Abstract
Understanding the environmental factors that regulate the biosynthesis of antimicrobial compounds by disease-suppressive strains of Pseudomonas fluorescens is an essential step toward improving the level and reliability of their biocontrol activity. We used liquid culture assays to identify several minerals and carbon sources which had a differential influence on the production of the antibiotics 2,4-diacetylphloroglucinol (PHL), pyoluteorin (PLT), and pyrrolnitrin and the siderophores salicylic acid and pyochelin by the model strain CHA0, which was isolated from a natural disease-suppressive soil in Switzerland. Production of PHL was stimulated by Zn2+, NH4Mo2+, and glucose; the precursor compound mono-acetylphloroglucinol was stimulated by the same factors as PHL. Production of PLT was stimulated by Zn2+, Co2+, and glycerol but was repressed by glucose. Pyrrolnitrin production was increased by fructose, mannitol, and a mixture of Zn2+ and NH4Mo2+. Pyochelin production was increased by Co2+, fructose, mannitol, and glucose. Interestingly, production of its precursor salicylic acid was increased by different factors, i.e., NH4Mo2+, glycerol, and glucose. The mixture of Zn2+ and NH4Mo2+ with fructose, mannitol, or glycerol further enhanced the production of PHL and PLT compared with either the minerals or the carbon sources used alone, but it did not improve siderophore production. Extending fermentation time from 2 to 5 days increased the accumulation of PLT, pyrrolnitrin, and pyochelin but not of PHL. When findings with CHA0 were extended to an ecologically and genetically diverse collection of 41 P. fluorescens biocontrol strains, the effect of certain factors was strain dependent, while others had a general effect. Stimulation of PHL by Zn2+ and glucose was strain dependent, whereas PLT production by all strains that can produce this compound was stimulated by Zn2+ and transiently repressed by glucose. Inorganic phosphate reduced PHL production by CHA0 and seven other strains tested but to various degrees. Production of PLT but not pyrrolnitrin by CHA0 was also reduced by 100 mM phosphate. The use of 1/10-strength nutrient broth-yeast extract, compared with standard nutrient broth-yeast extract, amended with glucose and/or glycerol resulted in dramatically increased accumulations of PHL (but not PLT), pyochelin, and salicylic acid, indicating that the ratio of carbon source to nutrient concentration played a key role in the metabolic flow. The results of this study (i) provide insight into the biosynthetic regulation of antimicrobial compounds, (ii) limit the number of factors for intensive study in situ, and (iii) indicate factors that can be manipulated to improve bacterial inoculants.
There exists unquestionable potential for managing plant diseases incited by soilborne phytopathogens and increasing crop productivity with the application of certain root-associated microorganisms, particularly fluorescent Pseudomonas spp. (3, 6). Interest in biological control has recently intensified because of imminent bans on effective chemical controls such as methyl bromide, widespread development of fungicide resistance in pathogens, and a general need for more sustainable disease control strategies. Unfortunately, the seemingly inherent variable performance of most biocontrol strains between field locations and cropping seasons has hampered commercial development, and relatively few biological agents are registered for use in production agriculture (3). Much of this variability has been attributed to differences in physical and chemical properties found in natural environments where biocontrol agents are applied (12, 53). Understanding which environmental factors are important and how these influence disease suppression is widely recognized as a key to improving the level and reliability of biocontrol.
Considerable progress has been made over the past two decades to elucidate the mechanisms by which fluorescent pseudomonads suppress disease. In many crop-pathogen systems, the primary mechanism of biocontrol by fluorescent pseudomonads is production of antibiotics such as 2,4-diacetylphloroglucinol (PHL), pyoluteorin (PLT), pyrrolnitrin, and phenazine-1-carboxylate (53). Under certain conditions, antibiotics improve the ecological fitness of these bacteria in the rhizosphere, which can further influence long-term biocontrol efficacy (36). Siderophores, including salicylic acid, pyochelin, and pyoverdine, which chelate iron and other metals, also contribute to disease suppression by conferring a competitive advantage to biocontrol agents for the limited supply of essential trace minerals in natural habitats (18, 29). Siderophores may indirectly stimulate the biosynthesis of other antimicrobial compounds by increasing the availability of these minerals to the bacteria. Antibiotics and siderophores may further function as stress factors or signals inducing local and systemic host resistance (27). Biosynthesis of antibiotics and other antifungal compounds is regulated by a cascade of endogenous signals, including sensor-kinase and response regulators encoded by lemA and gacA (4, 16, 26), sigma factors encoded by rpoD (46) and rpoS (45), and quorum-sensing autoinducers such as N-acyl-homoserine lactones (43).
Determining the exogenous environmental signals that modulate the biosynthetic regulation of antifungal compounds has been comparatively slow, largely because isolating and quantifying metabolites produced in the soil and rhizosphere is tedious (53). Numerous reporter systems for gene expression have been described which ultimately may help identify conditions triggering antibiotic biosynthetic genes. Reporter systems in biocontrol pseudomonads have also been used as a preliminary investigative tool to examine the influence of iron availability on the expression of pyoverdine genes (29) and the influence of Pythium culture filtrates on the expression of trehalase genes (15) and genes thought to be involved in rhizosphere competence (14).
Liquid culture screening is an attractive alternative approach for identifying putative environmental signals because it requires little knowledge of biosynthetic loci and because it is more adaptable to the simultaneous detection of multiple metabolites. This is an important advantage because many of the most effective biocontrol strains produce several antimicrobial compounds, the relative importance of which probably depends on the types of soil, host, and pathogen; the stage of disease development; and other environmental conditions (53, 55). Recent studies suggest that factors identified in vitro by using liquid culture screening do indeed act as important environmental signals in natural habitats. For example, we used liquid culture screening to identify fusaric acid produced by the phytopathogenic fungus, Fusarium oxysporum f. sp. radicis-lycopersici as a repressor of antibiotic production by biocontrol pseudomonads (10). It was then possible to demonstrate that fusaric acid acts as a negative signal in the biocontrol of fusarium crown and root rot of tomato inhibiting antibiotic production in situ (10) and that fusaric acid-insensitive strains are more suitable for controlling this disease (11).
In the current study, we screened minerals and carbon sources for stimulation or repression of biosynthesis of several antibiotics (PHL, PLT, and pyrrolnitrin) and siderophores (pyochelin and salicylic acid) by Pseudomonas fluorescens. Initially, we tested the influence of these factors on P. fluorescens CHA0 isolated from a Swiss soil naturally suppressive to black root rot of tobacco caused by Chalara elegans (synanamorph Thielaviopsis basicola, 55). P. fluorescens CHA0 is a model biocontrol strain for which the importance of antimicrobial metabolites in disease suppression has been demonstrated in several crop-pathogen systems and for which the genetics of antibiotic and siderophore biosynthesis has been well characterized (55). We then tested glucose, inorganic phosphate, and zinc, three of the most influential factors with strain CHA0, for influences on antibiotic production by an ecologically and genetically diverse collection of P. fluorescens biocontrol strains (22). We focused on minerals and carbon sources because (i) they have long been known to influence the activity of phytopathogenic microorganisms (13), (ii) they contribute to the variability of biocontrol in different soils and on host crops that differ in root exudate composition (25, 53), and (iii) they have been reported to influence production of other antibiotics in biocontrol strains (17, 36, 37, 49, 50). Minerals and carbon sources are also appealing because they are easy and economical to provide during liquid fermentation of inoculants or as fertilizer amendments to improve the biocontrol activity of indigenous and introduced bacteria.
MATERIALS AND METHODS
Strains and cultural conditions.
P. fluorescens strains used in this study were isolated from six crop species grown in soils from Ghana, Ireland, Italy, Oklahoma, Switzerland, and Washington (Table 1) and have been genetically characterized by using amplified ribosomal DNA restriction analysis (ARDRA) and PCR-based fingerprinting with randomly amplified polymorphic DNA (RAPD) markers (22). Bacteria were stored in 0.8% nutrient broth plus 0.5% yeast extract (NBY) broth (Difco, Detroit, Mich.) plus 40% glycerol at −80°C. Starter cultures were grown in 10-ml dilute (1/10-strength) NBY broth in 20-ml screw top vials for 8 to 12 h at 27°C at 140 rpm, yielding approximately 109 CFU/ml. Test cultures of 20 ml of NB or NBY broth (unbuffered) in 100-ml Erlenmeyer flasks were inoculated with 10 μl of starter culture. Chemical analysis indicated that NBY broth contained (mg/liter): total nitrogen, 1441.0; amino nitrogen, 604.0; total phosphate, 600.1; potassium, 597.9; sodium, 259.7; chloride, 121.7; sulfate, 54.9; magnesium, 22.9; calcium, 6.1; zinc, 0.5; and boron, cobalt, copper, iron, lithium, manganese, and molybdenum, <0.1. Autoclaved medium was amended with filter-sterilized mineral solutions to give 1 mM BH3O3, CaCl2 · 2H2O, FeSO4 · 7H2O, LiCl, MgSO4 · 7H2O, MnCl2 · 4H2O, Mo7(NH4)6O24 · 4H2O, NaCl, 0.7 mM CuSO4, ZnSO4 · 7H2O, or 0.1 mM CoCl2 · 6H2O and with autoclaved stock solutions of carbon sources to give 1% (wt/vol). Cultures were incubated 48 h at 24°C with shaking at 140 rpm in darkness, unless otherwise indicated. Culture pH for all media was 6.5 to 6.7 at inoculation and 7.7 to 7.9 after 48 h of bacterial growth. Bacterial growth after 48 h was approximately 109 CFU/ml in NBY or in NBY with any of the mineral amendments, and it was approximately 108 CFU/ml in dilute NBY.
TABLE 1.
Origin of P. fluorescens strains with ARDRA and RAPD groupinga
| Strain | Origin (host, soil source) | Group no.
|
|
|---|---|---|---|
| ARDRA | RAPD | ||
| CHA0 | Tobacco, Morens, Switzerland | 1 | 1 |
| Pf1 | Tobacco, Morens, Switzerland | 1 | 1 |
| Pf-5 | Cotton, Texas | 1 | 2 |
| PF | Wheat leaves, Oklahoma | 1 | 2 |
| PGNR1 | Tobacco, Ghana | 1 | 1 |
| PGNR2 | Tobacco, Ghana | 1 | 1 |
| PGNR3 | Tobacco, Ghana | 1 | 1 |
| PGNR4 | Tobacco, Ghana | 1 | 1 |
| PGNL1 | Tobacco, Ghana | 1 | 1 |
| PINR2 | Tobacco, Albenga, Italy | 1 | 1 |
| PINR3 | Tobacco, Albenga, Italy | 1 | 1 |
| C∗1A1 | Cucumber, Morens, Switzerland | 2 | 3 |
| CM1′A2 | Cucumber, Morens, Switzerland | 2 | 3 |
| CΔ1′B2 | Cucumber, Morens, Switzerland | 2 | 3 |
| PILH1 | Tomato, Albenga, Italy | 2 | 5 |
| PITR2 | Wheat, Albenga, Italy | 2 | 5 |
| PITR3 | Wheat, Albenga, Italy | 2 | 5 |
| Q1-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q2-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q4-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q5-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q6-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q7-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q8-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q9-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q12-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q13-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q37-87 | Wheat, Quincy, Wash. | 2 | 6 |
| Q65-87 | Wheat, Quincy, Wash. | 2 | 3 |
| Q86-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q88-87 | Wheat, Quincy, Wash. | 2 | 4 |
| Q95-87 | Wheat, Quincy, Wash. | 2 | 3 |
| Q112-87 | Wheat, Quincy, Wash. | 2 | 3 |
| Q128-87 | Wheat, Quincy, Wash. | 2 | 3 |
| Q139-87 | Wheat, Quincy, Wash. | 2 | 3 |
| TM1A3 | Tomato, Morens, Switzerland | 2 | 3 |
| TM1′A4 | Tomato, Morens, Switzerland | 2 | 3 |
| TM1A5 | Tomato, Morens, Switzerland | 2 | 3 |
| TM1′A5 | Tomato, Morens, Switzerland | 2 | 3 |
| TM1B2 | Tomato, Morens, Switzerland | 2 | 3 |
| P12 | Tobacco, Morens, Switzerland | 3 | 8 |
| F113 | Sugarbeet, Ireland | 3 | 7 |
Strains were isolated from the roots of the host plant grown in soil collected from Ghana, Ireland, Italy, Switzerland, and the United States and were characterized by ARDRA and RAPD (22).
Metabolite extraction and detection.
Antibiotics and siderophores were extracted from cultures and quantified with high-performance liquid chromatography (HPLC) as previously described (10). Metabolites were identified by comparison with the UV spectra of reference compounds. Metabolite quantity was estimated from standard curves of reference compounds and normalized for the number of culturable cells present, which was estimated by spreading appropriate dilutions on King’s B medium agar prior to extraction. Liquid cultures of 20 ml were acidified to pH 2 with 400 to 700 μl of 1 N HCl and extracted with 20 ml of ethyl acetate for 30 min with vigorous shaking at 150 to 200 rpm. Phase separation was accelerated with 15 min of centrifugation at 4,500 rpm (2,790 × g). The organic phase was transferred to a round-bottom glass flask and flash evaporated, and the residue was dissolved in 1 ml of HPLC-grade methanol. Aliquots of 10 μl were injected into a reversed-phase column (4 by 100 mm) packed with Nucleosil 120-5-C18 and thermostatically controlled at 50°C. Samples were eluted with a three-step linear methanol gradient from 18 to 23% (0 to 5 min), from 23 to 53% (5 to 6 min), and from 53 to 68% (6 to 15 min) in 0.43% o-phosphoric acid. The flow rate was 1 ml/min. Maximum UV absorbances and approximate retention times for detection were, respectively, 270 nm and 11.4 min for PHL (molecular weight of 210), 313 nm and 9.4 min for PLT (molecular weight of 268), 254 nm and 12.1 min for pyrrolnitrin (molecular weight of 257.1), 300 nm and 8.3 min for salicylic acid (molecular weight of 138), and 254 nm and 10.1 and 10.8 min for the characteristic twin peaks of pyochelin (molecular weight of 325).
Effect of nutrients, minerals, and carbon source on CHA0 metabolism.
Strain CHA0 was grown 48 h in 20-ml portions of NBY, 11 different NBY plus mineral amendments, dilute NBY, and dilute NBY plus 30 mM NaCl. All treatments were tested alone and with 1% (wt/vol) glycerol or 1% (wt/vol) glucose added. Treatments were arranged as a 14 × 3 factorial in a split-plot design with a main plot of nutrient treatment (none, copper, zinc, cobalt, ammonium molybdate, manganese, magnesium, iron, boron, calcium, sodium, lithium, 1/10-dilute NBY, and dilute NBY plus sodium chloride) and a subplot of carbon source amendment (none, glycerol, and glucose). The effect of a range of zinc sulfate concentrations, from 0 to 1.75 mM, on PHL and PLT production was evaluated in NBY broth. Metabolite production and bacterial growth were quantified as described above.
Effect of carbon sources, used alone and in combination with ammonium molybdate and zinc sulfate, on CHA0 metabolism.
Strain CHA0 was grown in NBY broth and in NBY broth amended with carbon sources at 1% (wt/vol). Carbon source amendments were tested alone and with a mineral mixture of 0.5 mM ammonium molybdate and 0.35 mM zinc sulfate. Metabolite extractions were made after 48 and 120 h of incubation. Treatments were arranged as a 6 × 2 × 2 factorial with a main plot of carbon source amendment (none, glucose, glycerol, fructose, sucrose, and mannitol), a subplot of mineral amendment (plus or minus), and a subsubplot of incubation time (2 and 5 days).
Effect of zinc, inorganic phosphate, and glucose amendments on growth and antibiotic production by diverse biocontrol strains of P. fluorescens.
A collection of 42 PHL-producing strains (22) was grown in broths of NB, NB amended with zinc sulfate, and NB amended with glucose. Yeast extract, which contributed most of the trace amounts of zinc to NBY, was omitted in these trials without having any effect on bacterial growth. Zinc sulfate was added to NB at 0.7 mM for ARDRA 1 strains and at 0.2 mM for ARDRA 2 and 3 strains (Table 1). Treatments were arranged as a 3 × 42 factorial with a main plot of medium amendment (none, zinc, and glucose) and a subplot of strain. The production of PHL was determined for all strains, and PLT production was determined for ARDRA 1 strains. In a separate experiment, CHA0 and eight additional strains from this collection were grown for 48 h in NB broth containing 1% (wt/vol) glucose (except F113, which was grown with 1% [wt/vol] sucrose) and amended with 0 and 100 mM inorganic phosphate (equimolar stock solution of K2HPO4 and KH2PO4). Production of PHL was determined by HPLC. For CHA0, additional treatments of 10 and 200 mM phosphate were included. Production of PLT and pyrrolnitrin was determined for CHA0 grown 5 days in NB plus 1% (wt/vol) glycerol.
Statistical analysis.
All experiments were conducted at least twice. Treatments consisted of three to four replicate broths. Data from repeated trials were pooled after confirming the homogeneity of variances and/or determining there was no significant treatment × trial interaction, except in the experiment to determine the influence of zinc and glucose on antibiotic production by diverse PHL-producing strains. In this case, the large number of treatments required the experiment to be replicated over time, with three replicates per treatment. Bacterial growth data were normalized with a log10 plus 1 transformation prior to analysis. The metabolite quantity was expressed relative to bacterial growth prior to analysis. The significance of main effects and interactions was determined by using the SAS general linear models procedure (Statistical Analysis Systems, Cary, N.C.). When appropriate, mean comparisons were made by using Fisher’s protected (P ≤ 0.05) least-significant-difference (LSD) test. The relationship between zinc concentration and antibiotic production was evaluated by using SAS linear regression analysis (Pearson coefficient). Relationships between strain ARDRA or RAPD groupings and PHL production in response to zinc and glucose were evaluated by using SAS correlation analysis.
RESULTS
Influence of nutrients, minerals, and carbon sources on antibiotic production.
In the first experiment, 11 minerals were added to NBY medium alone or in combination with carbon source amendment of 1% (wt/vol) glucose or glycerol to evaluate their influence on antibiotic production by CHA0 after 2 days of growth. Mineral amendment had a significant influence on PHL with or without glycerol or glucose amendment (P ≤ 0.0006) and on PLT with or without glycerol (P = 0.0001) but not with glucose. When added to NBY, zinc sulfate and ammonium molybdate increased PHL production (Table 2). In general, glycerol increased PHL production. The combination of zinc, copper, and ammonium molybdate with glycerol significantly (P ≤ 0.042) increased PHL production compared with the minerals or the carbon source alone. None of the other minerals influenced PHL production in NBY broth plus glycerol. Glucose generally increased PHL production in all mineral treatments, but there were no significant interactions with mineral amendments. There was a dramatic increase in PHL production when glucose was added to dilute NBY broth with or without sodium chloride. Production of PLT was significantly increased by zinc sulfate and cobalt chloride in normal NBY broth and in NBY plus glycerol compared with the no mineral controls (Table 2). In general, glycerol increased PLT production. However, glycerol reduced PLT production in zinc and cobalt media compared with the minerals used alone. Glucose repressed the production of PLT. Mineral amendments did not significantly reduce bacterial growth compared with the nonamended control; the cell number was reduced approximately 1 log unit by a 10-fold medium dilution (data not shown).
TABLE 2.
Influence of minerals and carbon source amendment on antibiotic production by P. fluorescens CHA0a
| Culture medium | Antibiotic production (ng/108 CFU)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PHL with (carbon source added):
|
LSD | PLT with (carbon source added):
|
LSD | |||||
| None | Glycerol | Glucose | None | Glycerol | Glucose | |||
| NBY alone | <0.1 | 2.6 | 98.4 | 67.5 | 12.8 | 27.0 | 0.9 | 10.5 |
| NBY amended with: | ||||||||
| Cu2SO4 | <0.1 | 469.6 | 913.9 | 673.6 | 1.1 | 19.1 | 5.8 | 14.3 |
| ZnSO4 | 6.0 | 665.1 | 79.5 | 509.7 | 265.8 | 114.8 | 13.3 | 108.9 |
| CoCl2 | <0.1 | 0.5 | 773.0 | 706.8 | 418.2 | 60.9 | 5.4 | 128.8 |
| (NH4)6Mo7O24 | 9.8 | 186.0 | 80.1 | 79.5 | 26.4 | 18.7 | 7.5 | 17.4 |
| MnCl2 | <0.1 | 6.4 | 113.4 | 86.2 | <0.1 | 22.7 | 4.0 | 5.6 |
| LiCl | 0.7 | 2.1 | 94.5 | 73.1 | 34.4 | 14.5 | 1.5 | 16.6 |
| FeSO4 | 0.2 | 8.2 | 17.7 | 8.8 | 40.4 | 45.7 | 1.9 | 15.3 |
| BH3O3 | <0.1 | 38.2 | 80.0 | NS | 38.1 | 14.2 | 1.4 | 13.2 |
| MgSO4 | 0.1 | 1.4 | 241.0 | 174.5 | 10.0 | 20.4 | 2.1 | 7.2 |
| NaCl | <0.1 | 3.6 | 102.2 | 75.1 | 23.6 | 20.3 | 1.5 | 10.1 |
| CaCl2 | <0.1 | 4.5 | 59.8 | 38.6 | 13.5 | 10.6 | 0.9 | 5.1 |
| Dilute NBY | <0.1 | 58.6 | 1,939.5 | 1,373.8 | 6.2 | 5.9 | 7.7 | NS |
| Dilute NBY with NaCl | <0.1 | 3.2 | 1,646.9 | 1,463.6 | 9.2 | 53.0 | 10.0 | 12.3 |
| LSD | 2.5 | 311.9 | 948.2 | 66.0 | 36.7 | NS | ||
Within a row, for each medium, the antibiotic concentration was significantly different according to Fisher’s LSD test (P ≤ 0.05 for PHL and P ≤ 0.02 for PLT), except where the analysis of variance was not significant (NS). Within a column, the antibiotic concentration was significantly different according to the LSD at the bottom (P ≤ 0.0006), except for where the analysis of variance was not significant.
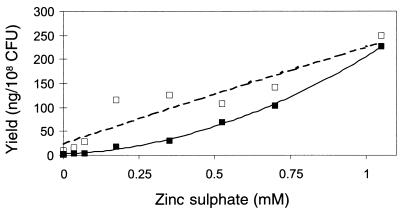
A follow-up experiment examined the relationship between zinc sulfate concentration and antibiotic production. Zinc sulfate concentration, from 0 to 1.1 mM, had a significant (P = 0.0001) positive relationship with production of both PHL and PLT (Fig. 1). Antibiotic production, normalized for the number of cells in each culture, continued to increase at concentrations of up to 1.75 mM zinc sulfate (data not shown). However, while bacterial growth was not significantly affected at concentrations of up to 1.1 mM (approximately log 9 CFU), growth sharply declined to below log 7 CFU at higher concentrations. Data for concentrations above 1.1 mM were not included in the final regression analysis.
FIG. 1.
Relationship between zinc sulfate concentration and the production of PHL (□) and PLT (■) in nutrient broth by CHA0 after 48 h of growth at 27°C. Values represent the mean of six cultures. Regression lines approximate y = 177.1x2 + 26.2x + 2.8 for PHL (solid line; r2 = 0.9978) and y = −9.5x2 + 210.5x + 25.0 for PLT (dashed line; r2 = 0.8727).
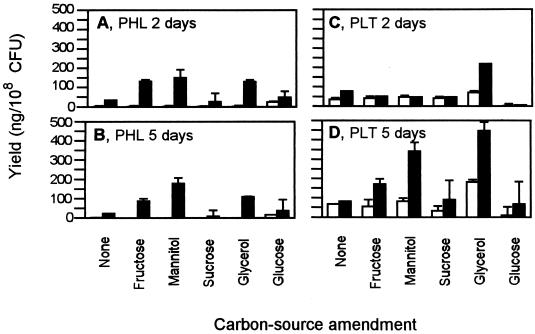
We then tested the effect of a wider range of carbon sources alone and looked at interactions between carbon source, a mineral mixture of zinc sulfate (0.35 mM) and ammonium molybdate (0.5 mM), and extended incubation time (2 and 5 days). The interaction of carbon source × mineral × time was significant for both PHL (P = 0.0334) and PLT (P = 0.0089). The effect of carbon sources was further analyzed based on response to mineral and time. Additionally, we examined the main effect of the minerals because there was a highly significant effect with an F value that was more than 10 times greater than that of the interaction. In this set of experiments, carbon sources used alone had no effect on PHL production at 2 and 5 days of incubation (Fig. 2A and B); only glycerol gave a slight increase in PLT production after 5 days growth compared to the no-carbon-source control (Fig. 2C and D). When carbon sources were tested in NBY amended with minerals, the addition of 1% (wt/vol) fructose, mannitol, or glycerol increased PHL production at 2 and 5 days and increased PLT production at 5 days compared with the no-carbon-source control. Across all carbon sources and regardless of time a mixture of zinc sulfate and ammonium molybdate significantly (P ≤ 0.0083) increased the production of PHL from 3.8 to 110.7 ng/108 CFU and the production of PLT from 55.1 to 203.8 ng/108 CFU compared with production in the absence of mineral amendment. Glucose almost completely repressed PLT production at 2 and 5 days. However, PLT began to accumulate in glucose-amended cultures after prolonged incubation. This was not uniformly observed with the other carbon sources, suggesting that repression was transient and that PLT production resumed as the glucose began to deplete. Incubation time had no consistent effect on PHL production, but PLT production was greater after 5 days. With the exception of sucrose which is not utilized by CHA0, carbon sources typically increased bacterial growth to 1010 CFU/ml compared to 109 CFU/ml in NBY alone (data not shown).
FIG. 2.
Influence of carbon source amendment (1% [wt/vol]) on production of PHL (A and B) and PLT (C and D) by CHA0 grown for 2 and 5 days, with (■) or without (□) zinc sulfate (0.35 mM) and ammonium molybdate (0.5 mM) amendments. Values represent the mean of six cultures (+ the standard error). Bars are not visible in some cases where values approach 0.
Production of monoacetyl-phloroglucinol was influenced by the same environmental conditions that influenced the production of PHL (data not shown). This further supports the findings of Shanahan et al. (47) that it is a precursor compound.
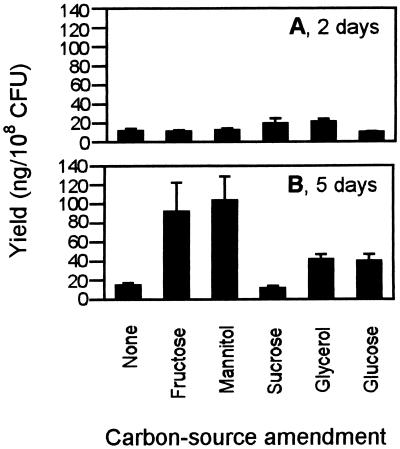
Production of pyrrolnitrin was greater at 5 days than at 2 days (P = 0.0267), and there were significant interactions between carbon source × time (P = 0.0001) and mineral amendment × time (P = 0.0187). Further analysis of these interactions indicated that mineral amendment significantly increased pyrrolnitrin production at 2 days (P = 0.0278) from 11.6 to 17.1 ng/108 CFU and at 5 days (P = 0.0165) from 32.4 to 68.9 ng/108 CFU compared to production in the absence of mineral amendment. At 2 days, although production was weak in all treatments, glycerol gave a slight but significant increase compared with the control (Fig. 3). At 5 days, production was virtually unchanged in the absence of carbon source amendment or when sucrose, which is not utilized by CHA0, was supplied. When fructose or mannitol were provided, pyrrolnitrin production was increased over fivefold relative to the control (Fig. 3).
FIG. 3.
Influence of carbon source amendment (1% [wt/vol]) on the production of pyrrolnitrin by CHA0 grown for 2 (A) and 5 (B) days. Values represent the mean of six cultures (+ the standard error).
Influence of minerals and carbon sources on siderophore production.
Cobalt chloride was the only mineral which increased pyochelin, and ammonium molybdate was the only mineral which increased salicylic acid production in nonamended NBY broth (Table 3). Copper and iron reduced pyochelin production and zinc increased salicylic acid production in the presence of glycerol. None of the minerals had an effect on the production of either siderophore in media amended with glucose. Glucose used alone and in combination with minerals generally increased pyochelin production, except in the presence of iron, when it reduced production. Glycerol used alone or in combination with minerals generally did not give a significant increase in pyochelin. When combined with dilute NBY broth, however, glycerol gave a slight increase and glucose gave a dramatic increase in the production of both pyochelin and salicylic acid (Table 3). The combination of each mineral with either glycerol or glucose generally increased salicylic acid production compared with NBY plus the minerals alone (Table 3).
TABLE 3.
Influence of minerals and carbon source amendment on siderophore production by P. fluorescens CHA0a
| Culture medium | Siderophore production (ng/108 CFU)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pyochelin with (carbon source added):
|
LSD | Salicylic acid with (carbon source added):
|
LSD | |||||
| None | Glycerol | Glucose | None | Glycerol | Glucose | |||
| NBY alone | 56.7 | 104.9 | 185.4 | 68.2 | <0.1 | 18.3 | 18.6 | 11.2 |
| NBY amended with: | ||||||||
| Cu2SO4 | 24.6 | 25.1 | 1,002.2 | 567.9 | 11.4 | 49.3 | 17.2 | 86.3 |
| ZnSO4 | 61.3 | 146.4 | 327.4 | 165.2 | <0.1 | 75.5 | 37.4 | 53.7 |
| CoCl2 | 150.2 | 107.9 | 945.5 | NS | <0.1 | 22.9 | 48.5 | 32.9 |
| (NH4)6Mo7O24 | 50.5 | 85.2 | 149.2 | 53.1 | 26.4 | 23.7 | 58.6 | 29.6 |
| MnCl2 | 74.7 | 72.3 | 170.7 | 87.1 | <0.1 | 9.6 | 51.7 | 22.5 |
| LiCl | 56.6 | 89.0 | 204.9 | 91.4 | <0.1 | 13.0 | 20.8 | 5.5 |
| FeSO4 | 39.4 | 11.6 | 5.9 | 24.6 | 0.9 | 10.4 | 21.4 | 11.5 |
| BH3O3 | 76.3 | 88.8 | 188.1 | 80.1 | 2.2 | 13.1 | 24.1 | 6.2 |
| MgSO4 | 81.9 | 89.8 | 362.0 | 172.6 | 8.2 | 12.6 | 46.8 | 26.5 |
| NaCl | 53.6 | 130.2 | 197.2 | 92.0 | 1.2 | 20.7 | 14.8 | 9.5 |
| CaCl2 | 66.0 | 67.0 | 139.5 | 43.7 | 8.4 | 8.8 | 22.4 | 11.8 |
| Dilute NBY | <0.1 | 174.9 | 2,361.9 | 1,756.9 | 4.9 | 78.7 | 416.5 | 268.6 |
| Dilute NBY with NaCl | <0.1 | 273.4 | 4,695.3 | 1,130.5 | <0.1 | 68.6 | 2,848.2 | 992.9 |
| LSD | 56.6 | 64.9 | 1,041.0 | 13.5 | 32.1 | 448.3 | ||
Within a row, for each medium, the siderophore concentration was significantly different according to Fisher’s LSD test (P ≤ 0.0471), except for where NS indicates a nonsignificant analysis of variance. Within a column, the siderophore concentration was significantly different according to the LSD at the bottom (P ≤ 0.0172).
We then evaluated the interactive effects of a larger range of carbon sources, incubation time, and a mineral mixture of zinc sulfate and ammonium molybdate on siderophore production. For pyochelin production, the highest-order interactions that were significant were carbon source × mineral (P = 0.0001) and carbon source × time (P = 0.0002). For salicylic acid production, the highest-order interaction that was significant was carbon source × mineral (P = 0.0064). These interactions were further evaluated.
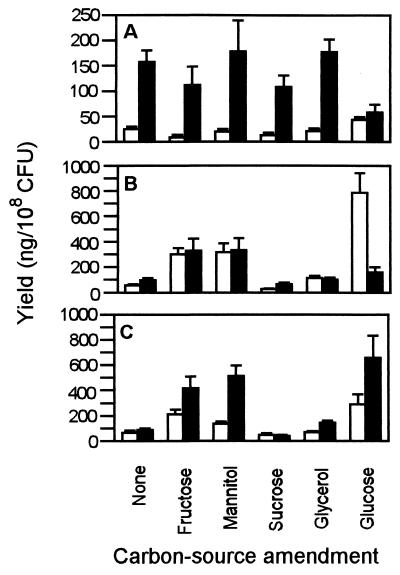
The carbon sources used alone had only a slight effect on salicylic acid production (Fig. 4A). However, mineral amendment significantly (P = 0.0001) increased production of salicylic acid by two- to threefold regardless of the carbon source amendment (Fig. 4A). In the absence of minerals, carbon source amendment had no effect on the production of salicylic acid. With the amendment of minerals, mannitol and glycerol increased salicylic acid production and glucose reduced production compared with the no-carbon-source control (Fig. 4A). In contrast, the carbon sources fructose, mannitol, and glucose significantly (P = 0.0001) increased pyochelin production from ca. 56 ng/108 CFU for the nonamended control without minerals to 302, 316, and 788 ng/108 CFU, respectively (Fig. 4B). Sucrose and glycerol had no effect on pyochelin production. Mineral amendment with zinc sulfate and ammonium molybdate reduced pyochelin production fourfold in the presence of glucose but had no effect on pyochelin production with other carbon sources. In treatments with a substantial yield of pyochelin (i.e., fructose, mannitol, and glucose), the yield was significantly greater (P = 0.0001) after 5 days of growth (Fig. 4C).
FIG. 4.
Influence of carbon source amendment (1% [wt/vol]) on siderophore production by CHA0. (A) Salicylic acid production with (■) or without (□) amendment of zinc sulfate (0.35 mM) and ammonium molybdate (0.5 mM). (B) Pyochelin production with (■) or without (□) mineral amendments. (C) Pyochelin production after 2 (□) and 5 (■) days. Values represent the mean of six cultures (+ the standard error).
Influence of zinc, inorganic phosphate, and glucose on growth and antibiotic production by diverse biocontrol strains.
Strains varied in tolerance to zinc sulfate. All ARDRA 1 strains could be grown in medium amended with 0.7 mM zinc sulfate without a reduction in CFU after 48 h of incubation; however, the maximum concentrations for strains in ARDRA groups 2 and 3 before growth was significantly reduced (>1 log10 CFU/ml) was approximately 0.2 mM. For the determination of antibiotic production, 0.7 mM zinc sulfate was used for ARDRA 1 strains and 0.2 mM was used for all other strains. Although the concentrations used differed they represented similar levels of toxicity (i.e., stress) to the strains which may be more important in antibiotic biosynthesis (2, 31). Amendment with 1% (wt/vol) glucose increased the growth of all strains by 0.5 to 1 log10 CFU/ml with no significant differences observed among strains or among the ARDRA or RAPD groups. The interaction between the strain and the treatment was not significant (P = 0.3222), indicating that the growth of the different strains was similar when grown in the same media.
Most strains produced only a low amount of PHL in unamended NB, and production was not correlated with either ARDRA or RAPD grouping in this medium. Zinc sulfate stimulated PHL production in CHA0 and all other ARDRA 1 strains (Table 4). Of all the ARDRA 2 and 3 strains only TM1′A4 and F113 were significantly stimulated. Zinc sulfate slightly reduced PHL production in PITR2, but it did not have a significant impact on other strains (Table 4). There were slight but significant negative correlations between PHL production by zinc sulfate and the ARDRA (P = 0.0005, r = −0.31) and RAPD (P = 0.0054, r = −0.25) groups. Glucose increased PHL production by all ARDRA 1 strains except PGNL1 and by all ARDRA 2 strains except PITR2, C∗1A1, CM1′A2, and Q128-87 (Table 4). There was no correlation between glucose response and ARDRA group. Strains in ARDRA groups 1 and 2 had similar positive responses to glucose. In contrast, only one of the two ARDRA 3 strains, P12 from tobacco in Switzerland, was stimulated by glucose.
TABLE 4.
Influence of zinc sulfate and glucose on the production of PHL by biocontrol strains of P. fluorescens in nutrient brotha
| Strain | PHL production (ng/108 CFU) with (nutrient broth amendment):
|
LSD | ||
|---|---|---|---|---|
| None | Zinc sulfateb | 1% Glucose | ||
| CHA0 | 1.3 | 48.7 | 84.2 | 36.0 |
| PF | 0.7 | 271.9 | 224.8 | 151.6 |
| Pf1 | 0.5 | 28.9 | 101.2 | 37.9 |
| Pf5 | 1.9 | 315.5 | 290.3 | 246.7 |
| PGNR1 | 1.0 | 46.3 | 78.6 | 52.0 |
| PGNR2 | 0.6 | 15.7 | 22.4 | 14.3 |
| PGNR3 | 1.1 | 39.4 | 56.3 | 34.0 |
| PGNR4 | 0.2 | 37.6 | 78.6 | 44.3 |
| PGNL1 | 1.2 | 22.6 | 56.4 | NS |
| PINR2 | 0.4 | 12.2 | 130.8 | 114.9 |
| PINR3 | 0.5 | 9.7 | 132.1 | 106.2 |
| C∗1A1 | 71.4 | 60.2 | 97.8 | NS |
| CM1′A2 | 31.0 | 83.1 | 143.0 | NS |
| CΔ1′B2 | 22.0 | 8.8 | 101.3 | 69.0 |
| PILH1 | 0.7 | 2.6 | 88.4 | 68.5 |
| PITR2 | 82.6 | 6.4 | 82.8 | 62.2 |
| PITR3 | 1.0 | 0.3 | 103.2 | 68.1 |
| Q1-87 | 1.0 | 1.1 | 102.7 | 55.8 |
| Q2-87 | 0.6 | 0.4 | 151.4 | 78.5 |
| Q4-87 | 1.6 | 0.7 | 97.5 | 23.6 |
| Q5-87 | 0.8 | 1.2 | 162.7 | 69.9 |
| Q6-87 | 0.5 | 5.1 | 150.8 | 52.4 |
| Q7-87 | 0.7 | 1.0 | 129.8 | 87.7 |
| Q8-87 | 0.1 | 22.1 | 174.0 | 74.8 |
| Q9-87 | 0.1 | 2.1 | 168.4 | 79.1 |
| Q12-87 | 0.9 | 1.0 | 162.1 | 77.7 |
| Q13-87 | 0.5 | 0.6 | 129.8 | 122.9 |
| Q37-87 | 0.9 | 1.7 | 187.9 | 142.8 |
| Q65-87 | 12.9 | 58.4 | 122.2 | 62.3 |
| Q86-87 | 0.4 | 1.8 | 169.7 | 118.2 |
| Q88-87 | 0.1 | 0.1 | 134.0 | 85.6 |
| Q95-87 | 10.4 | 3.9 | 95.2 | 19.0 |
| Q112-87 | 4.2 | 8.1 | 102.9 | 53.4 |
| Q128-87 | 46.9 | 46.8 | 167.6 | NS |
| Q139-87 | 3.1 | 2.9 | 199.6 | 99.5 |
| TM1A3 | 5.1 | 1.1 | 86.4 | 57.4 |
| TM1′A4 | 17.6 | 108.3 | 112.0 | 70.0 |
| TM1A5 | 4.7 | 1.7 | 137.3 | 78.4 |
| TM1′A5 | 55.6 | 97.0 | 123.0 | NS |
| TM1B2 | 51.4 | 96.0 | 120.1 | NS |
| P12 | 1.4 | 1.5 | 52.5 | 42.2 |
| F113 | 9.9 | 58.6 | 12.0 | 13.5 |
| LSD | 19.4 | 52.3 | 108.3 | |
| ARDRA 1 | 0.8 | 77.2 | 114.2 | 42.3 |
| ARDRA 2 | 14.8 | 21.5 | 131.2 | 13.5 |
| ARDRA 3 | 5.6 | 30.0 | 32.3 | NS |
| LSD | 16.2 | 49.1 | 53.8 | |
For each amendment, the main effects of strain and ARDRA group were significant at P = 0.050 and P = 0.008, respectively, except for where NS indicates a nonsignificant analysis of variance test for an amendment with no LSD test applied.
ZnSO4 · 7H2O was used at 0.7 mM for strains CHA0 to PINR3 and at 0.2 mM for strains C∗1A1 to F113.
Only the ARDRA 1 strains produced PLT, and data from the other strains were not included in the analysis. Among the ARDRA 1 strains, the quantity of PLT produced varied in NB and in NB amended with zinc sulfate (Table 5). Strains PF and Pf-5, the only ARDRA 1 strains in RAPD group 2, were the most productive in both media. Zinc sulfate amendment significantly increased PLT production by most strains by three- to sevenfold compared with production in nonamended media. The only strains which did not have a significant response to zinc sulfate were PINR2 and PINR3, the only ARDRA 1 strains isolated from Albenga soil from Italy (Table 5). In contrast, glucose reduced pyoluteorin production by all strains to below the detection limit.
TABLE 5.
PLT production by P. fluorescens biocontrol strains in nutrient broth with or without zinc sulfate amendmenta
| Strain | PLT production (ng/108 CFU)
|
LSD | |
|---|---|---|---|
| Alone | With zinc sulfate | ||
| CHA0 | 13.3 | 66.5 | 36.3 |
| PF | 39.8 | 274.7 | 128.0 |
| Pf1 | 29.9 | 144.7 | 102.8 |
| Pf5 | 39.7 | 408.7 | 294.2 |
| PGNR1 | 13.7 | 32.6 | 17.4 |
| PGNR2 | 11.3 | 31.7 | 13.7 |
| PGNR3 | 15.3 | 63.0 | 40.3 |
| PGNR4 | 12.7 | 48.8 | 7.2 |
| PGNL1 | 16.0 | 41.1 | 20.9 |
| PINR2 | 15.9 | 14.2 | NS |
| PINR3 | 25.1 | 69.3 | NS |
| LSD | 13.0 | 110.6 | |
Within a row, for each strain, the PLT concentration was significantly greater in nutrient broth amended with 0.7 mM zinc sulfate (P = 0.0391), except for where NS indicates a nonsignificant analysis of variance with no LSD test applied. Within a column, strains varied significantly in PLT production (P = 0.0003).
Inorganic phosphate inhibited PHL production by strains in all ARDRA groups but to various degrees (Table 6). For example, PHL production by CHA0 was almost abolished by 10 mM phosphate, whereas 100 mM phosphate reduced production by Q2-87 by only 10-fold (Table 6). No strain was insensitive to 100 mM phosphate. Production of PLT by CHA0 was completely inhibited by 100 mM but only slightly reduced by 10 mM phosphate (data not shown). Pyrrolnitrin production by CHA0 was not affected by 200 mM phosphate (data not shown). Bacterial growth was increased 5- to 10-fold by 100 mM phosphate amendment (data not shown).
TABLE 6.
Phosphate repression of PHL production by P. fluorescens biocontrol strainsa
| Strain | ARDRA group | Antibiotic yield (ng/108 CFU) with (phosphate amendment):
|
||
|---|---|---|---|---|
| None | 10 mM | 100 mM | ||
| CHA0 | 1 | 35.4 ± 8.9 | 1.1 ± 0.5 | 0 |
| Pf5 | 1 | 252.7 ± 49.9 | ND | 28.1 ± 9.9 |
| PITR3 | 2 | 279.0 ± 38.1 | ND | 31.8 ± 21.3 |
| Q2-87 | 2 | 108.4 ± 8.6 | ND | 13.2 ± 3.4 |
| Q65-87 | 2 | 164.3 ± 29.1 | ND | 2.1 ± 0.4 |
| TM1A3 | 2 | 276.4 ± 74.7 | ND | 1.9 ± 0.4 |
| TM1A5 | 2 | 271.9 ± 102.7 | ND | 1.9 ± 0.7 |
| P12 | 3 | 100.5 ± 21.6 | ND | 0 |
| F113 | 3 | 550.8 ± 51.4 | ND | 5.1 ± 2.3 |
Bacteria were grown for 48 h in NB plus 1% glucose, except for F113, which was grown in NB plus 1% sucrose. The antibiotic yield was determined by HPLC as described in Materials and Methods. Values represent the means of three trials ± the standard error. ND, not determined.
DISCUSSION
Bacterial gene expression in the rhizosphere is regulated both by endogenous and exogenous signals. Exogenous regulatory signal(s) activate LemA, a membrane-bound sensor-kinase, which in turn regulates the production of bacterial autoinducers that control the biosynthesis of antibiotics critical for the biocontrol of soilborne fungal pathogens (43). However, such signals have not yet been determined. Using a liquid culture assay, we identified several putative environmental signals that influenced the production of antifungal metabolites by an ecologically diverse collection of biocontrol strains.
We observed that carbon sources commonly found in plant root exudates had a differential influence on the spectrum of antibiotics produced by individual biocontrol strains irrespective of their effects on bacterial growth. For example, the production of PLT and PHL by strain CHA0 was stimulated by glycerol and glucose, respectively. Glucose, however, repressed PLT, with antibiotic accumulating only after prolonged growth when glucose began to deplete. Evidence suggests that glucose may block antibiotic production through repression of dehydrogenases that catalyze glucose oxidation, a reaction that transfers electrons from the enzyme cofactor PQQ to the electron transport chain (17). A PqqF− mutant of CHA0, which lacks glucose dehydrogenase activity, overproduces PLT (46). The use of 1/10-strength NBY, compared with NBY, which was amended with glucose and/or glycerol resulted in dramatically increased accumulations of PHL (but not PLT), pyochelin, and salicylic acid, indicating that the ratio of carbon source to nutrient concentration played a key role in metabolic flow. Slininger and Shea-Wilbur (50) reported that cell yield and phenazine production by P. fluorescens biocontrol strain 2-79 was increased at high molar C/N ratios (e.g., 16:1).
We confirmed that CHA0 also produced pyrrolnitrin in the presence of fructose and mannitol, albeit after incubation times considerably longer than are typically used to monitor PHL and PLT production and at concentrations much lower than those for other biocontrol strains (e.g., Pf5) (8a, 45). Weak production by this strain may reflect competition for the common substrate l-tryptophan in the pyrrolnitrin and indole-3-acetic acid biosynthetic pathways (23, 39). Although carbon sources differentially influence medium acidification during bacterial growth (7), which may then indirectly affect antibiotic production (50) and biocontrol activity (41), we did not observe such pH changes with the medium amendments used in this study.
Plant specificity of biocontrol strains has been demonstrated at both the species and cultivar level (34, 52). This has generally been attributed to differential utilization of the various carbon and nitrogen compounds found in exudates and its effects on bacterial growth and population structure (28, 40, 57). Our results suggest that another factor in plant specificity may be the influence of root exudate components on the biosynthesis of antimicrobial metabolites. Quantitative and/or qualitative differences in the sugar component of root exudates could determine the predominant biocontrol mechanism expressed in given crop-pathogen systems. This concept could explain results of genetic studies that have demonstrated a role for PHL (but not PLT) in biocontrol in wheat and cucumber plants and a role for PLT in cress and cotton plants (30, 33). Using an ice-nucleation reporter gene system, Kraus and Loper (24) recently observed differential expression of PLT biosynthetic genes in P. fluorescens Pf-5 on cotton and cucumber seeds.
Differences were also observed in the production of particular antibiotics by diverse strains. For example, glucose stimulated PHL production in almost all of the 42 strains screened, with the notable exception of P. fluorescens F113 from Ireland. In this strain, sucrose stimulated PHL production but glucose had no effect, confirming the previous observations of Shanahan et al. (48). Incidentally, F113 was the only strain isolated from sugarbeet, the roots of which tend to have an unusually high sucrose content. This suggests that evolutionary relationships may exist between biocontrol strains and their original host plants and further supports the notion of breeding for closer plant-biocontrol agent interactions to achieve improved disease suppression. A natural example of such selection is the decline of take-all disease due to the accretion of PHL-producing Pseudomonas spp. in the soil and rhizosphere after wheat monoculture (44). Many of the strains used in this study were isolated from a take-all decline soil in the United States (22).
We further demonstrated a differential effect of minerals on antibiotic biosynthesis. Zinc sulfate stimulated production of both PHL and PLT by CHA0, while ammonium molybdate stimulated only PHL and cobalt chloride stimulated only PLT. Other sulfate and chloride compounds did not have this effect, indicating that Zn2+, Co2+, and NH4-Mo2+ were the active cations. When we tested an ecologically and genetically diverse collection of biocontrol strains, zinc also stimulated PHL but in a strain-specific manner. Zinc increased PLT production in all strains able to produce this antibiotic, but the level of stimulation varied. In strain CHA0, pyrrolnitrin production was stimulated by a mixture of zinc and ammonium molybdate. Inorganic phosphate repressed PHL production by CHA0 and to a lesser extent repressed PLT but had no effect on pyrrolnitrin. Phosphate repression has been reported for other polyketide antibiotics (e.g., anthracycline and tetracycline) and phenazines in Pseudomonas spp. (32, 54) and for zwittermycin A and kanosamine in Bacillus (37, 38) and may be a common phenomenon in soil bacteria. Strains, however, differed in their sensitivity to phosphate repression. This may explain why Keel et al. (22) detected PHL production in some but not all strains on King’s medium B that contains ca. 9 mM K2HPO4. Surprisingly, iron, which stimulates production of a variety of antifungal metabolites (e.g., zwittermycin A [37], kanosamine [38], phenazine [49], and cyanide [21]), affected neither PHL nor PLT in strain CHA0. This does not exclude a role for iron or other ions not found to be regulatory in biosynthesis since trace amounts found in NBY and on glassware may have been sufficient.
How minerals influence antibiotic production by biocontrol pseudomonads is uncertain. In other bacteria, minerals repress antibiotic synthases, interrupt the transcription and promotion of biosynthetic genes, and may indirectly affect nutrient availability and pH (2, 5, 32). Also, zinc and other minerals are essential for growth, they influence cell membrane integrity, and they are key components and/or catalysts of over 300 enzymes and other proteins (56). It has been suggested that increased antibiotic biosynthesis is a response to environmental stress conditions (e.g., phosphate starvation or heavy metal toxicity) that decrease bacterial growth (2, 31). Further study to confirm this is particularly relevant to biocontrol bacteria introduced into soil where conditions can be extreme.
From a practical perspective, mineral effects on antibiotic biosynthesis may explain the association between soil chemical and physical properties and the variable performance of biocontrol strains between field sites (8a, 53). For example, zinc, which stimulated antibiotic production in CHA0, is typically more abundant in the naturally disease-suppressive soils from which this strain was isolated, and CHA0 is not effective when added to disease-conducive soils that contain less zinc (6). Ownley and coworkers (42) similarly found that zinc soil content was positively correlated with the biocontrol activity of P. fluorescens 2-79. Independently, Slininger and Jackson (49) demonstrated that zinc stimulated production of phenazine-1-carboxylate, the primary biocontrol determinant in strain 2-79 (53). In contrast, we found no effect of zinc on PHL production by Q2-87, a strain for which biocontrol was not correlated with zinc soil content (11a). Identifying factors favorable to biocontrol will facilitate the targeted deployment of specific strains and strain mixtures in field locations more suitable to their activity, so-called “prescription biocontrol” (3). Such an approach was taken by Duffy et al. (12), who identified soil factors favorable to take-all suppression by Trichoderma koningii and then applied this information to develop a model enabling its performance to be predicted at field sites in the United States and China. Another potential application of our work is the development of mineral amendments to improve biocontrol under unfavorable conditions. Preliminary work has shown that zinc-EDTA amendments improved the biocontrol of Gaeumannomyces graminis var. graminis by 2-79 in a zinc-deficient soil (53). Our finding that biocontrol strains differ in zinc tolerance, however, emphasizes the need to minimize the potential toxicity to other beneficial microorganisms. Providing minerals directly in biocontrol formulations would be one way to reduce the total dosage applied to the environment and optimize availability to the target agent. Our finding that inorganic phosphates repress antibiotic production by diverse strains raises important questions about potential adverse effects of phosphate fertilizers commonly used in agriculture on not only introduced biocontrol agents but also indigenous populations of antagonists.
Modulating the production of antimicrobial metabolites during growth may also improve the quality of inoculants. Lowering the PHL and PLT concentrations in inoculants with phosphate amendments would avoid potential phytotoxicity problems (33, 51, 53) and at the same time increase bacterial growth (31). On the other hand, increasing antibiotic concentrations with zinc and other amendments may provide a bridge of protection against diseases with a rapid onset (e.g., pythium damping-off) that outpace the ability of introduced bacteria to become established in the rhizosphere and commence in situ antibiotic production. Zinc and other minerals have the extra benefit of improving the genetic stability in inoculants (9). We have identified a number of mineral and/or carbon source amendments that stimulate siderophore production in P. fluorescens. Siderophores, particularly salicylic acid, have been implicated in the ability of certain strains to trigger induced resistance in plants (8, 35), and increasing their supply via inoculants may be advantageous. Zinc has previously been reported to stimulate the production of pyochelin and pyoverdin in the Pseudomonas aeruginosa biocontrol strain 7NSK2 (19) and plant-associated Azotobacter vinelandii (20). Interestingly, zinc stimulation relieves bacterial siderophore production from iron repression (19), which might allow a greater role for siderophores in microbial interactions under iron-sufficient conditions (29).
Identifying differential responses to signals sheds new light on the regulation of antibiotic biosynthesis and its evolution. By screening strains together we avoided differences that could be attributed to variations in experimental conditions in different laboratories working with single strains. The strains we studied have a conserved biosynthetic region (phlD) for antibiotic production (22) but are genetically different and have been characterized into three ARDRA and seven PCR-RAPD groups (22). Responses to zinc and glucose were not linked to any particular group of strains, which may reflect adaptation to specific local conditions. We recently reported that fusaric acid repression of PHL is ARDRA group dependent (11), suggesting that this is a more general adaptation. At this point we cannot say whether adaptations occurred in signal uptake and/or recognition, global activation, autoinduction, biosynthetic gene promotion, or antibiotic processing. Export was not a factor though, since our extraction procedures involved cell lysis and the release of intracellular antibiotics. Sequencing the phl operon and the flanking regions of several strains will shed more light on environmental regulation; currently, the complete sequence is available only for Q2-87 (1). Relieving strains or making them more responsive to certain environmental signals has been exploited for increased antibiotic production in pharmaceutical fermentations (31), and we believe it presents new opportunities to improve biocontrol.
ACKNOWLEDGMENTS
We are grateful to David Weller and two anonymous reviewers for constructive criticism of the manuscript and to Ulrich Burger and Joyce Loper for generously providing standards of PHL, pyrrolnitrin, and PLT.
This work was funded by the Swiss National Foundation of Science (grant no. 3100-50522.97).
REFERENCES
- 1.Bangera M G, Thomashow L S. Characterization of a genomic locus required for synthesis of the antibiotic 2,4-diacetylphloroglucinol by the biological control agent Pseudomonas fluorescens Q2-87. Mol Plant-Microbe Interact. 1996;9:83–90. doi: 10.1094/mpmi-9-0083. [DOI] [PubMed] [Google Scholar]
- 2.Behal V, Hunter I S. Tetracyclines. In: Vining L C, Stuttard C, editors. Genetics and biochemistry of antibiotic production. Boston, Mass: Butterworth-Heinemann; 1995. pp. 359–384. [Google Scholar]
- 3.Cook R J. Making greater use of introduced microorganisms for biological control of plant pathogens. Annu Rev Phytopathol. 1993;31:53–80. doi: 10.1146/annurev.py.31.090193.000413. [DOI] [PubMed] [Google Scholar]
- 4.Corbell N, Loper J E. A global regulator of secondary metabolite production in Pseudomonas fluorescens Pf-5. J Bacteriol. 1995;177:6230–6236. doi: 10.1128/jb.177.21.6230-6236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cousins R J. Metal elements and gene expression. Annu Rev Nutr. 1994;14:449–469. doi: 10.1146/annurev.nu.14.070194.002313. [DOI] [PubMed] [Google Scholar]
- 6.Défago G, Haas D. Pseudomonads as antagonists of soilborne plant pathogens: modes of action and genetic analysis. Soil Biochem. 1990;6:249–291. [Google Scholar]
- 7.Dekleva M L, Strohl W R. Glucose-stimulated acidogenesis by Streptomyces peucetius. Can J Microbiol. 1987;33:1129–1132. doi: 10.1139/m87-198. [DOI] [PubMed] [Google Scholar]
- 8.De Meyer G, Höfte M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology. 1997;87:588–593. doi: 10.1094/PHYTO.1997.87.6.588. [DOI] [PubMed] [Google Scholar]
- 8a.Duffy, B. K. Unpublished data.
- 9.Duffy B K, Défago G. Influence of cultural conditions on spontaneous mutations in Pseudomonas fluorescens CHA0. Phytopathology. 1995;85:1146. [Google Scholar]
- 10.Duffy B K, Défago G. Zinc improves biocontrol of Fusarium crown and root rot of tomato by Pseudomonas fluorescens and represses the production of pathogen metabolites inhibitory to bacterial antibiotic biosynthesis. Phytopathology. 1997;87:1250–1257. doi: 10.1094/PHYTO.1997.87.12.1250. [DOI] [PubMed] [Google Scholar]
- 11.Duffy B K, Défago G. A Fusarium pathogenicity factor blocks antibiotic biosynthesis by antagonistic pseudomonads. Phytopathology. 1997;87:S26. doi: 10.1094/PHYTO.1997.87.12.1250. [DOI] [PubMed] [Google Scholar]
- 11a.Duffy, B. K., B. H. Ownley, and D. M. Weller. Unpublished data.
- 12.Duffy B K, Ownley B H, Weller D M. Soil chemical and physical properties associated with suppression of take-all of wheat by Trichoderma koningii. Phytopathology. 1997;87:1118–1124. doi: 10.1094/PHYTO.1997.87.11.1118. [DOI] [PubMed] [Google Scholar]
- 13.Engelhard A W. Soilborne plant pathogens: management of diseases with macro- and microelements. St. Paul, Minn: American Phytopathological Society; 1989. [Google Scholar]
- 14.Fedi S, Tola E, Moënne-Loccoz Y, Dowling D N, Smith L M, O’Gara F. Evidence for signaling between the phytopathogenic fungus Pythium ultimum and Pseudomonas fluorescens F113: P. ultimum represses the expression of genes in P. fluorescens F113, resulting in altered ecological fitness. Appl Environ Microbiol. 1997;63:4261–4266. doi: 10.1128/aem.63.11.4261-4266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaballa A, Abeysinghe P D, Uhrich G, Matthijs S, De Greve H, Cornelis P, Koedam N. Trehalose induces antagonism towards Pythium debaryanum in Pseudomonas fluorescens ATCC 17400. Appl Environ Microbiol. 1997;63:4340–4345. doi: 10.1128/aem.63.11.4340-4345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaffney T D, Lam S T, Ligon J, Gates K, Frazelle A, Di Maio J, Hill S, Goodwin S, Torkewitz N, Allshouse A M, Kempf H J, Becker J O. Global regulation of expression of anti-fungal factors by a Pseudomonas fluorescens biological control strain. Mol Plant-Microbe Interact. 1994;7:455–463. doi: 10.1094/mpmi-7-0455. [DOI] [PubMed] [Google Scholar]
- 17.Gutterson N. Microbial fungicides: recent approaches to elucidating mechanisms. Crit Rev Biotechnol. 1990;10:69–91. [Google Scholar]
- 18.Höfte M, Boelens J, Verstraete W. Survival and root colonization of mutants of plant growth-promoting pseudomonads affected in siderophore biosynthesis or regulation of siderophore production. J Plant Nutr. 1992;15:2253–2262. [Google Scholar]
- 19.Höfte M, Dong Q, Kourambas S, Krishnapillai V, Sherratt D, Mergeay M. The sss gene product, which affects pyoverdine production in Pseudomonas aeruginosa 7NSK2, is a site-specific recombinase. Mol Microbiol. 1994;14:1011–1020. doi: 10.1111/j.1365-2958.1994.tb01335.x. [DOI] [PubMed] [Google Scholar]
- 20.Huyer M, Page W J. Ferric reductase activity in Azotobacter vinelandii and its inhibition by Zn2+ J Bacteriol. 1989;171:4031–4037. doi: 10.1128/jb.171.7.4031-4037.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keel C, Voisard C, Berling C H, Kahr G, Défago G. Iron sufficiency, a prerequisite for the suppression of tobacco black root rot by Pseudomonas fluorescens strain CHA0 under gnotobiotic conditions. Phytopathology. 1989;79:584–589. [Google Scholar]
- 22.Keel C, Weller D M, Natsch A, Défago G, Cook R J, Thomashow L S. Conservation of the 2,4-diacetylphloroglucinol biosynthesis locus among fluorescent Pseudomonas strains from diverse geographic locations. Appl Environ Microbiol. 1996;62:552–563. doi: 10.1128/aem.62.2.552-563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirner S, Hammer P E, Hill D S, Altmann A, Fischer I, Weislo L H, Lanahan M, van Pée K-H, Ligon J M. Functions encoded by pyrrolnitrin biosynthetic genes from Pseudomonas fluorescens. J Bacteriol. 1998;180:1939–1943. doi: 10.1128/jb.180.7.1939-1943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraus J, Loper J E. Characterization of a genomic region required for production of the antibiotic pyoluteorin by the biological control agent Pseudomonas fluorescens Pf-5. Appl Environ Microbiol. 1995;61:849–854. doi: 10.1128/aem.61.3.849-854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latour X, Corberand T, Laguerre G, Allard F, Lemanceau P. The composition of fluorescent pseudomonad populations associated with roots is influenced by plant and soil type. Appl Environ Microbiol. 1996;62:2449–2556. doi: 10.1128/aem.62.7.2449-2456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laville J, Voisard C, Keel C, Maurhofer M, Défago G, Haas D. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA. 1992;89:1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leeman M, Den Ouden F M, Van Pelt J A, Dirkx F P M, Steijl H, Bakker P A H M, Schippers B. Iron availability affects induction of systemic resistance to Fusarium wilt of radish in commercial greenhouse trials by seed treatment with Pseudomonas fluorescens WCS374. Phytopathology. 1996;85:149–155. [Google Scholar]
- 28.Lemanceau P, Corberand T, Gardan L, Latour X, Laguerre G, Boeufgras J M, Alabouvette C. Effect of two plant species flax (Linum usitatissinum L.) and tomato (Lycopersicon esculentum Mill.) of the diversity of soilborne populations of fluorescent pseudomonads. Appl Environ Microbiol. 1995;61:1004–1012. doi: 10.1128/aem.61.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loper J E, Henkels M D. Availability of iron to Pseudomonas fluorescens in rhizosphere and bulk soil evaluated with an ice nucleation reporter gene. Appl Environ Microbiol. 1997;63:99–105. doi: 10.1128/aem.63.1.99-105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loper J E, Nowak-Thompson B, Whistler C A, Hagen M J, Corbell N A, Henkels M D, Stockwell V O. Biological control mediated by antifungal metabolite production and resource competition: an overview. In: Ogoshi A, Kobayashi K, Homma Y, Kodama F, Kondo N, Akino S, editors. Plant growth-promoting rhizobacteria: present status and future prospects. Japan-OECD Paris workshop. Sapporo, Japan: Hokkaido University; 1997. pp. 108–115. [Google Scholar]
- 31.Martín J F, Demain A L. Control of antibiotic biosynthesis. Microbiol Rev. 1980;44:230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martín J F, Marcos A T, Martín A, Asturias J A, Liras P. Phosphate control of antibiotic biosynthesis at the transcriptional level. In: Torriani-Gorini A, Yagil E, Silver S, editors. Phosphates in microorganisms: cellular and molecular biology. Washington, D.C: ASM Press; 1994. pp. 140–147. [Google Scholar]
- 33.Maurhofer M, Keel C, Haas D, Défago G. Pyoluteorin production by Pseudomonas fluorescens strain CHA0 is involved in the suppression of Pythium damping-off of cress but not of cucumber. Eur J Plant Pathol. 1994;100:221–232. [Google Scholar]
- 34.Maurhofer M, Keel C, Haas D, Défago G. Influence of plant species on disease suppression by Pseudomonas fluorescens CHA0 with enhanced antibiotic production. Plant Pathol. 1995;44:44–50. [Google Scholar]
- 35.Maurhofer M, Reimmann C, Schmidli-Sacherer P, Heeb S, Haas D, Defago G. Salicylic acid biosynthetic genes expressed in Pseudomonas fluorescens strain P3 improve the induction of systemic resistance in tobacco against tobacco necrosis virus. Phytopathology. 1998;88:678–684. doi: 10.1094/PHYTO.1998.88.7.678. [DOI] [PubMed] [Google Scholar]
- 36.Mazzola M, Cook R J, Thomashow L S, Weller D M, Pierson L S. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol. 1992;58:2616–2624. doi: 10.1128/aem.58.8.2616-2624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milner J L, Raffel S J, Lethbridge B J, Handelsman J. Culture conditions that influence accumulation of zwittermicin A by Bacillus cereus UW85. Appl Microbiol Biotechnol. 1995;43:685–691. doi: 10.1007/BF00164774. [DOI] [PubMed] [Google Scholar]
- 38.Milner J L, Silo-Suh L, Lee J C, He H, Clardy J, Handelsman J. Production of kanosamine by Bacillus cereus UW85. Appl Environ Microbiol. 1996;62:3061–3065. doi: 10.1128/aem.62.8.3061-3065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oberhänsli T, Défago G, Haas D. Indole-3-acetic acid (IAA) synthesis in the biocontrol strain CHA0 of Pseudomonas fluorescens: role of tryptophan side chain oxidase. J Gen Microbiol. 1991;137:2273–2279. doi: 10.1099/00221287-137-10-2273. [DOI] [PubMed] [Google Scholar]
- 40.O’Connell K P, Goodman R M, Handelsman J. Engineering the rhizosphere: expressing a bias. Trends Biotechnol. 1996;14:83–88. [Google Scholar]
- 41.Ownley B H, Weller D M, Thomashow L S. Influence of in situ and in vitro pH on suppression of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens 2-79. Phytopathology. 1992;82:178–184. [Google Scholar]
- 42.Ownley B H, Weller D M, Alldredge J R. Relation of soil chemical and physical factors with suppression of take-all by Pseudomonas fluorescens 2-79. IOBC/WPRS Bull. 1991;14(8):299–301. [Google Scholar]
- 43.Pierson L S, III, Wood D W, Pierson E A, Chancey S T. N-Acyl-homoserine lactone-mediated gene regulation in biological control by fluorescent pseudomonads: current knowledge and future work. Eur J Plant Pathol. 1998;104:1–9. [Google Scholar]
- 44.Raaijmakers J M, Weller D M, Thomashow L S. Frequency of antibiotic-producing Pseudomonas spp. in natural environments. Appl Environ Microbiol. 1997;63:881–887. doi: 10.1128/aem.63.3.881-887.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarniguet A, Kraus J, Henkels M D, Muehlchen A M, Loper J E. The sigma factor ςs affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc Natl Acad Sci USA. 1995;92:12255–12259. doi: 10.1073/pnas.92.26.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnider U, Keel C, Blumer C, Troxler J, Défago G, Haas D. Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J Bacteriol. 1995;177:5387–5392. doi: 10.1128/jb.177.18.5387-5392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shanahan P, Glennon J D, Crowley J J, Donnelly D F, O’Gara F. Liquid chromatographic assay of microbially derived phloroglucinol antibiotics for establishing the biosynthetic route to production, and the factors affecting their regulation. Anal Chim Acta. 1993;272:271–277. [Google Scholar]
- 48.Shanahan P, O’Sullivan D J, Simpson P, Glennon J D, O’Gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992;58:353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slininger P J, Jackson M A. Nutritional factors regulating growth and accumulation of phenazine 1-carboxylic acid by Pseudomonas fluorescens 2-79. Appl Microbiol Biotechnol. 1992;37:388–392. [Google Scholar]
- 50.Slininger P, Shea-Wilbur M A. Liquid-culture pH, temperature, and carbon (not nitrogen) source regulate phenazine productivity of the take-all biocontrol agent Pseudomonas fluorescens 2-79. Appl Microbiol Biotechnol. 1995;43:794–800. doi: 10.1007/BF02431910. [DOI] [PubMed] [Google Scholar]
- 51.Slininger P J, Van Cauwenberger J E, Bothast R J, Weller D M, Thomashow L S, Cook R J. Effect of growth culture physiological state, metabolites, and formulation on the viability, phytotoxicity, and efficacy of the take-all biocontrol agent Pseudomonas fluorescens 2-79 stored encapsulated on wheat seeds. Appl Microbiol Biotechnol. 1996;45:391–398. [Google Scholar]
- 52.Smith K P, Handelsman J, Goodman R M. Modeling dose-response relationships in biological control: partitioning host responses to the pathogen and biocontrol agent. Phytopathology. 1997;87:720–729. doi: 10.1094/PHYTO.1997.87.7.720. [DOI] [PubMed] [Google Scholar]
- 53.Thomashow L S, Weller D M. Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites. In: Stacey G, Keen N T, editors. Plant-microbe interactions. Vol. 1. New York, N.Y: Chapman and Hall; 1996. pp. 187–235. [Google Scholar]
- 54.Turner J M, Messenger A J. Occurrence, biochemistry and physiology of phenazine pigment production. Adv Microb Physiol. 1986;27:211–275. doi: 10.1016/s0065-2911(08)60306-9. [DOI] [PubMed] [Google Scholar]
- 55.Voisard C, Bull C T, Keel C, Laville J, Maurhofer M, Schnider U, Défago G, Haas D. Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches. In: O’Gara F, Dowling D N, Boesten B, editors. Molecular ecology of rhizosphere microorganisms: biotechnology and the release of GMO’s. Weinheim, Germany: VCH; 1994. pp. 67–89. [Google Scholar]
- 56.Weinberg E D. Mineral element control of microbial secondary metabolism. In: Weinberg E D, editor. Microorganisms and minerals. New York, N.Y: Marcel Dekker; 1977. pp. 289–316. [Google Scholar]
- 57.Westover K M, Kennedy A C, Kelley S E. Patterns of rhizosphere microbial community structure associated with co-occurring plant species. J Ecol. 1997;85:863–873. [Google Scholar]