Abstract
Gestational alloimmune liver disease is a rare complication associated with reactive maternal immunoglobulins resulting in neonatal liver pathology. The mainstay treatment for prevention in future pregnancies is intravenous immunoglobulins. Although relatively well tolerated, adverse reactions may occur. In this report, we highlight a case of intravenous immunoglobulin induced pancytopenia diagnosed by exclusion after thorough work-up. The patient was counseled on options and an informed decision was made to proceed with re-trial of intravenous immunoglobulin without systemic prednisone. This resulted in the delivery of a healthy neonate. We propose that future adverse reactions to intravenous immunoglobulin in pregnancy may warrant the trial of a new medication lot and use of systemic steroids only if subsequently indicated.
Keywords: Case report, Intravenous immunoglobulin, Gestational alloimmune liver disease, Pancytopenia
Highlights
-
•
Suspected that intravenous immunoglobulin used to prevent gestational alloimmune liver diseaseinduced pancytopenia.
-
•
The pancytopenia resolved with a medication lot change without systemic steroid use.
-
•
A healthy neonate was delivered without evidence of liver pathology.
-
•
Management of intravenous immunoglobulin induced pancytopenia without steroids should be considered.
1. Introduction
Gestational alloimmune liver disease (GALD – formerly known as neonatal hemochromatosis) is caused by transplacental passage of reactive maternal immunoglobulins resulting in neonatal hepatic failure and demise [1,2]. Its pathologic onset is intrauterine and newborns present with signs of liver failure, including coagulopathy, ascites, and hypoalbuminemia [3]. Definitive diagnosis can be made only in the neonatal period and recurrence is up to 90% [4]. The mainstay treatment for GALD prevention in future pregnancies is antenatal therapy with high-dose intravenous immunoglobulin (IVIG), which has been shown to significantly reduce the risk of fetal and neonatal complications [5]. Despite benefits for GALD prevention, 20–50% of individuals receiving IVIG therapy experience adverse effects, with the majority of reactions being classified as mild, such as headaches, chills, or flushing [6]. IVIG associated maternal hemolysis and other cytopenias have also been noted in the literature [[7], [8], [9]]. Utilizing appropriate dosing and frequency, monitoring drug therapy, ensuring adequate hydration, avoiding formulation or lot changes, and utilizing pre-medication regimens have been shown to reduce the rates of adverse reactions.
IVIG induced maternal pancytopenia during antenatal treatment is one of the most severe adverse reversible reactions noted in the literature [10]. In one reported case, IVIG induced pancytopenia was suspected and was subsequently managed by decreasing the IVIG dose and starting maintenance prednisone, which ultimately resulted in the uncomplicated delivery of a healthy neonate [10]. With our patient, we highlight another case of suspected IVIG induced pancytopenia during treatment for a history of GALD that spontaneously resolved after discontinuation of IVIG followed by an informed decision to attempt a second trial of IVIG without prednisone, which similarly resulted in the uncomplicated delivery of a healthy neonate.
2. Case Presentation
The case concerns a 33-year-old woman, gravida 4 para 0–1–2-0, with a history of neonatal demise secondary to GALD and otherwise no contributory past medical history. A 21-week anatomy ultrasound scan during the patient's previous pregnancy's showed the fetus to have significant ascites, pericardial effusion, bilateral lower extremity clubbing, and dilated rectum. Cytomegalovirus, toxoplasmosis, parvovirus, and genetic workup were unremarkable and thus pregnancy followed expectantly. At 28 weeks, ultrasound findings were consistent with progressive non-immune hydrops (massive ascites, pericardial effusion, scalp edema) and anhydramnios. Given these findings, the patient was admitted for a course of betamethasone and subsequent urgent delivery via primary cesarean section. Apgar scores were 0, 3, and 4 at 1, 5, and 10 min, respectively. Neonatal demise followed unsuccessful resuscitation and pulmonary hemorrhage. Autopsy was consistent with GALD.
Prior to this current pregnancy, the patient had pre-conception counseling with discussion of a high likelihood of recurrence with a similar outcome if left untreated. A recommendation was made for weekly IVIG treatment starting early in pregnancy, around 14–18 weeks, owing to early-onset hydrops in the prior pregnancy. The patient was informed that IVIG treatment increases neonatal survival to greater than 90% as opposed to 30% if untreated [5]. The patient acknowledged counseling and a plan was made for weekly IVIG with repeat cesarean section for delivery.
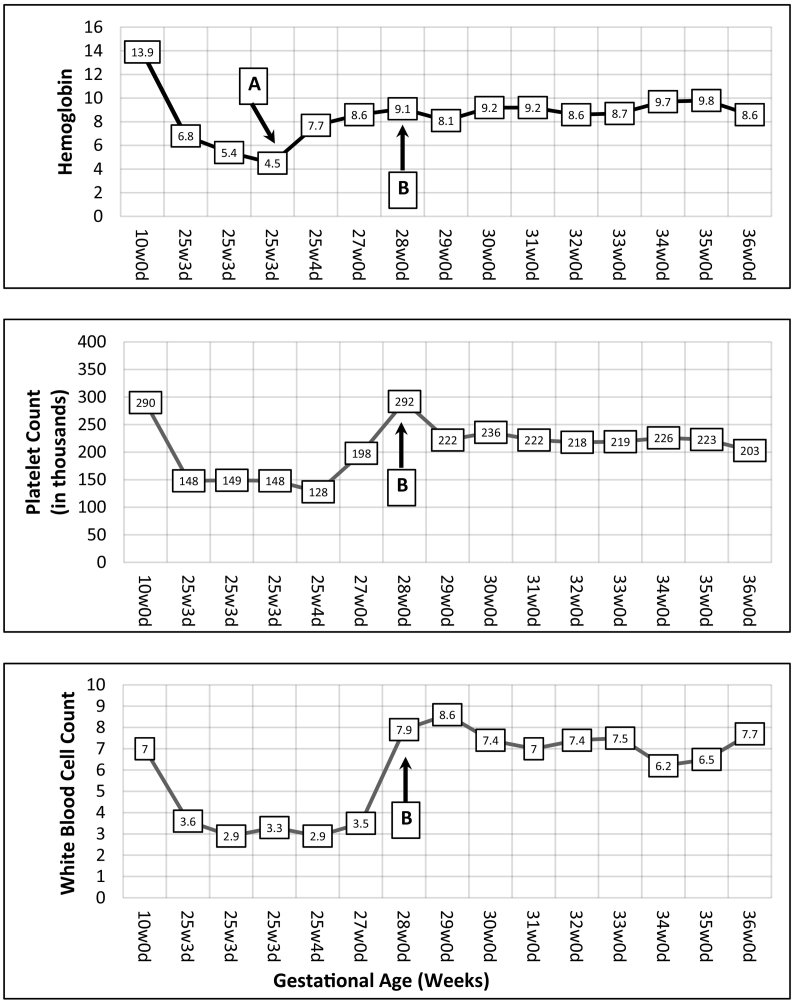
During the current pregnancy, a 15-week early anatomy scan showed normal findings and IVIG weekly treatments were initiated with 60 g infusion – 1 g/kg dosing. A follow-up anatomy scan at 18 weeks was reassuring, as was a normal fetal echocardiogram at 23 weeks. The patient continued to have unremarkable IVIG treatments until 25 weeks, when she began complaining of a 1-week history of nausea/vomiting, muscles aches/weakness, and subjective fever (negative for COVID). During this evaluation, it was noted that her hemoglobin, platelet, and white blood cell count trended from 13.9, 290 k, and 6.7 pre-pregnancy down to 6.8, 148 k, and 3.6, respectively. Shortly thereafter, repeat bloodwork showed further downtrends of her hemoglobin, platelet, and white blood cell count to 5.4, 149 k, and 2.9. Given severe anemia, 2 units of packed red blood cells (pRBCs) were administered and the case was discussed with the hematology team and a full work-up was performed for primary and secondary anemia, hemolysis, and pancytopenia.
Viral work-up was negative for cytomegalovirus, Ebstein-Barr virus, human immunodeficiency virus, hepatitis B, and parvovirus (IgG pos, IgM neg); hemolysis work-up revealed low haptoglobin (<8), elevated lactate dehydrogenase (557), elevated reticulocyte (3.8%), and negative direct Coombs test; and anemia work-up showed normal vitamin B12, folate, and iron studies. Further work-up with the hematology team revealed no schistocytes on blood smear, bone marrow biopsy with mild relative hyperplasia but no evidence of leukemia/lymphoma, peripheral and bone marrow flow cytometry normal, and normal glucose-6-phosphate dehydrogenase testing. After thorough review, IVIG induced pancytopenia was suspected.
At the 26-week visit, the patient felt symptomatically improved and after counseling, she opted to re-trial therapy at the same dose but with a new pooled IVIG lot number. From 26 weeks to 37 weeks, she continued with weekly IVIG and complete blood count screening (Fig. 1). Hemodynamic status remained stable with hemoglobin 8.6–9.7, platelet 198 k–292 k, and white blood cell count 6.2–8.6. She underwent uncomplicated repeat cesarean section at 37 weeks with delivery of 2675 g neonate, Apgar scores 8 and 9 and 1 and 5 min, respectively. In the immediate neonatal period, there was no evidence of hypoglycemia, jaundice, abdominal distension, or ascites and liver function tests all remained within normal limits with no clinical concern for GALD.
Fig. 1.
Trend of blood counts throughout pregnancy. Initial blood work-up during first trimester. IVIG treatments started at 15 weeks. Antepartum admission for pancytopenia 25w3d-25w5d and transfused 2u pRBCs 25w4d (Point A). IVIG re-started at 28 weeks (Point B). Delivery at 37w0d.
3. Discussion
We present a case of suspected IVIG induced maternal pancytopenia during antenatal management for history of GALD. During the patient's initial symptomatic presentation at 25 weeks, the pancytopenia differential diagnosis included viral etiology, malignancy, hemolysis, IVIG induced, or unspecified. Immediate work-up for primary and secondary anemia, hemolysis, and pancytopenia showed anemia predominant pancytopenia with hemolysis and thus the differential diagnosis narrowed to disseminated intravascular coagulation, autoimmune hemolytic anemia, IVIG induced hemolysis/pancytopenia, or concurrent marrow suppression. After more invasive work-up by the hematology team, by diagnosis of exclusion, the etiology was most consistent with IVIG induced pancytopenia.
Hemolysis, neutropenia, and leukopenia are known adverse effects of IVIG treatment [[7], [8], [9]]. Additionally, there are a few reported cases of IVIG induced maternal pancytopenia [10]. The etiology of these adverse reactions remains largely unknown. As mentioned by Herrmann et al., possible explanations include presence of antibodies (ex. antineutrophil or anti-AB), increased plasma viscosity and erythrocyte aggregation, or unintended components within the IVIG product, such as coagulant factors [10]. Based on the current literature, this patient's IVIG treatments were limited to the same lot number to help reduce the introduction of unintended antibodies or factors. Currently there is no reported benefit of prophylactic steroid administration to prevent serious adverse reactions and typically steroids are utilized after an adverse reaction has occurred.
Given the patient's prior intrauterine onset of non-immune hydrops at 21 weeks, her severe pancytopenia at 25 weeks was concerning for the prognosis of this pregnancy. Options to reduce risk of recurrent adverse reaction included: 1) reducing frequency/dose of IVIG, 2) co-administering prednisone IV, 3) limiting pooled IVIG product from a single lot number, 4) pre-screening the IVIG product for antibodies, and 5) discontinuing IVIG in favor of expectant management. Our patient was already being treated with the lowest recommended IVIG dose of 1 g/kg bodyweight and lowest frequency (weekly IVIG starting at 18 weeks). Additionally, our patient was already receiving pooled IVIG from a single lot and, currently, it is not possible to extensively screen IVIG beyond the manufacturer's baseline screening.
Therefore, our primary counseling focused on whether to restart IVIG with or without supplementation of prednisone. We again discussed with the patient that she was 26 weeks with a greater than 90% chance of GALD recurrence if untreated. We informed her that the risk of pancytopenia recurrence with repeat IVIG challenge is unknown, and discussed how prednisone treatment is often utilized to prevent severe adverse reactions, while changing the pooled IVIG lot may in and of itself limit the onset of pancytopenia.
After thorough discussion, the patient made an informed decision to opt for IVIG re-challenge without prednisone followed by transfusion mediated support as needed. She preferred to assume maternal risk (i.e., pancytopenia, hemolysis, hyponatremia) for fetal benefit – decreased risk of non-immune hydrops or fetal or neonatal demise from recurrent GALD. A plan was made to proceed with weekly IVIG from a different lot number with regular weekly blood work-up to monitor for recurrence. In the event of recurrence, prednisone IV with acute transfusion support would be utilized as needed. She did not require any additional transfusions beyond the 2 units pRBCs during initial pancytopenia presentation.
4. Conclusion
With this report, we hope to highlight a rare occurrence of IVIG induced maternal pancytopenia managed with a re-trial of IVIG from a different pooled medication lot without use of systemic prednisone. Although prednisone is relatively common, utilization of weekly IV steroids is not a benign intervention and carries the risk of multiple adverse events and significant cost to the patient. As such, we propose that future IVIG adverse reactions in pregnancy may warrant trial of a new medication lot and only utilize prednisone as the next level of management if indicated.
5. Patient Perspective
During the initial admission to the hospital, although I was nervous for my own health, my greatest area of concern was for my baby and avoiding another delivery with complications. I am thankful the team at Mount Sinai, along with Dr. Strong, were able to come to a diagnosis that then allowed me to safely restart IVIG treatments. Most importantly, I have nothing but gratitude towards all the teams involved in guiding me through this pregnancy and giving me a healthy baby.
Acknowledgments
Contributors
Minhazur Sarker participated directly in patient care, performed the literature review, and authored the manuscript and all revisions.
Chelsea DeBolt participated directly in patient care and oversaw all steps of the literature review and manuscript writing and revisions.
Noel Strong primarily managed the patient and actively participated in every step of manuscript writing and revision.
Funding
No funding from an external source supported the publication of this case report.
Patient consent
Informed and written consent was obtained from the patient prior to formulation of this case report.
Provenance and peer review
This article was not commissioned and was peer reviewed.
Acknowledgments
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this case report.
References
- 1.Whitington P.F., Malladi P. Neonatal hemochromatosis: is it an alloimmune disease? J. Pediatr. Gastroenterol. Nutr. 2005;40(5):544–549. doi: 10.1097/01.MPG.0000162004.44971.92. [DOI] [PubMed] [Google Scholar]
- 2.Pan X., Kelly S., Melin-Aldana H., Malladi P., Whitington P.F. Novel mechanism of fetal hepatocyte injury in congenital alloimmune hepatitis involves the terminal complement cascade. Hepatology. 2010;51(6):2061–2068. doi: 10.1002/HEP.23581. [DOI] [PubMed] [Google Scholar]
- 3.Vohra P., Haller C., Emre S., et al. Neonatal hemochromatosis: the importance of early recognition of liver failure. J. Pediatr. 2000;136(4):537–541. doi: 10.1016/S0022-3476(00)90020-9. [DOI] [PubMed] [Google Scholar]
- 4.Hemochromatosis N., Feldman A.G., Whitington P.F. 2013. Seminar-Pediatric Hepatology. Published online. [DOI] [Google Scholar]
- 5.Whitington P.F., Kelly S., Taylor S.A., et al. Antenatal treatment with intravenous immunoglobulin to prevent gestational alloimmune liver disease: comparative effectiveness of 14-week versus 18-week initiation. Fetal Diagn. Ther. 2018;43(3):218–225. doi: 10.1159/000477616. [DOI] [PubMed] [Google Scholar]
- 6.Pierce L.R., Jain N. 2003. Risks Associated With the Use of Intravenous Immunoglobulin. Published online. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M., Hosoda W., Sekijima Y., et al. Neutropenia as a complication of high-dose intravenous immunoglobulin therapy in adult patients with neuroimmunologic disorders. Clin. Neuropharmacol. 2003;26(6):306–311. doi: 10.1097/00002826-200311000-00009. https://oce-ovid-com.eresources.mssm.edu/article/00002826-200311000-00009/HTML Published November 2003. Accessed January 29. [DOI] [PubMed] [Google Scholar]
- 8.Rink B.D., Gonik B., Chmait R.H., O’Shaughnessy R. Maternal hemolysis after intravenous immunoglobulin treatment in fetal and neonatal alloimmune thrombocytopenia. Obstet. Gynecol. 2013;121(2 PART 2):471–473. doi: 10.1097/AOG.0B013E3182765C63. [DOI] [PubMed] [Google Scholar]
- 9.Daw Z., Padmore R., Neurath D., et al. Vol. 48. Transfusion; 2008. T R A N S F U S I O N C O M P L I C A T I O N S Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann A., Samelson-Jones B.J., Brake S., Samelson R. IVIG-associated maternal pancytopenia during treatment for neonatal alloimmune thrombocytopenia. Am. J. Perinatol. Rep. 2017;7:197–200. doi: 10.1055/s-0037-1607055. [DOI] [PMC free article] [PubMed] [Google Scholar]