in an inflated estimate for males with BPD, as males who receive the correct diagnosis might have a more severe symptom presentation.
Individuals with a sibling with BPD had higher rates of psychiatric disorders, trauma, and adverse behaviors but not somatic disorders. HRs were higher in individuals with a brother diagnosed with BPD compared to those with a sister diagnosed for the majority of indicators. Families with a male diagnosed with BPD appear to have a more severe phenotype and vulnerability to psychiatric disorders [45]. However, this must be interpreted with caution as the CIs overlapped for having a brother or sister diagnosed with BPD. Follow-up on these associations is needed.
As the associations for the time-varying analyses were largely consistent with the main analysis, ignoring time of BPD diagnosis did not introduce bias that invalidated our inferences.
Strengths, weaknesses
In the largest and most detailed BPD study to date, we were able to capture all Sweden-born individuals with BPD with prospective follow-up using national records. This considerable sample size allowed us to thoroughly examine a variety of understudied variables in a representative sample.
However, this study comes with caveats. First, although the NPR and BPD diagnostic codes are well-validated, the extent to which these comorbidities may be misdiagnosed is unclear [29, 32, 33]. However, one may argue that diagnoses, if not correctly diagnosed, may reflect levels of symptom presentation even if the full criteria of diagnosis is not met. Further, the prevalence of a BPD diagnosis in our sample (0.6%) is conservative compared to the projected median estimate of 1.7% [1]. This likely indicates that many individuals who meet the criteria for a BPD diagnosis remain undiagnosed. And, as a BPD diagnosis generally takes multiple, intensive clinical interviews, it is likely that we are capturing individuals with more severe BPD symptoms.
Next, we were only able to identify the instances of the indicators identifiable in the register, this means the true estimate of these incidences may be higher in under-reported or less severe cases. Some individuals with BPD may be less willing to seek certain types of medical care due to distrust or stigmatization from the health care system, which may attenuate our results [46]. Additionally, trauma is both an objective and deeply subjective experience; and we are unable to capture the subjective experience [21]. Finally, symptoms of BPD, like all psychiatric disorders, are continuously distributed across the population rather than a binary diagnosis. We are unable to examine specific BPD symptoms which limits the information gained from this study.
Future directions for research
This study identifies many avenues for needed research. The comorbidities between BPD and psychotic disorders should be examined further. Moreover, the association with death of a close family member opens the question of the heritability of premature mortality in families [47]. Also, studies should examine these associations in other severe psychiatric disorders, such as bipolar disorder, to determine what associations are specific to BPD rather than general to severe psychiatric disorders [48, 49]. Finally, further research should carefully expand on any causal assumptions about the nature of BPD.
Clinical implications
Our results indicate individuals with BPD frequently use health care services for a variety of psychiatric and somatic conditions. Unfortunately, despite their needs, the interpersonal difficulties and stigma surrounding individuals with BPD can be challenging for clinicians, which often results in poor care or premature ending of treatment [46, 50]. Thus, there is a clear need for support and advocates for individuals with BPD navigating the health care system. Clinicians should be aware of these difficulties within the health care system and offer adapted health education or referrals, e.g., a dietitian to prevent type 2 diabetes. Additionally, implementing already developed anti-stigmatization methods could help improve the doctor–patient relationship [50, 51].
Conclusions
BPD was associated with nearly all of the more than 30 indicators of psychiatric disorders, somatic illnesses, trauma, and adverse behavior. The associations were consistent across sex and temporality. This paper can serve as an atlas for associations within the aforementioned categories, many of which have previously been un- or under-reported, and can lead the way towards further causal and etiological research. Critically, the clinical implications indicate that increased support is needed for patients surrounding health care visits. It is hopeful that this provides the groundwork towards an understanding and increased awareness for this patient group.
Supplementary information
Author contributions
AET, RK-H, and HS conceptualized the study. AET completed the analysis and wrote the manuscript. SL, YL, SL, PL, HS, RK-H provided feedback on the conceptualization and manuscript.
Funding
Tate has received funding from the European Union’s Horizon 2020 Research and Innovation Program under the Marie Sklodowska-Curie CAPICE Project grant agreement number 721567. (https://www.capice-project.eu/) Lu is in part supported by a 2018 NARSAD Young Investigator Grant from the Brain & Behaviour Research Foundation and US NIMH (R01 MH123724). Open access funding provided by Karolinska Institute.
Competing interests
Larsson reports: Evolan (speaker); Shire (speaker, grant recipient); Takeda (speaker, grant recipient) all outside the submitted work. Tate, Sahlin, Liu, Lu, Lundström, Lichtenstein, Kuja-Halkola report no financial relationships with commercial interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01503-z.
References
- 1.Gunderson JG, Herpertz SC, Skodol AE, Torgersen S, Zanarini MC. Borderline personality disorder. Nat Rev Dis Prim. 2018;4:1–20.. doi: 10.1038/nrdp.2018.29. [DOI] [PubMed] [Google Scholar]
- 2.APA. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Association Publication; 2013.
- 3.Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004. [DOI] [PubMed]
- 4.Arola R, Antila H, Riipinen P, Hakko H, Riala K, Kantojärvi L. Borderline personality disorder associates with violent criminality in women: a population based follow-up study of adolescent psychiatric inpatients in Northern Finland. Forensic Sci Int. 2016;266:389–95. doi: 10.1016/j.forsciint.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Zanarini MC, Frankenburg FR, Hennen J, Reich DB, Silk KR. Axis I comorbidity in patients with borderline personality disorder: 6-year follow-up and prediction of time to remission. Am J Psychiatry. 2004;161:2108–14. doi: 10.1176/appi.ajp.161.11.2108. [DOI] [PubMed] [Google Scholar]
- 6.Czajkowski N, Aggen SH, Krueger RF, Kendler KS, Neale MC, Knudsen GP, et al. A twin study of normative personality and DSM-IV personality disorder criterion counts: evidence for separate genetic influences. Am J Psychiatry. 2018;175:649–56. doi: 10.1176/appi.ajp.2017.17050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Røysamb E, Neale M, et al. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychol Med. 2008;38:1617–25. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- 8.Skoglund C, Tiger A, Rück C, Petrovic P, Asherson P, Hellner C, et al. Familial risk and heritability of diagnosed borderline personality disorder: a register study of the Swedish population. Mol. Psychiatry. 2019:1–10. [DOI] [PMC free article] [PubMed]
- 9.Zapolski TC, Settles RE, Cyders MA, Smith GT. Borderline personality disorder, bulimia nervosa, antisocial personality disorder, ADHD, substance use: common threads, common treatment needs, and the nature of impulsivity. Independent. Practitioner. 2010;30:20. [PMC free article] [PubMed] [Google Scholar]
- 10.Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Personal Disord. 2014;28:734–50. doi: 10.1521/pedi_2012_26_093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welander-Vatn A, Ystrom E, Tambs K, Neale M, Kendler K, Reichborn-Kjennerud T, et al. The relationship between anxiety disorders and dimensional representations of DSM-IV personality disorders: a co-twin control study. J Affect Disord. 2016;190:349–56. doi: 10.1016/j.jad.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnow S, Arens EA, Sieswerda S, Dinu-Biringer R, Spitzer C, Lang S. Borderline personality disorder and psychosis: a review. Curr Psychiatry Rep. 2010;12:186–95. doi: 10.1007/s11920-010-0107-9. [DOI] [PubMed] [Google Scholar]
- 13.Doering S. Borderline personality disorder in patients with medical illness: a review of assessment, prevalence, and treatment options. Psychosom Med. 2019;81:584–94. doi: 10.1097/PSY.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 14.Østby KA, Czajkowski N, Knudsen GP, Ystrom E, Gjerde LC, Kendler KS, et al. Personality disorders are important risk factors for disability pensioning. Soc Psychiatry Psychiatr Epidemiol. 2014;49:2003–11. doi: 10.1007/s00127-014-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Gabalawy R, Katz LY, Sareen J. Comorbidity and associated severity of borderline personality disorder and physical health conditions in a nationally representative sample. Psychosom Med. 2010;72:641–7. doi: 10.1097/PSY.0b013e3181e10c7b. [DOI] [PubMed] [Google Scholar]
- 16.Sharma TR. Polycystic ovarian syndrome and borderline personality disorder: 3 case reports and scientific review of literature. J Psychiatry. 2015;18:1–4. doi: 10.4172/2378-5756.1000336. [DOI] [Google Scholar]
- 17.De Genna NM, Feske U, Larkby C, Angiolieri T, Gold MAPregnancies. abortions, and births among women with and without borderline personality disorder. Women’s Health Issues. 2012;22:e371–e377. doi: 10.1016/j.whi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sansone RA, Sansone LA. Borderline personality: a primary care context. Psychiatry. 2004;1:19. [PMC free article] [PubMed] [Google Scholar]
- 19.de Aquino Ferreira LF, Pereira FHQ, Benevides AMLN, Melo MCA. Borderline personality disorder and sexual abuse: a systematic review. Psychiatry Res. 2018;262:70–77. doi: 10.1016/j.psychres.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Conway CC, Boudreaux M, Oltmanns TF. Dynamic associations between borderline personality disorder and stressful life events over five years in older adults. Personal Disord: Theory Res Treat. 2018;9:521. doi: 10.1037/per0000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldwin JR, Degli Esposti M. Triangulating evidence on the role of perceived versus objective experiences of childhood adversity in psychopathology. JCPP Adv. 2021;1:e12010. doi: 10.1111/jcv2.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sansone RA, Sansone LA. Gender patterns in borderline personality disorder. Innov Clin Neurosci. 2011;8:16. [PMC free article] [PubMed] [Google Scholar]
- 23.Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69:533. doi: 10.4088/JCP.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnow S, Herpertz SC, Spitzer C, Stopsack M, Preuss UW, Grabe HJ, et al. Temperament and character in patients with borderline personality disorder taking gender and comorbidity into account. Psychopathology. 2007;40:369–78. doi: 10.1159/000106467. [DOI] [PubMed] [Google Scholar]
- 25.Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci. 2013;110:5258–62. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Källén B, Källén K. The Swedish Medical Birth Register—a summary of content and quality. 2003.
- 27.Careja R, Bevelander P. Using population registers for migration and integration research: examples from Denmark and Sweden. Comp Migr Stud. 2018;6:19. doi: 10.1186/s40878-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–73. doi: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;344:1–15. doi: 10.1007/s10654-019-00511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tampe U, Frank S, Weiss RJ, Jansson K-Å. Diagnosis of open tibial fracture showed high positive predictive value in the Swedish National Patient Register. Clin Epidemiol. 2020;12:1113. doi: 10.2147/CLEP.S271173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouppis E, Ekselius L. Validity of the personality disorder diagnosis in the Swedish National Patient Register. Acta Psychiatr Scand. 2020;141:432–8. doi: 10.1111/acps.13166. [DOI] [PubMed] [Google Scholar]
- 33.Kuja-Halkola R, Juto KL, Skoglund C, Rück C, Mataix-Cols D, Pérez-Vigil A, et al. Do borderline personality disorder and attention-deficit/hyperactivity disorder co-aggregate in families? A population-based study of 2 million Swedes. Mol Psychiatry. 2018;261:1–9. doi: 10.1038/s41380-018-0248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhaskaran K, Rachet B, Evans S, Smeeth L. Re: Helene Hartvedt Grytli, Morten Wang Fagerland, Sophie D. Fosså, Kristin Austlid Taskén. Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Beta-Blockers and Prostate Cancer Survival-Interpretation of Competing Risks Models. Eur Urol. 2022;64:e86–e87. doi: 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 36.Therneau T. A package for survival analysis in R. R package version 3.2-3. Computer software] Rochester, MN: Mayo Clinic. https://CRAN.R-project.org/package=survival. 2020.
- 37.Slotema CW, Daalman K, Blom JD, Diederen KM, Hoek HW, Sommer I. Auditory verbal hallucinations in patients with borderline personality disorder are similar to those in schizophrenia. Psychological Med. 2012;42:1873. doi: 10.1017/S0033291712000165. [DOI] [PubMed] [Google Scholar]
- 38.Strålin P, Hetta J. First episode psychosis: register-based study of comorbid psychiatric disorders and medications before and after. Eur Arch Psychiatry Clin Neurosci. 2020;271:303–13. doi: 10.1007/s00406-020-01139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momen NC, Plana-Ripoll O, Agerbo E, Benros ME, Børglum AD, Christensen MK, et al. Association between mental disorders and subsequent medical conditions. N Engl J Med. 2020;382:1721–31. doi: 10.1056/NEJMoa1915784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanska DJ. The Klüver-Bucy syndrome. Neurologic-psychiatric syndromes in focus—Part I. 41. Karger Publishers; 2018. p. 77–89. [DOI] [PubMed]
- 41.Weaver TL, Clum GA. Early family environments and traumatic experiences associated with borderline personality disorder. J Consulting Clin Psychol. 1993;61:1068. doi: 10.1037/0022-006X.61.6.1068. [DOI] [PubMed] [Google Scholar]
- 42.Cohen P, Chen H, Gordon K, Johnson J, Brook J, Kasen S. Socioeconomic background and the developmental course of schizotypal and borderline personality disorder symptoms. Dev Psychopathol. 2008;20:633–50. doi: 10.1017/S095457940800031X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amstadter AB, Aggen SH, Knudsen GP, Reichborn-Kjennerud T, Kendler KS. Potentially traumatic event exposure, posttraumatic stress disorder, and Axis I and II comorbidity in a population-based study of Norwegian young adults. Soc Psychiatry Psychiatr Epidemiol. 2013;48:215–23. doi: 10.1007/s00127-012-0537-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White CN, Conway CC, Oltmanns TF. Stress and personality disorders. The Oxford handbook of stress and mental health 2019:183.
- 45.Neale MC, Røysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G × E interaction. Twin Res Hum Genet. 2006;9:481–9. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall K, Moran P. Borderline personality disorder: an update for neurologists. Pract Neurol. 2019;19:483–91. doi: 10.1136/practneurol-2019-002292. [DOI] [PubMed] [Google Scholar]
- 47.Temes CM, Frankenburg FR, Fitzmaurice GM, Zanarini MC. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80:0–0. doi: 10.4088/JCP.18m12436. [DOI] [PubMed] [Google Scholar]
- 48.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2:119–37. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kjær JN, Biskin R, Vestergaard C, Munk-Jørgensen P. All-cause mortality of hospital-treated borderline personality disorder: a nationwide cohort study. J Personal Disord. 2020;34:723–35. doi: 10.1521/pedi_2018_32_403. [DOI] [PubMed] [Google Scholar]
- 50.Healthcare Management Forum. Mental illness-related stigma in healthcare: barriers to access and care and evidence-based solutions. Healthc Manage Forum 2017. SAGE Publications Sage CA: Los Angeles, CA. [DOI] [PMC free article] [PubMed]
- 51.Knaak S, Szeto AC, Fitch K, Modgill G, Patten S. Stigma towards borderline personality disorder: effectiveness and generalizability of an anti-stigma program for healthcare providers using a pre-post randomized design. Borderline Personal Disord Emot Dysregul. 2015;2:1–8. doi: 10.1186/s40479-014-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.