Abstract
A 76-year-old woman who was treated with lorlatinib for postoperative recurrent anaplastic lymphoma kinase-positive lung adenocarcinoma visited our hospital with massive hemoptysis. Chest computed tomography showed massive bleeding from the right upper lobe; however, the cause of bleeding was unclear. After bronchial artery embolization (BAE), bronchial occlusion was performed using an Endobronchial Watanabe Spigot (EWS) that was easily placed because BAE had reduced the bleeding volume. Treatment with BAE alone was inadequate; however, additional therapy with EWS after BAE successfully controlled the massive hemoptysis, especially in this patient who underwent lobectomy to prevent respiratory dysfunction.
Keywords: Hemoptysis, Bronchial artery embolization, Endoscopic bronchial occlusion, Endobronchial Watanabe Spigot
1. Introduction
Massive hemoptysis is sometimes fatal, with a mortality rate greater than 50%. Approximately 95% of hemoptysis is due to bleeding from arteries, such as the bronchial artery. Although bronchial artery embolization (BAE), the first-line treatment for hemoptysis, is safe and has a high control rate, it is sometimes difficult to perform if there is a risk of renal dysfunction, embolization of important blood vessels, or anatomical problems [1]. On the contrary, bronchial occlusion using Endobronchial Watanabe Spigot (EWS) has been developed for the treatment of intractable pneumothorax and thoracic empyema with bronchopleural fistula; however, evidence also exists on its usefulness in the management of hemoptysis [2]. BAE and EWS are both effective treatments for hemoptysis, and we encountered a case of massive hemoptysis successfully treated with EWS after BAE. We herein report a patient who underwent right lower lobectomy for lung cancer 18 years ago; because of the poor lung function, massive hemoptysis could be fatal. We attempted local treatment with EWS after BAE, thus avoiding total right pneumonectomy. Herein, we also performed a review of the literature following a MEDLINE database search on EWS for hemoptysis and its clinical presentations.
2. Case presentation
A 58-year-old female patient was referred to our hospital because of a nodule in the right lower lobe; she was diagnosed with advanced lung adenocarcinoma. She underwent right lower lobectomy at 58 years of age. Nine years later, at 67 years of age, the patient had recurrence of lung cancer in the cervical lymph node. We performed a re-biopsy of the enlarged cervical lymph nodes, and immunohistochemistry found the tumor to be anaplastic lymphoma kinase (ALK)-positive. Crizotinib, alectinib, and lorlatinib were sequentially administered to control the lung cancer. After the initiation of lorlatinib treatment, the tumor shrank with a partial response.
After 33 months of lorlatinib treatment, at 76 years of age, the patient presented with hemoptysis a day before admission. She had tachypneia and dyspneia on arrival to our hospital with 92% oxygen saturation under an oxygen flow rate of 8 L/min. Initial test findings revealed a hemoglobin level of 11.2 g/dL and normal coagulation profile (Table 1). Chest radiography revealed decreased permeability of the right lung, while chest contrast-enhanced computed tomography (CT) showed diffuse infiltrative shadows and ground-glass opacity in the right lung, and mild ground-glass opacity in the left lung, without active contrast extravasation. Shading was particularly severe in the right upper lobe (Fig. 1A and B). She was admitted to the intensive care unit and intubated the same day due to worsening oxygenation from massive hemoptysis (Fig. 1C and D). Because bleeding from the right lung was strongly suspected, unilateral intubation using a double-lumen tube in the non-bleeding left lung was performed by the anesthesiologist. On the same day, an attempt at BAE was abandoned after failing to identify the culprit vessels. She was deeply sedated for the next two days to prevent exacerbation of the bleeding. On day 3 of ventilation, we observed the right airway through a side tube of the double-lumen tube, and the right main bronchus was filled with blood clots; however, no active bleeding was observed (Fig. 2A). Thereafter, gradual clot removal was performed using a flexible therapeutic bronchoscope (Olympus BF-260), and on the fifth day, clot removal of the right upper lobe branch was performed. When the blood clot in the apical segment of the right upper lobe was removed, bleeding re-occurred. Therefore, we determined the source of the bleeding to be the apical segment of the right upper lobe (Fig. 2B and C). The bleeding point could not be observed, and there were no malignant findings in the bronchial mucosa or on histology of the removed clots. Therefore, the detailed cause of bleeding was unknown. On day 10 of ventilation, BAE was re-attempted; CT angiography using a 4-Fr pigtail catheter placed in the ascending aorta showed two right bronchial arteries. A microcatheter could be easily inserted into one branching bronchial artery from the right third intercostal artery, and bronchial arteriography and CT angiography from the same site showed that the blood flow distributed mainly on the right dorsal side of the bronchi; hence, we performed embolization using gelatin sponge. The other bronchial artery diverged inward from the bottom of the aorta at a steep angle, and the diameter of the blood vessel was small; therefore, it was difficult to insert a microcatheter, and embolization was not performed. We then attempted an endoscopic bronchial occlusion. There was less active bleeding because of the prior BAE, and we could perform the treatment without losing intraoperative vision. Because a small amount of hemorrhage from the apical and the anterior segment of the right upper lobe, two 5 mm EWS in total were successfully embolized, one each in the apical and anterior segment of the right upper lobe (Fig. 2D). We approached the apical segment of the right upper lobe using a curette. We made a small cut with a 20-gauge injection needle at the thick end of the EWS and inserted the tip of the cytology curette (Olympus Medical Systems). Then, we inserted the EWS by venting the curette to the upper angle. In addition, we approached the anterior segment of the right upper lobe in the traditional way by using a forceps. There was no recurrence of hemoptysis, and she was extubated on the 23rd day after admission. She continued rehabilitation and was discharged on the 43rd day.
Table 1.
Laboratory data on admission.
| Hematology | Biochemistry | ||||
|---|---|---|---|---|---|
| WBC | 6350 | /μL | T-Bil | 0.61 | mg/dL |
| Neu | 53.4 | % | TP | 5.4 | g/dL |
| Lym | 37.2 | % | Alb | 3.6 | g/dL |
| Mon | 4.5 | % | AST | 25 | IU/L |
| Eos | 4.6 | % | ALT | 22 | IU/L |
| Bas | 0.4 | % | γ-GTP | 46 | IU/L |
| Hb | 11.2 | g/dL | Amy | 66 | IU/L |
| Plt | 23.5 | ×104/μL | LDH | 208 | IU/L |
| CPK | 84 | IU/L | |||
| Coagulation | BUN | 24.2 | mg/dL | ||
| PT-INR | 0.95 | Cre | 0.88 | mg/dL | |
| APTT | 27.3 | sec | Na | 141 | mEq/L |
| D-dimer | 0.5 | μg/mL | K | 4.0 | mEq/L |
| Cl | 107 | mEq/L | |||
| Serology | CEA | 3.44 | ng/dL | ||
| CRP | 0.16 | mg/dL | |||
| HbA1c | 6.5 | % | Urine test | ||
| (1->3)-β-D-glucan | 6 | pg/mL | Protein | + | |
| T-SPOT | – | Occult blood | + | ||
| MAC antibody | – | ||||
| MPO-ANCA | – | Sputum culture | – | ||
| PR3-ANCA | – | ||||
Abbreviations: WBC, white blood cell; Neu, neutrophil; Lym, lymphocyte; Mon, monocyte; Eos, eosinophil; Bas, basophil; Hb, hemoglobin; Plt, platelet; PT-INR, prothrombin time-international normalized ratio; APTT, activated partial thromboplastin time; CRP, C-reactive protein; MAC, Mycobacterium avium complex; MPO-ANCA, myeloperoxidase-anti-neutrophil cytoplasmic antibody; PR3-ANCA, cytoplasmic-anti-neutrophil cytoplasmic antibody; T-Bil, total bilirubin; TP, total protein; Alb, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; Amy, amylase; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; BUN, blood urea nitrogen; Cre, creatinine; CEA, carcinoembryonic antigen.
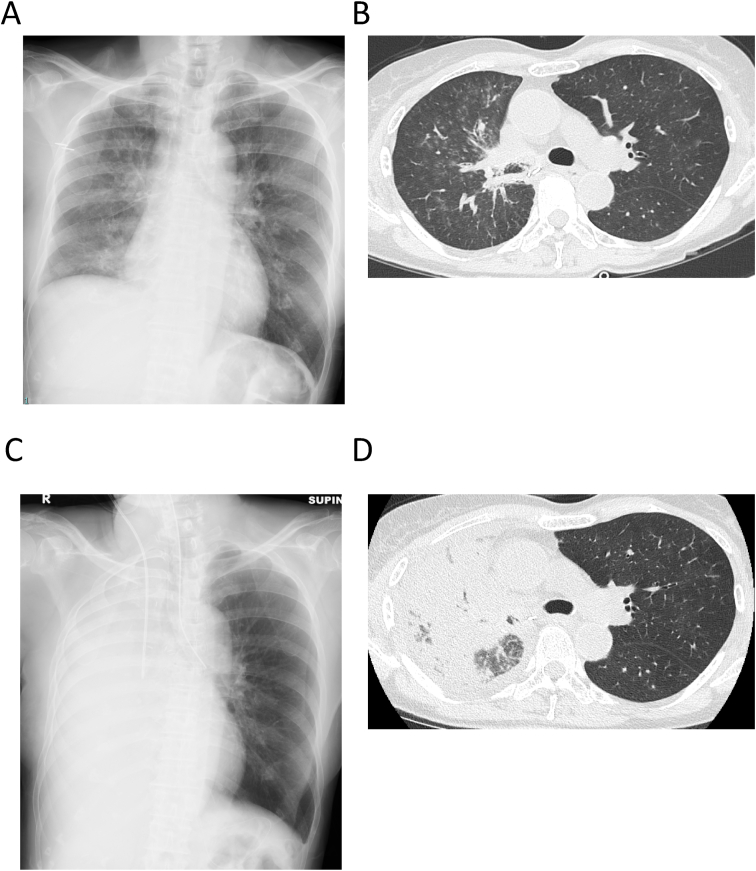
Fig. 1.
(A) Chest radiography on admission reveals decreased permeability of the right lung. (B) Chest computed tomography (CT) on admission shows diffuse infiltrative shadows and ground-glass opacity in the right lung, and mild ground-glass opacity in the left lung. The shading is particularly severe in the right upper lobe. (C) Chest radiography on admission to the intensive care unit (ICU) reveals that the permeability has further deteriorated. (D) Chest CT on admission to the ICU shows deterioration of diffuse infiltrative shadows and ground-glass opacity in the right lung; however, aeration of the left lung is well maintained.
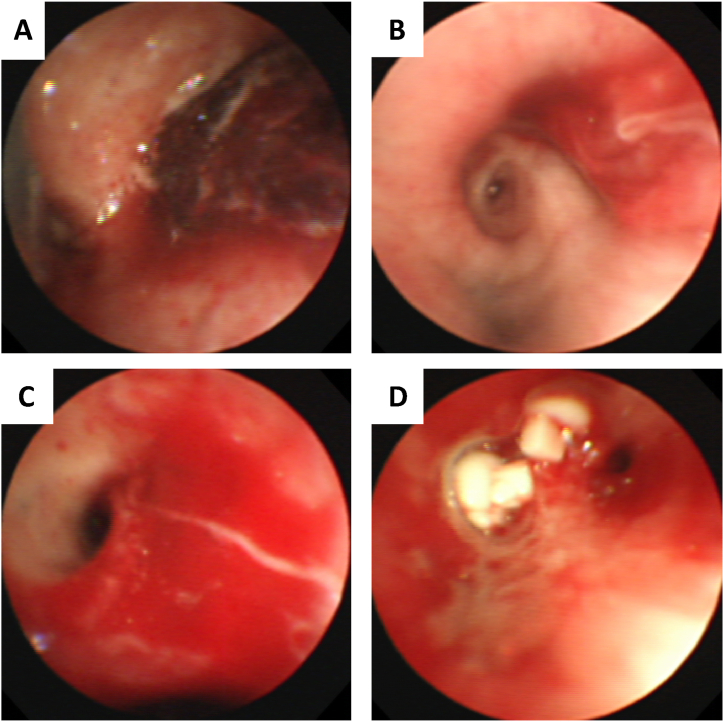
Fig. 2.
Bronchoscopic findings. (A) The right main bronchus is filled with blood clots. (B) Blood clot in the apical segment of the right upper lobe. (C) After removal of the blood clot, bleeding re-occurred. (D) Two EWSs were firmly inserted into the target bronchi.
EWS, Endobronchial Watanabe Spigot.
A chest CT scan performed after extubation did not show any recurrence of lung cancer. CT also showed two EWSs in optimal positions, and the aeration of the upper right lobe was well maintained (Fig. 3). Bronchial wall thickening had been observed in the apical segment of the right upper lobe for many years, suggesting antimicrobial or fungal infection or bronchial metastasis of the lung cancer. However, serological tests, culture findings, cytology, and endoscopic findings did not reveal any obvious cause of the bleeding. Because the cause was unclear, removal of EWS should be carefully assessed in the future. After two months of discharge, there has been no recurrence of hemoptysis or lung cancer.
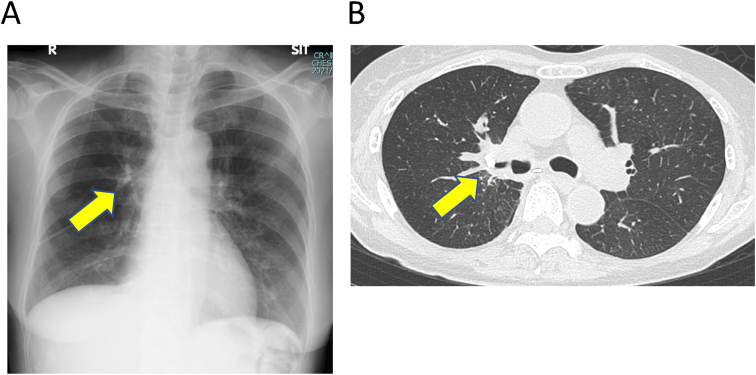
Fig. 3.
(A, B) Chest radiography and computed tomography images show two EWSs in optimal positions (arrow) and the aeration of the upper right lobe was well maintained.
EWS, Endobronchial Watanabe Spigot.
3. Discussion
We herein report a case of successful treatment with BAE followed by EWS for massive hemoptysis in a patient with postoperative recurrent lung cancer. In a nationwide study in Japan using the Diagnosis Procedure Combination (DPC) database, three main causes of hemoptysis (idiopathic hemoptysis, lung cancer, and respiratory tract infection) are reported. In Japan, an extremely high response rate was reported in BAE for idiopathic hemoptysis (cases of hemoptysis with no abnormalities in the lung field on CT or only emphysema) and infectious diseases that are not difficult to control [3]. In such cases, BAE monotherapy is considered sufficient. On the other hand, for uncontrolled nontuberculous mycobacteriosis or pulmonary aspergillosis and lung cancer, it is often difficult for BAE monotherapy to stop the bleeding in the long term [4]. Thus, surgical treatment and bronchoscopic intervention are considered if the lesion is localized. Palliative radiation therapy and chemotherapy are also considered for bleeding caused by recurrence of lung cancer. In our case, the patient presented with hemoptysis during treatment for lung cancer, but it was unclear whether lung cancer recurrence was the source of the bleeding. Based on these results, we concluded that BAE for idiopathic hemoptysis be performed first.
This case report presents two novelties. One of them was that the patient underwent a right lower lobectomy and had poor lung function. We had to attempt avoiding respiratory dysfunction following pneumonectomy. Guggino et al. reported postoperative respiratory dysfunction following pneumonectomy in 26.3% of patients with lung cancer with operative mortality rates of 0%–17.6% for cancer [5]. On the other hand, lobectomy of the right upper lobe and preservation of the middle lobe in the absence of the upper and lower lobes may produce torsion [6]. Therefore, we managed to administer local treatments. The data and clinical findings of previous studies and for the present case of hemoptysis treated with bronchial occlusion using EWS are described in Table 2 [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]]. Six of 18 patients had lung cancer as the underlying disease; however, in no other patients apart from our ours’, there was a report on hemoptysis after lobectomy. This is one of the few reports in which EWS was performed in a case of hemoptysis after lobectomy, and we could control bleeding by occlusion to prevent spilling over of blood into the remaining segments.
Table 2.
Summary of the published cases of bronchial occlusion with endobronchial Watanabe spigots for hemoptysis.
| Case | Age (years) | Sex | Etiology | Mechanical ventilation | Localization | Number of spigots | Hemoptysis cessation by BO | Combined with BAE | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| Detau (2007) | 39 | F | idiopathic | yes | Rt. B3 | 1 | yes | after BO | NR |
| Bylicki (2012) | 48 | M | overdose | yes | LLL | 1 | yes | no | no |
| 56 | F | idiopathic | yes | LUL | 1 | yes | no | no | |
| 83 | F | idiopathic | yes | LUL | 3 | Yes | no | no | |
| 55 | F | idiopathic | yes | LUL | 1 | yes | no | yes | |
| 72 | M | lung cancer | yes | RUL | 2 | yes | no | NR | |
| 77 | F | lung cancer | yes | LUL | 1 | no | no | yes | |
| 75 | F | bronchiectasis | yes | RML | 1 | yes | no | no | |
| 66 | M | lung cancer | yes | RUL | 1 | no | no | no | |
| 61 | M | lung cancer | yes | RUL | 2 | yes | no | no | |
| Kawaguchi (2013) | 77 | M | metastatic thymoma | yes | Lt. upper division bronchus | 1 | yes (cessation by BAE) | before BO | NR |
| Coiffard (2014) | 65 | M | lung cancer | no | Lt. B1+2 | 1 | yes | after BO | NR |
| Adachi (2016) | 57 | F | NTM | no | Rt. B5 | 2 | yes | after BO | no |
| Adachi (2016) | 63 | M | idiopathic | no | Lt. B1+2,3 | 2 | yes | after BO | no |
| Uzuka (2016) | 85 | F | idiopathic | yes | all peripheral bronchi | NR | no | before BO | - * |
| Kho (2017) | 78 | F | bronchiectasis | yes | Lt lingular, B10 | 2 | yes | no | no |
| Yagyu (2018) | 73 | F | pulmonary tumorlets | no | RLL | NR | no | before BO | NR |
| Oda (2018) | 62 | M | aspergillosis | yes | Rt. B3, RML | 2 | yes | no | no |
| 66 | M | aspergillosis | yes | Rt. B1 | 2 | yes | no | no | |
| Hozumi (2018) | 88 | M | Aorto-pleural fistula | yes | Lt. B1+2,3 | 4 | yes | no | no |
| Sakaguchi (2019) | 68 | M | idiopathic | yes | Rt. B3,4,5 | 3 | yes | no | no |
| Present case | 76 | F | idiopathic | yes | Rt. B1,3 | 2 | yes | before BO | no |
BO, bronchial occlusion; Rt, right; NR, not reported; LLL, left lower lobectomy; LUL, left upper lobectomy; RUL, right upper lobectomy; RML, right middle lobectomy; Lt, left; NTM, nontuberculous mycobacteriosis; seg, segment.
Idiopathic: The computed tomography scan shows no abnormalities but shows emphysema only in the lung field. *Death from hemoptysis.
Another novelty of this report is that we performed bronchial occlusion using EWS after BAE. EWS is usually employed as a temporary measure in the management of massive hemoptysis while awaiting surgery or BAE. According to previous reports (Table 2), seven cases were treated with both BAE and EWS, besides our case. Bleeding control was achieved in all three cases in which BAE was performed after EWS. In contrast, besides our case, BAE was conducted before EWS in three cases. One patient underwent EWS to prevent recurrence after hemostasis was achieved by BAE [9]. One patient underwent EWS because of difficulty in securing hemostasis using BAE; because it was still difficult to stop the bleeding even with EWS, lobectomy was performed [13]. The remaining patient eventually died due to difficulty in securing hemostasis using a combination of BAE and EWS [15]. Ando et al. reported the effectiveness of BAE for cryptogenic hemoptysis of 97.0% [3]; however, EWS for cases in which BAE is completely ineffective appears to have a high risk of failure. EWS is considered an effective alternative when BAE is slightly more effective but unable to achieve complete hemostasis, as in our case.
In our case, we encountered two difficulties. First, to control bleeding, BAE proved to be anatomically difficult, and bronchoscopic intervention was difficult because of the lack of a field of view. Because the patient underwent right lower lobectomy, the respiratory surgeon offered a total right lung resection as an alternative plan. However, we repeated BAE to avoid pneumonectomy and successfully embolized part of the bronchial artery responsible. Second, the insertion of EWS in the apical segment of the upper lobe was anatomically difficult. In most cases, bronchoscopists use grasping forceps to hold a spigot, to insert it into the target bronchus. However, by this method, EWS cannot be brought to the target bronchus due to insufficient flexion of the bronchoscope in the upper lobe or superior segment of the lower lobe; even if this succeeds, it often takes a long time. There is a special technique such as the “heel-kick method” to plug the spigot into any target bronchus; however, the operator needs to be proficient in the technique. Using a curette, we were able to insert an EWS easily within a short time. The cytology curette, a device that has been used for cytopathology in Japan for a long time, is also used as a guiding device by taking advantage of its bent tip characteristic. A curette equipped with an EWS was inserted through a hole made using an injection needle; this curette can be rotated in any direction and bent at any angle. This may have resulted in good results, even when performed by less experienced bronchoscopists [19].
Regarding the appropriate period of EWS placement, the risk of obstructive pneumonia associated with long-term indwelling has been a concern; however, some reports suggest that long-term placement of EWS is possible [16,20]. Therefore, it is necessary to carefully follow the course after treatment and comprehensively consider age, prognosis, hemoptysis risk, and obstructive pneumonia risk in each case.
4. Conclusion
We report a case of massive hemoptysis in a 76-year-old patient with postoperative recurrent lung cancer who was successfully treated using a combination therapy of EWS and BAE. There are three bulleted learning points in this report. First, there is a possibility that EWS will be effective in cases where hemostasis is difficult or insufficient with BAE alone. Second, treatment with EWS allowed us to avoid surgical resection. Third, using a curette, we could insert an EWS easily into the apical segment of the lung within a short time.
Statement of ethics
This case report was prepared and completed in accordance with the guidelines of the revised Helsinki Declaration of 2013. Written informed consent was obtained from the patient for publication of the case report and the accompanying images.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All of the authors contributed to the treatment of the patient.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
We would like to thank the patient for providing consent to publish her clinical information and data. We also thank Dr. Shuji Okahara for the anesthetic management in the intensive care unit. We also thank Editage (www.editage.jp) for the English language editing of this manuscript.
References
- 1.Yoon W., Kim J.K., Kim Y.H., Chung T.W., Kang H.K. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395–1409. doi: 10.1148/rg.226015180. [DOI] [PubMed] [Google Scholar]
- 2.Davidson K., Shojaee S. Managing massive hemoptysis. Chest. 2020;157(1):77–88. doi: 10.1016/j.chest.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Ando T., Kawashima M., Masuda K., Takeda K., Okuda K., Suzuki J., Ohshima N., Matsui H., Tamura A., Nagai H., Akagawa S., Ohta K. Clinical and angiographic characteristics of 35 patients with cryptogenic hemoptysis. Chest. 2017;152(5):1008–1014. doi: 10.1016/j.chest.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Serasli E., Kalpakidis V., Iatrou K., Tsara V., Siopi D., Christaki P. Percutaneous bronchial artery embolization in the management of massive hemoptysis in chronic lung diseases. Immediate and long-term outcomes. Int. Angiol. J Int Union Angiol. 2008;27(4):319–328. [PubMed] [Google Scholar]
- 5.Guggino G., Doddoli C., Barlesi F., Acri P., Chetaille B., Thomas P., Giudicelli R., Fuentes P. Completion pneumonectomy in cancer patients: experience with 55 cases. Eur. J. Cardio. Thorac. Surg. 2004;25(3):449–455. doi: 10.1016/j.ejcts.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Cable D.G., Deschamps C., Allen M.S., Miller D.L., Nichols F.C., Trastek V.F., Pairolero P.C. Lobar torsion after pulmonary resection: presentation and outcome. J. Thorac. Cardiovasc. Surg. 2001;122(6):1091–1093. doi: 10.1067/mtc.2001.117839. [DOI] [PubMed] [Google Scholar]
- 7.Dutau H., Palot A., Haas A., Decamps I., Durieux O. Endobronchial embolization with a silicone spigot as a temporary treatment for massive hemoptysis: a new bronchoscopic approach of the disease. Respiration. 2006;73(6):830–832. doi: 10.1159/000092954. [DOI] [PubMed] [Google Scholar]
- 8.Bylicki O., Vandemoortele T., Laroumagne S., Astoul P., Dutau H. Temporary endobronchial embolization with silicone spigots for moderate hemoptysis: a retrospective study. Respiration. 2012;84(3):225–230. doi: 10.1159/000339421. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi Y., Hanaoka J., Teramoto K., Kitamura S., Hashimoto M., Kaku R., Ishida K., Asakura S. Pulmonary metastasis of invasive thymoma, showing endobronchial polypoid growth: report of a case. Surg. Today. 2014;44(7):1371–1374. doi: 10.1007/s00595-013-0678-2. [DOI] [PubMed] [Google Scholar]
- 10.Coiffard B., Laroumagne S., Plojoux J., Astoul P., Dutau H. Endobronchial occlusion for massive hemoptysis with a guidewire-assisted custom-made silicone spigot: a new technique. J Bronchol Interv Pulmonol. 2014;21(4):366–368. doi: 10.1097/lbr.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 11.Adachi T., Ogawa K., Yamada N., Nakamura T., Nakagawa T., Tarumi O., Hayashi Y., Nakahara Y. Bronchial occlusion with Endobronchial Watanabe Spigots for massive hemoptysis in a patient with pulmonary Mycobacterium avium complex infection. Respir Invest. 2016;54(2):121–124. doi: 10.1016/j.resinv.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Adachi T., Oki M., Saka H. Management considerations for the treatment of idiopathic massive hemoptysis with endobronchial occlusion combined with bronchial artery embolization. Intern Med. 2016;55(2):173–177. doi: 10.2169/internalmedicine.55.5261. [DOI] [PubMed] [Google Scholar]
- 13.Yagyu K., Miyamoto A., Matsushita H., Okimora A. A case of lung tumorlets secondary to pulmonary hypoplasia with recurrent haemoptysis. Respirol Case Rep. 2018;6(8) doi: 10.1002/rcr2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kho S.S., Chan S.K., Yong M.C., Tie S.T. Endobronchial embolization for life-threatening hemoptysis with endobronchial Watanabe spigot. BMC Res. Notes. 2017;10(1):304. doi: 10.1186/s13104-017-2635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uzuka T., Nakamura M., Nakajima T., Kusudoh S., Usubuchi H., Tanaka A., Watanabe N. Idiopathic bronchial hemorrhage: a rare but catastrophic complication in cardiac surgery. J. Cardiothorac. Surg. 2016;11(1):78. doi: 10.1186/s13019-016-0477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oda N., Sakugawa M., Hosokawa S., Fukamatsu N., Bessho A. Successful long-term management of two cases of moderate hemoptysis due to chronic cavitary pulmonary aspergillosis with bronchial occlusion using silicone spigots. Intern Med. 2018;57(16):2389–2393. doi: 10.2169/internalmedicine.0553-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hozumi T., Kajiura K., Nakamura K., Taniguchi H., Goto T., Nasu M. Aorto-pleural fistula successfully treated by one-lung ventilation and Endobronchial Watanabe Spigots. Respirol Case Rep. 2019;7(1) doi: 10.1002/rcr2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi T., Kida H., Kanno Y., Oyama B., Inoue T., Miyazawa T., Mineshita M. Bronchial occlusion with endobronchial Watanabe spigot for hemoptysis in a mechanically ventilated patient with extracorporeal circulation. Intern Med. 2019;58(2):267–269. doi: 10.2169/internalmedicine.1176-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morikawa S., Okamura T., Minezawa T., Goto Y., Hayashi M., Yamaguchi T., Isogai S., Mieno Y., Yamamoto N., Uozu S., Nakanishi T., Okazawa M., Imaizumi K. A simple method of bronchial occlusion with silicone spigots (Endobronchial Watanabe Spigot; EWS®) using a curette. Ther. Adv. Respir. Dis. 2016;10(6):518–524. doi: 10.1177/1753465816664862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneda H., Minami K., Nakano T., Taniguchi Y., Saito T., Konobu T., Saito Y. Efficacy and long-term clinical outcome of bronchial occlusion with endobronchial Watanabe spigots for persistent air leaks. Respir Invest. 2015;53(1):30–36. doi: 10.1016/j.resinv.2014.09.002. [DOI] [PubMed] [Google Scholar]