Abstract
Aim: The coronavirus disease 2019 (COVID-19) pandemic has left negative spillover effects on the entire health care system. Previous studies have suggested significant declines in cases of acute coronary syndrome (ACS) and primary percutaneous coronary intervention (PCI) during the COVID-19 pandemic.
Methods: We performed a quasi-experimental, retrospective cohort study of ACS hospitalisations by using a multi-institutional administrative claims database in Japan. We used interrupted time series analyses to ascertain impacts on cases, treatment approaches, and in-hospital mortality before and after Japan’s state of emergency to respond to COVID-19. The primary outcome was the change in ACS cases per week.
Results: A total of 30,198 ACS cases (including 21,612 acute myocardial infarction and 8,586 unstable angina) were confirmed between 1st July 2018 and 30th June 2020. After the state of emergency, an immediate decrease was observed in ACS cases per week (-18.3%; 95% confidence interval, -13.1 to -23.5%). No significant differences were found in the severity of Killip classification (P=0.51) or cases of fibrinolytic therapy (P=0.74). The impact of the COVID-19 pandemic on in-hospital mortality in ACS patients was no longer observed after adjustment for clinical characteristics (adjusted odds ratio, 0.93; 95% confidence interval, 0.78 to 1.12;P=0.49).
Conclusions: We demonstrated the characteristics and trends of ACS cases in a Japanese population by applying interrupted time series analyses. Our findings provide significant insights into the association between COVID-19 and decreases in ACS hospitalisations during the pandemic.
Keywords: Coronavirus disease 2019, Acute coronary syndrome, Acute myocardial infarction, Unstable angina, Primary percutaneous coronary intervention, Fibrinolytic therapy, In-hospital mortality
See editorial vol. 29: 565-566
Introduction
Coronavirus disease 2019 (COVID-19) burden has been increasing worldwide and has been accompanied by significant excess deaths during the pandemic 1) . The COVID-19 pandemic not only poses an immediate menace from acute respiratory syndrome to individuals, but also leaves negative spillover effects on the entire health care system. In both Europe 2 - 6) and the United States 7 - 9) , significant declines in the number of admissions for acute coronary syndrome (ACS) and primary percutaneous coronary intervention (PCI) procedures have been reported during the COVID-19 pandemic.
Growing evidence has been accumulated/gathered during the pandemic on the unprecedented reduction of emergent cardiovascular disease hospital admissions and unpredictable obstacles to identify and perform primary PCI in a timely manner. Previous studies have suggested that wide variations and trends exist in the affected number of ST-elevation myocardial infarction (STEMI), non-STEMI and revascularisation procedures depending on countries, regions and time period 2 - 11) . Primary PCI remained the standard of reperfusion therapy for ACS patients during the COVID-19 pandemic 12) . Although fibrinolysis serves as an alternative strategy in non-PCI capable hospitals and in regions with limited primary PCI centers, there is a concern that the quality of ACS care provided will deteriorate and, in turn, worsen the in-hospital mortality of inpatients 13) .
There is a paucity of data on the trends and management of ACS cases during the pandemic in Japan and East Asia 12 , 13) , the region most affected by the COVID-19 at the early stage of the pandemic. Therefore, we assessed characteristics and prognosis of ACS cases in Japan using the nationwide administrative claim database during the COVID-19 pandemic. We performed a quasi-experimental, retrospective cohort study of all ACS hospitalisations by using a multi-institutional administrative claims database in Japan. We sought to define the impacts on cases, treatment approaches and in-hospital outcomes for patients with ACS during COVID-19 pandemic. We used interrupted time series analyses to ascertain volume changes before and after Japan’s state of emergency to respond to COVID-19.
Methods
Data Source and Study Subjects
We used Diagnosis Procedure Combination (DPC) data from the Quality Indicator/Improvement Project (QIP) database, the program administered by the Department of Healthcare Economics and Quality Management, Kyoto University. QIP regularly collects administrative claims data from voluntary participant acute care hospitals in Japan. Participant hospitals voluntarily provide DPC data for analyses. There are >500 cumulative number of QIP participant hospitals, which covers all over Japan and includes both public and private hospitals. The cumulative participating institutions are listed on the web (http://med-econ.umin.ac.jp/QIP/sanka_byouin.html) only if they have agreed to be made public in advance. In the current study, administrative data were available from 265 hospitals throughout the entire study period. The clinical summary data include hospital identifiers, patient demographics; admission and discharge date; diagnoses classified as main, most- and second-most-resource-intensive; trigger; comorbidities and complications.
We used the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, codes to define the population (ICD-10). Claims codes for surgical procedures from the Ministry of Health and Welfare in Japan were used to identify the PCI procedure and coronary artery bypass graft surgery ( Supplementary Table 1 ) . Fibrinolytic therapy was defined as the use of thrombolytic agents (alteplase or monteplase) as an initial treatment for acute myocardial infarction (AMI). This study included patients aged 18 years or older with a diagnosis of ACS. We defined ACS cases as ICD-10; I.21.x and I.20.0. who were discharged between 1st July 2018 and 30th June 2020. The study was approved by the Ethics Committee, Kyoto University Graduate School and Faculty of Medicine (approval number: R0135). The informed consent requirement was waived because of the retrospective nature of the study.
Supplementary Table 1. Variables, Types of Codes, and Corresponding Codes.
| Variables | Types of Codes | Codes |
|---|---|---|
| AMI | ICD-10 | I.21.x |
| Unstable angina | ICD-10 | I.20.0 |
| Hypertension | ICD-10 | I.10.x, I.11.x–I13.x, I15.x |
| Diabetes mellitus | ICD-10 |
E.10.0, E.10.1, E.10.9, E.11.0, E.11.1, E.11.9, E.12.0, E.12.1, E.12.9, E.13.0, E.13.1, E.13.9, E.14.0, E.14.1, E.14.9, E.11.2–E.11.8, E12.2–E.12.8, E.13.2–E.13.8, E.14.2–E.14.8 |
| Dyslipidemia | ICD-10 | E.78.x |
| Previous MI | ICD-10 | I.22.x, I.25.2 |
| PAD | ICD-10 | I.70.2, I.70.9, I.73.9, I.74.2, I.74.3, I.74.4, I.74.5 |
| Heart failure | ICD-10 | I.50.x |
| Stroke | ICD-10 | I.60, I.61.0, I.61.1, I.61.3, I.61.4, I.61.5, I.61.6, I.61.8, I.61.9, I.63 |
| PCI | claims code (K-code) | K5461, K5462, K5463, K547, K5481, K5482, K5491, K5492, K5493, K550, K550-2 |
| CABG | claims code (K-code) | K5521, K5522, K5523, K552-21, K552-22 |
AMI, acute myocardial infarction; ICD, international classification of disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft.
The primary outcome was a change of the weekly rate of ACS (acute AMI or unstable angina pectoris (UAP)) hospitalisations before and after Japan’s state of emergency to respond to COVID-19. The secondary outcomes were patient characteristics (demographics and disease severity), treatment approaches (primary PCI or fibrinolytic therapy) and in-hospital mortality of ACS patients.
Positive cases for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and deaths due to COVID-19 were counted based on the press statement by Ministry of Health, Labour and Welfare, Japan. All COVID-19 cases confirmed by reverse transcription-PCR and the cumulative number of deaths in Japan must be reported to the Ministry of Health, Labour and Welfare. Choropleth maps were also illustrated the geographic distribution of COVID-19 in Japan.
Statistical Analysis
Continuous values were expressed as median [interquartile range]. Comparisons between the two groups of continuous variables were performed by Mann–Whitney U tests. Categorical data were compared using chi-square, Fisher’s exact, or Kruskal–Wallis tests, as appropriate. A logistic regression model was used to examine the relationship between each variable and in-hospital mortality. Multivariable adjustment was determined a priori for age, sex, hypertension, diabetes mellitus, dyslipidemia, heart failure, Killip classification, peripheral artery disease, stroke, recalibrated Elixhauser comorbidity index 14 - 16) and the start of COVID-19 pandemic to avoid overfitting. Statistical significance was set at P<0.05 (two-tailed). Statistical analyses were performed with R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) using R packages ‘forecast’, ‘tsModel’ and ‘tseries’.
Interrupted Time Series Analyses for Changes in Case Numbers
Weekly volumes of ACS hospitalisations (categorised as AMI or UAP) are presented as line graphs. Segmented regression analysis was used to ascertain volume changes over time 7 , 17) . The time unit chosen was 1 week to provide optimal precision to the model. We used an identified a break point (7th April 2020), because the Japanese government announced a state of emergency and requested people to quarantine themselves and promote social distancing on this date. To provide insight into whether changes in ACS cases was related to the introduction of state of emergency, a sensitivity analysis was done by varying the break point and evaluating the segmented regression model for each break point. Candidate break points were as follows; 1) March 11, 2020, The World Health Organization has declared the COVID-19 outbreak a global pandemic. 2) End-March, 2020, in Japan, COVID-19 infections significantly increased in this period. 3) April 7, 2020, the Japanese government declared the state of emergency in 7 prefectures. 4) April 16, 2020, the area of state of emergency was expanded to nationwide level in Japan. The segmented regression model with the break point of 7th April, 2020 was used because this models showed the best Akaike information criteria. We grouped cases into 1 of 2 periods for analysis: before Japan’s state of emergency to respond to COVID-19 (1st July 2018, to 6th April 2020) and after Japan’s state of emergency to respond to COVID-19 (7th April 2020 to 30th June 2020). We hypothesised that the pandemic would have an immediate impact, considering the effect of Japan’s state of emergency. The impact was estimated by comparing the observed number of ACS admissions in the post-pandemic period to the expected estimates had the state of emergency not occurred. Segmented regression analysis was used to identify the level change in weekly hospitalisations during the pandemic, with consideration of time dependence in the model. Outcomes were analysed by accounting for seasonality and secular trends before and after Japan’s state of emergency. Seasonality was taken into account by including harmonic terms (sines and cosines) to adjust for the seasonal pattern. The validity of the model was assessed by the correlograms (autocorrelation and partial autocorrelation functions) and residual plots. Post-hoc subgroup analyses were performed for elderly (>75 years) and young (<55 years) populations and for 7 prefectures where a state of emergency was firstly declared and other 40 prefectures subpopulations to ascertain the consistency of findings.
Results
Impact of COVID-19 Outbreak in Japan
By the end of June, 18,721 SARS-CoV-2 positive cases and 973 deaths were identified by the press statement of Ministry of Health, Labour and Welfare, Japan. As shown in the Choropleth maps in Supplementary Fig.1 , High prevalence of confirmed SARS-CoV-2-positive cases and deaths were noted in areas of Tokyo, Hokkaido, Saitama, Chiba, Osaka, Hyogo, and Fukuoka.
Supplementary Fig.1. Choropleth are maps for COVID-19 infections and deaths in Japan.
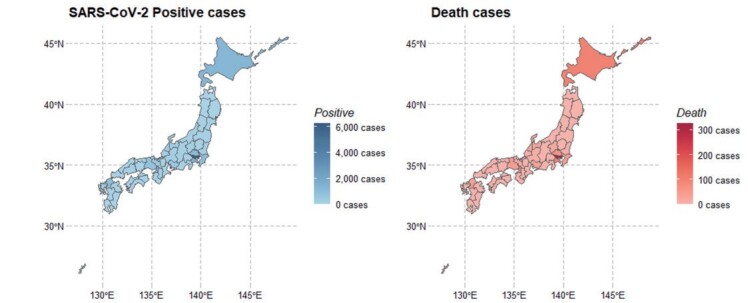
(Left) Geographic distribution of SARS-CoV-2 positive cases for during the initial phase of the epidemic in Japan
(Right) Geographic distribution of COVID-19 deaths in Japan.
High prevalence of confirmed SARS-CoV-2-positive cases and deaths were noted in areas of Tokyo, Hokkaido, Saitama, Chiba, Osaka, Hyogo, and Fukuoka.
SARS-Cov-2; severe acute respiratory syndrome coronavirus 2, COVID-19; coronavirus disease 2019
Patient Demographics
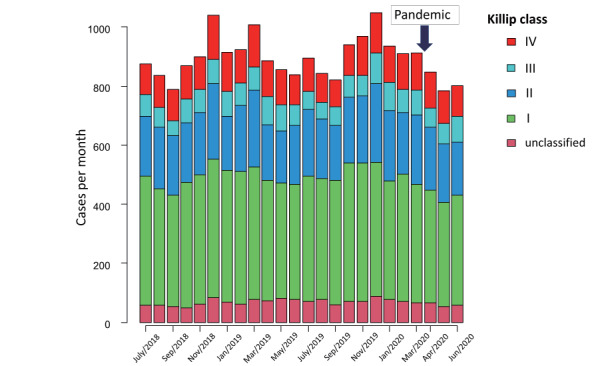
Table 1 displays the baseline characteristics of patients with ACS and a comparison of those characteristics before and after Japan’s state of emergency to respond to COVID-19. A total of 30,198 cases (median age, 72.0 years; 8,423 (27.9%) women) were included in this analysis. Among them, UAP cases accounted for 27.3%, and AMI was present in 2,441 cases (72.7%). Although patient demographics and treatment approaches were fairly consistent across periods, patients with ACS hospitalised during the COVID-19 period were on average 1 year older and more likely to have ehigher Elixhauser indices and diabetes mellitus, whereas cases with hypertension were less frequent during the pandemic. Length of hospital stay for ACS was similar across periods ( Table 1 ) . A comparison of disease severity between the two periods by Killip classification at admission remained at the same degree (P=0.51) ( Table 1 and Fig.1 ) .
Table 1. Patient demographics.
| All ACS (N = 30,198) | Before April 2020 (n = 26,841) | After April 2020 (n = 3,357) | P-value | |
|---|---|---|---|---|
| AMI/UAP, n (%) | 21,612 (71.6)/8,586 (28.4) | 19,171 (71.4)/7,670 (28.6) | 2,441 (72.7)/916 (27.3) | 0.12 |
| Demographics | ||||
| Age, years* | 72.0 [63.0, 81.0] | 72.0 [63.0, 81.0] | 73.0 [63.0, 82.0] | 0.04 |
| Sex (male), n (%) | 21,775 (72.1) | 19,361 (72.1) | 2,414 (71.9) | 0.79 |
| Comorbidities | ||||
| Hypertension, n (%) | 19,431 (64.3) | 17,332 (64.6) | 2,099 (62.5) | 0.02 |
| DM, n (%) | 9,590 (31.8) | 8,453 (31.5) | 1,137 (33.9) | <0.01 |
| Dyslipidemia, n (%) | 19,170 (63.5) | 17,012 (63.4) | 2,158 (64.3) | 0.31 |
| Smoking history, n (%) | 17,482 (57.9) | 15,563 (58.0) | 1,919 (57.2) | 0.37 |
| Prior MI, n (%) | 1,214 (4.0) | 1,080 (4.0) | 134 (4.0) | 0.96 |
| Stroke, n (%) | 295 (1.0) | 253 (0.9) | 42 (1.3) | 0.09 |
| PAD, n (%) | 61 (0.2) | 53 (0.2) | 8 (0.2) | 0.54 |
| Heart failure, n (%) | 9,071 (30.0) | 8,015 (29.9) | 1,056 (31.5) | 0.06 |
| Elixhauser Comorbidity Index* | 3 [0, 7] | 2 [0, 7] | 3 [0, 7] | 0.04 |
| Killip classification | 0.51 | |||
| I, n (%) | 10,062 (46.9) | 8,952 (47.1) | 1,110 (45.7) | |
| II, n (%) | 5,107 (23.8) | 4,519 (23.8) | 588 (24.2) | |
| III, n (%) | 1,827 (8.5) | 1,607 (8.5) | 220 (9.1) | |
| IV, n (%) | 2,804 (13.1) | 2,470 (13.0) | 334 (13.7) | |
| Treatment approaches | ||||
| PCI, n (%) | 24,112 (79.8) | 21,419 (79.8) | 2693 (80.2) | 0.58 |
| CABG, n (%) | 843 (2.8) | 757 (2.8) | 86 (2.6) | 0.43 |
| Fibrinolytic therapy, n (%) | 59 (0.2) | 55 (0.2) | 4 (0.1) | 0.40 |
| Medical management, n (%) | 5,411 (17.9) | 4,815 (17.9) | 596 (17.8) | 0.81 |
| Length of hospital stay, days* | 11.0 [6.0, 16.0] | 11.0 [6.0, 16.0] | 11.0 [6.0, 16.0] | 0.47 |
ACS; acute coronary syndrome, AMI; acute myocardial infarction, CABG; coronary artery bypass graft, DM; diabetes mellitus, PAD; peripheral artery disease, PCI; percutaneous coronary intervention, UAP; unstable angina pectoris.
Medical management other than PCI, CABG and fibrolytic therapy.
*Indicates values that are medians [1st quartile, 3rd quartile].
Fig.1. Serial changes of Killip classifications for acute myocardial infarction cases/month.
Interrupted Time Series Analyses for Changes in ACS Cases
Fig.2 presents the trend of ACS cases per week before and after the breakpoint in time of the COVID-19 pandemic. Fig.2 provides the result of the interrupted time series analyses for changes in ACS cases. The interrupted time series analysis showed a significant change in intercepts of the fitted curve between the two periods [intercept change -18.3%; 95% CI, -13.1 to -23.5%]. Beginning on 7th April 2020, ACS hospitalisations decreased for 12 weeks, marking the early COVID-19 period. Thereafter, ACS hospitalisations remained at a lower level than those of the previous year. The COVID-19 pandemic was accompanied by a decrease in absolute numbers of ACS cases with a reduction of 48.2 cases per week [95% CI, -18.5 to -77.8 cases per week] between the two periods. The pandemic affected both elderly (≥ 75 years) and young populations (<55 years) equally, with a similar impact on levels of acute coronary syndrome hospitalisations (elderly population: [intercept change -20.1%; 95% CI, -13.6 to -26.6%] and young population: [intercept change -20.5%; 95% CI, -8.0 to -33.2%]) ( Supplementary Fig.2 ) . The pandemic also affected both 7 prefectures where a state of emergency was firstly declared and other 40 prefectures subpopulations equally, with a similar impact on levels of acute coronary syndrome hospitalisations (7 prefectures: [intercept change -14.7%; 95% CI, -8.2 to -21.1%] and 40 prefectures population: [intercept change -17.9%; 95% CI, -12.4 to -23.4%]) ( Supplementary Fig.3 ) .
Fig.2. Interrupted time series analysis for ACS cases/week.
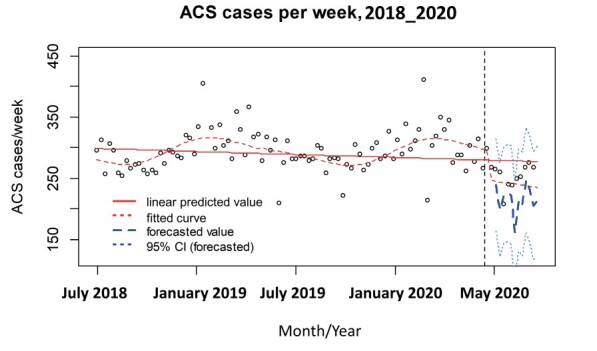
ACS; acute coronary syndrome.
Supplementary Fig.2. Interrupted time series analysis for ACS cases/week in elderly and young subpopulations.
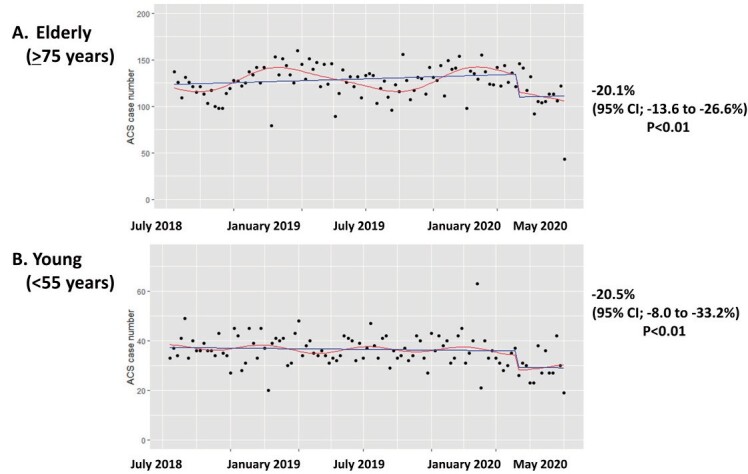
Red line; fitted curve, blue line; linear fitted value.
ACS; acute coronary syndrome.
Supplementary Fig.3. Interrupted time series analysis for ACS cases/week in 7 prefectures and other 40 prefectures.
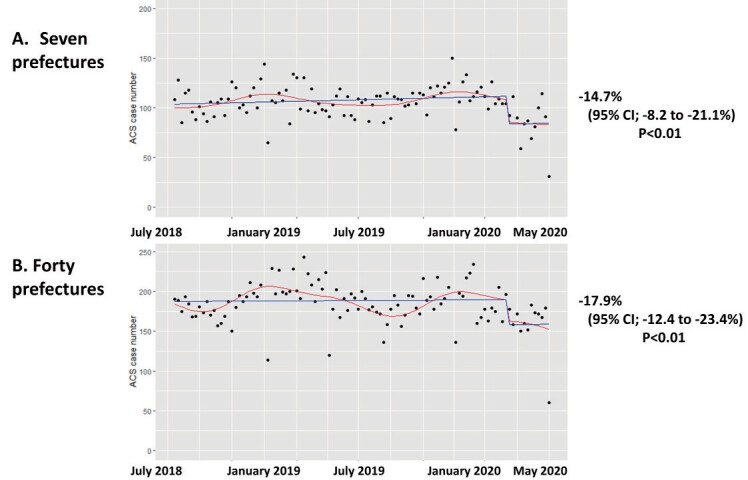
Seven prefectures (Tokyo, Hokkaido, Saitama, Chiba, Osaka, Hyogo, and Fukuoka), where a state of emergency was firstly declared in Japan.
Red line; fitted curve, blue line; linear fitted value.
ACS; acute coronary syndrome.
Primary PCI Procedures and Fibrinolytic Therapy
For all types of ACS combined, the percentage reduction in patients receiving primary PCI was 9.9%, with 231 [interquartile range, IQR; 219, 251] procedures per week in 2019 and 208 [IQR; 196, 213] procedures per week by the end of June 2020 (P<0.01). Coronary artery bypass graft (CABG) surgery during the pandemic had largely ceased by June 2020, with a percentage reduction of 31.3% and 8.0 [IQR; 6.0, 11.0] procedures per week in 2019 and 5.5 [IQR; 4.8, 7.3] procedures per week by the end of June 2020 (P<0.01). Cases of fibrinolytic therapy remained stable and did not increase during the COVID-19 pandemic, with 0.0 [IQR; 0.0, 1.0] procedures per week in 2019 and 0.0 [IQR; 0.0, 0.3] procedures per week by the end of June 2020 (P=0.19). Treatment approaches for cases with ACS did not differ from before and after the breakpoint in time of the COVID-19 pandemic (P=0.74) ( Table 2 and Fig.3 ) .
Table 2. Monthly case volumes from July 2018 to June 2020.
| Month | ACS cases, n | PCI cases, n (%) | CABG cases, n (%) | Fibrinolytic therapy cases, n (%) |
|---|---|---|---|---|
| Before COVID-19 | ||||
| Jul 2018 | 1,279 | 1,024 (80.1) | 26 (2.0) | 1 (0.1) |
| Aug 2018 | 1,200 | 956 (79.7) | 39 (3.2) | 2 (0.2) |
| Sep 2018 | 1,129 | 904 (80.1) | 29 (2.6) | 1 (0.1) |
| Oct 2018 | 1,218 | 969 (79.6) | 34 (2.8) | 2 (0.2) |
| Nov 2018 | 1,262 | 1,012 (80.2) | 39 (3.1) | 1 (0.1) |
| Dec 2018 | 1,501 | 1,205 (80.3) | 50 (3.3) | 4 (0.3) |
| Jan 2019 | 1,274 | 1,001 (78.6) | 33 (2.6) | 4 (0.3) |
| Feb 2019 | 1,280 | 1,023 (79.9) | 35 (2.7) | 3 (0.2) |
| Mar 2019 | 1,405 | 1,139 (81.1) | 40 (2.8) | 0 (0.0) |
| Apr 2019 | 1,276 | 1,024 (80.3) | 51 (4.0) | 4 (0.3) |
| May 2019 | 1,208 | 972 (80.5) | 25 (2.1) | 2 (0.2) |
| Jun 2019 | 1,213 | 947 (78.1) | 34 (2.8) | 5 (0.4) |
| Jul 2019 | 1,262 | 978 (77.5) | 31 (2.5) | 4 (0.3) |
| Aug 2019 | 1,174 | 955 (81.3) | 23 (2.0) | 2 (0.2) |
| Sep 2019 | 1,191 | 936 (78.6) | 30 (2.5) | 2 (0.2) |
| Oct 2019 | 1,322 | 1,061 (80.3) | 37 (2.8) | 1 (0.1) |
| Nov 2019 | 1,341 | 1,061 (79.1) | 36 (2.7) | 2 (0.1) |
| Dec 2019 | 1,491 | 1,164 (78.1) | 48 (3.2) | 3 (0.2) |
| Jan 2020 | 1,300 | 1,035 (79.6) | 42 (3.2) | 5 (0.4) |
| Feb 2020 | 1,268 | 1,012 (79.8) | 38 (3.0) | 1 (0.1) |
| Mar 2020 | 1,247 | 1,041 (83.5) | 37 (3.0) | 6 (0.4) |
| After COVID-19 | ||||
| Apr 2020 | 1,166 | 920 (78.9) | 38 (3.3) | 2 (0.2) |
| May 2020 | 1,071 | 866 (80.9) | 22 (2.1) | 1 (0.1) |
| Jun 2020 | 1,120 | 907 (81.0) | 26 (2.3) | 1 (0.1) |
ACS; acute coronary syndrome, CABG; coronary artery bypass graft, COVID-19; coronavirus disease 2019, PCI; percutaneous coronary intervention.
Fig.3. Serial changes of treatment approaches for ACS cases/month.
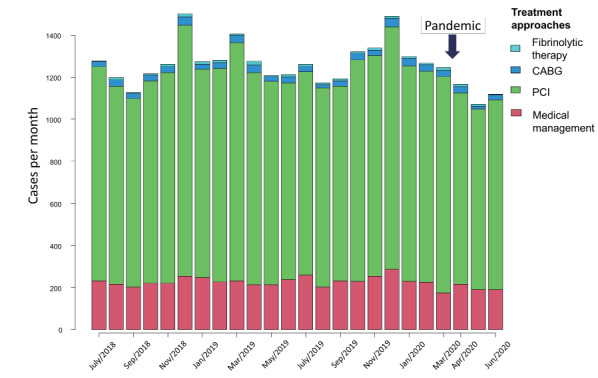
ACS; acute coronary syndrome, CABG; coronary artery bypass graft, PCI; percutaneous coronary intervention.
In-Hospital Mortality in Patients with ACS during the COVID-19 Pandemic
Overall, there were 2,363 (7.8%) in-hospital deaths during the study period. In-hospital mortality after the pandemic was similar to that before the pandemic in our study population. Patients who suffered from ACS during the pandemic had a similar in-hospital mortality as those who suffered before the pandemic (2,095/26,841 (7.8%) vs. 268/3,357 (8.0%) cases; P=0.70). In-hospital mortality within the first 24 hours was also unchanged from before and after the pandemic (979/26,841 (7.8%) vs. 126/3,357 (8.0%) cases; P=0.73). In the risk-adjusted logistic modeling, the COVID-19 crisis was not a statistically significant predictor of in-hospital mortality (odds ratio, 0.93; 95% CI, 0.78 to 1.12; P=0.49) ( Table 3 ) .
Table 3. Logistic regression analysis for in-hospital mortality.
| OR (95% CI) | P-value | |
|---|---|---|
| COVID-19 pandemic | 0.93 (0.78–1.12) | 0.49 |
| Age per year | 1.06 (1.05–1.06) | <0.01 |
| Sex (male) | 1.17 (1.03–1.33) | 0.01 |
| Hypertension | 0.32 (0.29–0.37) | <0.01 |
| Diabetes mellitus | 0.65 (0.57–0.75) | <0.01 |
| Dyslipidemia | 0.18 (0.16–0.21) | <0.01 |
| Heart failure | 0.53 (0.45–0.63) | <0.01 |
| Killip classification I | 0.06 (0.04–0.07) | <0.01 |
| Killip classification II | 0.12 (0.10–0.14) | <0.01 |
| Killip classification III | 0.36 (0.29–0.45) | <0.01 |
| Killip classification IV | 1.60 (1.35–1.88) | <0.01 |
| Stroke | 0.78 (0.48–1.25) | 0.30 |
| Peripheral artery disease | 2.20 (0.75–6.47) | 0.15 |
| Elixhauser Comorbidity Index | 1.01 (0.99–1.02) | 0.50 |
CI; confidence interval, COVID-19; coronavirus disease 2019, OR; odds ratio.
Discussion
Our interrupted time series study showed important changes in ACS hospitalisations and the trend of primary PCI during the COVID-19 pandemic in Japan. The main findings of our study were as follows: first, a significant decline in hospital admissions for ACS after Japan’s state of emergency to respond to COVID-19; second, a remarkable reduction of primary PCI during the pandemic, whereas the number of fibrinolytic therapy remain stationary; and third, in a Japanese population, a similar in-hospital mortality before and after the pandemic.
First, our data indicated that the COVID-19 pandemic was associated with a notable reduction in ACS cases across the entire spectrum of ACS: AMI and UAP. Our findings were consistent with previous reports in other regions, which showed a notable decrease in ACS hospitalisations during the COVID-19 pandemic 2 , 3 , 5 - 8 , 10 , 11 , 13 , 18) . Possible explanations for the marked decline in ACS cases included a reluctance to seek immediate medical attention of the elderly patients, attempts for hospitals to maintain bed availability and patient preference for staying home due to fear of COVID-19 contamination of the emergency departments of acute care hospitals or clinics 12 , 19 , 20) . Other plausible explanations were related to the situation of the state of emergency. People refrained from non-essential and non-urgent outings, potentially leading to less physical and business activity that might trigger an ACS, coupled with self-quarantine and reduced air pollution 20 , 21) .
Second, our data revealed a marked decline of primary PCI during the pandemic, whereas cases of fibrinolytic therapy remained unchanged compared to before the COVID-19 pandemic. We presume that resources and healthcare providers were properly prioritised in PCI-capable centers to ensure appropriate acute care for all ACS patients requiring primary PCI, suggesting that there is no need to promote fibrinolytic therapy. Our finding is consistent with the previous report from the Japanese Association of Cardiovascular Intervention and Therapeutics 22) , which indicated that primary PCI for ACS was performed as usual during the COVID-19 pandemic. The result of this Japanese nation-wide survey 22) also support our speculations. Although fibrinolysis could be an alternative therapeutic approach during the COVID-19 pandemic, there is a concern that half of patients receiving fibrinolysis might require the rescue PCI afterwards 12 , 23) .
Third, our study showed that even during the COVID-19 pandemic, in-hospital mortality did not differ from the period before the state of emergency, neither in the population of all ACS patients nor in the elderly or women. The lack of deterioration in in-hospital mortality from the previous year can be explained by the fact that the primary PCI system was maintained in Japan during the pandemic. Also, the results ought to reflect the efforts of facilities and healthcare providers to maintain readiness for primary PCI with limited human and medical resources. In addition, we could not capture the number of excess deaths reported as unknown causes or out-of-hospital cardiac arrest. Thus, we may have underestimated the in-hospital mortality due to survival bias.
Our study had several limitations. First, the administrative data were only available from QIP participating hospitals, not from the entire nation-level DPC data and depended on voluntary participation by individual institutions and hospitals. Our study samples were restricted to DPC participating hospitals, and hence utilizing QIP database may result in the limited generalizability to expand our findings to non-DPC hospitals. Thus, due to the retrospective and observational nature of this study with voluntarily participating hospitals, selection bias cannot be excluded. Second, the present study consisted of only patients with established ACS. Therefore, we may have underestimated ACS cases where the cause of death has not been determined. Our study lacked information about several factors associated with in-hospital mortality, such as severity of coronary artery lesion, ejection fraction, details of PCI procedures, chronic kidney disease, and troponin values. Lead time or survival bias is also considered in patients hospitalised with ACS. Men and young patients are likely to be diagnosed at an early stage, owing to their typical symptoms of ACS, which may lead to immediate primary PCI. Finally, the cause of the substantial reduction of ACS cases is still open to debate. Further investigations are warranted to identify and address the cause of the remarkable reduction of cardiovascular emergencies. The global impact of COVID-19 on burdens of cardiovascular emergencies and long-term adverse effects on morbidity and mortality and collateral damage to the healthcare system need to be addressed in cooperation with nationwide and worldwide research. It is a challenging task to reconcile the prevention of unjustified exposure of healthcare workers to COVID-19 infections with the provision of medical care to ACS patients in need of urgent treatment. We need to be ready for the next pandemic.
Conclusion
In conclusion, we demonstrated and validated the characteristics and trends of ACS cases in a Japanese population before and after the COVID-19 pandemic, by applying segmented regression and interrupted time series analyses. Our findings also provide significant insights into the association between COVID-19 and the decrease in ACS hospitalisations during the pandemic.
Source of Funding
This study was supported by JSPS KAKENHI [Grant Number JP19H01075] from the Japan Society for the Promotion of Science, by the GAP Fund Program of Kyoto University type B and by Health Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare, Japan [20HA2003] to Yuichi Imanaka. The funders played no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Acknowledgements
We gratefully acknowledge the participating hospitals in the QIP (Quality Indicator/Improvement Project) and their staffs.
Conflicts of Interest
None declared.
Data Availability Statements
Data cannot be shared for ethical/privacy reasons.
References
- 1).Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L, Noursadeghi M, Pillay D, Sebire N, Holmes C, Pagel C, Wong WK, Langenberg C, Williams B, Denaxas S, Hemingway H. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet, 2020; 395: 1715-1725 [Google Scholar]
- 2).De Filippo O, D’Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A, Secco GG, Campo G, Gallone G, Verardi R, Gaido L, Iannaccone M, Galvani M, Ugo F, Barbero U, Infantino V, Olivotti L, Mennuni M, Gili S, Infusino F, Vercellino M, Zucchetti O, Casella G, Giammaria M, Boccuzzi G, Tolomeo P, Doronzo B, Senatore G, Grosso Marra W, Rognoni A, Trabattoni D, Franchin L, Borin A, Bruno F, Galluzzo A, Gambino A, Nicolino A, Truffa Giachet A, Sardella G, Fedele F, Monticone S, Montefusco A, Omedè P, Pennone M, Patti G, Mancone M, De Ferrari GM. Reduced Rate of Hospital Admissions for ACS during Covid-19 Outbreak in Northern Italy. N Engl J Med, 2020; 383: 88-89 [Google Scholar]
- 3).Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, Hollings S, Roebuck C, Gale CP, Mamas MA, Deanfield JE, de Belder MA, Luescher TF, Denwood T, Landray MJ, Emberson JR, Collins R, Morris EJA, Casadei B, Baigent C. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet, 2020; 396: 381-389 [Google Scholar]
- 4).Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J, 2020; 41: 1852-1853 [Google Scholar]
- 5).Piccolo R, Bruzzese D, Mauro C, Aloia A, Baldi C, Boccalatte M, Bottiglieri G, Briguori C, Caiazzo G, Calabrò P, Cappelli-Bigazzi M, De Simone C, Di Lorenzo E, Golino P, Monda V, Perrotta R, Quaranta G, Russolillo E, Scherillo M, Tesorio T, Tuccillo B, Valva G, Villari B, Tarantini G, Varricchio A, Esposito G, Collaborators. Population Trends in Rates of Percutaneous Coronary Revascularization for Acute Coronary Syndromes Associated With the COVID-19 Outbreak. Circulation, 2020; 141: 2035-2037 [Google Scholar]
- 6).Toniolo M, Negri F, Antonutti M, Masè M, Facchin D. Unpredictable Fall of Severe Emergent Cardiovascular Diseases Hospital Admissions During the COVID-19 Pandemic: Experience of a Single Large Center in Northern Italy. J Am Heart Assoc, 2020; 9: e017122 [Google Scholar]
- 7).Gluckman TJ, Wilson MA, Chiu ST, Penny BW, Chepuri VB, Waggoner JW, Spinelli KJ. Case Rates, Treatment Approaches, and Outcomes in Acute Myocardial Infarction During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol, 2020 Aug 7: e203629 [Google Scholar]
- 8).Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic. J Am Coll Cardiol, 2020; 75: 2871-2872 [Google Scholar]
- 9).Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, Ambrosy AP, Sidney S, Go AS. The Covid-19 Pandemic and the Incidence of Acute Myocardial Infarction. N Engl J Med, 2020; 383: 691-693 [Google Scholar]
- 10).Scholz KH, Lengenfelder B, Thilo C, Jeron A, Stefanow S, Janssens U, Bauersachs J, Schulze PC, Winter KD, Schröder J, Vom Dahl J, von Beckerath N, Seidl K, Friede T, Meyer T. Impact of COVID-19 outbreak on regional STEMI care in Germany. Clin Res Cardiol, 2020 Jul 16: 1-11 [Google Scholar]
- 11).Tam CF, Cheung KS, Lam S, Wong A, Yung A, Sze M, Fang J, Tse HF, Siu CW. Impact of coronavirus disease 2019 (COVID-19) outbreak on outcome of myocardial infarction in Hong Kong, China. Catheter Cardiovasc Interv, 2020 May 5: 10.1002/ccd.28943 [Google Scholar]
- 12).Mahmud E, Dauerman HL, Welt FGP, Messenger JC, Rao SV, Grines C, Mattu A, Kirtane AJ, Jauhar R, Meraj P, Rokos IC, Rumsfeld JS, Henry TD. Management of Acute Myocardial Infarction During the COVID-19 Pandemic. J Am Coll Cardiol, 2020 Sep 15; 76: 1375-1384 [Google Scholar]
- 13).Xiang D, Xiang X, Zhang W, Yi S, Zhang J, Gu X, Xu Y, Huang K, Su X, Yu B, Wang Y, Fang W, Huo Y, Ge J. Management and Outcomes of Patients With STEMI During the COVID-19 Pandemic in China. J Am Coll Cardiol, 2020 Sep 15; 76: 1318-1324 [Google Scholar]
- 14).Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care, 2005; 43: 1130-1139 [Google Scholar]
- 15).Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care, 1998; 36: 8-27 [Google Scholar]
- 16).Shin JH, Kunisawa S, Imanaka Y. New outcome-specific comorbidity scores excelled in predicting in-hospital mortality and healthcare charges in administrative databases. J Clin Epidemiol, 2020; 126: 141-153 [Google Scholar]
- 17).Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol, 2017; 46: 348-355 [Google Scholar]
- 18).Bhatt AS, Moscone A, McElrath EE, Varshney AS, Claggett BL, Bhatt DL, Januzzi JL, Butler J, Adler DS, Solomon SD, Vaduganathan M. Fewer Hospitalizations for Acute Cardiovascular Conditions During the COVID-19 Pandemic. J Am Coll Cardiol. 2020; 76: 280-288 [Google Scholar]
- 19).Roffi M, Guagliumi G, Ibanez B. The Obstacle Course of Reperfusion for ST-Segment-Elevation Myocardial Infarction in the COVID-19 Pandemic. Circulation, 2020; 141: 1951-1953 [Google Scholar]
- 20).Ebinger JE, Shah PK. Declining Admissions for Acute Cardiovascular Illness: The COVID-19 Paradox. J Am Coll Cardiol, 2020; 76: 289-291 [Google Scholar]
- 21).Pranata R, Vania R, Tondas AE, Setianto B, Santoso A. A time-to-event analysis on air pollutants with the risk of cardiovascular disease and mortality: A systematic review and meta-analysis of 84 cohort studies. J Evid Based Med, 2020; 13: 102-115 [Google Scholar]
- 22).Ishii H, Amano T, Yamaji K, Kohsaka S, Yokoi H, Ikari Y. Implementation of Percutaneous Coronary Intervention During the COVID-19 Pandemic in Japan - Nationwide Survey Report of the Japanese Association of Cardiovascular Intervention and Therapeutics for Cardiovascular Disease. Circ J, 2020; 84: 2185-2189 [Google Scholar]
- 23).O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2013; 61: e78-e140 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared for ethical/privacy reasons.


