Abstract
Purpose
Sorafenib is an oral multikinase inhibitor with regulatory approval in advanced renal cell carcinoma (RCC), hepatocellular carcinoma (HCC) and refractory differentiated thyroid carcinoma (DTC). Vascular endothelial growth factor receptor (VEGFR) inhibitors like sorafenib may cause proteinuria. This study aimed to analyze the effectiveness and safety of sorafenib in RCC, HCC and DTC patients with chronic kidney disease (CKD).
Methods
This retrospective study analyzed integrated data from prospective post-marketing surveillance studies for advanced RCC, HCC and DTC. Background factors considered to affect patients’ prognosis were balanced by propensity score matching using eGFR cut-off values of 60 mL/min/1.73 m2.
Results
In the combined matched population (N = 2430), sorafenib was equally effective in patients with lower and higher eGFR values. Sorafenib had an overall response rate (ORR: complete + partial responses) of 18.9% and a disease control rate (DCR: complete + partial responses + stable disease) of 67.0%. There were no significant differences between lower and higher eGFR groups for response rates. Renal function was maintained throughout the 12-month study period in the combined population and in each indication. Adverse events (AEs) and serious AEs were reported in 91.6% and 58.2% of propensity score-matched patients, and with no significant differences between lower and higher eGFR groups.
Conclusion
The effectiveness and safety of sorafenib were similar in patients with eGFR < 60 and ≥ 60 mL/min/1.73 m2 during the 12-month observation period, and without impairing renal function.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00280-022-04428-0.
Keywords: Differentiated thyroid carcinoma, Hepatocellular carcinoma, Propensity score, Renal cell carcinoma, Sorafenib
Introduction
Sorafenib is an oral multikinase inhibitor with inhibitory effects on angiogenesis and tumor cell growth [1]. Following completion of a large phase 3 trial [2], sorafenib was approved by the U.S. Food and Drug Administration (FDA) in December 2005 for the treatment of patients with advanced renal cell carcinoma (RCC) [3]. FDA approval of sorafenib for advanced hepatocellular carcinoma (HCC) in November 2007 [4] followed the completion of multinational phase 3 trials of sorafenib in advanced HCC [5], including patients in the Asia–Pacific region [6]. In November 2013, sorafenib was approved by the FDA for advanced and metastatic radioactive iodine-refractory differentiated thyroid carcinoma (DTC) [7, 8].
Sorafenib targets the RAF/MEK/ERK pathway through potent inhibition of RAF kinase and inhibits receptor tyrosine kinases including vascular endothelial growth factor receptor (VEGFR)-2, VEGFR-3, platelet-derived growth factor receptor beta (PDGFRB), FLT3, RET and c-KIT [1, 9]. VEGFR is highly expressed in vascular endothelial cells and glomerular epithelial cells (podocytes), and there is evidence that VEGFR inhibitors (e.g., sunitinib, sorafenib, axitinib, lenvatinib or bevacizumab), which target circulating VEGF, may cause proteinuria [10–13].
KDIGO (Kidney Disease: Improving Global Outcomes) defines chronic kidney disease (CKD) “as abnormalities of kidney structure or function, present for > 3 months, with implications for health” [14]. Both KDIGO and The Japanese Society of Kidney Disease consider that the diagnostic threshold for CKD is an estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2 lasting for more than 3 months [14–16]. CKD is associated with an increased incidence of myocardial infarction, heart failure, stroke and risk of death [17–20]. Reduced eGFR has a graded association with all-cause mortality, brain vascular events and sharp rises in hospitalization rates [17, 18].
Although sorafenib has been reported to be effective and safe for CKD patients in RCC [21], the safety of sorafenib for CKD in HCC [22] and thyroid carcinoma [23] remains unclear. The aim of the study was to investigate the safety and effectiveness of sorafenib in patients with reduced eGFR (< 60 ml/min/1.73 m2) in an integrated analysis of three indications—RCC, HCC and DTC—using propensity score matching to adjust baseline factors affecting patient prognosis.
Methods
Study design
This retrospective study analyzed integrated data from prospective post-marketing surveillance (PMS) studies conducted after the approval of sorafenib for each indication, at the request of the Pharmaceuticals and Medical Devices Agency (PMDA), the Japanese regulatory authority: Japanese patients with metastatic RCC [24], unresectable HCC [22], or metastatic DTC were included. There were no dose restrictions or dose reduction criteria for sorafenib administration, including patients with severe disease (eGFR < 30 mL/min/1.73 m2). Background factors considered to affect patients’ prognosis were balanced by propensity score matching using eGFR cut-off values of 60 mL/min/1.73 m2 (i.e., eGFR < 60 and ≥ 60 mL/min/1.73 m2). We set the eGFR cut-off value at 60 mL/min/1.73 m2 because we suspected CKD in patients with eGFR < 60 mL/min/1.73 m2. Analysis of data using this selected cut-off value did not produce extreme data bias. Propensity score matching was calculated by logistic modelling for each disease population (RCC, HCC and DTC) and then applied to the combined population.
Criteria for propensity score matching for RCC were age, TNM (tumor, node, metastasis) stage, prior surgery, primary disease (unresectable/metastatic), C-reactive protein (CRP) level, and disease subtype (clear cell/non-clear cell carcinoma). Before propensity score matching, numbers of RCC patients in the eGFR < 60 and ≥ 60 mL/min/1.73 m2 groups were 1930 and 933, respectively; following matching, there were 583 patients in each group.
Criteria for propensity score matching for HCC were age, TNM stage, prior surgery, metastatic site (bone, lung), presence/absence of lymphatic metastases; comorbidities (cardiac, hypertension, diabetes); weight by sex; Child–Pugh score; baseline alanine aminotransferase (ALT), aspartate aminotransferase (AST) and hemoglobin concentrations, baseline platelet count; hepatitis B and hepatitis C status; alcohol consumption, liver cirrhosis, blood biomarker risk factors; treated with transcatheter arterial infusion (TAI), percutaneous ethanol injection (PEIT), percutaneous radiofrequency ablation (RFA), transarterial infusion chemotherapy (TAE) or transcatheter arterial chemoembolization (TACE) and/or radiotherapy. The number of HCC patients before propensity score matching in the eGFR < 60 and ≥ 60 mL/min/1.73 m2 groups was 423 and 1023, respectively; after matching, there were 364 patients in each group.
Criteria for propensity score matching for DTC were age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), any prior systemic anti-cancer therapy, any metastatic site, cardiac comorbidity, initial sorafenib dose, median baseline ALT, AST and hemoglobin concentrations, median baseline platelet count; days from diagnosis, and anti-hypertensive dose. Before propensity score matching, there were 129 and 263 patients in the eGFR < 60 and ≥ 60 mL/min/1.73 m2 groups, respectively; after matching, there were 98 patients in each group.
For the combined population (n = 4834), criteria for propensity score matching were age, ECOG performance status, prior systemic anticancer therapy, metastasis at any site, cardiac comorbidity, initial sorafenib dose, median baseline ALT, AST and hemoglobin concentrations, median baseline platelet count, weight by sex, time from diagnosis, and anti-hypertensive dose. Numbers of patients with eGFR of < 60 and ≥ 60 mL/min/1.73 m2 were 2531 and 2303, respectively; following matching, there were 1215 patients per group.
Disease-specific adverse events (AEs) were excluded from the integrated analysis. Remaining AEs were recorded according to MedDRA System Organ Class (SOC) and preferred term.
Statistical analysis
Continuous variables were summarized using mean, standard deviation (SD), median and interquartile range (IQR), and categorical data by number and percentage. Statistical comparisons of continuous variables were performed using Student’s t-test and the Mann–Whitney U-test; and for categorical data, Pearson χ2 test was used. Kaplan–Meier survival curves for PFS were constructed and hazard ratios (HRs) with 95% confidence interval (CI) calculated. Significance of HRs was determined using log-rank tests. When selecting the background factors used in propensity score matching for all cancer types, consideration was given to the following points: clinically important variables, whilst excluding variables with an extremely skewed distribution and those with a high proportion of missing data (even if clinically important); multicollinearity; correlations between variables.
Results
Patients’ baseline demographics in the combined population, before and after propensity score matching, are summarized in Table 1. Before propensity score matching, there were significant differences between eGFR < 60 mL/min/1.73 m2 (n = 2482) and eGFR ≥ 60 mL/min/1.73 m2 (n = 2219) groups for age, ECOG PS, baseline eGFR, TNM stage, prior surgery, prior systemic anticancer therapy, metastases at any site, lung metastases, cardiac and renal comorbidities, baseline AST, ALT, total bilirubin, albumin and creatinine concentrations, number of observation days, baseline platelet count, initial sorafenib dose, initial antihypertensive dose, distribution of indications (all p < 0.0001), and baseline hemoglobin concentration (p = 0.0009). After propensity score matching (n = 1215 in both groups), there were no significant differences between these variables except for renal-associated variables: baseline eGFR, renal comorbidity, and baseline creatinine (all p < 0.0001); and prior surgery (p < 0.0001), baseline albumin (p = 0.0145), and distribution of indications (p = 0.0150). Demographics and clinical characteristics of RCC, HCC and DTC cohorts are summarized in Supplementary Tables S1, S2 and S3, respectively.
Table 1.
Demographics of integrated data from 3 indications: renal cell carcinoma (RCC), hepatocellular carcinoma (HCC) and differentiated thyroid carcinoma (DTC)
| Variables | Before propensity score matching | After propensity score matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) | p-value | eGFR (mL/min/1.73 m2) | p-value | |||||||
| < 60 (N = 2482) | ≥ 60 (N = 2219) | < 60 (N = 1215) | ≥ 60 (N = 1215) | |||||||
| Gender, n (%) | ||||||||||
| Male | 1857 (74.8) | 1628 (73.4) | 0.2563 | 894 (73.6) | 897 (73.8) | 0.8901 | ||||
| Female | 625 (25.2) | 591 (26.6) | 321 (26.4) | 318 (26.2) | ||||||
| Age (y), mean ± SD | 68.4 ± 9.4 | 64.7 ± 10.7 | < 0.0001 | 66.9 ± 10.1 | 66.6 ± 10.3 | 0.5930 | ||||
| Weight (kg), mean ± SD | 59.15 ± 11.17 | 58.98 ± 12.07 | 0.6308 | 59.14 ± 11.64 | 58.69 ± 11.96 | 0.3496 | ||||
| BMI (kg/m2), mean ± SD | 22.61 ± 3.36 | 22.45 ± 3.77 | 0.1500 | 22.54 ± 3.50 | 22.43 ± 3.77 | 0.4695 | ||||
| ECOG Performance Status*, n (%) | ||||||||||
| 0 | 1620 (65.3) | 1384 (62.4) | < 0.0001 | 778 (64.0) | 772 (63.5) | 0.9191 | ||||
| 1 | 768 (30.9) | 690 (31.1) | 382 (31.4) | 384 (31.6) | ||||||
| ≥ 2 | 93 (3.8) | 145 (6.5) | 55 (4.5) | 59 (4.9) | ||||||
| Baseline eGFR (mL/min/1.73 m2), mean ± SD | 44.15 ± 12.25 | 80.75 ± 25.64 | < 0.0001 | 45.57 ± 11.96 | 77.46 ± 17.23 | < 0.0001 | ||||
| TNM stage, n (%) | ||||||||||
| I/II/III | 197 (7.9) | 336 (15.1) | < 0.0001 | 145 (11.9) | 161 (13.3) | 0.1034 | ||||
| IVA | 2042 (82.3) | 1266 (57.1) | 877 (72.2) | 819 (67.4) | ||||||
| IVB | 169 (6.8) | 458 (20.6) | 143 (11.8) | 167 (13.7) | ||||||
| IVC | 21 (0.9) | 63 (2.8) | 16 (1.3) | 22 (1.8) | ||||||
| Unknown | 53 (2.1) | 96 (4.3) | 34 (2.8) | 46 (3.8) | ||||||
| Prior surgery, n (%) | 2202 (88.7) | 1834 (82.7) | < 0.0001 | 1076 (88.6) | 991 (81.6) | < 0.0001 | ||||
| Prior systemic anticancer therapy*, n (%) | 1656 (66.7) | 922 (41.6) | < 0.0001 | 671 (55.2) | 634 (52.2) | 0.1322 | ||||
| Metastases, n (%) | ||||||||||
| Any site* | 2095 (84.4) | 1501 (67.6) | < 0.0001 | 927 (76.3) | 890 (73.3) | 0.0840 | ||||
| Bone | 604 (24.3) | 541 (24.4) | 0.9713 | 290 (23.9) | 324 (26.7) | 0.1125 | ||||
| Brain | 93 (3.8) | 72 (3.2) | 0.3502 | 42 (3.5) | 52 (4.3) | 0.2928 | ||||
| Lung | 1476 (59.5) | 962 (43.4) | < 0.0001 | 623 (51.3) | 604 (49.7) | 0.4408 | ||||
| Comorbidity, n (%) | ||||||||||
| Cardiac* | 942 (38.0) | 462 (20.8) | < 0.0001 | 360 (29.6) | 316 (26.0) | 0.0464 | ||||
| Pulmonary | 98 (4.0) | 86 (3.9) | 0.8977 | 50 (4.1) | 45 (3.7) | 0.6008 | ||||
| Renal | 257 (10.4) | 29 (1.3) | < 0.0001 | 130 (10.7) | 13 (1.1) | < 0.0001 | ||||
| Baseline AST (IU/L), mean ± SD | 32.89 ± 41.88 | 48.38 ± 56.76 | < 0.0001 | 40.30 ± 54.43 | 39.45 ± 46.20 | 0.6770 | ||||
| Baseline ALT (IU/L), mean ± SD | 25.40 ± 33.24 | 34.08 ± 31.19 | < 0.0001 | 29.62 ± 42.20 | 29.42 ± 29.42 | 0.8922 | ||||
| Total bilirubin (mg/dL), mean ± SD | 0.65 ± 0.53 | 0.84 ± 0.87 | < 0.0001 | 0.71 ± 0.58 | 0.73 ± 0.53 | 0.3797 | ||||
| Baseline albumin (g/dL), mean ± SD | 3.69 ± 0.97 | 3.49 ± 0.64 | < 0.0001 | 3.64 ± 1.19 | 3.54 ± 0.65 | 0.0145 | ||||
| Baseline creatinine (mg/dL), mean ± SD | 1.48 ± 1.38 | 0.73 ± 0.15 | < 0.0001 | 1.41 ± 1.29 | 0.75 ± 0.15 | < 0.0001 | ||||
| BMI ≥ 25 kg/m2, n (%) | 459 (18.5) | 418 (18.8) | 0.7623 | 252 (20.7) | 248 (20.4) | 0.8409 | ||||
| Observation days, median (IQR) | 245.0 (298.0) | 195.0 (291.0) | < 0.0001 | 207.0 (301.0) | 217.0 (287.0) | 0.4389 | ||||
| Initial sorafenib dose*, n (%) | ||||||||||
| 800 mg | 1880 (75.8) | 1666 (75.1) | 0.5962 | 923 (76.0) | 936 (77.0) | 0.5339 | ||||
| < 800 mg | 602 (24.3) | 553 (24.9) | 292 (24.0) | 279 (23.0) | ||||||
| Weight by sex*, n (%) | ||||||||||
| < Median | 1077 (48.5) | 1025 (50.6) | 0.1720 | 609 (50.1) | 618 (50.9) | 0.7150 | ||||
| ≥ Median | 1145 (51.5) | 1002 (49.4) | 606 (49.9) | 597 (49.1) | ||||||
| Baseline AST*, n (%) | ||||||||||
| < Median | 1277 (51.9) | 1064 (48.1) | 0.0105 | 603 (49.6) | 616 (50.7) | 0.5979 | ||||
| ≥ Median | 1186 (48.2) | 1148 (51.9) | 612 (50.4) | 599 (49.3) | ||||||
| Baseline ALT*, n (%) | ||||||||||
| < Median | 1330 (54.1) | 999 (45.1) | < 0.0001 | 632 (52.0) | 603 (49.6) | 0.2393 | ||||
| ≥ Median | 1130 (45.9) | 1215 (54.9) | 583 (48.0) | 612 (50.4) | ||||||
| Baseline platelet count*, n (%) | ||||||||||
| < Median | 1326 (53.7) | 975 (44.2) | < 0.0001 | 575 (47.3) | 549 (45.2) | 0.2901 | ||||
| ≥ Median | 1145 (46.3) | 1232 (55.8) | 640 (52.7) | 666 (54.8) | ||||||
| Baseline haemoglobin*, n (%) | ||||||||||
| < Median | 1272 (51.5) | 1040 (47.1) | 0.0030 | 617 (50.8) | 612 (50.4) | 0.8392 | ||||
| ≥ Median | 1198 (48.5) | 1166 (52.9) | 598 (49.2) | 603 (49.6) | ||||||
| Time from diagnosis (d)*, n (%) | ||||||||||
| < median | 61 (49.2) | 124 (50.6) | 0.7968 | 42 (49.4) | 52 (53.6) | 0.5719 | ||||
| ≥ median | 63 (50.8) | 121 (49.4) | 43 (50.6) | 45 (46.4) | ||||||
| Application at initial dose*, n (%) | 283 (11.4) | 425 (19.2) | < 0.0001 | 179 (14.7) | 199 (16.4) | 0.2630 | ||||
| Hypertension medicine at initial dose*, n (%) | 372 (15.0) | 432 (19.5) | < 0.0001 | 229 (18.9) | 245 (20.2) | 0.4127 | ||||
| Distribution of indications, n (%) | ||||||||||
| Renal cell carcinoma | 1930 (77.8) | 933 (42.1) | < 0.0001 | 761 (62.6) | 691 (56.9) | 0.0150 | ||||
| Hepatocellular carcinoma | 423 (17.0) | 1023 (46.1) | 365 (30.0) | 420 (34.6) | ||||||
| Differentiated thyroid carcinoma | 129 (5.2) | 263 (11.9) | 89 (7.3) | 104 (8.6) | ||||||
ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, ECOG PS Eastern Cooperative Oncology Group performance status, eGFR estimated glomerular filtration rate, IQR interquartile range, TNM tumor, node, metastasis
*Indicates variable used for propensity score matching
Before propensity score matching, patients in the eGFR < 60 mL/min/1.73 m2 group received significantly lower mean daily sorafenib doses (491.8 vs. 529.3 mg/d) than patients with eGFR ≥ 60 mL/min/1.73 m2, had lower relative sorafenib doses (66.5% vs. 69.7%) and prolonged treatment (median 5.22 vs. 3.71 months) (all p < 0.0001). After propensity score matching, duration of treatment was similar in each group whereas there were significant differences for mean daily sorafenib doses (p = 0.0028) and relative sorafenib doses (p = 0.0034). Before propensity score matching, patients with lower eGFR values had significantly higher rates of dose reductions (52.9% vs. 43.5%; p < 0.0001), similar rates of treatment interruption (41.4% vs. 39.2%) and lower rates of treatment discontinuation (74.1% vs. 82.8%; p < 0.0001) compared with patients with higher eGFR. Following propensity score matching, patients in the lower eGFR group had higher rates of dose reductions (50.8% vs. 46.3%; p = 0.0256), and comparable rates of treatment interruption and treatment discontinuation. Before propensity score matching, patients in the lower eGFR group had higher rates of discontinuation due to AEs (55.7% vs. 50.5%; p = 0.0011) and lower rates due to insufficient effectiveness (34.4% vs. 39.7%; p = 0.0007). After propensity score matching, rates for reasons for discontinuation were comparable in each group (Table 2).
Table 2.
Distribution of initial and median sorafenib dose, dose modification, and reason for treatment discontinuation
| Variable | Before propensity score matching | After propensity score matching | ||||
|---|---|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) | p-value | eGFR (mL/min/1.73 m2) | p-value | |||
| < 60 (N = 2531) | ≥ 60 (N = 2303) | < 60 (N = 1215) | ≥ 60 (N = 1215) | |||
| Starting sorafenib dose (mg), median (IQR) | 800.0 (0.0) | 800.0 (200.0) | 0.7524 | 800.0 (0.0) | 800.0 (0.0) | 0.3952 |
| Daily sorafenib dose, (mg/d), median (IQR) | 491.8 (415.9) | 529.3 (400.0) | < 0.0001 | 496.6 (415.8) | 525.3 (400.0) | 0.0028 |
| Relative sorafenib dose intensity (%), mean ± SD | 66.5 ± 27.1 | 69.7 ± 26.0 | < 0.0001 | 66.5 ± 27.1 | 69.7 ± 26.0 | 0.0034 |
| Duration of treatment (mo), median (IQR) | 5.22 (10.41) | 3.71 (8.77) | < 0.0001 | 4.30 (9.79) | 4.53 (9.66) | 0.2473 |
| Dose modification, n (%) | ||||||
| Reduction | 1338 (52.9) | 1001 (43.5) | < 0.0001 | 617 (50.8) | 562 (46.3) | 0.0256 |
| Interruption | 1048 (41.4) | 903 (39.2) | 0.1200 | 498 (41.0) | 511 (42.1) | 0.5925 |
| Discontinuation | 1875 (74.1) | 1907 (82.8) | < 0.0001 | 960 (79.0) | 972 (80.0) | 0.5465 |
| Reason for discontinuation, n (%) | ||||||
| Adverse events | 1045 (55.7) | 962 (50.5) | 0.0011 | 523 (54.5) | 511 (52.6) | 0.4007 |
| Insufficient effectiveness | 644 (34.4) | 757 (39.7) | 0.0007 | 347 (36.2) | 372 (38.3) | 0.3338 |
| Others | 281 (15.0) | 310 (16.3) | 0.2825 | 148 (15.4) | 134 (13.8) | 0.3101 |
IQR interquartile range
In propensity score matched patients (N = 2430), AEs were recorded in 2225 (91.6%) patients and included 1413 (58.2%) serious AEs (SAEs). There were no significant between-group differences in the prevalence of AEs or SAEs. The most common AE was hand-foot skin reaction (HFSR; n = 1351; 55.6%), including 124 SAEs (5.1%); then hypertension (n = 748; 30.8%), with 7 SAEs (0.3%); and rash (n = 566; 23.3%), with 139 SAEs (5.7%). Patients in the higher eGFR group had a higher rate of HFSR than those with lower eGFR (53.6% vs. 57.6%; p = 0.0454) (Table 3).
Table 3.
Adverse events (AEs) and serious adverse events (SAEs) in propensity score-matched patients
| Preferred term | All patients (N = 2430) | eGFR (mL/min/1.73 m2) | p-value (AEs) | p-value (SAEs) | ||||
|---|---|---|---|---|---|---|---|---|
| AEs n (%) |
SAEs | < 60 (N = 1215) | ≥ 60 (N = 1215) | |||||
| AEs | SAEs | AEs | SAEs | |||||
| Any | 2225 (91.6) | 1413 (58.2) | 1110 (91.4) | 711 (58.5) | 1115 (91.8) | 702 (57.8) | 0.7152 | 0.7113 |
| Hand and foot skin reaction | 1351 (55.6) | 124 (5.1) | 651 (53.6) | 66 (5.4) | 700 (57.6) | 58 (4.8) | 0.0454 | 0.4608 |
| Hypertension | 748 (30.8) | 7 (0.3) | 370 (30.5) | 4 (0.3) | 378 (31.1) | 3 (0.3) | 0.7252 | 0.7051 |
| Rash | 566 (23.3) | 139 (5.7) | 301 (24.8) | 80 (6.6) | 265 (21.8) | 59 (4.9) | 0.0840 | 0.0666 |
| Diarrhoea | 514 (21.2) | 33 (1.4) | 273 (22.5) | 19 (1.6) | 241 (19.8) | 14 (1.2) | 0.1119 | 0.3808 |
| Hepatic dysfunction | 508 (20.9) | 270 (11.1) | 261 (21.5) | 126 (10.4) | 247 (20.3) | 144 (11.9) | 0.4849 | 0.2453 |
| Lipase/amylase increased | 401 (16.5) | 11 (0.5) | 223 (18.4) | 6 (0.5) | 178 (14.7) | 5 (0.4) | 0.0139 | 0.7625 |
| Dysphonia | 106 (4.4) | 1(0.0) | 60 (4.9) | 0 | 46 (3.8) | 1 (0.1) | 0.1644 | 0.3172 |
| Alopecia | 359 (14.8) | 1 (0.0) | 188 (15.5) | 0 | 171 (14.1) | 1 (0.1) | 0.3311 | 0.3172 |
| Cytopenia | 273 (11.2) | 92 (3.8) | 136 (11.2) | 44 (3.6) | 137 (11.3) | 48 (4.0) | 0.9488 | 0.6707 |
| Appetite decreased | 254 (10.5) | 44 (1.8) | 138 (11.4) | 27 (2.2) | 116 (9.6) | 17 (1.4) | 0.1446 | 0.1282 |
| Bleeding | 189 (7.8) | 159 (6.5) | 104 (8.6) | 84 (6.9) | 85 (7.0) | 75 (6.2) | 0.1501 | 0.4603 |
| Mucositis | 159 (6.5) | 8 (0.3) | 82 (6.8) | 5 (0.4) | 77 (6.3) | 3 (0.3) | 0.6817 | 0.4788 |
| Hypophosphatemia | 158 (6.5) | 3 (0.1) | 84 (6.9) | 2 (0.2) | 74 (6.1) | 1 (0.1) | 0.4106 | 0.5635 |
| Fever | 152 (6.3) | 42 (1.7) | 78 (6.4) | 22 (1.8) | 74 (6.1) | 20 (1.7) | 0.7376 | 0.77556 |
| Fatigue | 49 (2.0) | 5 (0.2) | 24 (2.0) | 3 (0.3) | 25 (2.1) | 2.0 (0.2) | 0.8852 | 0.6544 |
| Renal failure/dysfunction | 45 (1.9) | 26 (1.1) | 38 (3.1) | 20 (1.7) | 7 (0.6) | 6 (0.5) | < 0.0001 | 0.0058 |
| Proteinuria or protein urine, n (%) | 21 (0.9) | 1 (0.0) | 14 (1.2) | 1 (0.1) | 7 (0.6) | 0 | 0.1250 | 0.3172 |
In all propensity score-matched patients (N = 1881), sorafenib had a complete response rate of 1.3%, and rates for partial response, stable disease and progressive disease were 17.5%, 48.1% and 22.1%, respectively, producing an overall response rate (ORR: complete + partial responses) of 18.9% and a disease control rate (DCR: complete + partial responses + stable disease) of 67.0%. There were no significant differences between lower and higher eGFR groups for response rates (Table 4). The ORR and DCR in propensity score-matched RCC patients (N = 1079), was 26.4% and 83.6%, respectively; and in propensity score-matched HCC patients (N = 490), was 7.6% and 54.7%, respectively. No comparable data for DTC were collected.
Table 4.
Tumor response for sorafenib treatment
| Variable, n (%) | All (N = 2109) | eGFR (mL/min/1.73 m2) | p-value* | p-value† | |
|---|---|---|---|---|---|
| < 60 (n = 1053) | ≥ 60 (n = 1056) | ||||
| Complete response | 28 (1.3) | 15 (1.4) | 13 (1.2) | 0.6980 | 0.2759 |
| Partial response | 370 (17.5) | 181 (17.2) | 189 (17.9) | 0.6687 | |
| Stable disease | 1015 (48.1) | 517 (49.1) | 498 (47.2) | 0.3729 | |
| Progressive disease | 465 (22.1) | 212 (20.1) | 253 (24.0) | 0.0341 | |
| Non-evaluable | 231 (11.0) | 128 (12.2) | 103 (9.8) | 0.0774 | |
| Overall response rate (ORR) | 398 (18.9) | 196 (18.6) | 202 (19.1) | 0.7623 | |
| Disease control rate (DCR) | 1413 (67.0) | 713 (67.7) | 700 (66.3) | 0.4870 | |
*2 × 2 Pearson χ2 tests for each type of response
†Pearson χ2 test for overall independence (excludes non-evaluable data)
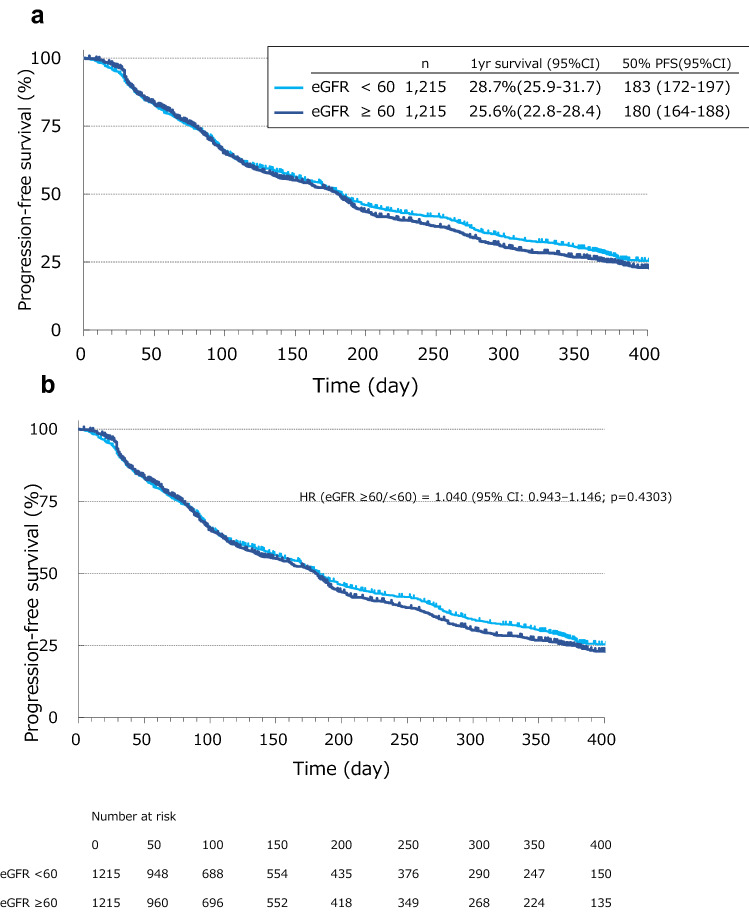
Combined analysis of propensity score matched patients showed no significant difference between lower and higher eGFR groups in PFS with a HR (eGFR ≥ 60/ < 60) of 1.040 (95% CI: 0.943–1.146; p = 0.4303). One-year PFS in the lower and higher eGFR groups was 28.7% and 25.6%, respectively (Fig. 1). In RCC patients, PFS was not significantly prolonged between eGFR groups (1-year PFS: 34.5% vs. 29.5%), with an HR of 1.096 (95% CI, 0.946–1.270; p = 0.2198) (Supplementary Figure S1A). In HCC patients, PFS was similar in each group (1-year PFS: 16.6% vs. 13.8%) with HR = 0.977 (95% CI, 0.823–1.160; p = 0.7889) (Supplementary Figure S1B). Similarly, in DTC patients, PFS was not significantly different between groups (1-year PFS: 46.5% vs. 48.4%) with HR = 0.770 (95% CI, 0.550–1.077; p = 0.1250) (Supplementary Figure S1C).
Fig. 1.
Progression-free survival in eGFR < 60 and ≥ 60 mL/min/1.73 m2 groups in the combined analysis population (N = 2430)
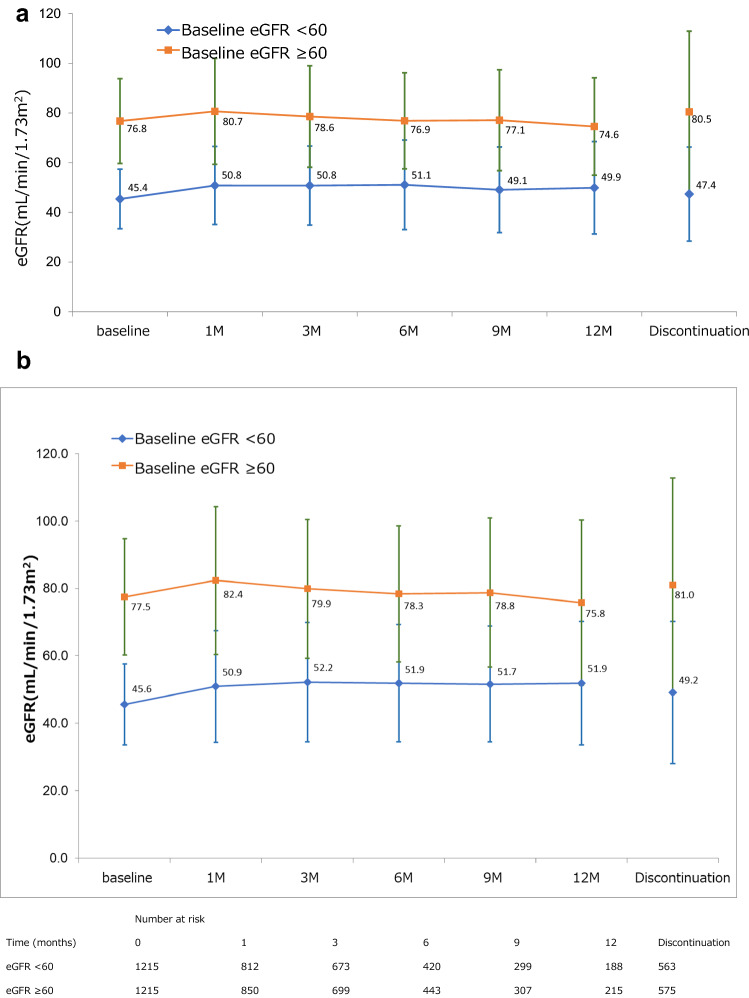
In the combined propensity score-matched population (N = 2430), mean ± SD baseline eGFR in the lower and higher eGFR groups was 45.6 ± 12.0 and 77.5 ± 17.2 mL/min/1.73 m2, respectively. The degree of change in eGFR from baseline was relatively constant throughout the 12-month observation period (Fig. 2).
Fig. 2.
Time course of change in renal function in eGFR < 60 and ≥ 60 mL/min/1.73 m2 groups in the combined analysis population (N = 2430)
In RCC propensity score matched patients (n = 1166) mean ± SD baseline eGFR values were 44.6 ± 12.1 and 73.6 ± 16.0 mL/min/1.73 m2, respectively; respective baseline eGFR values in HCC propensity score matched patients (n = 726) were 46.3 ± 10.9 and 81.9 ± 17.6 mL/min/1.73 m2; and in DTC propensity score matched patients (n = 196), values were 48.7 ± 10.1 and 78.1 ± 15.6 mL/min/1.73 m2. In common with the combined population, changes in eGFR from baseline for RCC (Supplementary Figure S2A), HCC (Supplementary Figure S2B), and DTC (Supplementary Figure S2C) were relatively constant throughout the 12-month observation period.
Discussion
Since the multi-kinase inhibitor sorafenib inhibits VEGF, it may worsen renal function in CKD patients as has been described for VEGF inhibitors such as bevacizumab [25, 26]. This is a particular concern for Japanese patients as biopsy results from living kidney donors indicate that Japanese donors have around 25% fewer total nephrons than American donors [27]. Although there are reports investigating the effects of molecular-targeted agents, including sorafenib, on changes in renal function [28], there are no large-scale studies covering multiple indications. This study examined the effectiveness and safety in CKD patients by integrating PMS data for sorafenib in RCC, HCC and DTC which were analyzed using propensity score matching. In contrast to a previous real-world study which showed that sorafenib had similar safety and efficacy in advanced RCC stratified using an eGFR cut-off of 45 mL/min/1.73 m2 [21], we used an eGFR cut-off of 60 mL/min/1.73 m2 in the present study. Generally, propensity score matching removed imbalances in baseline parameters between lower eGFR (< 60 mL/min/1.73 m2) and higher eGFR (≥ 60 mL/min/1.73 m2) groups. Renal-associated variables (baseline eGFR, renal comorbidity, and baseline creatinine) remained very highly statistically significant between groups (p < 0.0001), as did prior surgery which was mainly attributable to nephrectomy in RCC patients. In propensity-matched RCC patients, nephrectomy was performed in 538 of 583 (92.3%) cases with lower eGFR values and 528 of 583 (90.6%) with higher eGFR values. Differences in baseline albumin (p = 0.0145) and distribution of indications (p = 0.0150) after propensity score matching were also recorded, but with much lower levels of statistical significance.
The number of patients with CKD both in Japan and globally, is increasing within an aging population due to lifestyle-related diseases such as diabetes and hypertension [15, 29]. The results of this study showed that in the combined population, sorafenib was equally effective in patients with lower and higher eGFR values, with no significant differences in PFS found between the two groups. In RCC, the 1-year PFS for each group was 34.5% (95% CI 30.2–38.9) and 29.5% (95% CI 25.4–33.7), respectively, and there was no statistically significant difference between the survival curves of the two groups according to the log-rank test (p = 0.2198). The HR of the eGFR > 60 mL/min/1.73 m2 group compared to the eGFR < 60 mL/min/1.73 m2 group was 1.096 (95% CI 0.946–1.270), which was also not statistically significant. Renal function was maintained throughout the 12 month study period in both lower and higher eGFR groups in the combined population and also in each indication. As mean eGFR was lower in RCC than in HCC and DTC patients, the rate of change of eGFR from baseline was used for assessing the effect of sorafenib on renal function. No new safety concerns were identified.
Current European (ESMO) and NCCN guidelines for first-line advanced clear cell RCC recommend combination axitinib plus pembrolizumab in all prognostic groups and ipilimumab plus nivolumab in patients with poor/intermediate prognosis [30, 31]. In addition, ESMO guidelines also recommend first-line cabozantinib plus nivolumab in all risk groups [30] and additional first-line options recommended by NCCN guidelines are single-agent pazopanib and sunitinib in patients with a favorable prognosis and cabozantinib monotherapy in the poor/intermediate-risk group [31]. Although sorafenib has been shown to be effective and safe in PMS [22], it is primarily used in patients who have difficulty using sunitinib or pazopanib as first-line treatment. A retrospective analysis of the effectiveness and safety of sorafenib in RCC, compared patients with eGFR of < 45 and ≥ 45 mL/min/1.73 m2, and used propensity score matching to match patients’ background (n = 613 per group) [21]. PFS, tumor response rates, mean daily dose, median treatment duration, the incidence of SAEs, and dose modification rates were similar between groups [21]. These robust data support the use of sorafenib in RCC patients with impaired renal function.
Sunitinib and pazopanib, like sorafenib, inhibit VEGF and may affect renal function. A retrospective, registry-based study compared the effectiveness and safety of sunitinib in RCC patients with severe (< 30 mL/min/1.73 m2), moderate (30–60 mL/min/1.73 m2) and mild renal insufficiency or normal renal function (> 60 mL/min/1.73 m2), although the study was limited by a low number of cases (n = 22) with severe renal insufficiency compared with moderate (n = 234), and mild renal insufficiency/normal renal function (n = 534). No significant differences in PFS, OS or disease control rates were found, but patients with renal insufficiency were more likely to discontinue treatment due to AEs and had a significantly shorter duration of therapy [32]. A retrospective study of pazopanib in RCC patients (n = 229) found no significant difference in PFS, OS and incidence of AEs between patients with GFR ≤ 60 mL/min/1.73 m2 or > 60 mL/min/1.73 m2, but dose reductions were significantly more frequent in the lower GFR group [33].
In advanced unresectable HCC, sorafenib [5] and, more recently, lenvatinib [13], were approved for first-line treatment. A recent phase 3 trial showed that atezolizumab and bevacizumab combination therapy significantly prolonged OS and PFS compared with sorafenib [34]. This combination therapy was approved for first-line therapy. Other systemic treatment options are ramucirumab [35] and regorafenib which showed survival benefit in HCC patients progressing on sorafenib treatment [36]. The present study demonstrated the safety and effectiveness of sorafenib in HCC patients with CKD.
Sorafenib [7] and lenvatinib [12] are approved for first-line therapy of unresectable DTC. Although there are no studies comparing sorafenib with lenvatinib directly, NCCN guidelines recommend the administration of lenvatinib or sorafenib to patients with progressive or symptomatic DTC refractory to radioactive iodine therapy. Lenvatinib is described as the preferred agent due to its higher efficacy [37], but the decision of whether to use lenvatinib or sorafenib should be individualized for each patient based on the likelihood of response and comorbidities. Both of these multiple kinase inhibitors have different side effect profiles. Treatment-related AEs occurring in 50% or more patients with lenvatinib were hypertension, diarrhea, fatigue or asthenia, and decreased appetite [12], while high-frequency AEs with sorafenib were skin toxicity (HFSR, alopecia, rash or desquamation) and diarrhea [7]. The results presented here may help with the selection of treatment options in DTC cases with renal dysfunction.
There are three main limitations of this study. First, the study was retrospective and used propensity score matching. Propensity score matching aligns patients’ background to reduce bias in observational studies, but confounding factors may still remain after matching. In addition, data from some patients may be excluded during matching which potentially is another source of bias. In this integrated analysis, propensity score matching was applied to three different indications, but matching factors common to each indication was not always possible and this may have introduced a degree of bias into the results. Second, potential bias may have resulted from prescribing physicians being unfamiliar with sorafenib, as data were collected immediately after approval of sorafenib for each indication. This may have led to suboptimal side effect management and treatment. Third, the observation period in this study was up to 1 year after administration for each indication and further studies are needed on the efficacy and eGFR transition for longer sorafenib treatment periods.
Conclusion
Integrated analysis of RCC, HCC and DTC showed that the effectiveness and safety of sorafenib were similar in patients with eGFR < 60 and ≥ 60 mL/min/1.73 m2, during the 12-month observation period, and without impairing renal function.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplementary Figure 1A. Progression-free survival in eGFR <60 and ≥60 mL/min/1.73 m2 group in renal cell carcinoma patients (n = 1,166). Figure 1B. Progression-free survival in eGFR <60 and ≥60 mL/min/1.73 m2 group in hepatocellular carcinoma patients (n = 728). Figure 1C. Progression-free survival in eGFR <60 and ≥60 mL/min/1.73 m2 group in differentiated thyroid carcinoma patients (n = 196). Figure 2A. Time course of change in renal function in eGFR <60 and ≥60 mL/min/1.73 m2 groups in renal cell carcinoma patients. Figure 2B. Time course of change in renal function in eGFR <60 and ≥60 mL/min/1.73 m2 groups in hepatocellular carcinoma patients. Figure 2C. Time course of change in renal function in eGFR <60 and ≥60 mL/min/1.73 m2 groups in differentiated thyroid carcinoma patients (DOCX 285 KB)
Supplementary file2 Supplementary Table 1. Demographics and clinical characteristics of the renal cell carcinoma (RCC) cohort (DOCX 24 KB)
Supplementary file3 Supplementary Table 2. Demographics and clinical characteristics of the hepatocellular carcinoma (HCC) cohort (DOCX 24 KB)
Supplementary file4 Supplementary Table 3. Demographics and clinical characteristics of the differentiated thyroid carcinoma (DTC) cohort (DOCX 23 KB)
Acknowledgements
Under the direction of the authors, medical writing assistance was provided by Robert A. Furlong PhD and David P. Figgitt PhD, ISMPP CMPP™, Content Ed Net, with funding from Bayer Yakuhin, Ltd.
Author contributions
MO provided medical advice on RCC and renal function; SK provided medical advice on HCC and TI provided medical advice on DTC. TT provided an outline for the draft paper. TS analyzed the data and YO implemented each survey.
Funding
The study was funded by Bayer Yakuhin, Ltd.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Dr. Oya has received honoraria from Pfizer, Novartis, Bayer, Ono, BMS, Takeda, and MSD. Dr. Kaneko has received honoraria from Eisai, Bayer, Lilly, Takeda, MSD, and Ono. Dr. Imai declared no competing interests. Toshiaki Tsujino, Toshiyuki Sunaya and Yutaka Okayama are employees of Bayer.
Ethical approval and consent to participate
The authors deeply appreciate the cooperation/contribution of all people who were involved in this survey, including a large number of patients (and their families) with RCC (3335), HCC (1619) or DTC (427). The authors also thank members of the advisory board for the proper use of Nexavar for reviewing our propensity score-matched data. This study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: 10.1007/s00280-023-04587-8
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/16/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s00280-023-04587-8
References
- 1.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 3.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, Sridhara R, Garvey P, Justice R, Pazdur R. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 4.Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008;134:379. doi: 10.1053/j.gastro.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ, DECISION investigators Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitoia F, Jerkovich F. Selective use of sorafenib in the treatment of thyroid cancer. Drug Des Devel Ther. 2016;10:1119–1131. doi: 10.2147/DDDT.S82972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, Santoro M. BAY 43–9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 10.Launay-Vacher V, Deray G. Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs. 2009;20:81–82. doi: 10.1097/CAD.0b013e3283161012. [DOI] [PubMed] [Google Scholar]
- 11.Launay-Vacher V, Aapro M, De Castro JG, Cohen E, Deray G, Dooley M, Humphreys B, Lichtman S, Rey J, Scotté F, Wildiers H, Sprangers B. Renal effects of molecular targeted therapies in oncology: a review by the Cancer and the Kidney International Network (C-KIN) Ann Oncol. 2015;26:1677–1684. doi: 10.1093/annonc/mdv136. [DOI] [PubMed] [Google Scholar]
- 12.Schlumberger M, Tahara M, Wirth LJ. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:1868. doi: 10.1056/NEJMc1503150. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 14.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 15.Japan Nephrology Society Special issue: clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai Shi. 2012;54:1034–1191. [PubMed] [Google Scholar]
- 16.Japanese Society of Nephrology Essential points from Evidence-based Clinical Practice Guidelines for Chronic Kidney Disease 2018. Clin Exp Nephrol. 2019;23:1–15. doi: 10.1007/s10157-018-1648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 19.Bouchi R, Babazono T, Yoshida N, Nyumura I, Toya K, Hayashi T, Hanai K, Tanaka N, Ishii A, Iwamoto Y. Association of albuminuria and reduced estimated glomerular filtration rate with incident stroke and coronary artery disease in patients with type 2 diabetes. Hypertens Res. 2010;33:1298–1304. doi: 10.1038/hr.2010.170. [DOI] [PubMed] [Google Scholar]
- 20.Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Ide T, Takeshita A, Tsutsui H, JCARE-CARD Investigators Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) Circ J. 2009;73:1442–1447. doi: 10.1253/circj.cj-09-0062. [DOI] [PubMed] [Google Scholar]
- 21.Tatsugami K, Oya M, Kabu K, Akaza H. Efficacy and safety of sorafenib for advanced renal cell carcinoma: real-world data of patients with renal impairment. Oncotarget. 2018;9:19406–19414. doi: 10.18632/oncotarget.24779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko S, Ikeda I, Matsuzaki Y, Furuse J, Minami H, Okayama Y, Sunaya T, Ito Y, Inuyama L, Okita K. Safety and effectiveness of sorafenib in Japanese patients with hepatocellular carcinoma in daily medical practice: interim analysis of a prospective postmarketing all-patient surveillance study. J Gastroenterol. 2016;51:1011–1121. doi: 10.1007/s00535-016-1173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito Y, Suzuki S, Ito K, Imai T, Okamoto T, Kitano H, Sugitani I, Sugino K, Tsutsui H, Hara H, Yoshida A, Shimizu K. Tyrosine-kinase inhibitors to treat radioiodine-refracted, metastatic, or recurred and progressive differentiated thyroid carcinoma. Endocr J. 2016;63:597–602. doi: 10.1507/endocrj.EJ16-0064. [DOI] [PubMed] [Google Scholar]
- 24.Akaza H, Oya M, Iijima M, Hyodo I, Gemma A, Itoh H, Adachi M, Okayama Y, Sunaya T, Inuyama L. A large-scale prospective registration study of the safety and efficacy of sorafenib tosylate in unresectable or metastatic renal cell carcinoma in Japan: results of over 3200 consecutive cases in post marketing all-patient surveillance. Jpn J Clin Oncol. 2015;45:953–962. doi: 10.1093/jjco/hyv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.den Deurwaarder ES, Desar IM, Steenbergen EJ, Mulders PF, Wetzels JF, van Herpen CM. Kidney injury during VEGF inhibitor therapy. Neth J Med. 2012;70:267–271. [PubMed] [Google Scholar]
- 26.Izzedine H, Escudier B, Lhomme C, Pautier P, Rouvier P, Gueutin V, Baumelou A, Derosa L, Bahleda R, Hollebecque A, Sahali D, Soria JC. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine (Baltimore) 2014;93:333–339. doi: 10.1097/MD.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki T, Tsuboi N, Kanzaki G, Haruhara K, Okabayashi Y, Koike K, Kobayashi A, Yamamoto I, Ogura M, Hoy WE, Bertram JF, Shimizu A, Yokoo T. Biopsy-based estimation of total nephron number in Japanese living kidney donors. Clin Exp Nephrol. 2019;23:629–637. doi: 10.1007/s10157-018-01686-2. [DOI] [PubMed] [Google Scholar]
- 28.Miyake H, Muramaki M, Imai S, Harada K, Fujisawa M. Changes in renal function of patients with metastatic renal cell carcinoma during treatment with molecular-targeted agents. Target Oncol. 2016;11:329–335. doi: 10.1007/s11523-015-0395-4. [DOI] [PubMed] [Google Scholar]
- 29.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powles T, Guidelines Committee ESMO. Recent eUpdate to the ESMO Clinical Practice Guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer: renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:422–423. doi: 10.1016/j.annonc.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Jonasch E, Boyle S, Carlo MI, Manley B, Agarwal N, Alva A, Beckermann K, Choueiri TK, Costello BA, Derweesh IH, Desai A, George S, Gore JL, Haas N, Hancock SL, Kyriakopoulos C, Lam ET, Lau C, Lewis B, Madoff DC, McCreery B, Michaelson MD, Mortazavi A, Nandagopal L, Pierorazio PM, Plimack ER, Ponsky L, Ramalingam S, Shuch B, Smith ZL, Somer B, Sosman J, Dwyer MA, Motter AD. NCCN Guidelines Insights: Kidney Cancer, Version 1.2021. J Natl Compr Canc Netw. 2020;18:1160–1170. doi: 10.6004/jnccn.2020.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poprach A, Bortlicek Z, Melichar B, Lakomy R, Svoboda M, Kiss I, Zemanova M, Fiala O, Kubackova K, Coufal O, Pavlik T, Dusek L, Vyzula R, Buchler T. Efficacy of sunitinib in patients with metastatic or unresectable renal cell carcinoma and renal insufficiency. Eur J Cancer. 2015;51:507–513. doi: 10.1016/j.ejca.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Masini C, Vitale MG, Maruzzo M, Procopio G, de Giorgi U, Buti S, Rossetti S, Iacovelli R, Atzori F, Cosmai L, Vignani F, Prati G, Scagliarini S, Guida A, Berselli A, Pinto C. Safety and efficacy of pazopanib in first-line metastatic renal-cell carcinoma with or without renal failure: CORE-URO-01 study. Clin Genitourin Cancer. 2019;17:e150–e155. doi: 10.1016/j.clgc.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, IMbrave150 Investigators Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 35.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M, REACH-2 Study Investigators Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 36.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G, RESORCE Investigators Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 37.NCCN Clinical Practice Guidelines in Oncology. Thyroid Carcinoma version 1.2021 (2021) https://www.nccn.org/professionals/physician_gls/default.aspx
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplementary Figure 1A. Progression-free survival in eGFR <60 and ≥60 mL/min/1.73 m2 group in renal cell carcinoma patients (n = 1,166). Figure 1B. Progression-free survival in eGFR <60 and ≥60 mL/min/1.73 m2 group in hepatocellular carcinoma patients (n = 728). Figure 1C. Progression-free survival in eGFR <60 and ≥60 mL/min/1.73 m2 group in differentiated thyroid carcinoma patients (n = 196). Figure 2A. Time course of change in renal function in eGFR <60 and ≥60 mL/min/1.73 m2 groups in renal cell carcinoma patients. Figure 2B. Time course of change in renal function in eGFR <60 and ≥60 mL/min/1.73 m2 groups in hepatocellular carcinoma patients. Figure 2C. Time course of change in renal function in eGFR <60 and ≥60 mL/min/1.73 m2 groups in differentiated thyroid carcinoma patients (DOCX 285 KB)
Supplementary file2 Supplementary Table 1. Demographics and clinical characteristics of the renal cell carcinoma (RCC) cohort (DOCX 24 KB)
Supplementary file3 Supplementary Table 2. Demographics and clinical characteristics of the hepatocellular carcinoma (HCC) cohort (DOCX 24 KB)
Supplementary file4 Supplementary Table 3. Demographics and clinical characteristics of the differentiated thyroid carcinoma (DTC) cohort (DOCX 23 KB)
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.