Abstract
Trichoderma (Ascomycetes, Hypocreales) strains that have warted conidia are traditionally identified as T. viride, the type species of Trichoderma. However, two morphologically distinct types of conidial warts (I and II) have been found. Because each type corresponds to a unique mitochondrial DNA pattern, it has been questioned whether T. viride comprises more than one species. Combined molecular data (sequences of the internal transcribed spacer 1 [ITS-1] and ITS-2 regions and of part of the 28S rRNA gene along with results of restriction fragment length polymorphism analysis of the endochitinase gene and PCR fingerprinting), morphology, physiology, and colony characteristics distinguish type I and type II as different species. Type I corresponds to “true” T. viride, the anamorph of Hypocrea rufa. Type II represents a new species, T. asperellum, which is, in terms of molecular characteristics, close to the neotype of T. hamatum.
Species of the genus Trichoderma, among them T. viride, are well known for their production of several lytic enzymes (32) and/or antibiotics (3). Strains of those species are widely used in biocontrol of soilborne plant-pathogenic fungi (see reference 38). The exact characterization and identification of strains to the species level is the first step in utilizing the full potential of fungi in specific applications. T. viride is one of the most commonly reported and widely distributed of all soil fungi (5), occurring in the most extreme to the most mundane of habitats. Many physiological, antifungal, and insecticidal activities have been attributed to this species (for a review, see reference 5). Unfortunately, literature published before 1969 is likely to be unreliable because the name “Trichoderma viride” was applied to most strains of Trichoderma spp. (2). Rifai (36) revised the genus Trichoderma and characterized T. viride by its warted conidia. Since 1969 all Trichoderma strains having globose, subglobose, or ellipsoidal warted conidia have been identified as T. viride. However, in 1989, Meyer and Plaskowitz (27) observed two different types of warts on conidia identified as T. viride, which they termed types I and II (to which we refer here). Later Meyer (28) found that the respective types of conidial ornamentation corresponded to types of specific mitochondrial DNA, and he suggested that two species were involved but did not propose a taxonomy.
Owing to the fact that the name T. viride is applied to many strains that are used in experimental or economically important applications, it is highly desirable that the name used by so many different individuals refer to a single organism. From this perspective, the suggestion by Meyer (28) that the current concept of T. viride comprises more than one species is unsettling. As part of our ongoing studies of Trichoderma systematics (9, 16, 19–23, 29, 39, 40, 47) we have used a polyphasic approach to test Meyer’s two-species hypothesis. In the present work, we report on the application of combined molecular (sequences of the internal transcribed space 1 [ITS-1] and ITS-2 regions and of part of the 28S rRNA gene, along with restriction fragment length polymorphism [RFLP] analysis of the endochitinase gene and PCR fingerprinting), morphometric, and physiological approaches in a study of T. viride. The mixed-type data sets combining information from morphological, physiological, and molecular studies were used in a correspondence analysis (CA) (10, 44) to explore the taxonomic interrelationships within what has been called T. viride.
MATERIALS AND METHODS
Fungal cultures.
In the present study, we included all of the strains that were previously studied by Meyer (28), additional strains of T. viride, anamorphs of Hypocrea collections that could be determined as T. viride, and—for preliminary observations with respect to the position of T. viride in the section Trichoderma—also strains of T. atroviride and T. koningii. Sources and designations of the 71 investigated strains are listed in Table 1. All strains were cultivated on 2% cornmeal dextrose agar (CMD; Sigma). For DNA isolation, strains were grown for 48 h in a liquid medium (19) at room temperature on a shaker (120 rpm).
TABLE 1.
List of strains investigated in this study, including EMBL and GenBank accession numbers for ITS sequences and taxon labels used in UPGMA and CA
| Strain or species | Culture collection no. | Origin | EMBL/GenBank accession no. (ITS; 28S) | Taxon label in CA |
|---|---|---|---|---|
| T. viride (lectotype) | 910-263-877c | Germany (?), wood | ||
| T. hamatum | DAOM 167057 | Canada, soil | ITS, Z48816; 28S, AF127151 | Ha |
| T. viridea | Tr 48 = NRRL 5242 | United States, lab strain | ITS, AJ230669; 28S, AF127145 | Na1d |
| T. viridea | Tr 31 | United States, lab strain | ITS, AJ230669 | Na2d |
| T. viridea | Tr 32 | Korea, soil | ITS, AJ230669 | Na3d |
| T. viridea | Tr 50 | N.C. | ITS, AJ230669 | Na4d |
| T. viridea | GJS 90-14 | Vietnam | ITS, AJ230669 | Na5d |
| T. viridea | GJS 91-1 | South Africa | ITS, AJ230669 | Na6d |
| T. viridea | BBA 68646R | Germany, compost | ITS, AJ230680 | Nb1 |
| T. viridea | Tr 3 = CBS 433.97 = BBA 70684 | Md., Sclerotinia sp. | ITS, AJ230668; 28S, AF127153 | Nb2d |
| T. viridea | Tr 7 | United States, lab strain | ITS, AJ230668 | Nb3d |
| T. viridea | Tr 13 | Md., mutant of Tr 3 | ITS, AJ230668 | Nb4d |
| T. viridea | Tr 44 | Ga., soil | ITS, AJ230668 | Nb5d |
| H. vinosa | GJS 94-81 | New Zealand, Hoheria sp. | ITS, AJ230668 | Nb6d |
| T. viridea | BBA 68543 | Germany, Dieffenbachia sp. | ITS, AJ230668 | Nb7 |
| T. viridea | GJS 91-160 | Brazil, soil | ITS, AJ230668 | Nb8d |
| T. viridea | GJS 90-7 | Vietnam | ITS, AJ230668 | Nb9d |
| T. viridea | GJS 91-162 | Brazil, soil | ITS, AJ230668 | Nb10d |
| T. viridea | GJS 91-24 | ? | ITS, AJ230668 | Nb11d |
| H. spec. | Hy 42 | Wash. | ITS, X93982 | At1 |
| T. atroviride | DAOM 165779 | N.C. | ITS, Z48817; 28S, AF127151 | At2 |
| Hypocrea sp. cf. rufa | GJS 96-200 | Costa Rica | ITS, AJ230659 | At3d |
| H. muroiana | GJS 97-26 | Japan, Quercus sp. with Lentinus edodes | ITS, AJ230659 | At4d |
| H. rufa | GJS 90-134 | Ga., decorticated wood | ITS, AJ230659 | At5d |
| H. rufa | Hy 5 | Ind. | ITS, AJ230659 | At6d |
| T. atroviride | BBA 68768 | Germany, compost | ITS, AJ230659 | At7 |
| T. atroviride | BBA 68600 | Germany, compost | ITS, AJ230659 | At8 |
| T. atroviride | BBA 68601 | Germany, compost | ITS, AJ230659 | At9 |
| T. atroviride | BBA 70227 | Denmark, wet carpet | ITS, AJ230659 | At10 |
| T. atroviride | BBA 70228 | Denmark, wet carpet | ITS, AJ230659 | At11 |
| T. atroviride | BBA 65348R | Sweden, conifer | ITS, AJ230659 | At12 |
| T. virideb | Tr 8 = WSF 2023 | Wis., soil | ITS, X93986 | Vb1d |
| T. virideb | Tr 2 = ATCC 18652 | Colombia, soil | ITS, X93978; 28S, AF127150 | Vb2d |
| T. virideb | Tr 22 = ATCC 28020 | Wash., soil | ITS, AJ230678 | Vb3d |
| T. virideb | GJS 91-62 | Va., Acer wood | ITS, AJ230678 | Vb4d |
| T. virideb | BBA 70238 | Germany, turf | ITS, AJ230678 | Vb5 |
| H. rufa | Hy 70 | New Zealand, Fomes sp. | ITS, AJ230678 | Vb6d |
| T. virideb | GJS 92-14 | New Zealand, pine | ITS, AJ230678 | Vb7d |
| T. virideb | GJS 92-15 | Canada, peat | ITS, AJ230678 | Vb8d |
| Hypocrea sp. cf. rufa | GJS 89-127 | N.C., Indet tree | ITS, X93980 | Vb9d |
| T. virideb | Tr 21 = ATCC 28038 | Va., soil | ITS, AJ230675 | Vb10d |
| T. virideb | GJS 90-95 | N.C., decorticated wood | ||
| H. vinosa | GJS 94-9 | Taiwan, bark | ITS, AJ230670 | Ko1d |
| H. vinosa | GJS 94-10 | Taiwan, bark | ITS, AJ230671 | Ko2d |
| H. vinosa | GJS 94-11 | Taiwan, bark | ITS, AJ230671 | Ko3d |
| T. koningii | ATCC 64262 | Hungary | ITS, Z79628; 28S, AF127149 | Ko4 |
| T. koningii | CBS 457.96 = GJS 96-117 | The Netherlands, soil | ITS, Z79628 | |
| T. koningii | CBS 458.96 = GJS 96-118 | The Netherlands, soil | ITS, Z79628 | Ko5d |
| T. koningii | CBS 459.96 = GJS 96-120 | The Netherlands, soil | ITS, Z79628 | |
| T. koningii | CBS 460.96 = GJS 96-119 | The Netherlands, soil | ITS, Z79628 | |
| T. koningii | GJS 89-122 | Md., wood | ITS, Z79628 | Ko6d |
| H. rufa | GJS 97-243 | Ga., oak wood | ITS, AJ230667 | Ko7d |
| H. rufa | GJS 96-47 | Puerto Rico, wood | ITS, AJ230685 | Ko8d |
| T. atroviride | DAOM 165782 | N.C. | ITS, Z48818 | Ko9 |
| T. koningii | GJS 90-18 | Wis., burned wood | ||
| Trichoderma sp. cf. virideb | BBA 68432 | Russia/Petrograd, wheat | ITS, AJ230686 | Vd1 |
| Trichoderma sp. cf. virideb | BBA 70470 | Germany, wet building | ITS, AJ230682 | Vd2 |
| T. virideb | GJS 92-11 | New Zealand, pine | ITS, AJ230682 | Vd3d |
| Hypocrea sp. cf. rufa | GJS 89-142 | N.C., decorticated log | ITS, X93987 | Vd4d |
| H. rufa | GJS 94-118 | France, bark | ITS, AJ230679 | Vd5d |
| T. virideb | Tr 4 | Oreg., roots of Douglas fir infected with Phellinus weirii | ITS, AJ230682 | Vd6d |
| T. virideb | Tr 5 | Oreg., see Tr4 | ITS, AJ230682 | Vd7d |
| T. virideb | Tr 6 | Oreg., see Tr4 | ITS, AJ230682; 28S, AF127147 | Vd8d |
| T. virideb | Tr 26 = ATCC 32630 | Sweden, beach wood | ITS, AJ230682 | Vd9d |
| Trichoderma sp. cf. virideb | BBA 66069R | Germany, soil | ITS, AJ230681 | Vd10d |
| Trichoderma sp. cf. virideb | BBA 65450 | Germany, soil | ITS, AJ230673 | Vd11d |
| Trichoderma sp. cf. virideb | BBA 70471 | ? | ITS, AJ230682 | Vd12 |
| H. vinosa | GJS 96-163 | Taiwan, wood | ITS, AJ230672 | Ve1d |
| H. rufa | Hy 9 | Fla., wood | ITS, AJ230674; 28S, AF127146 | Ve2 |
| T. viride | GJS 90-20 | Wis., wood | ITS, AJ230676 | Ve3d |
| H. rufa | GJS 90-125 | N.C., oak wood | ITS, AJ230677 | Ve4d |
Morphological studies.
All measurements of anamorph characteristics were taken on cultures grown on CMD for about 1 week at 20 to 22°C. Conidiophores and conidia were measured in 3% KOH or water; KOH was used first in all cases to aid in wetting the conidia. Measurements of teleomorph characteristics were taken on herbarium material that was rehydrated briefly in 3% KOH. When possible, 30 measurements of each parameter in each collection were made.
With the permission of the director of Persoon’s Herbarium (Leiden, The Netherlands), we studied conidial ornamentation in the 200-year-old lectotype collection of T. viride (specimen number 910 263 877 [33]), using scanning electron microscopy (SEM) and light microscopy.
We determined growth rates and colony characteristics at 20, 25, 30, and 35°C on potato dextrose agar (PDA; Difco) and on synthetic low-nutrient agar (SNA) (31). Data were obtained as described by Lieckfeldt et al. (24).
Material for SEM studies was obtained from cultures that were grown on PDA for 2 weeks at 20°C. Agar blocks with abundant conidia were prepared for SEM according to the method of Meyer and Plaskowitz (27). Specimens were examined with a JEOL T300 scanning electron microscope.
DNA isolation and PCR fingerprinting.
PCR fingerprinting, RFLP analysis of the endochitinase gene, and sequencing of the ITS regions of the nuclear rRNA gene (rDNA) cluster of Trichoderma and Hypocrea strains were undertaken. DNA isolation and PCR fingerprinting were carried out as specified by Kuhls et al. (19) with a slight modification of the reaction volume of the PCR assay that amounted to only 25 μl. As primers, the sequence (GACA)4 and phage M13 core sequence 5′-GAGGGTGGCGGTTCT were used.
rDNA amplification and sequencing of the rDNA fragments.
Fragments containing (i) ITS-1, the 5.8S rDNA, and ITS-2 or (ii) the first part of the 28S rRNA gene, including the variable domains D1 and D2 (13), were amplified in a 100-μl-volume reaction as described by Kuhls et al. (21). Parts of the conserved regions of the small-subunit rDNA (primer SR6R [5′-AAGTAGAAGTCGTAACAAGG]) and the large-subunit rDNA (primers LR1 [5′-GGTTGGTTTCTTTTCCT], LR7 [5′-TACTACCACCAAGATCT], and LROR [5′-ACCCGCTGAACTTAAGC]) flanking the ITSs were used as primers (49). For direct cycle sequencing, fragments were purified (QIAquick PCR purification kit; Qiagen). The sequencing was done with an automatic sequencer (model A373; Applied Biosystems). Both DNA strands were sequenced, and reactions were carried out with a BigDye Terminator Cycle Sequencing Kit (Applied Biosystems), using 50 ng of purified PCR fragments and 10 pmol of the respective primer (SR6R, 5.8S, 5.8SR, LR1, or LR3 [49]). Cycle sequencing was performed in a DNA engine (MJ Research) programmed for 50 cycles of 30 s at 95°C, 20 s at 50°C, and 4 min at 60°C after an initial step of 1 min at 95°C. The assay reaction mixture had a volume of 10 μl. Surplus dye terminators were removed by sodium acetate-ethanol precipitation of the DNA.
The 28S rDNA fragments amplified with the primer pair LROR and LR7 were also used for RFLP analysis with the restriction enzymes HaeIII, HhaI, MspI, and Sau96I. The assay followed exactly the conditions described for the RFLP analysis of the endochitinase gene (see below).
PCR amplification and RFLP analysis of the 42-kDa endochitinase gene.
PCRs were performed with the oligonucleotides 5′-CACTTCACCATGTTGGGCTTCCTC and 5′-GATCTCTAGTTGAGACCGCTTCGG as primers (6). The 50-μl reaction volume contained 25 ng of genomic DNA and 0.2 μM each respective primer. The amplification program included an initial denaturation for 4 min at 94°C; 35 cycles of 30 s at 94°C, 1 min at 60°C, and 1 min at 72°C; and a final extension step of 10 min at 72°C. Amplification products from 5 μl of the assay reaction mixture were first checked by electrophoresis in a 1.2% agarose gel buffered with 1× Tris-borate-EDTA (37). For those strains that exhibited two endochitinase gene fragments, the 1.4-kb fragment was purified from the agarose gel by using a Jetsorb purification kit (Genomed, Bad Oeynhausen, Germany) according to the protocol provided by the supplier. For RFLP analysis, 15 μl of the amplification product was completely digested with 12 U of restriction enzyme (HaeIII, HhaI, MspI, or Sau96I) at 37°C according to the manufacturer’s (New England Biolabs) instructions. Digested DNA was electrophoresed in 2.0% agarose gels for 5 h at 110 V in 1× Tris-borate-EDTA buffer.
Data analysis.
For RFLP data, the presence or absence of bands was coded in binary form (0/1) and the matrix was used in both parsimony analysis (PAUP 3.1.1) (46) and cluster analysis with the UPGMA algorithm (Treecon) (48).
The PCR fingerprint patterns of the groups that were revealed by the other molecular methods were compared visually only.
The analysis of sequence data was performed with the PAUP 3.1.1 software after an initial alignment of the nucleotide sequences comprising the entire 650-bp sequence determined with the multiple-alignment program CLUSTAL V, using visual optimization when needed. (Sequence alignments are available upon request.) Parsimony analysis was carried out in two steps. A first analysis included all of the sequences and was performed with the heuristic search option of PAUP 3.1.1 (data not shown). The second search was done on a subset of 10 sequences, representing the main sequence types, by using the branch-and-bound algorithm with single gaps treated as a fifth base. In both analyses, T. longibrachiatum (Z31019) and T. polysporum (Z48815) were used as outgroups. Bootstrap analysis with 1,000 replications was done to test the robustness of the internal branches (7) of the single-most-parsimonious tree that was found in the branch-and-bound analysis.
Exploratory data analysis was carried out on the morphological data, using either analysis of variance or nonparametric tests when appropriate, and included box plot displays, growth curves, and statistical testing. Exploratory analysis and statistical tests were performed with the software package Systat 6.0 (51). In addition, a CA was carried out on the molecular data set as described in detail by Sieber et al. (44). Briefly, a matrix was constructed that contained all molecular data, expressed as the presence or absence (0 or 1, respectively) of a given molecular character or band. The resulting matrix was then subjected to multiple CA, using the package SimCA 2.1 (10).
To test the fitting of the data to the models, CA was also carried out on a combined matrix of the molecular and phenetic data of the individual isolates versus the species centroids (i.e., “ideal species”) (for details of the method, see reference 44). The morphological and molecular characteristics were coded as detailed in Table 2, and then the codes were transformed in class variables as described by Sieber et al. (44). The resulting matrix was then subjected to multiple CA (44). Classes were formed in accordance with the method of Sieber et al. (44). A statistical approach was adopted to define classes within each variable. Means and standard deviations (SD) of the measurements of all collections were calculated. The central class was then defined as the mean ± 0.5 SD, and all other classes were subsequently 1 SD in width. For the computation of the CA shown in Fig. 2, only the data for the isolates studied by morphological and molecular methods were used as active variables, while the data for the ideal species (ATRO, KONI, TV1B, TV1D, TV1E, TV2A, and TV2B) were inserted in the CA as supplementary variables and thus did not influence the outcome of the analysis.
TABLE 2.
Description of morphological and molecular characteristics, classes, and variables used for UPGMA and CA of the T. viride data set
| Characteristic | Characteristic described | Class | Variable |
|---|---|---|---|
| 1 | Angle subtending the phialide (A) | 1 = >100° | A1 |
| 2 = <100° | A2 | ||
| 3 = unknown | A3 | ||
| 2 | Length of conidia from CMD (CL) | 1 = <3.9 μm | CL1 |
| 2 = >3.9 μm | CL2 | ||
| 3 = unknown | CL3 | ||
| 3 | Width of conidia from CMD (CW) | 1 = <3.1 μm | CW1 |
| 2 = >3.1 μm | CW2 | ||
| 3 = unknown | CW3 | ||
| 4 | Length of conidia from SNA (SL) | 1 = 2.5–3.5 μm | SL1 |
| 2 = 3.6–4.0 μm | SL2 | ||
| 3 = >4.0 μm | SL3 | ||
| 4 = unknown | SL4 | ||
| 5 | Width of conidia from SNA (SW) | 1 = 2.5–3.0 μm | SW1 |
| 2 = 3.1–3.5 μm | SW2 | ||
| 3 = >3.5 μm | SW3 | ||
| 4 = unknown | SW4 | ||
| 6 | Phialide length (PH) | 1 = <8.2 μm | PH1 |
| 2 = 8.2–8.5 μm | PH2 | ||
| 3 = >8.5 μm | PH3 | ||
| 7 | Phialide middle (M) | 1 = 2.0 to <3.0 μm | M1 |
| 2 = >3.0 μm | M2 | ||
| 8 | Phialide base (B) | 1 = <2.0 μm | B1 |
| 2 = >2.0 and <2.9 μm | B2 | ||
| 3 = >2.9 μm | B3 | ||
| 9 | Hypha below phialide, axis (AX) | 1 = <2.0 μm | AX1 |
| 2 = 2.0–2.9 μm | AX2 | ||
| 3 = >2.9 μm | AX3 | ||
| 10 | Chlamydospore diameter (CY) | 1 = <9.0 μm | CY1 |
| 2 = 9–9.5 μm | CY2 | ||
| 3 = >9.5 μm | CY3 | ||
| 4 = unknown | CY4 | ||
| 11 | l/w ratio of phialides (PR) from CMD | 1 = <3.0 | PR1 |
| 2 = >3.0 | PR2 | ||
| 3 = unknown | PR3 | ||
| 12 | l/w ratio of conidia (CR) from CMD | 1 = 1.0–1.2 | CR1 |
| 2 = 1.3–1.7 | CR2 | ||
| 3 = unknown | CR3 | ||
| 13 | Ascus length (AL) | 1 = <85 μm | AL1 |
| 2 = >85 μm | AL2 | ||
| 3 = unknown | AL3 | ||
| 14 | Ascus width (AW) | 1 = <6.0 μm | AW1 |
| 2 = >6.0 μm | AW2 | ||
| 3 = unknown | AW3 | ||
| 15 | Length, distal part, ascospore (DL) | 1 = <4.5 μm | DL1 |
| 2 = >4.5 μm | DL2 | ||
| 3 = unknown | DL3 | ||
| 16 | Width, distal part, ascospore (DW) | 1 = <3.9 μm | DW1 |
| 2 = >3.9 μm | DW2 | ||
| 3 = unknown | DW3 | ||
| 17 | Length, proximal part, ascospore (XL) | 1 = 4.0–4.9 μm | XL1 |
| 2 = >4.9 μm | XL2 | ||
| 3 = unknown | XL3 | ||
| 18 | Width, proximal part, ascospore (XW) | 1 = <3.6 μm | XW1 |
| 2 = >3.6 μm | XW2 | ||
| 3 = unknown | XW3 | ||
| 19 | Stromal diameter (ST) | 1 = <1.5 mm | ST1 |
| 2 = >1.5 mm | ST2 | ||
| 3 = unknown | ST3 | ||
| 20 | Perithecial height (PI) | 1 = <232 μm | PI1 |
| 2 = >232 μm | PI2 | ||
| 3 = unknown | PI3 | ||
| 21 | Perithecial width (PD) | 1 = 140–170 μm | PD1 |
| 2 = 171–180 μm | PD2 | ||
| 3 = >180 | PD3 | ||
| 4 = unknown | PD4 | ||
| 22 | Width of surface layer of stroma (SR) | 1 = <20 μm | SR1 |
| 2 = >20 μm | SR2 | ||
| 23 | Diameter of surface cells of stroma seen in section (SC) | 1 = 4.5–5.0 μm | SC1 |
| 2 = >5.0 μm | SC2 | ||
| 3 = unknown | SC3 | ||
| 24 | Surface cells of stroma seen in surface view (SV) | 1 = <5.0 μm | SV1 |
| 2 = 5.0–5.5 μm | SV2 | ||
| 3 = >5.5 μm | SV3 | ||
| 4 = unknown | SV4 | ||
| 25 | Cells of interior of stroma below perithecia (IN) | 1 = 5.0–6.0 μm | IN1 |
| 2 = 6.1–7.0 μm | IN2 | ||
| 3 = >7.0 μm | IN3 | ||
| 26 | Hairs on stroma surface, length (HL) | 1 = <12 μm | HL1 |
| 2 = >12 μm | HL2 | ||
| 3 = unknown | HL3 | ||
| 27 | Hairs on stroma surface, width (HW) | 1 = 3.5–4.1 μm | HW1 |
| 2 = >4.1 μm | HW2 | ||
| 3 = unknown | HW3 | ||
| 28 | Conidial shape (CT) | 1 = subglobose, strongly warted | CT1 |
| 2 = subglobose, weakly warted | CT2 | ||
| 3 = subglobose, smooth | CT3 | ||
| 4 = oblong to ellipsoidal, smooth | CT4 | ||
| 29 | Presence of coconut odor (OD) | 1 = absent | OD1 |
| 2 = present | OD2 | ||
| 30 | Formation of chlamydospores on CMD within 1 week (CH) | 1 = present | CH1 |
| 2 = absent | CH2 | ||
| 31 | Growth on PDA at 72 h, 25°C (P25) | 1 = weak growth | P251 |
| 2 = moderate growth | P252 | ||
| 3 = strong growth | P253 | ||
| 32 | Growth on PDA at 72 h, 30°C (P30) | 1 = weak growth | P301 |
| 2 = moderate growth | P302 | ||
| 3 = strong growth | P303 | ||
| 4 = no growth | P304 | ||
| 33 | Time (h) at which conidia first observed on PDA at 25°C (PF) | 1 = 32 h | PF1 |
| 2 = 40 h | PF2 | ||
| 3 = 64 h | PF3 | ||
| 4 = 72 h | PF4 | ||
| 5 = conidia not formed | PF5 | ||
| 34 | Time (h) at which conidia first observed on SNA at 25°C (SF) | 1 = 32 h | SF1 |
| 2 = 40 h | SF2 | ||
| 3 = 64 h | SF3 | ||
| 4 = 72 h | SF4 | ||
| 5 = conidia not formed | SF5 | ||
| 35 | Conidial color at 25°C on PDA (CC) | 1 = green | CC1 |
| 2 = pale green | CC2 | ||
| 3 = white | CC3 | ||
| 4 = conidia not formed | CC4 | ||
| 36 | Colony reverse on PDA (PS) | 1 = cream | PS1 |
| 2 = cream and folded | PS2 | ||
| 3 = none | PS3 | ||
| 37 | Margin of colony on SNA, 10 days (SE) | 1 = smooth | SE1 |
| 2 = lobed | SE2 | ||
| 3 = fringed, thick ends | SE3 | ||
| 4 = fringed | SE4 | ||
| 5 = loose | SE5 | ||
| 6 = none | SE6 | ||
| 38 | Colony radius on PDA, 25°C, 24 h (P3) | 1 = <5 mm | P31 |
| 2 = >5–10 mm | P32 | ||
| 3 = >10–15 mm | P33 | ||
| 4 = >15 mm | P34 | ||
| 39 | Colony radius on SNA, 25°C, 48 h (S3) | 1 = <5 mm | S31 |
| 2 = <5–10 mm | S32 | ||
| 3 = >10–20 mm | S33 | ||
| 4 = >20–25 mm | S34 | ||
| 5 = >25 mm | S35 | ||
| 40 | Surface of aggregate on CMD (AS) | 1 = conidia formed at surface; projecting, terminally fertile conidiophores conspicuous | AS1 |
| 2 = conidia formed from plumose, projecting conidiophores that are entirely fertile | AS2 | ||
| 3 = aggregates uniformly cottony with no projecting conidiophores | AS3 | ||
| 41 | Morphology of phialides (PM) | 1 = hooked and/or sinuous phialides present | PM1 |
| 2 = phialides straight | PM2 | ||
| 3 = unknown | PM3 | ||
| 42 | Morphology of branches (BT) | 1 = regular with branches tending to be paired and regularly spaced | BT1 |
| 2 = irregular with long internodes between branches, fertile aggregate tending to be sinuous; phialides tending to be solitary | BT2 | ||
| 3 = unknown | BT3 | ||
| 43 | RAPD pattern M13 (RAPD) | Patterns 1–8 | RAPD1–RAPD8 |
| No data | RAPD9 | ||
| 44 | RAPD pattern (GACA)4 (TYPE) | Patterns 1–8 | TYPE1–TYPE8 |
| No data | TYPE9 | ||
| 45 | ITS sequence type (ITS) | Types 1–8 | ITS1–ITS8 |
| 46 | Endochitinase gene fragment (EGF) | 1 = 1 fragment | EGF1 |
| 2 = 2 fragments | EGF2 | ||
| 3 = unknown | EGF3 | ||
| 47 | Endochitinase gene RFLP, HaeIII (EH) | Patterns 1–12 | EH1–EH12 |
| No data | EH13 | ||
| 48 | Endochitinase gene RFLP, HhaI (HhaI) | Patterns 1–6 | HhaI1–HhaI6 |
| No data | HhaI7 | ||
| 49 | Endochitinase gene RFLP, MspI (MspI) | Patterns 1–11 | MspI1–MspI11 |
| No data | MspI12 | ||
| 50 | Endochitinase gene RFLP, Sau96I (Sau96I) | Patterns 1–5 | Sau96I1–Sau96I5 |
| No data | Sau96I6 | ||
| 51 | 28S sequence type (D28) | Types 1–6 | D281–D286 |
| No data | D280 | ||
| 52 | 28S RFLP, HaeIII (DH28) | Pattern 1 | DH281 |
| No data | DH280 | ||
| 53 | 28S RFLP, HhaI (RHh) | Pattern 1 | RHh1 |
| No data | RHh0 | ||
| 54 | 28S RFLP, MspI (PM) | Pattern 1 | PM1 |
| Pattern 2 | PM2 | ||
| No data | PM0 | ||
| 55 | 28S RFLP, Sau96I (PS) | Pattern 1 | PS1 |
| No data | PS0 |
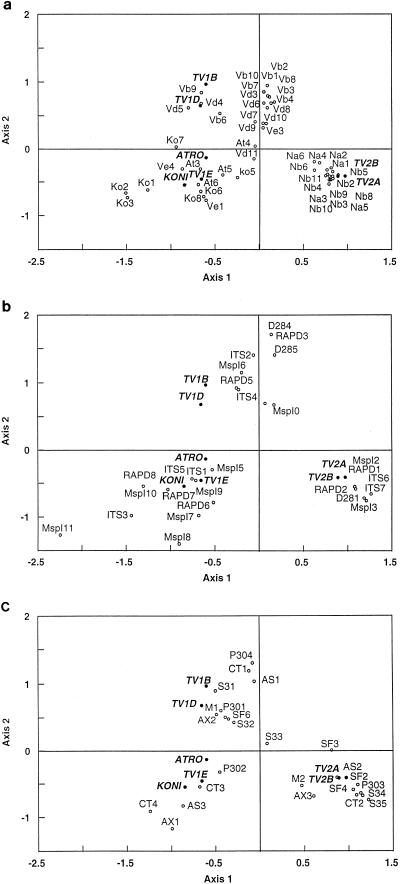
FIG. 2.
Discrimination of T. viride taxa by simple CA. (a) Graph of all 47 isolates and the ideal species supplemented after the analysis; (b) graph of important molecular characteristics and ideal species; (c) graph of important morphological characteristics of the anamorphs and ideal species. The scales of the two axes in all panels are the same, and the black dots of the ideal species are added to provide orientation.
Nucleotide sequence accession numbers.
The ITS nucleotide sequences determined in this study have been submitted to the GenBank and EMBL databases, and their accession numbers are listed in Table 1.
RESULTS
Morphology.
Fifty-seven strains of Trichoderma and Hypocrea (Table 1) were characterized with respect to a total of 42 morphological and physiological characteristics (Table 2). For the Persoon collection (lectotype material), SEM of conidia was performed. Conidia from this collection were measured, and the few remaining phialides were observed.
(i) Anamorph characteristics.
The most important characteristics are presented in Table 3. A more-detailed description of morphological characteristics is given elsewhere (41). Conspicuous differences in conidial shape and ornamentation, arrangement of conidiophores within conidial aggregates, branching of the conidiophores (regular or irregular), the arrangement of phialides on conidiophores, and the shape of the phialides were seen.
TABLE 3.
Salient phenotypic characteristics of strains of T. viride M type I (true T. viride) and type II (T. asperellum)
| T. viride group | Characteristic
|
|||||
|---|---|---|---|---|---|---|
| Conidial warts | Conidiophores | Phialides | Chlamydospores | Temp optimum | Colony radius (mm)a | |
| T. viride | Conspicuous | Irregularly branched; branches usually not paired | Tending to be sigmoidal or hooked; l/w ratio, 3.3 | Typically absent | 25 | 11–33 |
| T. asperellum | Inconspicuous | Regularly branched; branches typically paired | Straight; l/w ratio, 2.4 | Typically present | 30 | 31–47 |
Radii of colonies on PDA after incubation at optimum temperature for 48 h.
Three basic types of conidial ornamentation were observed by light microscopy and SEM. Some collections had conspicuous, grossly warted conidia with broadly rounded warts (T. viride type I [27]). Conidial warts of other collections were slightly more irregular and pyramidal (T. viride type II [27]). A number of collections had smooth conidia (T. atroviride or T. koningii). Conidia of Persoon specimens of T. viride had conspicuous, broadly rounded warts of type I.
Phialides of T. viride types I and II had a mean length of 9 μm or more, while those of T. atroviride and T. koningii tended to be shorter, with a median length of less than 8.5 μm. The length/width (l/w) ratio of phialides was greater than 3 for T. viride type I and T. koningii and less than 3 for T. viride type II and T. atroviride.
The median lengths and widths of the conidia of all the strains were between 3.0 and 4.5 μm and between 2.0 and 4.0 μm, respectively. The most obvious difference was in conidial shape, which is to some extent reflected by the l/w ratio of the conidia. Conidia of T. koningii are conspicuously oblong to ellipsoidal and have an l/w ratio of 1.6, whereas conidia of T. viride types I and II and T. atroviride are globose to subglobose, with l/w ratios of between 1.1 and 1.2. There is a tendency for conidia of type II to have a slightly larger l/w ratio than those of type I, and therefore many conidia of type II tend to appear ovoidal rather than subglobose.
Chlamydospores were produced within 1 week by some members of all groups but were more frequently found in T. viride type II than in type I; T. koningii and T. atroviride had intermediate positions.
(ii) Cultural observations.
Strains of T. viride type II had a much higher growth rate than type I strains, T. atroviride, or T. koningii. This was especially evident at or above 30°C. At 30°C, T. viride type I, T. atroviride, and T. koningii colonies had radii of less than 10 mm, whereas the radii of colonies of T. viride type II reached nearly 30 mm within 40 h. Only strains of type II were able to grow at 35°C.
A coconut-like odor was detected for all T. atroviride strains and for some strains of T. viride types I and II but not for any of the T. koningii strains.
None of the colonies produced a diffusing pigment. All produced dark-green conidia (18) (28D8) on CMD within 1 week when grown at 20°C under conditions of alternating cool white fluorescent light and darkness. There were, however, differences between T. viride types I and II in the speed with which conidia were formed and became green. Conidia formed in PDA cultures of T. viride type II strains grown at 20 or 25°C were already dark green within 40 to 48 h, whereas the type I strains and most T. atroviride and T. koningii strains grown at 20 or 25°C formed conidia very slowly, and within 40 to 48 h there were either no conidia or only a few pale-green conidia.
(iii) Teleomorph characteristics.
Each of the distinct groups that we studied included strains that were derived, or were said to have been derived, from ascospores of Hypocrea species. Unfortunately, only one strain derived from ascospores was included in T. viride type II (GJS 94-81; ICMP 5411 as Hypocrea vinosa), but we were not able to locate a specimen from which that culture was derived (PDD) and thus cannot confirm that connection.
Stromata of all groups formed either on decorticated wood, less frequently on bark, of hardwood trees and other fungi. They were pulvinate to discoidal, 0.5 to 1.5 mm in diameter, and brown; when young, stromata tended to be slightly effused and were light tan at the periphery. The surface was velvety, especially when young, and ostiolar openings were not visible. We could not distinguish the teleomorphs of the various Trichoderma groups based only on their anatomy or gross morphology.
DNA data.
For a total number of 65 strains, we obtained PCR fingerprinting, RFLP, and sequence data.
(i) ITS sequencing.
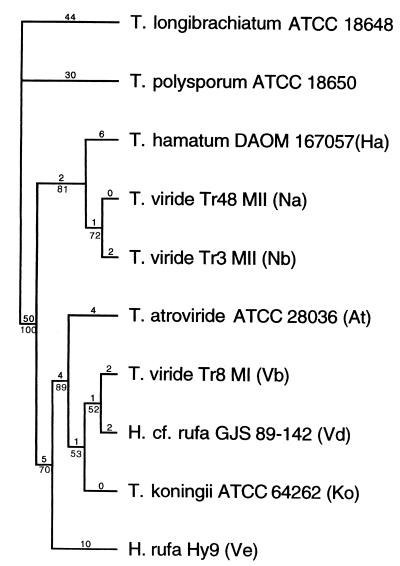
Sequencing of the ITSs of the ribosomal gene complex revealed a total of 13 to 17 base pair differences in ITS-1 and ITS-2 sequences between T. viride types I and II (Fig. 1). Each of those two sequence groups included two subgroups. Conidial ornamentation type I includes ITS groups Vb and Vd, and type II includes ITS groups Na and Nb. Moreover, subgroups Vb and Vd clustered together with strains that conformed morphologically, and in their DNA characteristics as well, to T. atroviride (subgroup At) and T. koningii (subgroup Ko), respectively. In addition, there was a fifth ITS subgroup, Ve, that did not conform to any described Trichoderma species. Ve was basal to the cluster including Vb, Vd, At, and Ko (Fig. 1). Sequence variation among the subgroups was found to be generally very low. For example, Na and Nb differed in 1 bp each in ITS-1 and ITS-2. Interestingly, there was also little sequence variation (ITS-1, 1 or 2 bp; ITS-2, 2 to 5 bp) among T. viride type I subgroups Vb and Vd, T. atroviride (At), and T. koningii (Ko). The only exception was subgroup Ve, which showed sequence differences from all other subgroups that were in the same range (12 to 17 bp) as was found for the main groups of types I and II. This was due to a greater within-subgroup sequence variation in Ve. In the parsimony tree (Fig. 1), T. viride type I (Vb and Vd), Ve, At, and Ko form one clade whereas T. viride type II (Na and Nb) forms a second clade, both supported by high bootstrap values. The separation of subgroups in this tree is not so clear. Notable is the position of the ex neotype strain of T. hamatum as a sister clade of the clade including the type II strains of T. viride.
FIG. 1.
Phylogenetic relationships among subgroups of T. viride. The cladogram is the single most parsimonious tree inferred by parsimony analysis based on rDNA sequences, including the ITS-1, 5.8S, and ITS-2 regions, using the branch-and-bound algorithm of PAUP 3.1.1 (164 steps, CI = 0.951; RI = 0.896; RC = 0.852). T. longibrachiatum and T. polysporum were used as outgroups. The numbers below the branches indicate the percentage at which a given branch was supported in 1,000 bootstrap replications. The total number of nucleotide changes assigned to each branch is shown above the branch.
An attempt, done with permission, to isolate DNA from the lectotype specimen of T. viride was not successful.
(ii) 28S RFLPs and sequencing.
For a total of 20 strains, representing all seven ITS subgroups, a 569-bp part of the 5′ end of the 28S rDNA spanning the variable domains D1 and D2 (13) was first analyzed by RFLP with four restriction enzymes and then sequenced. Although the base pair differences in D1 and D2 in general were less numerous than those found in the ITS regions, the same groups and subgroups were resolved in a PAUP tree (data not shown) (alignments are available upon request).
(iii) PCR fingerprinting.
PCR fingerprinting with two different primers gave patterns that distinguished T. viride type I strains from those of type II. Moreover, the patterns show intragroup variation reflecting the subgroups defined by ITS sequence variation in both groups (data not shown).
(iv) The 42-kDa endochitinase gene.
PCR amplification of the 42-kDa endochitinase gene resulted in a product of the expected size (1,450 bp) for all investigated strains. Strikingly, there was an additional reproducible fragment (900 bp) in all 11 strains of ITS subgroup Nb. The 900-bp fragment was also present in strain Na3 (the second subgroup of type II). RFLP analysis of the 1,450-bp fragment revealed 5 to 12 patterns for the four enzymes used. The calculated 1/0 data for all enzymes were combined and used in parsimony as well as cluster analysis of 20 strains representing all ITS subgroups. The trees resulting from both analyses yielded essentially the same results as did ITS sequences (data not shown). There was a clear separation of T. viride types I and II into two clusters with a distance value of more than 0.6 between them (UPGMA analysis). RFLP analysis of the endochitinase gene revealed the same subgroups of types I and II as were revealed by ITS sequencing. However, one of the strains that was placed in subgroup Ve (Ve3 = GJS 90-20) by ITS sequencing was placed in Vb by the endochitinase analysis. Although Ve is morphologically and genetically diverse, strain Ve3 is morphologically consistent with T. viride type I.
Combined data analysis. (i) CA of molecular data.
The result of a simple CA performed on the weighted matrix of molecular data, including the information from ITS and 28S rDNA sequencing, PCR fingerprinting, and RFLP analysis of the endochitinase gene, revealed a good discrimination of the two subgroups Na and Nb of T. viride type II on the first axis. All other isolates, representing T. viride type I, T. atroviride, and T. koningii, were not separated by the numerical analysis (data not shown).
(ii) CA of combined morphological and molecular data.
The results of the analysis using both morphological and molecular data are detailed in Fig. 2. The analysis was carried out on a standardized data matrix that included 13 molecular and 42 morphological characteristics. For each of the characteristics we defined classes, resulting in a total of 217 classes, which are described in Table 2. To minimize the influence of missing data, CA of combined data was carried out on 47 strains only (indicated in Table 1). The inertia explained by the first four factors was approximately 38% for this analysis and shows a good fit of the data to the model. In Fig. 2a, the positions of all isolates studied and of the ideal species (as constructed by using the results of the original analysis) are shown. All isolates belonging to one of the two subgroups of T. viride type II (Na or Nb) group closely to the ideal species TV2A and TV2B (as defined for T. viride type II) and are clearly separated from all other entities (discrimination on the first axis). The morphological data, however, do not distinguish the two groups that were discriminated in the molecular analysis, as can be seen from the compact group that they form. On the other hand, the other entities that could not be separated very clearly by molecular data seem to be more clearly separated according to morphological data in this analysis. Strains of subgroups At and Ko are no longer included in the big group that comprises Vb and Vd but form one group together with two isolates of Ve. This group also includes the ideal species ATRO, KONI, and TV1E (as defined for T. atroviride, T. koningii, and an as-yet-unnamed group of Hypocrea and Trichoderma collections that showed similarities to T. viride [Fig. 2a]). A few isolates of the group (Ko1, Ko2, and Ko3) have a more isolated position in the graph and could be considered distinct from all other isolates of this group. Isolates At4, Ko7, and Vd11 are also somewhat distant from the main groups, and very close to one of the axes, but are clearly separated from group Na-Nb. Strikingly, one member of subgroup Ve (Ve3) is very close to a third group of taxa including all isolates of Vb and Vd except Vb6, Vb9, Vd4, and Vd5. The last four isolates form a clear group centered on the ideal species TV1B and TV1D and close to the main Vb-Vd cluster.
Figure 2 gives the positions of the important molecular (Fig. 2b) and morphological (Fig. 2c) characteristics of the anamorphs that are significant for the characterization of the three main entities including the ideal species that we found: (i) TV1B and TV1D; (ii) TV2A and TV2B; and (iii) ATRO, KONI, and TV1E. With respect to molecular characteristics, we found randomly amplified polymorphic DNA (RAPD) patterns, ITS and 28S rDNA sequences, and RFLP patterns of the endochitinase gene to be group specific. Among the large number of physiological and morphological characteristics, we found that the growth rate on PDA at 30°C, time when conidia developed first on SNA at 25°C, colony radius after 48 h of growth on SNA at 25°C, surface of aggregates on CMD, width of the hypha below each phialide, phialide width, and conidial shape were significant for each of the three groups. We had no teleomorph data for the group Na-Nb, but some teleomorph characteristics were useful for the characterization and separation of groups Vb-Vd and At-Ko-Ve from each other.
Biogeography.
The strains that we used in this study were obtained from culture collections or were isolated by us. Thus, the geographic representation of the set of strains is limited. All groups have a cosmopolitan distribution, each including one or more strains that originated from Europe or North America as well as from either eastern Asia or the southwest Pacific region. With regard to types I (Vb and Vd) and II (Na and Nb) of T. viride, all strains of type I occurred in northern or southern temperate locations whereas there seemed to be a tendency for strains of type II to occur in warm regions.
DISCUSSION
We have used a polyphasic approach that combines data sets from morphological, physiological, and molecular investigations to study relationships among strains of T. viride. Formal, traditional taxonomy is based solely on morphology or, more recently, and alternatively, on molecular data only. Although this has been a major point of criticism by fungal taxonomists (43), there are only a few examples of the use of combined data sets in studies of fungi (34, 35, 45). Perhaps this is due to the complexity of mathematical analyses using mixed types of data. Cluster analysis (used to detect groupings in data) and CA (with the main advantage of obtaining corresponding characteristics and taxon ordination simultaneously) were used in our study to handle the large data sets. Initially, all data were evaluated independently and then combined in a single analysis to find out whether groupings of strains were still supported and to deduce characteristics that are significant for species or group recognition.
The results of DNA sequencing clearly support Meyer’s (28) contention that T. viride is paraphyletic. The base pair sequence differences between T. viride types I and II were in the range of interspecies variability compared to data for other species of the same genus (21, 24). Additional molecular data as well as morphological characteristics unanimously confirm the existence of T. viride types I and II, with type I representing Vb and Vd and type II representing Na and Nb. T. viride type I (Vb plus Vd) can be distinguished from T. viride type II (Na plus Nb) by ITS sequences, PCR fingerprinting patterns, RFLPs of the 42-kDa endochitinase gene, growth rate (especially at 35°C), colony characteristics, branching patterns of conidiophores, morphology of phialides, and conidial ornamentation. These differences indicate that T. viride type II is a distinct species, for which we have formally proposed the name T. asperellum in another publication (41). Basically, T. viride can be recognized under a light microscope by its strongly warted, subglobose to globose conidia.
Meyer’s type II accounts for collections that have finely ornamented, subglobose to broadly ellipsoidal conidia. ITS sequencing resolved type II into two groups, which differed from each other by 1 bp each in ITS-1 and ITS-2. In comparison to the ITS sequence variability noted for several species of Trichoderma (reviewed in reference 23), this is in the range of intraspecies variation. To test our findings from ITS sequencing (e.g., the possibility of a subgroup splitting in T. viride type II), other molecular characteristics were used. The final CA suggests that, according to the molecular data, (i) type II may be considered a separate entity and (ii) subgroups (Na and Nb) are resolved. We could not find, however, any justification morphologically to recognize the groups Na and Nb as independent taxa, and thus in the CA of combined data the subgroups Na and Nb of T. viride type II form only a single species (Fig. 2).
T. viride type I is not as straightforward as type II. We found groups that could be readily recognized by conidial morphology, ornamentation, the branching pattern of conidiophores, and phialide morphology. Strikingly, molecular data did not resolve subgroups. This might be due to highly similar ITS sequences: each of the morphologically characterized groups differed from each other by no more than 7 bp, values that are common for intraspecies variation in the genus Trichoderma (23).
Our examination of the specimen of T. viride from the Persoon herbarium, which Bisby (2) designated the lectotype, shows subglobose conidia that have large, conical warts and are consistent with most of the representatives of T. viride type I subgroups Vb and Vd. We agree with Meyer (27, 28) that his T. viride type I is true T. viride. The Persoon material is too old to show how the phialides are arranged, but a few hooked phialides, typical of T. viride type I subgroups Vb and Vd, were seen, and there was an indication of a branching pattern that we described for type I. Unfortunately, we were unable to obtain DNA from conidia of this specimen in two attempts.
ITS sequences of subgroups At and Ko are identical to sequences published by Kuhls et al. (21) for T. atroviride and for T. koningii/H. koningii (24), respectively. Strains of both groups differed from T. viride in having smooth conidia that were subglobose (At) or oblong and at most slightly ornamented (Ko).
A number of isolates had a somewhat isolated position in the CA display. In most cases, the small amount of molecular data available for the isolates could be a reason for the positions of the isolates in the graph (At4, Vd11, and Ko7). The position of Ve3 close to Vb and Vd is supported by morphological and most of the molecular data, except the ITS-1 sequence, which strongly differs from the types found for Vb and Vd. The three isolates Ko1, Ko2, and Ko3 were all Hypocrea strains collected in Taiwan; morphologically, they fit the description of H. vinosa. In Fig. 2a, a slight splitting of strains of subgroups Vb and Vd is visible. Interestingly, the four strains (Vb6, Vb9, Vd4, and Vd5) that are closest to the ideal species TV1B and TV1D are all anamorphs of H. rufa collections, whereas the main and very large group of Vb and Vd represents only T. viride collections. This last interpretation, however, must be taken with caution, since the distances on the map barely allow one to draw firm conclusions. The findings, however, do not contradict the general outcome of the CA of morphological, physiological, and molecular characteristics with respect to the characterization of two species of T. viride. In ongoing experiments, emphasis is on a detailed characterization of the relationship between the true T. viride—subgroups Vb and Vd, T. atroviride, and T. koningii—and the more diverse group Ve by extending the number of Trichoderma and Hypocrea strains that morphologically fit into this complex. We have checked the possibility that group Ve is similar or even identical to T. pubescens or T. strigosum, both of which have been shown recently to cluster near T. viride (16). We have also tested whether Ve could be one of the Hypocrea sp. cf. muroiana sequence types that have been described previously (24). In all sequence comparisons so far, strains of Ve remained a separate cluster, but it must be emphasized again that the level of genetic variability in the genus Trichoderma, as concluded from molecular data, is very low. The results of the extended investigations will perhaps answer the question of whether all strains that we studied and that exhibit a low level of genetic diversity and intergrading phenotypic characteristics should be recognized as distinct taxa on some level. The high degree of genetic similarity of species within section Trichoderma with overlapping sequence variation in ITS-1 and ITS-2 of the rDNA nicely reflects the processes of evolution and speciation.
The teleomorphs encountered in this study are phenotypically homogeneous. We detected small differences in ascospore measurements, but while these differences were statistically significant, their biological significance is doubtful. These collections agree in most details with the description of H. rufa that was published by Webster (50). The main differences are that Webster reported larger ascospores (distal, 5.5 to 7.2 by 4.5 to 5.0 μm; proximal, 5.5 to 7.0 by 3.6 to 4.5 μm) for one of his English specimens than we have observed (3.9 to 5.3 by 4.1 to 4.6 μm and 4.1 to 5.7 by 3.0 to 4.2 μm, respectively). Other specimens listed by Webster had smaller ascospores. The anamorph reported by Webster as T. viride fits in with our concept of T. viride but produced abundant chlamydospores.
The cosmopolitan distribution of Trichoderma species makes them ideal candidates for biocontrol applications in different habitats, but the sensitivity of single strains to abiotic environmental factors must be considered (15). Köhl and Schlösser (17) reported differences in antagonistic activity at different temperatures among Trichoderma isolates within a species group. Temperature tolerance of biocontrol isolates relative to that of the pathogen could be critical to the success of an application. Because T. viride is often cited as producing antibiotics and as acting as a fungal antagonist and potential biocontrol agent against soilborne plant-pathogenic fungi (1, 38), it was especially interesting and important to learn about the temperature optimum of T. viride type I and II strains. Types I and II of T. viride are easily separated on the basis of temperature optima. Type II (T. asperellum) has an optimum of 30°C and a maximum of >35°C, and true T. viride has an optimum temperature of 22.5°C and a maximum of 30°C. The temperature tolerance of strains might be an important factor with respect to T. viride strains that act as promoters or inhibitors of plant growth (25, 26) or even as weed pathogens in mushroom cultures (42). The strikingly different temperature optima among T. viride strains was also noted by Fujimori and Okuda (8), who described two groups clearly separated by RAPD analysis. Furthermore, both groups were characterized as producers or nonproducers of isonitrile antibiotics. We have analyzed representatives of both groups morphologically and by RFLP analysis of the ITS-1–5.8S–ITS-2 rDNA region and the endochitinase gene. T. viride NR 5566 and NR 6937 (isonitrile antibiotic producers) are strains of T. asperellum, the new species, whereas NR 5510, NR 5541, NR 6898, NR 6955, NR 6896, NR 6969, FP 5563, and FP 5564 (nonproducers) are true T. viride.
Of the strains that we originally investigated, only Tr48 has been used in published physiological or biocontrol studies (14); we have investigated a larger number of T. viride strains with biocontrol application listed in the American Type Culture Collection catalogue (ATCC 20900, ATCC 20905, ATCC 90200, ATCC 34650, ATCC 38717, and ATCC 52439). Only one of the six strains had finely warted conidia and was identified as T. asperellum. The remaining five strains have molecular characters similar to those of members of Trichoderma section Pachybasium, which includes T. inhamatum and T. harzianum. In a list of commercial biocontrol products for use against soilborne crop diseases (47a), we found only one T. viride strain. We have analyzed the substratum Ecofit in terms of isolating DNA from conidia as well as from mycelium of the included fungus. The fungus was identified as T. asperellum, too. In a search of sequence data banks, we found two T. harzianum strains used in biocontrol which have the same sequence types as subgroup a of type II, viz. T. harzianum T-203 (accession no. AF04971 [3a]) and T. harzianum strain 3 (accession no. Y13575 [12]). Moreover, a number of sequences submitted for strains identified as T. viride that have biocontrol activities are actually T. asperellum (accession no. AF059515 (4). In several cases, the strains were T. atroviride (ATCC 32173 [30]; DB 35916, IMI 296237, and IMI 304531 [1]; and IMI 110150 [12]). All search results strongly indicate that T. viride biocontrol strains reported to have high temperature optima are actually T. asperellum. No true T. viride strain was found to produce antibiotics.
ACKNOWLEDGMENTS
This work was supported by grant EL 627/4 from the Deutsche Forschungsgemeinschaft (Bonn, Germany) to E.L. and by a grant from the Fond der Chemischen Industrie (Frankfurt am Main, Germany) to Thomas Börner (Humboldt-Universität, Berlin, Germany). G.J.S. was supported in part by NSF-97-12308 (a PEET grant) to the Pennsylvania State University.
We are grateful to R. Vilgalys and T. G. Mitchell (Duke University, Durham, N.C.) for providing the rDNA primers. We also thank B. Liebe, Y. Claußner, and Yolaine Covignac (Berlin) for technical assistance; J. Müller (Berlin) for sequencing with the automatic sequencer; and J. Plaskowitz (USDA-ARS, Beltsville, Md.) for preparing the SEM samples.
REFERENCES
- 1.Arisan-Atac I, Heidenreich E, Kubicek C P. Randomly amplified polymorphic DNA fingerprinting identifies subgroups of Trichoderma viride and other Trichoderma sp. capable of chestnut blight biocontrol. FEMS Microbiol Lett. 1995;126:249–256. doi: 10.1111/j.1574-6968.1995.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 2.Bisby G R. Trichoderma viride Pers. ex Fries, and notes on Hypocrea. Trans Br Mycol Soc. 1939;23:149–168. [Google Scholar]
- 3.Chet I. Trichoderma—application, mode of action, and potential as biocontrol agent of soilborne plant pathogenic fungi. In: Chet I, editor. Innovative approaches to plant disease control. New York, N.Y: John Wiley & Sons; 1987. pp. 137–160. [Google Scholar]
- 3a.Chet, I. 1998. Personal communication.
- 4.Dodd, S., R. N. Crowhurst, A. G. Rodrigo, G. J. Samuels, R. A. Hill, and A. Stewart. Examination of Trichoderma phylogenies derived from ribosomal DNA sequence data. Mycol. Res., in press.
- 5.Domsch K H, Gams W, Anderson T-H. Compendium of soil fungi. Vol. 1. London, United Kingdom: Academic Press; 1980. [Google Scholar]
- 6.Fekete C, Weszely T, Hornok L. Assignment of a PCR-amplified chitinase sequence cloned from Trichoderma hamatum to resolved chromosomes of potential biocontrol species of Trichoderma. FEMS Microbiol Lett. 1996;145:385–391. doi: 10.1111/j.1574-6968.1996.tb08605.x. [DOI] [PubMed] [Google Scholar]
- 7.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujimori F, Okuda T. Application of the random amplified polymorphic DNA using the polymerase chain reaction for efficient elimination of duplicate strains in microbial screening. I. Fungi. J Antibiot. 1994;47:173–182. doi: 10.7164/antibiotics.47.173. [DOI] [PubMed] [Google Scholar]
- 9.Gams W, Meyer W. What exactly is Trichoderma harzianum Rifai? Mycologia. 1998;90:904–915. [Google Scholar]
- 10.Greenacre M J. SimCA: a program to perform simple correspondence analysis. Am Stat. 1986;40:230–231. [Google Scholar]
- 11.Greenacre M J. Correspondence analysis in medical research. Stat Methods Med Res. 1992;1:97–117. doi: 10.1177/096228029200100106. [DOI] [PubMed] [Google Scholar]
- 12.Grondona I, Hermosa R, Tejada M, Gomis M D, Mateos P F, Bridge P D, Monte E, Garcia-Acha I. Physiological and biochemical characterization of Trichoderma harzianum, a biological control agent against soilborne fungal plant pathogens. Appl Environ Microbiol. 1997;63:3189–3198. doi: 10.1128/aem.63.8.3189-3198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gueho E, Improvis L, Christen R, De Hoog G S. Phylogenetic relationships of Cryptococcus neoformans and some related basidiomycetous yeasts determined from partial large subunit rRNA sequences. Antonie Leewenhoek. 1993;63:175–189. doi: 10.1007/BF00872392. [DOI] [PubMed] [Google Scholar]
- 14.Hau C T, Ciegler A, Hesseltine C W. New mycotoxin, a trichotoxin from Trichoderma viride isolated from southern leaf blight-infected corn. Appl Microbiol. 1972;23:183–185. doi: 10.1128/am.23.1.183-185.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hjeljord L, Tronsmo A. Trichoderma and Gliocladium in biocontrol: an overview. In: Kubicek C P, Harman G E, editors. Trichoderma and Gliocladium. London, United Kingdom: Taylor & Francis Ltd.; 1998. pp. 131–151. [Google Scholar]
- 16.Kindermann J, El-Ayouti Y, Samuels G J, Kubicek C P. Phylogeny of the genus Trichoderma based on sequence analysis of the internal transcribed spacer 1 of the rDNA cluster. Fungal Genet Biol. 1998;24:298–309. doi: 10.1006/fgbi.1998.1049. [DOI] [PubMed] [Google Scholar]
- 17.Köhl J, Schlösser E. Decay of sclerotia of Botrytis cinerea by Trichoderma spp. at low temperatures. J Phytopathol. 1989;125:320–326. [Google Scholar]
- 18.Kornerup A, Wanscher J H. Methuen handbook of colour. London, United Kingdom: Methuen; 1978. [Google Scholar]
- 19.Kuhls K, Lieckfeldt E, Börner T. PCR-fingerprinting used for comparison of ex type strains of Trichoderma species deposited in different culture collections. Microbiol Res. 1995;150:363–371. doi: 10.1016/S0944-5013(11)80017-0. [DOI] [PubMed] [Google Scholar]
- 20.Kuhls K, Lieckfeldt E, Samuels G J, Kovacs W, Meyer W, Petrini O, Gams W, Börner T, Kubicek C P. Molecular evidence that the asexual industrial fungus Trichoderma reesei is a clonal derivative of the ascomycete Hypocrea jecorina. Proc Natl Acad Sci USA. 1996;93:7755–7760. doi: 10.1073/pnas.93.15.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhls K, Lieckfeldt E, Samuels G J, Meyer W, Kubicek C P, Börner T. Revision of Trichoderma sect. Longibrachiatum including related teleomorphs based on analysis of ribosomal DNA internal transcribed spacer sequences. Mycologia. 1997;89:442–460. [Google Scholar]
- 22.Leuchtmann A, Petrini O, Samuels G J. Isozyme subgroups in Trichoderma section Longibrachiatum. Mycologia. 1996;88:384–394. [Google Scholar]
- 23.Lieckfeldt E, Kuhls K, Muthumeenakshi S. Molecular taxonomy of Trichoderma and Gliocladium and their teleomorphs. In: Kubicek C P, Harman G E, editors. Trichoderma and Gliocladium. London, United Kingdom: Taylor & Francis Ltd.; 1998. pp. 35–56. [Google Scholar]
- 24.Lieckfeldt E, Samuels G J, Börner T, Gams W. Trichoderma koningii: neotypification and Hypocrea teleomorph. Can J Bot. 1998;76:1507–1522. [Google Scholar]
- 25.Lindsey D L, Baker R. Effect of certain fungi on dwarf tomatoes grown under gnotobiotic conditions. Phytopathology. 1967;57:1262–1263. [Google Scholar]
- 26.Menzies J G. A strain of Trichoderma viride pathogenic to germinating seedlings of cucumber, pepper and tomato. Plant Pathol. 1993;42:784–791. [Google Scholar]
- 27.Meyer R, Plaskowitz J S. Scanning electron microscopy of conidia and conidial matrix of Trichoderma. Mycologia. 1989;81:312–317. [Google Scholar]
- 28.Meyer R J. Mitochondrial DNAs and plasmids as taxonomic characteristics in Trichoderma viride. Appl Environ Microbiol. 1991;57:2269–2276. doi: 10.1128/aem.57.8.2269-2276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer W, Morawetz R, Börner T, Kubicek C P. The use of DNA-fingerprint analysis in the classification of some species of the Trichoderma aggregate. Curr Genet. 1992;21:27–30. [Google Scholar]
- 30.Neethling D, Nevalainen H. Mycoparasitic species of Trichoderma produce lectins. Can J Microbiol. 1995;42:141–146. doi: 10.1139/m96-022. [DOI] [PubMed] [Google Scholar]
- 31.Nirenberg H I. Untersuchungen über die morphologische und biologische Differenzierung inder Fusarium-Sektion Liseola. Mitt Biol Bundesanst Land- Forstwirtsch Berl-Dahl. 1976;169:1–117. [Google Scholar]
- 32.Papavizas G C. Trichoderma and Gliocladium: biology, ecology, and potential for biocontrol. Annu Rev Phytopathol. 1985;23:23–54. [Google Scholar]
- 33.Persoon C H. Dispositio methodica fungorum. Römer’s neues Bot Mag. 1794;1:81–128. [Google Scholar]
- 34.Petrini L E. Rosellinia species of the temperate zones. Sydowia. 1992;44:169–281. [Google Scholar]
- 35.Petrini O, Petrini L E, Laflamme G, Oulette G B. Taxonomic position of Gremmeniella abietina and related species: a reappraisal. Can J Bot. 1989;67:2805–2814. [Google Scholar]
- 36.Rifai M A. A revision of the genus Trichoderma. Mycol Papers. 1969;116:1–56. [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Samuels G J. Trichoderma: a review of biology and systematics of the genus. Mycol Res. 1996;100:923–935. [Google Scholar]
- 39.Samuels G J, Petrini O, Manguin S. Morphological and macromolecular characterization of Hypocrea schweinitzii and its Trichoderma anamorph. Mycologia. 1994;86:421–435. [Google Scholar]
- 40.Samuels G J, Petrini O, Kuhls K, Lieckfeldt E, Kubicek C P. The Hypocrea schweinitzii complex and Trichoderma sect. Longibrachiatum. Stud Mycol. 1998;41:1–54. [Google Scholar]
- 41.Samuels G J, Lieckfeldt E, Nirenberg H I. Description of T. asperellum sp. nov. and comparison to T. viride. Sydowia. 1999;51:71–88. [Google Scholar]
- 42.Seaby D. Trichoderma as a weed mould or pathogen in mushroom cultivation. In: Kubicek C P, Harman G E, editors. Trichoderma and Gliocladium. London, United Kingdom: Taylor & Francis Ltd.; 1998. pp. 267–288. [Google Scholar]
- 43.Seifert K A, Wingfield B D, Wingfield M J. A critique of DNA sequence analysis in the taxonomy of filamentous Ascomycetes and ascomycetous anamorphs. Can J Bot. 1995;73(Suppl. 1):S760–S767. [Google Scholar]
- 44.Sieber T N, Petrini O, Greenacre M J. Correspondence analysis as a tool in fungal taxonomy. Syst Appl Microbiol. 1998;21:442–449. doi: 10.1016/S0723-2020(98)80053-2. [DOI] [PubMed] [Google Scholar]
- 45.Sieber-Canavesi F, Petrini O, Sieber T N. Endophytic Leptostroma species on Piecea abies, Abies alba, and Abies balsamea: a cultural, biochemical, and numerical study. Mycologia. 1991;83:89–96. [Google Scholar]
- 46.Swofford D L. PAUP, Phylogenetic Analysis Using Parsimony, version 3.1.1. Computer program distributed by the Illinois Natural History Survey, Champaign. 1993. [Google Scholar]
- 47.Turner D, Kovacs W, Kuhls K, Lieckfeldt E, Peter B, Arisan-Atac L, Strauss J, Samuels G J, Börner T, Kubicek C P. RAPD-analysis of world-wide distribution and genetic variation of Trichoderma spp. and Hypocrea schweinitzii (Fr.:Fr.) Sacc. belonging to Trichoderma section Longibrachiatum. Mycol Res. 1997;101:449–459. [Google Scholar]
- 47a.USDA, Beltsville Agricultural Research Center. 1999. Commercial biocontrol products for use against soilborne crop diseases. [Online.] http://www.barc.usda.gov/psi/bpdl/bioproduct/htm. [23 April 1999, date last accessed.]
- 48.van de Peer Y. User manual for Treecon, version 3.0, a software package for the construction and drawing of evolutionary trees. Antwerp, Belgium: University of Antwerp; 1994. [Google Scholar]
- 49.Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webster J. Culture studies on Hypocrea and Trichoderma. I. Comparison of perfect and imperfect states of H. gelatinosa, H. rufa, and Hypocrea sp. 1. Trans Br Mycol Soc. 1964;47:75–96. [Google Scholar]
- 51.Wilkinson L. Systat 6.0 for Windows: statistics. Chicago, Ill: SPSS Inc.; 1996. [Google Scholar]