Abstract
Coronavirus Disease 2019 (COVID-19) has been a significant cause of global mortality and morbidity since it was first reported in December 2019 in Wuhan, China. COVID19 like previous coronaviruses primarily affects the lungs causing pneumonia, interstitial pneumonitis, and severe acute respiratory distress syndrome (ARDS). However, there is increasing evidence linking COVID-19 to cardiovascular complications such as arrhythmias, heart failure, cardiogenic shock, fulminant myocarditis, and cardiac death. Given the novelty of this virus, there is paucity of data on some cardiovascular complications of COVID-19, specifically myocarditis. Myocarditis is an inflammatory disease of the heart muscle with a heterogenous clinical presentation and progression. It is mostly caused by viral infections and is the result of interaction of the virus and the host's immune system. There have been several case reports linking COVID-19 with myocarditis, however the true mechanism of cardiac injury remains under investigation. In this paper we review the clinical presentation, proposed pathophysiology, differential diagnoses and management of myocarditis in COVID-19 patients.
Introduction
Coronaviruses (CoVs) are a family of human and animal viral pathogens who target multiple organ systems including the respiratory and cardiovascular systems.1 , 2 Zoonotic transmission of CoVs have been responsible for previous outbreaks of severe acute respiratory syndrome (SARS)-CoV and Middle East respiratory syndrome (MERS)-CoV.1 , 3
Novel Coronavirus Disease 2019 (COVID-19) was first reported in December 2019 in Wuhan, China and as of May, 2022, has led to greater than 500 million cases worldwide with greater than 6 million deaths. In the US there has been over 81million reported cases and greater than 900,000 deaths.4
Myocarditis is a known complication associated with viral illnesses and is the result of interaction of the viral and the host's immune system. Mortality rates with myocarditis vary widely depending on the etiology, subclass and degree of cardiac dysfunction developed. however, some studies have demonstrated a 5-year mortality as high as 56% in endomyocardial biopsy proven myocarditis.5 While a broad range of infectious etiologies are associated with myocarditis, preceding viral infection is most common in the development of myocarditis.6 Myocarditis can clinically mimic acute coronary syndrome, however, by definition, myocarditis is myocyte injury in the absence of obstructing coronary lesions.
While acute respiratory distress was documented and studied in both SARS and MERS, there is scant data on cardiovascular complications from these viruses. Specifically, the true incidence of COVID-19 induced myocarditis remains unknown but there have been several case reports on myocarditis or myocardial injury.7 , 8 Observational studies of COVID-19 patients report myocardial damage, often in the absence of obstructing coronary disease that is suggestive of myocarditis, cardiogenic shock and ventricular arrhythmias.9 , 10
We present a detailed review of the clinical presentation of these patients with respect to the broad differential diagnoses, inpatient management, and the pathophysiology of myocarditis in COVID-19 patients.
Pathophysiology
The pathophysiology of viral myocarditis can be described in four distinct phases. The first phase is direct myocardial injury, during which the virus binds to its receptor on the cardiomyocyte. This leads to endocytosis of the virus and subsequent degradation of cellular structures and overall myocyte injury.11, 12, 13, 14, 15 Release of proinflammatory cytokines and activation of the innate immune system occurs because of this cardiac injury. Most patients recover fully after this phase.12 Phase 2 involves viral clearing via release of inflammatory cytokines such as interferon-ɣ, tumor necrosis factor (TNF), interferon 1 & 2.11 , 14 , 15 These inflammatory cytokines however are also responsible for the development of myocarditis, in part induced by immune dysregulation and molecular mimicry.13 , 15 Molecular mimicry is caused by similarities between viral antigens and cardiac antigens resulting in an activation of autoreactive T-cells. The third phase is acquired immune response, where CD4+ T helper (Th) cells and cytotoxic CD8+ cells are recruited to the site of injury and exercise their effect by lysing virus infected cells and neutralizing antiviral antibodies and autoantibodies.14 , 15 In the final stage, the virus is either cleared effectively with the resolution of inflammation or there is ineffective viral clearance leading to persistent low level inflammation with concomitant myocardial remodeling and left ventricular dilation known as inflammatory cardiomyopathy.11, 12, 13, 14, 15
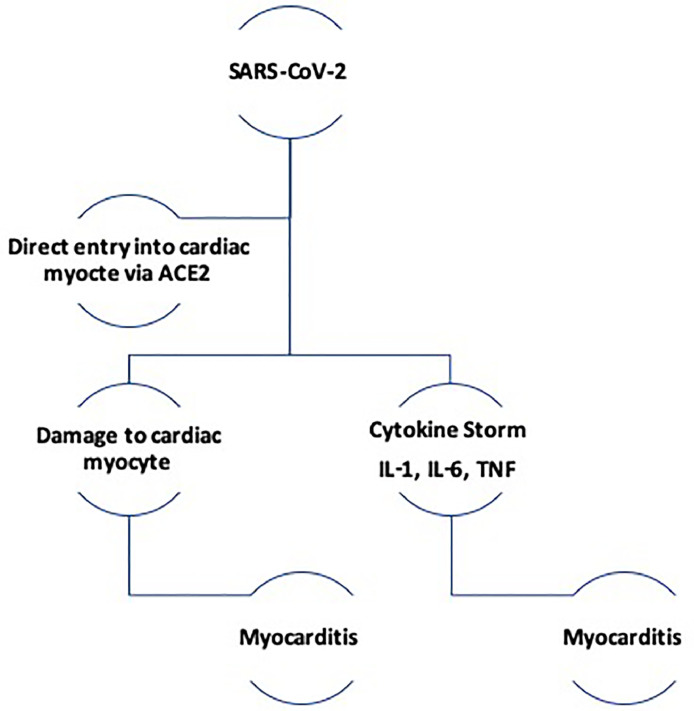
Proposed pathophysiology of COVID 19 myocarditis
There have been several case reports linking COVID-19 with myocarditis, however the true mechanism of cardiac injury remains under investigation. Different mechanisms have been postulated to explain the COVID-19 related cardiac injury including direct myocardial injury mediated via ACE2, immune dysregulation mediated by cytokine storm and hypoxia from imbalance in oxygen supply and demand.15, 16, 17(Fig 1 ).
FIG 1.
Proposed pathophysiology of COVID-19 induced myopericarditis.
Direct Myocardial Injury
SARS-CoV-2 infects host cells by binding its surface spike protein to the human ACE2 receptor. Prior to binding, the spike protein must be activated by transmembrane protease serine 2 (TMPRSS2), this allows for fusion of viral and cellular membranes thereby facilitating viral entry into the cell.16, 17, 18 It is proposed that interaction of SARS-CoV-2 and the ACE2 receptor can lead to alteration of ACE2 pathway and consequential cardiac injury. Similarly, previous research on SARS demonstrated that ACE2 dependent SARS-COV infection led to a decreased myocardial ACE2 protein expression and was associated with macrophage infiltration and myocardial damage.19
Immune dysregulation & Cytokine Storm
Multiple studies have shown an increase in proinflammatory cytokines such as IL-2, IL-6, IL-7, tumor necrosis factor - a (TNF-a), C-X-C motif cytokine 10 (CXCL10), and C-C motif ligand 2 (CCL2) in patients with severe COVID-19 infection.15 , 16 The release of these cytokines into circulation in what is described as a cytokine release syndrome (CRS) leads to an overactivation of T-lymphocytes that can result in myocardial damage. Elevated serum levels of IL-6 observed in CRS, has been identified as a predictor of mortality and morbidity in retrospective studies from COVID-19.15
Clinical Manifestations
The clinical signs of SARS-COV-2 myocarditis are highly variable, and often nonspecific, making it challenging to distinguish it from other cardiopulmonary conditions. The presenting complaints are usually part of a continuum of symptoms associated with the viral prodrome. These may include fever, myalgias, diarrhea, nausea, and other respiratory symptoms. In a retrospective study of SARS-COV-2 infected patients with possible myocarditis, dyspnea, chest tightness, cough and fever were the most common symptoms at presentation.8 , 20
Some patients may also exhibit a more severe clinical presentation including ventricular arrhythmia, pericardial effusion or fulminant myocarditis with subsequent cardiogenic shock and death.7 , 21 , 22 In a single center retrospective study of 112 patients with SARS-COV-2 infection, 12.5% had abnormalities suggestive of myocarditis.23 SARS-COV-2 myocarditis can occur unexpectedly at any stage during hospitalization and is increasingly being recognized as a late complication that can develop after improvements in a patient's respiratory condition.7 Therefore, a high index of suspicion for myocarditis is required to allow for prompt investigation and timely initiation of appropriate treatment.
Diagnosis
Electrocardiography (ECG)
Arrhythmias are a common occurrence in SARS-CoV-2. A retrospective study out of Wuhan, China, Hubei showed that among 138 COVID-19 patients enrolled, arrhythmia was observed in 7% of patients who did not require ICU treatment and in 44% of patients who did require ICU admission.24 These arrhythmias included but were not limited to, atrial fibrillation, conduction block, ventricular tachycardia, and ventricular fibrillation.24 In addition to arrhythmias, several studies and case reports have showed findings of dynamic diffuse ST Elevation or T wave inversion as was also seen with MERS-CoV.25, 26, 27 Conventionally, ST changes occur as a result of atherothrombotic type 1 MI or type 2 MI, vasospasms and acute pericarditis. However, recent data suggest that dynamic ST elevation can be observed in SARS-CoV-2 likely related to myopericarditis.26 In this regard, ECG remains a very important and readily available test that allows for timely recognition of life-threatening conduction block abnormalities and arrhythmias.28 Despite its low sensitivity, it is a useful test for risk stratification.
Transthoracic Echocardiography
Echocardiographic findings of myocarditis may include reduced left ventricular ejection fraction (LVEF) (<50%), segmental wall motion abnormalities, or left ventricular wall thickening (>10 mm) and/or presence of pericardial effusion (≥5 mm).29 , 30 Case reports of patients with confirmed SARS-COV-2 and suspected myocarditis have documented the presence of pericardial effusion, left ventricular systolic dysfunction, and wall motion abnormalities.31 , 32 Although these findings are nonspecific, they are often crucial for proper diagnosis and prognosis.
Cardiac MRI
Cardiac MRI (CMR) is also useful in diagnosing myocarditis in COVID-19 patients most especially among young patients. One of the earlier cases of COVID associated myocarditis documented by CMR was in a young adult who tested positive for COVID and presented with chest pain and fatigue without fever or cough or elevated troponin and normal left ventricular ejection fraction by echocardiography.33 Cardiac MRI showed late subepicardial late gadolinium enhancement predominantly in the inferior and lateral walls.33
Coronary Angiography
The role of coronary angiography in the diagnosis of SARS-CoV-2 induced myocarditis is to rule out coronary pathology and most importantly type 1 myocardial infarction. However, performing coronary angiography during the COVID-19 pandemic has posed unique challenges.34 To reduce the spread of infection, elective catheterizations are being deferred and the risk of staff exposure vs patient benefit is weighed individually. In stable COVID 19 patients presenting with STEMI or NSTEMI conservative management such as fibrinolytic therapy is now being considered in lieu of percutaneous coronary intervention.35 This creates a diagnostic challenge as up to 17% of patients hospitalized with COVID-19 may present with acute myocardial injury.36 Distinguishing between acute myocarditis and acute coronary syndrome can be challenging particularly when angiography cannot be performed.35 A study of COVID-19 patients with suspected STEMI who underwent coronary angiography showed that approximately 40% of the cases had no identifiable culprit lesion.36 However, it was unclear if this presentation was due to SARS-COV-2 induced myocarditis or coronary vasospasm.35 In patients with clinical presentation of myocardial infarction without evidence of obstructive coronary disease on angiography, myocarditis is a likely diagnosis given previous studies have shown up to 40% of patients with the former presentation have diffuse myocarditis or focal myocarditis.36
Histology/Endomyocardial Biopsy
Per gold standard diagnostic test set by the “Dallas Criteria” in 1987, myocarditis is diagnosed based on endomyocardial biopsies (EMB).37 Active myocarditis requires the presence of inflammatory infiltrates of non-ischemic origin in myocardial tissue associated with necrosis and/or degeneration of adjacent cardiomyocytes.38 Previously, biopsy proven studies of myocarditis have shown that viral antigens such as Enteroviruses, Adenovirus, Parvovirus, and Human Herpes Virus genomics persist in the myocardium postinfectivity state.39 , 40
Only limited reports exist on the histological presentation of SARS-CoV-2 related myocarditis.
Earlier this year, a case of a 43-year-old COVID-19 patient with diagnosed acute myocarditis was reported in whom the EMB results were consistent with myocarditis with evidence of myocardial inflammation and diffuse T-lymphocytic inflammatory infiltrates, interstitial edema, and limited focal necrosis.41 More recently, biopsy proven viral genomes was detected in a child diagnosed with COVID-19-related multisystem inflammatory syndrome.42
Management
Patients with left ventricular dysfunction should be treated with current guideline recommended heart failure therapy including angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs)/angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoids receptor antagonists, and beta blockers.43 However, caution should be used in the acute setting and one should insure hemodynamic stability before starting these therapies.43 Temporary mechanical circulatory support is often used in cases of fulminant viral myocarditis with clinical deterioration despite optimal pharmacological therapy.44 , 30
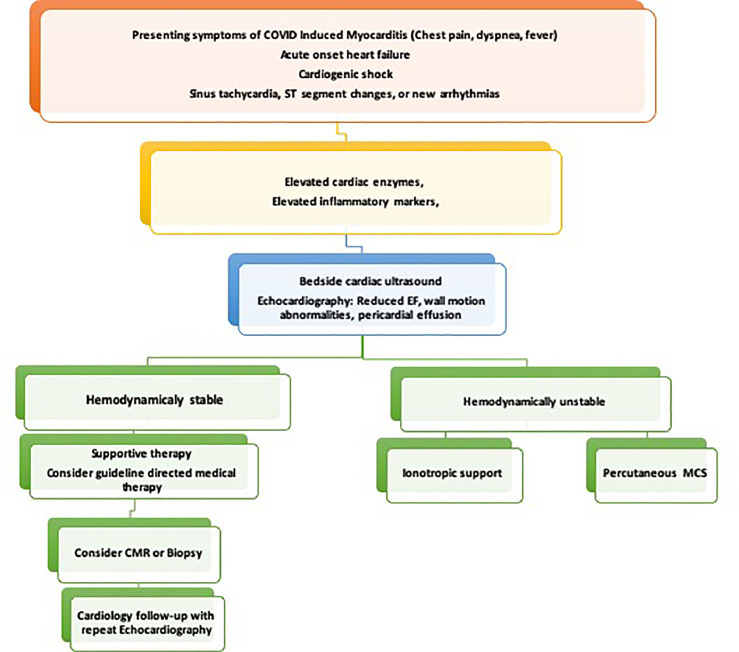
Similarly, data on treatment of myocarditis is limited and current management consists of symptomatic supportive treatment and experimental drug therapies. A multidisciplinary team (shock team) approach is recommended to improve patient outcomes. Treatment algorithms have been suggested to assist with diagnosis, risk stratification, and to aid with clinical decision making (Fig 2 ).44 , 30
FIG 2.
Flow chart for evaluation and management of suspected COVID-19 induced myocarditis.
Recently Investigated Therapies for COVID 19 induced Myocarditis
Based on the proposed mechanisms of COVID 19 induced myocardial injury, various clinical therapies have been investigated including anti-inflammatory agents, antivirals, and cytokine and immune modulators however randomized trials supporting the sue of specific therapies are still lacking.
Colchicine
Colchicine has been used as a nonsteroidal anti-inflammatory treatment, particularly in the setting of pericarditis and gout. It has been hypothesized to have potential antiviral effects based on prior studies in which treatment showed decreased levels of cytokines including interleukins (IL) 1 and 6, granulocyte macrophage colony stimulating factor, and the nucleotide-binding oligomerization leucine-rich repeat and pyrin domain (NLRP3) inflammasome.45 , 46 It is suggested that treatment with colchicine therefore may be beneficial in suppressing the cytokine storm that results in ARDS secondary to COVID-19 infection.46
The recently concluded GRECCO-19 trial47 sought to evaluate the effect of colchicine on cardiac and inflammatory biomarkers in the setting of hospitalized COVID-19 patients. This open label randomized clinical trial in Greece randomized patients in a 1:1 allocation to either standard medical treatment or colchicine with standard medical treatment. The study showed similar biochemical evidence of myocardial injury, based on high sensitivity troponin, between the 2 groups but those that received colchicine had a statistically significant reduced rate of clinical deterioration.47 The results showed similar levels of C-reactive protein (CRP) but did show a significant reduction in dimerized plasma fragment D (D-Dimer) levels in the treatment group which raises the possibility of an antithrombotic in addition to an anti-inflammatory mechanism.47
Corticosteroids
Although corticosteroids have been used empirically in severe cases of patients with biopsy proven myocarditis48 , 49 the benefit of this approach in COVID 19 myocarditis remain unknown.
The RECOVERY trial50 demonstrated accelerated ventilation weaning in addition to reduced mortality in patients with ARDS secondary to COVID-19 with short term use of dexamethasone.
In the dexamethasone group, 482 patients (22.9%) compared to 1110 patients (25.7%) in the usual care group died within 28 days after randomization (age-adjusted rate ratio, 0.83; 95% confidence interval [CI], 0.75-0.93; P < 0.001).50 In those receiving invasive mechanical ventilation, the differences were more pronounced. In the dexamethasone group, the incidence of death was lower than that in the usual care group among patients receiving invasive mechanical ventilation (29.3% vs 41.4%; rate ratio, 0.64; 95% CI, 0.51-0.81).50 Interestingly, no clear benefit of dexamethasone was shown among patients who did not require oxygen at randomization (17.8% vs 14.0%; rate ratio, 1.19; 95% CI, 0.91-1.55).50 Despite these promising results, more data is needed to better understand the role of corticosteroids in COVID-19 induced myocarditis.
Intravenous Immunoglobulin (IVIG)
IVIG has shown benefit in various inflammatory settings, both clinically and experimentally. Suggested mechanisms of action include anti-inflammatory effects and neutralization of toxins, oxidative stress, and reduction of complement factors and cytokines.51 IVIG has been showed to reduce parvovirus B19 viral load, improve ejection fraction, and improve NYHA classification in patient infected with Parvovirus B19 dilated cardiomyopathy.52 IVIG has been associated with improvement in LVEF only when used in combination with other treatment modalities but not as a monotherapy.53 , 54
Considering the proposed association between COVID 19 induced myocarditis and autoantibodies, Yang et al used IVIG in 28/52 ICU patients (53.8%).55 Shi et al noted use of IVIG was higher in patients with cardiac injury (68 [82.9%] vs 191 [57.2%]) compared to patient without cardiac injury.56 However, neither study was able to show statistically significant benefit in the use of IVIG.
Cytokine Modulators
Given the known association between cytokine activation and COVID 19 mediated inflammatory response, various cytokine inhibitors have been studied including Toclizumab, Siltuximab, and Sarilumab.57 These are all interleukin 6 inhibitors and may have a potential therapeutic role in COVID 19 induced myocarditis.57 In the REMAP-CAP study,58 Toclizumab and Sarilumab were shown to have some efficacy with improved outcomes, including mortality in critically ill patients.58 However, the role of these medications in cytokine induced myocardial injury remains unclear and until more substantial data is accessed on the clinical efficacy of these therapies, it should only be used in an individualized case by case basis.
Temporary Mechanical Circulatory Support
Fulminant viral myocarditis is rare and the use of temporary mechanical circulatory support (MCS) devices has only been documented in case reports without support of randomized control trials59, 60, 61. However, several case series have shown that MCS may be useful as a bridge to recovery.59, 60, 61, 62 In the event of non-recoverable myocardial injury, MCS can be a short-term stabilizing support system as a bridge to cardiac transplantation or permanent durable left ventricular assist device (LVAD) placement.62, 63, 64, 65
In one of the early reports of COVID-19 fulminant myocarditis,27 the patient was treated with antivirals and mechanical ventilation. Ultimately the patient required veno-venous (VV) ECMO for hypoxia. After being treated with these supportive measures, the LVEF returned to normal. The patient ultimately succumbed to secondary infection and septic shock.27
Unique Challenges of COVID 19 Myocarditis
Delay in Diagnosis
Early diagnosis of COVID 19 myocarditis relies on our clinical skills and judgment in identifying patients who are at risk.32 , 34 Diagnosing COVID 19 myocarditis can be challenging especially in the setting of acute patient decompensation or inability to perform key diagnostic tests such as cardiac MRI or endomyocardial biopsy.34 Cardiac biomarkers can be helpful in the diagnosis but fail to differentiate between myocarditis secondary to infection of cardiac tissue from myocardial injury related to ischemia.66 Therefore we believe in initiation of empiric therapy based on clinical suspicion.
Ethical Considerations
There is an inherent duty to care for patients, particularly during the setting of a pandemic. Yet, we are placed at a high risk to provide patient care and have a duty to care for ourselves and loved ones to prevent the spread of disease. This includes judicious use of personal protective equipment. The ASE (American society of Echocardiography) released a COVID statement regarding who and when to order an ECHO.67 Briefly, the statement recommends imaging those in which imaging is expected to provide clinical benefit which includes following established appropriate use criteria (AUC).67 Transesophageal echocardiography (TEE) has a heightened risk of spreading COVID 19 and may provoke aerosolization of the virus due to coughing of gagging during examination.68 With use of QTC prolonging medications, often in combination, there is increased personnel exposure to EKG technicians due to necessity for serial ECGs and equipment exposure, further straining limited supply of personal protective equipment. Some FDA-approved consumed mobile ECG devices are capable of generating accurate QTc measurements and therefore may be a reasonable alternative during the COVID-19 pandemic to minimize patient and health exposure and improve social distancing measures.69
Myocarditis after Vaccination
Cases of acute myocarditis have been reported after a variety of vaccinations.70, 71, 72, 73 Smallpox and seasonal influenza vaccines have resulted in rare cases of vaccine-associated immune eosinophilic myocarditis in healthy individuals who received these vaccines.71 Recently, there have been reported cases of acute myocarditis after mRNA covid-19 vaccine administration.73, 74, 75 According to the US Center for Disease Control and Prevention, it commonly occurs in males with the highest frequency between ages 12-29 following 2nd dose with an incidence of 0.48 cases per 100,000.72 Similar pattern was reported in a study conducted at a large healthcare organization in Israel although at a higher estimated incidence of 2.13 cases per 100,000.74 In Australia, there were 50 reports of suspected myocarditis and/ or pericarditis out of 288 total adverse events after 3.7 million doses of Pfizer mRNA vaccines as at July 11th 2021.75 Most cases were mild or moderate in severity.74 , 75 A high index of suspicion is required in identifying patients presenting with acute symptoms following covid-19 vaccination. Typical presentations include chest pain in 85-95% of cases, dyspnea in 19%-49% and syncope in 6% of cases with fever accompanying these symptoms in 65% of patients.76 There is uncertainty regarding a causal relationship between vaccinations and myocarditis. Potential mechanisms such as hypersensitivity to vaccine components, inflammatory reaction, or inappropriate activation of the immune system have been proposed. However, it is difficult to establish a true causal relationship due to the rarity of this cases.76
Future Directions
Our understanding of the cardiovascular effects of COVID-19 continues to improve and should translate into better diagnostic, therapeutic and management protocols for patients. While there have been case reports on COVID-19 induced myocarditis,27 , 32 , 33 the true incidence remains unknown. Hence, the importance of a registry at this time cannot be understated as this will assist in collection of structured data to aid research and inform the medical community.
Given that most patients with acute myocarditis require supportive treatment, patients who do develop symptomatic heart failure would require current guideline-directed medical therapy. However, further research is hereby warranted to assess the role of these medications in COVID-19 patients. Additionally, the role of antiviral agents and immunosuppressive drugs in COVID-19 are currently under investigation. It is our hope that ongoing clinical trials may provide support for immunosuppressive therapies in myocardial inflammation.
Finally, due to the novelty of this disease, the long-term complications that may arise from COVID-induced myocarditis remain unknown. The assumption from traditional viral myocarditis is that there is a possibility of long term complications such as progressive heart failure with dilatated cardiomyopathy, and in some cases, the need for ventricular assist devices and heart transplantation. Therefore, future research studies focusing on outcome of COVID 19 myocarditis patients is paramount.
Conclusion
Myocarditis is an important consideration for patients with COVID-19 given the propensity for significant morbidity and mortality. Different mechanisms have been proposed to explain the COVID-19 related cardiac injury including direct myocardial injury mediated via ACE2, immune dysregulation mediated by cytokine storm and hypoxia from imbalance in oxygen supply and demand. Given that there may be other concomitant cardiovascular complications with similar presentations associated with COVID-19, other diagnosis such as Acute Coronary Syndrome and Takotsubo syndrome/Stress cardiomyopathy must be considered. Currently, treatment remains mostly supportive, in cases of left ventricular dysfunction current heart failure guidelines should be applied. There may also be a role for mechanical circulatory support & extracorporeal membrane oxygenation as a bridge to recovery in patients who present with acute cardiogenic shock. We hope that ongoing clinical trials may provide more direction regarding the pathophysiology of this disease and targeted therapeutic options.
Footnotes
Financial disclosure: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest: None.
References
- 1.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Wilde AH, Snijder EJ, Kikkert M, et al. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge X-Y, Li J-L, Yang X-L, et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Map. Johns Hopkins coronavirus resource center. Accessed October 24, 2022. https://coronavirus.jhu.edu/map.html
- 5.Sagar S, Liu PP, Cooper LT. Myocarditis. Lancet Lond Engl. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol Mech Dis. 2008;3(1):127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 7.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;27 doi: 10.1001/jamacardio.2020.1096. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim I-C, Kim JY, Kim HA, et al. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochi AN, Tagliari AP, Forleo GB, et al. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grogan M, Redfield MM, Bailey KR, et al. Long-term outcome of patients with biopsy-proved myocarditis: comparison with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1995;26:80–84. doi: 10.1016/0735-1097(95)00148-s. [DOI] [PubMed] [Google Scholar]
- 11.Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52:274–288. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. Eur Heart J. 2008;29:2073–2082. doi: 10.1093/eurheartj/ehn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack A, Kontorovich AR, Fuster V, et al. Viral myocarditis–diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 14.Shauer A, Gotsman I, Keren A, et al. Acute viral myocarditis: current concepts in diagnosis and treatment. Isr Med Assoc J IMAJ. 2013;15:180–185. [PubMed] [Google Scholar]
- 15.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2022 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clerkin KJ., Fried J.A., Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 17.Basu-Ray I, Soos MP. StatPearls. StatPearls Publishing; 2020. Cardiac manifestations of coronavirus (COVID-19)http://www.ncbi.nlm.nih.gov/books/NBK556152/ Accessed June 12020. [PubMed] [Google Scholar]
- 18.Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;0 doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng J-H, Liu Y-X, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;10:1–5. doi: 10.1007/s15010-020-01424-5. Published online April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu H, Ma F, Wei X, et al. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020;16 doi: 10.1093/eurheartj/ehaa190. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K, Fang Y-Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;27 doi: 10.1001/jamacardio.2020.1096. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng J-H, Liu Y-X, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;10:1–5. doi: 10.1007/s15010-020-01424-5. Published online April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seecheran R, Narayansingh R, Giddings S, et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620925571. 2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. PMID: 32556199; PMCID: PMC7337658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kociol RD, Cooper LT, Fang JC, et al. Recognition and initial management of fulminant myocarditis. Circulation. 2020;141 doi: 10.1161/CIR.0000000000000745. e69 e92. [DOI] [PubMed] [Google Scholar]
- 31.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul JF, Charles P, Richaud C, Caussin C, Diakov C. Myocarditis revealing COVID-19 infection in a young patient. Eur Heart J. 2020;21:776. doi: 10.1093/ehjci/jeaa107. VolumeIssuePage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from the ACC's Interventional Council and SCAI. J Am Coll Cardiol. 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefanini GG, Montorfano M, Trabattoni D, et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarda L, Colin P, Boccara F, et al. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol. 2001;37:786–792. doi: 10.1016/s0735-1097(00)01201-8. [DOI] [PubMed] [Google Scholar]
- 37.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 38.Shauer A, Gotsman I, Keren A, et al. Acute viral myocarditis: current concepts in diagnosis and treatment. Isr Med Assoc J IMAJ. 2013;15:180–185. [PubMed] [Google Scholar]
- 39.Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 40.Matthias Pauschinger, Andrea Doerner, Uwe Kuehl, et al. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation. 1999;99(7):889–895. doi: 10.1161/01.CIR.99.7.889. [DOI] [PubMed] [Google Scholar]
- 41.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolhnikoff M, Ferranti JF, Monteiro RA de A, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;0 doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Writing Committee. Gluckman TJ, Bhave NM, et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;79:1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ammirati E., Frigerio M., Adler E.D., et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasiripour S, Zamani F, Farasatinasab M. Can colchicine as an old anti-inflammatory agent be effective in COVID-19? J Clin Pharmacol. 2020 doi: 10.1002/jcph.1645. Published online May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gendelman O, Amital H, Bragazzi NL, Watad A, Chodick G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: Insights from a large healthcare database analysis. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.1313. e2013136-e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chau V.Q., Giustino G., Mahmood K., et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007485. [DOI] [PubMed] [Google Scholar]
- 49.Hekimian G., Kerneis M., Zeitouni M., et al. Coronavirus disease 2019 acute myocarditis and multisystem inflammatory syndrome in adult intensive and cardiac care units. Chest. 2021;159:657–662. doi: 10.1016/j.chest.2020.08.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maisch B, Alter P. Treatment options in myocarditis and inflammatory cardiomyopathy : focus on i. v. immunoglobulins. Herz. 2018;43:423–430. doi: 10.1007/s00059-018-4719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dennert R, Velthuis S, Schalla S, et al. Intravenous immunoglobulin therapy for patients with idiopathic cardiomyopathy and endomyocardial biopsy-proven high PVB19 viral load. Antivir Ther. 2010;15:193–201. doi: 10.3851/IMP1516. [DOI] [PubMed] [Google Scholar]
- 53.Kishimoto C, Shioji K, Hashimoto T, et al. Therapy with immunoglobulin in patients with acute myocarditis and cardiomyopathy: analysis of leukocyte balance. Heart Vessels. 2014;29:336–342. doi: 10.1007/s00380-013-0368-4. [DOI] [PubMed] [Google Scholar]
- 54.McNamara DM, Holubkov R, Starling RC, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Zhu K, Dai R, et al. Specific interleukin-1 inhibitors, specific interleukin-6 inhibitors, and GM-CSF blockades for COVID-19 (at the edge of sepsis): a systematic review. Front Pharmacol. 2022;12 doi: 10.3389/fphar.20. Published 2022 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.REMAP-CAP Investigators. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. Epub 2021 Feb 25. PMID: 33631065; PMCID: PMC7953461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howell E, Paivanas N, Stern J, Vidula H. Treatment of acute necrotizing eosinophilic myocarditis with immunosuppression and mechanical circulatory support. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003665. [DOI] [PubMed] [Google Scholar]
- 60.Steinhaus D, Gelfand E, VanderLaan PA, Kociol RD. Recovery of giant-cell myocarditis using combined cytolytic immunosuppression and mechanical circulatory support. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2014;33:769–771. doi: 10.1016/j.healun.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 61.Raasch H, Simpson RJ. Biventricular assist device terminates polymorphic ventricular tachycardia in giant cell myocarditis. Tex Heart Inst J. 2012;39:719–721. [PMC free article] [PubMed] [Google Scholar]
- 62.den Uil CA, Akin S, Jewbali LS, et al. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: a systematic review and meta-analysis. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2017;52:14–25. doi: 10.1093/ejcts/ezx088. [DOI] [PubMed] [Google Scholar]
- 63.Asaumi Y, Yasuda S, Morii I, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005;26:2185–2192. doi: 10.1093/eurheartj/ehi411. [DOI] [PubMed] [Google Scholar]
- 64.Diddle JW, Almodovar MC, Rajagopal SK, Rycus PT, Thiagarajan RR. Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Crit Care Med. 2015;43:1016–1025. doi: 10.1097/CCM.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 65.Lorusso R, Centofanti P, Gelsomino S, et al. Venoarterial extracorporeal membrane oxygenation for acute fulminant myocarditis in adult patients: a 5-year multi-institutional experience. Ann Thorac Surg. 2016;101:919–926. doi: 10.1016/j.athoracsur.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 66.Stefanini GG, Chiarito M, Ferrante G, et al. COVID-19 Task Force. Early detection of elevated cardiac biomarkers to optimize risk stratification in patients with COVID-19. Heart. 2020;106:1512–1518. doi: 10.1136/heartjnl-2020-317322. Epub 2020 Aug 14. PMID: 32817312. [DOI] [PubMed] [Google Scholar]
- 67.ASE Statement on COVID-19. Accessed August 2, 2020. https://www.asecho.org/ase-statement-covid-19/.
- 68.Bracco D. Safe(r) transesophageal echocardiography and COVID-19. Can J Anaesth. 2020:1–3. doi: 10.1007/s12630-020-01667-8. Published online April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hudson B, Mantooth R, DeLaney M. Myocarditis and pericarditis after vaccination for COVID-19. J Am Coll Emerg Physicians Open. 2021;2:e12498. doi: 10.1002/emp2.12498. Jul 26PMID: 34337595; PMCID: PMC8313036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckart RE, Love SS, Atwood JE, et al. Department of Defense Smallpox Vaccination Clinical Evaluation Team. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–205. doi: 10.1016/j.jacc.2004.05.004. PMID: 15234435. [DOI] [PubMed] [Google Scholar]
- 72.Wallace, M., Oliver, S., & Meeting, A. (2021). cdc.gov/coronavirus COVID-19 mRNA vaccines in adolescents and young adults: benefit-risk discussion.
- 73.Pepe S, Gregory AT, Myocarditis Denniss AR. Pericarditis and Cardiomyopathy After COVID-19 Vaccination. Heart Lung Circ. 2021;30:1425–1429. doi: 10.1016/j.hlc.2021.07.011. Epub 2021 Jul 31. PMID: 34340927; PMCID: PMC8324414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;0 doi: 10.1056/NEJMoa2109730. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110737. NEJMoa2110737Epub ahead of print. PMID: 34614329; PMCID: PMC8531986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]