Abstract
Vascular aging has a central role in the pathogenesis of cardiovascular diseases contributing to increased mortality of older adults. There is increasing evidence that, in addition to the documented role of cell-autonomous mechanisms of aging, cell-nonautonomous mechanisms also play a critical role in the regulation of vascular aging processes. Our recent transcriptomic studies (Kiss T. et al. Geroscience. 2020;42(2):727–748) demonstrated that circulating anti-geronic factors from young blood promote vascular rejuvenation in aged mice. The present study was designed to expand upon the results of this study by testing the hypothesis that circulating pro-geronic factors also contribute to the genesis of vascular aging phenotypes. To test this hypothesis, through heterochronic parabiosis, we determined the extent to which shifts in the vascular transcriptome (RNA-seq) are modulated by the old systemic environment. We reanalyzed existing RNA-seq data, comparing the transcriptome in the aorta arch samples isolated from isochronic parabiont aged (20-month-old) C57BL/6 mice [A–(A); parabiosis for 8 weeks] and young isochronic parabiont (6-month-old) mice [Y–(Y)] and also assessing transcriptomic changes in the aortic arch in young (6-month-old) parabiont mice [Y–(A); heterochronic parabiosis for 8 weeks] induced by the presence of old blood derived from aged (20-month-old) parabionts. We identified 528 concordant genes whose expression levels differed in the aged phenotype and were shifted towards the aged phenotype by the presence of old blood in young Y–(A) animals. Among them, the expression of 221 concordant genes was unaffected by the presence of young blood in A–(Y) mice. GO enrichment analysis suggests that old blood-regulated genes may contribute to pathologic vascular remodeling. IPA Upstream Regulator analysis (performed to identify upstream transcriptional regulators that may contribute to the observed transcriptomic changes) suggests that the mechanism of action of pro-geronic factors present in old blood may include inhibition of pathways mediated by SRF (serum response factor), insulin-like growth factor-1 (IGF-1) and VEGF-A. In conclusion, relatively short-term exposure to old blood can accelerate vascular aging processes. Our findings provide additional evidence supporting the significant plasticity of vascular aging and the existence of circulating pro-geronic factors mediating pathological remodeling of the vascular smooth muscle cells and the extracellular matrix.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00519-1.
Keywords: Heterochronic parabiosis, Aging, Vascular aging, Transcriptome, Aneurysm, Atherosclerosis, Aorta
Introduction
Diseases of the large arteries (including aorta aneurysm and large vessel atherosclerosis and its complications [e.g., ischemic stroke, ischemic heart disease]) are among the most common causes of serious long-term disability and cardiovascular mortality among older adults in the developed world [1]. These diseases account for approximately one-third of all deaths in the United States in those over 65 and nearly two-thirds of all deaths in those over the age of 85 [2].
Epidemiological studies demonstrate that the impact of traditional risk factors (including high blood pressure, hypercholesterolemia, tobacco smoking, obesity, diabetes mellitus, etc.) on the incidence of the aforementioned large vessel diseases are dwarfed by the single most important risk factor for these vascular pathologies: advanced aging [1]. In order to develop novel approaches for the prevention and treatment of age-related large vessel pathologies, it is essential to elucidate the contribution of shared biological mechanisms of aging to the functional and phenotypic changes that are manifested in the old vasculature [3, 4]. Previous studies have characterized several important cell-autonomous mechanisms that drive functional decline in the aging vasculature [1], including mitochondrial dysfunction [5, 6], increased oxidative stress [1, 7–17], impaired production and bioavailability of NO [11, 18–20], cellular NAD + depletion, and energetic dysfunction [5, 21–24] and dysregulation of sirtuin pathways [8, 25].
Over the past decade, major advances in the field of geroscience, coupled with advances in animal model development and multiomics technologies, have led to explosive growth in biological information that implicates cell-nonautonomous mechanisms as also playing critical roles in driving organismal aging processes and pathogenesis of age-related diseases [26–31]. It was also recognized that aging is characterized by complex alterations of inter-organ communication, which also modulate cell-autonomous processes of aging in the cardiovascular system, exacerbating the genesis of vascular pathologies [1]. Accordingly, there is growing evidence that there are factors present in the circulation, which are derived from other organ systems (including the adipose tissue, the endocrine system, the gastrointestinal tract, the central nervous system, the immune system) and regulate cellular aging processes in the vasculature. The view has emerged that there are multiple circulating anti-geronic factors extant, which can reverse or prevent the genesis of cardiovascular aging phenotypes and whose production declines with age [4].
Parabiosis is a surgical procedure for joining two animals to allow for blood exchange via their shared circulatory systems [32–35]. By comparing heterochronic (young–old) and isochronic (young–young and old–old) parabiont pairs of animals [29, 31, 34–55], it can be investigated how circulating (humoral and/or cellular) factors derived from one animal contribute to the genesis of aging phenotypes in various organ systems in the other parabiont. Using heterochronic parabiosis in mice as an experimental tool, our recent studies [54] provided critical evidence that the presence of young blood derived from young parabionts significantly improves endothelium-dependent vasorelaxation and attenuates the production of reactive oxygen species in vessels of heterochronic parabiont aged mice. Using RNA-seq, we also assessed transcriptomic changes in the aortic arch associated with aging and heterochronic parabiosis [54]. We have identified 212 discordant genes whose expression levels differed in the aged phenotype but have shifted back toward the young phenotype by the presence of young blood in aged heterochronic parabiont animals [54]. Pathway analysis suggested that vasoprotective effects conferred by young blood include mitochondrial rejuvenation. On the basis of the transcriptomic changes, insulin-like growth factor-1 (IGF-1) has been identified as a putative circulating anti-geronic factor that contributes to the vasoprotective effects mediated by exposure to young blood [54]. This finding accords with the results of earlier studies, which demonstrated that circulating levels of IGF-1 decline in older adults [56] and aged mice [27]. Importantly, IGF-1 exerts multifaceted vasoprotective effects, maintaining a youthful vascular phenotype and function [27, 56–63].
Experiments using heterochronic parabiosis also suggested that in addition to anti-geronic factors, there are also pro-geronic factors present in the circulation that can accelerate cellular aging processes. The production of circulating pro-geronic factors increases with age and they mediate deleterious cell-nonautonomous effects in multiple organs, including the skeletal muscle, central nervous system, and the heart [29, 39, 47, 48, 50, 52, 64].
The present study was designed as a follow-up investigation to test the hypothesis that age-related changes in circulating pro-geronic factors also contribute to vascular aging. To test this hypothesis, we reanalyzed our previously published transcriptomic dataset [54] assayed from the aortas of heterochronic (young–old) and isochronic (young–young and old–old) parabiont pairs of experimental mice. We determined to what extent, if any, transposition of aging phenotypes could be observed in the young aorta by exposure to an old systemic environment.
Methods
Animals and parabiosis surgery
To elucidate the effects of old blood on the vascular transcriptome, in the present study we reanalyzed our previously published transcriptomic dataset [54] assayed from the aortas of heterochronic (young–old) and isochronic (young–young and old–old) parabiont pairs of experimental mice. Here we provide a short description of the experimental procedures based on the description of the methods in our original publication [54]. As previously described [54], young (4-month-old) and aged (18-month-old) male C57BL/6 mice were obtained from the aging rodent colony maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA). Mice were housed under specific pathogen-free conditions at the rodent barrier facility at Albert Einstein College of Medicine under a controlled photoperiod (12 h light; 12 h dark) with unlimited access to water and were fed a standard chow diet (ad libitum). Parabiosis surgery in young and aged animals was carried out by the Einstein Health Span Core [65], according to published protocols [66, 67] as reported previously [30]. Surgical unions were performed between young animals (isochronic; young Y–(Y); n = 4 pairs), aged animals (isochronic old; A–(A); n = 4 pairs), and young and aged mice (heterochronic Y–(A) and A–(Y); n = 5 pairs) as described [30]. Following surgery, animals were kept on a partial heating pad overnight. Pairs were then intensively monitored and received subcutaneous (s.c) injections of Banamine (2 mg/kg each) immediately post-op and twice a day for three days and then once daily for four days. Animals also received 1 mL of Ringer’s lactate (s.c.) immediately after, daily for three days post-op to prevent dehydration. Animals remained joined for ~ 8 weeks prior to sacrifice. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the Albert Einstein College of Medicine and the University of Oklahoma Health Sciences Center. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
RNA isolation, cDNA synthesis, library construction, and next-generation sequencing
RNA was isolated from the aortic arch samples using AllPrep DNA/RNA Mini Kit (Qiagen) as previously described [68, 69]. Prior to 3′-tag RNA-seq analysis quality control measures were implemented. The concentration of RNA was ascertained via fluorometric analysis on a Thermo Fisher Qubit fluorometer. The overall quality of RNA was verified using an Agilent Tapestation instrument. Following initial QC steps sequencing libraries were generated using the Lexogen Quantseq FWD library prep kit according to the manufacturers’ protocol by the Clinical Genomics Core of the Oklahoma Medical Research Foundation [70]. Briefly, the first strand of cDNA was generated using 5′-tagged poly-T oligomer primers. Following RNase digestion, the second strand of cDNA was generated using 5′-tagged random primers. A subsequent PCR step with additional primers added the complete adapter sequence to the initial 5′ tags, added unique indices for demultiplexing of samples, and amplified the library. Final libraries for each sample were assayed on the Agilent Tapestation for appropriate size and quantity. These libraries were then pooled in equimolar amounts as ascertained via fluorometric analyses. Final pools were absolutely quantified using qPCR on a Roche LightCycler 480 instrument with Kapa Biosystems Illumina Library Quantification reagents. Sequencing was performed using custom primers on an Illumina NextSeq 500 instrument with High Output chemistry and 75 bp single-ended reads.
RNA-seq data analysis and visualization
Raw sequencing reads were trimmed of their Illumina TruSeq adapter sequences using Trimmomatic v0.35 [71], filtered for contaminants of ribosomal, mitochondrial, and hemoglobin transcripts, then aligned to the mouse genome version GRCm38 using Kallisto v0.43.03 [72]. Samples were checked for outliers and separation by principle components analysis (PCA) with the R function prcomp. Raw expression counts were summarized at the gene level to transcript-length adjusted, library-size scaled counts per million (CPM) with the R/Bioconductor package tximport [73]. Differential expression analysis was performed using the empirical Bayes approach implemented in the R/Bioconductor package DESeq2 [74]. Significantly differentially expressed (DE) genes had an absolute log2 fold change ≥ 0.585 (corresponding to a change of 50% or more in the expression) and the False Discovery Rate FDR-adjusted p-value ≤ 0.05. Gene annotation was done using biomaRt [75] in the R/Bioconductor package. The R package pheatmap v1.0.12 was used to perform hierarchical clustering and to generate the heat maps. The org.Mm.eg.db v3.8.2 R/Bioconductor package was used to collect Gene Ontology, KEGG, and Reactome terms associated with the DE (differentially expressed) genes. The same package was used to translate Ensemble IDs to Entrez IDs when it was required by the statistical packages.
Functional annotation
To collect gene annotation data the g:Profiler service version e102_eg49_p15_e7ff1c9 was used via the R interface and with fdr correction method applying significance threshold of 0.05 [76]. The results were plotted using the ggplot2 v3.3.5 package [https://ggplot2.tidyverse.org] in the R v4.1.1 environment and the EnrichmentMap v3.3.3 tool [77] in the Cytoscape v3.8.2 environment [78].
To identify upstream regulators that potentially explain the observed gene expression changes in our samples we used upstream regulator analysis (URA) algorithm [79] implemented in the ingenuity pathway analysis (IPA; QIAGEN) software [79]. IPA is a commercial software package most commonly utilized to intersect DE genes with known biological functions and pathways maintained in the ingenuity knowledge base, a collection of nearly 5 million experimental findings manually curated from either literature or third-party databases. Detailed information can be found at http://qiagen.force.com/KnowledgeBase/.
Gene set enrichment analysis
Gene set enrichment analysis was performed using the fgsea v1.18.0 package implemented in R [80]. Results were plotted in ggplot2.
Results
Exposure to old blood mimics age-related changes in vascular mRNA expression profile
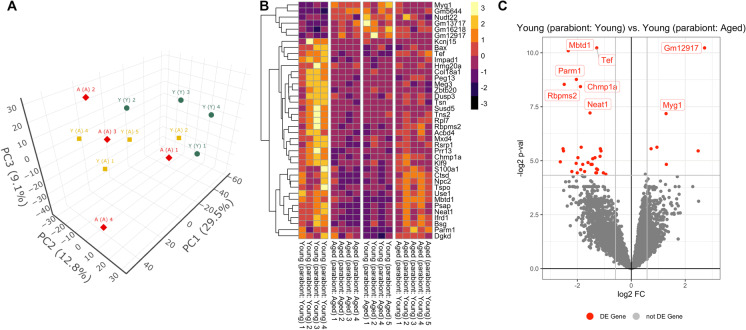
By re-analyzing the previously collected transcriptomic data [81] data we assessed transcriptomic changes in the aorta arch associated with aging and exposure to aged blood via heterochronic parabiosis. Biological replicates from the isochronic parabiosis mice [Y–(Y) and A–(A)] exhibited distinct clustering by PCA, suggesting distinct aortic transcriptomes (Fig. 1A). Transcriptomic profiles of aortas of young mice exposed to aged blood [Y–(A)] clustered between the isochronic pairs in the PCA, suggesting old blood shifted the aortic transcriptome of young mice toward an aged phenotype. In our original publication of the transcriptomic data [54] we documented the presence of an outlier (Y–(A) sample #3 in the original dataset). This sample was excluded from the present re-analysis of the dataset.
Fig. 1.
Old blood induces aging-like changes in vascular mRNA expression profile Panel A: Principal component analysis (PCA) plot of mRNA expression profiles in aorta samples derived from isochronic parabiont young mice [Y–(Y)], isochronic parabiont aged mice [A–(A)] and heterochronic parabiont young mice [Y–(A)]. The profiles from Y–(Y) mice (green) cluster separately from clusters representing A–(A) mice (red) and Y–(A) mice (yellow) in the space of the first three principal components. The A–(A) and Y–(A) expression profiles were more similar and clustered less discriminately in the PCA, indicating the impact of old blood on the aorta transcriptome in young mice. PC1, PC2, and PC3: Principal components 1, 2, and 3, respectively. Panel B: The heat map is a graphic representation of normalized expression values of genes that are differentially expressed both in aorta samples derived from isochronic parabiont aged mice [A–(A)] and heterochronic parabiont young mice [Y–(A)] as compared to those in aorta samples obtained from isochronic parabiont young mice [Y–(Y)]. The expression values for these genes in aorta samples obtained from heterochronic parabiont aged mice [A–(Y)] are also shown. Hierarchical clustering analysis reveals groups of genes whose expression is similarly up- or down-regulated in aortas of both A–(A) mice and Y–(A) mice but is unaffected by the presence of young blood in A–(Y) mice. Panel C: Volcano plot depicting differentially expressed genes comparing aortic samples derived from Y–(Y) and Y–(A) mice. Stratified p-values are plotted against expression fold changes for results obtained in Y–(A) samples normalized to Y–(Y) samples. Colored points refer to genes whose expression is significantly altered in Y–(A) mice
In Fig. 1B a heat map is shown as a graphical representation of normalized expression values of genes that are differentially expressed both in aorta samples derived from isochronic parabiont aged mice [A-(A)] and heterochronic parabiont young mice [Y–(A)] as compared to those in aorta samples obtained from isochronic parabiont young mice [Y–(Y)]. Hierarchical clustering analysis reveals groups of genes whose expression is similarly up- or down-regulated in aortas of both A–(A) mice and Y–(A) mice relative to Y–(Y) mice but is unaffected by exposure to young blood in aged mice [A–(Y)].
We then determined the number of genes that were significantly upregulated or downregulated (“differentially expressed”; DE; fold-change ≥ 1.5 or ≤ 0.67; p < 0.05 adjusted for multiple comparisons) in the aorta by aging or by heterochronic parabiosis. We then filtered for genes that are significantly altered (adjusted p < 0.05), expressed at an appreciable level (fragments per kilobase of transcript per million mapped reads > 1). There were 347 DE genes in A–(A) animals compared to Y–(Y) controls [81]. We further identified 39 DE genes in Y–(A) mice compared to A–(A) controls. In Fig. 1C a volcano plot shows statistical significance (p-value) versus magnitude of age-related change in gene expression. Red symbols denote genes, whose expression levels significantly differed in Y–(A) mice.
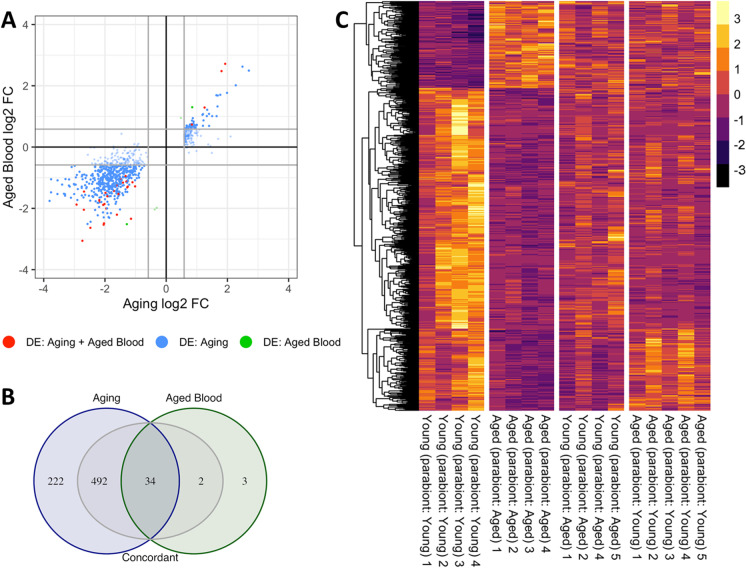
We realized that significance cut-offs to identify DE genes shared between the age-effect [A–(A) vs. Y–(Y)] and old blood-effect [Y–(A) vs. Y–(Y)] datasets might be too stringent with the result that the analysis in Fig. 1C might miss concordant patterns of gene expression with important biological relevance for old blood-induced accelerated vascular aging and age-related vascular pathologies. Thus, we also used a slightly relaxed approach to detect concordant transcriptional patterns (here termed “pro-geronic shifts”) by comparing the age-effect and old blood-effect gene expression datasets using combination criteria that took into account the effect direction (Fig. 2). Specifically, genes were ranked by their effect size direction and ranked lists were compared to identify overlapping genes across a continuous significance gradient. Genes with “pro-geronic shifts” were defined as concordant genes that (1) were DE based on both p-value and fold-change criteria either in the age-effect or the old-blood-effect comparison, (2) satisfied a fold-change criterion with a cutoff of ≥ 1.5 or ≤ 0.67.
Fig. 2.
Old blood promotes aging-like changes in vascular mRNA expression profile: identification of concordant genes Panel A: Old blood-induced changes in gene expression (log2 fold changes; heterochronic parabiont young [Y–(A)] mice vs. isochronic parabiont young [Y–(Y)] mice) plotted against age-related changes (log2 fold changes; isochronic parabiont aged [A-–(A)] mice vs. isochronic parabiont young [Y–(Y)] mice) in the aortic transcriptome. Red symbols indicate concordant differentially expressed genes, whose expression significantly changes with age, and the direction of this effect is mimicked by exposure to old blood. Blue and green symbols denote concordant genes, whose expression similarly changes in aging and by old blood exposure, but only the aging (blue) or the old blood effect (green) reaches the cutoff for statistical significance. Panel B: Venn diagrams showing the numbers of differentially expressed mRNAs in each group. The blue circle represents genes, which are significantly up- or down-regulated in aged mice as compared to young mice. The green circle represents genes, which are significantly up- or down-regulated by the presence of old blood in young mice. The intersection of the two circles represents concordant differentially expressed genes. Grey areas represent concordant genes, whose expression similarly changes in aging and by old blood exposure, but only the aging or the old blood effect reaches the cutoff for statistical significance. Panel C: The heat map is a graphic representation of normalized expression values of concordant genes in aorta samples derived from Y–(Y), A–(A), and Y–(A) mice. Data for aorta samples derived from A–(Y) mice are shown for comparison. Note that the expression of the majority of concordant genes, which are down-regulated by old blood in young mice, is unaffected by the presence of young blood in old mice. These data indicate that many of the effects of pro-geronic factors, which induce accelerated aging phenotypes in young mice, cannot be rescued by the presence of young blood in old mice
in the comparison in which the statistical significance criterion was not met, and (3) satisfied the criterion that the effect directions of the age-effect and old blood-effect are the same. We found that these combination criteria found more biologically meaningful sets of genes showing pro-geronic shift (n = 528) than the analysis requiring concordant genes meet both fold change and statistical significance criteria (Fig. 2A, blue dots; Fig. 2B, three internal intersections in gray). These data suggest that changes in circulating pro-geronic factor(s) contribute to age-related dysregulation of vascular gene expression.
Old blood induces aging-like transcriptomic changes by regulating genes expressed in endothelial cells and smooth muscle cells in the mouse aorta
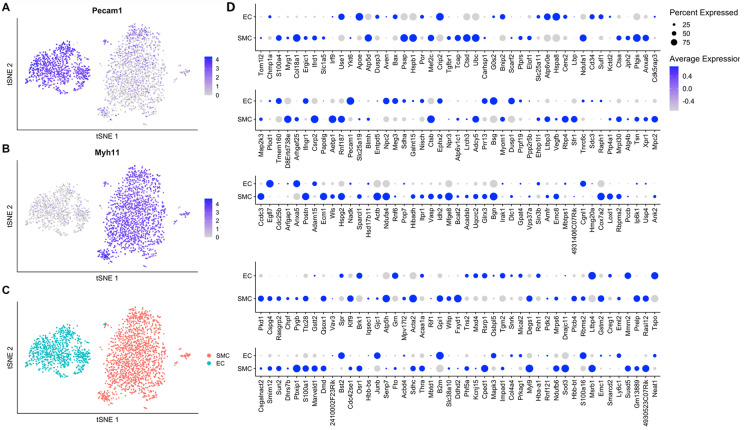
Next, we aimed to determine whether old blood factors promote aging-like gene expression changes in endothelial cells (EC) or vascular smooth muscle cells (SMC). To identify genes that are enriched in endothelial cells and SMCs, we reanalyzed single-cell data RNA-seq data obtained from the intima and madia leyers of wild-type mouse aortas, published by Deng et al [82].
Unbiased Louvain clustering of cells resolved 2 robust, transcriptionally distinct clusters of aorta-derived cells (Fig. 3A–C). Clusters of endothelial cells and vascular smooth muscle cells (SMCs) were identified by the significant, cluster-specific markers calculated by the MAST method as described [83]. Then, we determined which concordant genes from our dataset showing pro-geronic shifts are expressed in the endothelial cells and/or the SMCs (Fig. 3D). We found that old blood induces aging-like transcriptomic changes in young mouse aortas by regulating genes expressed both in endothelial cells and SMCs.
Fig. 3.
Old blood induces aging-like transcriptomic changes by regulating genes expressed in endothelial cells and smooth muscle cells in the mouse aorta To determine the expression pattern of concordant genes in endothelial cells (ECs) and vascular smooth muscle cells (SMCs), single-cell RNA-seq data were obtained in ECs, and SMCs derived from the mouse aorta and published by Deng et al [82] were reanalyzed. Panel A–C: Identification of ECs and SMCs based on differentially expressed marker genes. Panel C depicts two-dimensional tSNE plots based on differentially expressed marker genes, colored by cluster. Panels A and B depict the expression of a canonical endothelial cell marker (Pecam1) and a canonical smooth muscle cell marker (Myh11), respectively. Relative expression values for each cell in each cluster identified in the two-dimensional tSNE plots are shown. Panel D: Bubble plot shows the relative expression of the concordant genes in mouse aorta ECs and SMCs. Bubble size is proportional to the percentage of cells expressing a gene, and color intensity are proportional to average scaled gene expression within a cluster. Note that old blood induces aging-like transcriptomic changes in young mouse aortas by regulating genes expressed both in endothelial cells and smooth muscle cells
Old blood-induced vascular transcriptomic changes in young mice predict pathologic vascular remodeling, suggesting a possible role for circulating factors present in old blood in the pathogenesis of aorta aneurysms and atherosclerosis.
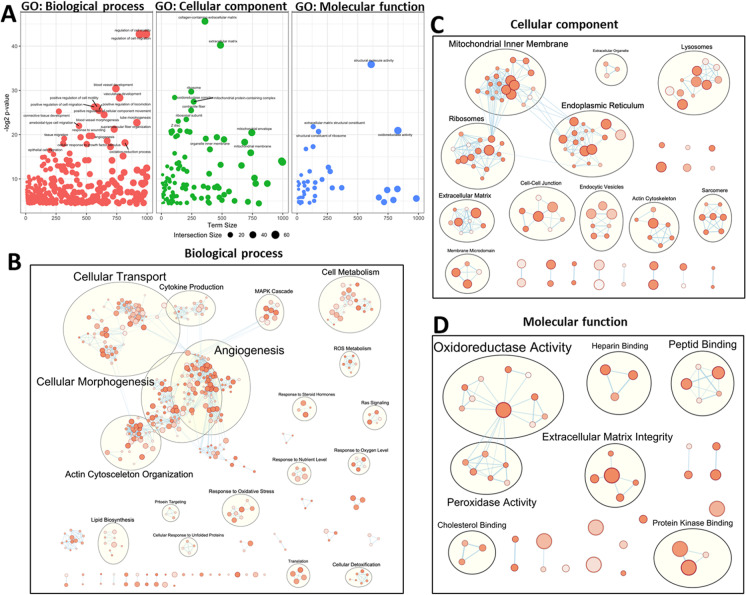
We performed GO (gene ontology) enrichment analysis to explore potential biological functions of the old blood-induced concordant genes with pro-geronic shifts. GO enrichment analysis of concordant genes identified functions in vascular SMC and extracellular matrix remodeling (Fig. 4A; Table S1).
Fig. 4.
GO enrichment analysis results: old blood induces a gene expression signature indicating pathological remodeling of the aorta Panel A: Bubble plots visualizing gene ontology (GO) representatives of biological processes (BP, left panel), cellular components (CC, middle panel), and molecular functions (MF, right panel), which are over-represented (enriched) in the concordant genes induced by old blood. Terms are shown at –log2 (adjusted p-value; cut off > 0.05) vs. term size. Bubble size indicates the number of genes annotated with a term in the list of concordant genes. Note that the presence of old blood in young animals is associated with transcriptional changes indicating multifaceted biological processes related to vascular remodeling. Panels B, C: Enrichment maps visualizing shared, significantly enriched GO terms (Panels B, C: Biological processes, cellular components, and molecular functions, respectively) for concordant genes organized into a network. Note that mutually overlapping GO terms cluster together
Overrepresentation analysis of GO terms revealed that genes regulating vascular SMC function and processes involved in vascular remodeling (including the GO terms “regulation of cell migration” [GO:0030334] and “blood vessel development” [GO:0001568]), genes regulating oxidative stress responses (including the GO terms “response to oxidative stress” [GO:0006979] and “reactive oxygen species biosynthetic process” [GO:1903409]), extracellular matrix related genes (including the GO terms “connective tissue development” [GO:0061448]; “extracellular matrix organization” [GO:0030198] and “collagen metabolic process” [GO:0032963]), and genes regulating apoptosis (including the GO terms “regulation of cell death” [GO:0010941] and “apoptotic process” [GO:0006915] and “positive regulation of cell death” [GO:0010942]) were overrepresented among the concordant genes.
For functional enrichment visualization, we used the EnrichmentMap Cytoscape App [77]. Figure 4B–D shows the enrichment maps in which significantly enriched GO terms associated with concordant genes are organized into a network. Nodes represent gene-sets (GO terms), edges represent mutual overlap, and, in this way, mutually overlapping GO terms cluster together. Note that the pro-geronic shift in gene expression profile in aortas of Y–(A) animals are associated with transcriptional changes indicating pathological vascular remodeling and oxidative stress-related responses.
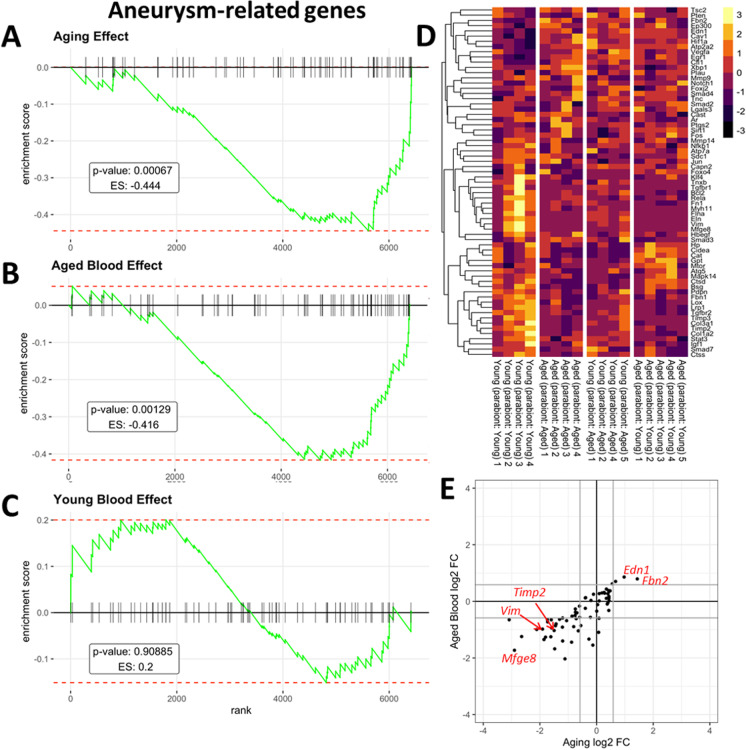
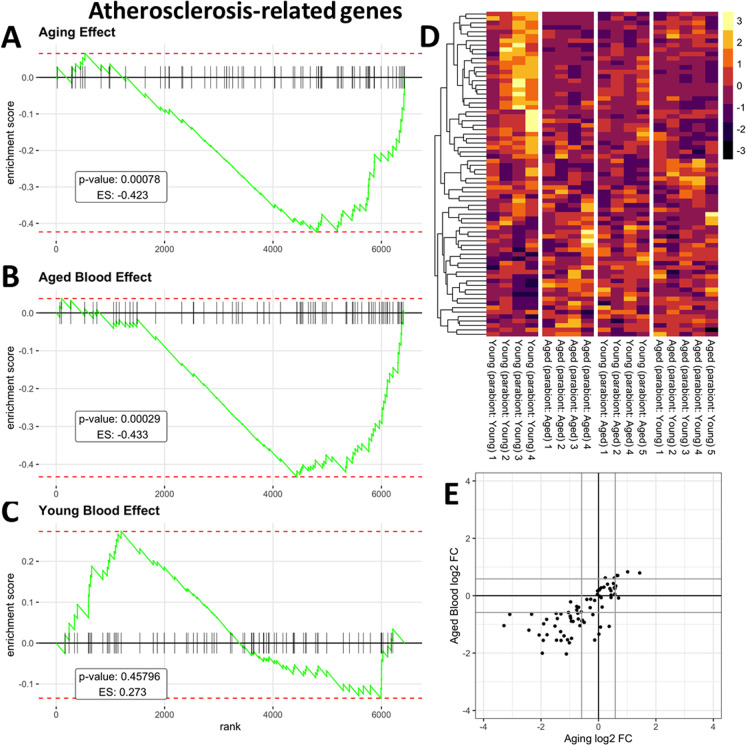
In humans, aging is known to promote the pathogenesis of aorta aneurysms and atherosclerosis [1]. In the present study, we aimed to elucidate how aorta aneurysm- and atherosclerosis-related gene expression is altered by circulating factors present in old blood. To compile a list of genes with known functions related to the pathogenesis of aorta aneurysms and atherosclerosis we identified genes associated with aorta aneurysms and atherosclerosis using the IRIDESCENT text mining package [84]. IRIDESCENT's database contains over 730,000 recognized terms and phrases, representing over 162,000 unique concepts obtained from public databases (OMIM, Entrez Gene, Gene Ontology, ChemID, FDA approved drugs, and disease ontology terms). Over 22 million MEDLINE abstracts were processed to identify co-occurring terms. This creates a network of concepts, weighted by their frequency of co-occurrence. Concepts such as “aorta aneurysm” and “atherosclerosis” were then queried for frequently co-occurring gene names. We used Gene Set Enrichment Analysis (GSEA) for interpreting the expression of aorta aneurysm- and atherosclerosis-related genes [85] in all of our comparisons of mouse pairs. GSEA of these aorta aneurysms- and atherosclerosis-related genes was performed using a ranked gene list for each comparison of mouse pairs based on the gene-level signed statistics (Figs. 5 and 6, respectively). Figure 5A–C depicts a running-sum statistic (enrichment score) based on Fig. 5D, increasing when a gene is a member of the aorta aneurysm-related gene set and decreasing when it is not. Note that in aged mice, enrichment scores increased predominantly on the right indicating age-related down-regulation of genes related to extracellular matrix homeostasis (Fig. 5A). In response to the presence of old blood in young mice enrichment scores showed similar increases on the right, indicating that presence of old blood in young mice mimics the effects of aging on extracellular matrix homeostasis (Fig. 5B). In contrast, young blood had no consistent effect on aneurysm-related genes (Fig. 5C). Figure 5E shows that many aneurysm-related genes are dysregulated both in aging and by the presence of old blood in young animals as well. GSEA of atherosclerosis-related genes yielded similar results (Fig. 6), showing that many genes involved in the regulation of atherogenesis are similarly dysregulated both in aging and by the presence of old blood in young animals.
Fig. 5.
Presence of old blood in young mice mimics age-related changes in vascular expression of genes associated with the pathogenesis of aorta aneurysms Panel A: Gene set enrichment analysis (GSEA) to test the effect of aging on the enrichment of the set of genes associated with the pathogenesis of aorta aneurysms by comparing aorta samples derived from isochronic parabiont young mice [parabiont: young; Y–(Y)] and isochronic parabiont aged mice [parabiont: aged; A–(A)]. Aging-induced gene expression changes were ranked from most up-regulated (left) to most down-regulated (right). Ticks represent genes encoding aneurysm-related factors. Shown is a running-sum statistic (enrichment score) based on panel D, increasing when a gene is a member of the aneurysm-related gene set and decreasing when it is not. Panel B: GSEA showing the effect of exposure to old blood on the enrichment of aneurysm-related genes. Aorta samples derived from isochronic parabiont young mice [parabiont: young; Y–(Y)] and heterochronic parabiont young mice [parabiont: aged; Y–(A)] were compared. Note that in aged mice, enrichment scores increased predominantly on the right indicating age-related down-regulation of genes related to extracellular matrix homeostasis. In response to the presence of old blood in young mice, enrichment scores showed similar increases on the right, indicating that the presence of old blood in young mice mimics the effects of aging on aneurysm-related genes. Panel C: Youngblood had no consistent effect on aneurysm-related genes (comparison: A–(Y) vs. A–(A)). Panel D: The heat maps are graphical representations of normalized expression values of aneurysm-related genes. Hierarchical clustering analysis revealed the similarities on aortic expression profiles of aneurysm-related genes in aged mice and old blood exposed young mice. Panel E: Old blood-induced changes in aneurysm-related gene expression (log2 fold changes; Y–(A) vs. Y–(Y)) plotted against age-related changes (log2 fold changes; A–(A) vs. Y–(Y)) in aneurysm-related gene expression. Note that many aneurysm-related genes are dysregulated both in aging and by the presence of old blood in young animals as well
Fig. 6.
Presence of old blood in young mice mimics age-related changes in vascular expression of genes associated with the pathogenesis of atherosclerosis Panel A: Gene set enrichment analysis (GSEA) to test the effect of aging on the enrichment of the set of genes associated with the pathogenesis of atherosclerosis by comparing aorta samples derived from isochronic parabiont young mice [parabiont: young; Y–(Y)] and isochronic parabiont aged mice [parabiont: aged; A–(A)]. Aging-induced gene expression changes were ranked from most up-regulated (left) to most down-regulated (right). Ticks represent genes encoding atherosclerosis-related factors. Shown is a running-sum statistic (enrichment score) based on panel D, increasing when a gene is a member of the atherosclerosis-related gene set and decreasing when it is not. Panel B: GSEA showing the effect of exposure to old blood on enrichment of atherosclerosis-related genes. Aorta samples derived from isochronic parabiont young mice [parabiont: young; Y–(Y)] and heterochronic parabiont young mice [parabiont: aged; Y–(A)] were compared. Note that in aged mice enrichment scores increased predominantly on the right indicating age-related dysregulation of genes related to atheroprotection. In response to the presence of old blood in young mice enrichment scores showed similar increases on the right, indicating that the presence of old blood in young mice mimics the effects of aging. Panel C: Youngblood had no consistent effect on atherosclerosis-related genes (comparison: A–(Y) vs. A–(A)). Panel D: The heatmaps are graphic representations of normalized expression values of atherosclerosis-related genes. Hierarchical clustering analysis revealed the similarities on aortic expression profiles of atherosclerosis-related genes in aged mice and old blood exposed young mice. Panel E: Old blood-induced changes in atherosclerosis-related gene expression (log2 fold changes; Y–(A) vs. Y–(Y)) plotted against age-related changes (log2 fold changes; A–(A) vs. Y–(Y)) in atherosclerosis-related gene expression. Note that many genes are similarly dysregulated both in aging and by the presence of old blood in young animals as well
Ingenuity upstream regulator analysis
We have performed IPA upstream regulator analysis [54] to identify upstream transcriptional regulators that may contribute to the observed transcriptomic changes in our dataset, which can help to identify the mechanism of action of pro-geronic factors present in the old blood. The upstream regulator analysis is based on information in the ingenuity knowledge base (a curated relational database of the available biomedical literature) on the expected effects between transcriptional regulators and their target genes. Using the IPA Upstream Regulator analysis, it was examined how many known targets of each transcriptional regulator were differentially expressed in our samples and the direction of these gene expression changes were compared to what is expected from the literature. On the basis of the observed direction of change, a prediction of the activation state of the predicted transcriptional regulators (“activated” or “inhibited”) was made. For each potential transcriptional regulator two statistical measures, an overlap p‐value and an activation z‐score were computed. The overlap p‐value calls likely upstream regulators based on the significant overlap between the DE genes and known targets regulated by that particular transcriptional regulator. The activation z‐score is used to infer the activation state of the predicted transcriptional regulators (“activated” or “inhibited”) based on comparison with a model that assigns random regulation directions. The results of the IPA upstream regulator analysis are shown in Table 1. In particular, the IPA upstream regulator analysis predicts that old blood-induced accelerated vascular aging is associated with inhibition of pathways mediated by SRF (serum response factor), insulin-like growth factor-1 (IGF-1) and VEGF-A (Table 1).
Table 1.
Predicted upstream transcriptional regulators that may mediate the observed aging-like vascular effects of circulating pro-geronic factors present in old blood Results of the IPA Upstream Regulator analysis. Shown are predicted upstream transcriptional regulators that may contribute to the observed old blood-induced transcriptomic changes in our dataset. The overlap p-value calls likely upstream regulators based on the significant overlap between dataset genes and known targets regulated by a transcriptional regulator
| Upstream regulator | Name/alias | Remark | Molecule type | Predicted activation state | Activation z-score | P-value of overlap |
|---|---|---|---|---|---|---|
| TGFB1 | Transforming growth factor-β | Regulates VSMC differentiation and vascular remodeling; regulates VSMC aging [146]; TGF-β1 increases in aged humans and mice [147] | Growth factor | Inhibited | − 3.8 | 4.35E-14 |
| TP53 | Tumor protein P53 | tumor suppressor; atheroprotection in aorta [148], prevents pathological remodeling [149] | Transcription regulator | Inhibited | − 3.55 | 3.87E-13 |
| PRL | Prolactin | Serum prolactin increases with age in men [150] | Cytokine | Inhibited | − 3.665 | 9.96E-11 |
| Alpha catenin | Links cadherins to the actin cytoskeleton; promotes cell–cell adhesions; α-catenin regulates Wnt/β-catenin signaling [151]; Wnt/beta-catenin pathway is activated during advanced arterial aging in humans [152] | Group | Activated | 3.266 | 1.19E-08 | |
| AGT | Angiotensinogen | angiotensin signaling regulates VSMC remodeling and arterial aging [153, 154] | Growth factor | Inhibited | − 3.927 | 0.000000045 |
| ROR1 | Receptor tyrosine kinase-like orphan receptor 1 | Ror1/2 knockout mice exhibit cardiovascular hyperinflammation [155] | Kinase | Inhibited | − 2.414 | 0.000000352 |
| SEMA7A | Semaphorin 7A | SEMA7A regulates atherogenesis [156]; TGBβ regulates SEMA7A | Transmembrane receptor | Inhibited | − 2.236 | 0.0000016 |
| UBA1 | Ubiquitin-like modifier activating enzyme 1 | Plays central role in ubiquitination; regulates cell cycle, endocytosis, signal transduction, apoptosis, DNA damage repair, and transcription; pro-atherogenic [157] | Enzyme | Activated | 2.236 | 0.000002 |
| CD44 | Homing cell adhesion molecule | Regulates cell–cell interactions, cell adhesion and migration; regulates atherogenesis [158] | Cell-surface glycoprotein | Inhibited | − 2.165 | 0.00000315 |
| CCR2 | C–C chemokine receptor type 2 | Receptor for MCP-1; regulates aneurysm formation [159]; circulating MCP-1 increases with age [160] | G-protein coupled receptor | Inhibited | − 2.138 | 0.0000081 |
| LARP1 | La-related protein 1 | Novel target of the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway. In response to cellular mTOR activity, LARP1 serves as a phosphorylation-sensitive molecular switch regulating ribosome biogenesis [161] | Translation regulator | Activated | 2.646 | 0.0000104 |
| CR1L | Complement C3b/C4b receptor 1 like | Cofactor for complement factor I | Other | Activated | 2.2 | 0.0000105 |
| IKZF1 | Ikaros | Up-regulated in aorta aneurysm [162] | Transcription regulator | Activated | 2.177 | 0.0000177 |
| MRTFB | MKL/myocardin-like protein 2 | Binds to SRF and act as coactivators; regulates VSMC differentiation; regulated in vascular injury | Transcription regulator | Inhibited | − 2.043 | 0.0000254 |
| IL10RA | Interleukin 10 receptor, alpha subunit | Mediate the immunosuppressive signal of IL-10, and thus inhibits the synthesis of proinflammatory cytokines; IL-10 attenuates neointimal proliferation; IL-10 levels positively associate with CVD risk [163] | Transmembrane receptor | Inhibited | − 2.887 | 0.0000266 |
| SNAI1 | Transcription factor that promotes the repression of E-cadherin to regulate epithelial to mesenchymal transition | Transcription regulator | Inhibited | − 2.608 | 0.0000946 | |
| SNAI2 | Transcription factor that regulates cell differentiation and migration; regulates epithelial to mesenchymal transition; mediates anti-apoptotic effects | Transcription regulator | Inhibited | − 2.387 | 0.0000275 | |
| MKNK1 | MAP kinase-interacting serine/threonine-protein kinase 1 | Regulates cardiovascular remodeling [164] | Kinase | Inhibited | − 2.646 | 0.0000369 |
| ITGA11 | Integrin alpha 11 | Binds to type I collagen; regulates tissue fibrosis [165]; | Integrin | Inhibited | − 2 | 0.0000399 |
| CCN2 | CTGF/connective tissue growth factor | Regulates cell adhesion, migration and proliferation, angiogenesis, and vascular remodeling [166–169] | growth factor | Inhibited | − 2.611 | 0.0000599 |
| SRF | Serum response factor | Regulates VSMC growth, migration and proliferation [170]; binds to a serum response element (SRE) associated with a variety of genes including immediate early genes such as c-fos, fosB, junB, egr-1, and -2, and muscle genes such as actins and myosins. Regulates cell cycle, apoptosis, cell growth, and cell differentiation [170]. It is a downstream target of mitogen-activated protein kinase pathway (MAPK). SRF is down-regulated in aging skeletal muscle in mice and humans [121]. Muscle specific loss of SKF promotes muscle atrophy [121]. Endothelium-specific knockdown of SRF promotes aorta aneurysm formation [171] | Transcription regulator | Inhibited | − 2.613 | 0.0000761 |
| SMAD7 | Mothers against decapentaplegic homolog 7 | Inhibits TGFβ1 signaling | Transcription regulator | Activated | 2.759 | 0.0000791 |
| VEGFA | Vascular endothelial growth factor A | Induces angiogenesis, endothelial cell growth, promotes cell migration, and inhibits apoptosis; VEGF signaling decreases with age; an increase in circulatory VEGF in aged mice exerts rejuvenating effects on the aged vasculature and alleviates multiple adverse age-related processes [122] | Growth factor | Inhibited | − 2.764 | 0.000103 |
| PRKG1 | cGMP-dependent protein kinase 1, alpha isozyme | Humans heterozygous for an activating mutation in PRKG1 develop thoracic aortic aneurysms and dissections as young adults. Mice heterozygous for this activating mutation also develop age-dependent aortic dilation with increased smooth muscle cell apoptosis, elastin fiber breaks, and oxidative stress [172] | Kinase | Activated | 2 | 0.000183 |
| HIF1A | Hypoxia-inducible factor 1-alpha | Transcription factor; regulates cellular responses to systemic oxygen levels; regulates tissue regeneration and repair | Transcription regulator | Inhibited | − 2.032 | 0.000219 |
| AR | Androgen receptor | Activated by testosterone and dihydrotestosterone; plasma testosterone varies considerably in aging C57BL/6 J mice [173]; testosterone protects against aortic aneurysms [174, 175] | Ligand-dependent nuclear receptor | Inhibited | − 2.843 | 0.000528 |
| ERG | ETS-related gene | Regulates cell proliferation, differentiation, angiogenesis, inflammation, and apoptosis; genetic variant associates with aorta aneurysm [176] | Transcription regulator | Inhibited | − 2.183 | 0.000704 |
| ANGPT2 | Angiopoietin-2 | Antagonist for both ANGPT1 and TIE2; vascular remodeling; inhibits development of aortic aneurysm and atherosclerosis [177] | Growth factor | Inhibited | − 2.085 | 0.000771 |
| FAS | FasR, tumor necrosis factor receptor superfamily member 6 (TNFRSF6) | Death receptor; activation by its ligand (Fas ligand, FasL) leads to apoptosis; apoptosis of VSMCs through the Fas-FasL pathway contribute to the pathogenesis of aorta aneurysms [178]; circulating FasL increases with age in humans [179] | Transmembrane receptor | Activated | 2.226 | 0.00217 |
| AHR | Aryl hydrocarbon receptor | AhR promotes aging phenotypes across species [180]; compounds produced by the microbiota can activate AhR | Ligand-dependent nuclear receptor | Activated | 2.657 | 0.0025 |
| IGF1 | Insulin-like growth factor-1 | In aging circulating levels of IGF-1 decline and circulating levels of IGF-1 binding proteins increase; IGF-1 is critical for maintenance of a youthful vascular phenotype [56–62, 181, 182] | Growth factor | Inhibited | − 2.075 | 0.00299 |
Effect of old blood on SRF-regulated pathways
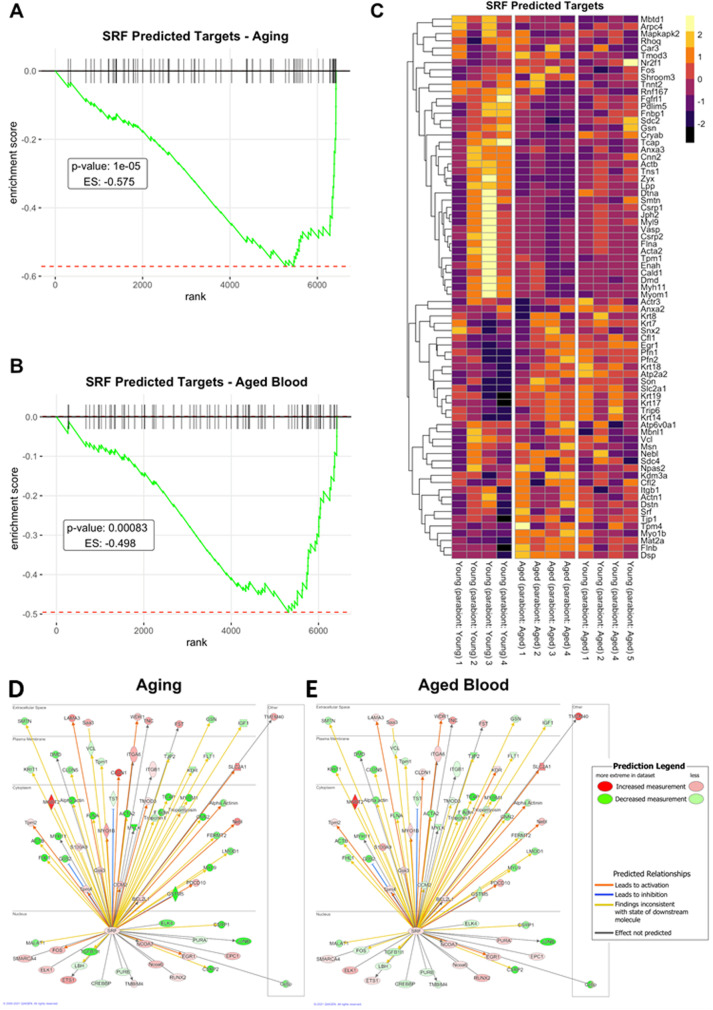
To investigate the contribution of SRF-regulated pathways to the effects induced by old blood we analyzed the expression of known SRF-regulated genes (Table S2). SRF-regulated genes were identified using the MotifMap database [86], and a literature search [87]. A list of SRF-regulated genes was also retrieved from the ingenuity knowledge base.
We used GSEA for interpreting the expression of SRF-driven genes. Figure 7A,B depicts a running-sum statistic (enrichment score) based on Fig. 7C. Note that in aged mice, enrichment scores increased predominantly on the right indicating age-related down-regulation of SRF-driven genes (Fig. 7A). In response to the presence of old blood in young mice, enrichment scores showed similar increases on the right, indicating that the presence of old blood in young mice mimics the effects of aging on SRF-driven genes (Fig. 7B). We also performed comparison analysis using the Ingenuity Pathway Analysis tool to depict aging- and old-blood-effect changes in the SRF-driven genes (Fig. 7D and E, respectively). Note that many SRF-driven genes are similarly dysregulated both in aging and by the presence of old blood in young animals as well.
Fig. 7.
Old blood induces aging-like transcriptomic changes, in part, by dysregulating SRF-driven gene expression Panel A: Gene set enrichment analysis (GSEA) to test the effect of aging on the enrichment of the set of genes whose expression is regulated by SRF (serum response factor) by comparing aorta samples derived from isochronic parabiont young mice [parabiont: young; Y–(Y)] and isochronic parabiont aged mice [parabiont: aged; A–(A)]. Aging-induced gene expression changes were ranked from most up-regulated (left) to most down-regulated (right). Ticks represent genes encoding SRF-regulated proteins. Shown is a running-sum statistic (enrichment score) based on panel C, increasing when a gene is a member of the SRF-driven gene set and decreasing when it is not. Panel B: GSEA showing the effect of exposure to old blood on the enrichment of SRF-regulated genes. Aorta samples derived from isochronic parabiont young mice [parabiont: young; Y–(Y)] and heterochronic parabiont young mice [parabiont: aged; Y–(A)] were compared. Note that in aged mice, enrichment scores increased predominantly on the right indicating age-related down-regulation of SRF-driven genes. In response to the presence of old blood in young mice, enrichment scores showed similar increases on the right, indicating that the presence of old blood in young mice mimics the effects of aging on SRF-driven genes. Panel C: The heat maps are graphical representations of normalized expression values of SRF-driven genes. Hierarchical clustering analysis revealed the similarities on aortic expression profiles of SRF-driven genes in aged mice and old blood exposed young mice. Panel D-E: Comparison analysis using the ingenuity pathway analysis (IPA) tool. The upstream regulator heat maps depict age- and old blood-related changes in the expression of SRF-driven genes. Each symbol on the map represents a gene product in the SRF pathway. The symbols are set to color by age-related (panel D) and old blood exposure-induced (panel E) changes in gene expression (fold change). Red color indicates up-regulation, green color indicates down-regulation
Discussion
The key finding of this study is that relatively short-term exposure to an old humoral environment in young mice can promote the acquisition of accelerated vascular aging phenotypes, including dysregulation of the expression of genes related to pathological vascular remodeling.
Although significant progress has been made in recent years to understand the genesis of cardiovascular aging phenotypes that arise as the consequence of spontaneous, stochastic damage [4, 16, 18, 21, 70, 88–97], the relative contribution of cell-autonomous and non-autonomous mechanisms to vascular aging remained unclear. Here we show for the first time that circulating factors present in the blood of old mice promote accelerated vascular aging mimicking select transcriptional changes associated with old age in the aorta. Our findings support the concept that cell-nonautonomous mechanisms play important roles in driving vascular aging processes and thereby likely promote the pathogenesis of age-related cardiovascular diseases [1]. Previous studies also demonstrated that the presence of old blood in the circulation of young heterochronic parabionts also promotes aging-like phenotypic changes in the liver, heart, and brain [31, 47, 49, 52, 55, 98]. Interestingly, circulating noncellular factors present in the old blood were also shown to confer pro-geronic effects on the central nervous system [99, 100], suggesting that key circulating anti-geronic factor(s) may penetrate the blood–brain barrier and/or exert their deleterious effects on the brain by promoting accelerated aging in the cerebral microcirculation. The remarkable level of the malleability of vascular aging phenotypes in response to both anti-geronic [7, 101–103] and pro-geronic circulating factors highlight the potential for therapeutic interventions to reverse the deleterious effects of aging in the circulatory system via targeting the systemic milieu directly or indirectly.
Aging is associated with structural remodeling of the aorta both in humans [104, 105] and laboratory rodents [106–108]. These aging-induced structural and cellular changes alter the mechanical properties of the aorta and contribute to the genesis of age-related large vessel diseases, including atherosclerosis and aneurysm formation. Here we report that circulating factors present in the blood of old mice induce aging-like changes in vascular remodeling-related gene expression in the aorta of young parabionts. On the basis of previous findings [108–112] we posit that old blood-induced dysregulation of genes driving production, assembly, and deposition of extracellular matrix components, SMC proliferation, and apoptosis contribute to the genesis of accelerated vascular aging phenotypes. In addition, dysregulation of genes regulating ROS metabolism may also contribute to old blood-mediated accelerated vascular aging.
Our GSEA results provide additional transcriptomic insight into the contribution of circulating factors to the pathogenesis of specific age-related vascular diseases. Specifically, we predict that circulating factors present in old blood may exacerbate biological processes involved in the pathogenesis of aorta aneurysm and atherosclerosis. Further studies are evidently needed to test these predictions experimentally.
Importantly, the vasculature comes in contact with each plasma constituent derived from the old blood (including circulating hormones, cytokines, other proteins, peptides, lipid mediators, micropeptides, metabolites, and circulating exosomes) as well as circulating cellular factors, all of which may confer important pro-geronic effects. The exact nature and the cellular origins of circulating pro-geronic factors responsible for the induction of accelerated vascular aging in our studies remain obscure. In the present study, we performed upstream regulator analysis to gain insight into the cellular pathways induced by pro-geronic factors present in old blood. This analysis highlighted the possible role of TGFβ signaling and other pathways involved in vascular remodeling. The possibility indicated by this analysis that pro-geronic factors present in old blood may inhibit the tumor suppressor and vasculoprotective p53 pathway is also highly intriguing.
It is also potentially interesting for the discovery of novel cell-nonautonomous mechanisms of aging that the upstream regulator analysis identified inhibition of SRF-driven pathways as a putative mediator of the effects of old blood on the aorta. Greenberg discovered in 1984 that the addition of young serum to quiescent cells in culture rapidly stimulates c-fos and thereby promotes cell growth [113]. Subsequently, it was demonstrated that several growth factors can have synergistic effects and a short DNA sequence was identified in the promoter region of c-fos and other serum-responsive genes (termed serum response element or SRE), which renders the cells responsive to stimulation by young serum samples [114]. Treisman identified the transcription factor that binds to SRE and named it Serum Response Factor or SRF. [115, 116]. Interestingly, cells of the cardiovascular system, including VSMCs, were found to be particularly sensitive to stimulation by young serum, and SRF was shown to regulate several VSMC-specific genes [87, 117–120]. SRF is down-regulated in aging skeletal muscle in mice and humans [121]. Importantly, muscle-specific knockdown of SRF was shown to promote muscle atrophy [121]. Future studies should determine the roles of dilution of young blood factors by old blood [99, 100] and the effects of specific pro-geronic factors in old blood, which interfere with the young blood factors activating SRF-driven pathways.
Interestingly, our analysis also suggests that the presence of old blood inhibits VEGF-A and IGF-1 signaling in young vessels. These findings are potentially significant as VEGF-A signaling in the vasculature is known to decrease with age, and recent studies show that an increase in circulatory VEGF-A in aged mice exerts rejuvenating effects on the aged vasculature [122]. Similarly, circulating free IGF-1 also declines in aging [123–126], in part, due to an increased presence of IGF-1 binding proteins in the circulation [127–129]. Previous studies provided ample evidence that decreases in circulating levels of free IGF-1 [59] contribute to the genesis of vascular aging phenotypes, including endothelial dysfunction [60], impaired autoregulation of cerebral blood flow [61], pathological remodeling of the extracellular matrix and the media [57], increased atherogenesis [130], impaired vascular oxidative stress resistance [131, 132], impaired angiogenesis, and capillary rarefaction [133]. Follow-up investigations should further interrogate experimentally the role of disrupted VEGF-A and IGF-1 signaling in the deleterious vascular effects of old blood transfer.
Other circulating factors, whose levels are altered in young mice by heterochronic parabiosis or systemic administration of old plasma or blood and which may confer aging-like effects in multiple organs (e.g., brain, heart, and skeletal muscle) include β2-microglobulin and TGFβ family member cytokines [31, 52]. There is also evidence that TNFα [134] and other inflammatory cytokines (e.g., CCL11) may serve as circulating pro-geronic factors. However, transcriptomic analysis of the aortas did not indicate that these cytokines play a central role in old blood-mediated accelerated aging in the mouse aorta. Factors secreted by senescent cells in various organs of aged mice (the senescence-associated secretory phenotype [SASP] [135, 136]), which also are present in the systemic circulation, have also been proposed to drive age-related dysfunction [4].
Perspectives
The present results support the remarkable plasticity of vascular aging in preclinical models and its amenability to modulation by circulating pro-geronic/old blood factors. Follow-up investigations are warranted to define the exact nature of the circulating old blood factors that regulate critical pathways involved in vascular aging. Studies using blood exchange paradigms, in which pro-geronic factors are diluted by replacing half of the plasma in mice with saline containing 5% albumin, suggest that noncellular factors play a key role in the mediation of the old blood effects in many organs [99]. Nevertheless, future studies are warranted to determine the potential roles of aged leukocytes that exhibit a pro-inflammatory phenotype in the pathogenesis of age-related vascular diseases [137]. Interesting in that regard is that 10 to 20% of adults aged 70 or older exhibit clonal hematopoiesis of indeterminate potential (CHIP), characterized by somatic mutations in leukocytes, which associates with a pro-inflammatory status and increased risk for atherogenesis [138–141]. Future studies should also determine whether the observed cell non-autonomous mechanisms of vascular aging are not strain- or species-dependent and elucidate their role in humans. We used an 8-week parabiosis protocol, which represents ~ 6% of the maximum lifespan potential of the mouse strain used. This is biologically equivalent to ~ 6 years for human life. Studies (e.g., using a serum transfer paradigm) are warranted to determine how shorter exposures to circulating pro-geronic and/or anti-geronic factors affect vascular phenotypes. Subsequent studies are also warranted to identify the critical organ(s), tissues, and cell types, which contribute to the synthesis/release of the circulating pro-geronic factors that drive vascular aging processes. A potentially highly promising area of research includes the investigation of the role of factors secreted by senescent cells in distant organs (e.g., the adipose tissue [142–145]). Finally, studies targeting cell non-autonomous mechanisms of vascular aging identified using the aforementioned approaches may lead to the development of new treatments for the prevention of age-related vascular diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the American Heart Association, the American Federation for Aging Research (DMH; Irene/Diamond Postdoctoral Transition Award to PB), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, K01AG073614), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS, the Presbyterian Health Foundation, the Reynolds Foundation, the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (P20GM125528), the Einstein Nathan Shock Center (P30AG038072) and by the NKFIH (Nemzeti Kardiovaszkularis Laboratorium) and the TKP 2021. Analysis help was provided by the Oklahoma Medical Research Foundation Quantitative Analysis Core supported by COBRE grant 1 P30 GM110766-01 and the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS. The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Heart Association, or the Presbyterian Health Foundation.
Declarations
Competing interest
Dr. Anna Csiszar serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences, and GeroScience. Dr. Andriy Yabluchanskiy serves as Guest Editor for The American Journal of Physiology-Heart and Circulatory Physiology. Dr. Derek Huffman serves as Associate Editor for GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience and as Consulting Editor for The American Journal of Physiology-Heart and Circulatory Physiology.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tamas Kiss, Ádám Nyúl-Tóth, Rafal Gulej, Stefano Tarantini, Tamas Csipo, Derek M. Huffman, Anna Csiszar, and Zoltan Ungvari contributed equally to this work.
Contributor Information
Derek M. Huffman, Email: derek.huffman@einstein.yu.edu
Anna Csiszar, Email: anna-csiszar@ouhsc.edu.
References
- 1.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health, United States, 2016: with chartbook on long-term trends in health Hyattsville (MD); 2017. [PubMed]
- 3.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 4.Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75:931–941. doi: 10.1016/j.jacc.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A and Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17. [DOI] [PMC free article] [PubMed]
- 6.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 11.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 12.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2.- and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Lung Cell Mol Physiol. 2006;291:H2698–704. doi: 10.1152/ajpheart.00534.2006. [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Lung Cell Mol Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 14.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 15.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE and Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response Am J Physiol Heart Circ Physiol. 2011;301:H363-72. [DOI] [PMC free article] [PubMed]
- 18.Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A, Ungvari Z. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019;41:533–542. doi: 10.1007/s11357-019-00101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE and Ungvari Z. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci. 2014. [DOI] [PMC free article] [PubMed]
- 20.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A and Ungvari Z. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for prevention of vascular cognitive impairment GeroScience. 2019;41:619–630. [DOI] [PMC free article] [PubMed]
- 23.Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Csipo T, Nyul-Toth A, Lipecz A, Szabo C, Farkas E, Wren JD, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41:419–439. doi: 10.1007/s11357-019-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L and sinclair DA. Impairment of an Endothelial NAD(+)-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell. 2018;173:74–89 e20. [DOI] [PMC free article] [PubMed]
- 25.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashpole NM, Herron JC, Mitschelen MC, Farley JA, Logan S, Yan H, Ungvari Z, Hodges EL, Csiszar A, Ikeno Y, Humphrey MB and Sonntag WE. IGF-1 regulates vertebral bone aging through sex-specific and time-dependent mechanisms. J Bone Miner Res. 2015. [DOI] [PMC free article] [PubMed]
- 27.Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison EJ, Champagne DP, Dzieciatkowska M, Nemkov T, Zimring JC, Hansen KC, Guan F, Huffman DM, Santambrogio L and D'Alessandro A. Parabiosis incompletely reverses aging-induced metabolic changes and oxidant stress in mouse red blood cells. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed]
- 31.Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun. 2016;7:13363. doi: 10.1038/ncomms13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludwig FC, Elashoff RM. Mortality in syngeneic rat parabionts of different chronological age. Trans N Y Acad Sci. 1972;34:582–587. doi: 10.1111/j.2164-0947.1972.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 33.Horrington EM, Pope F, Lunsford W, Mc CC. Age changes in the bones, blood pressure, and diseases of rats in parabiosis. Gerontologia. 1960;4:21–31. doi: 10.1159/000210970. [DOI] [PubMed] [Google Scholar]
- 34.Lunsford WR, Mc CC, Lupien PJ, Pope FE, Sperling G. Parabiosis as a method for studying factors which affect aging in rats. Gerontologia. 1963;7:1–8. doi: 10.1159/000211170. [DOI] [PubMed] [Google Scholar]
- 35.McCay CM, Pope F, Lunsford W, Sperling G, Sambhavaphol P. Parabiosis between old and young rats. Gerontologia. 1957;1:7–17. doi: 10.1159/000210677. [DOI] [PubMed] [Google Scholar]
- 36.Bitto A, Kaeberlein M. Rejuvenation: it's in our blood. Cell Metab. 2014;20:2–4. doi: 10.1016/j.cmet.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannata A, Marcon G, Cimmino G, Camparini L, Ciucci G, Sinagra G, Loffredo FS. Role of circulating factors in cardiac aging. J Thorac Dis. 2017;9:S17–S29. doi: 10.21037/jtd.2017.03.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan X, Wheatley EG, Villeda SA. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu Rev Neurosci. 2017;40:251–272. doi: 10.1146/annurev-neuro-072116-031357. [DOI] [PubMed] [Google Scholar]
- 40.Flemming A. Cardiovascular disease: rejuvenating the ageing heart. Nat Rev Drug Discov. 2013;12:503. doi: 10.1038/nrd4064. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh AK, O'Brien M, Mau T, Qi N, Yung R. Adipose tissue senescence and inflammation in aging is reversed by the young milieu. J Gerontol A Biol Sci Med Sci. 2019;74:1709–1715. doi: 10.1093/gerona/gly290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gontier G, Iyer M, Shea JM, Bieri G, Wheatley EG, Ramalho-Santos M, Villeda SA. Tet2 Rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep. 2018;22:1974–1981. doi: 10.1016/j.celrep.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982;156:1767–79. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirayama R, Takemura K, Nihei Z, Ichikawa W, Takagi Y, Mishima Y, Utsuyama M, Hirokawa K. Differential effect of host microenvironment and systemic humoral factors on the implantation and the growth rate of metastatic tumor in parabiotic mice constructed between young and old mice. Mech Ageing Dev. 1993;71:213–221. doi: 10.1016/0047-6374(93)90085-6. [DOI] [PubMed] [Google Scholar]
- 45.Katsimpardi L, Kuperwasser N, Camus C, Moigneu C, Chiche A, Tolle V, Li H, Kokovay E and Lledo PM. Systemic GDF11 stimulates the secretion of adiponectin and induces a calorie restriction-like phenotype in aged mice. Aging Cell. 2019:e13038. [DOI] [PMC free article] [PubMed]
- 46.Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, Soifer I, van Bruggen N, Kolumam GA, Villeda SA, Lamba DA, Jasper H. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab. 2019;1:276–290. doi: 10.1038/s42255-018-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Cherian R, Jin K. Systemic milieu and age-related deterioration. Geroscience. 2019;41:275–284. doi: 10.1007/s11357-019-00075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeCarolis NA, Kirby ED, Wyss-Coray T and Palmer TD. The role of the microenvironmental niche in declining stem-cell functions associated with biological aging. Cold Spring Harb Perspect Med. 2015;5. [DOI] [PMC free article] [PubMed]
- 51.Middeldorp J, Lehallier B, Villeda SA, Miedema SS, Evans E, Czirr E, Zhang H, Luo J, Stan T, Mosher KI, Masliah E, Wyss-Coray T. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 2016;73:1325–1333. doi: 10.1001/jamaneurol.2016.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA. Beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karin O, Alon U. Senescent cell accumulation mechanisms inferred from parabiosis. Geroscience. 2020;43(1):329–41. 10.1007/s11357-020-00286-x. [DOI] [PMC free article] [PubMed]
- 54.Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727–748. doi: 10.1007/s11357-020-00180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yousefzadeh MJ, Wilkinson JE, Hughes B, Gadela N, Ladiges WC, Vo N, Niedernhofer LJ, Huffman DM, Robbins PD. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience. 2020;42:951–961. doi: 10.1007/s11357-020-00185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, Ungvari Z and Csiszar A. IGF-1 deficiency promotes pathological remodeling of cerebral arteries: a potential mechanism contributing to the pathogenesis of intracerebral hemorrhages in aging. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed]
- 58.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, Ungvari A, Nyul-Toth A, Schwartzman ML, Benyo Z, Sonntag WE, Csiszar A, Ungvari Z. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: implications for brain aging. Geroscience. 2021;43:901–911. doi: 10.1007/s11357-021-00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castellano JM, Palner M, Li SB, Freeman GM, Jr, Nguyen A, Shen B, Stan T, Mosher KI, Chin FT, de Lecea L, Luo J, Wyss-Coray T. In vivo assessment of behavioral recovery and circulatory exchange in the peritoneal parabiosis model. Sci Rep. 2016;6:29015. doi: 10.1038/srep29015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cuervo AM, Huffman DM, Vijg J, Milman S, Singh R, Barzilai N. Einstein-Nathan Shock Center: translating the hallmarks of aging to extend human health span. Geroscience. 2021;43:2167–2182. doi: 10.1007/s11357-021-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris RB. Loss of body fat in lean parabiotic partners of ob/ob mice. Am J Physiol. 1997;272:R1809–R1815. doi: 10.1152/ajpregu.1997.272.6.R1809. [DOI] [PubMed] [Google Scholar]
- 67.Harris RB. Parabiosis between db/db and ob/ob or db/+ mice. Endocrinology. 1999;140:138–145. doi: 10.1210/endo.140.1.6449. [DOI] [PubMed] [Google Scholar]
- 68.Imperio CG, McFalls AJ, Colechio EM, Masser DR, Vrana KE, Grigson PS, Freeman WM. Assessment of individual differences in the rat nucleus accumbens transcriptome following taste-heroin extended access. Brain Res Bull. 2016;123:71–80. doi: 10.1016/j.brainresbull.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Galvan V, Ballabh P, Richardson A, Freeman WM, Wren JD, Deak F, Ungvari Z and Csiszar A. Obesity in aging exacerbates neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed]
- 70.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, Csipo T, Farkas E, Wren JD, Garman L, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020;42:527–546. doi: 10.1007/s11357-020-00165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 73.Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2015;4:1521. doi: 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the r/bioconductor package biomaRt. Nat Protoc. 2009;4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kramer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korotkevich G, Sukhov V, Budin N, Shpak B, Artyomov MN and Sergushichev A. Fast gene set enrichment analysis. https://wwwbiorxivorg/content/101101/060012v3. 2021.
- 81.Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727–748. doi: 10.1007/s11357-020-00180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng J, Ni Z, Gu W, Chen Q, Nowak WN, Chen T, Issa Bhaloo S, Zhang Z, Hu Y, Zhou B, Zhang L, Xu Q. Single-cell gene profiling and lineage tracing analyses revealed novel mechanisms of endothelial repair by progenitors. Cell Mol Life Sci. 2020;77:5299–5320. doi: 10.1007/s00018-020-03480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A, Ungvari Z. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience. 2020;42:429–444. doi: 10.1007/s11357-020-00177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wren JD, Garner HR. Shared relationship analysis: ranking set cohesion and commonalities within a literature-derived relationship network. Bioinformatics. 2004;20:191–198. doi: 10.1093/bioinformatics/btg390. [DOI] [PubMed] [Google Scholar]
- 85.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daily K, Patel VR, Rigor P, Xie X, Baldi P. MotifMap: integrative genome-wide maps of regulatory motif sites for model species. BMC Bioinformatics. 2011;12:495. doi: 10.1186/1471-2105-12-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 88.Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA and Ungvari ZI. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;316(6):H1253–H1266. 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed]
- 89.Rojas-Vazquez S, Blasco-Chamarro L, Lopez-Fabuel I, Martinez-Manez R, Farinas I. Vascular senescence: a potential bridge between physiological aging and neurogenic decline. Front Neurosci. 2021;15:666881. doi: 10.3389/fnins.2021.666881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ungvari Z, Tarantini S, Nyul-Toth A, Kiss T, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Benyo Z, Csiszar A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: from increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience. 2019;41:727–738. doi: 10.1007/s11357-019-00107-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ, Clark JE, Punjabi PP, Awad W, Torella D, Tchkonia T, Kirkland JL, Ellison-Hughes GM. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell. 2019;18:e12931. doi: 10.1111/acel.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y, Bloom SI and Donato AJ. The role of senescence, telomere dysfunction and shelterin in vascular aging. Microcirculation. 2018:e12487. [DOI] [PMC free article] [PubMed]
- 93.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313:H890–H895. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gevaert AB, Shakeri H, Leloup AJ, Van Hove CE, De Meyer GRY, Vrints CJ, Lemmens K and Van Craenenbroeck EM. Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ Heart Fail. 2017;10. [DOI] [PubMed]
- 95.Uryga AK, Bennett MR. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J Physiol. 2016;594:2115–2124. doi: 10.1113/JP270923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Regina C, Panatta E, Candi E, Melino G, Amelio I, Balistreri CR, Annicchiarico-Petruzzelli M, Di Daniele N, Ruvolo G. Vascular ageing and endothelial cell senescence: molecular mechanisms of physiology and diseases. Mech Ageing Dev. 2016;159:14–21. doi: 10.1016/j.mad.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Gonzalez-Armenta JL, Li N, Lee RL, Lu B, Molina AJA. Heterochronic parabiosis: old blood induces changes in mitochondrial structure and function of young mice. J Gerontol A Biol Sci Med Sci. 2021;76:434–439. doi: 10.1093/gerona/glaa299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mehdipour M, Mehdipour T, Skinner CM, Wong N, Liu C, Chen CC, Jeon OH, Zuo Y, Conboy MJ and Conboy IM. Plasma dilution improves cognition and attenuates neuroinflammation in old mice. Geroscience. 2021;43(1):1–18. 10.1007/s11357-020-00297-8. [DOI] [PMC free article] [PubMed]
- 100.Mehdipour M, Skinner C, Wong N, Lieb M, Liu C, Etienne J, Kato C, Kiprov D, Conboy MJ, Conboy IM. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin. Aging (Albany NY) 2020;12:8790–8819. doi: 10.18632/aging.103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:235–249. doi: 10.1093/gerona/gls158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mehdipour M, Etienne J, Chen CC, Gathwala R, Rehman M, Kato C, Liu C, Liu Y, Zuo Y, Conboy MJ, Conboy IM. Rejuvenation of brain, liver and muscle by simultaneous pharmacological modulation of two signaling determinants, that change in opposite directions with age. Aging (Albany NY) 2019;11:5628–5645. doi: 10.18632/aging.102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, Mitchell GF, Benjamin EJ, Vasan RS. Aortic root remodeling over the adult life course: longitudinal data from the Framingham Heart Study. Circulation. 2010;122:884–890. doi: 10.1161/CIRCULATIONAHA.110.937839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Redheuil A, Yu WC, Mousseaux E, Harouni AA, Kachenoura N, Wu CO, Bluemke D, Lima JA. Age-related changes in aortic arch geometry: relationship with proximal aortic function and left ventricular mass and remodeling. J Am Coll Cardiol. 2011;58:1262–1270. doi: 10.1016/j.jacc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spronck B, Ferruzzi J, Bellini C, Caulk AW, Murtada SI, Humphrey JD. Aortic remodeling is modest and sex-independent in mice when hypertension is superimposed on aging. J Hypertens. 2020;38:1312–1321. doi: 10.1097/HJH.0000000000002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hawes JZ, Cocciolone AJ, Cui AH, Griffin DB, Staiculescu MC, Mecham RP, Wagenseil JE. Elastin haploinsufficiency in mice has divergent effects on arterial remodeling with aging depending on sex. Am J Physiol Heart Circ Physiol. 2020;319:H1398–H1408. doi: 10.1152/ajpheart.00517.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wheeler JB, Mukherjee R, Stroud RE, Jones JA, Ikonomidis JS. Relation of murine thoracic aortic structural and cellular changes with aging to passive and active mechanical properties. J Am Heart Assoc. 2015;4:e001744. doi: 10.1161/JAHA.114.001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/S0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 110.Bruel A, Oxlund H. Growth hormone influences the content and composition of collagen in the aorta from old rats. Mech Ageing Dev. 2002;123:627–635. doi: 10.1016/S0047-6374(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 111.Rammos C, Hendgen-Cotta UB, Deenen R, Pohl J, Stock P, Hinzmann C, Kelm M, Rassaf T. Age-related vascular gene expression profiling in mice. Mech Ageing Dev. 2014;135:15–23. doi: 10.1016/j.mad.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 112.Gao P, Gao P, Choi M, Chegireddy K, Slivano OJ, Zhao J, Zhang W, Long X. Transcriptome analysis of mouse aortae reveals multiple novel pathways regulated by aging. Aging (Albany NY) 2020;12:15603–15623. doi: 10.18632/aging.103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greenberg ME, Ziff EB. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 114.Chai J, Tarnawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol. 2002;53:147–157. [PubMed] [Google Scholar]
- 115.Norman C, Runswick M, Pollock R, Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 116.Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987;6:2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Galmiche G, Labat C, Mericskay M, Aissa KA, Blanc J, Retailleau K, Bourhim M, Coletti D, Loufrani L, Gao-Li J, Feil R, Challande P, Henrion D, Decaux JF, Regnault V, Lacolley P, Li Z. Inactivation of serum response factor contributes to decrease vascular muscular tone and arterial stiffness in mice. Circ Res. 2013;112:1035–1045. doi: 10.1161/CIRCRESAHA.113.301076. [DOI] [PubMed] [Google Scholar]
- 118.Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype. Proc Natl Acad Sci U S A. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Werth D, Grassi G, Konjer N, Dapas B, Farra R, Giansante C, Kandolf R, Guarnieri G, Nordheim A, Heidenreich O. Proliferation of human primary vascular smooth muscle cells depends on serum response factor. Eur J Cell Biol. 2010;89:216–224. doi: 10.1016/j.ejcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 121.Lahoute C, Sotiropoulos A, Favier M, Guillet-Deniau I, Charvet C, Ferry A, Butler-Browne G, Metzger D, Tuil D, Daegelen D. Premature aging in skeletal muscle lacking serum response factor. PLoS One. 2008;3:e3910. doi: 10.1371/journal.pone.0003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grunewald M, Kumar S, Sharife H, Volinsky E, Gileles-Hillel A, Licht T, Permyakova A, Hinden L, Azar S, Friedmann Y, Kupetz P, Tzuberi R, Anisimov A, Alitalo K, Horwitz M, Leebhoff S, Khoma OZ, Hlushchuk R, Djonov V, Abramovitch R, Tam J and Keshet E. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021;373. [DOI] [PubMed]
- 123.Di Somma C, Brunelli V, Savanelli MC, Scarano E, Savastano S, Lombardi G, Colao A. Somatopause: state of the art. Minerva Endocrinol. 2011;36:243–255. [PubMed] [Google Scholar]
- 124.Sanders JL, Guo W, O'Meara ES, Kaplan RC, Pollak MN, Bartz TM, Newman AB, Fried LP, Cappola AR. Trajectories of IGF-I predict mortality in older adults: the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2018;73:953–959. doi: 10.1093/gerona/glx143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaplan RC, Buzkova P, Cappola AR, Strickler HD, McGinn AP, Mercer LD, Arnold AM, Pollak MN, Newman AB. Decline in circulating insulin-like growth factors and mortality in older adults: cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97:1970–1976. doi: 10.1210/jc.2011-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bann D, Holly JM, Lashen H, Hardy R, Adams J, Kuh D, Ong KK, Ben-Shlomo Y. Changes in insulin-like growth factor-I and -II associated with fat but not lean mass in early old age. Obesity (Silver Spring) 2015;23:692–698. doi: 10.1002/oby.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van den Beld AW, Carlson OD, Doyle ME, Rizopoulos D, Ferrucci L, van der Lely AJ, Egan JM. IGFBP-2 and aging: a 20-year longitudinal study on IGFBP-2, IGF-I, BMI, insulin sensitivity and mortality in an aging population. Eur J Endocrinol. 2019;180:109–116. doi: 10.1530/EJE-18-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mohan S, Farley JR, Baylink DJ. Age-related changes in IGFBP-4 and IGFBP-5 levels in human serum and bone: implications for bone loss with aging. Prog Growth Factor Res. 1995;6:465–473. doi: 10.1016/0955-2235(95)00027-5. [DOI] [PubMed] [Google Scholar]
- 129.Alessio N, Squillaro T, Di Bernardo G, Galano G, De Rosa R, Melone MA, Peluso G and Galderisi U. Increase of circulating IGFBP-4 following genotoxic stress and its implication for senescence. Elife. 2020;9. [DOI] [PMC free article] [PubMed]
- 130.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lahteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res. 2012;110:1252–1264. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schafer MJ, Zhang X, Kumar A, Atkinson EJ, Zhu Y, Jachim S, Mazula DL, Brown AK, Berning M, Aversa Z, Kotajarvi B, Bruce CJ, Greason KL, Suri RM, Tracy RP, Cummings SR, White TA and LeBrasseur NK. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed]
- 136.Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H, Weigand BM, Patel AD, Pirtskhalava T, Inman CL, Johnson KO, Dickinson SL, Rocha A, Schafer MJ, Zhu Y, Allison DB, von Zglinicki T, LeBrasseur NK, Tchkonia T, Neretti N, Passos JF, Kirkland JL, Jurk D. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20:e13296. doi: 10.1111/acel.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 138.Fuster JJ, Walsh K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age-related cardiovascular disease. Circ Res. 2018;122:523–532. doi: 10.1161/CIRCRESAHA.117.312115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khetarpal SA, Qamar A, Bick AG, Fuster JJ, Kathiresan S, Jaiswal S, Natarajan P. Clonal hematopoiesis of indeterminate potential reshapes age-related CVD: JACC review topic of the week. J Am Coll Cardiol. 2019;74:578–586. doi: 10.1016/j.jacc.2019.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Libby P, Ebert BL. CHIP (Clonal hematopoiesis of indeterminate potential): potent and newly recognized contributor to cardiovascular risk. Circulation. 2018;138:666–668. doi: 10.1161/CIRCULATIONAHA.118.034392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dou H, Feher A, Davila AC, Romero MJ, Patel VS, Kamath VM, Gooz MB, Rudic RD, Lucas R, Fulton DJ, Weintraub NL, Bagi Z. Role of adipose tissue endothelial ADAM17 in age-related coronary microvascular dysfunction. Arterioscler Thromb Vasc Biol. 2017;37:1180–1193. doi: 10.1161/ATVBAHA.117.309430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. doi: 10.1093/gerona/gls238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhu W, Kim BC, Wang M, Huang J, Isak A, Bexiga NM, Monticone R, Ha T, Lakatta EG, An SS. TGFbeta1 reinforces arterial aging in the vascular smooth muscle cell through a long-range regulation of the cytoskeletal stiffness. Sci Rep. 2018;8:2668. doi: 10.1038/s41598-018-20763-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mercer J, Figg N, Stoneman V, Braganza D, Bennett MR. Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ Res. 2005;96:667–674. doi: 10.1161/01.RES.0000161069.15577.ca. [DOI] [PubMed] [Google Scholar]
- 149.George SJ, Angelini GD, Capogrossi MC, Baker AH. Wild-type p53 gene transfer inhibits neointima formation in human saphenous vein by modulation of smooth muscle cell migration and induction of apoptosis. Gene Ther. 2001;8:668–676. doi: 10.1038/sj.gt.3301431. [DOI] [PubMed] [Google Scholar]
- 150.Sawin CT, Carlson HE, Geller A, Castelli WP, Bacharach P. Serum prolactin and aging: basal values and changes with estrogen use and hypothyroidism. J Gerontol. 1989;44:M131–M135. doi: 10.1093/geronj/44.4.M131. [DOI] [PubMed] [Google Scholar]
- 151.Daugherty RL, Serebryannyy L, Yemelyanov A, Flozak AS, Yu HJ, Kosak ST, deLanerolle P, Gottardi CJ. alpha-Catenin is an inhibitor of transcription. Proc Natl Acad Sci U S A. 2014;111:5260–5265. doi: 10.1073/pnas.1308663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marchand A, Atassi F, Gaaya A, Leprince P, Le Feuvre C, Soubrier F, Lompre AM, Nadaud S. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011;10:220–232. doi: 10.1111/j.1474-9726.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 153.Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, Lakatta EG. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS ONE. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chavkin NW, Sano S, Wang Y, Oshima K, Ogawa H, Horitani K, Sano M, MacLauchlan S, Nelson A, Setia K, Vippa T, Watanabe Y, Saucerman JJ, Hirschi KK, Gokce N, Walsh K. The Cell surface receptors ror1/2 control cardiac myofibroblast differentiation. J Am Heart Assoc. 2021;10:e019904. doi: 10.1161/JAHA.120.019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu S, Liu Y, You T, Heath J, Xu L, Zheng X, Wang A, Wang Y, Li F, Yang F, Cao Y, Zhang H, van Gils JM, van Zonneveld AJ, Jo H, Wu Q, Zhang Y, Tang C, Zhu L. Vascular semaphorin 7A upregulation by disturbed flow promotes atherosclerosis through endothelial beta1 integrin. Arterioscler Thromb Vasc Biol. 2018;38:335–343. doi: 10.1161/ATVBAHA.117.310491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liao J, Yang X, Lin Q, Liu S, Xie Y, Xia Y, Li HH. Inhibition of the ubiquitin-activating enzyme UBA1 suppresses diet-induced atherosclerosis in apolipoprotein e-knockout mice. J Immunol Res. 2020;2020:7812709. doi: 10.1155/2020/7812709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao L, Hall JA, Levenkova N, Lee E, Middleton MK, Zukas AM, Rader DJ, Rux JJ, Pure E. CD44 regulates vascular gene expression in a proatherogenic environment. Arterioscler Thromb Vasc Biol. 2007;27:886–892. doi: 10.1161/01.ATV.0000259362.10882.c5. [DOI] [PubMed] [Google Scholar]
- 159.MacTaggart JN, Xiong W, Knispel R, Baxter BT. Deletion of CCR2 but not CCR5 or CXCR3 inhibits aortic aneurysm formation. Surgery. 2007;142:284–288. doi: 10.1016/j.surg.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 160.Yousefzadeh MJ, Schafer MJ, Noren Hooten N, Atkinson EJ, Evans MK, Baker DJ, Quarles EK, Robbins PD, Ladiges WC, LeBrasseur NK and Niedernhofer LJ. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell. 2018;17. [DOI] [PMC free article] [PubMed]
- 161.Hong S, Freeberg MA, Han T, Kamath A, Yao Y, Fukuda T, Suzuki T, Kim JK and Inoki K. LARP1 functions as a molecular switch for mTORC1-mediated translation of an essential class of mRNAs. Elife. 2017;6. [DOI] [PMC free article] [PubMed]
- 162.Lindquist Liljeqvist M, Hultgren R, Bergman O, Villard C, Kronqvist M, Eriksson P, Roy J. Tunica-specific transcriptome of abdominal aortic aneurysm and the effect of intraluminal thrombus, smoking, and diameter growth rate. Arterioscler Thromb Vasc Biol. 2020;40:2700–2713. doi: 10.1161/ATVBAHA.120.314264. [DOI] [PubMed] [Google Scholar]
- 163.Welsh P, Murray HM, Ford I, Trompet S, de Craen AJ, Jukema JW, Stott DJ, McInnes IB, Packard CJ, Westendorp RG, Sattar N, PS Group Circulating interleukin-10 and risk of cardiovascular events: a prospective study in the elderly at risk. Arterioscler Thromb Vasc Biol. 2011;31:2338–44. doi: 10.1161/ATVBAHA.111.231795. [DOI] [PubMed] [Google Scholar]
- 164.Yuan Y, Yan L, Wu QQ, Zhou H, Jin YG, Bian ZY, Deng W, Yang Z, Shen DF, Zeng XF, Wang SS, Li H, Tang QZ. Mnk1 (mitogen-activated protein kinase-interacting kinase 1) deficiency aggravates cardiac remodeling in mice. hypertension. 2016;68:1393–1399. doi: 10.1161/HYPERTENSIONAHA.116.07906. [DOI] [PubMed] [Google Scholar]
- 165.Bansal R, Nakagawa S, Yazdani S, van Baarlen J, Venkatesh A, Koh AP, Song WM, Goossens N, Watanabe H, Beasley MB, Powell CA, Storm G, Kaminski N, van Goor H, Friedman SL, Hoshida Y, Prakash J. Integrin alpha 11 in the regulation of the myofibroblast phenotype: implications for fibrotic diseases. Exp Mol Med. 2017;49:e396. doi: 10.1038/emm.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Branchetti E, Poggio P, Sainger R, Shang E, Grau JB, Jackson BM, Lai EK, Parmacek MS, Gorman RC, Gorman JH, Bavaria JE, Ferrari G. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc Res. 2013;100:316–324. doi: 10.1093/cvr/cvt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fan WH, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur J Cell Biol. 2000;79:915–923. doi: 10.1078/0171-9335-00122. [DOI] [PubMed] [Google Scholar]
- 168.Ruperez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003;108:1499–1505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- 169.Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fulop GA, Kiss T, Csiszar A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience. 2017;39:491–498. doi: 10.1007/s11357-017-9995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Miano JM. Role of serum response factor in the pathogenesis of disease. Lab Invest. 2010;90:1274–1284. doi: 10.1038/labinvest.2010.104. [DOI] [PubMed] [Google Scholar]
- 171.Franco CA, Mericskay M, Parlakian A, Gary-Bobo G, Gao-Li J, Paulin D, Gustafsson E, Li Z. Serum response factor is required for sprouting angiogenesis and vascular integrity. Dev Cell. 2008;15:448–461. doi: 10.1016/j.devcel.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 172.Schwaerzer GK, Kalyanaraman H, Casteel DE, Dalton ND, Gu Y, Lee S, Zhuang S, Wahwah N, Schilling JM, Patel HH, Zhang Q, Makino A, Milewicz DM, Peterson KL, Boss GR, Pilz RB. Aortic pathology from protein kinase G activation is prevented by an antioxidant vitamin B12 analog. Nat Commun. 2019;10:3533. doi: 10.1038/s41467-019-11389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nelson JF, Latham KR, Finch CE. Plasma testosterone levels in C57BL/6J male mice: effects of age and disease. Acta Endocrinol (Copenh) 1975;80:744–752. doi: 10.1530/acta.0.0800744. [DOI] [PubMed] [Google Scholar]
- 174.Son BK, Kojima T, Ogawa S, Akishita M. Testosterone inhibits aneurysm formation and vascular inflammation in male mice. J Endocrinol. 2019;241:307–317. doi: 10.1530/JOE-18-0646. [DOI] [PubMed] [Google Scholar]
- 175.Yeap BB, Hyde Z, Norman PE, Chubb SA, Golledge J. Associations of total testosterone, sex hormone-binding globulin, calculated free testosterone, and luteinizing hormone with prevalence of abdominal aortic aneurysm in older men. J Clin Endocrinol Metab. 2010;95:1123–1130. doi: 10.1210/jc.2009-1696. [DOI] [PubMed] [Google Scholar]
- 176.Marsman J, Gimenez G, Day RC, Horsfield JA, Jones GT. A non-coding genetic variant associated with abdominal aortic aneurysm alters ERG gene regulation. Hum Mol Genet. 2020;29:554–565. doi: 10.1093/hmg/ddz256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu H, Moran CS, Trollope AF, Woodward L, Kinobe R, Rush CM, Golledge J. Angiopoietin-2 attenuates angiotensin II-induced aortic aneurysm and atherosclerosis in apolipoprotein E-deficient mice. Sci Rep. 2016;6:35190. doi: 10.1038/srep35190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pisano C, Balistreri CR, Ricasoli A, Ruvolo G. Cardiovascular disease in ageing: an overview on thoracic aortic aneurysm as an emerging inflammatory disease. Mediators Inflamm. 2017;2017:1274034. doi: 10.1155/2017/1274034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang S, Moriarty-Craige SE, Li C, Lynn MJ, Cai J, Jones DP, Sternberg P. Associations of plasma-soluble fas ligand with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:1345–1349. doi: 10.1167/iovs.07-0308. [DOI] [PubMed] [Google Scholar]
- 180.Brinkmann V, Ale-Agha N, Haendeler J, Ventura N. The aryl hydrocarbon receptor (AhR) in the aging process: another puzzling role for this highly conserved transcription factor. Front Physiol. 2019;10:1561. doi: 10.3389/fphys.2019.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age (Dordr) 2016;38:239–258. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, Ungvari Z and Csiszar A. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43(5):2387–2394. 10.1007/s11357-021-00405-2. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.