Abstract
Background
A meta-analysis of randomized controlled trials (RCTs) was conducted to systematically evaluate the effects of berberine on the inflammatory markers of metabolic syndrome (MetS) and related disorders.
Method
Databases that were searched from inception to October 2020 included PubMed, Web of Science, the Cochrane Library, CNKI, VIP, WanFang Data, and ClinicalTrials.gov. Two reviewers independently selected articles and extracted data. The pooled evaluations were entered and analyzed in Review Manager 5.3.
Results
Of the 7387 publications screened, 52 studies were included, and the related trials involved 4616 patients. Pooled estimates showed that the use of berberine could significantly reduce the concentration level of C-reactive protein (CRP) [standardized mean difference (SMD) = − 1.54, 95% confidence intervals (CI) − 1.86, − 1.22, p < 0.05], tumor necrosis factor-α (TNF-α) [SMD = − 1.02, 95% CI − 1.27, − 0.77, p < 0.05], and interleukin 6 (IL-6) [SMD = − 1.17, 95% CI − 1.53, − 0.81, p < 0.05] among patients with MetS and related disorders. However, it did not affect the level of interleukin 1β (IL-1β) [SMD = − 0.81, 95% CI − 1.80, 0.17, p = 0.11].
Conclusion
Overall, the use of berberine in patients with MetS and related disorders appeared to significantly decrease several inflammatory markers. Further multi-center and rigorous investigations with larger patient populations are encouraged to confirm the effect of berberine on MetS and related disorders.
Keywords: Berberine, Metabolic syndrome, Inflammatory markers, Meta-analysis
Introduction
Metabolic syndrome (MetS) is a cluster of interconnected physiological and metabolic abnormalities characterized by obesity, insulin resistance, hypertension, and hyperlipidemia (Lee and Herceg 2017). The prevalence of MetS in adults worldwide is reportedly about 20–25% (Ranasinghe et al. 2017). MetS patients have increased risks of cardiovascular disease, diabetes, and some other chronic diseases (Grundy et al. 2006; Arnlöv et al. 2010; Noda et al. 2009). Previous reports have suggested that the development of MetS is associated with increased levels of inflammatory markers, including C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), interleukin 1 (IL-1), etc. (Festa et al. 2000; Wisse 2004; Akbari et al. 2018; Tabrizi et al. 2018, 2019). Pharmacological strategies to reduce inflammation have become more widespread and more useful in treating MetS and related disorders (Esser et al. 2015).
Berberine is an isoquinoline quaternary alkaloid that can be found in plant extracts produced from Berberis vulgaris and some traditional Chinese medicinal herbs, and it has been found to perform well in managing blood sugar, blood lipids, blood pressure, and without causing serious adverse events (Lan et al. 2015; Liang et al. 2019; Ju et al. 2018). Given that berberine costs less than many other drugs, it could have great potential for use in the management and control of MetS and related disorders. As for the effects of berberine on the concentration level of inflammatory markers, the results of randomized controlled trials (RCTs) have been inconsistent. A systematic review conducted by Beba et al. suggested that berberine could reduce the concentration level of CRP, but only five studies were included in the analysis, and the experimental and control groups of included studies were based on different populations (Beba et al. 2019; Chen et al. 2016; Hu et al. 2012). A more thorough evaluation of the effects of berberine on inflammatory markers in patients with MetS and related disorders needs to be further analyzed with multiple outcomes and evidence from more RCTs. To our knowledge, there are no RCTs relative to this study field in other nations, but many in China. Besides, these RCTs have not been included in systematic reviews or meta-analyses for qualitative or quantitative research.
The present study summarizes a meta-analysis that systematically reviewed and quantified the effects of berberine use on inflammatory markers in Chinese patients with MetS and related disorders to provide special evidence for supporting pharmacists’ and physicians’ clinical actions and decisions in China’s MetS and related disorders management.
Materials and methods
Search strategy and study selection
The meta-analysis was conducted based on the recommendations of the Cochrane Collaboration (Higgins et al. 2020), and has been reported according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Page et al. 2021). The databases of PubMed, Web of Science, the Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP Chinese periodical service platform, WanFang Data, and ClinicalTrials.gov (http://www.clinicaltrial.gov) were searched from the date of their inception to October 2020. Medical Subject Headings and text search words included patients [“metabolic syndrome” or “acute coronary syndromes” or “coronary artery disease” or “CVD” or “diabetic” or “T1DM” or “T2DM” or “overweight” or “obese” or “chronic kidney disease” or “end-stage renal disease” or “dialysis” or “heart failure” or “myocardial infarction” or “atherosclerotic” or “hypercholesterolemic” or “hypertension” or “high blood pressure” or “dyslipidemia” or “hyperlipidemia” or “polycystic ovary syndrome” or “stable angina” or “unstable angina” or “diabetic nephropathy” or “obesity” or “stable atherosclerotic plaques” or “atherosclerotic”] (Akbari et al. 2018, 2019; Tabrizi et al. 2018, 2019; Hamedifard et al. 2019), intervention [“berberine”], and outcomes [“CRP” or “IL-6” or “TNF-α” or “IL-1” or “inflammatory”]. References cited by the included studies were traced to uncover relevant additional studies.
Inclusion and exclusion criteria
All clinical trials that met the following criteria which were defined according to the PICO strategy recommended by Cochrane were included: (1) the study population consisted of Chinese patients diagnosed with MetS and related disorders. The MetS-related disorders included acute coronary syndrome, coronary artery disease, cerebrovascular disease, diabetes, obesity, chronic kidney disease, heart failure, myocardial infarction, atherosclerosis, hypercholesterolemia, hypertension, dyslipidemia, hyperlipidemia, polycystic ovary syndrome, angina pectoris, diabetic nephropathy, and stable atherosclerotic plaques; (2) the experimental group was treated with berberine or berberine combined with other treatments, and placebo or non-berberine treatments were used as the control group; (3) RCTs comparing outcomes in CRP, TNF-α, IL-6, and IL-1.
Studies with the following criteria were excluded from this meta-analysis: (1) duplicate and non-full-text publications; (2) reviews, non-human studies, and retrospective and observational studies; and (3) published in languages other than Chinese or English.
Data extraction and risk-of-bias assessment
Studies were independently selected by two authors (XWZ and JFH), and they achieved good agreement (κ = 0.879). Conflicts between the two authors were resolved by the opinion of a third author (YQL). Eligibility screening was performed in two steps: (1) title and abstract screening for relevance to the study objective, and (2) full-text screening for eligibility for meta-analysis. For each eligible study, the following information was extracted (1) basic information (e.g., first author, year of publication, sample size); (2) baseline characteristics of intervention and study population; and (3) relevant outcomes, including CRP, TNF-α, IL-6, and IL-1. Two authors (XWZ and JFH) independently extracted data from each selected RCTs using a standard abstraction excel sheet (κ = 0.962).
The methodological quality of each RCT was evaluated by two independent investigators (XWZ and JFH) using the Cochrane Risk of Bias assessment tool (κ = 0.973). The assessment domains of the Cochrane Risk of Bias assessment tool include selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases (Higgins et al. 2020).
Statistical analysis
The meta-analysis was undertaken in Review Manager version 5.3 (Cochrane Collaboration, Oxford, UK). Standardized mean differences (SMDs) and 95% confidence intervals (CIs) were used to assess continuous outcomes. p values ≤ 0.05 were considered to be statistically significant. Heterogeneity among the included studies was assessed using the I2 estimate and the p value of the Chi-square test. I2 values < 50% and p value > 0.10 were determined to indicate no significant heterogeneity, and the fixed-effect (FE) model was used for meta-analysis. When significant heterogeneity was determined, its source was further evaluated by sensitivity analyses or subgroup analyses. Sensitivity analyses were conducted to assess the effect of each trial on the validity of the pooled overall SMDs using the leave-one-out method. Subgroup analyses were conducted according to the following variables: dosage of berberine (< 0.9 g daily vs. ≥ 0.9 g daily), type of condition (metabolic syndrome vs. type 2 diabetes vs. diabetic nephropathy vs. cardiovascular disease vs. polycystic ovary syndrome vs. other), duration of study (< 3 months vs. ≥ 3 months vs. unclear), and sample size (< 30 vs. 30–60 vs. > 60). In the absence of clinical and methodological heterogeneity, the random-effects (RE) model was used to analyze the outcomes. The results of the meta-analysis were shown in forest plots. Publication bias was detected by funnel plot symmetry tests and Egger’s regression tests. Egger’s regression test was undertaken in Stata /MP version 16.0 (Stata Corp., College Station, TX, USA).
Results
Search results
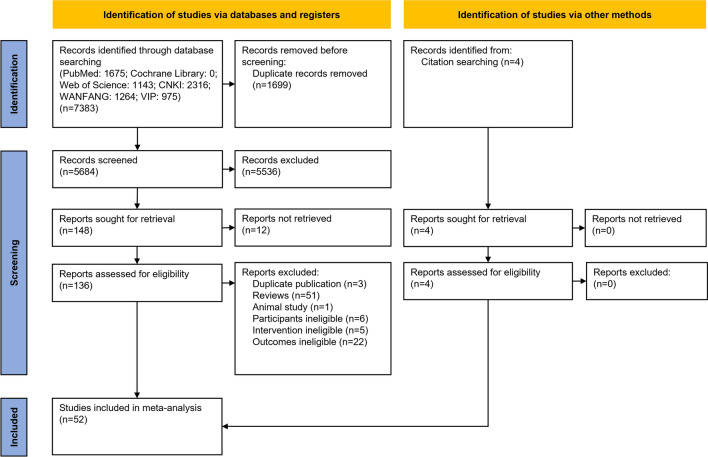
A total of 7387 articles were retrieved from the initial search. After screening titles and abstracts, 152 studies were potentially eligible, and these were retrieved for full-text review. After reading the full text, 100 were excluded, because they failed to meet the inclusion criteria. Ultimately, 52 studies that fully satisfied the pre-established inclusion criteria of this meta-analysis were included. The search procedure and reasons for exclusion can be found in the flowchart presented in Fig. 1.
Fig. 1.
Flowchart of the search, inclusion, and exclusion study selection
Study characteristics
The 52 included studies were published between 2008 and 2020 (Liu and Hu 2008; Xu et al. 2008; Zhang et al. 2008, 2010, 2014; Liu et al. 2010; Sheng and Xie 2010; Zhu 2010; Meng et al. 2011; Xiang et al. 2011; Zhou and Huang 2011, 2012; Deng et al. 2012; Dou et al. 2012; Liu and Wang 2012; Yu et al. 2012; Shu 2014; Dai et al. 2015; Chen et al. 2015, 2017; Zhan et al. 2015; Zhu et al. 2015; Li et al. 2016; Sun 2016, 2017; Wang 2016; Zhou et al. 2016; Dong et al. 2017; Li 2017a, b, c Yuan et al. 2017; Bai et al. 2018; Du and Zhang 2018; Fan et al. 2018; He et al. 2018; He 2018; Huang et al. 2018; Li and Deng 2018; Lie et al. 2018a, b Lu et al. 2018; Ning 2018; Wang et al. 2018; Yang et al. 2018, 2020; Cao and Su 2019; Lai et al. 2019; Lan et al. 2019; Xie and Huang 2019; Yang and Yin 2019; Ye and You 2019). The collective patient population comprised 2304 individuals in the experimental group and 2312 individuals in the control group. There were 41 studies that reported the level of CRP, 26 that reported the level of TNF-α, 25 that reported the level of IL-6, and three studies that reported the level of IL-1β. The main characteristics of these studies are presented in Table 1
Table 1.
Characteristics of included studies
| Study | Population | Sample size (C/E) | Age (years) | Intervention | Duration | Presented data | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | E | C | E | ||||||||||
| Liu and Hu (2008) | Type 2 diabetes | 30/30 | 53.07 ± 8.51 | 52.00 ± 9.81 | Metformin 1.5 g/d | Metformin + berberine 0.9–1.5 g/d | 8 weeks | CRP | |||||
| Xu et al. (2008) | Diabetic nephropathy | 40/40 | 51 ± 3.5a | 51 ± 3.5a | Pioglitazone 30 mg/d | Pioglitazone + berberine 0.9 g/d | 12 weeks | CRP | |||||
| Zhang et al. (2008) | Type 2 diabetes and dyslipidemia | 58/52 | N/A | 51 ± 10 | Placebo | Berberine 1.0 g/d | 3 months | CRP, IL-6 | |||||
| Liu et al. (2010) | Type 2 diabetes | 20/20 | 59.40 ± 15.40 | 62.80 ± 12.20 | Basic treatmentsb | Basic treatmentsb + berberine 0.9 g/d | 3 months | CRP, TNF-α, IL-6 | |||||
| 20/20 | 64.45 ± 14.40 | Basic treatmentsb + Rosiglitazone 4 mg/d | |||||||||||
| Sheng and Xie (2010) | Type 2 diabetes | 30/30 | 51 ± 8 | 52 ± 11 | Glipizide 10 mg/d + metformin 1.5 g/d | Glipizide + metformin + berberine 1.5 g/d | 3 months | CRP, TNF-α, IL-1β, IL-6 | |||||
| Zhang et al. (2010) | Acute coronary syndromes | 20/20 | 61.42 ± 8.60a | 61.42 ± 8.60a | Basic treatmentsb | Basic treatmentsb + berberine 0.9 g/d | 30 days | CRP | |||||
| Zhu (2010) | Diabetic nephropathy | 48/44 | 66.69 ± 8.32 | 65.71 ± 8.41 | Irbesartan 0.15 g/d | Irbesartan + berberine 1.2 g/d | 12 weeks | CRP, TNF-α | |||||
| Meng et al. (2011) | Type 2 diabetes | 30/30 | 53 ± 13.9 | 51 ± 13.3 | Insulin | Insulin + berberine 0.9 g/d | 12 weeks | TNF-α, IL-6 | |||||
| Xiang et al. (2011) | Type 2 diabetes | 20/20 | N/A | N/A | Placebo | Berberine 1.2 g/d | 12 weeks | CRP, TNF-α, IL-6 | |||||
| 20/20 | N/A | Aspirin 0.1 g/d | |||||||||||
| Zhou and Huang (2011) | Hyperlipidemia | 60/60 | N/A | N/A | No treatment | Berberine 0.9 g/d | 4 months | CRP | |||||
| Deng et al. (2012) | Polycystic ovary syndrome and insulin resistance | 28/31 | 26.75 ± 2.62 | 25.74 ± 2.66 | Ethinylestradiol cyproterone 2 mg: 0.035 mg/d + placebo | Ethinylestradiol cyproterone + berberine 0.9 g/d | 3 menstrual cycles | CRP, TNF-α | |||||
| Dou et al. (2012) | Obesity | 60/58 | 47.68 ± 8.40 | 48.42 ± 8.60 | Vitamin C 0.9 g/d | Berberine 0.9 g/d | 4 weeks | CRP | |||||
| Liu and Wang (2012) | Ischemic heart disease and heart failure | 44/50 | 69.6 ± 8.2 | 67.5 ± 10.3 | Basic treatmentsb | Basic treatmentsb + berberine 0.9 g/d | 8 weeks | TNF-α | |||||
| Yu et al. (2012) | Type 2 diabetes | 24/24 | 45.6 ± 5.4a | 45.6 ± 5.4a | Glibenclamide 5 mg/d | Exenatide 5 μg/d + berberine 0.9 g/d | 12 weeks | CRP | |||||
| Zhou and Huang (2012) | Obesity and type 2 diabetes | 46/46 | 46.67 ± 8.52a | 46.67 ± 8.52a | Metformin 1.5 g/d | Metformin + berberine 0.6 g/d | 12 weeks | CRP | |||||
| Shu (2014) | Type 2 diabetes | 32/32 | 61.21 ± 13.52 | 62.80 ± 12.20 | Insulin | Insulin + berberine 0.9 g/d | 24 weeks | CRP | |||||
| Zhang et al. (2014) | Cerebral infarction | 30/30 | 54.1 ± 4.6 | 55.6 ± 5.2 | Basic treatmentsb | Basic treatmentsb + atorvastatin + berberine 0.4 g/d | 4 weeks | CRP | |||||
| 30/30 | 54.3 ± 4.9 | Basic treatmentsb + atorvastatin 40 mg/d | |||||||||||
| Dai et al. (2015) | Hypertension and type 2 diabetes | 33/39 | 53.06 ± 10.36 | 55.31 ± 11.79 | Basic treatmentsb | Basic treatmentsb + berberine 0.3 g/d | 2 years | CRP | |||||
| Chen et al. (2015) | Coronary artery disease and hypercholesteremia | 40/40 | 51.5 ± 10.4 | 52.1 ± 9.8 | Simvastatin 20 mg/d | Simvastatin 10 mg/d + berberine 0.5 g/d | 1 month | CRP | |||||
| Zhan et al. (2015) | Type 2 diabetes with hyperlipidemia | 40/40 | 51.6 ± 3.8a | 51.6 ± 3.8a | Basic treatmentsb + metformin 1.5 g/d | Basic treatmentsb + metformin + berberine 0.6 g/d | 3 months | CRP | |||||
| Zhu et al. (2015) | Acute ischemic stroke | 28/16 | 66.25 ± 8.83 | 63.31 ± 8.10 | Atorvastatin 20 mg/d + aspirin 0.1 g/d | Atorvastatin 20 mg/d + aspirin + berberine 0.4 g/d | 3 months | CRP | |||||
| 11/16 | 66.45 ± 8.86 | Atorvastatin 40 mg/d + aspirin 0.1 g/d | |||||||||||
| Li et al. (2016) | Insulin resistance with schizophrenia | 33/31 | 40.18 ± 12.21 | 40.14 ± 9.40 | Risperidone 3.85 ± 0.94 mg/d + placebo | Risperidone 3.77 ± 0.85 mg/d + berberine 0.9 g/d | 12 weeks | TNF-α, IL-1β, IL-6 | |||||
| Sun (2016) | Obesity and type 2 diabetes | 48/48 | 52.37 ± 4.48 | 52.32 ± 4.45 | Sitagliptin 0.1 g/d | Sitagliptin + berberine 0.9 g/d | 12 weeks | CRP, IL-6 | |||||
| Wang (2016) | Type 2 diabetes | 25/25 | N/A | N/A | Basic treatmentsb | Basic treatmentsb + berberine 0.3 g/d | 3 months | CRP, IL-6 | |||||
| Zhou et al. (2016) | Obesity and type 2 diabetes | 30/30 | 55.6 ± 12.7 | 56.4 ± 10.9 | Basic treatmentsb | Basic treatmentsb + berberine 0.6 g/d | 3 months | CRP, TNF-α, IL-6 | |||||
| Chen et al. (2017) | Metabolic syndrome with renal damage | 10/10 | 40.20 ± 5.89 | 38.70 ± 10.3 | Losartan 0.1 g/d | Losartan + berberine 0.9 g/d | 8 weeks | TNF-α | |||||
| Dong et al. (2017) | Type 2 diabetes | 49/49 | 51.34 ± 4.43 | 52.23 ± 4.41 | Metformin 1.5 g/d | Metformin + berberine 0.9 g/d | 12 weeks | CRP, TNF-α, IL-6 | |||||
| Li (2017a) | Metabolic syndrome with schizophrenia | 42/40 | 42.14 ± 11.61 | 41.86 ± 10.22 | Olanzapine + metformin 0.75 g/d | Olanzapine + berberine 0.9 g/d | 12 weeks | TNF-α, IL-1β, IL-6 | |||||
| Li (2017b) | Obesity and type 2 diabetes | 30/30 | 51.24 ± 3.91 | 50.54 ± 3.78 | Sitagliptin 0.1 g/d | Sitagliptin + berberine 0.9 g/d | 3 months | CRP, IL-6 | |||||
| Li (2017c) | Acute cerebral ischemic stroke | 60/60 | 61.94 ± 3.77 | 62.84 ± 4.67 | Basic treatmentsb | Basic treatmentsb + berberine 0.9 g/d | 14 days | CRP, IL-6 | |||||
| Sun (2017) | Type 2 diabetes | 91/91 | 58.34 ± 11.21 | 58.95 ± 10.57 | Metformin 1.5 g/d | Metformin + berberine 0.09 g/d | 8 weeks | CRP, TNF-α, IL-6 | |||||
| Yuan et al. (2017) | Type 2 diabetes | 41/41 | 65.78 ± 8.96 | 66.13 ± 9.06 | Glimepiride 1 mg/d | Glimepiride + Gegen Qinlian Decoction + berberine 0.6 g/d | 2 weeks | CRP, TNF-α | |||||
| Bai et al. (2018) | Hyperlipidemia | 75/75 | 63.38 ± 7.24 | 63.29 ± 7.85 | Ezetimibe 10 mg/d | Ezetimibe + berberine 0.4 g/d | 1 month | CRP | |||||
| Du and Zhang (2018) | Coronary heart disease | 12/18 | 66 ± 10 | 60 ± 6 | Basic treatmentsb | Basic treatmentsb + berberine 0.9 g/d | 3 months | CRP, TNF-α, IL-6 | |||||
| Fan et al. (2018) | Type 2 diabetes | 40/40 | 52.71 ± 7.89 | 53.27 ± 8.15 | Metformin 1.5 g/d | Metformin + berberine 1.5 g/d | 3 months | CRP, TNF-α, IL-6 | |||||
| He et al. (2018) | Diarrhea with hyperlipidemia | 62/62 | 55.16 ± 6.79 | 56.78 ± 6.74 | Basic treatmentsb + levofloxacin 0.5 g/d | Basic treatmentsb + berberine 0.36 g/d | 8 weeks | CRP, TNF-α, IL-6 | |||||
| He (2018) | Diabetic nephropathy | 52/52 | 56.4 ± 7.3 | 56.2 ± 7.5 | Basic treatmentsb valsartan 80 mg/d | Basic treatmentsb + valsartan + berberine 1.2 g/d | 12 weeks | CRP, TNF-α | |||||
| Huang et al. (2018) | Type 2 diabetes | 65/65 | 67.16 ± 8.54 | 66.09 ± 8.67 | Insulin | Insulin + berberine 1.8 g/d | 1 month | TNF-α | |||||
| Li and Deng (2018) | Nonalcoholic fatty liver disease | 53/53 | 74.68 ± 4.32 | 74.07 ± 5.16 | Polyene phosphatidyl choline 1.368 g/d | Polyene phosphatidyl choline + berberine 0.36 g/d | 12 weeks | TNF-α | |||||
| Lie et al. (2018a) | Polycystic ovary syndrome | 38/38 | N/A | N/A | Ethinylestradiol cyproterone 2 mg: 0.035 mg/d + placebo | Ethinylestradiol cyproterone + berberine 0.9 g/d | 21 days | CRP | |||||
| Lie et al. (2018b) | Type 2 diabetes | 57/57 | 57 ± 12 | 53 ± 15 | Basic treatmentsb | Basic treatmentsb + berberine 1.2 g/d | 6 months | CRP | |||||
| Lu et al. (2018) | Acute ischemic cerebral infarction | 60/60 | 60.7 ± 5.2 | 59.9 ± 6.1 | Basic treatmentsb + rosuvastatin 10 mg/d | Basic treatmentsb + rosuvastatin + berberine 0.9 g/d | N/A | CRP | |||||
| Ning (2018) | Acute cerebral infarction | 39/39 | 61.00 ± 1.26 | 60.00 ± 1.47 | Basic treatmentsb + atorvastatin 40 mg/d | Basic treatmentsb + atorvastatin + berberine 0.9 g/d | 15 days | CRP, IL-6 | |||||
| Wang et al. (2018) | Metabolic syndrome with renal damage | 10/10 | 35.62 ± 1.43 | 37.30 ± 1.96 | Basic treatmentsb | Basic treatmentsb + berberine 0.9 g/d | 8 weeks | IL-6 | |||||
| Yang et al. (2018) | Symptomatic atherosclerotic intracranial artery stenosis | 60/60 | 61.98 ± 4.09 | 61.98 ± 4.09 | Simvastatin 40 mg/d + aspirin 0.1 g/d | Simvastatin + aspirin + berberine 1.2 g/d | 6 months | CRP | |||||
| Cao and Su (2019) | Metabolic syndrome and insulin resistance | 40/40 | 65.6 ± 1.8 | 65.5 ± 1.8 | Basic treatmentsb | Basic treatmentsb + berberine 1.2 g/d | 1 month | CRP, TNF-α, IL-6 | |||||
| Lai et al. (2019) | Polycystic ovary syndrome and insulin resistance | 48/48 | 28.48 ± 6.34 | 29.53 ± 5.21 | Metformin 1 g/d | Peikun pills 18 g/d + berberine 0.9 g/d | 3 months | CRP, TNF-α, IL-6 | |||||
| Lan et al. (2019) | Hypertensive atherosclerosis | 40/40 | 63.3 ± 6.2 | 64.2 ± 5.5 | Basic treatmentsb | Basic treatmentsb + berberine 0.9 g/d | 8 weeks | TNF-α, IL-6 | |||||
| 40/40 | 65.1 ± 5.0 | Basic treatments + berberine 1.8 g/d | |||||||||||
| Xie and Huang (2019) | Diabetic nephropathy | 53/53 | 61.3 ± 1.2 | 62.1 ± 1.6 | Basic treatmentsb + tripterygium wilfordii polyglycosides 60 mg/d | Basic treatmentsb + tripterygium wilfordii polyglycosides + berberine 1.5 g/d | 90 days | TNF-α, IL-6 | |||||
| Yang and Yin (2019) | Coronary heart disease | 30/40 | 61.37 ± 8.79 | 60.63 ± 8.53 | Basic treatmentsb + rosuvastatin 10 mg/d | Basic treatmentsb + berberine 0.9 g/d | 4 weeks | CRP, TNF-α | |||||
| Ye and You (2019) | Acute ischemic cerebral infarction | 33/33 | 56.65 ± 7.12 | 57.36 ± 6.79 | Rosuvastatin 10 mg/d | Rosuvastatin + berberine 0.9 g/d | 12 days | CRP, IL-6 | |||||
| Yang et al. (2020) | Type 2 diabetes | 96/96 | 49.7 ± 7.4 | 49.9 ± 7.8 | Metformin 2 g/d | Metformin + berberine 1.5 g/d | 3 months | TNF-α, IL-6 | |||||
N/A The date was not reported, CRP C-reactive protein, TNF-α tumor necrosis factor-alpha, IL-6 interleukin-6, IL-1β interleukin-1 beta, C control group, E experimental group, X g/d X g daily
aOnly demographic characteristics of the total sample population were reported
bDifferent patients used different drugs for basic treatments
Risk-of-bias assessment
Two studies exhibited a high risk of bias in the “random sequence generation” domain (Chen et al. 2017; Yang et al. 2018), since their methods taken to generate random sequences and arrange groups did not accord with the randomization standard. Twenty-four studies exhibited an unclear risk without information about concealment of the allocation sequence. All included studies exhibited an unclear risk in the “allocation concealment” domain because of the lack of detailed description of allocation. Only six studies illustrated the details of blinding (Zhang et al. 2008; Xiang et al. 2011; Deng et al. 2012; Li et al. 2016; Du and Zhang 2018; Li and Deng 2018). Forty-three studies exhibited a low risk of attrition bias without incomplete outcome data. The domain “reporting bias” exhibited an unclear risk of bias, because the measurement of the concentration of inflammatory markers was not mentioned. The domain “other bias” exhibited an unclear risk of bias due to insufficient information. In general, many domains were assessed as “unclear risk”, which indicated that the included studies were likely to be at risk of bias. The risks of bias in each study are summarized in Fig. 2
Fig. 2.
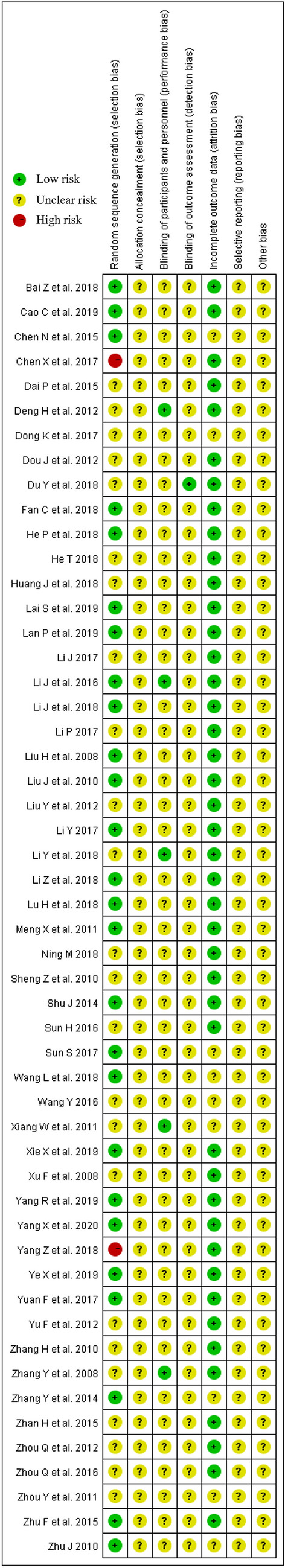
Quality assessment of included studies
Main outcomes
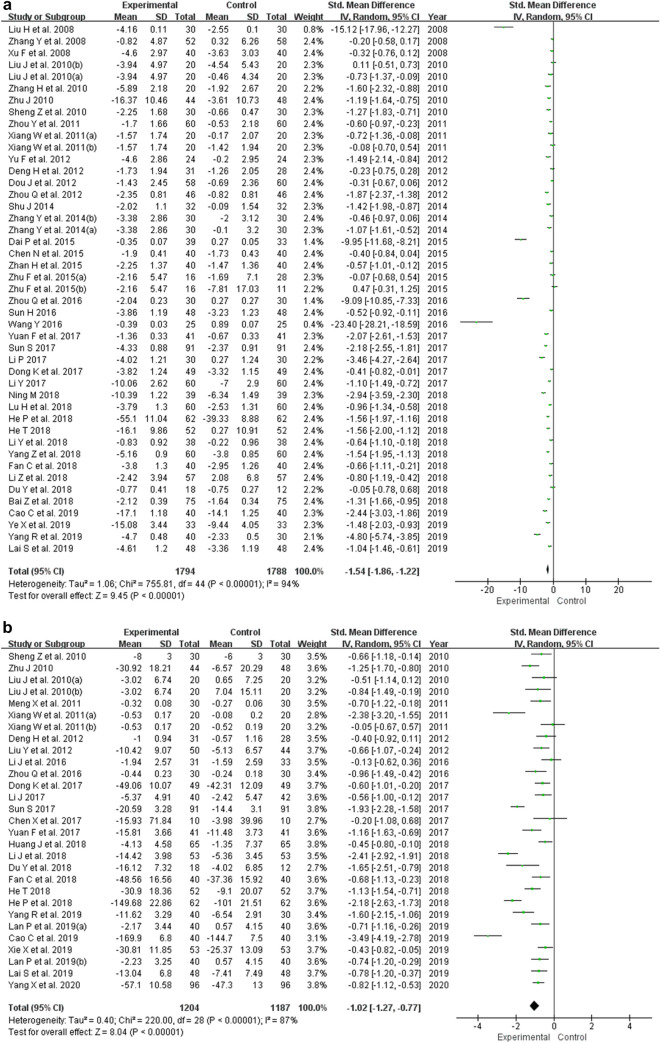
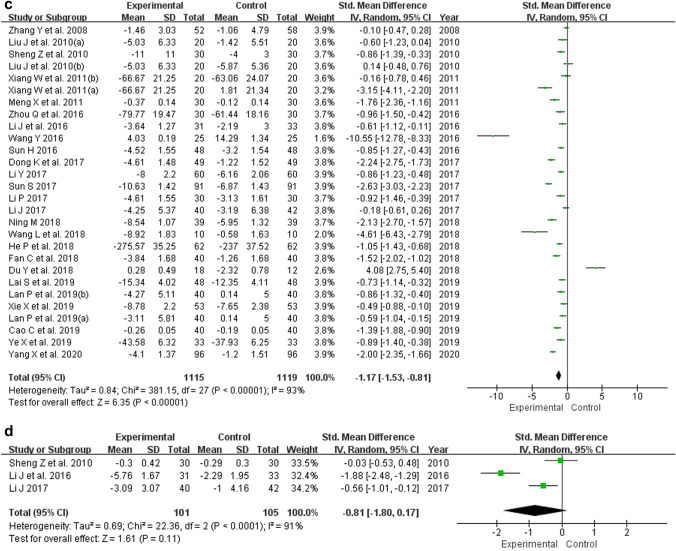
Forest plots that demonstrate the effects of berberine use on the evaluated inflammatory markers are shown in Fig. 3. The pooled findings using random-effects model showed that berberine use in patients with MetS and related disorders significantly decreased the concentration level of CRP (SMD = − 1.54; 95% CI − 1.86, − 1.22; p < 0.05), TNF-α (SMD = − 1.02; 95% CI − 1.27, − 0.77; p < 0.05), and IL-6 (SMD = − 1.17; 95% CI − 1.53, − 0.81; p < 0.05). Moreover, pooled findings from the random-effects model showed that there was no significant impact of berberine on the level of IL-1β (SMD = − 0.81; 95% CI − 1.80, 0.17; p = 0.11).
Fig. 3.
Forest plots of the effect of berberine on a CRP, b TNF-α, c IL-6, and d IL-1β. CRP C-reactive protein, TNF-α tumor necrosis factor-alpha, IL-6 interleukin-6, IL-1β interleukin-1 beta
Heterogeneity
The meta-analysis showed statistically significant heterogeneity for the outcomes of CRP (I2 = 94%; p < 0.10), TNF-α (I2 = 87%; p < 0.10), IL-6 (I2 = 93%; p < 0.10), and IL-1β (I2 = 91%; p < 0.10), as shown in Fig. 3. Following sensitivity analyses, the heterogeneity did not change significantly and only reduced by 1–4%, with the elimination of individual studies. And there was not any statistically significant difference between before and after sensitivity pooled SMDs for CRP, TNF-α, IL-6, and IL-1β concentration levels, as presented in Table 2.
Table 2.
Sensitivity analyses of berberine's influence on inflammation
| Outcomes | Pre-sensitivity analyses | Upper and lower of effect size | Post-sensitivity analyses | ||||
|---|---|---|---|---|---|---|---|
| No. of studies included | Pooled SMD (RE) | 95% CI | Pooled SMD (RE) | 95% CI | Excluded studies | ||
| CRP | 45 | − 1.54 | − 1.86, − 1.22 | Upper | − 1.39 | − 1.69, − 1.09 | Dai et al. (2015) |
| Lower | − 1.58 | − 1.90, − 1.26 | Zhu et al. (2015) | ||||
| TNF-α | 29 | − 1.02 | − 1.27, − 0.77 | Upper | − 0.94 | − 1.17, − 0.72 | Cao and Su (2019) |
| Lower | − 1.05 | − 1.30, − 0.80 | Xiang et al. (2011), Li et al. (2016) | ||||
| IL-6 | 28 | − 1.17 | − 1.53, − 0.81 | Upper | − 1.02 | − 1.35, − 0.69 | Wang (2016) |
| Lower | − 1.29 | − 1.63, − 0.95 | Du and Zhang (2018) | ||||
| IL-1β | 3 | − 0.81 | − 1.80, 0.17 | Upper | − 0.31 | − 0.84, 0.22 | Li et al. (2016) |
| Lower | − 1.21 | − 2.50, 0.08 | Sheng and Xie (2010) | ||||
CRP C-reactive protein, TNF-α tumor necrosis factor-alpha, IL-6 interleukin-6, IL-1β interleukin -1 beta, SMD standardized mean differences, RE, random effect
Following subgroup analyses, heterogeneity was changed among some of the strata of subgroups. The heterogeneity changed significantly in the strata of polycystic ovary syndrome (I2 = 19%; p = 0.27) and ≥ 3 months (I2 = 10%; p = 0.35) for TNF-α. Furthermore, there were significant differences between before and after subgroup analyses in the stratum of metabolic syndrome for TNF-α (SMD = − 1.42; 95% CI − 3.38, 0.55; p > 0.05) and the stratum of cardiovascular disease for IL-6 (SMD = − 0.42; 95% CI − 1.24, 0.39; p > 0.05). These results of subgroup analyses suggested that type of condition and duration of study may be the source of heterogeneity in the meta-analysis. Table 3 shows the subgroup analysis of the influence of berberine on CRP, TNF-α, and IL-6.
Table 3.
Subgroup analyses of the influence of berberine on CRP, TNF-α, and IL-6
| Variables | N | I2 (%) | SMD (95% CI) | p value |
|---|---|---|---|---|
| CRP | ||||
| Total | 45 | 94 | − 1.54 [− 1.86, − 1.22] | < 0.00001 |
| Dosage of berberine | ||||
| < 0.9 g/d | 14 | 96 | − 2.45 [− 3.23, − 1.67] | < 0.00001 |
| ≥ 0.9 g/d | 31 | 92 | − 1.24 [− 1.56, − 0.93] | < 0.00001 |
| Type of condition | ||||
| Metabolic syndrome | 1 | –- | − 2.44 [− 3.03, − 1.86] | < 0.00001 |
| Type 2 diabetes | 21 | 96 | − 2.38 [− 3.02, − 1.75] | < 0.00001 |
| Diabetic nephropathy | 3 | 92 | − 1.03 [− 1.75, − 0.30] | < 0.00001 |
| Cardiovascular disease | 13 | 92 | − 1.20 [− 1.72, − 0.67] | < 0.00001 |
| Polycystic ovary syndrome | 3 | 65 | − 0.65 [− 1.11, − 0.20] | 0.005 |
| Other | 4 | 89 | − 0.94 [− 1.51, − 0.37] | < 0.001 |
| Duration of study | ||||
| < 3 months | 25 | 93 | − 1.50 [− 1.88, − 1.12] | < 0.00001 |
| ≥ 3 months | 19 | 95 | − 1.72 [− 2.32, − 1.12] | < 0.00001 |
| Unclear | 1 | — | − 0.96 [− 1.34, − 0.58] | < 0.00001 |
| Sample size | ||||
| < 30 | 10 | 93 | − 1.02 [− 1.86, − 0.18] | 0.02 |
| 30–60 | 32 | 95 | − 1.71 [− 2.09, − 1.34] | < 0.00001 |
| > 60 | 3 | 83 | − 1.68 [− 2.21, − 1.16] | < 0.00001 |
| TNF-α | ||||
| Total | 29 | 87 | − 1.02 [− 1.27, − 0.77] | < 0.00001 |
| Dosage of berberine | ||||
| < 0.9 g/d | 5 | 84 | − 1.74 [− 2.25, − 1.22] | < 0.00001 |
| ≥ 0.9 g/d | 24 | 80 | − 0.85 [− 1.08, − 0.63] | < 0.00001 |
| Type of condition | ||||
| Metabolic syndrome | 3 | 96 | − 1.42 [− 3.38, 0.55] | 0.16 |
| Type 2 diabetes | 13 | 82 | − 0.89 [− 1.19, − 0.58] | < 0.00001 |
| Diabetic nephropathy | 3 | 78 | − 0.93 [− 1.44, − 0.41] | 0.0004 |
| Cardiovascular disease | 5 | 66 | − 1.00 [− 1.39, − 0.60] | < 0.00001 |
| Polycystic ovary syndrome | 2 | 19 | − 0.62 [− 0.99, − 0.26] | 0.0008 |
| Other | 3 | 94 | − 1.58 [− 2.97, − 0.18] | 0.001 |
| Duration of study | ||||
| < 3 months | 19 | 91 | − 1.16 [− 1.52, − 0.80] | < 0.00001 |
| ≥ 3 months | 10 | 10 | − 0.72 [− 0.86, − 0.57] | < 0.00001 |
| Sample size | ||||
| < 30 | 6 | 81 | − 0.92 [− 1.59, − 0.24] | 0.008 |
| 30–60 | 19 | 86 | − 0.98 [− 1.26, − 0.70] | < 0.00001 |
| > 60 | 4 | 95 | − 1.34 [− 2.12, − 0.55] | 0.0009 |
| IL-6 | ||||
| Total | 28 | 93 | − 1.17 [− 1.53, − 0.81] | < 0.00001 |
| Dosage of berberine | ||||
| < 0.9 g/d | 4 | 97 | − 3.16 [− 4.73, − 1.59] | < 0.0001 |
| ≥ 0.9 g/d | 24 | 91 | − 0.95 [− 1.29, − 0.61] | < 0.00001 |
| Type of condition | ||||
| Metabolic syndrome | 3 | 93 | − 1.72 [− 3.19, − 0.25] | 0.02 |
| Type 2 diabetes | 15 | 94 | − 1.57 [− 2.14, − 1.00] | < 0.00001 |
| Diabetic nephropathy | 1 | –- | − 0.49 [− 0.88, − 0.10] | 0.01 |
| Cardiovascular disease | 6 | 93 | − 0.42 [− 1.24, 0.39] | 0.31 |
| Polycystic ovary syndrome | 1 | –- | − 0.73 [− 1.14, − 0.32] | 0.0005 |
| Other | 2 | 85 | − 0.87 [− 1.29, − 0.44] | 0.0002 |
| Duration of study | ||||
| < 3 months | 16 | 91 | − 1.36 [− 1.78, − 0.95] | < 0.00001 |
| ≥ 3 months | 12 | 95 | − 0.91 [− 1.57, − 0.26] | 0.006 |
| Sample size | ||||
| < 30 | 7 | 97 | − 1.92 [− 3.77, − 0.06] | 0.04 |
| 30–60 | 18 | 83 | − 0.98 [− 1.24, − 0.71] | < 0.00001 |
| > 60 | 3 | 94 | − 1.89 [− 2.76, − 1.02] | < 0.0001 |
N number of SMD included, CRP C-reactive protein, TNF-α tumor necrosis factor alpha, IL-6 interleukin-6, SMD standardized mean differences, X g/d X g daily, –- not applicable
Publication bias
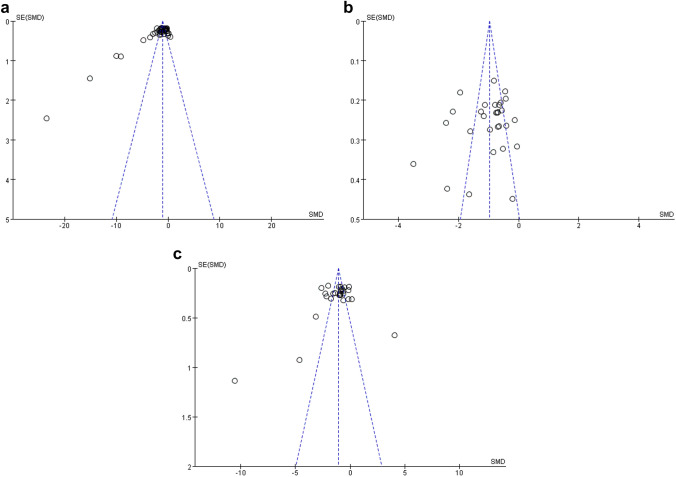
Funnel plots and Egger’s regression test were not evaluated for IL-1β levels due to the relatively small number of studies with this endpoint. These tests showed no significant evidence of publication bias for meta-analyses assessing the effect of berberine on TNF-α (p = 0.46; 95% CI − 1.38, 0.64) and IL-6 (p = 0.43; 95% CI − 0.48, 1.09) concentration levels. However, as shown in Fig. 4, the asymmetry displayed in the funnel plot, and Egger’s test (p < 0.05; 95% CI 1.27, 2.26) of CRP indicated some publication bias, which probably is attributed to unpublished studies with negative findings.
Fig. 4.
Funnel charts based on a CRP, b TNF-α, and c IL-6. CRP C-reactive protein, TNF-α tumor necrosis factor alpha, IL-6 interleukin-6
Discussion
Regulating inflammatory markers through various pathways to exert anti-inflammatory effects is one possible mechanism of action that berberine may have in the treatment of MetS and related disorders. An animal experiment conducted by Jeong HW found that berberine can restore damaged islet cells by activating the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway (Jeong et al. 2009). In the adipose tissue of obese mice, berberine was shown to significantly down-regulate the expression of pro-inflammatory genes, including IL-1β, IL-6, TNF-α, monocyte chemoattractant protein-1 (MCP-1), inducible nitric oxide synthase (iNOS), and cyclooxygenase 2 (COX-2), and continually inhibit peritoneal macrophages and RAW264.7 cell pro-inflammatory genes (IL-1β, IL-6, iNOS, MCP-1, COX-2, and alkaline metalloproteinase-9) expression induced by lipopolysaccharide (LPS) (Jeong et al. 2009). Additionally, berberine can reduce the phosphorylation of MAPK by intervening in the activation of TNF-α and other inflammatory markers on MAPK (Li et al. 2014). Wan Q reported that berberine inhibits the activation of the extracellular-signal-regulated kinase (ERK) signaling pathway, and down-regulates the expression of TNF-α and IL-6, through in-vitro experiments on human umbilical vein endothelial cells (HUVECs) (Wan et al. 2014). Inflammatory markers like IL-6 and IL-1β regulate and induce the expression of CRP. Furthermore, an increase in CRP levels can facilitate those inflammatory markers when inflammation occurs (Yang et al. 2012).
As an extract from traditional Chinese herbs, berberine has a long history of clinical application and many efficacy trials on humans in China. The meta-analysis included 52 RCTs involving 4616 Chinese patients with MetS and related disorders, which complemented the evidence of the effects of berberine use on inflammatory markers in humans and in China. The results suggested that berberine could reduce the concentration level of CRP significantly, which was consistent with the results of a previous study (Beba et al. 2019). Furthermore, this meta-analysis analyzed three other important inflammatory markers of metabolic syndrome (MetS) and related disorders. The results suggested that berberine could reduce the concentration level of TNF-α, and IL-6 significantly, but could not reduce the concentration level of IL-1β. Sensitivity analyses and subgroup analyses indicated that the results of the meta-analysis were relatively stable. The type of condition had the greatest impact on the heterogeneity and pooled estimates of the meta-analysis. However, due to the small number of included studies and the estimated heterogeneity, there were additional doubts about the pooled estimate result of IL-1β, which need to be resolved in further trials.
There are a few limitations to this meta-analysis. First, the result of the risk-of-bias assessment presented a large proportion of uncertain risks for insufficient information in trial methods. To a certain extent, the potential differences in the methods of random sequence generation, allocation concealment, and concentration measurement among the included studies have caused the high heterogeneity of the meta-analysis results. Second, the study population of all the included studies was Chinese patients, and the sample size of individual clinical trial was small. The results of this meta-analysis are accordingly hard to extrapolate to other ethnic populations or geographical regions. Therefore, more studies with larger sample size, ideally multi-centers, and rigorous design are needed to confirm the effect of berberine on the inflammatory markers of MetS and related disorders.
Conclusion
Despite the limitations of meta-analysis, the robust methodology followed in selecting RCTs for inclusion and in completing the evaluation does facilitate the conclusion that berberine use in patients with MetS and related disorders appears to have significantly decreased inflammatory markers, including CRP, TNF-α, and IL-6. This study provides new and useful evidence for supporting clinical medication decisions for MetS and related disorders and encourages undertaking further RCTs.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 71673298).
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Neither ethics approval nor participant consent was required as this study was based solely on the summary results of previously published articles. Individual patient data were not obtained or accessed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akbari M, Ostadmohammadi V, Tabrizi R, et al. The effects of melatonin supplementation on inflammatory markers among patients with metabolic syndrome or related disorders: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. 2018;26:899–907. doi: 10.1007/s10787-018-0508-7. [DOI] [PubMed] [Google Scholar]
- Akbari M, Tamtaji OR, Lankarani KB, et al. The effects of resveratrol supplementation on endothelial function and blood pressures among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc. 2019;26:305–319. doi: 10.1007/s40292-019-00324-6. [DOI] [PubMed] [Google Scholar]
- Arnlöv J, Ingelsson E, Sundström J, et al. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- Bai Z, Zhang D, Pu X. Clinical study of ezetimibe combined with berberine capsules in the treatment of hyperlipidemia. Neural Inj Funct Reconstr. 2018;13:469–471. [Google Scholar]
- Beba M, Djafarian K, Shab-Bidar S. Effect of berberine on C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2019;46:81–86. doi: 10.1016/j.ctim.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Cao C, Su M. Effects of berberine on glucose-lipid metabolism, inflammatory cytokines and insulin resistance in patients with metabolic syndrome. Exp Ther Med. 2019;17:3009–3014. doi: 10.3892/etm.2019.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Chen X, Yang H, et al. Simvastatin combined berberine in patients with coronary artery disease and hypercholesteremia. Cent South Pharm. 2015;13:203–205. [Google Scholar]
- Chen L, Lu W, Li Y. Berberine ameliorates type 2 diabetes via modulation of Bifidobacterium species, tumor necrosis factor-α, and lipopolysaccharide. Int J Clin Exp Med. 2016;9(6):9365–9372. [Google Scholar]
- Chen X, Fu T, Huang C, et al. Effect of berberine on expression of CHOP mRNA, GRP78mRNA and inflammatory cytokines in patients with metabolic syndrome and renal damage. J Nephrol Dial Transplant. 2017;26:333–338. [Google Scholar]
- Dai P, Wang J, Lin L, et al. Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus: evaluation via biochemical markers and color Doppler ultrasonography. Exp Ther Med. 2015;10:869–876. doi: 10.3892/etm.2015.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Wei W, Guan Y. The clinical study of berberine in patients with polycystic ovarian syndrome and insulin resistance. Tianjin Med J. 2012;40:1009–1011. [Google Scholar]
- Dong K, Shang J, Tao L. Effect of berberine combined with metformin on serum inflammatory factors and islet function in type 2 diabetes mellitus. J Clin Pathol Res. 2017;37:1418–1422. [Google Scholar]
- Dou J, Xing Y, Zhang R. Effect of berberine on serum C-reactive protein in obese patients. Pract Pharm Clin Rem. 2012;15:147–148. [Google Scholar]
- Du Y, Zhang J. Effect of berberine hydrochloride on plasma LDLR level in patients with coronary heart disease and its correlation analysis. Pract Pharm Clin Rem. 2018;21:1244–1248. [Google Scholar]
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24:283–307. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- Fan C, Li Y, Song H, et al. Effectiveness evaluation analysis on berberine tablets combination with metformin treating type 2 diabetes. Mod Hosp. 2018;18:731–733. [Google Scholar]
- Festa A, D'Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.CIR.102.1.42. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- Hamedifard Z, Milajerdi A, Reiner Ž, et al. The effects of spirulina on glycemic control and serum lipoproteins in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2019;33:2609–2621. doi: 10.1002/ptr.6441. [DOI] [PubMed] [Google Scholar]
- He T. Clinical efficacy and safety of berberine combined with valsartan in the treatment of diabetic nephropathy. Pract Clin J Integr Tradit Chin West Med. 2018;18:8–10. [Google Scholar]
- He P, Wang C, Liu S. Effects of berberine therapy on serum triglyceride, total cholesterol and inflammatory cytokines in patients with diarrhea complicated by hyperlipidemia. Hebei Med J. 2018;40:2276–2283. [Google Scholar]
- Higgins JPT, Thomas J, Chandler J et al (2020) Cochrane handbook for systematic reviews of interventions version 6.1. Cochrane. https://www.training.cochrane.org/handbook. Accessed on 27 May 2020
- Hu Y, Ehli EA, Kittelsrud J, et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19:861–867. doi: 10.1016/j.phymed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Huang J, Hu W, Lin X. The improving effect of berberine hydrochloride on insulin resistance in type 2 diabetes and its mechanism. Chin J Gerontol. 2018;38:4130–4132. [Google Scholar]
- Jeong HW, Hsu KC, Lee JW, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- Ju J, Li J, Lin Q, et al. Efficacy and safety of berberine for dyslipidaemias: a systematic review and meta-analysis of randomized clinical trials. Phytomedicine. 2018;50:25–34. doi: 10.1016/j.phymed.2018.09.212. [DOI] [PubMed] [Google Scholar]
- Lai S, Zhong Y, Liang J. Effects of berberine combined with Peikun pill on lipid and glucose metabolism, inflammatory factors and hormone levels in patients with insulin resistance of polycystic ovary syndrome. World Chin Med. 2019;14:2683–2687. [Google Scholar]
- Lan J, Zhao Y, Dong F, et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- Lan P, Zhong Y, Yang Y, et al. Effect of berberine on TNF-alpha and IL-6 in patients with hypertensive atherosclerosis. Mod Diagn Treat. 2019;30:1976–1978. [Google Scholar]
- Lee HS, Herceg Z. Chapter 30—nutritional epigenome and metabolic syndrome. In: Tollefsbol TO, editor. Handbook of epigenetics: the new molecular and medical genetics (2rd) Academic Press, US; 2017. pp. 465–475. [Google Scholar]
- Li J. A control study of efficacy and safety treated by berberine and metformin on metabolic syndrome in patients with schizophrenia. Tianjin Medical University; 2017. [Google Scholar]
- Li P. Effects of berberine combined with sitagliptin on blood glucose and inflammation indexes in obese patients with type 2 diabetes. Henan Med Res. 2017;26:903–904. [Google Scholar]
- Li Y. The Effects of berberine on anti-inflammatory and anti-atherosclerotic plaques in patients with acute cerebral ischemic stroke. Chengde Medical University; 2017. [DOI] [PubMed] [Google Scholar]
- Li J, Deng Z. Effect of compound berberine tablets combined with polyene phosphatidylcholine on elderly patients with non-alcoholic fatty liver. Chin J Gerontol. 2018;38:5224–5227. [Google Scholar]
- Li Z, Geng YN, Jiang JD, et al. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014;2014:1–12. doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao Y, Liu Y, et al. The impact of berberine on insulin resistance and cytokines in patients with schizophrenia. Tianjin Med J. 2016;44:1143–1146. [Google Scholar]
- Li Y, Lin Y, Wang X, et al. Effects of berberine on blood glucose, blood lipids and insulin resistance in patients with polycystic ovary syndrome. Chron Pathematol J. 2018;19:125–126. [Google Scholar]
- Li Z, Liu B, Zhuang X, et al. Effects of berberine on the serum cystatin C levels and urine albumin/creatine ratio in patients with type 2 diabetes mellitus. Natl Med J China. 2018;98:3756–3761. doi: 10.3760/cma.j.issn.0376-2491.2018.46.007. [DOI] [PubMed] [Google Scholar]
- Liang Y, Xu X, Yin M, et al. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: a systematic literature review and a meta-analysis. Endocr J. 2019;66:51–63. doi: 10.1507/endocrj.EJ18-0109. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu Q. Effect of berberine on islet β-cell function in type 2 diabetes mellitus (Damp-Heat Type) Chin J Inf TCM. 2008;15:18–20. [Google Scholar]
- Liu Y, Wang S. Observation of the curative effect of berberine in treatment of ischemic heart disease and heart failure. Chin J Integr Med Cardio-Cerebrovasc Dis. 2012;10:519–520. [Google Scholar]
- Liu J, Wang D, Lin Z, et al. Effects of berberine on serum TNF-α and IL-6 levels of type 2 diabetic patients. Zhejiang Med. 2010;32:207–209. [Google Scholar]
- Lu H, Cui H, Yang X, et al. Effects of risuvastatin combined with berberine hydrochloride on the indicators of acute ischemic cerebral infarction patients. J Clin Exp Med. 2018;17:1729–1732. [Google Scholar]
- Meng X, Zeng Y, Kong H, et al. Research on the effect of berberine on the function of endothelial vessels, IL-6 and TNF-a in newly diagnosed patients with type 2 diabetes mellitus. Chin J Prev Contr Chron Dis. 2011;19:504–505. [Google Scholar]
- Ning M. Clinical effects of berberine combined with atorvastatin in treatment of patients with acute cerebral infarction. Med J Chin People’s Health. 2018;30:16–20. [Google Scholar]
- Noda H, Iso H, Saito I, et al. The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: the Japan public health center-based study. Hypertens Res. 2009;32:289–298. doi: 10.1038/hr.2009.14. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe P, Mathangasinghe Y, Jayawardena R, et al. Prevalence and trends of metabolic syndrome among adults in the Asia-Pacific region: a systematic review. BMC Public Health. 2017;17:101. doi: 10.1186/s12889-017-4041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Xie D. The level of inflammatory cytokines in patients with type 2 diabetes and the effect of adjuvant berberine treatment on it. New Med. 2010;41:177–180. [Google Scholar]
- Shu J. Effects of berberine on serum adiponectin and high-sensitivity C-reactive protein levels in type 2 diabetic patients. China Pharm. 2014;23:33–34. [Google Scholar]
- Sun H. Clinical observation of berberine hydrochloride combined with sitagliptin in treatment of diabesity type 2 diabetes mellitus. Drugs Clin. 2016;31:1020–1023. [Google Scholar]
- Sun S. Effect of berberine on the serum levels of IL-10, IL-6 and CRP in patients with type 2 diabetes mellitus. Journal of Changchun University of Chinese Medicine. 2017;33:431–433. [Google Scholar]
- Tabrizi R, Tamtaji OR, Lankarani KB, et al. The effects of resveratrol supplementation on biomarkers of inflammation and oxidative stress among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Food Funct. 2018;9:6116–6128. doi: 10.1039/C8FO01259H. [DOI] [PubMed] [Google Scholar]
- Tabrizi R, Tamtaji OR, Mirhosseini N, et al. The effects of statin use on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;141:85–103. doi: 10.1016/j.phrs.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Wan Q, Cui X, Jia Y, et al. Berberine reduce TNF-α and IL-6 secreted in HUVEC induced by visfatin through ERK1/2 signal pathway. Chin J Exp Tradit Med Formulae. 2014;20:125–129. [Google Scholar]
- Wang Y. Berberine blood lipids in patients with type 2 diabetes mellitus and the effect of insulin resistance and mechanism research. Diabetes New World. 2016;19:27–28. [Google Scholar]
- Wang L, Huang C, Chen X, et al. Effects of berberine on intestinal bifidobacterium and inflammatory factors in metabolic syndrome patients with renal damage. Nephrol Dialy Transplant. 2018;27:440–444. [Google Scholar]
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- Xiang W, Huang X, Huang G. A study of two anti-inflammatory drugs in the treatment of newly diagnosed type 2 diabetes patients. J Pract Diabetol. 2011;7:51–52. [Google Scholar]
- Xie X, Huang G. Clinical effect of Tripterygium wilfordii polyglycosides combined with berberine on early diabetic patients with early diabetic nephropathy. Med Innov China. 2019;16:38–41. [Google Scholar]
- Xu F, Yu F, Li Q, et al. Observation of berberine plus pioglitazone in the treatment of early diabetic nephropathy and the level of adiponectin. Chongqing Med. 2008;37:2102–2103. [Google Scholar]
- Yang R, Yin J. Effects of berberine combined with rosuvastatin calcium on blood lipids, serum inflammatory cytokines and endothelial function in patients with coronary heart disease. J Mod Med Health. 2019;35:431–433. [Google Scholar]
- Yang J, Luo M, Liu C, et al. Effect of berberine on C-reactive protein in rats with diabetic nephropathy. Sichuan J Physiol Sci. 2012;34:51–53. [Google Scholar]
- Yang Z, Deng X, Wang D. Clinical study of the effect of berberine combined with simvastatin on Lp-PLA2 in patients with symptomatic atherosclerotic intracranial artery stenosis. China Med Pharm. 2018;8:83–85. [Google Scholar]
- Yang X, Liu Z, Yang H. Clinical study on berberine hydrochloride tablets combined with metformin in the treatment of primary type 2 diabetes. SH J TCM. 2020;54:59–62. [Google Scholar]
- Ye X, You L. Analysis of clinical efficacy of berberine hydrochloride combined with rosuvastatin calcium in the treatment of 33 patients with acute ischemic cerebral infarction. Heilongjiang Med Pharm. 2019;42:148–149. [Google Scholar]
- Yu F, Xu F, Li Q, et al. Effects of exenatide combinated with berberine therapy on early phase insulin secretion in the patients with new onset type 2 diabetes mellitus. Chin J Clin Ration Drug Use. 2012;5:32–33. [Google Scholar]
- Yuan F, Fang L, Dong G. Effect of Gegenqinlian decoction combined with berberine in the treatment of biguanides resistant type 2 diabetes in elderly patients in glycosylated hemoglobin and homocysteine. Chin J Biochem Pharm. 2017;37:55–58. [Google Scholar]
- Zhan H, Chen H, Lin C, et al. Effects of berberine on T2DM and high lipid serum NO level in patients with hyperlipidemia and the activity of SOD. J North Pharm. 2015;12:119–121. [Google Scholar]
- Zhang Y, Li X, Zou D, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xiao J, Zhang H, et al. Effect of berberine on high sensitivity C-reactive protein and homocysteine in patients with acute coronary syndromes. Chin J Esthetic Med. 2010;19:156–157. [Google Scholar]
- Zhang Y, Hu X, Zhou Y, et al. A study of berberine combined with atorvastatin in treatment of patients with cerebral infarction. Pract J Clin Med. 2014;11:80–83. [Google Scholar]
- Zhou Y, Huang S. Clinical observation on 60 cases of hyperlipemia treated by berberine. Chin J Clin Ration Drug Use. 2011;4:76–77. [Google Scholar]
- Zhou Q, Huang S. Clinical study of berberine combined with metformin in the treatment of obese type 2 diabetes. J Pract Diabetol. 2012;8:33–35. [Google Scholar]
- Zhou Q, Huang S, Yang X. The effect of comprehensive treatment on inflammatory cytokines in newly diagnosed obesity type 2 diabetes. J Pract Diabetol. 2016;12:42–43. [Google Scholar]
- Zhu J. Effects of berberine hydrochloride combined with irbesartan on middle-aged and elderly patients with early diabetic nephropathy. Clin Educ Gen Pract. 2010;8:512–515. [Google Scholar]
- Zhu F, Chen L, Zhu J. Influence of berberine combining with atorvastatin on serum high-sensitivity C-reactive protein and adipocyte fatty acid-binding protein in patients with acute ischemic stroke. Chin J Contemp Neurol Neurosurg. 2015;15:43–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.