Abstract
The accumulation of senescent cells contributes to aging pathologies, including neurodegenerative diseases, and its selective removal improves physiological and cognitive function in wild-type mice as well as in Alzheimer’s disease (AD) models. AD models recapitulate some, but not all components of disease and do so at different rates. Whether brain cellular senescence is recapitulated in some or all AD models and whether the emergence of cellular senescence in AD mouse models occurs before or after the expected onset of AD-like cognitive deficits in these models are not yet known. The goal of this study was to identify mouse models of AD and AD-related dementias that develop measurable markers of cellular senescence in brain and thus may be useful to study the role of cellular senescence in these conditions. We measured the levels of cellular senescence markers in the brains of P301S(PS19), P301L, hTau, and 3xTg-AD mice that model amyloidopathy and/or tauopathy in AD and related dementias and in wild-type, age-matched control mice for each strain. Expression of cellular senescence markers in brains of transgenic P301L and 3xTg-AD mice was largely indistinguishable from that in WT control age-matched mice. In contrast, markers of cellular senescence were differentially increased in brains of transgenic hTau and P301S(PS19) mice as compared to WT control mice before the onset of AD-like cognitive deficits. Taken together, our data suggest that P301S(PS19) and hTau mice may be useful models for the study of brain cellular senescence in tauopathies including, but not limited to, AD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00531-5.
Keywords: Aging, Inflammation, Tauopathies, Amyloidopathy
Introduction
Chronic inflammation underlies many age-associated impairments and pathologies [1, 2], including AD and other neurodegenerative diseases [3]. The mechanisms that drive increased inflammation during aging, however, are incompletely understood. Cellular senescence contributes to the development of chronic inflammation and tissue damage in a variety of tissues [4]; thus, it is a suitable target for therapeutic interventions in age-related diseases, including those of the central nervous system where inflammation is causally involved [5]. Cellular senescence in the central nervous system, and its occurrence in models of CNS disease, including but not limited to AD, however, is still poorly understood.
Cellular senescence is triggered in response to molecular damage or cellular stress. In these conditions, some somatic cells stop dividing and enter an irreversible state of cell cycle arrest [6, 7]. Senescent cells also activate a paracrine response which includes the coordinated expression of various pro-inflammatory cytokines, chemokines, and growth factors collectively known as the senescence-associated secretory phenotype (SASP) [8, 9] that is under control of mitogen-activated protein kinases and Nf-KB [10, 11] among other mediators. The expression of the pro-inflammatory SASP induces the recruitment of phagocytic immune cells to remove cells undergoing senescence and may at the same time activate growth of nearby progenitor cells [12]. However, with the progression of age or in the presence of persistent stress, the clearance of senescent cells is impaired or incomplete, allowing dysfunctional senescent cells to accumulate. While acute senescent cell generation is beneficial in wound healing and tumor suppression, chronic senescent cell accumulation with age increases inflammation in a variety of cell tissue types, leading to tissue dysfunction[1]. Thus the accumulation of senescent cells may substantially contribute to the emergence of a chronic pro-inflammatory environment causally involved in the etiology of age-related pathologies, including neurodegenerative disorders [13, 14]. The mechanisms and functional impact of cellular senescence have been studied in in vitro models (reviewed in [15] and [16]) and some in vivo models [17, 18]. Cellular senescence in the brain, where a large proportion of cells are post-mitotic or normally quiescent, however, remains largely unexplored. Bhat et al. [19] provided strong evidence that astrocytes in brain tissue from aged individuals and patients with Alzheimer’s disease (AD) undergo senescence measured as a significant increase in p16INK4a and matrix metalloproteinase-1 (MMP-1) expression [19, 20]. Another study by Zhang et al. [21] provided evidence that oligodendrocyte progenitor cells (OPCs) in human AD patients express elevated levels of cell cycle arrest markers and some SASP components. Robust cellular senescence in a model of AD-related dementia has been reported [22]. Importantly, the amelioration of tauopathy-associated pathology and cognitive impairment by elimination of senescent cells [22] suggests that, similar to its role in age-related dysfunction in peripheral organs [12, 23], cellular senescence may play a role in the etiology of AD.
While the accumulation of senescent cells is expected to increase inflammation, increased brain inflammation in late onset of AD, however, may not be completely explained by cellular senescence [24]. Thus, to better understand the role of senescent cell accumulation in the pathogenesis of AD and define the impact of potential interventions targeting senescent cells, an important question that needs to be answered is whether senescent cell accumulation is observed in different models of AD and if so whether it precedes onset of disease defined as appearance of cognitive deficits in these mouse models. Mice modeling AD or primary tauopathies that display senescent cell accumulation should provide useful models to define the role of senescent cell accumulation in AD and related dementias.
AD is characterized histopathologically by neuronal loss and the accumulation of extracellular β-amyloid plaques and intracellular neurofibrillary tangles in the brain and clinically by progressive cognitive impairment. Both amyloid-beta (Aβ) [25] and pathogenic forms of tau protein [26] have been causally implicated in the etiology of AD. Mouse models that model either amyloidosis or tauopathy of AD have been generated and characterized, with some that recapitulate both histopathological hallmarks [27, 28]. Very few models, however, recapitulate neuronal loss that is characteristic of human disease [29]. No single mouse model recapitulates all features of AD [30], and both the sequence of appearance and the time at emergence of AD-like deficits is frequently different in different mouse models of AD. Neuronal loss is absent in the majority of AD models except for some models of tauopathy and in those neuronal loss may emerge after the onset of cognitive impairment. Whether senescent cell accumulation starts before or after the onset of cognitive deficits in mouse models of AD is unknown.
It is widely accepted that soluble aggregated oligomeric forms of tau or amyloid-beta (Aβ), as opposed to large aggregates such as neurofibrillary tangles and Aβ plaques, are the major pathogenic toxic species in AD and primary tauopathies [31, 32]. Because of the enforced expression of their precursors (monomeric tau and amyloid precursor protein (APP)) as transgenes, soluble oligomeric forms of tau or Aβ appear early in the postnatal period or early adulthood (~ 4–12 weeks of age) in rodent models of Alzheimer’s disease [33–35], before age-associated changes such as cellular senescence are present. It is thus unlikely that activation of aging-associated cellular senescence precedes the increase in soluble aggregated forms of tau or Aβ in rodent models of AD. While frequent in models of tauopathy, neuronal loss, another central feature of AD, is absent in the vast majority of models of AD amyloidosis. We thus used emergence of cognitive impairment as the “time at disease onset” for our studies because cognitive impairment is a central and translationally relevant event in AD-like pathogenesis that emerges in adulthood when aging-associated processes are already at play, in all models tested. Since P301L mice do not develop cognitive deficits, we used neuronal loss as a relevant marker for disease onset only for this strain.
Our goal for the present study was to determine whether markers of cellular senescence are increased in brains of five independent AD mouse models as compared to relevant wild-type control mice before or at the time of disease onset (defined as emergence of cognitive deficits, except for P301L mice), thus indicative of senescence processes initiated during earlier pre-symptomatic stages in AD- and/or tauopathy-like progression. While it is possible that senescence may occur as a “wave” during the early stages of AD-like progression in mouse models, a more likely scenario is that once triggered in the pre-symptomatic stages, cellular senescence would increase continuously or until reaching a plateau after onset of disease in AD model mice. Assuming the latter scenario, we measured markers of senescence at time of onset of disease to maximize our chances to detect prior activation of the senescence response, initiated during the pre-symptomatic stage of AD-like progression. Thus, a limitation of our design is that we may have missed transient “waves” of senescence in brain that may occur in the pre-symptomatic stage of AD-like progression in model mice.
Cellular senescence is identified using three sets of biomarkers that represent biologically divergent aspects of the senescence program [36]. Senescent cells express elevated levels of cell damage/cell cycle arrest markers (such as P16, P21, or P53), release inflammatory mediators collectively referred to as SASP (including IL6, IL1α, and TNF-α among others), and undergo lysosomal expansion that can be quantified using SA-βgal activity as a marker. While individually these three sets of biomarkers are not unique to cellular senescence, a combination of the three is canonically used to identify senescent cells.
We measured markers of cell damage/cell cycle arrest, SASP, and increased activity of SA-βgal in brain tissues of three models of primary tauopathy (P301L, P301S(PS19)) or AD tauopathy (hTau mice), one model of amyloidopathy (Tg2576), and in one model where amyloidopathy and tauopathy co-occur (3xTg-AD mice) at the expected time of onset of AD-like cognitive dysfunction, except for P301L mice where we used onset of neuronal loss as the time of onset of AD-like disease. We found evidence of increased cellular senescence in the brains of P301S and hTau mice, to different degrees, but not in P301L and 3xTg-AD nor in Tg2576 animals, suggesting that senescent cells accumulate in the brains of P301S and hTau mice modeling non-AD tauopathy and AD tauopathy, respectively, before the expected onset of cognitive deficits during progression of AD-like disease. hTau and P301S mice may thus constitute useful models to study the mechanisms by which cellular senescence drives AD.
Methods
Mice
hTau mice expressing all six isoforms of WT human tau from a BAC containing the complete sequence of the human microtubule-associated protein tau (MAPT) gene under control of its promoter, in the mouse Mapt knockout C57BL/6 J background [37], as well as P301S Tg mice and NTg B6C3F1/J breeders were obtained from JAX (Bar Harbor, ME). P301S(PS19) mice express the human MAPT gene carrying the P301S mutation, with 4 microtubule-binding domains and one N-terminal insert (4R/1 N) driven by the mouse prion promoter [38]. Brain tissues from 3xTg-AD mice expressing a human APP transgene carrying the Swedish mutation plus a human MAPT transgene carrying the P301L mutation in the background of a knock-in M146V mutation in the mouse presenilin-1 gene and from non-transgenic littermates[27] were a gift from Dr. Salvatore Oddo. Brain tissues from JNLP3(P301L) mice expressing a human MAPT transgene carrying the P301L mutation with 4 microtubule-binding repeat domains and no N-terminal inserts, driven by the mouse prion promoter[39], were obtained from Dr. Rakez Kayed. Tg2576 mice expressing a human APP695 transgene carrying the Swedish (K670N, M671L) mutation under the control of the hamster prion promoter and non-transgenic littermates were obtained from Dr. Kelly Dineley (University of Texas Medical Branch at Galveston, Galveston, TX). All studies were performed under approval of the UT Health San Antonio Institutional Animal Care and Use Committee (IACUC, Animal Welfare Assurance Number: A3345-01) and in compliance with the ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments) for reporting animal experiments. Mice were housed and sacrificed using methods approved by the institutional IACUC. Brain tissues were dissected, flash frozen, and stored at − 80° C until they could be sectioned or dissected.
P301S behavior
Male and female P301S(PS19) mice at 7 months of age underwent cognitive testing, including acclimation (2 days), reference memory testing, including hidden trials, and a naive probe and other probes at the end of each day (2 days), reversal training (1 day) and visible (control) trials (1 day) as previously described [40]. Analysis was performed with repeated measures ANOVA. Hippocampus-dependent contextual memory was measured in male and female P301S(PS19) mice at 12 months of age using fear conditioning. Animals acclimated to the testing environment for 3 h prior to training were placed into test chambers contained within isolation chambers (Coulbourn Instruments, Holliston, MA, USA). Animals were subsequently exposed to two pairings of a 30-s auditory stimulus (white noise), which co-terminated with a 2-s foot shock (1 mA). Hippocampal-dependent contextual memory was assessed 24 h after the training session, during a 5-min exposure to the context itself, conducted in the absence of any tone or shock. FreezeFrame software (Coulbourn Instruments) was used to record and monitor freezing behavior within the isolation chambers. Data were exported, and percent freezing was calculated for each mouse. Data were analyzed using a one-way ANOVA followed by Tukey’s multiple comparison test among all means.
β-galactosidase assay
SA-βgal staining was performed on coronal cryosections of hemibrains using the SPiDER-βgal kit (Dojindo Molecular Technologies, Rockville, MD, USA). Briefly, brain sections were incubated with 2 ml of SPiDER-βgal working solution together with 4′,6-diamidino-2-phenylindole (DAPI), a fluorescent stain that binds tightly to adenine–thymine rich regions of DNA, incubated at 37 °C for 30 min, and washed twice with HBSS and imaged (excitation, 488 nm; emission, 500–600 nm) using a Keyence fluorescence microscope. Red fluorescence indicated the presence of SA-βgal activity. Ratios of total number of red over blue fluorescent pixels in hippocampus were calculated with ImageJ software and used as a measure of SA-βgal activation.
RT-qPCR
RNA was isolated from (1) frozen brain tissues that included cortex and hippocampus of 3xTg-AD (n = 5 per group, age 12 months old), hTau (Tg n = 7, ages 7–9 months; NTg n = 4, ages 9, 14, and 19 months), P301S (NTg n = 12, age 7 months; Tg, n = 13 at 7 months of age and 3 at 10 months of age.), and Tg2576 (Tg n = 6, age 19 months; NTg n = 6, age 19 months) transgenic and non-transgenic (WT) mice or (2) cryosections from P301L and WT mice (Tg n = 3, age 6 months; NTg n = 4, age 6 months) using the Qiagen RNeasy® Mini Kit. RNA was then reverse-transcribed to cDNA using SuperScript® III First-Strand Synthesis SuperMix as per the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). mRNA levels for SASP components IL-6, IL-1β, TNF-α, MCP-1, PAI-1, CCL4, and CD11b as well as cell damage/cell cycle arrest markers p16, p53, and p21 were measured by real-time PCR (RT-qPCR). mRNA was quantified by determining the point at which fluorescence accumulation entered the exponential phase (Ct), and the Ct ratio of the target gene to actb or GAPDH was calculated for each sample. qPCR primers are listed in Supplementary Table 1.
Statistical analyses
Data were analyzed using Prism 8 (GraphPad, San Diego, CA, USA). Significance of differences between experimental group means were defined using unpaired Student’s t test. Differences between means at P < 0.05 as indicated in the legends to the figures were considered statistically significant.
Results
To identify mouse models that recapitulate brain cellular senescence of AD and thus may be useful in studies to address the role of senescence in AD etiology, we quantitated markers of cellular senescence in brain tissues of several independent AD mouse models at or before the earliest documented age-at-onset of neuronal loss or AD-like cognitive deficits, thus at a time during disease progression when potentially causative senescent cell accumulation would have already emerged. We defined cellular senescence as the concomitant elevation in levels of markers of cell damage/cell cycle arrest together with increased levels of several components of the senescence-associated secretory phenotype (SASP) and augmented SA-βgal activity.
JNPL3(P301L) mice
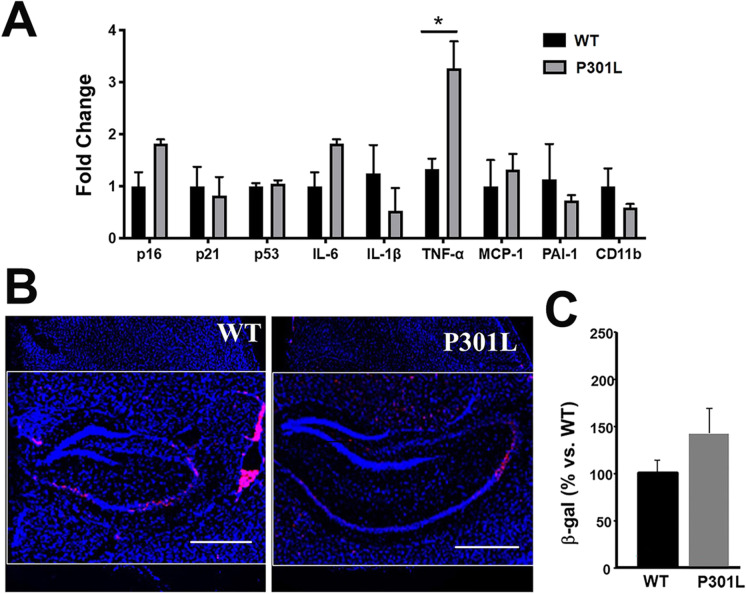
We first examined JNPL3(P301L) mice that express a human MAPT transgene carrying the P301L mutation associated with frontotemporal dementia [39]. P301L animals develop neurofibrillary tangles (NFT) between 4.5 and 6.5 months of age. Neuronal loss is detectable around 10 months of age in the absence of overt cognitive deficits [39, 41]. To determine whether cellular senescence occurs early during tauopathy in P301L mice, we measured cell damage/cell cycle arrest and SASP markers in brains of P301L transgenic animals at 6 months of age, thus after NFT development but before neuronal loss. Because these animals do not develop detectable cognitive impairment, we used expected onset of neuronal loss as the time at onset for disease. We found no significant differences in expression of cell damage/cell cycle arrest markers or of SASP components, with the exception of an increase in TNF-α transcript levels, in transgenic P301L brains as compared to WT non-transgenic littermates (Fig. 1A). Consistent with these observations, we found a trend, but no significant differences in SA-βgal levels in the CA2 region of the hippocampus of P301L transgenic animals as compared to non-transgenic mice (Fig. 1B). Taken together, these data indicate that the P301L mice do not show evidence of cellular senescence before the expected onset of disease defined as neuronal loss and thus may not be an ideal model to study the role of senescence in AD.
Fig. 1.
Increased leves of TNFα but not of markers of senescence, in brains of P301L mice modeling tauopathy. A Levels of markers of cell damage/cell cycle arrest p16, p21, and p52 and of components of the senescence-associated secretory phenotype (SASP; IL-6, IL-1α, PAI-1, MCP-1, and CD11-b) in cortices, including hippocampus, of 6-month-old P301L mice and WT controls measured using qRT-PCR. Transcript levels were normalized to those of GAPDH and are shown as fold changes compared to the WT group. n = 5, *P < 0.05, unpaired Student’s t test. B–C Quantitative analyses of SA-βgal activity, as percent red fluorescent area (indicating SA-βgal activity, panel B, representative images) normalized (panel C, quantitative analyses of data in B) to blue fluorescent area as a measure of total cell number in hippocampi of WT and P301S(PS19) mice. Scale bar, 500 µM. *P < 0.05, unpaired Student’s t test. WT, n = 4; P301L, n = 3 mice per group at 6 months of age. Data are means ± SEM
P301S(PS19) mice
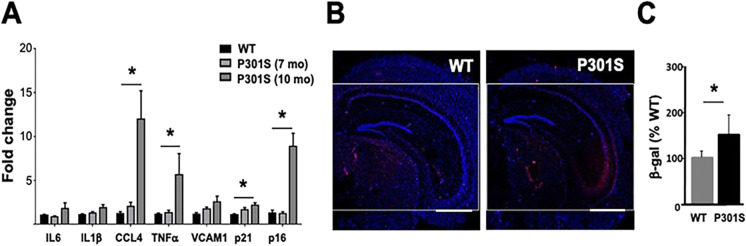
Next we examined another model of primary tauopathy, P301S(PS19) mice, that express a human MAPT transgene carrying the P301S mutation associated with frontotemporal dementia [38]. P301S mice develop NFT, and some reports indicate that this is accompanied by spatial learning deficits at 6 months of age [42], followed by neuronal loss starting at 9 months of age. Spatial learning and memory deficits in P301S mice, however, may not emerge until 8–9 [43] or 10–11 months of age [44]. A delay in the onset of pathology has also been reported for P301S mice [43, 45]. In our hands, P301S(PS19) mice did not display defects in spatial and contextual memory at 7 nor at 8 months of age, respectively (Figure S1). Because we did not observe cognitive changes nor evidence of activation of cellular senescence at 7–8 months of age, we measured markers of senescence at a later age (10 months). We found cell damage/cell cycle arrest markers P16 and P21 and some components of SASP such as CCL4 and TFNα in 10 month-old P301S animals compared to WT mice (Fig. 2A). Consistent with these observations, SA-β-gal activity was increased in hippocampal CA2 and CA3 regions of 10 month-old P301S mice as compared to WT controls (Fig. 2B–C). Taken together, these data indicate that selected markers of cellular senescence increase at the transition from pre-symptomatic to symptomatic stages of tauopathy in brains of P301S(PS19) mice.
Fig. 2.
Specific markers of cellular senescence are increased in brains of P301S mice modeling tauopathy. A Levels of cell damage/cell cycle arrest markers p16 and p21 and of components of the senescence-associated secretory phenotype (SASP; IL-6, IL-1α, CCL-4, VCAM1, and TNFα) in hippocampus of 7- and 10-month-old P301S(PS19) mice and non-transgenic littermates (WT) measured using qRT-PCR. Transcript levels were normalized to those of β-actin and are shown as fold changes compared to the WT group. n = 12–13 for 7-month-old WT and P301S(PS19) and n = 3 for 10-month-old P301S(PS19) groups. Scale bar, 500 µM. *P < 0.01 as a result of pairwise comparisons between groups indicated, unpaired Student’s t test. B–C Quantitative analyses of SA-βgal activity, as percent red fluorescent area (indicating SA-βgal activity, panel B, representative images) normalized (panel C, quantitative analyses of data in B) to blue fluorescent area as a measure of total cell number in hippocampi of WT and hTau mice. Scale bar, 500 µM. *P < 0.05, unpaired Student’s t test. NTg, n = 12, P301S(PS19), n = 13 at 7 months of age and 3 at 10 months of age. Data are means ± SEM
hTau mice
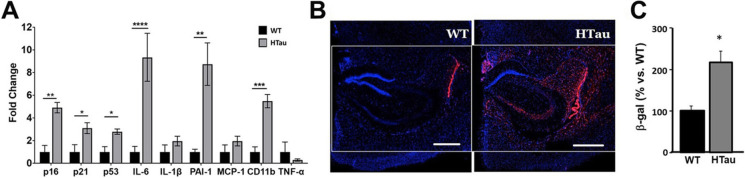
We next measured senescence markers in hTau mice modeling tauopathy of AD through expression of all 6 isoforms of non-mutant human tau from the MAPT gene driven by its endogenous promoter [37]. Accumulation of hyperphosphorylated human tau in hTau mice begins at 6 months of age, but low levels of soluble aggregated pathogenic tau may be detected as early at 4 months of age. Neuronal loss occurs around 10 months of age [37], and spatial memory deficits are detectable at 12 months of age [46]. We found that all cell damage/cell cycle arrest markers examined (p16, p21, and p53) and SASP components IL-6, PAI-1, and CD11b were increased in the brains of 7–9-month-old hTau mice as compared to WT control animals (Fig. 3A). Consistent with these observations, SA-β-gal activity was significantly increased in hippocampal CA1 and CA2 in hTau mice as compared to controls (Fig. 3B–C). These data indicate that cellular senescence is robustly activated in the brains of hTau mice at 7–9 months of age, indicating that senescent cells accumulate in the early stages of AD-like tauopathy in this model.
Fig. 3.
Markers of cellular senescence are increased in brains of hTau mice modeling tauopathy of AD. A Levels of cell damage/cell cycle arrest markers p16, p21, and p53 and of components of the senescence-associated secretory phenotype (SASP; IL-6, IL-1α, PAI-1, MCP-1, CD11-b, and TNFα) in cortical tissues, including hippocampus, of 7–9-month-old hTau and non-transgenic littermate mice (WT) measured with qRT-PCR. Transcript levels were normalized to those of GAPDH and are shown as fold changes compared to the WT group. WT, n = 4; hTau, n = 7; *P < 0.05; **P < 0.01; ***P < 0.001, and ****P < 0.0001 as a result of unpaired Student’s t test between the groups indicated. WT animals were 9–19-month-old; hTau were 7–9-month-old. B–C Quantitative analyses of SA-βgal activity, measured as percent red fluorescent area (indicating SA-βgal activity, panel B, representative images) normalized (panel C, quantitative analyses of data in B) to blue fluorescent area as a measure of total cell number in hippocampi of WT and P301S(PS19) mice. Scale bar, 500 µM. *P < 0.05, unpaired Student’s t test. WT, n = 4 aged 14–19 months; hTau, n = 7 aged 7–9 months. Data are means ± SEM
3xTg-AD and Tg2576 mice
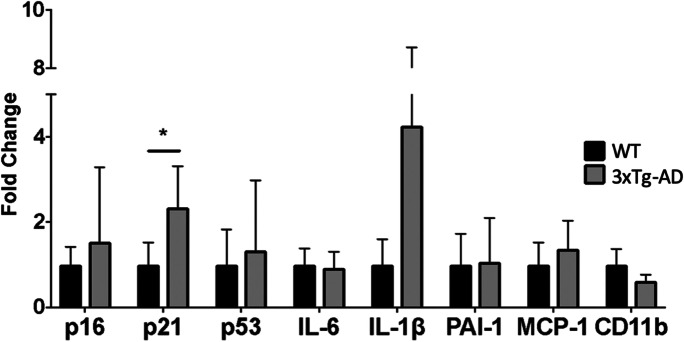
Because we found substantially elevated levels of markers of cellular senescence in brains of hTau and P301S(PS19) mice modeling tauopathy (Figs. 2 and 3), we next sought to define the impact of concomitant tauopathy and amyloidopathy, and of amyloidopathy alone, on brain cellular senescence in AD model mice. To define the impact of concomitant tauopathy and amyloidopathy on brain cellular senescence, we measured cellular senescence markers in 3xTg-AD mice that express a human APP transgene carrying the Swedish mutation together with a human MAPT transgene carrying the P301L mutation in a mouse presenilin-1 M146V knock-in background [27]. Aβ plaques form at 3–5 months of age in 3xTg-AD mice, while neurofibrillary tangles are evident at 12 months of age [27]. In the cohort that we used for these experiments, cognitive deficits emerged at 12 months of age (S. Oddo, personal communication). Our studies revealed a modest but significant increase in p21 levels but no significant change in SASP components in 12-month-old 3xTg-AD mice as compared to control animals (Fig. 4). Taken together, our data suggest that cellular senescence is absent in the brains of 12-month-old 3xTg-AD mice at 12 months of age.
Fig. 4.
p21 but not other markers of cell damage/cell cycle arrest or SASP are increased in the brains of 3xTg-AD mice modeling amyloidopathy and non-AD tauopathy. Levels of cell damage/cell cycle arrest markers p16, p21, and p53 and of components of the senescence-associated secretory phenotype (SASP; IL-6, IL-1α, PAI-1, MCP-1 and CD11-b) in cortical tissues of 12-month-old 3xTg-AD and non-transgenic mice (WT) measured with qRT-PCR. Transcript levels were normalized to those of GAPDH and are shown as fold changes compared to the WT group. Scale bar, 500 µM. *P < 0.05 as a result of unpaired Student’s t test between the groups indicated. WT, n = 5, 3xTg-AD, n = 5 at 12 months of age. Data are means ± SEM
Next, to define whether senescence develops in the brains undergoing amyloidosis in the absence of concomitant tauopathy, we measured markers of cellular senescence in Tg2576 mice that express a human APP transgene carrying the FAD Swedish mutation [30] and show cognitive impairment at 7–8 months of age [47] and substantial deposition of dense Aβ plaques at 9 months of age, but no neuronal loss [48, 49]. We found no changes in the levels of cell damage/cell cycle arrest markers but observed significant increases in levels of SASP components IL-6 and IL-1β and in SA-β-gal staining in the brains of 19-month-old Tg2576 mice at very late stages of AD-like disease (Fig. 5). Increases in IL-6 and IL-1β mRNA abundance confirm prior reports of widespread inflammation in Tg2576 brain [50, 51]. Because we did not observe any changes in markers of cell cycle arrest in the brains of Tg2576 mice at late stages of AD-like progression, however, our data indicate that cellular senescence is absent in the brains of Tg2576 mice at late stages of AD-like disease.
Fig. 5.
Increased inflammatory cytokines, but no change in markers of cell damage/cell cycle arrest in brains of Tg2576 mice. A Levels of cell damage/cell cycle arrest markers p16, p21, and p53 and of components of the senescence-associated secretory phenotype (SASP; IL-6, IL-1α, TNFα PAI-1, MCP-1, PAI-1, and CD11-b) in mRNA fractions isolated from vasculature-depleted brain homogenates of 19-month-old Tg2576 and non-transgenic (WT) mice measured with qRT-PCR. Transcript levels were normalized to those of GAPDH and are shown as fold changes compared to the WT group. WT, n = 6; Tg2576, n = 6; *P < 0.05 and ****P < 0.0001 as a result of unpaired Student’s t test between the groups indicated. B Quantitative analyses of SA-βgal activity, measured as percent red fluorescent area (indicating SA-βgal activity, panel B, representative images) normalized (panel C, quantitative analyses of data in B) to blue fluorescent area as a measure of total cell number in hippocampi of WT and Tg2576 mice. *P < 0.05, unpaired Student’s t test. WT, n = 6; Tg2576, n = 6, at 19 months of age. Data are means ± SEM
Discussion
Accumulating evidence indicates that cellular senescence is involved in the etiology of age-related diseases such as Alzheimer’s disease [52, 53]. Defining the impact of accumulation of senescent cells in the brain and their removal is of paramount importance for our understanding of AD and AD-related dementias [54]. A variety of mouse models of AD exist that show substantial differences in the development of various AD-related pathologies as well as in their severity and time of emergence. While it has been reported that a model of primary tauopathy displays features of cellular senescence [22, 55], to what extent different AD models recapitulate cellular senescence in AD is unclear. This is an important question, because the use of adequate models will be critical in studies that will clarify the role of brain cellular senescence in AD.
In the present studies, we sought to measure the activation of cellular senescence in brain tissues from a set of diverse mouse models of AD tauopathy at the end of the pre-symptomatic period that precedes the onset of disease, defined as the earliest time that cognitive deficits or neuronal loss can be detected in each model. We also measured markers of cellular senescence in a model of concomitant AD amyloidopathy and tauopathy, and in a model of AD amyloidopathy, at late stages of AD-like disease.
We show that markers of senescence were increased, albeit to different degrees, in two mouse models of tauopathy (hTau and P301S mice) at the time of onset of AD-like disease, indicating that accumulation of senescent cells preceded the onset of disease in these models. We found increased markers of senescence in the brains of P301S mice by 10 months of age but not at 7 months, in contrast to previous studies [22]. Consistent with this observation, we found that P301S mice in our cohorts do not show decline in spatial memory as measured by Morris water maze (MWM) or in contextual memory measured with the fear conditioning paradigm at 7 or 8 months of age, suggesting that the onset of cognitive impairment occurs after 8 months of age in P301S mice (Figure S1). Later times of emergence of cognitive deficits in P301S mice, however, have been reported previously [43, 44] [43, 45]. Taken together, these data suggest that accumulation of senescent cells in the brain may play a role in the development of AD-like disease in the P301S model (Fig. 2).
In contrast, hTau mice modeling tauopathy of AD showed a robust increase in markers of cellular senescence at 7–9 months of age, which precedes the defined onset of cognitive impairment and neuronal loss in this model by approximately 3–5 months. Because hTau mice overexpress WT human tau in a null mouse tau background [30], these data strongly suggest that expression of all 6 isoforms of WT human tau, as in human AD, potently triggers cellular senescence in brain during the early stages of AD-like progression.
In contrast to our observations in models of tauopathy, we did not find evidence of cellular senescence in the brains of 3xTg-AD mice modeling amyloidopathy together with mutant tau-associated tauopathy or in the brains of Tg2576 mice that model AD amyloidopathy exclusively. Brain tissues of 3xTg-AD mice that we examined were from animals that preceded the shift in phenotype that was recently reported for this model [56], and were at moderate stages of AD-like progression. In the case of Tg2576 mice, however, we used tissues from animals at late stages of AD-like disease. It is currently unknown how long individual senescent cells persist during aging or during disease progression [1, 57]. Thus, although we have not found evidence of cellular senescence in brains of 3xTg-AD or Tg2576 mice, whether cellular senescence develops in brain during the early pre-symptomatic stages of AD-like disease in these models cannot be unequivocally defined from our data since it is possible that senescent cells accumulate early in disease progression but either die or are cleared from the brain at later stages of disease. Additional studies at earlier stages of AD-like progression in 3xTg-AD and Tg2576 mice are necessary to define the potential impact of cellular senescence in these models of AD.
Because the elucidation of the role of brain cellular senescence in AD will require the use of AD models that recapitulate this feature of the disease, the present studies aimed to ascertain the incidence of cellular senescence in the brains of commonly used models of AD. Our data singled out hTau and P301S(PS19) mice as models of tauopathy where brain cellular senescence is detected during the pre-symptomatic phase of disease and may thus play a role in disease etiology. hTau mice may be useful to define the impact of brain cellular senescence in AD, while P301S(PS19) mice may provide a suitable model to study the impact of brain cellular senescence in neurodegenerative tauopathies associated with mutated tau. While we did not find evidence of cellular senescence in the brains of P301L, 3xTg-AD, and Tg2576 mice, it is possible that cellular senescence is transient or emerges at very late stages during AD-like progression in these models. Our studies, however, were not designed to address these hypotheses.
Prior studies in P301S mice [22] suggested that astrocytes are a major cell type undergoing senescence in P301S mice. Although our studies did not address cell type specificity of the senescence response, it is possible that the robust upregulation of cellular senescence markers that we observed in hTau mice is at least in part astrocyte-driven. The same may be possible for hTau mice. That astrocytes may be a major cell type undergoing senescence in AD was suggested by prior studies [19, 20]. Albeit quiescent in non-diseased states, astrocytes also retain the capacity to enter the cell cycle in conditions of injury or disease; thus, they can exist in a state that is permissive to the activation of senescence.
Overall, our data indicate that hTau and P301S(PS19) mice may be useful tools for the study of cellular senescence in AD and AD-related dementias. Future studies in AD and in models of AD will define the specific brain cell types that undergo senescence as well as the mechanisms that drive its activation and its time of emergence with respect to disease progression. This knowledge will inform urgently needed interventions to treat AD and other dementias.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
These studies were supported by NIH/NIA RF1AG057964-01 to VP and VG; Merit Review Award 5I0 1BX002211-05A1 from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service, 1RF1AG068283-01 the Robert L. Bailey and daughter Lisa K. Bailey Alzheimer’s Fund in memory of Jo Nell Bailey to VG; and a William & Ella Owens Medical Research Foundation Grant, the San Antonio Medical Foundation, and the JMR Barker Foundation to VG, and Diversity Supplement RF1AG057964-01 to RR. These studies were also supported by an award to VG through the NCATS/NIH Clinical and Translational Science Award grant UL1TR002645. AOD was’supported by NIA Training Grant T32AG021890.
Declarations
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors Angela O. Dorigatti and Ruben Riordan are contributed equally.
Contributor Information
Veronica Galvan, Email: veronica-galvanhart@ouhsc.edu.
Viviana I. Perez, Email: viviana.perez@oregonstate.edu
References
- 1.Childs BG, et al. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (review) Mol Med Rep. 2016;13(4):3391–3396. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song, P., J. An, and M.H. Zou, Immune clearance of senescent cells to combat ageing and chronic diseases. Cells, 2020. 9(3). [DOI] [PMC free article] [PubMed]
- 5.Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Invest. 2017;127(10):3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 8.Coppe JP, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anerillas C, Abdelmohsen K, Gorospe M. Regulation of senescence traits by MAPKs. Geroscience. 2020;42(2):397–408. doi: 10.1007/s11357-020-00183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes-Paciencia S, et al. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22. doi: 10.1016/j.cyto.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Prata L, et al. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin Immunol. 2018;40:101275. doi: 10.1016/j.smim.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon OH, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lok K, et al. Characterization of the APP/PS1 mouse model of Alzheimer’s disease in senescence accelerated background. Neurosci Lett. 2013;557 Pt B:84–9. doi: 10.1016/j.neulet.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 15.Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology. 2004;5(1):1–10. doi: 10.1023/B:BGEN.0000017682.96395.10. [DOI] [PubMed] [Google Scholar]
- 16.Pedro de Magalhães J. From cells to ageing: a review of models and mechanisms of cellular senescence and their impact on human ageing. Experimental Cell Research. 2004;300(1):1–10. doi: 10.1016/j.yexcr.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Prieto, L.I., S.I. Graves, and D.J. Baker, Insights from in vivo studies of cellular senescence. Cells, 2020. 9(4). [DOI] [PMC free article] [PubMed]
- 18.Yousefzadeh MJ, et al. Mouse models of accelerated cellular senescence. Methods Mol Biol. 2019;1896:203–230. doi: 10.1007/978-1-4939-8931-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat, R., et al., Astrocyte senescence as a component of Alzheimer’s disease. PLoS One, 2012. 7(9): p. e45069. [DOI] [PMC free article] [PubMed]
- 20.Cohen J, Torres C. Astrocyte senescence: evidence and significance. Aging Cell. 2019;18(3):e12937. doi: 10.1111/acel.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P, et al. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019;22(5):719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussian TJ, et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562(7728):578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadigh-Eteghad S, et al. Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Princ Pract. 2015;24(1):1–10. doi: 10.1159/000369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 27.Oddo S, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 28.Lewis J, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 29.Jankowsky JL, Zheng H. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol Neurodegener. 2017;12(1):89. doi: 10.1186/s13024-017-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall AM, Roberson ED. Mouse models of Alzheimer’s disease. Brain Res Bull. 2012;88(1):3–12. doi: 10.1016/j.brainresbull.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 32.Chen XQ, Mobley WC. Alzheimer disease pathogenesis: insights from molecular and cellular biology studies of oligomeric abeta and Tau species. Front Neurosci. 2019;13:659. doi: 10.3389/fnins.2019.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger Z, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27(14):3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsia AY, et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeder S, et al. Oligomeric tau-targeted immunotherapy in Tg4510 mice. Alzheimers Res Ther. 2017;9(1):46. doi: 10.1186/s13195-017-0274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noren Hooten, N. and M.K. Evans, Techniques to induce and quantify cellular senescence. J Vis Exp, 2017(123). [DOI] [PMC free article] [PubMed]
- 37.Andorfer C, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86(3):582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- 38.Yoshiyama Y, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Lewis J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 40.Zamzow DR, et al. Higher levels of phosphorylated Y1472 on GluN2B subunits in the frontal cortex of aged mice are associated with good spatial reference memory, but not cognitive flexibility. Age (Dordr) 2016;38(3):50. doi: 10.1007/s11357-016-9913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent BA, et al. Longitudinal evaluation of Tau-P301L transgenic mice reveals no cognitive impairments at 17 months of age. Brain Behav. 2018;8(1):e00896. doi: 10.1002/brb3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi H, et al. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS One. 2011;6(6):e21050. doi: 10.1371/journal.pone.0021050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang B, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32(11):3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Min SW, et al. SIRT1 deacetylates Tau and reduces pathogenic Tau spread in a mouse model of tauopathy. J Neurosci. 2018;38(15):3680–3688. doi: 10.1523/JNEUROSCI.2369-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iba M, et al. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci. 2013;33(3):1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polydoro M, et al. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci. 2009;29(34):10741–10749. doi: 10.1523/JNEUROSCI.1065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez CM, et al. Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2010;30(7):2442–2453. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irizarry MC, et al. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56(9):965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Liu J, Suo WZ. GRK5 deficiency exaggerates inflammatory changes in TgAPPsw mice. J Neuroinflammation. 2008;5:24. doi: 10.1186/1742-2094-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30(1):121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saez-Atienzar S, Masliah E. Author Correction: Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nat Rev Neurosci. 2020;21(10):587. doi: 10.1038/s41583-020-0366-3. [DOI] [PubMed] [Google Scholar]
- 53.DiBattista AM, Sierra F, Masliah E. NIA workshop on senescence in brain aging and Alzheimer’s disease and its related dementias. Geroscience. 2020;42(2):389–396. doi: 10.1007/s11357-020-00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):18–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musi N, et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17(6):e12840. doi: 10.1111/acel.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belfiore R, et al. Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell. 2019;18(1):e12873. doi: 10.1111/acel.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker DJ, Sedivy JM. Probing the depths of cellular senescence. J Cell Biol. 2013;202(1):11–13. doi: 10.1083/jcb.201305155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.