Abstract
The liver contribution to the biological network underlying physical frailty in aging is underestimated. How best to measure this contribution magnitude and impact on health risk trajectories in frail individuals is not yet entirely clear. We analyzed the association of a novel liver frailty phenotype with the risk of death in older participants of the Salus in Apulia Study cohort. Clinical and physical examination, routine biomarkers, medical history, and anthropometry were analyzed in 1929 older adults (65 +). Physical frailty was classified by Cardiovascular Health Study criteria, and liver fibrosis risk by fibrosis-4 (FIB-4). The liver frailty phenotype was defined as physical frailty plus high-risk liver fibrosis (score > 2.67). Physical frailty, high-risk liver fibrosis, and liver frailty subjects were compared to subjects without these conditions (non-frail). Proportional Cox regression tested the adjusted association between liver frailty and all-cause mortality for each category. The liver frailty prevalence was relatively low (3.8%), but higher in men (58.1%). Compared to non-frail older subjects, liver frailty subjects were significantly older (effect size (ES) − 1.11, 95% confidence interval (CI) − 1.35 to − 0.87), with a lower education (ES 0.48, 95%CI 0.24 to 0.71) and higher multimorbidity (ES 15.81, 95%CI 4.20 to 27.41). Cox multivariate analyses showed a two-fold increased risk of overall mortality (hazard ratio 2.09, 95%CI 1.16–3.74) even after the adjustment for age, sex, education, and alcohol consumption. The liver frailty phenotype runs twice the risk of overall mortality compared with the non-frail population. This clinical tool, validated in a Southern Italian population, is based on simple sets of measures that can conveniently be assessed also in the primary care setting.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00434-x.
Keywords: Liver frailty, Aging phenotype, Primary care, Older population, Mortality
Introduction
Trends in socio-economic development, along with advances in medical care, have prolonged human life expectancy but also raised concerns about health care, and especially the management of aging to meet the projected health demand burden [1]. Research efforts are attempting to chart clear directions in preventive medicine to anticipate the primary adverse health-related outcomes of aging populations. Along these lines, the global Burden of Disease (GBD) epidemiological overview works well as a filter for intervention areas. Based on the latest register, multimorbidity and polypharmacy affect more than half of the aging population, and chronic diseases top the list of health burdens to be faced [2].
Of note, liver disease has accounted for the most alarming epidemiological data among non-communicable conditions over the last decade [3]. Especially in Western countries, non-alcoholic fatty liver disease (NAFLD) contributes significantly to liver mortality and morbidity. It is likely that unhealthy lifestyle changes, equally involved in increasing metabolic overeating disorders such as obesity and diabetes mellitus, may have contributed to this situation. In addition, advancing age itself causes an increased vulnerability to acute liver damage and susceptibility to fibrotic responses. Age is a prognostic determinant of several liver disease pathways, including NAFLD, alcoholic liver disease, hepatitis C, and liver transplantation [4].
Worsening the adverse life trajectories overburdening public health care, older populations experience a decline in the physiological reserves of several systems, leading to the homeostatic imbalance known as frailty [5]. This intermediate status of the aging process can be defined as either a unidimensional entity, based on physical or biological factors according to the construct derived from the Cardiovascular Health Study (CHS) [5], or as a non-specific multidimensional status, based on a deficit accumulation model with interconnected domains [6]. Clinically speaking, frailty is a distinct, multifaceted construct that consists specifically, but not exclusively, of functional decline, a loss of physical condition associated with malnutrition, impaired cognition, poor walking speed, and muscle strength [7–9]. Therefore, given the multidimensional nature of frailty, both clinicians and researchers must consider different domains, including physical, cognitive, social, or biopsychosocial, and nutritional frailty phenotypes, although a universal consensus on this issue is lacking [5, 7–9]. All these domains embody an intriguing biological pathway that delineates a worrying aging phenotype now included among the geriatric giants to be faced. Increased risks of falls, disability, institutionalization, hospitalization, dementia, and mortality are typical hallmarks of the destiny of a frail older adult. A recent meta-analysis with population level data estimated a worldwide prevalence of 12% frail older adults, applying using the physical frailty phenotype of the CHS [10], and this prevalence rose to 14.8% in the Southern Italian population [11].
At present, frailty is an ever-expanding concept, and its multidimensional nature opens a large window onto research into many underlying pathophysiological pathways. Liver health in the aging population is an intriguing new field: 17 to 43% of patients with advanced liver disease awaiting liver transplantation are frail [12]. This points to the existence of common underlying pathophysiological pathways, leading to a multidimensional phenotype that places an even greater burden on public healthcare. Despite this valuable prognostic information, frailty is not routinely acknowledged in older patients living with chronic liver disease. Especially in the primary care setting, it is becoming ever more important to account for subclinical and high-risk phenotypes of liver damage in order to stratify by risk of adverse outcomes. Capturing this feature is critical in addressing the insidious challenge of liver aging, especially when considering the synergistic role played by a risk cluster such as alcohol overuse and polypharmacy in shaping the physiologic trajectories of declining proliferative and metabolic functions. In the present study, we hypothesized a novel liver frailty phenotype, assessed by combining the fibrosis-4 (FIB-4) liver fibrosis risk assessment tool with the CHS criteria for physical frailty. We tested its association and predictive power on overall mortality in older participants of the Salus in the Apulia cohort study, comparing it with any major confounding condition over a 9-year observation period.
Methods
Study population
Participants in the present study had been recruited from the electoral rolls of Castellana Grotte (Apulia, Southern Italy). The sampling framework was the health registry office list until 31st December 2011, which included 19,675 subjects, 4021 aged 65 + years. All the subjects had been recruited to the “Salus in Apulia Study,” a public health initiative funded by the Italian Ministry of Health and Apulia Regional Government and conducted at the National Institute of Gastroenterology IRCCS “Saverio De Bellis” Research Hospital. The mortality data were obtained from the Regione Puglia Electronic Health Records, updated until May 31, 2020. This study used data on a subpopulation of the Salus in Apulia Study including 1929 older subjects who underwent all the assessments. All participants signed informed consent before the examinations. The study was approved by the IRB of the head institution, the National Institute of Gastroenterology and Research Hospital “Saverio de Bellis” in Castellana Grotte (Apulia, Southern Italy). The study met the principles of the Helsinki Declaration and adhered to the “Standards for Reporting Diagnostic Accuracy Studies” (STARD) guidelines (http://www.stard-statement.org/) and the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines.
Clinical and laboratory examination
Education was defined by the total years of schooling. For the blood tests, a blood sample was collected in the morning after overnight fasting to measure the levels of fasting blood glucose, glycated haemoglobin (HbA1c), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides using standard automated enzymatic colorimetric methods (AutoMate 2550, Beckmann Coulter, Brea, Ca, US) under strict quality control. LDL cholesterol was calculated using the Friedewald equation. Plasma glucose was determined using the glucose oxidase method (Sclavus, Siena, Italy). Blood cell count was determined by a Coulter Hematology analyzer (Beckman–Coulter, Brea, CA). The clinical evaluation included extemporaneous ambulatory systolic blood pressure and diastolic blood pressure, determined in a sitting position after at least a 10-min rest, at least three different times, using the OMRON M6 automatic blood pressure monitor. Alanine amino transferase (ALT), aspartate amino transferase (AST), and gamma glutamyl transferase (GGT) were measured using automatic enzyme procedures. Serum insulin concentrations were measured by radioimmunoassay (Behring, Scop-pito, Italy), and serum 25(OH) vitamin D was quantified by a chemiluminescence method (Diasorin Inc, Stillwater, OK, USA); all samples were analyzed in duplicate. Serum high-sensitivity C-reactive protein (CRP) was assayed using a latex particle-enhanced immunoturbidimetric assay (Kamiya Biomedical Company, Seattle, WA) (reference range: 0–5.5 mg/L; interassay coefficient of variation: 4.5%). Serum interleukin (IL)-6 and tumor growth factor- (TNF-α) were assayed using the quantitative sandwich enzyme technique, ELISA (QuantiKine High Sensitivity Kit, R&D Systems, Minneapolis, MN, and QuantiGlo immunoassay from R&D Systems, Minneapolis, MN). The interassay coefficient of variations was 11.7% for IL-6 and 13.0% for TNF-α. Inflammatory marker assays were analyzed at the same laboratory following strict quality control procedures.
Multimorbidity and non-communicable diseases
Multimorbidity was defined as the co-presence at the baseline examination of two or more major non-communicable diseases, i.e., diabetes mellitus, hypertension, peripheral age-related hearing loss (ARHL), vision loss, cognitive impairment (as defined by the Mini-Mental State Examination, MMSE) [13], asthma, chronic obstructive pulmonary disease (COPD), and late-life depression (LLD), as described elsewhere [8, 11, 14].
Anthropometric assessment
Clinical procedures were carried out under the supervision of a senior nutritionist (RZ). All anthropometric measurements were taken with participants dressed in lightweight clothing and without shoes. Variables were all collected at the same time between 7:00 and 10:00 a.m. in the morning, after overnight fasting. Height was measured to the nearest 0.5 cm using a wall-mounted stadiometer (Seca 711; Seca, Hamburg, Germany). Body weight was determined to the nearest 0.1 kg using a calibrated balance beam scale (Seca 711; Seca, Hamburg, Germany). The body mass index (BMI) was calculated by dividing body weight (kg) by the square of height (m2) and classified according to World Health Organization criteria [15]. Waist circumference (WC) was measured at the narrowest part of the abdomen or in the area between the tenth rib and the iliac crest (minimum circumference).
Alcohol intake assessment
Dietary habits over the previous year, assessed by a self-administered Food Frequency Questionnaire (FFQ) as described elsewhere [16], were extracted from the Salus in Apulia dataset and used to derive data on alcohol consumption of all participants. Daily alcohol intake estimates were obtained using Italian food composition tables [17]. A threshold of 20 g/day in females and 30 g/day in males was applied, according to American and European guidelines for daily alcohol consumption [18, 19].
Non-invasive liver fibrosis assessment
A liver fibrosis score was calculated according to the pre-existing FIB-4 equation including age, AST, ALT, and platelets [20]. Importantly, as suggested by McPherson and colleagues [21], 3 age-specific cut-off points for subjects aged 65 + years were used to assess the liver fibrosis risk (low-risk: < 2, intermediate-risk: 2–2.66, high-risk: > 2.67). We applied the new intermediate cut-off for subjects aged ≥ 65 years, while maintaining the cut-off for the highest risk group, given its proven effectiveness in improving specificity for advanced fibrosis, effectively controlling the false positive rate, and avoiding an unfavorable increase in the false-negative rate of the test. Only the high-risk (> 2.67) group subjects were considered when operationalizing the liver frailty phenotype, as described in detail below.
Assessment of physical frailty and liver frailty
Assessment of the physical frailty status was performed using the operational CHS criteria, slightly modified for the present study, namely, positivity to three or more of the following components: weight loss, exhaustion, low levels of physical activity, weakness, and slow movement, as detailed elsewhere [11]. The whole sample was subdivided into two different groups based on the number of physical frailty components. Subjects who met three or more criteria were included in the frailty group; all the others were classified as non-frail subjects. Liver frailty was defined as the co-presence of physical frailty and a high-risk liver fibrosis score, exceeding 2.67 points.
Statistical analysis
The whole sample was subdivided according to the exposure variables. Three categories of FIB-4 scoring (low-risk or “fibrosis excluded” if ≤ 2; intermediate-risk or “needs further investigation” if ranging between 2 and 2.67; high-risk or “fibrosis likely” if above 2.67) were applied to describe clinical and functional differences in terms of frequency and associations between groups. Normal distributions of quantitative variables were tested using the Kolmogorov–Smirnov test. Data are reported as mean ± standard deviations (M ± SD) for continuous measures and frequency and percentages (%) for all categorical variables. In order to focus on the practical differences between the groups in terms of effect size (ES), a statistical approach based on the null hypothesis significance test (NHST) was not used. Differences in the prevalence of exposure groups (FIB-4 categories), and other categorical variables and their 95% confidence intervals (CI) were calculated and used to assess important differences in the magnitude of association, i.e., ES. Differences between continuous variables were calculated using Cohen’s d difference between means, Hedge’s g when the assumption of a similar variance was violated, and their ES using the confidence intervals [22]. To study the time between study entry and a subsequent event, the non-parametric Kaplan–Meier method was used to explore the survival probability, and the log-rank test was applied to evaluate the equality of survival among categories. Three nested Cox multivariable models were used to estimate the hazard ratio (HR) of death for principal variables (physical frailty and higher FIB-4) since this also allows estimation of the HR of survival for an individual, given prognostic variables (measured continuously or categorically). The Cox proportional hazard model was fitted to the data. Cox model assumptions were applied by plotting the Martingale residuals against continuous covariates to assess the functional form of covariates (linearity assumption) and using statistical tests based on the scaled Schoenfeld residuals (cox.zph in survival package) to evaluate proportional hazards (PH) assumptions. All well-fitting models were assessed using Akaike Information Criteria (AIC) and the Bayesian information criterion (BIC). Risk estimators were expressed as HR and 95% CI. The multicollinearity of models was evaluated through the variance inflation factor (VIF), using the score of 2 as a cut-off for exclusion. The Cox model’s prediction power was assessed by using the C-statistics score (concordance), to quantify the capacity of the estimated risk score in discriminating among subjects with different event times. Confounders were selected among the retained factors related both to exposure (liver frailty) and to overall mortality factors such as age, sex, education, and alcohol consumption [23].
Results
The examined population (N = 1,929, aged 73.56 ± 6.30 years) appeared to be sex-balanced, (males 50.5% n = 974). Table 1 summarizes the main population differences according to the health conditions, i.e., normal/non-frail (no physical frailty nor liver fibrosis risk), physical frailty, high-risk liver fibrosis (FIB-4 > 2.67), and both (subjects with physical frailty plus high-risk liver fibrosis, i.e., liver frailty phenotype). A slight majority of the population (66.5%, N = 1282) was classified as normal, neither frail nor at high-risk of liver fibrosis. There was an 11% prevalence of physical frailty and 3.8% of high-risk liver fibrosis plus physical frailty, i.e., liver frailty. Supplementary Table 1 shows effect size comparisons between different conditions. Age showed a clear upward trend from the normal to the liver frailty phenotype (ES − 1.11, 95%CI − 1.35 to − 0.87). Alcohol consumption, smoking habit, and BMI showed no significant differences between groups, unlike sex (ES − 10.02, 95%CI − 15.78 to − 4.25 from the normal to the high-risk liver fibrosis group). While physical frailty was significantly more prevalent in women (57.5% versus 42.5%), high-risk liver fibrosis was higher in men (59% versus 41%), and ultimately the liver frailty phenotype was significantly more prevalent in men (58.1% versus 41.9%). Education showed a downtrend from the normal to the liver frailty subjects, who had the least schooling (ES 0.48, 95%CI 0.24 to 0.71 from the normal/non-frail to the liver frailty phenotype).
Table 1.
Sociodemographic, clinical variables, multimorbidity in non-frail, physical frailty, liver fibrosis, and liver frailty older subjects. The Salus in Apulia Study (n = 1929)
| Non-frail | Physical frailty | Liver fibrosis | Liver frailty | |
|---|---|---|---|---|
| Prevalences (%) | 1282 (66.50) | 212 (11.00) | 361 (18.70) | 74 (3.80) |
| Sociodemographic variables | ||||
| Age (years) | 72.37 ± 5.78a | 74.14 ± 6.27 | 76.35 ± 6.54 | 78.85 ± 6.59 |
| BMI (Kg/m2) | 28.38 ± 4.77 | 28.58 ± 5.15 | 28.37 ± 5.02 | 29.42 ± 5.3 |
| Sex | ||||
| Male | 628 (49.00) | 90 (42.50) | 213 (59.00) | 43 (58.10) |
| Female | 654 (51.00) | 122 (57.50) | 148 (41.00) | 31 (41.90) |
| Education (years) | 7.2 ± 3.84 | 6.62 ± 4.12 | 6.51 ± 3.57 | 5.39 ± 3.41 |
| Smoking | 111 (8.70) | 13 (6.10) | 23 (6.40) | 4 (5.40) |
| Alcohol consumptiona | 48 (5.10) | 6 (3.90) | 16 (6.20) | 1 (1.90) |
| Metabolic biomarkers | ||||
| Fasting blood glucose (mg/dl) | 105.09 ± 27.66 | 109.1 ± 33.3 | 106.84 ± 28.71 | 105.08 ± 22.25 |
| Hb A1c (mmol/mol) | 40.2 ± 10.56 | 41.81 ± 10.73 | 40.93 ± 10.57 | 39.92 ± 8.44 |
| Insulin (UI) | 9.23 ± 6.74 | 9.13 ± 6.51 | 8.11 ± 6.35 | 7.63 ± 4.96 |
| Total cholesterol (mg/dl) | 186.84 ± 37.04 | 183.79 ± 39.13 | 174.67 ± 34.39 | 171.93 ± 39.57 |
| HDL cholesterol (mg/dl) | 49.17 ± 13.19 | 48.21 ± 12.25 | 47.27 ± 12.81 | 46.92 ± 12.92 |
| LDL cholesterol (mg/dl) | 114.82 ± 31.15 | 112.93 ± 34.15 | 107.15 ± 28.58 | 101.97 ± 30.46 |
| Triglycerides (mg/dl) | 108.98 ± 61.08 | 108.58 ± 61.5 | 95.84 ± 60.73 | 107.81 ± 59.76 |
| Systolic blood pressure (mmHg) | 132.69 ± 14.4 | 133.77 ± 14.21 | 133.46 ± 14.39 | 135.41 ± 17.04 |
| Diastolic blood pressure (mmHg) | 78.38 ± 7.62 | 78.49 ± 7.64 | 76.97 ± 8.98 | 77.36 ± 8.33 |
| Inflammatory Biomarkers | ||||
| Interleukin 6 (pg/ml) | 3.68 ± 6.3 | 4.25 ± 6.26 | 4.66 ± 8.34 | 4.09 ± 6.09 |
| Tumor necrosis factor-alpha (pg/ml) | 2.72 ± 3.56 | 2.58 ± 1.61 | 3.17 ± 4.81 | 3.17 ± 2.69 |
| Red blood cells (106 cells/mm3) | 4.82 ± 1.13 | 4.8 ± 0.53 | 4.69 ± 0.56 | 4.72 ± 0.57 |
| Hemoglobin (g/dl) | 13.84 ± 1.44 | 13.76 ± 1.63 | 13.55 ± 1.61 | 13.78 ± 1.56 |
| Platelets (106 cells/mm3) | 233.47 ± 56.26 | 237.15 ± 65.94 | 184.36 ± 53.76 | 182.73 ± 53.07 |
| White blood cells (106 cells/mm3) | 6.2 ± 1.85 | 6.43 ± 2.04 | 5.73 ± 1.53 | 5.92 ± 2.65 |
| C reactive protein (mg/l) | 0.59 ± 0.88 | 0.58 ± 0.93 | 0.57 ± 0.73 | 0.68 ± 0.82 |
| Vitamin D (ng/ml) | 38.59 ± 17.85 | 40.79 ± 17.44 | 39.43 ± 17.37 | 40.14 ± 17.49 |
| Liver biomarkers | ||||
| AST (U/L) | 24.14 ± 10.2 | 24.66 ± 7.9 | 56.44 ± 45.05 | 60.73 ± 51.19 |
| ALT (U/L) | 24.98 ± 18.45 | 26.92 ± 21.06 | 27.56 ± 26.26 | 26.89 ± 20.28 |
| GGT (U/L) | 29.57 ± 32.34 | 31.56 ± 32.91 | 46.37 ± 43.9 | 47.27 ± 44.87 |
| FIB-4 | 1.64 ± 0.48 | 1.68 ± 0.51 | 4.74 ± 3.09 | 5.29 ± 3.72 |
| Multimorbidity assessment | ||||
| Diabetes mellitus | 149 (11.60) | 38 (17.90) | 47 (13.00) | 11 (14.90) |
| Hypertension | 886 (69.10) | 159 (75.00) | 254 (70.50) | 48 (64.90) |
| Age-related hearing loss | 249 (19.40) | 51 (24.10) | 93 (25.80) | 32 (43.20) |
| Late-life depression | 90 (7.90) | 16 (8.60) | 28 (8.50) | 6 (8.70) |
| Vision loss | 43 (3.40) | 9 (4.20) | 18 (5.00) | 2 (2.70) |
| Chronic obstructive pulmonary disease | 232 (18.10) | 42 (19.80) | 54 (15.00) | 15 (20.30) |
| Asthma | 118 (9.20) | 28 (13.20) | 27 (7.50) | 5 (6.80) |
| Multimorbidity score | 525 (41.00) | 113 (53.30) | 166 (46.00) | 42 (56.80) |
| Time of observation (months) | 56.87 ± 22.41 | 48.49 ± 18.69 | 56.58 ± 22.05 | 51.89 ± 24.43 |
All data are shown as mean ± SD for continuous variables and as n (%) for proportions. Significance shown in bold
BMI body mass index, HbA1c glycated hemoglobin, AST aspartate amino transferase, ALT alanine amino transferase, GGT gamma glutamyl transferase, FIB-4 fibrosis-4 score
aAlcohol consumption Yes if > 50 g/day or 18,250 g/year (males), and > 25 g/day or 9125 g/year (females)
As regard metabolic biomarkers, the glycemic profile follows a consistent trend according to the worsening pathological condition. HbA1c levels were lower, on average, in the liver frailty phenotype compared to the normal/non-frail group (ES 0.02, 95%CI − 0.23 to 0.23), although the difference was not significant. Blood insulin levels showed a similar downward trend, with significant differences between the normal group and the high-risk liver fibrosis (ES 0.17, 95%CI 0.05 to 0.29) or the liver frailty group (ES 0.24, 95%CI 0.01 to 0.48). The same significant downward trajectories were observed for total cholesterol (ES 0.33, 95%CI 0.22 to 0.45 from the normal to the high-risk liver fibrosis group, and ES 0.40, 95%CI 0.17 to 0.64 from the normal to the liver frailty group), HDL-cholesterol (ES 0.14, 95%CI 0.03 to 0.26 from the normal to the high-risk liver fibrosis group), and LDL-cholesterol (ES 0.25, 95%CI 0.13 to 0.37 from the normal to the high-risk liver fibrosis group, and ES 0.41, 95%CI 0.18. to 0.65 from the normal to the liver frailty group). Hemoglobin and red blood cells were on average significantly lower in the high-risk liver fibrosis group compared to the normal/non-frail group (ES 0.18, 95%CI 0.06 to 0.30, and ES 0.12, 95%CI 0.01 to 0.24, respectively).
In terms of the inflammatory profile, IL-6 showed higher average values in the high-risk liver fibrosis group, but the difference was not statistically significant (ES − 0.12, 95%CI − 0.24. to 0.01). Similarly, TNF-α levels were on average higher among the high-risk liver fibrosis subjects, but the difference was not statistically significant (ES − 0.12, 95%CI − 0.23 to 0.01). Platelet count was found to drop considerably from the normal to the physical frailty and liver frailty groups (ES 0.88, 95%CI 0.76 to 1.00 and ES 0.90, 95%CI 0.67 to 1.14). In contrast, CRP and white blood cell mean values did not show a trend of significance across groups, despite being higher overall in the liver frailty group compared to normal/non-frail subjects (ES − 0.11, 95%CI − 0.34 to 0.12, and ES − 0.09, 95%CI − 0.32 to 0.15).
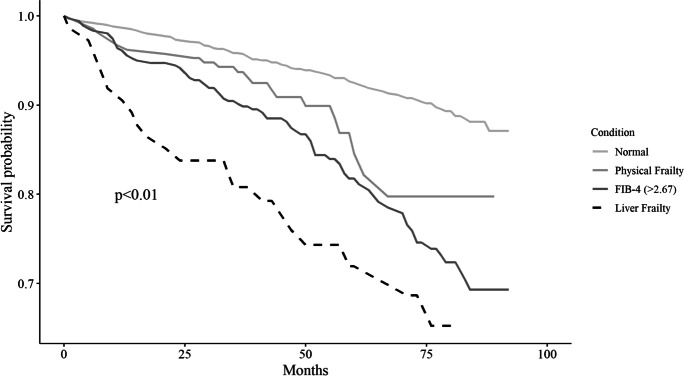
The liver biomarkers, AST and GGT were significantly higher in subjects at high risk for liver fibrosis (ES − 0.72, 95%CI − 0.84 to − 0.60) compared to normal/non-frail subjects (ES − 0.38, 95%CI − 0.50 to − 0.27). The rating of chronic diseases showed, on average, a significantly higher burden of multimorbidity for subjects with liver frailty compared to the normal/non-frail group (ES 15.81, 95%CI 4.20 to 27.41). Combined Kaplan–Meier survival probability analyses of the liver frailty category (Fig. 1) showed that this novel phenotype was significantly related to an increased risk of all-cause mortality.
Fig. 1.
Kaplan Meier survival curves for the four categories: non-frail older subjects, older subjects with physical frailty, older subjects with a high-risk fibrosis-4 (FIB-4) score, and older subjects with physical frailty and a high-risk FIB-4, i.e., the liver frailty phenotype. The Salus in Apulia Study (n = 1929)
Table 2 shows results of Cox survival multi-variable analysis for each category (physical frailty, high-risk liver fibrosis, and liver frailty) adjusted for major confounders, i.e., age, sex, education, and alcohol consumption, as reported in the footnotes. Physical frailty alone was associated with an almost 50% greater risk of death (HR 1.47, 95%CI 0.98 to 2.16), whereas high-risk liver fibrosis, as defined by FIB-4 > 2.67, showed a nearly 70% greater risk of all-cause mortality (HR 1.63, 95%CI 1.23 to 2.17). Thus, including the liver frailty condition within a model mutually adjusted for other conditions and the main confounders was found to be associated with a nearly two-fold increased risk of all-cause mortality as compared to normal condition (HR 2.09, 95%CI 1.16 to 3.74). Furthermore, the fully adjusted model (Model 3) showed a good prediction power measured using the model concordance index (c-statistic 0.732; standard error 0.019). In addition, the standard error of the single covariates of the Cox model defined a marked absence of overfitting.
Table 2.
Multivariable Cox models (unadjusted and adjusted) for all-cause mortality using the three phenotype categories (physical frailty, liver fibrosis, and liver frailty). The Salus in Apulia Study (n = 1929)
| HR | CI 95% | Std. Err | |
|---|---|---|---|
| Model 1 [C statistic:0.723 (std.err:0.019)] | |||
| Physical frailty | 1.47 | 0.98 to 2.16 | 0.20 |
| Age (years) | 1.11 | 1.09 to 1.14 | 0.01 |
| Sex (female) | 0.38 | 0.35 to 0.63 | 0.17 |
| Education (years) | 0.94 | 0.89 to 0.98 | 0.02 |
| Alcohol consumptiona | 0.22 | 0.05 to 0.89 | 0.71 |
| Model 2 [C statistics: 0.757 (std. err:0.018)] | |||
| FIB-4 (> 2.67) | 1.63 | 1.23 to 2.17 | 0.14 |
| Age (years) | 1.10 | 1.08 to 1.13 | 0.01 |
| Sex (female) | 0.49 | 0.37 to 0.66 | 0.14 |
| Education (years) | 0.97 | 0.93 to 1.01 | 0.02 |
| Alcohol consumptiona | 0.21 | 0.05 to 0.85 | 0.03 |
| Model 3 [C statistics: 0.732 (std. err.:0.019)] | |||
| Physical frailty | 1.63 | 0.96 to 2.77 | 0.27 |
| FIB-4 (> 2.67) | 1.70 | 1.18 to 2.45 | 0.18 |
| Liver frailty | 2.09 | 1.16 to 3.74 | 0.29 |
| Age (years) | 1.10 | 1.07 to 1.13 | 0.01 |
| Sex (female) | 0.41 | 0.29 to 0.58 | 0.17 |
| Education (years) | 0.94 | 0.90 to 0.99 | 0.02 |
| Alcohol consumptiona | 0.21 | 0.05 to 0.88 | 0.71 |
Model 1: Results of Cox multi-variable analysis on physical frailty as regressor, corrected for age, sex, education, and alcohol consumption. Model 2: Results of Cox multi-variable analysis on fibrosis-4 (FIB-4) score (> 2.67] as regressor, corrected for age, sex, education, and alcohol consumption. Model 3: Results of Cox multi-variable analysis on physical frailty, FIB-4 (> 2.67] and liver frailty as regressors, corrected for age, sex, education, and alcohol consumption
aAlcohol consumption Yes if > 50 g/day or 18,250 g/year (males), and > 25,g/day or 9125,g/year (females)
Discussion
The present study provided evidence that a phenotype featuring physical frailty plus liver fibrosis risk placed the older population at a nearly two-fold increased risk of all-cause mortality over 9 years of observation. Of note, the liver frailty phenotype was found to trigger a greater effect on overall mortality in both Cox models (equal to its major confounders) and survival analyses.
The operationalization of this novel liver frailty phenotype encompassed assessment of the physical function performance status using CHS criteria to objectively capture the physical frailty construct, together with a liver fibrosis risk assessment algorithm to estimate the degree of liver disease progression using rapid, and effective clinical measures, i.e., the FIB-4 scoring algorithm. The novel liver frailty phenotype can conveniently be translated into a population-based validated clinical tool consisting of two simple sets of measures that can also be assessed by primary care professionals. This is one way to address the challenge of preventive medicine for frail populations; a simple scoring approach would allow further skimming of the frail population, thus identifying high-risk older subjects with chronic liver diseases to be closely followed and monitored.
From a dissemination perspective, 3.8% of our population showed the liver frailty phenotype, among 11% identified as physically frail. Despite the low prevalence of liver frailty, the phenotype has peculiar hallmarks, differentiating this group from the physical frailty group, as shown in the results. Subjects with liver frailty were older, consistently with the biological mechanism underlying that construct, thus corroborating to some extent the internal validity of our data. Males were more likely to show liver frailty, and this finding fits well with the latest National Center for Health Statistics metrics, reporting men to be two-fold more likely to die of chronic liver disease and cirrhosis than women [24]. Presumably, adult females undergo marked switches in their hormonal status, and the drop in estrogens appears to play a protective role for the liver [25]. Furthermore, the lower education observed among older subjects with the liver frailty phenotype is in line with a sizable body of evidence describing a clear link between education and chronic liver disease, i.e., an independent association linking educational level with virus and alcohol-related etiologies [26]. Beyond that, the liver frailty phenotype metabolic profile was marked by significantly lower total and LDL cholesterol levels than those in older subjects with only physical frailty. This finding seems likely justified by the mediating confounding effect of age, consistent with the small number of hypolipidemic medications prescribed in older adults and the data supporting positive changes in the lipid balance during the aging process [27], greatly influenced by a substantial impairment of the synthetic liver function due to parenchymal damage [28]. No less importantly, reduced platelet counts and a significantly greater occurrence of ARHL within the multimorbidity cluster appeared to delineate this phenotype. On one hand, the reduced platelet count may result from thrombocytopenia, which is commonly observed in advanced liver disease, and may be expressed by a decreased thrombopoietin production and accelerated platelet depletion driven by hypersplenism [29]. On the other, a link between ARHL and the physical frailty phenotype complicated by chronic liver disease may be speculated, in view of the liver failure to detoxify gut-derived neuroactive compounds, such as ammonia, promoting neural degeneration. This indicates a form of sensory loss characterized by neurovascular and neurophysiological changes, including dysregulation of cerebral blood flow and disruption of the neurovascular unit and blood–brain barrier, similar to those observed in dementia and age-related cognitive decline [8].
From a pathophysiological perspective, this novel phenotype in aging adults suggests overlapping pathways between physical frailty and a risk of chronic liver disease, and their shared etiologic history strengthens this assumption. Indeed, they share a clustered etiology involving aging, male gender, multimorbidity and/or polypharmacy, oxidative stress, education, genetics, environment, and lifestyle. Moreover, the evidence suggests a clear pattern of co-occurrence, as physical frailty affects about 17–43% of patients with advanced liver disease [12]. Furthermore, since both involve multiple intermediary systems dysfunctions, including neuromuscular, endocrine, immune, and musculoskeletal deficits, potential overlapping mechanisms are conceivable. A chronic state of systemic inflammation, together with a dysfunctional immune system unable to respond adequately to stimuli, has been observed in both frailty and advanced liver disease [30, 31]. The chronic rise in inflammatory cytokines levels, especially TNF-α and IL-6, results in muscle degradation and progressive loss of physical endurance leading to sarcopenia [32]. This state of immune dysfunction is further worsened in chronic liver disease due to the altered hepatic protein synthesis and damage to the reticuloendothelial system, gut-associated lymphoid tissue, and circulating immune cells that contribute to the acquired immunodeficiency and worsen as portal hypertension progresses.
Some potential study limitations must be borne in mind. Firstly, mortality was not attributed to a specific disease, and so we could not analyze the association of the liver frailty phenotype with cause-specific death. Then, although factors related to the specific etiology of chronic liver disease (i.e., alcohol consumption or virus exposure) may influence the observed association with overall mortality, accounting for this was limited to our available data. Thus, we completely adjusted our analyses for alcohol use that yielded no significant changes in risk estimates. In addition, we had no information about viral hepatitis markers. However, the prevalence of viral hepatitis among older rural populations in Southern Italy has been reported to be around 4% [33], and thus likely may have had a limited impact on our results. In addition, information on liver fibrosis status, as detected by transient elastography (FibroScan), was not available. To address this limitation, we used FIB-4 as a functional surrogate, to provide a more accessible screening tool for physicians, especially general practitioners serving the community. The strengths of this study were the long-term prospective observation time, the fairly large sample size, and the generalizability of the results to Southern Mediterranean populations.
In conclusion, multidimensional screening of frail older adults for risks of adverse health-related events in later life, employing simple diagnostic algorithms to predict adverse outcomes, is the best way to act early. The present novel physical frailty and high-risk liver fibrosis phenotype places the older population at a nearly two-fold increased risk of overall mortality over 4 years. The operationalization of this novel frailty phenotype translates to the clinical screening level, offering potential scenarios of application in order to more promptly address the preventive medicine challenge for the frail population. Thus, applying a simple scoring approach to the frail population would allow further skimming, so as to identify high-risk individuals needing to be more closely monitored. Last but not least, the easy-to-use FIB-4 scoring unlocks a window for use also by non-physician health care providers.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the “Salus in Apulia” Research Team. This manuscript is the result of the research work on frailty undertaken by the “Italia Longeva: Research Network on Aging” team, supported by the resources of the Italian Ministry of Health—Research Networks of National Health Institutes. We thank the General Practitioners of Castellana Grotte for their fundamental role in the recruitment of participants to these studies: Cecilia Olga Maria Campanella, Annamaria Daddabbo, Giosuè Dell’aera, Rosalia Francesca Giustiniano, Massimo Guzzoni Iudice, Savino Lomuscio, Rocco Lucarelli, Antonio Mazzarisi, Mariana Palumbo, Maria Teresa Persio, Rosa Vincenza Pesce, Gabriella Puzzovivo, Pasqua Maria Romano, Cinzia Sgobba, Francesco Simeone, Paola Tartaglia, and Nicola Tauro.
Funding
Italian Ministry of Health with “Ricerca Corrente 2020” funds.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Francesco Panza, Email: f_panza@hotmail.com.
Rodolfo Sardone, Email: rodolfo.sardone@irccsdebellis.it.
References
- 1.Chang AY, Skirbekk VF, Tyrovolas S, Kassebaum NJ, Dieleman JL. Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. Lancet Public Health. 2019;4(3):e159–e167. doi: 10.1016/S2468-2667(19)30019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72(5):1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 4.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31(3):184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 6.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zupo R, Castellana F, Bortone I, Griseta C, Sardone R, Lampignano L, et al. Nutritional domains in frailty tools: working towards an operational definition of nutritional frailty. Ageing Res Rev. 2020;64:101148. doi: 10.1016/j.arr.2020.101148. [DOI] [PubMed] [Google Scholar]
- 8.Sardone R, Castellana F, Bortone I, Lampignano L, Zupo R, Lozupone M, et al. Association between central and peripheral age-related hearing loss and different frailty phenotypes in an older population in Southern Italy. JAMA Otolaryngol Head Neck Surg. 2021;147(6):561–571. doi: 10.1001/jamaoto.2020.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortone I, Sardone R, Lampignano L, Castellana F, Zupo R, Lozupone M, et al. How gait influences frailty models and health-related outcomes in clinical-based and population-based studies: a systematic review. J Cachexia Sarcopenia Muscle. 2021;12(2):274–297. doi: 10.1002/jcsm.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Caoimh R, Sezgin D, O’Donovan MR, Molloy DW, Clegg A, Rockwood K, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50(1):96–104. doi: 10.1093/ageing/afaa219. [DOI] [PubMed] [Google Scholar]
- 11.Castellana F, Lampignano L, Bortone I, Zupo R, Lozupone M, Griseta C, et al. Physical frailty, multimorbidity, and all-cause mortality in an older population from Southern Italy: results from the Salus in Apulia Study. J Am Med Dir Assoc. 2021;22(3):598–605. doi: 10.1016/j.jamda.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Laube R, Wang H, Park L, et al. Frailty in advanced liver disease. Liver Int. 2018;38(12):2117–2128. doi: 10.1111/liv.13917. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Sardone R, Lampignano L, Guerra V, Zupo R, Donghia R, Castellana F, et al. Relationship between inflammatory food consumption and age-related hearing loss in a prospective observational cohort: results from the Salus in Apulia Study. Nutrients. 2020;12(2):426. doi: 10.3390/nu12020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulijaszek SJ. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. pp. 252. (World Health Organization, Geneva, 2000.). J Biosoc Sci. 2003;35:624–5. 10.1017/S0021932003245508. [PubMed]
- 16.Zupo R, Sardone R, Donghia R, Castellana F, Lampignano L, Bortone I, et al. Traditional dietary patterns and risk of mortality in a longitudinal cohort of the Salus in Apulia Study. Nutrients. 2020;12(4):1070. doi: 10.3390/nu12041070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnovale E, Miuccio FC. Tabelle di composizione degli alimenti. In: Arab L, Wittler M, Schettler G, editors. European Food Composition Tables in Translation. Berlin: Springer; 1987. pp. 63–67. [Google Scholar]
- 18.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. 10.1016/j.jhep.2015.11.004. [DOI] [PubMed]
- 19.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 20.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 21.McPherson S, Hardy T, Dufour J-F, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112(5):740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grissom R, Kim JJ. Effect sizes for research: A broad practical approach. 2005.
- 23.Villén N, Guisado-Clavero M, Fernández-Bertolín S, Troncoso-Mariño A, Foguet-Boreu Q, Amado E, et al. Multimorbidity patterns, polypharmacy and their association with liver and kidney abnormalities in people over 65 years of age: a longitudinal study. BMC Geriatr. 2020;20(1):206. doi: 10.1186/s12877-020-01580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy J, Peters MG. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol. 2013;9(10):633–639. [PMC free article] [PubMed] [Google Scholar]
- 25.Burra P, De Martin E, Gitto S, Villa E. Influence of age and gender before and after liver transplantation. Liver Transpl. 2013;19(2):122–134. doi: 10.1002/lt.23574. [DOI] [PubMed] [Google Scholar]
- 26.Stroffolini T, Sagnelli E, Sagnelli C, Morisco F, Babudieri S, Furlan C, et al. The association between education level and chronic liver disease of any etiology. Eur J Intern Med. 2020;75:55–59. doi: 10.1016/j.ejim.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Upmeier E, Lavonius S, Heinonen P, Viitanen M, Isoaho H, Arve S, et al. Longitudinal changes in serum lipids in older people the Turku elderly study 1991–2006. Age Ageing. 2011;40(2):280–283. doi: 10.1093/ageing/afq180. [DOI] [PubMed] [Google Scholar]
- 28.Jármay K, Karácsony G, Nagy A, Schaff Z. Changes in lipid metabolism in chronic hepatitis C. World J Gastroenterol. 2005;11(41):6422–6428. doi: 10.3748/wjg.v11.i41.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurokawa T, Ohkohchi N. Platelets in liver disease, cancer and regeneration. World J Gastroenterol. 2017;23(18):3228–3239. doi: 10.3748/wjg.v23.i18.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Stahl EC, Haschak MJ, Popovic B, Brown BN. Macrophages in the aging liver and age-related liver disease. Front Immunol. 2018;9:2795. doi: 10.3389/fimmu.2018.02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurosawa T, Goto M, Kaji N, Aikiyo S, Mihara T, Ikemoto-Uezumi M, et al. Liver fibrosis-induced muscle atrophy is mediated by elevated levels of circulating TNFα. Cell Death Dis. 2021;12(1):11. doi: 10.1038/s41419-020-03353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cozzolongo R, Osella AR, Elba S, Petruzzi J, Buongiorno G, Giannuzzi V, et al. Epidemiology of HCV infection in the general population: a survey in a southern Italian town. Am J Gastroenterol. 2009;104(11):2740–2746. doi: 10.1038/ajg.2009.428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.