Abstract
The xanthan-degrading bacterium Paenibacillus alginolyticus XL-1, isolated from soil, degrades approximately 28% of the xanthan molecule and appears to leave the backbone intact. Several xanthan-degrading enzymes were excreted during growth on xanthan, including xanthan lyase. Xanthan lyase production was induced by xanthan and inhibited by glucose and low-molecular-weight enzymatic degradation products from xanthan. A xanthan lyase with a molecular mass of 85 kDa and a pI of 7.9 was purified and characterized. The enzyme is specific for pyruvated mannosyl side chain residues and optimally active at pH 6.0 and 55°C.
Xanthan, the extracellular polysaccharide (eps) produced by Xanthomonas campestris, has many industrial applications as a thickener of aqueous solutions and as a stabilizer of emulsions, foams, and particulate suspensions. Xanthan is used in many foods, e.g., juices, drinks, ice cream, salad dressings, and dry mix formulations such as desserts. The bulk of xanthan, however, is used for enhancing oil recovery and in the manufacturing of explosives, paints, polishes, fire-fighting liquids, and cosmetics (18).
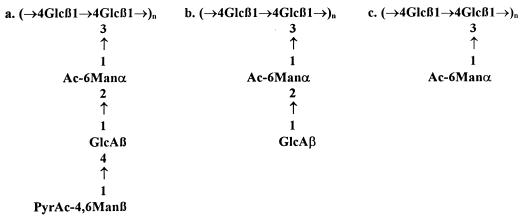
Xanthan has a pentasaccharide repeating unit: the β-1,4-glucan backbone is substituted on alternate glucosyl residues with a trisaccharide side chain consisting of α-mannose, β-glucuronic acid, and β-mannose (Fig. 1a). Also “variant xanthans” with truncated side chains have been described (2, 25). These variants, consisting of tetrasaccharide or trisaccharide repeating units (Fig. 1b and c), are produced by X. campestris mutants. Truncation of the side chain affects the viscometric properties of xanthan. Compared to the polypentamer, the acetylated polytetramer is a weaker viscosifier (15), whereas the polytrimer is reported to be a superior viscosifier on a weight basis (10). However, these variant xanthans, especially the polytrimer, are produced at low yields (26). An attractive alternative method to produce xanthans with truncated side chains could be enzymatic modification of polypentamer xanthan.
FIG. 1.
Native (a) and mutant xanthan structures (b, polytetramer; c, polytrimer). The extent of acetylation and pyruvation varies with bacterial source and culture conditions.
Xanthan-modifying enzymes can be obtained from xanthan-degrading microorganisms. Xanthan-degrading pure cultures (22, 23), as well as mixed cultures (6), have been described. In some cases pure cultures were isolated from mixed cultures but, compared to the mixed cultures, the growth rates and production of xanthan-degrading enzymes were considerably lower (5, 12, 23).
Xanthan lyase is one of the enzymes that can be used for xanthan modification. This enzyme removes the terminal mannosyl residue via β-elimination, yielding a free mannose and a tetrasaccharide repeating unit as in Fig. 1b; however, with a 4,5-ene-glucuronyl residue on the side chain. Xanthan lyase activity can easily be monitored by measuring the increase of A235 caused by the conjugation of the formed C=C bond with the carboxylate group in the uronic acid residue. Alternatively, the double bond introduced by xanthan lyase can be oxidized with periodate. This yields an oxidation product that reacts with thiobarbituric acid (TBA) to a chromophore at 590 nm (24). Xanthan lyases were first obtained by Sutherland (23) from a Bacillus sp., a Corynebacterium sp., and a mixed culture. The action of these enzymes was independent of the degree of pyruvation and acetylation of xanthan. Pyruvated mannose-specific xanthan lyases have been purified from a salt-tolerant mixed culture by Ahlgren (1) and, very recently, from a Bacillus sp. by Hashimoto et al. (9).
In this study, the isolation of Paenibacillus alginolyticus XL-1 from an enrichment culture on xanthan is described. This strain was tested for xanthan-degrading enzyme activities. Several xanthan-degrading enzymes were excreted, including a pyruvated mannose-specific xanthan lyase that was purified and characterized.
MATERIALS AND METHODS
Enrichment of xanthan-degrading bacteria.
Xanthan-degrading bacteria were enriched on mineral salts medium, pH 6.9, with 3 g of xanthan (Sigma G-1253, practical grade) liter−1. Mineral salts medium contained the following (in milligrams per liter): EDTA, 10.0; ZnSO4 · 7H2O, 2.0; CaCl2 · 2H2O, 1.0; FeSO4 · 7H2O, 5.0; Na2MoO4 · 2H2O, 0.2; CuSO4 · 5H2O, 0.2; CoCl2 · 6H2O, 0.4; MnCl2 · 4H2O, 1.0; (NH4)2SO4: 2,000; MgCl2 · 6H2O, 100; K2HPO4, 1,550; and NaH2PO4 · H2O, 850. In a 500-ml Erlenmeyer flask, 100 ml of xanthan medium was inoculated with 1 ml of a 1:1 (wt/vol) mixture of soil and 0.9% (wt/vol) NaCl. The cultures were incubated with shaking at 30°C, and 1 ml was transferred daily to 100 ml of fresh medium. After repeated transfers, pure cultures were isolated and maintained on solid mineral salts medium containing 5 g of mannose or xanthan liter−1 and yeast extract (0.05 g liter−1, added after autoclaving from a filter-sterilized 5 g liter−1 stock solution).
Strain and culture conditions.
P. alginolyticus XL-1, isolated from a mixed culture growing on xanthan, was maintained on solid xanthan medium supplemented with filter-sterilized yeast extract. Liquid cultures were incubated at 30°C with shaking, on mineral salts medium containing 5 g of carbon source and 0.05 g of filter-sterilized yeast extract liter−1. For enzyme production, 1 liter of xanthan medium in a 5-liter Erlenmeyer flask was inoculated with 5 ml of a xanthan-grown overnight culture. After 20 h of incubation, the culture was centrifuged (15,000 × g, 15 min, 4°C), and the supernatant was used for enzyme purification.
Polysaccharides.
Practical-grade xanthan was obtained from Sigma (G-1253). Native and chemically modified xanthans (eps of X. campestris X646 and Xanthomonas phaseoli X556, Kelzan [Kelco], and Flocon 4800C [Pfizer]) were kind gifts of Ian Sutherland (Division of Biological Sciences, Institute of Cell and Molecular Biology, University of Edinburgh, Edinburgh, United Kingdom). The capsular polysaccharide of Klebsiella serotype K5 (8) was kindly provided by Harm Snippe (Eijkman-Winkler Institute for Microbiology, Infectious Diseases and Inflammation, Academic Hospital Utrecht, Utrecht, The Netherlands).
Purification of xanthan.
Practical-grade xanthan was dissolved to 20 g liter−1 in demineralized water, and 17% (vol/vol) ice-cold trichloroacetic acid solution (80% [wt/vol]) was added to precipitate proteins. The mixture was stirred for 20 min at 4°C and centrifuged (25,000 × g, 15 min, 4°C). After neutralization of the supernatant with 5 M NaOH, xanthan was precipitated by adding 3 volumes of ice-cold absolute ethanol. The precipitate was collected by filtration and dissolved in demineralized water. After extensive dialysis at 4°C against demineralized water, the purified xanthan solution was stored at −20°C or lyophilized.
Chemical modification of xanthan.
Pyruvic acetals were removed from xanthan by using the procedure of Bradshaw et al. (4). Purified xanthan (5 g liter−1) was heated at 100°C for 90 min in 5 mM trifluoroacetic acid (TFA). After dialysis against demineralized water, the preparation was stored at −20°C. Acetyl groups were removed according to the method of Shatwell et al. (20). Purified xanthan (2.5 g liter−1) in 0.1 M NH4OH was incubated at 60°C for 1 h. After dialysis, the solution was stored at −20°C.
Preparation of LMW enzymatic degradation products of xanthan.
To obtain low-molecular-weight (LMW) degradation products released from xanthan by the enzyme system of P. alginolyticus XL-1, filter-sterilized supernatant (15 ml) of a xanthan-grown overnight culture was incubated at 30°C with 250 ml of an autoclaved xanthan solution (10 g liter−1 in 15 mM phosphate buffer [pH 6.9]). Reducing sugars were measured every 3 to 4 days to monitor xanthan degradation. Between days 13 and 17, the reducing sugar formation ceased, and the incubation mixture was centrifuged to remove insoluble xanthan residues. The supernatant was dialyzed against 300 ml of demineralized water, and the dialysate containing the LMW fraction was autoclaved for use as a medium component.
To obtain the LMW product released from xanthan by purified xanthan lyase, 2 ml of purified xanthan solution (7 g liter−1) was incubated with 50 mU of xanthan lyase for 4 h at 30°C. The reaction mixture was dialyzed against 50 ml of demineralized water. The dialysate containing the LMW fraction was lyophilized and stored at −20°C.
Xanthan lyase purification.
P. alginolyticus XL-1 was cultured as described above. The supernatants of 2 cultures (1 liter each) were pooled, and the extracellular enzymes were concentrated by ammonium sulfate precipitation (60% saturation). After centrifugation, the pellet was resuspended in 24 ml of 10 mM Tris (pH 8.0) and centrifuged to remove insoluble materials. To the supernatant, solid (NH4)2SO4 was added to a final concentration of 1 M. Subsequent purification steps were carried out on an FPLC system (Pharmacia) operated at room temperature. The enzyme solution was applied to a hydrophobic interaction chromatography (HIC) column (Pharmacia HiTrap Phenyl Sepharose HP; 1 ml) in 6-ml batches. Proteins were eluted with a linear gradient of 1 to 0 M (NH4)2SO4 in 10 mM Tris (pH 8.0) at a flow rate of 0.5 ml min−1 in a total volume of 10 ml. The xanthan lyase-containing fractions of four subsequent runs were pooled and dialyzed overnight at 4°C against 10 mM Tris (pH 8.0). Subsequently, the HIC pool was applied to an anion-exchange chromatography (AEC) column (Source 15Q HR 5/5). Proteins were eluted with a linear gradient of 0 to 0.15 M NaCl in 10 mM Tris (pH 8.0) at a flow rate of 1 ml min−1 in a total volume of 25 ml. Xanthan lyase-containing fractions were pooled and stored at −20°C.
Protein electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out according to the method of Laemmli (14) by using a Hoeffer Mighty Small system (Pharmacia). Gels were stained with Coomassie brilliant blue. Isoelectric focusing (IEF) was carried out on a Phast Gel system (Pharmacia).
Enzyme assays.
To assess total xanthan-degrading enzyme (“xanthanase”) activity, equal volumes of culture supernatant and purified xanthan solution (5 g liter−1 in 15 mM phosphate buffer [pH 6.9]) were mixed and incubated at 30°C. Samples were drawn at set intervals and assayed for reducing sugars. β-1,4-Glucanase activity was measured like xanthanase by using carboxymethyl cellulose (CM-cellulose) instead of xanthan as the substrate. Similar incubations were carried out to determine enzyme activity releasing uronic acid-containing fragments from xanthan. In these samples, high-molecular-weight xanthan residues were precipitated with 3 volumes of ice-cold absolute ethanol. After centrifugation, the supernatant containing the LMW degradation products was concentrated by lyophilization and assayed for uronic acids. To determine glycosidase activities, equal volumes of culture supernatant and a 10 mM solution of p-nitrophenyl-d-glycoside (10 mM) were incubated. Released p-nitrophenol was measured at 410 nm after the reaction was stopped with 3 volumes of ice-cold 0.2 M Na2CO3. Xanthan lyase activity was determined spectrophotometrically in a Perkin-Elmer λ-l spectrophotometer. Xanthan lyase (100 μl of crude enzyme or 0.5 to 1 μg of purified enzyme) was added to 500 μl of purified xanthan solution (0.05 g liter−1 in 15 mM phosphate buffer [pH 6.9]) and mixed quickly, and the A235 value was recorded continuously. One unit of xanthan lyase activity is defined as the amount of enzyme that forms 1 μmol of 4,5-ene-glucuronyl residues per minute (ɛ235 = 8.0 cm2 μmol−1). To determine the optimal pH for xanthan lyase, activity was measured as described above but xanthan was dissolved in McIlvaine buffer of different pH values. Tenfold-concentrated McIlvaine buffer was prepared by mixing 0.1 M citric acid and 0.2 M Na2HPO4 to obtain the desired pH. To assess the thermal stability of xanthan lyase, the purified enzyme was diluted to 40 μg ml−1 in McIlvaine buffer at pHs 5, 6, and 7. The enzyme solutions were incubated for 15 min at different temperatures and immediately stored on ice. Subsequently, activity was measured according to the standard procedure.
Determination of ɛ235 of the xanthan lyase reaction product.
Purified xanthan lyase (150 mU) was incubated with 1.2 ml of purified xanthan (6 g liter−1). Both A235 and the reducing sugar concentration relative to a mannose standard were measured at set intervals. The A235 was measured after inactivating the enzyme with 0.5 volume of 5 M NaOH and an appropriate dilution with water. A235 was plotted against the concentration of reducing sugars, and from the slope an ɛ235 of 8.0 cm2 μmol−1 was determined.
Calculation of xanthan degradation.
To determine the extent of xanthan degradation by P. alginolyticus XL-1, the bacterium was cultured in closed 500-ml serum bottles containing 40 ml of xanthan medium. Total sugar, reducing sugar, CO2 evolution, and biomass formation were monitored during incubation. The following assumptions were made to be able to calculate xanthan degradation on a C-mol basis: the number of xanthan repeating units equals the total sugar content, corrected for the reducing (i.e., nonpolysaccharide) sugars, divided by 5; the average molecular formula for a xanthan repeating unit is C32.7H48.9O26.8 (molar mass, 869.3 g; 96% acetylation and 26% pyruvation; see Table 3); the carbon content of the biomass is 50% (wt/wt); and the reducing sugars released are hexoses.
TABLE 3.
Activity of purified P. alginolyticus XL-1 xanthan lyase on various substratesa
| Substrate | Acetylation (%)b | Pyruvation (%)b | Relative activity |
|---|---|---|---|
| Xanthan (Sigma) | 96 | 26 | 100 |
| Xanthan (Sigma), DAd | 21 | 21 | 100 |
| Xanthan (Sigma), DPd | 83 | 12 | 0 |
| Flocon 4800Cc | 40 | 61 | 80 |
| Flocon 4800C, DPc | 54 | 12 | 0 |
| X646c | 90 | 54 | 50 |
| X646, DPc | 88 | 7 | 0 |
| X646, DAc | 26 | 44 | 0 |
| X646, DAPc | 20 | 7 | 0 |
| X556c | 32 | 74 | 67 |
| X556, DPc | 22 | 12 | 0 |
| Kelzan | 54 | 23 | 80 |
| K5 | 58 | 23 | 0 |
All polysaccharides were dissolved to 0.05 g liter−1 except X556-eps (dissolved to 0.025 g liter−1 due to high background absorption at 235 nm). A relative activity of 100 is equal to 0.7 mU ml−1.
Expressed as a percentage of the number of repeating units. The number of repeating units was determined from total sugar measurements divided by 5 for xanthans and 3 for K5-cps.
Data adapted from Shatwell et al. (20).
DP, chemically depyruvated; DA, chemically deacetylated.
Analytical procedures.
The growth of P. alginolyticus XL-1 on xanthan was monitored by measuring CO2 evolution in closed 500-ml serum bottles containing 40 ml of medium with an HP 6890 gas chromatograph (Hewlett-Packard) equipped with a Poraplot Q column (25 m). Growth on carbon sources other than xanthan was determined by measuring the optical density at 660 nm. Dry biomass was measured gravimetrically after the cells were collected by centrifugation, washed twice with demineralized water, and dried overnight at 110°C.
Protein was measured by using the bicinchoninic acid protein assay kit (Pierce) according to the supplier’s instructions. Reducing sugars were determined with the dinitrosalicylic acid method of Miller (16) by using glucose or mannose as the standard. Total sugar was determined with the phenol-sulfuric acid method of Dubois et al. (7) by using glucose as the standard. Uronic acids were determined by the method of Blumenkrantz and Asboe-Hansen (3) with glucuronic acid as the standard. For qualitative determination of 4,5-ene-glucuronyl residues formed by xanthan lyase, the TBA method of Weissbach and Hurwitz (27) was used. Glucose was determined enzymatically with the Boehringer Mannheim d-glucose test kit according to the supplier’s instructions.
To determine the pyruvate content of xanthan, xanthan (1 g liter−1) was hydrolyzed in 1 M HCl for 1.5 h at 100°C. Subsequently, free pyruvate was determined enzymatically by using the Sigma Diagnostics pyruvate kit according to the supplier’s instructions. The acetyl content of xanthan was determined by the method of Hestrin (11) with acetylcholine as the standard.
Thin-layer chromatography (TLC) of the LMW xanthan lyase product was carried out on silica gel plates (Merck Kieselgel 60 F254). The eluent was 2-propanol–acetone–1 M lactic acid (2:2:1). For the detection of sugars, the plates were sprayed with phenol-sulfuric acid reagent (3 g of phenol and 5 ml of concentrated sulfuric acid in 95 ml of ethanol) and heated at 110°C for 5 to 10 min.
RESULTS
Isolation of a xanthan lyase-producing P. alginolyticus.
Microorganisms were enriched from soil samples in liquid medium with xanthan as the sole source of carbon and energy. After repeated transfers, a xanthan-degrading mixed culture was obtained that grew to a high optical density within 24 h. The presence of xanthan lyase activity in the supernatant of the mixed culture was demonstrated by incubating the supernatant with xanthan and measuring an increase of the A235 as well as the formation of TBA-reactive material. No β-1,4-glucanase activity was observed.
After 30 daily transfers, dilutions of the mixed culture were plated on solid mineral salts medium with mannose and filter-sterilized yeast extract. Initial attempts to isolate xanthan lyase-producing bacteria failed when yeast extract was autoclaved with the medium, suggesting a requirement for a heat-labile component from yeast extract. After 2 days of incubation at 30°C, 12 visibly different colonies were selected and obtained as pure cultures. The pure cultures were grown in mineral salts medium with xanthan and filter-sterilized yeast extract. After overnight incubation at 30°C, cultures were assayed for xanthan lyase production. One of the isolated strains produced xanthan lyase activity, and this strain was designated strain XL-1.
Identification of strain XL-1 was carried out by the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany (data not shown). Physiological tests pointed to P. alginolyticus and/or P. chondroitinus, and fatty acid analysis showed the pattern for the Bacillus circulans and/or Paenibacillus group. Partial 16S ribosomal DNA sequencing showed 97.8% similarity to P. alginolyticus. Although strain XL-1 did not degrade alginate, the name P. alginolyticus will be maintained, as the description of the species (21, 19) does not exclude strains that are incapable of alginate degradation.
P. alginolyticus XL-1 was able to use xanthan, glucose, glucuronic acid, mannose, mannitol, and glycerol as the sole carbon and energy source; no growth was observed on CM-cellulose. Strain XL-1 did not grow without filter-sterilized yeast extract or with autoclaved yeast extract. Filter-sterilized yeast extract could be replaced by a mixture of vitamins (containing p-aminobenzoic acid, folic acid, lipoic acid, riboflavin, biotin, Ca-pantothenic acid, nicotinic acid, pyridoxamine, pyridoxin, thiamine, and cobalamin). To determine which vitamins were essential, P. alginolyticus XL-1 was cultured in media in each of which a single vitamin was omitted. No growth occurred when thiamine or biotin was absent, the latter probably being the heat-labile compound that is destroyed when yeast extract is autoclaved.
Xanthan utilization by P. alginolyticus XL-1.
When plates with strain XL-1 on solid medium with xanthan were stained with Congo red, red haloes were observed around the colonies, indicating the presence of β-1,4-glucan. This suggested that long stretches of the xanthan backbone remained intact whereas the side chains were removed, exposing the backbone and allowing interaction with Congo red. To determine the extent of xanthan degradation, P. alginolyticus XL-1 was cultured on xanthan in liquid medium, and total amounts of sugar, reducing sugar, CO2 evolution, and biomass formation were monitored during incubation. CO2 evolution stopped after 24 h, but the incubation was carried on for another 72 h to allow growth-independent xanthan degradation to proceed to completion.
At the start of the incubation, 1.37 mmol of total xanthan sugar (8.96 mmol of C) was present. After 96 h, 1.00 mmol of CO2 (1.00 mmol of C), 19.6 mg of dry biomass (0.82 mmol of C), and 0.11 mmol of reducing sugars (0.66 mmol of C) were formed. The amount of total soluble sugar at 96 h was 0.57 mmol, of which 0.11 mmol was reducing sugar. Therefore, only 0.46 mmol of soluble polysaccharide sugar (3.0 mmol of C if intact pentasaccharide repeating units are assumed) was still present after incubation. Thus, 28% of the xanthan C initially present was converted to CO2, biomass, and reducing sugar but only 33% was recovered as remaining polysaccharide sugar. The unrecovered 39% was probably modified xanthan with a decreased solubility, present in the slime-like layer that was observed on top of the cell pellet after centrifugation. This layer was lost upon washing of the cells for biomass determination.
The extent of xanthan degradation by the extracellular enzyme system of P. alginolyticus XL-1 was also determined. A sterile xanthan solution was incubated with 2.5% (vol/vol) filter-sterilized crude supernatant of a xanthan-grown culture of P. alginolyticus XL-1. Reducing sugar formation was monitored weekly, and by week 4 no further increase in reducing sugar concentration was observed. The final amount of reducing sugars formed was 26% of the total sugar initially present. This value is in accordance with the 28% breakdown calculated from CO2, biomass, and reducing sugar formation in the P. alginolyticus XL-1 culture and suggests that xanthan is degraded to monosaccharides that are subsequently metabolized.
Production of xanthan-degrading enzymes by P. alginolyticus XL-1.
P. alginolyticus XL-1 produces various xanthan-degrading enzyme activities. Table 1 summarizes the activities observed in the supernatant of a 20-h culture of P. alginolyticus XL-1 grown on xanthan. Xanthan lyase activity could be detected with unmodified, depyruvated, and deacetylated xanthan as substrates. Also LMW compounds reacting as uronic acids were released from xanthan upon incubation with culture supernatant. These compounds could be either uronic acids or uronic acid-containing oligosaccharides. β-1,4-Glucanase, β-mannosidase, α-mannosidase, β-glucuronidase, and β-glucosidase activities could not be detected.
TABLE 1.
Activities of xanthan-degrading enzymes present in a 20-h culture of P. alginolyticus XL-1 grown on xanthan
| Enzyme | Substrate | Activity (mU ml−1)a |
|---|---|---|
| Total xanthanase | Xanthan (Sigma) | 39.0 |
| Xanthan lyase | Xanthan (Sigma) | 15.6 |
| Xanthan DPb (Sigma) | 1.7 | |
| Xanthan DAb (Sigma) | 15.6 | |
| Uronic acid-releasing activity | Xanthan (Sigma) | 2.9 |
One unit is defined as the amount of enzyme releasing 1 μmol of the assayed compound per min.
DP, chemically depyruvated; DA, chemically deacetylated.
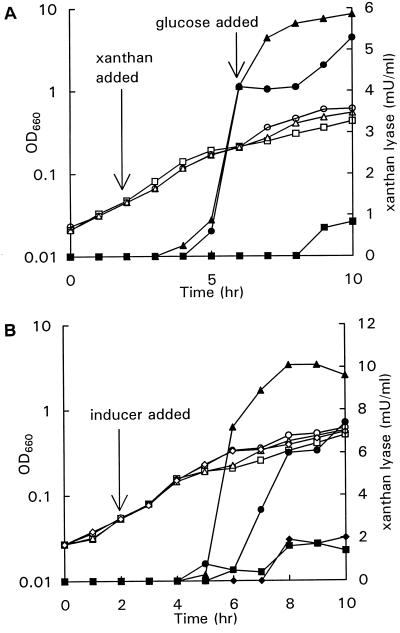
With carbon sources other than xanthan, very low titers of xanthan lyase were produced, indicating that xanthan lyase was induced during growth on xanthan. To demonstrate the inducing effect of xanthan, xanthan (0.2 g liter−1) was added to two cultures of P. alginolyticus XL-1 growing exponentially on mannose. Figure 2A shows that xanthan lyase production started 2 h after the addition of xanthan. At 4 h after the addition of xanthan, glucose (0.25 g liter−1) was added to one of the induced cultures, causing an immediate stop in xanthan lyase production. When glucose was exhausted, xanthan lyase production started again. In the induced culture without additional glucose, xanthan lyase production stopped 6 h after the addition of xanthan, although growth continued. In the control culture without xanthan, a small amount of xanthan lyase activity was produced toward the end of the exponential growth phase.
FIG. 2.
(A) Growth (open symbols) and xanthan lyase activity (closed symbols) in mannose-grown cultures of P. alginolyticus XL-1. Symbols: ■, control; ▴, xanthan (0.2 g liter−1) added after 2 h; ●, xanthan (0.2 g liter−1) added after 2 h and glucose (0.25 g liter−1) added after 6 h. (B) Growth (open symbols) and xanthan lyase activity (closed symbols) in mannose-grown cultures of P. alginolyticus XL-1. Symbols: ■, control; ▴, xanthan (0.2 g liter−1) added after 2 h; ●, xanthan (0.2 g liter−1) and LMW xanthan degradation products (0.54 mM) added after 2 h; ⧫, LMW xanthan degradation products (0.54 mM) added after 2 h.
It is unlikely that xanthan is the true xanthan lyase inducer since xanthan molecules are too large to enter a bacterial cell. Probably, xanthan lyase production is triggered by an LMW enzymatic degradation product of xanthan. This was tested by monitoring xanthan lyase activity after the addition of LMW xanthan degradation products (0.54 mM reducing sugars), xanthan (0.2 g liter−1), or both xanthan and LMW xanthan degradation products to log-phase cultures of P. alginolyticus XL-1 growing on mannose. Figure 2B shows that rather than having an inducing effect, the enzymatic xanthan-hydrolysate appeared to inhibit xanthan lyase production. In the culture with only LMW xanthan degradation products, xanthan lyase was not produced above the level in the control culture without additional potential inducers.
Xanthan lyase purification.
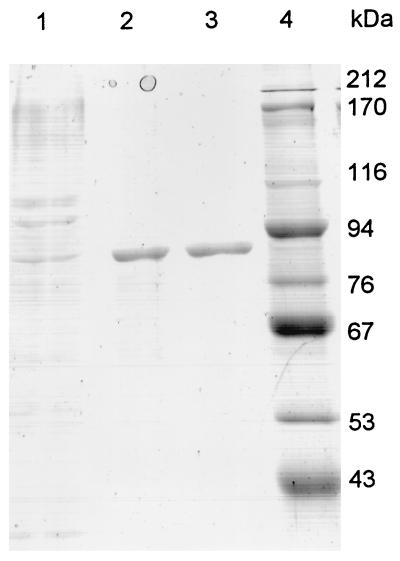
Xanthan lyase was purified 26-fold from 2 liters of culture supernatant. The enzyme eluted from the Phenyl Sepharose column at approximately 0.7 M (NH4)2SO4 in three fractions. The enzyme eluted from the Source 15Q column at approximately 0.05 M NaCl in a single peak at A280 in two subsequent fractions. The purification results are summarized in Table 2. SDS-PAGE and subsequent staining of the gel with Coomassie brilliant blue showed a single band (Fig. 3). The molecular mass was estimated to be 85 kDa compared to the relative mobilities of the protein standards in the SDS-PAGE gel. IEF showed a single band at pH 7.9.
TABLE 2.
Purification of xanthan lyase from 2 liters of culture fluid of P. alginolyticus XL-1 grown on xanthan
| Purification step | Total protein (mg) | Total Ua | Sp act (U mg−1 of protein) | Purification factor |
|---|---|---|---|---|
| Cell-free culture broth | 517 | 50.0 | 0.10 | 1 |
| (NH4)2SO4 precipitation (60% saturation) | 75 | 32.8 | 0.44 | 4.4 |
| Phenyl Sepharose HP chromatography plus overnight dialysis | 8.5 | 19.5 | 2.29 | 22.9 |
| Source 15Q chromatography | 4.2 | 11.0 | 2.62 | 26.2 |
One unit is defined as the amount of enzyme that produces 1 μmol of 4,5-ene-glucuronic acid residues per min. Activity was calculated from the increase of A235; ɛ235 was 8.0 cm2 μmol−1.
FIG. 3.
SDS-PAGE gel of different purification stages of xanthan lyase. Lane 1, ammonium sulfate precipitate; lane 2, dialyzed HIC pool; lane 3, AEC pool; lane 4, protein standards.
Properties of purified xanthan lyase.
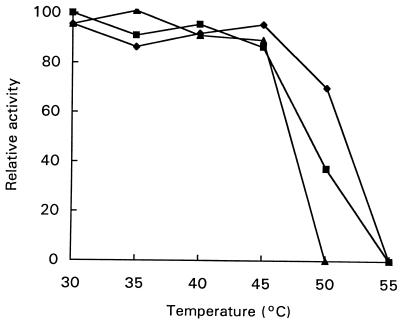
The purified xanthan lyase was active over a broad pH range with an optimum at pH 6.0. The optimum temperature for xanthan lyase was 55°C. Figure 4 shows, however, that the enzyme was not stable at temperatures higher than 45°C. The enzyme was a little more stable at acidic pH values, but at 55°C enzyme activity was lost at all pH values tested.
FIG. 4.
Residual xanthan lyase activity after 15 min of incubation at the indicated temperature at pHs 5 (⧫), 6 (■), and 7 (▴). A relative activity of 100 is equal to 105 mU ml−1.
Various salts affected xanthan lyase activity. CuCl2 caused a 70% activity drop at a concentration of 0.1 mM, whereas HgCl2 completely inhibited the enzyme at 0.1 mM. EDTA did not show any effect at a concentration of 10 mM. NaCl had no effect at 10 mM but higher concentrations of NaCl inhibited the enzyme: at 85 mM NaCl, 60% of the initial activity was found, and at 450 mM all activity was lost. CoCl2, MgCl2, and CaCl2 at a concentration of 1 mM and MnCl2 at a concentration of 0.1 mM had no effect on xanthan lyase activity.
To characterize the LMW reaction product of xanthan lyase, the purified enzyme was incubated with xanthan, and the LMW fraction was isolated. After it was freeze-dried, the material was dissolved in demineralized water and divided into two aliquots, and one aliquot was hydrolyzed with TFA. The untreated aliquot contained 5.7 mM reducing sugars (mannose equivalents) and 0.1 mM pyruvate. The TFA-hydrolyzed aliquot contained 5.9 mM mannose equivalents and 4.6 mM pyruvate. TLC analysis showed an unidentified spot and a slight mannose spot for the untreated aliquot, whereas the TFA-treated aliquot showed only a mannose spot. These results suggested that the enzyme preferentially removes pyruvated mannose from xanthan. To confirm that the purified xanthan lyase was specific for pyruvated mannosyl residues, the enzyme was incubated with various xanthans that were chemically depyruvated, deacetylated, or both depyruvated and deacetylated. The Klebsiella K5 capsular polysaccharide was tested as well. The K5 polysaccharide has the Pyr4,6Manβ(1→4)GlcAβ bond of the xanthan side chain in its backbone (8), but it was not a substrate for xanthan lyase. No xanthan lyase activity could be detected on chemically depyruvated xanthans (Table 3). The enzyme was active on chemically deacetylated Sigma xanthan, as well as on xanthan that is naturally low in acetyl content (Flocon 4800C and X556-eps). However, the enzyme was not active on deacetylated X646-eps.
DISCUSSION
The xanthan-degrading bacterium P. alginolyticus XL-1 was isolated from a mixed culture growing on xanthan. This organism requires both thiamine and biotin for growth. Since the organism proliferated in the mixed culture without the addition of biotin and thiamine, it is likely that these compounds were provided by other organisms. This phenomenon may explain other researchers’ findings that the growth rate and the production of xanthan-degrading enzymes are greatly reduced when pure xanthan-degrading cultures are separated from the mixed cultures they originated from (5, 6, 12, 23). Possibly, these mixed cultures also produced some growth factor required by the xanthan degraders. Alternatively, two or more strains may have acted concertedly in xanthan degradation.
Xanthan is not degraded to completion by P. alginolyticus XL-1. The formation of Congo red-stainable material around colonies on agar plates with xanthan and the semisoluble, slime-like material observed in liquid cultures indicate that the enzymes excreted by strain XL-1 only remove residues from the xanthan side chains, whereas long stretches of the β-1,4-glucan backbone remain intact. As only 28% of xanthan C was recovered as CO2, biomass, or reducing sugars, the side chains are apparently removed only partially.
The outer side chain mannosyl residue can be removed either by a xanthan lyase or a β-mannosidase. Only xanthan lyase activity was detected in the culture supernatant, but it cannot be excluded that a β-mannosidase is produced that is not active on p-nitrophenyl-β-d-mannoside. Extracellular xanthan lyase production by strain XL-1 is induced by xanthan and repressed by glucose. Also, LMW enzymatic degradation products of xanthan inhibited rather than induced xanthan lyase production. Possibly, the true xanthan lyase inducer is an intermediate enzymatic degradation product of xanthan (e.g., an oligosaccharide) that is further converted by other degrading enzymes to a repressor (e.g., a monosaccharide). This would explain the inhibiting effect of LMW-xanthan degradation products: degradation has proceeded to such an extent that all inducer has been converted to repressor. In cultures with xanthan as inducer, xanthan lyase production stopped after a relatively short period of time, while exponential growth continued. This may also be explained by the conversion of an inducing xanthan fragment to a repressor over time, resulting in a stop in xanthan lyase production.
The purified xanthan lyase of strain XL-1 is different from the xanthan lyases described by Ahlgren (1) and Sutherland (23) in a number of respects. The Paenibacillus enzyme (molecular mass, 85 kDa) is much larger than these xanthan lyases, which had a molecular mass in the range of 30 to 33 kDa. The optimal pH is in between the values reported for these enzymes: pH 7.25 for the Bacillus enzyme (23) and pH 5 for the xanthan lyase from the mixed culture (1). The pI of 7.9 is much higher than the pI of the xanthan lyase purified by Ahlgren (1) (pI 3.7). Furthermore, the xanthan lyase from P. alginolyticus XL-1 is not as salt tolerant as the enzyme described by Ahlgren (1), which is not surprising since strain XL-1 was not selected for its salt tolerance. The xanthan lyase of P. alginolyticus XL-1 is more similar to the recently described xanthan lyase of Bacillus sp. strain GL1 (9). The molecular mass of this enzyme is in the same order of magnitude (75 kDa), and the optimal pH (5.5) is near that of the Paenibacillus enzyme (pH 6.0). There are, however, also differences: the Paenibacillus enzyme is more stable at higher temperatures. Furthermore, the Paenibacillus xanthan lyase is not affected by 1 mM CoCl2, MgCl2, CaCl2, or 10 mM EDTA, whereas the Bacillus sp. strain GL1 xanthan lyase was stimulated by CoCl2 and inhibited by the other compounds. On the other hand, CuCl2 and HgCl2 had a much stronger inhibiting effect on the Paenibacillus enzyme.
Like the other xanthan lyases described in the literature, the Paenibacillus enzyme was active on intact, nondepolymerized xanthan. The enzymes described by Ahlgren (1) and Hashimoto et al. (9), however, probably act in conjunction with depolymerases. Also, the lyases described by Sutherland (23) were associated with endoglucanases and showed a higher activity on xanthan-derived oligosaccharides than on intact xanthan. The Paenibacillus xanthan lyase was not found to be associated with endoglucanases either in the pure culture or in the mixed culture from which strain XL-1 originated. Therefore, the true substrate for this xanthan lyase is probably intact xanthan.
Like the enzyme described by Ahlgren (1) and Hashimoto et al. (9), the purified xanthan lyase is specific for pyruvated mannosyl residues. The LMW fraction released by xanthan lyase from xanthan contained a little more mannose than pyruvate and on TLC plates a slight mannose spot in the untreated sample was visible. Possibly, xanthan lyase releases a small amount of unpyruvated mannose from xanthan during prolonged incubation. However, the purified enzyme showed no activity at all on chemically depyruvated xanthans. Therefore, it is clear that the enzyme prefers pyruvated xanthan to nonpyruvated xanthan. Unexpectedly, xanthan lyase was not active on pyruvated, deacetylated X646-eps. It is not likely that the acetyl group is required for activity, since the enzyme was active on xanthans that are originally low in acetyl substituents as well as on chemically deacetylated Sigma xanthan. Possibly, the deacetylated X646-eps has adopted a structure which renders the pyruvated side chains inaccessible to xanthan lyase. The Klebsiella K5 polysaccharide was not a substrate, suggesting that the purified xanthan lyase is a true “exolyase.”
In chemically depyruvated xanthans, ca. 12% of the repeating units are still pyruvated. Apparently, the pyruvate groups were removed incompletely by the acid hydrolysis treatment, which was mild to prevent the cleavage of glycosidic bonds. However, the purified xanthan lyase was not active on these substrates despite the presence of pyruvate groups. Possibly, removal of pyruvate groups was incomplete because parts of the polysaccharide molecules are inaccessible to H+ molecules, e.g., due to aggregate formation. If so, it would also be unlikely that enzymes can act on these parts of the polysaccharide molecules.
The 4,5-ene-glucuronyl residue in the xanthan side chain resulting from xanthan lyase activity is identical to the 4,5-ene-galacturonyl residue that is formed by pectate lyase in pectate oligomers. The ɛ235 for the 4,5-ene-glucuronyl residue determined in this study (8.0 cm2 μmol−1) is, however, higher than the values reported for products of pectate lyase, i.e., 4.6 (13) and 5.2 (17) cm2 μmol−1. This difference is probably due to the different molecular environments that surround the 4,5-ene-glucuronyl residue in modified xanthan and in pectate-derived oligomers, respectively.
With the xanthan lyase described here, the pyruvated mannosyl residues of xanthan side chains are preferentially removed, resulting in a modified xanthan consisting of tetrameric and pentameric repeating units. Obviously, the extent of modification is dependent on the extent of pyruvation of xanthan. Most xanthans are pyruvated to ca. 30%; therefore, if complete conversion to a polytetramer is desired, a second xanthan lyase or a β-mannosidase is required that is either specific for nonpyruvated mannosyl residues or nonspecific. Strain XL-1 probably produces a second xanthan lyase as the crude culture broth exhibited xanthan lyase activity with chemically depyruvated xanthan as a substrate (see Table 1). To further modify the tetrameric repeating units formed by xanthan lyase to trimers, an enzyme removing the unsaturated uronic acid residue is required. Such an enzyme, a “4,5-ene-β-d-glucuronidase,” has to our knowledge not yet been described in the literature. Since an enzyme activity releasing uronic acid(-containing fragment)s from xanthan was detected in the culture supernatant (see Table 1), it may be possible that strain XL-1 produces such an enzyme. Considering the various xanthan-degrading enzyme activities produced by P. alginolyticus XL-1, we think this strain is a valuable source of enzymes for structural modifications of xanthan.
ACKNOWLEDGMENTS
We thank Ian Sutherland for his generous gift of xanthan samples with different pyruvate and acetate contents and Harm Snippe for kindly providing the Klebsiella K5-cps. We thank André Pots for his assistance with IEF and Joost Wijnen for identification of the vitamins required for growth of P. alginolyticus XL-1.
This research was financially supported by ABON (Association of Biotechnological Research Centres in The Netherlands).
REFERENCES
- 1.Ahlgren J A. Purification and characterization of a pyruvated-mannose-specific xanthan lyase from heat-stable, salt-tolerant bacteria. Appl Environ Microbiol. 1991;57:2523–2528. doi: 10.1128/aem.57.9.2523-2528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betlach M R, Capage M A, Doherty D H, Hassler R A, Henderson N M, Vanderslice R W, Marellia J D, Ward M B. Genetically engineered polymers: manipulation of xanthan biosynthesis. In: Yalpani M, editor. Industrial polysaccharides: genetic engineering, structure/property relations and applications. Amsterdam, The Netherlands: Elsevier; 1987. pp. 35–80. [Google Scholar]
- 3.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Chem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw I J, Nisbet B A, Kerr M H, Sutherland I W. Modified xanthan: its preparation and viscosity. Carbohydr Polym. 1983;3:23–28. [Google Scholar]
- 5.Cadmus M C, Jackson L K, Burton K A, Plattner R D, Slodki M E. Biodegradation of xanthan gum by Bacillus sp. Appl Environ Microbiol. 1982;44:5–11. doi: 10.1128/aem.44.1.5-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadmus M C, Slodki M E, Nicholson J J. High-temperature, salt-tolerant xanthanase. J Ind Microbiol. 1989;4:127–133. [Google Scholar]
- 7.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 8.Dutton G S, Yang M-T. The structure of the capsular polysaccharide of Klebsiella K-type 5. Can J Chem. 1973;51:1826–1832. [Google Scholar]
- 9.Hashimoto W, Miki H, Tsuchiya N, Nankai H, Murata K. Xanthan lyase of Bacillus sp. strain GL1 liberates pyruvylated mannose from xanthan side chains. Appl Environ Microbiol. 1998;64:3765–3768. doi: 10.1128/aem.64.10.3765-3768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassler R A, Doherty D H. Genetic engineering of polysaccharide structure: production of variants of xanthan gum in Xanthomonas campestris. Biotechnol Prog. 1990;6:182–187. doi: 10.1021/bp00003a003. [DOI] [PubMed] [Google Scholar]
- 11.Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem. 1949;180:249–261. [PubMed] [Google Scholar]
- 12.Hou C T, Barnabe N, Greaney K. Biodegradation of xanthan by salt-tolerant aerobic microorganisms. J Ind Microbiol. 1986;1:31–37. doi: 10.1128/aem.52.1.37-44.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konno H. Endopectate lyase from Erwinia aroideae. Methods Enzymol. 1988;161:381–385. doi: 10.1016/0076-6879(88)61044-5. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Levy S, Schuyler S C, Maglothin R K, Staehelin L A. Dynamic simulations of the molecular conformations of wild type and mutant xanthan polymers suggest that conformational differences may contribute to observed differences in viscosity. Biopolymers. 1996;38:251–277. doi: 10.1002/(sici)1097-0282(199602)38:2<251::aid-bip10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Miller G L. Use of dinitrosalicylic acid for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 17.Moran F, Nasuno S, Starr M P. Extracellular and intracellular polygalacturonic acid trans-eliminases of Erwinia carotovora. Arch Biochem Biophys. 1968;123:298–306. doi: 10.1016/0003-9861(68)90138-0. [DOI] [PubMed] [Google Scholar]
- 18.Morris V J. Bacterial polysaccharides. In: Stephen A M, editor. Food polysaccharides and their applications. New York, N.Y: Marcel Dekker; 1995. pp. 341–375. [Google Scholar]
- 19.Nakamura L K. Bacillus alginolyticus sp. nov. and Bacillus chondroitinus sp. nov., two alginate-degrading species. Int J Syst Bacteriol. 1987;37:284–286. [Google Scholar]
- 20.Shatwell K P, Sutherland I W, Dea I C M, Ross-Murphy S B. The influence of acetyl and pyruvate substituents on the helix-coil transition behaviour of xanthan. Carbohydr Res. 1990;206:87–103. doi: 10.1016/0141-8130(90)90056-g. [DOI] [PubMed] [Google Scholar]
- 21.Shida O, Takagi H, Kadowaki K, Nakamura L K, Komagata K. Transfer of Bacillus alginolyticus, Bacillus chondroitinus, Bacillus curdlanolyticus, Bacillus glucanolyticus, Bacillus kobensis, and Bacillus thiaminolyticus to the genus Paenibacillus and emended description of the genus Paenibacillus. Int J Syst Bacteriol. 1997;47:289–298. doi: 10.1099/00207713-47-2-289. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland I W. An enzyme system hydrolysing the polysaccharides of Xanthomonas species. J Appl Bacteriol. 1982;53:385–393. [Google Scholar]
- 23.Sutherland I W. Xanthan lyases—novel enzymes found in various bacterial species. J Gen Microbiol. 1987;133:3129–3134. doi: 10.1099/00221287-133-11-3129. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland I W. Polysaccharide lyases. FEMS Microbiol Rev. 1996;16:323–347. doi: 10.1111/j.1574-6976.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 25.Tait M I, Sutherland I W. Synthesis and properties of a mutant type of xanthan. J Appl Bacteriol. 1989;66:457–460. [Google Scholar]
- 26.Vanderslice R W, Doherty D H, Capage M A, Betlach M R, Hassler R A, Henderson N M, Ryan-Graniero J, Tecklenburg M. Genetic engineering of polysaccharide structure in Xanthomonas campestris. In: Crescenzi V, Dea I C M, Paoletti S, Stivala S S, Sutherland I W, editors. Biomedical and biotechnological advances in industrial polysaccharides. New York, N.Y: Gordon and Breach; 1989. pp. 145–156. [Google Scholar]
- 27.Weissbach A, Hurwitz J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959;234:705–709. [PubMed] [Google Scholar]