Abstract
Background
SARS-CoV-2 continues to spread despite fast vaccine rollout, which could be attributed to waning immunity or to a reduced protection against some variants. A thorough characterization of vaccine protection and its duration in time is needed to inform vaccination policies and enhance public trust.
Methods
We linked three national databases with exhaustive information on screening, vaccination and hospitalizations in France from January 1st to December 12, 2021. We performed a two-step analysis to estimate vaccine effectiveness against severe outcomes of Covid-19 (requiring hospitalization) in people aged 50 years or over, combining: (i) a test-negative case–control design to assess vaccine effectiveness against symptomatic infections; and (ii) a survival analysis to assess the additional protection against severe outcomes (hospitalizations, ICU admissions and inpatient deaths) in infected individuals.
Findings
We found a high vaccine effectiveness in people aged 50 years or more, reaching 82% against symptomatic infections and 94% against hospitalizations, after a full vaccination scheme with the Covid-19 vaccines used in France.
Vaccine effectiveness against symptomatic infections decreased over time, dropping to 53% after six months, but remained high against severe outcomes (90% after six months). The booster dose allowed restoring protection levels above 90% against symptomatic infections. Vaccine protection and its evolution in time, showed little difference against the variants that circulated prior to December 2021 in France, including the Delta variant.
Interpretation
Though vaccine immunity decreases over time, vaccination remains crucial to provide individual protection against severe outcomes requiring hospitalization. This decline can be reversed by the receipt of a booster dose.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Effectiveness, Booster, Real-world estimates, Test-negative design
Introduction
Vaccination against Covid-19 began in late December 2020 in France. By the end of 2021, the full vaccination coverage reached 77% overall (datavaccin), and 91% for people aged 18 and over. Meanwhile, the French territory was hit by three epidemic waves, defined by an acceleration of Covid-19 infections and hospitalizations, each one of them being attributed to a distinct dominant SARS-CoV-2 variant strain: the Alpha variant for the wave that peaked in March–April 2021, the Delta variant for the one that peaked in August 2021, and the Omicron variant for the one that peaked in early 2022. The latter was detected in France in early December 2021 and spread more quickly than any previous SARS-CoV-2 variant. Despite the gradual vaccine rollout, the burden of Covid-19 in France remained heavy in 2021. While vaccine coverage reached high level, part of the population remained reluctant to get the vaccine. In this context, it is essential to get reliable evidence on the protection provided by vaccines against symptomatic infections, hospitalizations and inpatient deaths related to Covid-19, on the evolution of this protection over time and after the booster dose, and on the relative effectiveness of vaccines against the different variants of concern.
At first, due to limited availability of vaccine doses, vaccines were administered in priority to healthcare workers and those most likely to develop severe forms of the disease requiring hospitalization or leading to death, in particular to people aged 75 years and over, those living in retirement homes, and long-term care units. Vaccination was extended to all caregivers, and progressively to all age groups, starting with those with comorbidities, with a decreasing lower age limit for vaccine eligibility: 50 years, 18, 12 and five years (Appendix 1). Four different Covid-19 vaccine brands were used: Moderna, Pfizer/BioNTech, AstraZeneca and Janssen. A vaccination scheme was initially considered complete after two doses (or one dose for those infected by SARS-CoV-2), except for the Janssen vaccine, for which one dose was deemed sufficient. A full vaccination cycle became a sufficient condition required to get a French health pass that came into effect in June, and was first required to access events and places gathering many people. From August, the health pass was extended to grant entry into all museums, bars, restaurants, trains and other public spaces (irrespective of the number of people). The booster dose campaign started in early September. First limited to the most fragile people and people aged 65 years or over, it became available to all professionals caring for these vulnerable people, and to all adults. This booster became mandatory for the health pass to remain valid as of December 15, 2021, and January 15, 2022, for those aged respectively 65 years or over, and 18 years or over.
Vaccination coverage progressed at unprecedented speed worldwide, providing growing evidence on vaccine effectiveness against the risk of symptomatic infections and severe Covid-19 outcomes in the real world. The early estimates showed a good effectiveness of the first vaccine dose against the Alpha variant, with 61% (CI95%: 51–69) protection against symptomatic infections in people over 70 years of age, one month after the first dose of the Pfizer/BioNTech and AstraZenecca vaccines [20]. In people aged 80 years or over, vaccinated with Pfizer/BioNTech (resp. AstraZenecca), an additional reduction in the risk of hospitalization of 43% (33–52) (resp. 37%; 3–59) was observed; thus resulting in an effectiveness against the risk of hospitalization of about 80% after a single dose of one of the two vaccines. The protection provided by vaccination against the Alpha variant, increased after the second vaccine dose, reaching 89% (85–93) protection against symptomatic infections in people 80 years or older, with the Pfizer/BioNTech vaccine [20]. Two weeks after the second dose, vaccination provided a reduction in the risk of severe forms of Covid-19 requiring hospitalization of 92% (91; 93) for vaccination with the Pfizer/BioNTech vaccine, 96% (94–97) with the Moderna vaccine, and 96% (65–99) with the AstraZeneca vaccine, in people aged 75 years or older in France [6]. However, this protection seemed lower against the Delta variant (84%; 75–90) [5]. Vaccine effectiveness against symptomatic cases in individuals aged 16 years was lower by 12 to 19% with the Delta variant as compared with the Alpha variant, after one vaccine dose. Yet, these differences were smaller after two doses: 88% (85–90) with the Alpha variant and 80% (77–82) with the Delta variant [21].
Evidence started pointing towards a significant decline over time in vaccine efficacy or effectiveness against symptomatic infections, but to a lesser extent against severe cases [14,25,[26], [28]]. Vaccine effectiveness against symptomatic Delta variant infections dropped, 20 weeks after vaccination, to 47% (45–50) and 70% (69–71), for AstraZeneca and Pfizer/BioNTech vaccine respectively, with a stronger decline in those aged 65 years or older, but with a lower decline against hospitalizations [1]. However, studies find that the booster dose restores similar (or even better) levels of protection against symptomatic infections and severe cases than those prior to the waning of immunity [2,4,22].
This paper provides with complementary insights on vaccine effectiveness and its evolution over the year 2021 in the French context, using unprecedented data matching three National exhaustive databases containing exhaustive information on Covid-19 screening (SI-DEP), vaccination (VAC-SI) and hospitalizations (SI-VIC) in France. We used data from January 1st 2021 to December 12, 2021, thus stopping our analysis prior to the exponential growth of the Omicron variant. We focused on people aged 50 years or over, a population that concentrates the most severe forms of the disease and that became eligible for vaccination and then booster dose at an earlier stage (Appendix 1). We performed a two-step analysis of vaccine effectiveness against severe outcomes of Covid-19 (hospitalization, ICU admission, inpatient death) and estimated: (i) vaccine effectiveness against symptomatic forms of Covid-19; (ii) vaccine protection against the risk of hospitalization and death in individuals with symptomatic forms of Covid-19. In particular, we studied the evolution of vaccine protection under the combined effect of the emergence of the Delta variant, and of the decrease in immunity over time after the completion of the primary vaccination scheme. We additionally estimated the contribution of the booster dose in restoring a significant level of protection.
Material and methods
Study design
We used a two-step analysis to estimate vaccine effectiveness against severe outcomes of Covid-19, defined as symptomatic infections leading to hospitalizations, intensive care units (ICU) admissions, or inpatient deaths. First, we used a test-negative case–control design [17] to estimate vaccine effectiveness against symptomatic Covid-19 infections. This method, already used in the Covid-19 pandemic [10,20,21,25,[27], [29]], relies on the comparison of vaccination statuses between cases (individuals with confirmed SARS-CoV 2 infection) and controls (individuals who are not positively tested for SARS-CoV-2 infection), with cases and controls tested after reporting symptoms suggestive of Covid-19.
Then, we performed a survival analysis among individuals with symptomatic forms of Covid-19, to evaluate a possible additional risk reduction provided by vaccination against severe outcomes of the disease.
Data sources
Three National databases created to monitor the epidemic and the vaccination campaign were linked together at the individual level. This study provides the first use of this matched dataset in an international peer-reviewed journal.
SI-VIC, the information system for monitoring victims of attacks and exceptional health situations, records, for people infected with SARS-CoV-2, hospitalizations in general wards and ICU, and inpatient deaths. The diagnosis of infection relies on RT-PCR testing or thoracic CT scanning. This reporting system, maintained by the ANS (Agence du Numérique en Santé), is exhaustive and covers all healthcare structures (public and private) over the French territory. SI-VIC is meant to provide a real-time impact assessment of Covid-19 burden on healthcare structures.
SI-DEP, the screening information system, records the tests performed (RT-PCR, serology and antigenic tests) for SARS-CoV-2 and the results of these tests. This database, maintained by the AP-HP (Assistance Publique - Hôpitaux de Paris), is exhaustive for all tests performed on the French territory (but self-tests). Since mid-2020, RT-PCR testing was available to the population without prescription and covered by national health insurance (Appendix 1). As of January 2021, a molecular screening was performed on all RT-PCR positive samples: first to identify known variant strains (wild-type, alpha, beta, gamma); then, from June 2021, to identify some key mutations (E484K, E484Q, L452R). The presence or absence of symptoms in tested individuals should be systematically reported, but this information is missing for 20% of the RT-PCR tests performed in 2021.
VAC-SI, the Covid-19 vaccine information system, maintained by the CNAM, the French national Health Insurance (Caisse Nationale d'Assurance Maladie), provides administrated vaccines and vaccinated persons on the French territory. This dataset covers nearly the entire French population (all those affiliated to the French Health Care System [Social security]), whether vaccinated or not, and all individuals vaccinated in France. This database contains information on vaccination (dates of receipt of the vaccine dose, vaccine brand name), and information on vaccine priority populations (presence of comorbidities, healthcare professionals or social workers, retirement homes residents).
To match these databases, a pseudonym (non-meaningful character string identifying each person) was generated from the concatenation and encryption of identifying information (surname, first name, sex and date of birth). The pseudonym (but not the identifying information) is present in all the databases transmitted to the Statistics office of the French Ministry for Solidarity and Health (DREES) for statistical use, which allows the matching of data on screening, hospitalization and vaccination at the individual level. However, matching imperfections may remain ([12]; Appendix 5).
The deployment of these three databases was authorized by the French Data Protection Authority (Commission Nationale Informatique et Libertés). No consent of the patients is required, and the patients must be informed of their right to access, modify, rectify and delete any data concerning them. The French Ministry for Solidarity and Health is accountable to implement legal, technical and organizational measures to guarantee data protection.
Study period and study population
The data used were extracted on the 11th of January 2022, for observations from January 1st to December 12, 2021. In the last week of the study period, the Omicron variant represented less than 10% of the positive RT-PCR tests with molecular screening [24]. We excluded the last weeks of December when the Omicron variant started its exponential growth, as more data is needed to estimate vaccine effectiveness against this emerging variant.
We included tests performed on individuals: (i) aged 50 years or over, a population that concentrates the most severe outcomes of the disease and that became eligible for vaccination at an earlier stage; (ii) tested by RT-PCR, to focus on recent infections for which the causative variant could be identified; (iii) reporting symptoms in the seven days prior to the time of screening. When several positive tests were associated with the same pseudonym, we considered those performed less than 15 days apart as part of the same infectious episode. We thus identified distinct infectious episodes, and we included in the analysis only one positive test per episode and per individual (in priority a test with molecular screening and if needed the earliest test). For a given individual, we also excluded the negative tests that had been performed within 15 days of a confirmed infectious episode (either before or after). When a pseudonym had several negative tests associated with symptoms performed less than 15 days apart, the earliest was chosen.
We included only individuals present in the VAC-SI register and with non-missing information about the presence or absence of comorbidities considered for prioritizing vaccine administration in the 2021 French vaccination campaign. For our analysis, we defined four vaccination statuses: unvaccinated, one dose (D1), full primary vaccination cycle without (D2) or with booster (DB). They were further refined according to the time since the dose and the vaccine brand. In particular, individuals vaccinated with the Janssen vaccine completed their primary vaccination scheme after only one dose. The vaccination status was estimated as of the date of RT-PCR screening. In France, a full primary vaccination status is also achieved after only one dose in case of a confirmed infection in the three months preceding the first dose or 15 days after (set aside the Janssen vaccine). We excluded all individuals with a confirmed SARS-CoV-2 infection at least 60 days prior to the time of screening (in coherence with the European Surveillance System definition of suspected cases of SARS-CoV-2 reinfection), in order to estimate vaccine effectiveness in fully susceptible individuals. This choice implies that a full primary vaccination cycle only relates to a two doses vaccine course. We excluded individuals with atypical vaccination schemes: those vaccinated with two doses from two different vaccines when one of them was the Janssen vaccine, and those who never received their second dose of a bi-dose vaccine (they were excluded 28 days after their first dose).
To study severe outcomes of Covid-19, we included the inpatients aged 50 years or over that were present in all three databases (SI-VIC, SI-DEP and VAC-SI). We applied the filters already listed above on data from vaccination and screening. In addition, we did not consider inpatients: (i) infected by SARS-CoV-2 but whose admission was not attributable to Covid-19 (persons hospitalized for other conditions may have been systematically screened) based on the corresponding SI-VIC binary variable; and (ii) with no symptomatic positive RT-PCR test recorded within 15 days before hospital admission, or within two days after. In case of symptomatic positive RT-PCR test within two days after admission, inpatients are reclassified as tested on the day of admission to avoid negative durations in the survival analysis, and kept in the analysis.
Statistical analysis
We used a test-negative case-control design to estimate vaccine effectiveness against symptomatic Covid-19. Symptomatic positive individuals (respectively positive to a given variant for the analysis by variant) were randomly matched to controls (symptomatic negative individuals) on age (ten-year age brackets), sex, area of residence (NUTS-3 level), week of testing and presence or absence of a comorbidity qualifying for prioritization in the vaccination campaign. The odds ratios (OR) were estimated using a conditional logistic regression modelling the probability of testing positive with a strata-specific constant and vaccination status, with no additional control variables [18]. Vaccine effectiveness (VE(S+)) was given by the following formula: VE(S+) = 1 − OR [7].
We then estimated the risk of severe outcome (hospitalization, ICU admission, or death) among individuals with RT-PCR-confirmed SARS-CoV-2 symptomatic infection, according to their vaccination status. We fitted a Cox survival model on the time interval between the date of the test and the date of the hospital admission associated with the severe outcome, if any, or the end of the follow-up period. The latter was censored at 15 days post-test or at the end of the study period (December 12, 2021). A hazard ratio of hospitalization (respectively ICU admission or death) was then estimated according to the vaccination statuses (HR(H| S+)), controlling for the same variables as those used in the case-control study.
This two-step analysis allowed calculating the vaccine effectiveness against severe outcomes of Covid-19, VE(H), VE(ICU), VE(D), for hospitalization, ICU admission and inpatient death respectively, considering that, in addition to the risk reduction against symptomatic forms, vaccination also provides a risk reduction in the development of severe disease in symptomatic individuals. Vaccine effectiveness against severe outcomes of Covid-19 was deduced from the following formula: VE(i) = 1 − OR(S+) ∗ HR(i| S+), where i refers to either hospitalization, ICU admission or inpatient death. The estimated combined vaccine effectiveness may thus write , with each coefficient βk obtained from one stage. We assume follows a normal distribution with a variance corresponding to the sum of the two variances obtained in each stage, and apply the Delta method to obtain confidence intervals after taking the exponential.
We estimated the evolution of vaccine effectiveness over time, according to the time elapsed since each vaccine dose. We provided estimates up to six months after the second dose, except for the variant-specific analysis, that we truncated three months after the second dose. Indeed, for all variants but the Delta one, the variant-specific incidence was very low when the population reached this duration after a full vaccination scheme. In addition, we reported estimates relative to three frequent vaccine courses: (i) Cominarty, (ii) Spikevax, and (iii) Vaxzevria full primary course, followed (or not) by a booster dose of an mRNA vaccine (either Cominarty or Spikevax).
Results
Description of the study population
Over the period from January 1st to December 12, 2021, 8,881,107 individuals were tested by RT-PCR and reported symptoms at the time of screening. 2,413,356 (27%) of them were aged 50 years or over and 2,024,773 (84%) of the latter were successfully linked to vaccination data with non-missing data on comorbidities. We excluded 44,604 individuals with unusual vaccination schemes, and 67,134 individuals with a known past infection prior to the time of screening. Of these remaining individuals, 437,694 (23%) were tested positive for SARS-CoV-2.
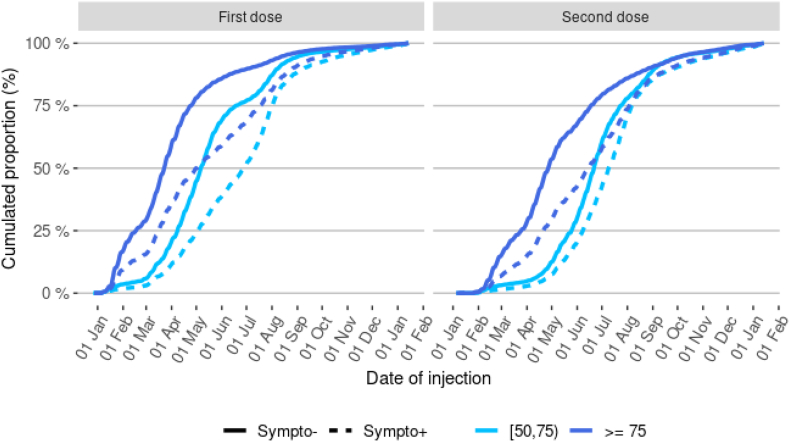
The study population for the test-negative design analysis consisted of 432,117 positive cases (5577 cases were excluded because of the lack of similar controls) and 864,234 controls (two matched controls for each case). Almost none of them was vaccinated at the beginning of the year 2021, 50% had received one dose by May, the 20th of 2021, and two doses by July, the 2nd of 2021. By the end of 2021, 86% of them had completed their primary vaccination cycle and 46% of them had received a booster dose. The distribution of vaccination statuses over time differed between cases and controls, with an earlier start of the primary vaccination scheme in the latter (Fig. 1, Appendix 2).
Fig. 1.
Distribution of dates of receipt for the first and second vaccine doses in control and cases, by age-group.
Abbreviations: Sympto+ (cases): Symptomatic individuals with a laboratory confirmed SARS-CoV-2 infection (cases). Sympto- (controls): Individuals with symptoms non-related to SARS-CoV-2 infection
Among the symptomatic positive cases in the sample, 67% had not yet been vaccinated when they got tested, 15% were older than 75 years, 52% were women, and 63% had a comorbidity (Appendix 2).
The study population for the survival analyses consisted of the 437,694 persons with confirmed SARS-CoV-2 symptomatic infection, among which there were 44,615 hospitalizations, 12,050 ICU admissions and 7476 inpatient deaths recorded in SI-VIC. We did not consider the 4813 hospitalizations that did not meet the criteria listed in the study population section.
Vaccine effectiveness of the primary vaccination cycle
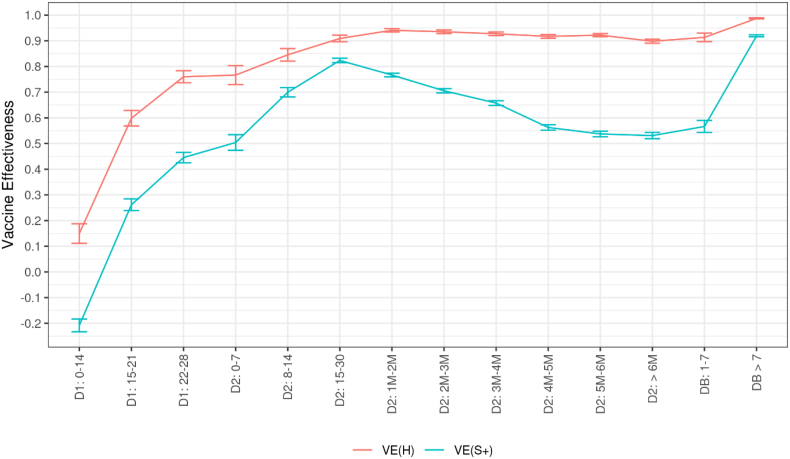
Among those aged 50 years and over, vaccine effectiveness against symptomatic infection grew quickly even after only one dose and further increased after the completion of the primary vaccination cycle. It reached 26% (24–28) two weeks after the first dose and 45% (43–47) one month after, and it peaked at 82% (81–83) two weeks after the second dose (Fig. 2). However, in the early days after the first dose, before protective immunity has been reached, we observe an increased risk of symptomatic infection in vaccinated persons versus comparable unvaccinated persons.
Fig. 2.
Covid-19 vaccine effectiveness against symptomatic infections and hospitalizations among persons aged 50 years or over, according to the time elapsed since the receipt of each vaccine dose, data collected from January 1st to December 12, 2021
Abbreviations: D1: First vaccine dose. D2: Second vaccine dose. DB: Booster dose. M: Month. S+: Symptomatic infection. H: Hospitalization. VE: Vaccine effectiveness. The numbers in the x-axis indicate the time (in days or months) elapsed since the receipt of the dose of interest. Precisely, thresholds used to define month intervals are 31–62, 63–90, 91–120, 121–150, 151–182, >182 in days
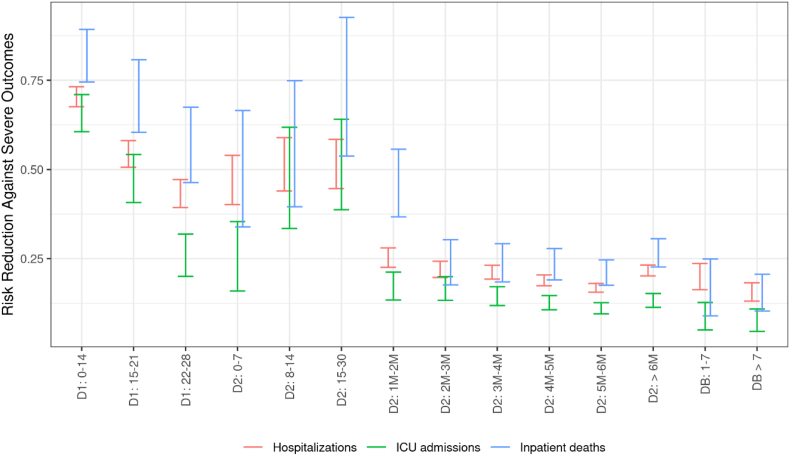
The risk of hospitalization, ICU admission, and inpatient death of infected symptomatic individuals decreased as the vaccination cycle progressed (Fig. 3). Among them, vaccination provided more than 75% (72–77) risk reduction against hospitalizations and ICU admissions, and 54% (44–63) risk reduction against inpatient deaths, one month after the receipt of the second dose.
Fig. 3.
Risk reduction against Covid-19 severe outcomes (hospitalizations, ICU admissions and inpatient deaths) among persons aged 50 years or over, according to the time elapsed since the receipt of each vaccine dose, data collected from January 1st to December 12, 2021
Abbreviations: D1: First vaccine dose. D2: Second vaccine dose. DB: Booster dose. M: Month
a The numbers in the x-axis indicate the time (in days or months) elapsed since the receipt of the dose of interest. Precisely, thresholds used to define month intervals are 31–62, 63–90, 91–120, 121–150, 151–182, >182 in days.
We then combined the estimates of vaccine effectiveness against symptomatic infections with the additional protection provided by vaccination against severe outcomes of the disease in those experiencing symptomatic infections. We thus obtained a vaccine effectiveness that peaked at 94% (93–95) against hospitalizations, 96% (95–97) against ICU admissions and 89% (87–91) against inpatient deaths after a primary vaccination cycle (Fig. 2).
Decline in vaccine effectiveness over time, before the booster shot
Among persons aged 50 years or over, the vaccine effectiveness against symptomatic infections peaked in the first month after the second dose, before declining sharply (Fig. 2), and falling to 53% (52–54) within six months. However, the additional risk reduction for ICU admissions and inpatient deaths among symptomatic individuals decreased only very little over time (Fig. 3), and remained constant for hospitalizations. As a result, vaccine effectiveness against severe disease declined less and slower. It was still about 90% (89–91) against the risk of hospitalization, more than six months after the second dose.
The booster dose seemed very efficient in restoring vaccine effectiveness to levels even higher than ever: reaching 92% against symptomatic forms and 99% against hospitalizations.
Vaccine effectiveness by age and comorbidities
Vaccine effectiveness against symptomatic infections was similar at the beginning of the vaccination cycle and up to five months after two doses for people aged 50 years or over with comorbidities, but was six percentage points lower after six months (Appendix 3). When studying severe Covid-19 outcomes, the presence of comorbidity, independently of vaccination status, was associated with a higher risk of complications with a hazard ratio of 1·57 (1·54–1·61) for hospitalization, 1·72 (1·65–1·79) for ICU admission, and 1·35 (1·27–1·43) with inpatient death. However, the vaccine reduced the risk of severe outcome in a similar way for people with comorbidities than for all people aged 50 years or more. As a result, vaccine effectiveness against severe Covid-19 after a full vaccination scheme appeared very similar for people with comorbidities. The booster dose then restored similar and high protection levels in both categories.
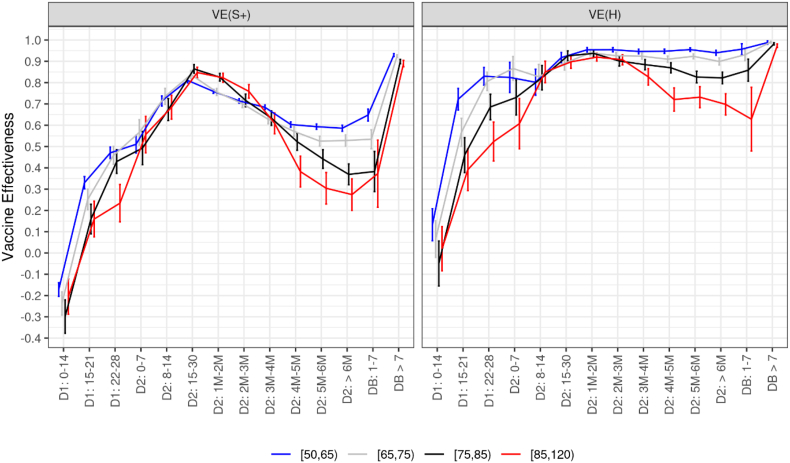
Vaccine effectiveness against symptomatic infections and severe cases showed no significant differences between age groups (for people aged 50 years or over) right after the completion of the primary vaccination cycle (Fig. 4). However, vaccine effectiveness against symptomatic cases, which significantly declined for all age groups, decreased stronger among the elderly. After six months, it dropped to 59% (57–60) in people aged 50 to 74, to 37% (32–41) in people aged 75 to 84, and to 27% (20–34) in those aged 85 and over. Vaccine effectiveness against hospitalizations declined moderately for all age groups but those aged 85 years or over, for whom it dropped to 70% (64–75) after six months. Again, high vaccine effectiveness in these frail and older populations was restored after the booster dose, up to levels comparable to those obtained in the younger age groups (97%; 96–98).
Fig. 4.
Covid-19 vaccine effectiveness against symptomatic infections and hospitalizations by age, according to the time elapsed since the receipt of each vaccine dose, data collected from January 1st to December 12, 2021.
Abbreviations: D1: First vaccine dose. D2: Second vaccine dose. DB: Booster dose. M: Month. S+: Symptomatic infection. H: Hospitalization. VE: Vaccine effectiveness
The numbers in the x-axis indicate the time (in days or months) elapsed since the receipt of the dose of interest. Precisely, thresholds used to define month intervals are 31–62, 63–90, 91–120, 121–150, 151–182, >182 in days.
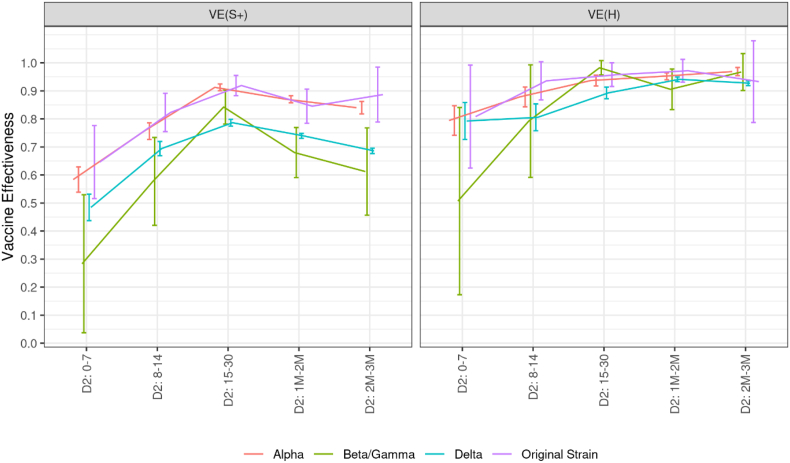
Vaccine effectiveness against different variants
Vaccine effectiveness did not differ significantly against the Alpha variant and the wild type SARS-Cov-2, reaching 91% (90–92) and 92% (88–96) respectively, 15 days after the second dose (Fig. 5 and Table 4 in Appendix 3). In comparison, vaccine effectiveness against the Beta/Gamma and Delta variants were lower against symptomatic infections, at all stages of the vaccination course, peaking respectively at 84% (78–90) and 79% (77–80), 15 days after the second dose. Point estimates suggest a lower effectiveness against the Beta/Gamma variants than against the Delta one after one month, but these differences are not significant. A decrease in vaccine effectiveness against symptomatic infections is observed over time for all variants (but the wild type). In contrast, vaccine effectiveness against hospitalization does not appear to decrease in the first three months, regardless of the variant. At the peak and from two to three months after the second dose, this effectiveness is significantly higher against the Alpha variant than against the Delta one by four percentage points.
Fig. 5.
Covid-19 vaccine effectiveness against symptomatic infections and hospitalizations related to various variants of concern, according to the time elapsed since the receipt of each vaccine dose, data collected from January 1st to December 12, 2021.
Abbreviations: D1: First vaccine dose. D2: Second vaccine dose. DB: Booster dose. M: Month. S+: Symptomatic infection. H: Hospitalization. VE: Vaccine effectiveness. The numbers in the x-axis indicate the time (in days or months) elapsed since the receipt of the dose of interest. Precisely, thresholds used to define month intervals are 31–62, 63–90, 91–120, 121–150, 151–182, >182 in days
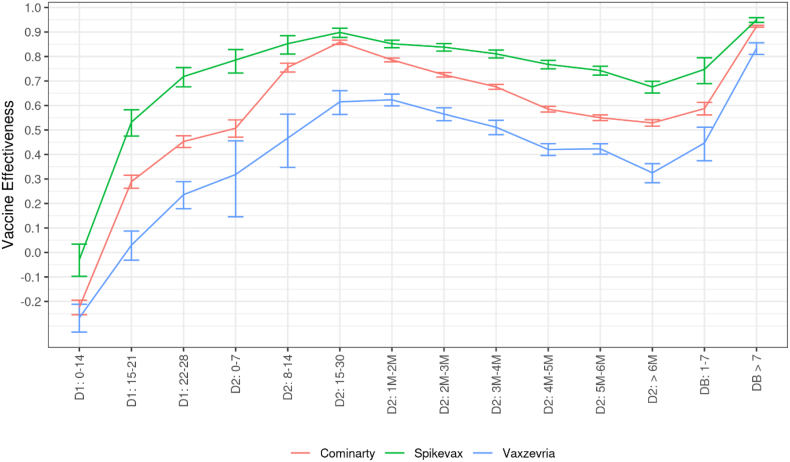
Vaccine effectiveness for the most frequent vaccine courses
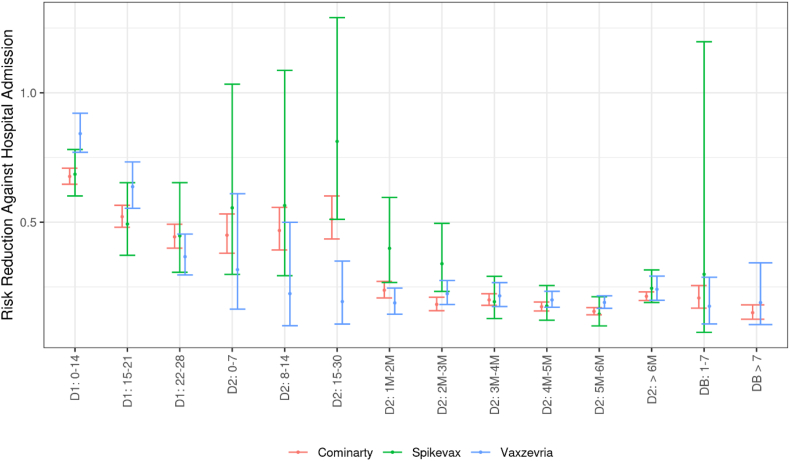
Vaccine effectiveness against symptomatic infections varied in level according to the vaccine used for the primary vaccine course (Fig. 6). The highest protection levels were reached with a Spikevax primary course (one or two doses), intermediate levels with Cominarty and the lowest levels with a Vaxzevria primary course. A parallel decrease in vaccine effectiveness following the second dose is observed for the three primary courses. The reduction in the risk of hospital admission in symptomatic individuals by time elapsed since vaccine doses hardly differs across primary vaccination courses (Fig. 7).
Fig. 6.
Covid-19 vaccine effectiveness against symptomatic infections among persons aged 50 years or over, according to received vaccine and to the time elapsed since the receipt of each vaccine dose, data collected from January 1st to December 12, 2021
Abbreviations: D1: First vaccine dose. D2: Second vaccine dose. DB: Booster dose. The numbers in the x-axis indicate the time (in days or months) elapsed since the receipt of the dose of interest. Precisely, thresholds used to define month intervals are 31–62, 63–90, 91–120, 121–150, 151–182, >182 in days. Cominarty: Primary vaccine course with Cominarty doses only. Spikevax: Primary vaccine course with Spikevax doses only. Vaxzevria: Primary vaccine course with Vaxzevria doses only. Booster dose are relative to mRNA-vaccine Cominarty or Spikevax
Fig. 7.
Risk reduction against Covid-19 severe hospitalizations among persons aged 50 years or over, according to received vaccine and to the time elapsed since the receipt of each vaccine dose, data collected from January 1st to December 12, 2021
Abbreviations: D1: First vaccine dose. D2: Second vaccine dose. DB: Booster dose. The numbers in the x-axis indicate the time (in days or months) elapsed since the receipt of the dose of interest. Precisely, thresholds used to define month intervals are 31–62, 63–90, 91–120, 121–150, 151–182, >182 in days. Cominarty: Primary vaccine course with Cominarty doses only. Spikevax: Primary vaccine course with Spikevax doses only. Vaxzevria: Primary vaccine course with Vaxzevria doses only. Booster dose are relative to mRNA-vaccine Cominarty or Spikevax.There was no hospital admission for the category “DB > 7” with a Spikevax primary course
Discussion
We have used three large and exhaustive datasets on Covid-19 screening, vaccination and hospitalizations in France to assess vaccine effectiveness from January to mid-December 2021. On the study population aged 50 years or more, we found a high vaccine effectiveness against Covid-19 symptomatic infections and severe outcomes, which increased as one progressed through the vaccination scheme. At its peak, the vaccine effectiveness of a primary vaccination cycle (without booster) reached 85% against symptomatic forms and 90% against severe outcomes, with little difference between age groups, and no difference for individuals with comorbidities.
Vaccine effectiveness against symptomatic forms then decreased over time after the completion of the primary vaccination cycle, dropping to 57% in those aged 50 years or over six months after completion of the vaccination cycle and to 39% in those aged 85 years or over. However, protection against severe outcomes declined much slower and vaccine effectiveness remained high (84% six months after completion of the vaccination course), except in the oldest age group where protection over time dropped stronger (70% after six months). These estimates are consistent with observational studies in other countries.
When the time elapsed since vaccination reached several months in the first vaccinated individuals, the Delta variant had become predominant. Our findings show that the drop in vaccine protection over time seems mainly due to a decline in vaccine protection, rather than to a greater capacity of the Delta variant to escape vaccine protection. Indeed, we found that vaccine effectiveness was only slightly lower against the Delta variant than against the wild type and Alpha variant. In addition, in the first three months after vaccination, a similar drop in vaccine effectiveness was observed for all variants, which suggests that immunity waning is not specific to the Delta variant. If the reduction in vaccine protection against the variants under study remains limited, our analyses were conducted prior to the rapid spread of the Omicron variant in France, for which vaccine escape may be of greater concern. Estimates on that matter, though still little documented to date, point towards very little (or no) protection against symptomatic infections [3,8], but partially restored protection with a booster [30] and a limited protection against severe cases [9].
We showed that the highest levels of protection against both symptomatic and severe outcomes of the disease were obtained one week after receiving a booster dose, without distinction on age groups. This confirms that the booster dose is essential to restore a high level of protection.
These real-world estimates are also consistent with laboratory analyses: although the amount of antibodies in vaccinated individuals decreases over time [19], memory B cells seem to remain numerous and capable of a better response [13], thus likely preventing severe cases. In addition, a rapid serological response was observed after the booster dose (Pfizer/BioNTech), with significantly higher antibody titers than those observed after the second dose [15].
The exhaustiveness of the databases and the possibility to match them together to get information at an individual scale are the great strengths of this study. We thus provide evidence of vaccine effectiveness in real-world conditions over a long period. The large sample size allows us: (i) to provide reliable estimates of vaccine protection against severe Covid-19, which remain rare events; and (ii) to detail these estimates for several sub-populations and with a precise time elapsed since the receipt of all vaccine doses, up to the booster dose. However, the observational nature of the data itself also brings about some limitations. We used a test-negative design in order to reduce selection biases that are difficult to measure such as health-seeking behavior, access to testing and case ascertainment. This method has been proven useful in our study context. In particular, it allowed limiting the effect of the evolution of screening policies on the propensity of getting tested (Appendix 1). Yet, test-negative designs rely on strong assumptions, the applicability of which is difficult to assess and may have varied over the study period [11,17]. The control variables that are used to limit the selection bias in the use of vaccination may not be sufficient, as a given vaccination status at a given time may reflect unobserved factors. For example, among people aged 50 years or over, those vaccinated at the beginning of the study period are likely to be more vulnerable (persons in retirement homes or with comorbidities), whereas those unvaccinated at the end of the study period are likely to be special (persons for whom vaccination is contraindicated, or persons against vaccination). In our study, we observed an increased risk of infection in the first days after vaccination, before protective immunity has been reached. This finding, observed elsewhere [10,20], seems to be due to a higher baseline risk of infection among those who were initially prioritized to receive the vaccine, suggesting missing unobserved controls (Appendix 4). In addition, being vaccinated could affect: (i) the perception of the need to be screened in case of symptoms and (ii) the probability of exposure to the virus, if being vaccinated leads to an increase in social interactions or to a lesser application of barrier gestures.
Beyond the biases inherent to observational studies, some limitations are linked to the data and variables available. Even though we excluded individuals with a known past infection based on virological and serological tests performed prior to the first vaccination dose, some past infections remained undetected and the vaccine effectiveness of the first dose of vaccine may partially reflect the stimulation of pre-existing immunity. The matching between the three databases was not perfect, which led to sample restrictions that could affect the representativeness of the results obtained. However, the corrections made to improve the matching between databases have not resulted in significant revisions of the results. Our findings only relate to vaccine effectiveness against symptomatic infections, but these symptoms were self-reported, without medical advice.
Overall, we found high levels of vaccine effectiveness against symptomatic infections and severe diseases after a full primary vaccination cycle. The protection was high against all the variants that circulated in France prior to December 2021, including the Delta variant that did not show a strong capacity to escape vaccine immunity. The decline of effectiveness overtime -which is strong against symptomatic infections but remains limited against severe diseases, is efficiently restored by a booster dose. Our findings underscore the importance of monitoring vaccine effectiveness over time, and of maximizing the vaccine uptake of the booster dose.
Funding
We acknowledge financial support from the French Ministry for Solidarity and Health.
Author contributions
All authors conceived the study, developed the methods, performed analyses, and co-wrote the paper.
Ethical considerations
The study was based on the analysis of pseudonymised data collected from health professionals. According to French law, such studies are not required to receive ethics committee approval, as the study was carried out as a contribution to the legal missions of the DREES (health surveillance and alert, healthcare performance assessment), and therefore benefited from the legal prerogatives vested in the French Ministry for Solidarity and Health to carry out these missions. The DREES is allowed to process personal health data in order to compute statistics, under article 65 of the law “Data processing and Liberties” (Informatique et Libertés) of January 6th, 1978. In that case, these statistics aim to prevent, manage and assess the consequences of the covid epidemic.
Research in context
Evidence before this study
Covid-19 vaccines were developed at unprecedented speed and most clinical trials provided estimates of vaccine effectiveness on relatively small samples and over short periods. Observational studies, providing insights in the real world, confirmed the strong protection conferred by a full vaccination scheme against symptomatic and severe infections. They also pointed towards a waning immunity over time, restored by a booster dose, and towards a reduced protection against some emerging variants
Added value of this study
Our study is the first to use the matching of three exhaustive nationwide French databases, providing information on vaccination, screening and hospitalization at the individual level, from January 1st to December 12, 2021. The quality of the data, and the broad time spectrum covered, allowed studying the evolution of vaccine effectiveness in real-world conditions under the influence of both immunity waning and the emergence of new variants. The exhaustiveness of the data allowed providing reliable estimates on the protection against severe Covid-19 outcomes (hospitalizations, ICU admissions, inpatient deaths), which remain rare events.
Implications of all the available evidence
Overall, we found high levels of vaccine effectiveness against symptomatic infections and severe outcomes after a full vaccination cycle. The decline in effectiveness over time appeared strong against symptomatic infections but to a lesser extent against severe cases. This seemed mostly due to waning immunity, rather than to the capacity of the Delta variant to escape vaccine protection. Our findings underscore the importance of monitoring vaccine effectiveness over time, and maximizing the vaccine uptake of the booster dose to restore immune levels.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Frédéric Tallet, Charlotte Geay, Mathilde Gaini, Gladys Baudet, Catherine Pollak, Benoit Ourliac, Daniel Lévy-Bruhl, Juliette Paireau and Diane Naouri.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gloepi.2022.100076.
Appendix A. Supplementary data
Supplementary material
References
- 1.Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., et al. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. N Engl J Med. 2022;386(340–350) doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N., Stowe J., Kirsebom F., Gower C., Ramsay M., Bernal J.L. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(831–837) doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews Nick, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386 doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouillon K., Baricault B., Botton J., Jabagi M.J., Bertrand M., Semenzato L., et al. 2021. Estimation de l’impact de la vaccination chez les personnes âgées de 75 ans et plus sur le risque de formes graves de Covid-19 en France à partir des données du Système national des données de santé (SNDS) – actualisation jusqu’au 20 juillet 2021. Rapport d’études EPI-PHARE-Groupement d’intérêt scientifique (GIS) ANSM-CNAM (octobre) [Google Scholar]
- 6.Botton J., Dray-Spira R., Baricault B., Drouin J., Bertrand M., Jabagi M.J., et al. Reduced risk of severe COVID-19 in more than 1.4 million elderly people aged 75 years and older vaccinated with mRNA-based vaccines. Vaccine. 2022 Jan 24;40(3):414–417. doi: 10.1016/j.vaccine.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond H.S., Sullivan S.G., Cowling B.J. Regression approaches in the test-negative study design for assessment of influenza vaccine effectiveness. Epidemiol Infect. 2016;144(8):1601–1611. doi: 10.1017/S095026881500309X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchan Sarah A., et al. Effectiveness of COVID-19 vaccines against omicron or delta infection. medRxiv. 2022 doi: 10.1001/jamanetworkopen.2022.32760. 2021–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collie Shirley, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2022;386(494–496) doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung H., He S., Nasreen S., Sundaram M.E., Buchan S.A., Wilson S.E., et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario: test negative design study. BMJ. 2021;374 doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean N.E., Hogan J.W., Schnitzer M.E. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385(1431–1433) doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DREES . 2021. Les appariements SI-VIC, SI-DEP et VAC-SI. [Google Scholar]
- 13.Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., et al. mRNA vaccination induces durable immune memory to SARS-CoV-2 with continued evolution to variants of concern. Science. 2021;374(6572) doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(e85) doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ireland G., Whitaker H., Shamez N., Ladhani S.N., Baawuah F., Subbarao S., et al. Serological responses to COVID-19 Comirnaty booster vaccine, London, United Kingdom, September to December 2021. Eurosurveillance. 2022;27(1) doi: 10.2807/1560-7917.ES.2022.27.1.2101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson M.L., Nelson J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 18.Breslow N.E., Day N.E., Halvorsen K.T., Prentice R.L., Sabai C. Am J Epidemiol. 1978;108(4):299–307. doi: 10.1093/oxfordjournals.aje.a112623. [DOI] [PubMed] [Google Scholar]
- 19.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(e84) doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattiuzzi C., Lippi G. Primary COVID-19 vaccine cycle and booster doses efficacy: analysis of Italian nationwide vaccination campaign. Eur J Public Health. 2022;32(2) doi: 10.1093/eurpub/ckab220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santé publique France . 2021. Point épidémiologique Covid-19 du 23 décembre 2021 (décembre) [Google Scholar]
- 25.Stowe J., Andrews N., Gower C., Gallagher E., Utsi L., Simmons R. Public Health England; 2021. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B. 1.617. 2) variant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenforde M.W., Patel M.M., Ginde A.A., Douin D.J., Talbot H.K., Casey J.D., et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States. Clin Infect Dis. 2021;74(9) doi: 10.1093/cid/ciab687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas Stephen J., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson M.G., Stenehjem E., Grannis S., Ball S.W., Naleway A.L., Ong T.C., et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385(15):1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willett Brian J., et al. The hyper-transmissible SARS-CoV-2 omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism. medRxiv. 2022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material