Abstract
Neuroimaging is widely used to aid in the diagnosis and clinical management of neonates with neonatal encephalopathy (NE). Yet, despite widespread use clinically, there are few published guidelines on neuroimaging for neonates with NE. This review outlines the primary patterns of brain injury associated with hypoxic-ischemic injury in neonates with NE and their frequency, associated neuropathological features, and risk factors. In addition, it provides an overview of neuroimaging methods, including the most widely used scoring systems used to characterize brain injury in these neonates and their utility as predictive biomarkers. Last, recommendations for neuroimaging in neonates with NE are presented.
Keywords: Advanced MRI techniques, Asphyxia, Diffusion-tensor imaging, Diffusion-weighted imaging, Hypoxic-ischemic brain injury, Magnetic resonance spectroscopy imaging, Neonatal encephalopathy, Neonates, Outcome prediction, Predictive values
1. Introduction
Neonatal encephalopathy (NE) in the term neonate is defined as a clinical syndrome of disturbed neurological function [1]. Of affected neonates, a substantial portion will present with perinatal acidosis and a degree of neurological dysfunction and meet diagnostic criteria for neonatal hypoxic-ischemic encephalopathy (HIE). Other neonates will present with NE due to other underlying etiologies, including perinatal stroke, genetic/metabolic syndromes, and infections. The incidence of NE is estimated as 3.0 per 1000 live births in high-income countries, with 1.5 per 1000 live births due to HIE and the remaining due other etiologies [2]. This incidence rises to 9.8–26.5 per 1000 live births in low- and middle-income countries [3]. NE is a major cause of morbidity and mortality [4]. While therapeutic hypothermia has improved the likelihood that neonates with NE due to moderate to severe HIE will survive with improved outcomes through at least school-age in high-income countries, the diagnosis and management of neonates with NE remains a challenge [5].
Neuroimaging is widely employed as both a diagnostic and prognostic tool for NE [6,7]. Over the last three decades, the role of neuroimaging for NE has progressed in parallel with advances in both research and clinical practice. Nearly 40 years ago, Magnetic Resonance Spectroscopy (MRS) first demonstrated that hypoxic-ischemic brain injury evolves, and that, critically, most neurons survive the primary insult only to die in the days and weeks that follow [8]. Indeed, this observation that there was a transient period – a latent phase – before cells died paved the way for neuroprotective therapies, which aim to interrupt the underlying injury cascade, thereby mitigating cell death and long-term neurodevelopment disabilities. Today, therapeutic hypothermia – the first empirically-supported neural rescue therapy for NE – is the standard of care for neonates with moderate to severe NE in high-income countries. Still, even with therapeutic hypothermia, 30% or more of these neonates suffer adverse outcomes [5,9]. What is not known is why these neonates are so severely injured. Are they suffering from subacute or chronic injuries at the time of birth? How does the trajectory of brain development after NE shape long-term neurodevelopmental outcomes? Neuroimaging studies can help address these questions.
The goal of this review is to provide an overview of the utility of neuroimaging for the diagnosis and management of term neonates with NE, concluding with recommendations. Although the focus of this review will be centered on the population of neonates with HIE, the neuroimaging recommendations are broadly applicable to all neonates with NE.
2. Injury patterns on neuroimaging and their relation to neuropathology
Hypoxia-ischemia is associated with several hallmark patterns of brain injury that have been characterized by neuroimaging and neuropathology. These patterns vary in accordance with numerous clinical factors, including the gestational age of the affected neonate, nature and severity of the insult, and timing and efficacy of interventions. Furthermore, these patterns inform our understanding of injury mechanisms and long-term prognosis.
The susceptibility of the developing brain to hypoxic-ischemic injury varies by cell type, with neurons and oligodendroglia typically more susceptible to hypoxia-ischemia, as well as associated astrogliosis and microgliosis [10]. The most common injury observed on neuropathology is selective neuronal necrosis. This term refers to necrosis of neurons in a characteristic, often widespread, distribution, with the topography depending in part on the nature and severity of the insult and gestational age of the neonate.
In this section, patterns of hypoxic-ischemic brain injury are discussed, along with their frequency, the associated neuropathological features, and the risk factors – providing an overall summary in Table 1. In later sections, the relation between these neuroimaging findings and the long-term neurodevelopmental outcomes will be described.
Table 1.
Brain injury patterns observed in neonates with neonatal encephalopathy (NE).
| Pattern of injury | Neuropathological findings |
MR Imaging | Frequency* | Associated risk factors |
|---|---|---|---|---|
| Central/BGT (also known as “cerebrocortical-deep nuclear”) | Neuronal necrosis in BGT, perirolandic cortex and hippocampus | Injury to BGT and perirolandic cortex, with hippocampal injury less well detected | Common 25–75% cases | Sentinel events; severe partial asphyxia with prolonged duration or combination of partial and near-total asphyxia |
| Watershed (also known as “parasagittal” or “borderzone”) | Neuronal necrosis in cerebral cortex in a parasagittal distribution along the borderzone between major cerebral vessels | Injury to the cerebral cortex and/or subadjacent white matter along a parasagittal convexity (intervascular borderzone) | Common 15–45% cases |
Hypotension |
| Punctate white matter (also known as “focal/multifocal”) | Focal necrotic lesions in the periventricular white matter and surrounded by larger areas of diffuse reactive gliosis | Punctate white matter lesions | Non-cystic white matter lesions: 10–20% Severe, cystic lesions: very uncommon. |
Prematurity, but also well documented in term neonates with NE; hypoxia without significant acidosis; inflammation |
| Global (also known as “near-total”) | Widespread neuronal necrosis across the entire neuraxis | Diffuse injury (almost always includes BGT) | 5–10% | Profound or prolonged insult |
| Brainstem ± deep nuclear gray | Neuronal necrosis in select brainstem nuclei and BGT | Brainstem tegmentum ± BGT | 5–20% | Acute total hypoxic-ischemic insult |
| Cerebellum | Cytotoxic edema; reduced neuronal cell density | Focal areas of restricted diffusion in severe cases; lower ADC values in vermis and dentate nuclei | 9% | Unknown |
| Perinatal arterial ischemic stroke | Focal arterial distribution infarct | Perinatal ischemic infarct. Rarely hemorrhagic. | <1–5% | Multifactorial |
| Other | Unknown | Mamillary body injury; hemorrhagic lesions; venous infarcts; sinovenous thrombosis | Unknown | Unknown |
A. Central/basal ganglia – thalamus (BGT) injury pattern.
This pattern is characterized by injury to the BGT and may extend to the cerebral cortex, usually localized to the perirolandic region. On neuropathology, it is often referred to as the “ cerebrocordcal-deep nuclear pattern ”. The BGT pattern is typically bilateral and symmetric. The predominant neuropathological features include selective neuronal necrosis in the basal ganglia and thalamus (especially ventrolateral nuclei of the thalamus and lentiform nuclei) and perirolandic cortex, as well as the hippocampus (pyramidal neurons), in particular, in Sommer’s sector (CA1) in term neonates and the subiculum in preterm neonates [11]. On neuroimaging, the predominant features include injury to the BGT and perirolandic cortex (Fig. 1A). Hippocampal injury is more difficult to detect acutely, even by diffusion-weighted imaging (DWI) [12]; nevertheless, it may be detected chronically as reduced volume by quantitative morphometry [13]. Clinically, the central/BGT pattern is commonly observed following perinatal sentinel events [14,15] and moderate to severe, relatively prolonged insults [11], while experimental studies in primates suggest that this pattern results from a combination of anoxia (e.g., near-total asphyxia) and hypoxia (e.g., partial asphyxia), which may be incurred during a single event or across serial events [16,17].
Fig. 1.
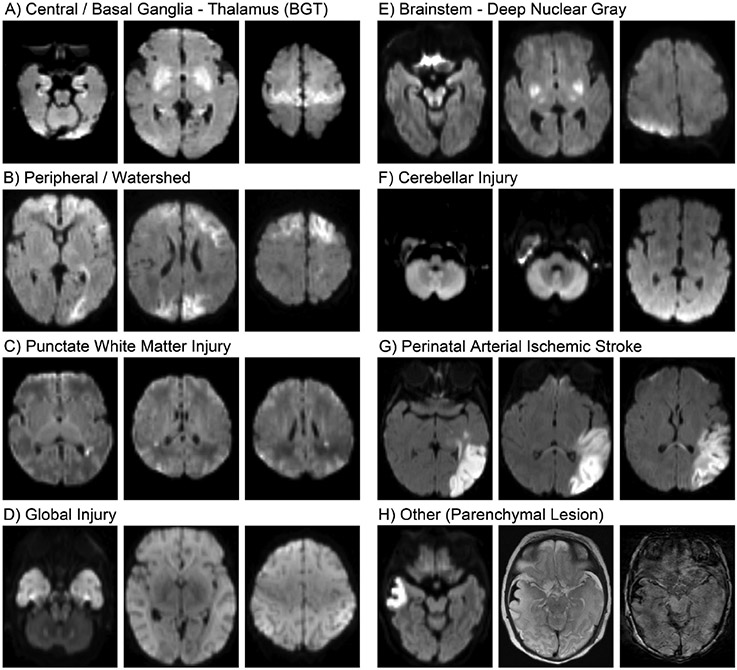
Patterns of brain injury in neonates with NE as shown on DWI images obtained at days 4–5 of life. Details regarding the patterns and associated neuropathological findings and clinical risk factors are found in the text and summarized in Table 1. Note that the parenchyma lesion (H) is associated with a subpial hemorrhage, which is better visualized on T2-weighted images (center) and SWI (right).
B. Watershed injury pattern.
The parasagittal pattern is characterized by injury to the cerebral cortex and/or subadjacent white matter in a parasagittal distribution along the vascular borderzone between the major cerebral arteries (Fig. 1B). For this reason, this pattern is also referred to as the “parasagittal” or “borderzone“ injury pattern. The injury is typically bilateral, although it may be asymmetric. The predominant neuropathological feature is selective neuronal necrosis involving cortical neurons along the parasagittal convexity [11]. Neurons in deeper cortical layers, and, in particular, localized in the depths of sulci are especially affected [18]. In the most severe cases, the area of necrosis extends across the entire parasagittal convexity; however, more commonly, it is localized to posterior parietal-occipital regions. This pattern is among the least common observed at autopsy, especially in isolation, considering many of the neonates with NE presenting with this injury pattern survive; however, it is among the most common patterns by neuroimaging. In clinical and experimental studies, parasagittal injury is associated with prolonged maternal hypotension [11,16], hypoglycemia [19], or sepsis [20].
C. Punctate white matter injury.
White matter injury may be primary or secondary, such as Wallerian degeneration. The primary white matter injury patterns are characterized by injury to the parasagittal white matter (described above) and the periventricular white matter. On neuropathology, this injury is often termed “periventricular leukomalacia (PVL)” and characterized by focal necrotic lesions in the periventricular white matter surrounded by larger areas of diffuse reactive gliosis. On neuroimaging, these are often referred to as “punctate white matter injury” or the “focal/multifocal injury pattern“ [21], and may be visualized on cranial ultrasound, or more often on MRI, as punctate foci of high signal on T1-weighted imaging with or without concomitant restricted diffusion (Fig. 1C). This pattern is relatively common, in particular, among premature neonates and those with a milder degree of NE [21]. Clinically, white matter injury in term neonates with NE has been associated with chorioamnionitis, decreased placental maturation [22], and hypoglycemia [23], as well as pathological genetic mutations [24]. Experimental studies in primates suggest that white matter injury, including PVL, results from clinical circumstances that lead to hypoxia, but without a significant degree of acidosis [16]. A recent study showed that the majority of term neonates with punctate white matter lesions have normal outcomes or mild and non-specific neurologic deficits. The ones who are severely affected usually have an underlying genetic disorder [24].
D. Global injury pattern.
The global pattern, also referred to as “near-total” injury pattern, is the most severe, characterized by diffuse brain injury. The predominant neuropathological feature is widespread neuronal necrosis across essentially all levels of the neuraxis (cortex, deep nuclear gray, midbrain, brainstem). On neuroimaging, the global pattern in characterized by diffuse signal abnormalities in the BGT, cortex, brainstem, and white matter (Fig. 1D). The cerebellum may appear relatively normal; however, quantitative imaging metrics (e.g., apparent diffusion coefficient) may be abnormal [25,26]. Clinically, the global pattern has been associated with very severe and prolonged insults and experimentally with prolonged asphyxia [11,16].
E. Brainstem injury.
This pattern is characterized by injury to the brainstem tegmentum, often associated with injury to the BGT, as described above. The predominant neuropathological features include selective neuronal necrosis in select brainstem nuclei, including the inferior colliculus, nuclei of the 3rd, 4th, 5th (motor), 7th, 8th (cochlear), 9th and 10th cranial nerves as well as the substantia nigra, reticular formation, pontine nuclei, inferior olive, and cuneate and gracilis nuclei, with involvement of the inferior olivary nuclei being the most common brainstem finding in both term and preterm neonates [11]. Injury to the BGT and brainstem tegmentum may be visualized on neuroimaging, in particular on MRI (Fig. 1E) [27]; however, damage to individual nuclei cannot be readily detected using conventional MRI sequences at clinical field strength. Furthermore, while isolated injury to the brainstem ± BGT is well documented on neuropathology, this pattern is relatively rare in neuroimaging studies; more commonly, there is also injury to the cerebral cortex [28]. Experimental studies in primates suggest that injury to the deep nuclear gray and brainstem results from an acute total hypoxic-ischemic event [11].
F. Cerebellar injury.
Cerebellar injury has been largely under-recognized in neonates with NE. Although experimental studies in primates and neuropathological studies in neonates have both demonstrated that cerebellar neurons are vulnerable to hypoxic-ischemic injury [11,26,29], this insult has received less attention in clinical studies. However, recent studies have demonstrated cerebellar abnormalities on DWI as well as a correlation between these abnormalities and microglia activation on neuropathology among neonates with NE (Fig. 1F) [25,26,30].
G. Perinatal arterial ischemic stroke.
A small proportion of neonates with NE display perinatal arterial ischemic stroke on imaging. Perinatal arterial ischemic stroke is characterized by an acute ischemic lesion localized within a cerebral artery distribution, most commonly the middle cerebral artery. On neuropathology, these lesions are characterized as infarcts, affecting all cell types. On neuroimaging, these lesions are best seen on diffusion-weighted imaging on early scans (i.e., first week), indicating they were acquired in the perinatal period and are readily recognized due to their wedge-like shape and localization to a vascular distribution (Fig. 1G). While seizures are the most common clinical manifestation of perinatal arterial ischemic stroke in the neonatal period, some neonates present with clinical features of NE. Clinically, the risk factors for perinatal arterial ischemic stroke include pregnancy complications (e.g., gestational diabetes, preeclampsia) and delivery complications (e.g., vacuum extraction, emergency cesarian section) [31]. Genetic polymorphisms such MTHFR (C677T), Factor V Leiden (G1691A), and prothrombin (G20210A) are no longer considered as risk factors [32].
H. Other injury.
In addition to the hallmark patterns of injury described above, other injury patterns have been identified by neuroimaging in neonates with NE. Mamillary body injury has been recently characterized in this context [33,34]. Other injury include hemorrhages [35], focal ischemic lesions (Fig. 1G) [21], venous infarcts, and sinovenous thrombosis [36].
Finally, it should be acknowledged that while A-H above are presented as distinct patterns, neonates with NE may evidence more than one pattern of injury at once. Furthermore, there is mounting preclinical evidence that inflammatory mechanisms may play a role in the pathogenesis of perinatal brain injury, including NE. Preclinical studies have demonstrated associations between neuroimaging findings and neuropathological features of inflammation [37,38]; however, further work is needed to validate these associations in neonates with NE.
3. Neuroimaging methods
3.1. Ultrasound
Currently, cranial ultrasound (cUS) is not the primary neuroimaging modality in neonates with NE due to presumed perinatal asphyxia. However, it is commonly used in the neonates with NE, with over 50% of all neonatal centers recently surveyed (n = 94) by the Newborn Brain Society (https://newbornbrainsociety.org/news/nncc-sig-survey-cus-ne/) reporting at least one cranial ultrasound during therapeutic hypothermia. The most frequent indication was related to concerns for major intracranial hemorrhage as a potential exclusion criterion for therapeutic hypothermia and to assess for lesions of antenatal onset or abnormalities suggestive of NE mimics.
Importantly, in relation to its neuroimaging diagnostic value, several studies compared cUS and MRI and showed good agreement. Leijser et al. compared data in 23 neonates with NE and found complete agreement in 12 and the same pattern, but more extensive injury, on MRI in 8 neonates [39]. Epelman et al. prospectively compared cUS and MRI performed within 2 h of each other in 76 neonates; MRI examinations were conclusive in 70 neonates and cUS was regarded as positive in 67 with a diagnostic accuracy of 96% [40]. The population, however, was mixed and only 53 neonates with NE showed patterns consistent with NE.
When cUS is performed as part of the admission procedure, it will help identify lesions that developed before birth (e.g., atrophy, porencephaly, ventriculomegaly, germinolytic cysts, lenticulostriate vasculopathy) and may suggest an underlying problem. In the study by Annink et al., these abnormalities were found in 14/83 neonates (17%) [15]. Furthermore, when severe echogenicity in the white matter is clearly present prior to the start of hypothermia, this is suggestive of onset of the insult prior to delivery, and this was significantly associated with an adverse outcome on univariate logistic regression analysis (OR = 5.0; 95% CI 1.4–18.4) [15].
Predominant injury to the thalami and basal ganglia can be recognized with cUS (Fig. 2), but this will take 48–72 h to develop and will not be present on the admission cUS examination if the insult occurred in the perinatal period. This may explain why the cUS data from the Vermont-Oxford database were rather disappointing when compared to MRI, as cUS was performed at a median of 2 days (IQR 1–3) [41]. In this study, deep nuclear gray matter abnormality was seen by early cUS in 140/1994 (7.0%) neonates, compared to 603/2671 (22.6%) on MRI. Increased echogenicity will first become apparent in the thalami and may, in severe cases, subsequently also be visible in the basal ganglia, resulting in the so called “four column” appearance with a line of reduced echogenicity of the PLIC in between (Fig. 2). This appearance is strongly associated with a poor outcome, similar to an abnormal (absent) signal intensity from myelin in the PLIC on MRI [15,42]. In neonates who show a near total pattern of injury on MRI, loss of gray-white matter differentiation with diffuse increased echogenicity and slit-like ventricles will be seen at 48–72 h (Fig. 2). A cUS score for neonates with NE was recently developed and validated and has been shown to be easy to use and predictive of an adverse outcome when performed on day 3–7 with an AUC of 0.90 [43].
Fig. 2.
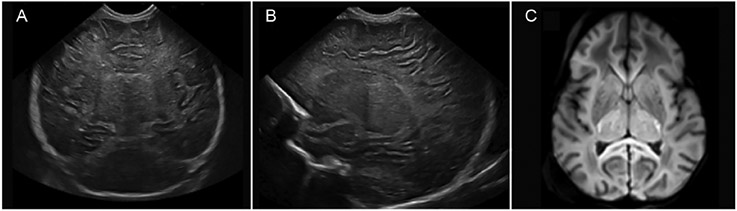
cUS performed on day 2 of life, including coronal (A) and parasagittal (B) views. Increased echogenicity is seen in the thalami and to a lesser degree in the basal ganglia, with reduced echogenicity in between suggestive of involvement of the PLIC. The ventricles are slit-like and there is increased echogenicity of the white matter as well. The axial MRI-DWI, performed on day 3 of life (C), shows extensive diffusion restriction in the thalami as well as in the subcortical white matter and cortex.
Doppler ultrasound can additionally be used to see whether there is luxury perfusion with an increased diastolic flow. The predictive value of the resistance index (S-D/S) during hypothermia is not as high as known from normothermia studies, but becomes comparable following rewarming [44,45]. A recent study performed dynamic colour Doppler sonography during hypothermia to study basal ganglia perfusion, with increased perfusion reported in those who died [46].
3.2. CT scan
Since the Quality Standard Subcommittee of the American Academy of Neurology (AAN) and the Practice Committee of the Child Neurology Society (CNS) jointly published a consensus statement referred to as the Practice Parameter [47] in 2002 suggesting CT as the first-line brain imaging modality in a subgroup of neonates with NE, MRI has been increasingly used in investigations of NE [41]. Although CT is sensitive to blood and calcifications, this modality provides less detailed evaluation of cerebral injury in areas of prognostic importance compared to MRI. Even if the risk of cancer due to radiation exposure has decreased by 50–70% due to dose reduction over the years, radiation-related malignancies and cognitive impairments remain of concern in the pediatric population [48,49]. CT should now be used only in urgent situations, such as evaluation for significant intracranial hemorrhage with midline shift needing a neurosurgical intervention, or for herniation or very severe injury to aid early direction of care, and only when cUS and MRI are not readily available. The 2002 Practice Parameter has since been retired in 2018 by the AAN and CNS.
3.3. Magnetic resonance imaging (MRI)
MRI is the most widely used technique for evaluating brain injury in neonates with NE. The application of MRI may provide the clinicians with the best diagnostic information with exclusion of other causes of NE, such as metabolic disorders or neonatal stroke, and provide help with prognostication and, thereby, guide clinical decision-making and counseling of families.
Conventional MRI sequences for the neonatal brain include anatomic sequences (T1- and T2-weighted imaging) and diffusion-weighted imaging (DWI). T1-weighted sequences assist in assessing myelination and detecting ischemia, subacute hemorrhage, and subacute venous sinus thrombosis. T2-weighted sequences are useful for delineating the gray and white matter interface in the neonatal brain and identifying any white matter signal intensity abnormalities [50]. The presence of acute injury is usually subtle on conventional imaging during the first few days of life, but the findings are gradually more apparent as the T1 hyperintense signal changes and T2 prolongation can be seen 6–10 days after the insult [50].
Diffusion-weighted imaging takes advantage of the random motion of water molecules within the brain to provide an indirect measure of the regional cerebral microstructure. When a strong and rapidly acting magnetic gradient is applied, the motion of water molecules causes a loss of signal on apparent diffusion coefficient (ADC) maps. DWI enables the detection of cytotoxic edema [51], sometimes days before the lesions can be identified with conventional T1 and T2 sequences [52]. Furthermore, quantification of ADC values improves prediction of outcome after a hypoxic-ischemic insult [53]. Diffusion-tensor imaging (DTI) utilizes the same principles as standard DWI, but samples a larger number of diffusion-encoding directions, and in some instances, b-values, to quantify the changes of the diffusion coefficient (mean diffusivity) and the overall directionality (fractional anisotropy [FA]) of the water diffusion to characterize brain microstructure. With increasing maturity, mean diffusivity decreases [54,55] presumably due to a decrease in water content and the development of cell membranes that restrict water diffusion [54,56]. In cortical gray matter, FA is high early in the third trimester (due to the radial organization of the cerebral cortex) and becomes undetectable by term [57,58]. In the white matter, it increases with maturation [59,60]. FA describes the degree of anisotropy of the diffusion signal and correlates with the structural integrity of white matter tracts. Decreases of FA in white matter tracts predict poor outcome after NE [61,62] and may even be sensitive to occult injury not apparent on conventional MRI sequences [63], as well as long-term changes to white matter microstructure [64,65].
In contrast to MRI, which measures the signal from protons in water, magnetic resonance spectroscopy (MRS) detects the signal from protons in different chemical metabolites. Among the metabolites measured by MRS, N-acetylaspartate (NAA) and lactate are the most useful in assessing metabolic changes associated with brain development and injury. In normal conditions, NAA is found in high concentrations in neurons and its level increases further with cerebral maturation [66,67]. With injury, however, the levels of NAA decrease in proportion to neuronal mitochondrial dysfunction and cell death [68]. Lactate is a byproduct of anaerobic metabolism and increases in concentration due to disturbances in the delivery of cerebral energy substrates and oxidative metabolism [69]. In neonates with NE, NAA declines over days to weeks and then remains low. In contrast, lactate peaks during the first few days after acute brain injury and then declines toward “normal” by the end of the first week of life [70]. Both NAA and the ratio of lactate to NAA show good accuracy for detecting brain injury in NE and predicting long-term outcomes [71-74]. In a recent retrospective study examining the prognostic accuracy of each MR biomarker, the concentration of tNAA (NAA + N-acetylaspartylglutamate) in the deep gray matter was the most accurate quantitative MR biomarker. Although ADC values, lactate levels, and lactate/tNAA ratios also showed high prognostic value during the first 24–96 h of life, only tNAA retained high prognostic value in the second week of life [75]. Other metabolites showing transient changes following NE includes phosphocreatine, myoinositol, glutamine, and choline [8,76-78].
Susceptibility-weighted imaging (SWI) is sensitive to venous blood, hemorrhage, and iron content (all of which cause increased susceptibility in affected brain tissue). Clinically, it is often used to demonstrate intraparenchymal, intraventricular, and extra-axial hemorrhages in neonates with NE. However, there is mounting evidence that SWI may have broader utility. A recent study demonstrated that SWI can depict abnormal cerebral venous contrast, indicative of abnormal perfusion in neonates with NE [79].
4. Timing of neuroimaging
Hypoxic-ischemic brain injury evolves over days and serial imaging has demonstrated this evolution is associated with varying signal abnormalities on cUS as well as conventional T1- and T2-weighted MRI, DWI, and MRS. As such, it is important to consider the timing of neuroimaging when interpreting it. The most common time-points are: (1) immediately following TH (~days 4–5 of life) and (2) during the second week of life (days 10–14 of life). Early imaging takes advantage of the diffusion signal as ADC reaches its nadir during the first week of life prior to pseudonormalizing early in the second week [80]. Importantly, injury may continue to evolve over the first days to week(s) and thus early imaging may not fully document the extent of brain injury [81]. However, studies comparing early (~4 days) to late (>7 days) scans have shown strong agreement, indicating that for most neonates, early MRI provides sufficient diagnostic and prognostic information [7,82-87]. Moreover, early MRI may be a more reliable predictor of adverse neurodevelopmental outcomes [88], and brain injuries identified during this early time appear to represent irreversible changes [83,87].
At the same time, several centers have demonstrated that MRI may be obtained safely even earlier during TH (days 2–3 of life) [87,89]. Methods to safely maintain hypothermia during the scan have been developed [90]. The American College of Obstetricians and Gynecologists in partnership with the American Academy of Pediatrics published a task force report referred to as the ACOG guidelines in 2014 and included recommendations for early MRI in the context of NE as it may be beneficial for identifying antenatal injuries, as well as cerebral hemorrhages and other pathologies, e.g., congenital infections or cerebral malformations. However, it is important to recognize that the full extent of hypoxic-ischemic injuries may not yet be well visualized on cUS or MRI, in particular during the first 24 h of life, thereby limiting the negative predictive value of a normal imaging during this period [83,87,91,92].
If a severe intraventricular hemorrhage is the primary concern, a head ultrasound at the bedside should be the first choice as it is not only more accessible, but also sensitive to intraventricular hemorrhage and other acute blood.
Late imaging (e.g., beyond one month of life) is rarely obtained clinically, unless there are ongoing clinical concerns. However, as a research tool, it may help elucidate the trajectory of neurodevelopment after NE, including repair and reorganization.
5. Predictive value of MRI
Brain MRI in neonates with NE was quickly recognized as an important tool for prognostication. Studies of early, serial imaging resulted in the development of various scoring systems [42,93-97].
The Barkovich system described two patterns of injury that correlated with the pathophysiology of acute profound and partial prolonged hypoxia-ischemia (see above), i.e., the BGT and Watershed (WS) patterns [93]. This system, initially based on T1- and T2-weighted images, later incorporated DWI and has been shown to be associated with outcome before but also during the cooling era [98-100].
In contrast, the Rutherford scoring system did not utilize DWI and was applied to imaging predominantly acquired after the 1st week of life. In this system, the BGT, posterior limb of the internal capsule (PLIC), white matter, and cortex are scored [94]. Abnormal signal intensity in the PLIC was demonstrated as an easy to interpret finding with high interobserver agreement and was a good predictor of motor outcome [42]. The predictive value of PLIC abnormalities for abnormal developmental outcome has been shown to be high (sensitivity 0.9, specificity 1). Further, almost all neonates with normal signal intensity in the PLIC had normal motor outcomes, and three with mild motor outcomes had white matter abnormalities. Neonates with equivocal PLIC findings had abnormal motor outcomes, but also had BGT injury. Finally, neonates with abnormal PLIC signal all had abnormal motor outcomes, and all had accompanying BGT injury. However, the use of this scoring system is limited by the fact that the abnormal PLIC signal only consistently becomes apparent at the end of the first week.
The Neonatal Research Network (NRN) developed a scoring system based on increasing amounts of injury (0 = normal, 1A = minimal cerebral lesions, 1B = more extensive lesions, 2A = BG, Thalamus or PLIC injury, 2B = 2A plus cerebral lesions, and 3 = cerebral hemispheric devastation) [95]. DWI results were not included. Imaging occurred late with mean age at scan of 15 ± 12 days. Images were also scored using the Barkovich and Rutherford systems. All three systems were associated with death or death/disability at 18 months of age. This system retained its predictive ability for outcome at 6–7 years of age [101].
Two additional systems, one developed at Washington University in St. Louis and one in Utrecht, the Netherlands, are more comprehensive and detailed. The score developed at Washington University in St. Louis by Bednarek et al. incorporates T1, T2, and DWI and evaluated five brain regions (deep gray matter (GM) and PLIC, white matter, cortex, cerebellum, and brainstem). Each region was scored by hemisphere and by sequence. The scores were summated for a final score ranging from 0 to 138. Scores can be categorized as no injury (0), mild (1–11), moderate (12–32), and severe (33–138) with increasing severity associated with increased odds of abnormal outcome [96]. An ROC curve for the score to predict outcome identified an AUC of 0.72 (95% CI 0.57–0.86), p = .007, with MRI score cut-off of 10.5–11.5 [96]. Cortical and white matter scores were associated with lower cognitive scores; deep gray nuclei, cortical and white matter scores were associated with lower motor scores [96]. The system proposed developed in Utrecht by Weeke et al. includes T1, T2, DWI and MRS. Regions scored included gray matter (including BGT, PLIC, perirolandic cortex, brainstem, and hippocampus), white matter/cortex (cortex, periventricular white matter, optic radiations, and corpus callosum), and cerebellum, with additional findings (e.g., hemorrhage) also noted [97]. This system was developed and tested using two international cohorts (N = 173), with the majority imaged in the first postnatal week and follow-up at 18–24 months and school age. The gray matter sub-score was the best predictor of outcome at both 18–24 months and school age.
Regardless of the scoring system applied, gray matter injury ± abnormal PLIC are consistently predictive of poor motor outcome. Extent of watershed injury is linked to cognitive outcome. When performed in the first week of life, DWI added to conventional sequences can facilitate the identification of injury. When implementing a scoring system to evaluate extent of brain injury, it is important to consider the expertise of the interpreter, availability of different modalities and timing of the imaging. MRS may be utilized as both a qualitative biomarker, as in the Weeke scoring system, or a quantitative biomarker based on measure neurometabolite concentrations (mmol/kg) or ratios. NAA and lactate are the primary predictive MRS metabolites and are typically measured from the BGT, as well as parietal white matter, in neonates with NE as shown in Fig. 3 (top panel). Of note, in a recent prospective multicenter study, decreases in NAA, as well as increases in the lactate/NAA ratio measured in the thalamus, were predictive of adverse outcomes at age 2 years (sensitivity 1.00 [95% CI 0.16–1.00]; specificity 0.95 (95% CI 0.84–0.99)) [74]. Overall, NAA and the ratio of lactate/NAA are among the most accurate predictive biomarkers, outperforming most, if not all, conventional imaging findings in meta-analyses and most recently, in the multicenter prospective Magnetic Resonance Biomarkers in Neonatal Encephalopathy (MARBLE) observational study [74].
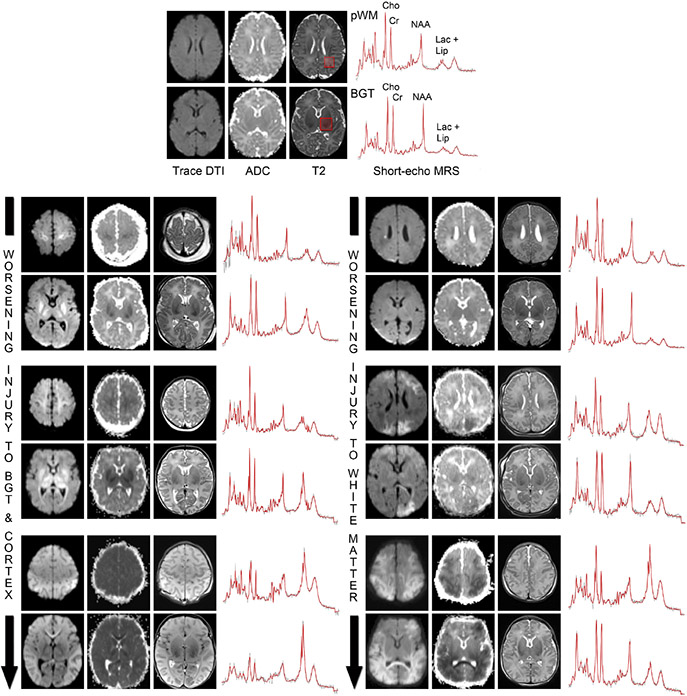
Fig. 3.
MRI and MRS biomarkers indicate the degree of injury to the BGT, cortex, and white matter. The top panel depicts Trace-DTI, ADC, and T2-weighted images obtained at the level of the parietal white matter (pWM, upper) and BGT (lower) from a typically developing term neonate, with the major metabolite peaks labeled for reference. Short-echo MRS spectra obtained from the corresponding voxels (red boxes on the anatomical images) in the BGT and pWM are shown to the right. The lower panels depict Trace-DTI, ADC, T2w and MRS spectra from six neonates with NE imaged at days 4–5 of life. Note how the lactate-lipid signals in the BGT and pWM voxels increase in line with the degree of injury to the BGT-cortex and white matter, respectively, while NAA decreases. Cho = choline; Cr = creatine; NAA = nacetylaspartate; lac + lip = combined signal from lactate and lipids.
Table 2 summarizes the MR biomarkers and their accuracy in predicting the combined outcome of death and neurodevelopmental disability at ages 18–30 months.
Table 2.
The accuracy of MR biomarkers in neonates with neonatal encephalopathy (NE) as predictors of the combined outcome of death and neurodevelopmental disability at 18–30 months of age.
| Scoring System/ Abnormality |
Biomarker cutoff | Design | Ref | Therapy | AUROC (95% CI) |
Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|
| Barkovich (BGT/WS score) | |||||||||
| Any MRI abnormality | BGT or WS ≥ 1 | Prospective, observational | [131] | No TH | 0.63 (0.57–0.68) | 0.92 | 0.33 | 0.44 | 0.88 |
| TH | 0.68 (0.56–0.81) | 0.73 | 0.63 | 0.19 | 0.95 | ||||
| BGT | BGT ≥ WS | No TH | 0.87 | 0.70 | 0.68 | 0.88 | |||
| TH | 0.69 | 0.88 | 0.45 | 0.95 | |||||
| Watershed | WS > BGT | No TH | 0.83 | 0.39 | 0.30 | 0.88 | |||
| TH | 0.33 | 0.69 | 0.05 | 0.95 | |||||
| Rutherford | |||||||||
| PLIC | PLIC equivocal or abn. | Prospective, observational | [42] | No TH | 0.9 | 1.0 | 1.0 | 0.87 | |
| Any mod or severe abnormal. | BGT ≥ 2; WMI = 3 or PLIC | Prospective, RCT substudy | [94] | No TH | 0.81 (0.71–0.91) | 0.94 | 0.68 | 0.74 | 0.92 |
| TH | 0.84 (0.74–0.94) | 0.88 | 0.82 | 0.76 | 0.91 | ||||
| NICHD NRN | Any abn (MRI > 0) | Prospective RCT substudy | [95] | Mixed | 0.90 | 0.65 | 0.62 | 0.91 | |
| Wash U | Total score > 10.5 | Prospective, observational | [96] | TH | 0.72 (0.57–0.86) | 0.77 | 0.46 | 0.47 | 0.76 |
| Weeke | |||||||||
| GM* + MRS | GM w/MRS ≥ 11.5 | Retrospective, multicenter | [97] | TH (Cohort 1) | 0.989 (0.973–1.0) | 0.923 | 0.953 | 0.889 | 0.968 |
| GM* | GM ≥ 9.5 | TH (Cohort 1) | 0.988 (0.973–1) | 0.923 | 0.958 | 0.889 | 0.971 | ||
| TH (Cohort 2) | 0.832 (0.708–0.955) | 0.421 | 0.982 | 0.889 | 0.836 | ||||
| MARBLE | |||||||||
| BGT | BGT ≥ 1# | Prospective, multicenter, observational | [74] | TH | 0.81 (0.75–0.87) | 0.71 | 0.88 | 0.54 | 0.94 |
| Cortex | WS ≥ 1# | 0.67 (0.60–0.73) | 0.48 | 0.81 | 0.33 | 0.89 | |||
| PLIC | PLIC ≥ 1 | 0.82 (0.76–0.87) | 0.71 | 0.90 | 0.58 | 0.94 | |||
| Lac/NAA | Lac/NAA > 0.22 | 0.94 (0.89–0.97) | 0.88 | 0.90 | 0.64 | 0.98 | |||
| NAA | NAA ≤ 5.6 mmol/kg | 0.99 (0.94–1.0) | 1.0 | 0.97 | 0.86 | 1.0 |
Notes:
GM as defined in Weeke scoring system includes BGT, PLIC, brainstem, perirolandic and hippocampus.
MARBLE utilized the Rutherford scoring system above for characterizing BGT and cortical injury on MRI, but applied a different cutoff.
Abbreviations: BGT, Basal Ganglia – Thalamus; GM, gray matter; MARBLE, Magnetic Resonance Biomarkers in Neonatal Encephalopathy; NAA, N-acetylaspartate; NICHD, National Institute of Child Health and Human Development; NRN, Neonatal Research Network; PLIC, posterior limb of the internal capsule; WS, watershed NAA, N-acetylaspartate
6. Future directions
6.1. Areas of research
There is mounting evidence that neonates with mild NE are at risk for adverse neurodevelopmental outcomes. These neonates were excluded from the first trials because the available data at that time suggested that neonates with mild NE were not at high risk for death or neurodevelopmental impairment. Recent data have shown this assumption to be incorrect. Nearly 20% of neonates with mild NE have evidence of brain injury on neonatal brain MRIs [102], with up to 25% showing clinically significant delays in cognitive, language, and/or motor development in the long-term [103]. What is not known is whether neonates with mild NE would benefit from therapeutic hypothermia. Moreover, it remains to be determined whether NE is a single continuum from mild to severe or whether mild NE represents a distinct subtype arising from different underlying cellular and molecular processes. While neuroimaging alone cannot address all of these questions, it can be used to better characterize mild NE with regard to nature and severity of the apparent injury, alterations in chemical metabolites, perfusion, tissue microstructure, and connectivity, and in turn, help to guide the development of neuroprotective therapies for this population.
There are now several adjuvant neuroprotective and neurorestorative therapies under development for NE, several of which have now been studied in phase I/II [104] or phase III clinical trials [105]. Yet, it is important to recognize that it took two decades and multiple clinical trials to translate therapeutic hypothermia from the laboratory into clinical practice [106], and it is neither practical nor feasible to replicate this approach for each of the potential therapies under development. It remains to be determined which are the most promising therapies and for which neonates. This type of targeted, and ultimately personalized, approach will rely heavily on neuroimaging biomarkers – not only to help identify candidate neonates, but also to provide surrogate endpoints, thereby eliminating the need to retain large samples over a long follow-up period.
6.2. MR biomarkers
While neuroimaging in neonates with NE has been widely used to investigate direct brain injury secondary to hypoxic-ischemic insults, MRI is now being used as a surrogate to predict long-term neurodevelopment [85], especially in trials testing alternative treatments for NE [107-110]. The most used markers for such trials are lactate/NAA ratio in the thalamus or fractional anisotropy in the PLIC [107-109]. While these markers may be useful to assess the efficacy of neuroprotective treatments, it may be necessary to develop further neuroimaging markers to test the efficacy of neurorestorative treatments that are currently under development.
Emerging biomarkers, including measures of structural connectivity such as tract-based spatial statistics (TBSS) [61], are being increasingly used in clinical research as diagnostic and prognostic biomarkers. Likewise, measures of functional connectivity have the potential to not only detect injury, but also elucidate the trajectory of recovery and reorganization after perinatal brain injury.
6.3. Emerging neuroimaging techniques
Arterial spin labeling (ASL) is a non-invasive sequence that can be used in neonates with NE to understand alterations of brain perfusion [111,112]. In the first 24 h after birth, ASL may show transient hypoperfusion in injured brain regions, which then evolves to hyperperfusion by days 2–3 of life [113]. Importantly, systemic cooling using current clinical guidelines does not mitigate brain injury in neonates who demonstrate perfusion abnormalities on early MRI, suggesting that ASL may be especially useful as an early prognostic biomarker [113].
Resting-state functional MRI (fMRI) uses blood-oxygenation-level-dependent (BOLD) imaging to identify synchronous changes in local blood oxygenation levels, which are used to infer the organization of intrinsic functional brain networks. Initial studies have demonstrated that canonical Resting State Networks (RSNs), including auditory, somatomotor, visual, and default mode networks are identifiable in neonates with NE, although they are more likely to be unilateral and/or localized, with limited long-range interhemispheric and intrahemispheric correlations [61]. Likewise, a recent study showed disrupted brain networks in the left rolandic operculum, left supramarginal gyrus, bilateral superior temporal gyrus, and right middle temporal gyrus among neonates with severe NE [114].
Neurite orientation dispersion and density imaging (NODDI) is another technique newly applied to neonates to study in more detail their white matter microstructure [115]. The advantage of NODDI over other diffusion-weighted imaging techniques is that it may be more sensitive to changes in the gray matter [116]. Likewise, NODDI and other DTI techniques can be used to interrogate the structural connectome – the macrostructural organization of structural connections in the human brain. Recent studies have demonstrated alterations in the connectome of neonates with NE, with these early disruptions in connectivity predictive of abnormal neurodevelopmental outcomes [117,118].
Another emerging technology is MR Thermometry, which provides a direct measure of brain temperature based on chemical shift differences observed by MRS [119-121]. This technique has already demonstrated applicability for monitoring brain temperature during therapeutic hypothermia and detecting brain injury; however, many unknowns remain, e.g., whether brain temperature is higher during seizures.
Many studies have previously detailed the types and patterns of brain injuries in neonates with NE (treated or not) with hypothermia. However, few studies have described how these injuries affect ongoing brain development (e.g., brain growth and myelination) during the first months/years of life in these neonates. Development of new MRI techniques is now allowing further study of this issue [122-124], and first studies have now been published demonstrating myelination abnormalities in these neonates [125].
In addition, portable MRI techniques are also emerging and may be available in the future to scan the critically ill neonates with NE at the bedside.
6.4. Neuroimaging harmonization
Because NE is a relatively rare disorder, it is extremely challenging to amass large sample sizes needed to characterize NE subgroups or treatment response using data from a single institution. At the same time, carrying out quantitative analyses of multisite MRI data requires harmonization. The primary goal of harmonization is to reduce measurement error by maximizing the consistency of neuroimaging data within and across sites. Minimizing measurement error is important because it will increase the standardized effect size and, therefore, statistical power [126]. Presently, there is no national or international standard for a neonatal MRI protocol or standardized sequences or sequence parameters for NE. While the development of such protocols would have a substantial impact on clinical research, there are both prospective and retrospective techniques that can be deployed to help maximize harmonization, in particular, in multisite studies. Prospectively, it is important to ensure consistency and compliance, as protocol compliance cannot be addressed post hoc using standard statistical techniques. Retrospectively, it is possible to include “site” as a covariate in statistical models, although these effects are often non-linear and non-uniform across the brain, as well as sequence-specific and thereby requiring advanced statistical methods such as ComBat (“combating batch effects when combining batches”) [127]. In addition, innovative machine learning and artificial intelligence methods are showing considerable promise for harmonizing diverse image sets, but none of these methods are currently ready for clinical use [128].
7. Recommendations
Neuroimaging has a key role to play in the diagnosis and management of neonates with NE. Still, despite widespread use clinically, there are few published guidelines on neuroimaging for NE.
The first joint practice parameter from the Quality Standard Subcommittee of the American Academy of Neurology (AAN) and Practice Committee of the Child Neurology Society (CNS) was published in 2002, an era in which computed tomography (CT) was the imaging modality of choice. These parameters were formally retired by the AAN and CNS in 2018 [47]. More recently, the American College of Obstetricians and Gynecologists in partnership with the American Academy of Pediatrics published a task force report in 2014, which was reaffirmed in 2019 [129]. Likewise, the Canadian Pediatric Society published a position statement in 2018. Main recommendations from each of these guidelines are summarized in Table 3.
Table 3.
Recommendations for neuroimaging in term neonates with neonatal encephalopathy (NE).
| Neuroimaging of the Neonate (2002 – retired on February 27, 2018) |
*Neonatal Encephalopathy and Neurologic Outcome 2nd edition (2014, reaffirmed in 2019) |
Imaging the term neonatal brain (2018) |
Neuroimaging in the term newborn with neonatal encephalopathy (2021) |
|
|---|---|---|---|---|
| Organization | Practice Parameter of the American Academy of Neurology (AAN) and Child Neurology Society (CNS) | American College of Obstetricians and Gynecologists (ACOG) and American Academy of Pediatrics (AAP) | Position Statement of the Canadian Pediatric Society (CPS) | Newborn Brain Society (NBS) Guidelines and Publications Committee |
| Objective | - To identify patterns consistent with hypoxic-ischemic brain injury - To inform management decisions - To prognosticate long-term outcome - To rule out other etiologies than hypoxic-ischemic brain injury |
|||
| Cranial ultrasound (cUS) | - No specific recommendation | - Cranial ultrasonography on admission and in the first 2–3 days of life to exclude major cerebral dysgenesis | - May be useful as first-line imaging if trained technologists and radiologists available - Not recommended as sole imaging modality |
- May be useful as a first-line imaging modality, if qualified personnel available for interpretation, to rule-out major intracranial hemorrhage and to assess for lesions of antenatal onset or abnormalities suggestive of NE mimics - Timing: during the first day of life as a screening tool - Should be complemented with MRI |
| CT scan | - Non-contrast CT should be performed as a first-line if NE in context of significant birth trauma and low hematocrit or abnormal coagulopathy - MRI should be performed if CT findings cannot explain the clinical status |
No specific recommendation, but the document acknowledges CT as a rapidly acquired neuroimaging technique that is sensitive to hemorrhage, but not to brain injury in the first 24–48 h | - May be useful in urgent situations when MRI is not available - At 72 ± 12 h |
- May be considered only in urgent situations (e.g., concern for significant intracranial hemorrhage or herniation) - Only when MRI or US are not available |
| MR imaging | - For all other neonates with NE between days 2–8 of life - Include single-voxel MRS and DWI to be added if available - CT should be performed if MRI is not available or if neonate is too unstable for MRI |
- Should be acquired in all term neonates with moderate to severe NE - MRI, MRS and DWI between 24 and 96 h provide the most useful guide on the potential timing of cerebral insult - MRI undertaken optimally at 10 days of life (acceptable 7–21 days) will best delineate the full extent of cerebral injury |
- Preferred imaging technique - Between days 3–5 of life if no hypothermia - If hypothermia, after rewarming has taken place - Consistent timing to facilitate recognition of injury - A repeat MRI on days 10–14 of life if discordance between imaging and clinical features |
- Preferred imaging technique - Sequences: at least T1- and T2-weighted sequences, DWI with ADC maps, and MRS - Timing: (A) early MRI (days 2–5 of life) recommended for diagnosis and prognosis; typically immediately after TH since the most practical in routine clinical practice; may consider during TH to inform the direction (or possible redirection) of care, even though the full extent of brain injury may not yet be visible (B) consider repeating MRI at 10–14 days of life when discrepancy between early imaging and clinical condition of the neonate, or if ambiguity persist |
| Additional recommendations | - When a qualified radiologist is not on site, images should be sent electronically elsewhere for interpretation | - If trained and experienced neuroradiologist not on site, send electronically images for interpretation to a center with such clinician on site |
Abbreviations: AAN, Academy of Neurology; ACOG, American College of Obstetricians and Gynecologists; ADC, apparent diffusion coefficient; CNS, Child Neurology Society; CPS, Canadian Pediatric Society; CT, computed tomography; cUS, cranial ultrasound; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NBS, Newborn Brain Society; NE, neonatal encephalopathy.
In line with the scientific evidence presented in this review, updated recommendations for neuroimaging of neonates with NE are presented here (Table 3). MRI is recommended as the preferred neuroimaging modality; however, cUS may be beneficial early, in particular, to rule-out major intracranial hemorrhage and to assess for lesions of antenatal onset or abnormalities suggestive of NE mimics. The MRI protocol should include at least conventional T1- and T2-weighted sequences, DWI with ADC maps, and MRS. Collectively, these sequences provide the best sensitivity for detecting acute as well as subacute and chronic brain injury. MRS is beneficial not only for its sensitivity to hypoxic-ischemic brain injury, but also because it can provide evidence of other syndromes including inborn errors of metabolism. Timing of MRI is important, and early MRI (days 2–5 of life) is optimal for diagnosis and prognosis. The ADC signal reaches its nadir within the first few days of injury, increasing thereafter with pseudonormalization early in the second week. To maximize the utility of DWI and its sensitivity to acute brain injury, MRI should be performed between 2 and 5 days when possible. Within this window, MRI during TH (i.e., days 23 of life) may be especially beneficial to inform the direction (or possible redirection) of care, even though the full extent of brain injury may not yet be visible, while MRI immediately after TH (i.e., days 45 of life) may be more practical in routine clinical practice. Repeat MRI is recommended at days 1014 of life when clinical concerns persist, including discrepancies between the early neuroimaging findings and the clinical condition of the neonate. The MRI images should be interpreted by a trained and experienced radiologist or other qualified physician with expertise in pediatric neuroimaging. When such expertise is not available, images should be sent electronically for interpretation to a center with such clinician.
The day of the MRI is an important day for parents of neonates with NE treated with hypothermia. However, interpreting and communicating brain MRI results for neonates with NE remains a challenging task for clinicians and parents. Developing a systemic and interdisciplinary approach to prognostication, standardizing reporting of brain MRI results and training further clinicians in their interpretation may help facilitate difficult decision-making discussions and make them more meaningful for parents.
8. Conclusion
Neuroimaging plays a crucial role in the diagnosis and management of neonates with NE. It can provide information on the nature and potential timing of brain injury, including important prognostic information, which may allow the clinicians to better counsel families, and if a severe outcome is expected, to assist in delineation of care models. Quantitative MRI techniques may play a more significant role in the future by refining our ability to prognosticate and help us study the prevention of secondary brain injury and the response to experimental treatment. The harmonization of imaging within and across institutions would facilitate international collaborations and ultimately improve care.
Practice points.
Neuroimaging in the term neonates with neonatal encephalopathy (NE) is useful to identify patterns consistent with hypoxic-ischemic brain injury, to inform management decisions, to prognosticate long-term outcome, and to rule out other etiologies than hypoxic-ischemic brain injury.
Magnetic resonance imaging is the preferred imaging technique for examining the brain of term neonates with NE. Sequences should include at least T1- and T2-weighted sequences, DWI with ADC maps, and MRS.
An early MRI (days 2–5 of life) is recommended for diagnosis and prognosis. MRI during TH may be especially beneficial to inform the direction (or possible redirection) of care, even though the full extent of brain injury may not yet be visible, while MRI immediately after TH may be more practical in routine clinical practice.
Repeat MRI is recommended at days 10–14 of life when clinical concerns persist, including discrepancies between the early neuroimaging findings and the clinical condition of the neonate.
Early ultrasound may be useful during the first day of life as a screening tool to rule-out major intracranial hemorrhage and to assess for lesions of antenatal onset or abnormalities suggestive of NE mimics.
Research directions.
New neuroimaging techniques are emerging to further study alterations of brain perfusion, intrinsic functional brain networks, white matter microstructure, myelination, and brain growth in the context of NE.
MRI is now being used as a surrogate to predict long-term neurodevelopment, especially in trials testing alternative treatments for NE. While these markers may be useful to assess the efficacy of neuroprotective treatments, it may be necessary to develop further neuroimaging markers to test the efficacy of neurorestorative treatments that are currently under development.
Presently, there is no international/national standard for a neonatal MRI protocol or standardized sequences or sequence parameters for NE. The development of such protocols would have a substantial impact on clinical research. Innovative machine learning and artificial intelligence methods are showing considerable promise for harmonizing diverse image sets.
Funding
JLW is supported by grants from the National Institutes of Health (K23HD099309, U01NS092764). PW is supported by grants from FRSQ Clinical Research Scholar Career Award Senior and a Canadian Institutes of Health Research (CIHR) Project Grant.
Abbreviations
- AAN
American Academy of Neurology
- ACOG
American College of Obstetricians and Gynecologists
- ADC
apparent diffusion coefficient
- BGT
Basal Ganglia – Thalamus
- CNS
Child Neurology Society
- CPS
Canadian Pediatric Society
- CT
computed tomography c
- cUS
cranial ultrasound
- DWI
diffusion-weighted imaging
- DTI
diffusion-tensor imaging
- GM
gray matter
- FA
fractional anisotropy
- MARBLE
Magnetic Resonance Biomarkers in Neonatal Encephalopathy
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- NAA
N-acetylaspartate
- NBS
Newborn Brain Society
- NE
neonatal encephalopathy
- NICHD
National Institute of Child Health and Human Development
- NODDI
neurite orientation dispersion and density imaging
- NRN
Neonatal Research Network
- PLIC
posterior limb of the internal capsule
- PVL
periventricular leukomalacia
- SWI
susceptibility-weighted imaging
- tNAA
NAA + N-acetylaspartylglutamate
- WS
watershed
Footnotes
Newborn Brain Society, PO Box 200783, Roxbury Crossing, MA 02120. publications@newbornbrainsociety.org.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
References
- [1].Nelson KB, Leviton A. How much of neonatal encephalopathy is due to birth asphyxia? Am J Dis Child 1991;145:1325–31. [DOI] [PubMed] [Google Scholar]
- [2].Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010;86:329–38. [DOI] [PubMed] [Google Scholar]
- [3].Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res 2013;74(Suppl 1):50–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering T. 4 million neonatal deaths: when? where? why? Lancet 2005;365:891–900. [DOI] [PubMed] [Google Scholar]
- [5].Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;1:CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sanchez Fernandez I, Morales-Quezada JL, Law S, Kim P. Prognostic value of brain magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: a meta-analysis. J Child Neurol 2017;32:1065–73. [DOI] [PubMed] [Google Scholar]
- [7].Ouwehand S, Smidt LCA, Dudink J, et al. Predictors of outcomes in hypoxic-ischemic encephalopathy following hypothermia: a meta-analysis. Neonatology 2020:1–17. [DOI] [PubMed] [Google Scholar]
- [8].Hope PL, Costello AM, Cady EB, et al. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet 1984;2:366–70. [DOI] [PubMed] [Google Scholar]
- [9].Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on death or disability at age 18 Months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 2017;318:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Volpe J. Neurology of the Newborn. 6 ed. Philadelphia: W.B. Saunders Company; 2017. [Google Scholar]
- [11].Kinney HC, Volpe JJ. Hypoxic-ischemic injury in the term infant: neuropathology. In: Volpe C, editor. Volpe’s Neurology of the Newborn, sixth ed. Elsevier; 2018. p. 484–99. [Google Scholar]
- [12].Alderliesten T, Nikkels PG, Benders MJ, de Vries LS, Groenendaal F. Antemortem cranial MRI compared with postmortem histopathologic examination of the brain in term infants with neonatal encephalopathy following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed 2013;98:F304–9. [DOI] [PubMed] [Google Scholar]
- [13].Annink KV, de Vries LS, Groenendaal F, et al. The long-term effect of perinatal asphyxia on hippocampal volumes. Pediatr Res 2019;85:43–9. [DOI] [PubMed] [Google Scholar]
- [14].Okereafor A, Allsop J, Counsell SJ, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 2008;121:906–14. [DOI] [PubMed] [Google Scholar]
- [15].Shankaran S, Laptook AR, McDonald SA, et al. Acute perinatal sentinel events, neonatal brain injury pattern, and outcome of infants undergoing a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2017;180:275–278 e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Myers RE. Four patterns of perinatal brain damage and their conditions of occurrence in primates. Adv Neurol 1975;10:223–34. [PubMed] [Google Scholar]
- [17].McAdams RM, McPherson RJ, Kapur RP, Juul SE. Focal brain injury associated with a model of severe hypoxic-ischemic encephalopathy in nonhuman primates. Dev Neurosci 2017;39:107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marin-Padilla M. Developmental neuropathology and impact of perinatal brain damage. III: gray matter lesions of the neocortex. J Neuropathol Exp Neurol 1999;58:407–29. [DOI] [PubMed] [Google Scholar]
- [19].Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 2008;122:65–74. [DOI] [PubMed] [Google Scholar]
- [20].Jenster M, Bonifacio SL, Ruel T, et al. Maternal or neonatal infection: association with neonatal encephalopathy outcomes. Pediatr Res 2014;76:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li AM, Chau V, Poskitt KJ, et al. White matter injury in term newborns with neonatal encephalopathy. Pediatr Res 2009;65:85–9. [DOI] [PubMed] [Google Scholar]
- [22].Harteman JC, Nikkels PG, Benders MJ, Kwee A, Groenendaal F, de Vries LS. Placental pathology in full-term infants with hypoxic-ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. J Pediatr 2013;163:968–995 e962. [DOI] [PubMed] [Google Scholar]
- [23].Martinez-Biarge M, Bregant T, Wusthoff CJ, et al. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr 2012;161:799–807. [DOI] [PubMed] [Google Scholar]
- [24].Hayman M, van Wezel-Meijler G, van Straaten H, Brilstra E, Groenendaal F, de Vries LS. Punctate white-matter lesions in the full-term newborn: underlying aetiology and outcome. Eur J Paediatr Neurol 2019;23:280–7. [DOI] [PubMed] [Google Scholar]
- [25].Annink KV, Meerts L, van der Aa NE, et al. Cerebellar injury in term neonates with hypoxic-ischemic encephalopathy is underestimated. Pediatr Res 2021;89:1171–8. [DOI] [PubMed] [Google Scholar]
- [26].Kwan S, Boudes E, Gilbert G, et al. Injury to the cerebellum in term asphyxiated newborns treated with hypothermia. AJNR Am J Neuroradiol 2015;36:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sarkar SS, Gupta S, Bapuraj JR, Dechert RE, Sarkar S. Brainstem hypoxic-ischemic lesions on MRI in infants treated with therapeutic cooling: effects on the length of stay and mortality. J Perinatol 2021;41:512–8. [DOI] [PubMed] [Google Scholar]
- [28].Volpe JJ. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol 2012;72:156–66. [DOI] [PubMed] [Google Scholar]
- [29].McAdams RM, Fleiss B, Traudt C, et al. Long-term neuropathological changes associated with cerebral palsy in a nonhuman primate model of hypoxic-ischemic encephalopathy. Dev Neurosci 2017;39:124–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hayakawa K, Tanda K, Koshino S, Nishimura A, Kizaki Z, Ohno K. Pontine and cerebellar injury in neonatal hypoxic-ischemic encephalopathy: MRI features and clinical outcomes. Acta Radiol 2020;61:1398–405. [DOI] [PubMed] [Google Scholar]
- [31].Fernandez-Lopez D, Natarajan N, Ashwal S, Vexler ZS. Mechanisms of perinatal arterial ischemic stroke. J Cerebr Blood Flow Metabol 2014;34:921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Curtis C, Mineyko A, Massicotte P, et al. Thrombophilia risk is not increased in children after perinatal stroke. Blood 2017;129:2793–800. [DOI] [PubMed] [Google Scholar]
- [33].Lequin MH, Steggerda SJ, Severino M, et al. Mammillary body injury in neonatal encephalopathy: a multicentre, retrospective study. Pediatr Res 2021:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Annink KV, de Vries LS, Groenendaal F, et al. Mammillary body atrophy and other MRI correlates of school-age outcome following neonatal hypoxic-ischemic encephalopathy. Sci Rep 2021;11:5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Al Yazidi G, Boudes E, Tan X, Saint-Martin C, Shevell M, Wintermark P. Intraventricular hemorrhage in asphyxiated newborns treated with hypothermia: a look into incidence, timing and risk factors. BMC Pediatr 2015;15:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med 2001;345:417–23. [DOI] [PubMed] [Google Scholar]
- [37].Lodygensky GA, West T, Stump M, Holtzman DM, Inder TE, Neil JJ. In vivo MRI analysis of an inflammatory injury in the developing brain. Brain Behav Immun 2010;24:759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lodygensky GA, Kunz N, Perroud E, et al. Definition and quantification of acute inflammatory white matter injury in the immature brain by MRI/MRS at high magnetic field. Pediatr Res 2014;75:415–23. [DOI] [PubMed] [Google Scholar]
- [39].Leijser LM, Vein AA, Liauw L, Strauss T, Veen S, Wezel-Meijler G. Prediction of short-term neurological outcome in full-term neonates with hypoxic-ischaemic encephalopathy based on combined use of electroencephalogram and neuroimaging. Neuropediatrics 2007;38:219–27. [DOI] [PubMed] [Google Scholar]
- [40].Epelman M, Daneman A, Kellenberger CJ, et al. Neonatal encephalopathy: a prospective comparison of head US and MRI. Pediatr Radiol 2010;40:1640–50. [DOI] [PubMed] [Google Scholar]
- [41].Barnette AR, Horbar JD, Soll RF, et al. Neuroimaging in the evaluation of neonatal encephalopathy. Pediatrics 2014;133:e1508–1517. [DOI] [PubMed] [Google Scholar]
- [42].Rutherford MA, Pennock JM, Counsell SJ, et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 1998;102:323–8. [DOI] [PubMed] [Google Scholar]
- [43].Annink KV, de Vries LS, Groenendaal F, et al. The development and validation of a cerebral ultrasound scoring system for infants with hypoxic-ischaemic encephalopathy. Pediatr Res 2020;87:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Elstad M, Whitelaw A, Thoresen M. Cerebral Resistance Index is less predictive in hypothermic encephalopathic newborns. Acta Paediatr 2011;100:1344–9. [DOI] [PubMed] [Google Scholar]
- [45].Skranes JH, Elstad M, Thoresen M, Cowan FM, Stiris T, Fugelseth D. Hypothermia makes cerebral resistance index a poor prognostic tool in encephalopathic newborns. Neonatology 2014;106:17–23. [DOI] [PubMed] [Google Scholar]
- [46].Faingold R, Cassia G, Morneault L, Saint-Martin C, Sant’Anna G. Basal ganglia perfusion using dynamic color Doppler sonography in infants with hypoxic ischemic encephalopathy receiving therapeutic hypothermia: a pilot study. Quant Imag Med Surg 2016;6:510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the quality standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2002;58:1726–38. [DOI] [PubMed] [Google Scholar]
- [48].Journy NM, Lee C, Harbron RW, McHugh K, Pearce MS, Berrington de Gonzalez A. Projected cancer risks potentially related to past, current, and future practices in paediatric CT in the United Kingdom, 1990–2020. Br J Cancer 2017;116:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hall P, Adami HO, Trichopoulos D, et al. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. BMJ 2004;328:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Krishnan P, Shroff M. Neuroimaging in neonatal hypoxic ischemic encephalopathy. Indian J Pediatr 2016;83:995–1002. [DOI] [PubMed] [Google Scholar]
- [51].Cowan FM, Pennock JM, Hanrahan JD, Manji KP, Edwards AD. Early detection of cerebral infarction and hypoxic ischemic encephalopathy in neonates using diffusion weighted magnetic resonance imaging. Neuropediatrics 1994;25:172–5. [DOI] [PubMed] [Google Scholar]
- [52].Robertson RL, Ben-Sira L, Barnes PD, et al. MR line-scan diffusion-weighted imaging of term neonates with perinatal brain ischemia. AJNR Am J Neuroradiol 1999;20:1658–70. [PMC free article] [PubMed] [Google Scholar]
- [53].Alderliesten T, de Vries LS, Benders MJ, Koopman C, Groenendaal F. MR imaging and outcome of term neonates with perinatal asphyxia: value of diffusion-weighted MR imaging and (1)H MR spectroscopy. Radiology 2011;261:235–42. [DOI] [PubMed] [Google Scholar]
- [54].Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol 2002;23:1445–56. [PMC free article] [PubMed] [Google Scholar]
- [55].Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imag 2002;16:621–32. [DOI] [PubMed] [Google Scholar]
- [56].Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 2002;15:435–55. [DOI] [PubMed] [Google Scholar]
- [57].Deipolyi AR, Mukherjee P, Gill K, et al. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage 2005;27:579–86. [DOI] [PubMed] [Google Scholar]
- [58].McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cerebr Cortex 2002;12:1237–43. [DOI] [PubMed] [Google Scholar]
- [59].Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci 2005;25:5988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Prayer D, Barkovich AJ, Kirschner DA, et al. Visualization of nonstructural changes in early white matter development on diffusion-weighted MR images: evidence supporting premyelination anisotropy. AJNR Am J Neuroradiol 2001;22:1572–6. [PMC free article] [PubMed] [Google Scholar]
- [61].Tusor N, Wusthoff C, Smee N, et al. Prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr Res 2012;72:63–9. [DOI] [PubMed] [Google Scholar]
- [62].Merhar SL, Gozdas E, Tkach JA, et al. Neonatal functional and structural connectivity are associated with cerebral palsy at two years of age. Am J Perinatol 2020;37:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lemmon ME, Wagner MW, Bosemani T, et al. Diffusion tensor imaging detects occult cerebellar injury in severe neonatal hypoxic-ischemic encephalopathy. Dev Neurosci 2017;39:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gosar D, Tretnjak V, Bregant T, Neubauer D, Derganc M. Reduced white-matter integrity and lower speed of information processing in adolescents with mild and moderate neonatal hypoxic-ischaemic encephalopathy. Eur J Paediatr Neurol 2020;28:205–13. [DOI] [PubMed] [Google Scholar]
- [65].Kwan S, Boudes E, Benseler A, et al. Evolution of apparent diffusion coefficient and fractional anisotropy in the cerebrum of asphyxiated newborns treated with hypothermia over the first month of life. Neural Plast 2015;2015:653727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 2002;48:949–58. [DOI] [PubMed] [Google Scholar]
- [67].Bluml S, Wisnowski JL, Nelson MD Jr, et al. Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cerebr Cortex 2013;23:2944–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pang R, Martinello KA, Meehan C, et al. Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 48 h predicts cell death following varied neuroprotective interventions in a piglet model of hypoxia-ischemia with and without inflammation sensitization. Front Neurol 2020;11:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 2004;305:99–103. [DOI] [PubMed] [Google Scholar]
- [70].Wu TW, Tamrazi B, Hsu KH, et al. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: in relation to time, characteristic of injury, and serum lactate concentration. Front Neurol 2018;9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 2010;125:e382–395. [DOI] [PubMed] [Google Scholar]
- [72].Alderliesten T, de Vries LS, Staats L, et al. MRI and spectroscopy in (near) term neonates with perinatal asphyxia and therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed 2017;102:F147–52. [DOI] [PubMed] [Google Scholar]
- [73].Mitra S, Kendall GS, Bainbridge A, et al. Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 2 weeks of birth accurately predicts 2-year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed 2019;104:F424–32. [DOI] [PubMed] [Google Scholar]
- [74].Lally PJ, Montaldo P, Oliveira V, et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol 2019;18:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Shibasaki J, Niwa T, Piedvache A, et al. Comparison of predictive values of magnetic resonance biomarkers based on scan timing in neonatal encephalopathy following therapeutic hypothermia. J Pediatr 2021:In press. [DOI] [PubMed] [Google Scholar]
- [76].Amess PN, Penrice J, Wylezinska M, et al. Early brain proton magnetic resonance spectroscopy and neonatal neurology related to neurodevelopmental outcome at 1 year in term infants after presumed hypoxic-ischaemic brain injury. Dev Med Child Neurol 1999;41:436–45. [PubMed] [Google Scholar]
- [77].Robertson NJ, Lewis RH, Cowan FM, et al. Early increases in brain myo inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr Res 2001;50:692–700. [DOI] [PubMed] [Google Scholar]
- [78].Wisnowski JL, Wu TW, Reitman AJ, et al. The effects of therapeutic hypothermia on cerebral metabolism in neonates with hypoxic-ischemic encephalopathy: an in vivo 1H-MR spectroscopy study. J Cerebr Blood Flow Metabol 2016;36:1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tang Z, Mahmoodi S, Dasmahapatra S, Darekar A, Vollmer B. Ridge detection and analysis of susceptibility-weighted magnetic resonance imaging in neonatal hypoxic-ischaemic encephalopathy. In: Papiez B, Namburete A, Yaqub M, Noble J, editors. Medical image understanding and analysis. Springer; 2020. [Google Scholar]
- [80].Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J, Shimony J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 2012;78:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gano D, Chau V, Poskitt KJ, et al. Evolution of pattern of injury and quantitative MRI on days 1 and 3 in term newborns with hypoxic-ischemic encephalopathy. Pediatr Res 2013;74:82–7. [DOI] [PubMed] [Google Scholar]
- [82].Agut T, Leon M, Rebollo M, Muchart J, Arca G, Garcia-Alix A. Early identification of brain injury in infants with hypoxic ischemic encephalopathy at high risk for severe impairments: accuracy of MRI performed in the first days of life. BMC Pediatr 2014;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Boudes E, Tan X, Saint-Martin C, Shevell M, Wintermark P. MRI obtained during versus after hypothermia in asphyxiated newborns. Arch Dis Child Fetal Neonatal Ed 2015;100:F238–42. [DOI] [PubMed] [Google Scholar]
- [84].Chakkarapani E, Poskitt KJ, Miller SP, et al. Reliability of early magnetic resonance imaging (MRI) and necessity of repeating MRI in noncooled and cooled infants with neonatal encephalopathy. J Child Neurol 2016;31:553–9. [DOI] [PubMed] [Google Scholar]
- [85].Charon V, Proisy M, Bretaudeau G, et al. Early MRI in neonatal hypoxic-ischaemic encephalopathy treated with hypothermia: prognostic role at 2-year follow-up. Eur J Radiol 2016;85:1366–74. [DOI] [PubMed] [Google Scholar]
- [86].Skranes JH, Cowan FM, Stiris T, Fugelseth D, Thoresen M, Server A. Brain imaging in cooled encephalopathic neonates does not differ between four and 11 days after birth. Acta Paediatr 2015;104:752–8. [DOI] [PubMed] [Google Scholar]
- [87].Wintermark P, Hansen A, Soul J, Labrecque M, Robertson RL, Warfield SK. Early versus late MRI in asphyxiated newborns treated with hypothermia. Arch Dis Child Fetal Neonatal Ed 2011;96:F36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].O’Kane A, Vezina G, Chang T, et al. Early versus late brain magnetic resonance imaging after neonatal hypoxic ischemic encephalopathy treated with therapeutic hypothermia. J Pediatr 2021;232:73–79 e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wu TW, McLean C, Friedlich P, Grimm J, Bluml S, Seri I. Maintenance of wholebody therapeutic hypothermia during patient transport and magnetic resonance imaging. Pediatr Radiol 2014;44:613–7. [DOI] [PubMed] [Google Scholar]
- [90].Wintermark P, Labrecque M, Warfield SK, DeHart S, Hansen A. Can induced hypothermia be assured during brain MRI in neonates with hypoxic-ischemic encephalopathy? Pediatr Radiol 2010;40:1950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Barkovich AJ, Westmark KD, Bedi HS, Partridge JC, Ferriero DM, Vigneron DB. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. AJNR Am J Neuroradiol 2001;22:1786–94. [PMC free article] [PubMed] [Google Scholar]
- [92].Imai K, de Vries LS, Alderliesten T, et al. MRI changes in the thalamus and basal ganglia of full-term neonates with perinatal asphyxia. Neonatology 2018;114:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- [94].Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2012;97:F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Trivedi SB, Vesoulis ZA, Rao R, et al. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatr Radiol 2017;47:1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Weeke LC, Groenendaal F, Mudigonda K, et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr 2018;192:33–40 e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr 2005;146:453–60. [DOI] [PubMed] [Google Scholar]
- [99].Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol 2006;27:533–47. [PMC free article] [PubMed] [Google Scholar]
- [100].Al Amrani F, Marcovitz J, Sanon PN, et al. Prediction of outcome in asphyxiated newborns treated with hypothermia: is a MRI scoring system described before the cooling era still useful? Eur J Paediatr Neurol 2018;22:387–95. [DOI] [PubMed] [Google Scholar]
- [101].Shankaran S, McDonald SA, Laptook AR, et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2015;167:987–993 e983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Prempunpong C, Chalak LF, Garfinkle J, et al. Prospective research on infants with mild encephalopathy: the PRIME study. J Perinatol 2018;38:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Conway JM, Walsh BH, Boylan GB, Murray DM. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome - a systematic review. Early Hum Dev 2018;120:80–7. [DOI] [PubMed] [Google Scholar]
- [104].McNally MA, Soul JS. Pharmacologic prevention and treatment of neonatal brain injury. Clin Perinatol 2019;46:311–25. [DOI] [PubMed] [Google Scholar]
- [105].Juul SE, Comstock BA, Heagerty PJ, et al. High-dose erythropoietin for asphyxia and encephalopathy (HEAL): a randomized controlled trial - background, aims, and study protocol. Neonatology 2018;113:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Gunn AJ, Laptook AR, Robertson NJ, et al. Therapeutic hypothermia translates from ancient history in to practice. Pediatr Res 2017;81:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Azzopardi D, Robertson NJ, Bainbridge A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol 2016;15:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Maiwald CA, Annink KV, Rudiger M, et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (ALBINO): study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III). BMC Pediatr 2019;19:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Thayyil S, Oliveira V, Lally PJ, et al. Hypothermia for encephalopathy in low and middle-income countries (HELIX): study protocol for a randomised controlled trial. Trials 2017;18:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics 2016;137. [DOI] [PubMed] [Google Scholar]
- [111].Massaro AN, Bouyssi-Kobar M, Chang T, Vezina LG, du Plessis AJ, Limperopoulos C. Brain perfusion in encephalopathic newborns after therapeutic hypothermia. AJNR Am J Neuroradiol 2013;34:1649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wintermark P. Injury and repair in perinatal brain injury: insights from non-invasive MR perfusion imaging. Semin Perinatol 2015;39:124–9. [DOI] [PubMed] [Google Scholar]
- [113].Wintermark P, Hansen A, Gregas MC, et al. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am J Neuroradiol 2011;32:2023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Li HX, Yu M, Zheng AB, et al. Resting-state network complexity and magnitude changes in neonates with severe hypoxic ischemic encephalopathy. Neural Regen Res 2019;14:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kansagra AP, Mabray MC, Ferriero DM, Barkovich AJ, Xu D, Hess CP. Microstructural maturation of white matter tracts in encephalopathic neonates. Clin Imag 2016;40:1009–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012;61:1000–16. [DOI] [PubMed] [Google Scholar]
- [117].Ziv E, Tymofiyeva O, Ferriero DM, Barkovich AJ, Hess CP, Xu D. A machine learning approach to automated structural network analysis: application to neonatal encephalopathy. PLoS One 2013;8:e78824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Jeong JW, Lee MH, Fernandes N, et al. Neonatal encephalopathy prediction of poor outcome with diffusion-weighted imaging connectome and fixel-based analysis. Pediatr Res 2021:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Owji ZP, Gilbert G, Saint-Martin C, Wintermark P. Brain temperature is increased during the first days of life in asphyxiated newborns: developing brain injury despite hypothermia treatment. AJNR Am J Neuroradiol 2017;38:2180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wu TW, McLean C, Friedlich P, et al. Brain temperature in neonates with hypoxic-ischemic encephalopathy during therapeutic hypothermia. J Pediatr 2014;165:1129–34. [DOI] [PubMed] [Google Scholar]
- [121].Bainbridge A, Kendall GS, De Vita E, et al. Regional neonatal brain absolute thermometry by 1H MRS. NMR Biomed 2013;26:416–23. [DOI] [PubMed] [Google Scholar]
- [122].Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 2009;29:1433–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Deoni SC, Mercure E, Blasi A, et al. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci 2011;31:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 2014;134:e444–453. [DOI] [PubMed] [Google Scholar]
- [125].Olivieri B, Rampakakis E, Gilbert G, Fezoua A, Wintermark P. Myelination may be impaired in neonates following birth asphyxia. Neuroimage Clin 2021;31:102678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Baguley T. Understanding statistical power in the context of applied research. Appl Ergon 2004;35:73–80. [DOI] [PubMed] [Google Scholar]
- [127].Fortin JP, Parker D, Tunc B, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017;161:149–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Grigorescu I, Vanes L, Uus A, et al. Harmonized segmentation of neonatal brain MRI. Front Neurosci 2021;15:662005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].ACOG. Executive summary: neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ task force on neonatal encephalopathy. Obstet Gynecol 2014;123:896–901. [DOI] [PubMed] [Google Scholar]
- [130].Cheong JL, Coleman L, Hunt RW, et al. Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: substudy of a randomized trial. Arch Pediatr Adolesc Med 2012;166:634–40. [DOI] [PubMed] [Google Scholar]
- [131].Bach AM, Fang AY, Bonifacio S, et al. Early magnetic resonance imaging predicts 30-month outcomes after therapeutic hypothermia for neonatal encephalopathy. J Pediatr 2021;238:94–101. [DOI] [PubMed] [Google Scholar]