Abstract
Repurposed drugs may reduce morbidity and mortality in patients with hematological disorders who develop COVID-19 illness. 112 patients with predominantly hematological illnesses were randomized to receive standard of care, ivermectin 12 mg [Iv 12] or 24 mg [Iv24] for asymptomatic, mild, or moderate COVID 19 illness. Serial respiratory samples for rRT-PCR samples were sent on Day 3, 5 and 7. rRT-PCR negativity and ≥ 2 log10 reduction in viral loads on day 3, 5 and 7 were similar between the 3 treatment groups across all disease categories. Symptom progression occurred in 26 patients [21.6%] with no difference across 3 treatment groups. Twenty-two patients [18.3%] have expired while 98 [81.7%] survived. Survival rates were similar across treatment groups [controls—80.5%, Iv12—77.5%, Iv24—87.2% respectively]. Overall, poorer survival was seen with moderate illness compared to others [51.6% vs 92.1%; p = 0.000] and was the only significant risk factor identified on multivariate analysis. In this Phase II randomised trial, single dose of 12 or 24 mg of ivermectin did not reduce viral loads, prevent symptom progression, or reduce mortality in patients with predominantly haematological illnesses who develop mild to moderate COVID 19 illness.
Keywords: Ivermectin, COVID-19, Symptom progression, Survival
Introduction
The novel virus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV-2), emerged towards the end of 2019 and caused an acute respiratory illness—Coronavirus Disease-19 [COVID-19], that spread throughout the world and was declared a pandemic by the World Health Organization [WHO]. This infection has been associated with mortality ranging from 1 to 5% in immunocompetent individuals but is associated with higher mortality in immunosuppressed patients especially in those with cancer. The COVID-19 and Cancer Consortium (CCC19) database involving centers from USA, Canada and Spain suggested mortality rates of 13% with higher rates of 25% in those who have progressive disease [1]. Data from China suggests that patients with cancer had a higher risk of severe events (patients requiring invasive ventilation, or death) [39%] compared with patients without cancer [8%] [2]. A prospective observational study by the UK Coronavirus Cancer Monitoring Project (UKCCMP) suggested a mortality of 28% in patients with cancer with mortality being driven mainly by age, gender, and comorbidities [3]. Data from both Italy and Spain involving patients with hematological malignancies have shown mortality rates ranging from 30 to 37% [4, 5]. Early studies from China showed that higher viral loads were associated with severe clinical outcomes [6, 7]. The median time to achieving a PCR negativity was 10 days ranging from 6 to 12 days [8]. Viral clearance was an important factor affecting mortality and a study from Wuhan China showed that the median duration of viral shedding was 20 days (range 17–24) in survivors, but SARS-CoV-2 was detectable until death in non- survivors [9]. It is therefore clear that any drug that results in rapid clearance of viral load will be useful in reducing morbidity and mortality associated with this infection.
Ivermectin is currently licensed for the treatment for strongyloidiasis, onchocerciasis, or river blindness, head lice, scabies and other diseases caused by soil-transmitted helminths globally. Ivermectin has a broad range of antiviral activity against many viruses including the human immunodeficiency virus 1 (HIV-1), Simian virus SV40, Dengue virus (DENV), West Nile Virus, Venezuelan equine encephalitis virus (VEEV) and Influenza. Investigators from Monash University showed that Ivermectin was a strong inhibitor of SARS-CoV-2 with laboratory data showing a 93% reduction in viral RNA at 24 h and a 5000-fold reduction at 48 h with no further reduction at 72 h with no evidence of drug toxicity at any of the timepoints tested. Ivermectin has a very good safety profile and is commonly used in the management of parasitic infections in patients following chemotherapy and stem cell transplantation.
We therefore undertook this study to determine whether a single dose of ivermectin will help in reducing viral loads in patients with predominantly hematological disorders who were admitted with COVID 19 infection.
Methods
This was a prospective Phase II randomized study that enrolled patients between June 2020 and February 2021 at the Christian Medical College, Vellore, India. This study was approved by the Institutional Review Board of CMC Vellore and was registered at the Clinical Trials Registry of India [CTRI/2020/05/025068].
Patients
All consecutive patients with hematological disorders admitted with COVID 19 illness, as defined by a positive rRT-PCR for SARS CoV-2, into the medical wards at Christian Medical College, Vellore, India were eligible to be randomized after informed consent. Patients with asymptomatic, mild, or moderate COVID-19 illness as per the interim WHO definitions in May 2020 were included. Asymptomatic patients were those found to be rRT-PCR positive for SARS CoV-2 on routine testing prior to any procedure. The mild illness group included patients with uncomplicated upper respiratory tract infection with non-specific symptoms such as fever, cough, anorexia, malaise, muscle pain, sore throat, nasal congestion, or headache. The moderate illness group included patients presenting with features of pneumonia not requiring supplemental oxygen or fulfilling criteria for severe pneumonia. Patients fulfilling the WHO definitions of severe and critical COVID 19 illness were excluded from this study.
Study Design
Patients were randomized to receive either institutional standard of care [SOC] [Control group] or a single dose of Ivermectin at 12 mg [Iv12 group] or 24 mg [Iv24 group] along with SOC though a computer-generated randomization table. Children received ivermectin dosages at 200 µg/kg or 400 µg/kg for single and double dose, respectively.
Clinical, Laboratory and Virological Monitoring
All patients, including asymptomatic patients, were hospitalized for supportive management as per government directives during the first wave. Patients were monitored daily for worsening or development of new symptoms and for adverse events directly related to Ivermectin. On enrollment, full blood counts, renal and liver functions, Prothrombin time [PT], Activated Partial Thromboplastin time [APTT] and plasma fibrinogen along with D-dimer, C reactive protein, procalcitonin, ferritin, lactic dehydrogenase, Quantitative immunoglobulin estimation, IL-6 levels and estimation of total amount of B lymphocytes and T lymphocytes was performed.
Following administration of Ivermectin, serial nasopharyngeal swabs were collected on days 3, 5, 7 for detection of SARS-CoV-2 by real time RT-PCR. After processing, total nucleic acid was extracted from samples on Qiacube HT automated nucleic acid extraction system [Qiagen Inc.]. Extracts were subjected to a realtime RT-PCR for the detection of SARS CoV-2 using the Real Star® SARS CoV-2 kit (Altona Diagnostics GmbH, Germany) as per manufacturers instruction. The triplex rRT-PCR assay detects the envelope (E) gene of lineage B-ß coronaviruses, spike (S) gene of SARS CoV-2 with a heterologous internal control (IC). Sample batches were tested with kit provided positive controls and additional internal quality controls to monitor assay performance. Sample results were considered valid if IC was detected. Sample was considered negative if no signal was detected in the E and S gene. Sample was declared positive if Ct value was < 40 for E and/or S gene. Samples were repeated if IC was not detected or if only single virus gene (E or S) were detected to confirm result. The Ct value of the E gene was used for analysis of primary outcomes.
Outcome Measures
Primary outcome measure was the proportion of patients negative for SARS-CoV-2 RNA by rRT-PCR on day 7 post-treatment. Secondary outcomes included viral load [Ct values of E gene] on days 3, 5 and 7 post treatment, proportion of patients with symptom progression as judged by the WHO ordinal score, incidence of adverse events attributable to ivermectin and all-cause mortality at discharge from the COVID ward. The criteria of discharge from the COVID ward were used for survival since a number of patients, during the same hospital admission, were subsequently transferred to the hematology wards for treatment of the primary illness.
Sample Size Justification
It was estimated that in patients with hematological illnesses, only 10% would become PCR negative on Day 7 with supportive care [SOC] while it was expected that 40% would become negative on Day 7 with ivermectin. Sample size was calculated to have 80% power at a 5% significance level to detect a negativity rate of 40% compared to 10% with SOC. The sample size was calculated as 31 in each arm with a 2:1 ratio for ivermectin compared to SOC for primary outcome analysis. Further recruitment into the study was stopped in March 2021 by the grant agency after initial analysis of the data suggested that the primary end point, which was a significant reduction in viral loads with ivermectin, was unlikely to be reached with additional recruitment. Hence only 84 patients were evaluable for the primary analysis.
Statistical Analysis
Overall survival (OS) included all patients who were alive on discharge from the COVID ward. Dichotomous variables were compared using a χ2 test and continuous variables were compared using a student's t-test or a Mann–Whitney U test as appropriate. The prognostic relevance of clinical and biological variables was studied using univariate and multivariate Cox regression analysis. For all the tests, a two-sided p value of 0.05 or less was considered statistically significant. Statistical analysis used the IBM SPSS 24.0 Software.
Results
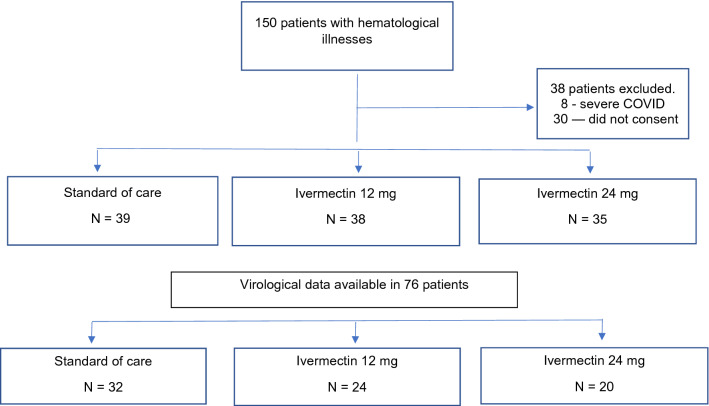
Between June 2020 and February 2021, 150 patients with hematological illnesses were assessed for recruitment. Eight patients with severe COVID 19 were excluded while 30 did not give consent and therefore 112 patients were randomized between the 3 groups (Fig. 1). Baseline characteristics are described in Table 1. The median age and distribution of pediatric patients were similar for all groups. No significant differences were observed between the 3 treatment groups in terms of malignant versus non-malignant illness, disease activity, number with significant neutropenia or thrombocytopenia at admission. Distribution of patients with normal, high, and low neutrophil–lymphocyte ratio [NLR] were similar as was the ratio of patients with normal versus high values of D-Dimer, C-reactive protein [CRP] and ferritin. At diagnosis, 14 [12.6%] had asymptomatic COVID, 67 [59.8%] had mild COVID and 31 [27.6%] had moderate COVID infection. The distribution of disease severity was similar between the 3 treatment groups.
Fig. 1.
CONSORT Statement for patient enrollment
Table 1.
Baseline characteristics of patients in the 3 treatment groups [N = 112]
| Standard of care group [Controls] |
Ivermectin1 2 mg group [Iv12] |
Ivermectin 24 mg group [Iv24] |
|
|---|---|---|---|
| Total number | 39 | 38 | 35 |
| Median age [years]; range | 43.2 [3–77] | 38.5 [6–70] | 42.3 [4–73] |
| Children < 15 years | 2 [5.2%] | 3 [7.9%] | 4 [11.4%] |
| Gender—Male:Female | 30:9 | 30:8 | 19:16 |
| Type of disease | |||
| Malignant | 30 [73.2%] | 29 [72.5%] | 26 [66.7%] |
| Acute leukemia | 11 | 13 | 10 |
| Chronic leukemia | 2 | 2 | 2 |
| Lymphoma | 9 | 9 | 14 |
| Myeloma | 8 | 1 | 3 |
| MPN | – | 1 | – |
| Non-malignant | 9 [21.9%] | 9 [22.5%] | 9 [23.1%] |
| Aplastic anemia | 2 | 4 | 6 |
| Megaloblastic anemia | – | 1 | 1 |
| ITP/AIHA/PRCA | 3 | 3 | 1 |
| Sickle cell disease | 1 | 1 | – |
| Bleeding disorders# | 2 | – | 1 |
| Disease activity | |||
| Remission | 17 [41.5%] | 14 [36.8%] | 13 [33.3%] |
| Active on treatment | 13 [36.6%] | 18 [47.3%] | 14 [46.2%] |
| Relapsed/refractory | 9 [21.9%] | 6 [15.9%] | 8 [20.5%] |
| Previous transplant [allo/auto] | 3 [1/2] | 6 [3/3] | 2 [2/0] |
| Platelet count < 50 × 109/L | 12 [29.2%] | 19 [50%] | 19 [54.2%] |
| ANC < 1 × 109/L | 13 [31.7%] | 16 [42.1%] | 18 [51.4%] |
| Neutrophil–lymphocyte ratio [NLR] | |||
| Normal [1–3] | 14 [39.1%] | 11 [28.9%] | 11 [31.6%] |
| High [> 3] | 16 [39.1%] | 18 [47.4%] | 12 [34.2%] |
| Low [< 1] | 9 [21.9%] | 9 [23.7%] | 12 [34.2%] |
| Increased D-dimer [n = 104] | 26 [68.2%] | 27 [71.1%] | 24 [68.5%] |
| Increased CRP [n = 96] | 28 [68.2%] | 25 [65.7%] | 26 [74.2%] |
| Increased Ferritin [n = 105] | 26 [63.4%] | 32 [84.2%] | 27 [77.1%] |
| COVID infection status | |||
| Asymptomatic | 6 [14.6%] | 4 [10.5%] | 4 [11.5%] |
| Mild | 20 [53.7%] | 26 [68.4%] | 21 [60%] |
| Moderate | 13 [31.7%] | 8 [21.1%] | 10 [28.5%] |
MPN, Myeloproliferative disorders; ITP, Immune thrombocytopenia; AIHA, Autoimmune hemolytic anemia; PRCA, Pure red cell aplasia; DM, Diabetes Mellitus; HT, Hypertension; IHD, Ischaemic heart disease; Allo, Allogeneic transplant; Auto, Autologous transplant; ANC, Absolute neutrophil count
#Consisted of severe hemophilia [1] and Afibrinogenemia [2]
COVID 19 Treatment and Side Effects of Ivermectin
Patients received a single dose of either 12 or 24 mg of Ivermectin along with SOC or SOC alone. Children received between 4 and 12 mg of ivermectin based upon their body weight. Based upon the treatment protocols prevalent in the institution for various stages of COVID-19 illness, patients in addition, received dexamethasone [n = 70], low molecular weight heparin [n = 38] or remdesivir [n = 21] with 43 [35.8%] receiving a combination of the above drugs. Two patients had mild nausea after ivermectin but were also on dexamethasone at the same time. There were no other toxicities that could be directly attributed to ivermectin reported in any of the patients.
Symptom Resolution and Survival
In this study, we evaluated whether ivermectin would help in faster symptom resolution and lower progression of symptoms (Table 2). The number of patients who received dexamethasone, low molecular weight heparin and remdesivir were similar between the 3 treatment groups.
Table 2.
Clinical resolution of symptoms and survival till hospital discharge
| Standard of care group [Controls] |
Ivermectin 12 mg group [Iv12] |
Ivermectin 24 mg group [Iv24] |
|
|---|---|---|---|
| Number of patients | 39 | 38 | 35 |
|
Time to symptom resolution [days/range] |
5.93 ± 5.93 [0–22] |
5.56 ± 5.42 [0–24] |
4.82 ± 4.35 [0–17] |
| Symptom progression | |||
| Yes | 10 [25.7%] | 8 [21.1%] | 6 [17.1%] |
| No | 29 [74.3%] | 30 [78.9%] | 29 [82.9%] |
| Symptom progression | |||
| Asymptomatic | 0/6 [0%] | 0/4 [0%] | 0/4 [0%] |
| Mild | 3/20 [15%] | 3/26 [11.5%] | 2/21 [9.5%] |
| Moderate | 7/13 [53.8%] | 5/8 [62.5%] | 4/10 [40%] |
| Symptom progression in children | |||
| Yes | 0 | 0 | 1/4 [25%] |
| No | 2/2 [100%] | 3/3 [100%] | 3/4 [75%] |
| Survival till discharge | |||
| Yes | 31 [79.4%] | 30 [78.9%] | 30 [85.7%] |
| No | 8 [20.6%] | 8 [21.1%] | 5 [14.3%] |
| Survival till discharge | |||
| Asymptomatic | 6/6 [100%] | 4/4 [100%] | 4/4 [100%] |
| Mild | 19/20 [95%] | 22/26 [84.6%] | 20/21 [95.2%] |
| Moderate | 6/13 [46.1%] | 4/8 [50%] | 6/10 [60%] |
| Additional treatment received for COVID 19 | |||
| Steroids | 26 [66.6%] | 26 [65%] | 18 [51.4%] |
| LMW heparin | 15 [38.4%] | 11 [27.5%] | 12 [35.2%] |
| Remdesivir | 9 [23.1%] | 8 [20%] | 4 [11.4%] |
The mean time to symptom resolution was similar between the 3 groups. Overall, 24 patients [21.4%] showed progression of symptoms while 94 [78.6%] did not show any evidence of progression. The number of patients who had symptom progression were similar between the 3 treatment groups [controls—25.7%, Iv12 group—21.1%, Iv 24 group—17.1%]. Symptom progression was similar among children between the 3 treatment groups. There was no significant difference observed in progression of symptoms among the 3 treatment groups even among the 3 different disease categories [asymptomatic, mild, and moderate COVID-19 illness]. Twenty-one patients [18.7%] have expired while 91 [81.3%] have survived till discharge. Survival rates were similar for the 3 treatment groups [79.4% for controls, 78.9% for Iv12 and 85.7% for Iv 24 respectively]. Survival rates were however significantly higher in patients with mild and asymptomatic illness compared to moderate illness [92.6% vs 51.6%; p < 0.001] and this was true in all 3 treatment categories.
Factors influencing symptom progression included age [HR 1.033; 95% CI − 1.006 to 1.060; p = 0.016], moderate illness [HR 6.827; 95% CI − 2.589 to 18.003; p = 0.000], high NLR ratio [HR 3.413; 95% CI 1.110 to 10.491; p = 0.032] and high D-dimer levels at admission [HR 13.596; 95% CI − 1.757 to 105.203; p = 0.012]. The use of ivermectin, gender, type of disease [malignant versus non-malignant versus medical illness], disease activity, presence of neutropenia and thrombocytopenia, procalcitonin and CRP levels and the presence of hypogammaglobulinemia did not influence progression. On multivariate analysis, only moderate illness at presentation [p = 0.032] continued to remain significant.
Factors influencing survival included age [HR 1.029; 95% CI − 1.001 to 1.058; p = 0.042], moderate illness at presentation [HR 8.973, 95% CI 3.140–25.640; p = 0.000] and high D-dimer levels at admission [HR 10.672, 95% CI 1.372 to 83.078; p = 0.024]. The use of ivermectin, gender, type of disease [malignant versus non-malignant], disease activity, presence of neutropenia and thrombocytopenia, NLR ratio, procalcitonin and CRP levels and the presence of hypogammaglobulinemia did not influence survival. On a multivariate analysis, only moderate illness at presentation [p = 0.012] again continued to remain significant.
Viral Clearance
The other objective of this study was to assess the effect of ivermectin on viral clearance. Of the 112 patients randomized, repeat rRT-PCR samples were available in only 76 patients and were evaluated for viral clearance. The median age, distribution of malignant and non-malignant disorders, disease activity and COVID infection status were similar between the 3 treatment groups (Table 3). Viral clearance as defined as PCR negativity on days 3, 5 and 7 were similar between the control and treatment groups. PCR negativity on Days 3, 5 and 7 were 16.1%, 34.6% and 50% with controls, 17.3%, 31.8% and 41.1% with ivermectin 12 mg and 15.7%, 22.2% and 33.3% with ivermectin 24 mg respectively. We studied whether ivermectin resulted in more patients having a ≥ 2 log10 reduction in viral loads [Ct value difference between the primary and subsequent samples; 1log10 = − 3.3 Ct value difference] at similar time points. The number of patients with ≥ 2 log10 reduction on days 3, 5 and 7 were similar between the control and treatment groups. The Day 7 rRT-PCR negativity for asymptomatic, mild, and moderate illness were similar for the control and the treatment groups as was the percentage of patients with a ≥ 2 log10 reduction on Day 7 in each of the disease groups.
Table 3.
Virological clearance in the three treatment groups
| Standard of care group [Controls] |
Ivermectin 12 mg group [Iv12] |
Ivermectin 24 mg group [Iv24] |
|
|---|---|---|---|
| Total number | 32 | 24 | 20 |
|
Median age [years] range |
42 [3–71] |
38.3 [6–68] |
43 [4–73] |
| Type of disease | |||
| Malignant | 25 [78.1%] | 19 [73.1%] | 16 [66.8%] |
| Non-malignant | 7 [21.9%] | 5 [19.2%] | 4 [16.6%] |
| Disease activity | |||
| Remission | 12 [37.5%] | 9 [37.6%] | 9 [45%] |
| Active on treatment | 11 [34.3%] | 10 [41.6%] | 6 [30%] |
| Relapsed/Refractory | 9 [28.2%] | 5 [20.8%] | 5 [25%] |
| COVID infection status | |||
| Asymptomatic | 2 [6.3%] | 4 [10.3%] | 4 [20%] |
| Mild | 18 [56.2%] | 17 [64.1%] | 13 [65%] |
| Moderate | 12 [37.5%] | 3 [25.6%] | 3 [15%] |
| rRT-PCR negativity | |||
| Day 3 | 5/31 [16.1%] | 4/23 [17.3%] | 3/19 [15.7%] |
| Day 5 | 9/26 [34.6%] | 7/22 [31.8%] | 4/18 [22.2%] |
| Day 7 | 11/22 [50%] | 7/17 [41.1%] | 5/15 [33.3%] |
| ≥ 2 log10 reduction | |||
| Day 3 | 6/23 [26.1%] | 8/19 [42.1%] | 4/14 [28.5%] |
| Day 5 | 11/22 [50%] | 11/18 [61.1%] | 6/15 [40%] |
| Day 7 | 14/19 [73.6%] | 11/15 [73.3%] | 6/14 [42.8%] |
| rRT-PCR negativity Day 7 | |||
| Asymptomatic | 1/1 [100%] | 1/1 [100%] | 1/2 [50%] |
| Mild | 8/15 [53.3%] | 5/13 [38.5%] | 4/10 [40%] |
| Moderate | 3/7 [42.8%] | 1/3 [33.3%] | 0/3 [0%] |
| ≥ 2 log10 reduction Day 7 | |||
| Asymptomatic | 1/1 [100%] | 1/1 [100%] | 2/2 [100%] |
| Mild | 11/13 [84.6%] | 8/11 [72.7%] | 4/9 [44.4%] |
| Moderate | 6/8 [75%] | 2/3 [66.6%] | 0/3 [0%] |
Discussion
This Phase II randomized trial, compared the efficacy of 2 different doses of ivermectin with standard of care in reducing viral loads and preventing progression of symptoms in patients with hematological and medical illnesses. In this analysis, a single dose of ivermectin did not result in faster reduction in viral loads [Negative rRT-PCR on day 7 or ≥ 2 log10 reduction in viral loads on Day 7] irrespective of the disease category. Ivermectin has been studied for both prophylaxis as well as treatment of mild to severe COVID 19 infections. In a study on 100 asymptomatic patients from Lebanon, Ivermectin was shown to improve Ct values and reduce progression of symptoms in a randomized controlled trial [10]. Meta-analysis of data, however, on ivermectin prophylaxis has not suggested any benefit [11]. Data on RCT studies have reported mixed utility of ivermectin on mild and moderate COVID. In another randomized study involving 24 patients with non-severe COVID-19 illness, the use of ivermectin was associated with a marked reduction of self-reported anosmia/hyposmia and a reduction of cough but only a tendency to lower viral loads [12].
The secondary end points in this study included adverse events, reduction in symptom progression and survival till discharge from the COVID ward. The use of ivermectin did not result in a significant reduction in symptom progression and survival. Studies on symptom resolution with the use of Ivermectin in COVID 19 have shown mixed results. In a randomized trial involving 400 patients with mild illness, a 5-day course of ivermectin did not improve the time to resolution of symptoms [13]. This is similar to data from Egypt where a RCT involving 164 patients showed no significant improvement in symptom resolution with ivermectin in patients with mild to moderate COVID 19 illness [14]. However, studies from Iran, involving 69 patients, and from Bangladesh, involving 400 patients, showed better symptom resolution with the use of ivermectin [15, 16]. In the study from Bangladesh, a combination of ivermectin and doxycycline was used.
Our data suggests that in patients with hematological disorders, the use of a single dose of ivermectin was not useful in reducing symptom progression or mortality following COVID-19 illness. There is very limited data on the use of drugs for the treatment of COVID 19 in patients with hematological disorders. A few studies, none of them RCTs, have explored the use of convalescent plasma in patients with hematological malignancies and COVID 19 infection with variable effect on survival [17–19]. Anakinra, a IL-1 receptor antagonist was not found to be useful in patients with hematological malignancies and severe SARS-Cov-2 infection [20]. There is an ongoing trial on Baricitinib to prevent respiratory insufficiency progression in onco-hematology patients.
Going forward, there is an urgent and important need to identify drugs that can reduce symptom progression and reduce mortality in patients with hematological disorders who develop COVID 19 infection. Our data suggests that older age, moderate illness at presentation and high D-dimer levels had an impact on symptom progression and survival. Therefore, patients with moderate to severe illness and those with mild illness but with high D-dimer levels at admission should be the target population for future studies. There has always been a concern regarding the therapeutic levels that can be achieved by a single dose of ivermectin against the SARs Cov-2 virus. In the study by Caly et al., the potential drug efficacy in vitro was observed at a high ivermectin concentration [21]. The IC50 reported (2190 ng/mL) is at least 50 times higher than the maximal concentration achievable with the standard dose of 200 μg/kg. A 5-day course of ivermectin at 12 mg daily for 5 days for 5 days either alone or in combination with other drugs was found to be safe and well tolerated and associated with reduction in viral loads and faster resolution of symptoms compared to placebo in 2 studies [22, 23]. At present, results of ongoing studies looking at higher doses of 600–1200 µg/kg/day for 5 days for COVID 19 infection are awaited. Higher doses of ivermectin in combination with other drugs needs to be studied further in patients with hematological disorders.
In conclusion, a single dose of ivermectin [12 mg or 24 mg] did not reduce viral loads or symptom progression and did not improve survival in patients with hematological disorders who developed mild or moderate COVID 19 illness. Moderate illness, age and high D-dimer levels were identified as variables affecting symptom progression and survival. Further studies involving higher doses and duration of ivermectin therapy either alone or in combination with other drugs are needed in patients with hematological disorders.
Acknowledgements
This study was funded by a COVID grant from the Science and Engineering Board [SERB], Department of Science and Technology, Government of India.
Author Contributions
BG, UK designed the study, interpreted the data and drafted the paper. MM designed the study, acquired of laboratory data and drafted the paper. KML analysed the data, provided critical revision of the paper and approved the final version. SS, PR, DJC, BT, WR, AJD, FNA, AK, SL, AA and VM helped in acquiring the clinical data, interpretation of data and revising the manuscript critically. All authors have approved the final submitted version of the manuscript.
Funding
This study was funded by a COVID grant from the Science and Engineering Board [SERB], Department of Science and Technology, Government of India.
Declarations
Conflict of interest
No conflicts of interest to declare for any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Biju George and Mahesh Moorthy are primary authors.
Contributor Information
Biju George, Email: biju@cmcvellore.ac.in.
Mahesh Moorthy, Email: maheshmoorthy@cmcvellore.ac.in.
References
- 1.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;20(395):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LYW, Cazier J-B, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;20(395):1907–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with hematological malignancies in Italy: a retrospective multicenter cohort study. Lancet Haematol. 2020;7(10):e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinana JL, Martino R, Garcia IG, et al. Risk factors and outcomes of COVID19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of infected patients. NEJM. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Yan L-M, Wan L, Xiang T-X, Le A, Liu J-M, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang D, Mo G, Yuan X, Tao Y, Peng X, Wang F, et al. Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection. Am J Respir Crit Care Med. 2020;201(9):1150–1152. doi: 10.1164/rccm.202003-0524LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samaha AA, Mouawia H, Fawaz M, et al. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS—Cov 2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021;13(6):989. doi: 10.3390/v13060989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against COVID-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. doi: 10.1136/bmj.n949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaccour C, Casellas A, Di Matteo AB, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. E Clin Med. 2021;32:100720. doi: 10.1016/j.eclinm.2020.100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Medina E, Lopez P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abd-Elsalam S, Noor RA, Badawi R, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J Med Virol. 2021 doi: 10.1002/jmv.27122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahbaznejad L, Davoudi A, Eslami G, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin Ther. 2021 doi: 10.1016/j.clinthera.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmud R, Rahman MM, Alam I, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49(5):3000605211013550. doi: 10.1177/03000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biernat MM, Kolasinska A, Kwiatkowski J, et al. Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID 19. Viruses. 2021;13(3):436. doi: 10.3390/v13030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hueso T, Pouderoux C, Pere H, et al. Convalescent plasma therapy for B cell depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeyaraman P, Agrawal N, Bhargava R, et al. Convalescent plasma therapy for severe COVID 19 in patients with hematological malignancies. Transfus Apher Sci. 2021;60:103075. doi: 10.1016/j.transci.2021.103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villegas C, Poza M, Talayero P, et al. IL-1R blockade is not effective in patients with hematological malignancies and severe SARS-Cov-2 infection. Ann Hematol. 2020;99(12):2953–2956. doi: 10.1007/s00277-020-04160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID 19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamus N, Nemirturk N, Cetinkaya RA, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID 19 patients. BMC Infect Dis. 2021;21(1):411. doi: 10.1186/s12879-021-06104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]