Abstract
Allylsulfide, an inhibitor of ammonia monooxygenase, was tested to determine its ability to inhibit nitrification and methane oxidation in pure cultures, in agricultural humisol enrichment cultures, and in humisol slurries. We confirmed that allylsulfide is a differential inhibitor of cultures of nitrifiers and methanotrophs at concentrations of 1 and 200 μM, respectively, which result in 50% inhibition. However, although a nitrifying enrichment culture added to sterilized humisol was inhibited 50% by 4 μM allylsulfide, 500 μM allylsulfide was necessary for 50% inhibition of the endogenous nitrifying activity in nonsterile humisol. We concluded that native nitrifiers were protected, possibly by being in colonial aggregates or sheltered microenvironments.
Methanotrophic bacteria are gram-negative aerobes that have the unique ability to use methane (CH4) as their sole carbon and energy source. The methane monooxygenase (MMO), which is responsible for oxidation of CH4, is of interest because of its broad substrate specificity. MMO oxidizes a variety of xenobiotic chemicals (for a review see reference 10), and it is also able to cooxidize ammonium (NH4+) to NH2OH (5, 15, 24), an integral step in the nitrogen cycle.
Chemoautotrophic nitrifiers are aerobic, gram-negative rods that oxidize NH4+ or nitrite (NO2−) and use CO2 as their carbon source. The conversion of NH4+ is catalyzed by ammonia monooxygenase (AMO) (7, 13). In addition to monooxygenase activity, AMO has a dehydrogenase/oxidase and reductive dehalogenation activity (14). As a result, the AMO of ammonia oxidizers has broad substrate specificity that includes aliphatic, aromatic, and halogenated molecules (14). Some ammonia oxidizers are also capable of oxidizing CH4 to CO2 and incorporating some of the carbon from CH4 into cellular components, but growth under these conditions has not been reported (19, 33).
Although methanotrophs can oxidize NH4+ to NO2− and nitrifiers can oxidize CH4, the interactions between nitrifiers and methanotrophs in natural systems are complex and not well-understood (29). Members of both groups are present and active on the aerobic side of the anoxic-oxic interface and are responsible for O2 depletion. Few studies have been conducted to determine to what extent these two kinds of microorganisms contribute to the metabolism of NH4+ and CH4 in natural systems (1). For such studies, a substance that inhibits only one of the two processes would be helpful. Allylsulfide shows potential as a differential inhibitor. Allylsulfide is a strong inhibitor of NH4+ oxidation in Nitrosomonas europaea, and it may act as an irreversible mechanism-based inactivator of AMO (21, 22). In contrast, allylsulfide inhibits CH4 oxidation at concentrations that are 2 to 3 orders of magnitude higher than the concentrations that result in similar levels of inhibition of nitrification (29).
In this study we used a variety of pure cultures and enrichment cultures obtained from an agricultural humisol to confirm that allylsulfide is a differential inhibitor of nitrifiers and methanotrophs. However, we found that nitrifiers in nonsterile humisol slurries were 2 orders of magnitude less sensitive to allylsulfide than were nitrifiers in nitrifying enrichment cultures alone or in the presence of sterile soil. We concluded that endogenous nitrifiers are protected from allylsulfide inhibition and that allylsulfide does not differentially inhibit the nitrifier and methanotroph populations in the humisol examined.
MATERIALS AND METHODS
Pure cultures and media.
Methylosinus trichosporium OB3b, a group II methanotroph (obtained from R. S. Hanson), and strain MWT2, a group II methanotroph (isolated from humisol by P. Dunfield and T. Ren), were grown in nitrate mineral salts (NMS) and ammonium mineral salts (AMS) media and assayed to determine CH4 oxidation as described by Roy and Knowles (29).
Nitrifier enrichment culture.
Humisol (the same humisol that was used in the study described in reference 9) was collected from the Central Experimental Farm of Agriculture and Agri-Food Canada in Ottawa, Canada, in August 1997. The humisol was sieved (sieve size, 2 mm) and stored at 4°C. To obtain a nitrifier enrichment culture from the humisol, an extinction dilution experiment was conducted as described by Schmidt and Belser (31). After 4 weeks, a positive tube at the highest dilution that produced NO2−, NO3−, and acid from NH4+ was used to inoculate a flask containing 100 ml of ammonia oxidizer medium (31). This flask was incubated for 11 days in the dark on a rotary shaker (200 rpm) at 25°C. After incubation, 90 ml of the culture was transferred to 1 liter of the same medium, and the preparation was incubated in the dark at 25°C with magnetic stirring. During incubation, the pH was adjusted periodically to 7.5 with sterile 1% (wt/vol) K2CO3. After 26 days the late-log- to early-stationary-phase cells were centrifuged (15,000 × g, 10 min, 4°C), resuspended in fresh medium, and used to inoculate an 8-liter batch culture that was used for experimental purposes. The batch culture was incubated in the dark and was sparged with filter-sterilized (pore size, 0.45 μm) air for 30 days at 25°C. This culture could be used for several weeks without any change in activity. Portions of the batch culture were centrifuged (20,000 × g, 10 min, 4°C) and then both washed and resuspended in an equal volume of fresh medium; the resulting preparations were used in nitrification experiments.
Allylsulfide inhibition of nitrifier enrichment culture.
Portions (50 ml) of a freshly resuspended nitrifier enrichment culture were placed in three 125-ml flasks. Sterile NH4+ oxidizer medium was added to another three flasks, which were used as uninoculated controls. Allylsulfide was dissolved in dimethyl sulfoxide (DMSO), 100-μl aliquots were added to the flasks, and the flasks were sealed with Suba-seals (William Freeman, Barnsley, United Kingdom). Picolinic acid (0.25 M), another potential differential inhibitor of nitrifiers and methanotrophs (23, 30), adjusted to pH 7.0 with NaOH and diluted in distilled deionized H2O, was added instead of allylsulfide in some experiments. All of the flasks were incubated at 25°C with shaking at 200 rpm. At suitable times, 2.5-ml liquid samples were withdrawn with a syringe from each flask. A 1.5-ml portion of each sample was added to a microcentrifuge tube, and the other 1 ml was used to determine the pH. The microcentrifuge tubes were centrifuged at 15,000 × g for 10 min. The supernatant was frozen at −70°C and used later for nitrogen oxide analyses. Percentages of inhibition were calculated by determining the slopes of NH4+ oxidation data as percentages of the control (no allylsulfide) activity.
The nitrifier enrichment culture was also examined for CH4 oxidation. Nitrifiers were resuspended in the medium described above supplemented with 0 or 1 mM NH4+ and 2 ppmv or 0.2 or 1% CH4 in the headspace. This was done to identify the optimal conditions for testing the sensitivity of nitrifier enrichment culture CH4 oxidation to allylsulfide.
Allylsulfide and humisol nitrification.
Inhibition of humisol nitrification by allylsulfide was studied by using a modification of the nitrification activity procedure of Schmidt and Belser (31). To 125-ml flasks, 45 ml of 0.5 mM potassium phosphate buffer (pH 7.0), 100 μl of 0.25 M (NH4)2SO4, and 10 g of humisol were added. Dilutions of allylsulfide in DMSO were added in 100-μl aliquots. High concentrations of allylsulfide (>500 μM) interfered with hydrazine-copper reduction of NO3− during analysis, so 0.5 ml of 1 M KClO3 was added to inhibit NO2− oxidation (3) and the NO2− content was measured as a product of nitrification in some experiments (31). Chlorate (10 mM) did not affect the rate of humisol NH4+ oxidation (data not shown). Picolinate, at appropriate dilutions in distilled deionized H2O, was added in some inhibition experiments instead of allylsulfide. The flasks were sealed with Suba-seals and incubated at 25°C with shaking at 200 rpm. At certain times, each flask was inverted, and a 1.5-ml liquid sample was withdrawn with a syringe and centrifuged at 15,000 × g for 10 min. The supernatant was frozen at −70°C and used later for analysis. Percentages of inhibition were calculated as described above.
In some experiments, the humisol slurry was blended prior to the procedure described above. Humisol (222 g, fresh weight) was suspended in 500 ml of 0.5 mM potassium phosphate buffer (pH 7.0) and blended with a Waring blender for a total of 10 min with resting in an ice bath for 3 to 5 min for every 2 min of blending. Another 500 ml of 0.5 mM potassium phosphate buffer (pH 7.0) was added, and 50 ml of the blended suspension was added to each 125-ml experimental flask. Next we added 100 μl of a 0.25 M (NH4)2SO4 solution, 100 μl of DMSO containing allylsulfide, and 0.5 ml of 1 M KClO3. The flasks were sealed with Suba-seals, and the rest of the experiment was performed as described above. Percentages of inhibition were calculated as described above.
Sterile humisol and nitrifiers.
Ten-gram samples of humisol were added to 125-ml flasks and autoclaved for 1 h on 3 consecutive days. Portions (45 ml) of a nitrifier enrichment culture (washed and resuspended in 0.5 mM potassium phosphate buffer [pH 7.0]) were added to the flasks containing sterile humisol. Then 100 μl of 0.25 M (NH4)2SO4, 0.5 ml of 1 M KClO3, and 100 μl of DMSO containing dissolved allylsulfide were added to each flask before it was sealed with a Suba-seal. The experimental cultures were incubated at 25°C with shaking at 200 rpm. Slurry suspensions were monitored to determine whether nitrification occurred by removing 1.5-ml portions, centrifuging them at 15,000 × g for 10 min, and storing the supernatants at −70°C; later the supernatants were used for NO2− analysis. Percentages of inhibition were calculated as described above.
Methane-enriched humisol.
Suspensions containing 10 g of humisol and 45 ml of 0.5 mM potassium phosphate buffer (pH 7.0) were shaken in a series of 125-ml flasks with Suba-seals at 200 rpm with 10% CH4 in air in the headspace for 4 days. The contents of eight flasks were combined and magnetically stirred, and 15-ml aliquots were added to 60-ml serum bottles. Then 30 μl of 0.25 M (NH4)2SO4 and 30 μl of DMSO containing allylsulfide were added to each bottle before the bottle was closed with a butyl rubber seal and a crimp. At time intervals over 24 h, 0.5-ml portions of the headspace gas were withdrawn and used for CH4 analysis. Percentages of inhibition were calculated by determining the slopes of CH4 oxidation and growth data as percentages of the control (no allylsulfide) activity.
Analytical procedures.
In the methanotroph, nitrifier, and humisol CH4 oxidation experiments, the headspaces of flasks containing NMS and AMS media were analyzed to determine their CH4 and CO2 contents by using a gas chromatograph (GC) equipped with a thermal conductivity detector (28). In the experiments in which the nitrifier enrichment culture was incubated with low CH4 concentrations (2 ppmv), samples of headspace gas were removed and then analyzed with a GC equipped with a flame ionization detector (8). In the nitrification experiments, slurry samples were analyzed colorimetrically with an autoanalyzer to determine their NO2− and NO3− contents (28). Culture growth was monitored by measuring the absorbance at 430 nm with a Spectronic 20 spectrophotometer.
RESULTS AND DISCUSSION
Methanotrophs.
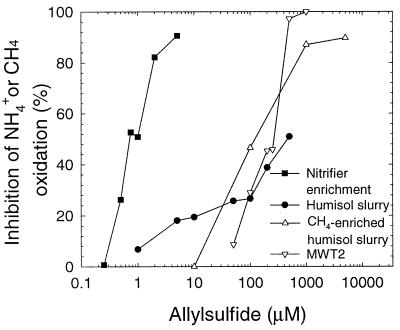
MWT2, a group II methanotroph (as determined by methanol dehydrogenase sequencing [12]), was isolated from the agricultural humisol used in this study. MWT2 was as sensitive to allylsulfide as M. trichosporium was in NMS medium (Table 1). All of the activities measured, including CH4 oxidation, CO2 production (data not shown), growth, and NH4+ oxidation, were inhibited at similar allylsulfide concentrations. This indicates that a methanotrophic bacterium from the humisol was inhibited by allylsulfide to the same extent as the known methanotroph M. trichosporium was. Over periods of 24 h, allylsulfide also inhibited CH4 oxidation by CH4-enriched humisol at concentrations that were within a factor of 2 of the concentrations required to inhibit strain MWT2 in AMS medium (Fig. 1 and Table 1).
TABLE 1.
Concentrations of allylsulfide that result in 50% inhibition of methanotroph activities
| System (medium) | Allylsulfide concn (μM) that results in 50% inhibition of:
|
Reference | ||
|---|---|---|---|---|
| CH4 oxidation | Growth | NH4+ oxidation | ||
| M. trichosporium (NMS) | >200 | 200 | NDa | 29 |
| 900 | 700 | ND | This study | |
| Lake sediment | 121 | 29 | ||
| Strain MWT2 (NMS) | 350 | 300 | ND | This study |
| Strain MWT2 (AMS) | 300 | 250 | 300 | This study |
| Methane-enriched humisol | 150 | ND | ND | This study |
ND, not determined.
FIG. 1.
Allylsulfide inhibition of NH4+ oxidation (solid symbols) and CH4 oxidation (open symbols) by cultures used in this study.
The results of our studies with methanotrophic cultures and CH4-oxidizing enrichment cultures showed that the activities of methanotrophic bacteria which we measured (CH4 oxidation, NH4+ oxidation, and growth) were inhibited by allylsulfide to the same extent. This finding is important for environmental studies of the contributions of methanotrophs and nitrifiers to N and C cycling in soils when allylsulfide is used as a differential inhibitor. It has been hypothesized that allylsulfide targets the MMO and also affects NO3− metabolism by methanotrophs, based on differential inhibition of M. trichosporium in NMS and AMS media (29). Our data suggest that at least in MWT2, a group II methanotroph, nitrate metabolism is an unlikely target since allylsulfide inhibited this isolate similarly in AMS and NMS media. Also, since growth was found to be as sensitive or slightly more sensitive to allylsulfide, it is likely that another methanotroph enzymatic system(s) is inhibited in addition to (or instead of) MMO.
Nitrifiers.
A nitrifier enrichment culture was prepared from the humisol and was exposed to allylsulfide. Addition of allylsulfide at a concentration of 1 μM resulted in 50% inhibition of oxidation of NH4+ in the enrichment culture (Fig. 1). This inhibition was constant for 24 h (data not shown). Low allylsulfide concentrations also inhibit N. europaea (22) and lake sediment slurry nitrification (29) (Table 2). This result confirms that allylsulfide is a differential inhibitor of at least some nitrifiers and methanotrophs. The allylsulfide concentrations that produced similar inhibition results for nitrifiers and methanotrophs in this study differed by at least 2 orders of magnitude. In a previous study of lake sediment, inhibition of nitrification and inhibition of CH4 oxidation by allylsulfide differed by as much as 2 to 3 orders of magnitude (29).
TABLE 2.
Concentrations of allylsulfide that result in 50% inhibition of nitrifier activity
| System | Allylsulfide concn that results in 50% inhibition of NH4+ oxidation (μM) | Reference |
|---|---|---|
| Lake sediment | 0.2 | 29 |
| Nitrifier enrichment culture | 1 | This study |
| Nitrifier enrichment culture in sterile humisol | 4 | This study |
| Humisol nitrification | 500 | This study |
The effect of allylsulfide on CH4 oxidation by nitrifiers was also examined. Oxidation of 2 ppmv of CH4 and 0.2 and 1% CH4 by the nitrifying enrichment culture was not detected either in the presence or in the absence of 1 mM NH4Cl. It may be that the rate of CH4 cooxidation was below the detection limit of our GC method and that CH4 cooxidation might require detection by 14C tracer methods. Previous researchers who described CH4 oxidation by nitrifier cultures used 14C tracer methods (19, 20, 33) or monitored methanol levels by gas-liquid chromatography and flame ionization detection (16, 32). Thus, the effect of allylsulfide on CH4 oxidation by nitrifying bacteria remains unknown.
Humisol.
The nitrification rates in the humisol slurries were similar to (differed by a factor of less than 2 from) the rates observed in the enrichment cultures. However, allylsulfide was a relatively ineffective inhibitor of nitrification in a humisol slurry (Fig. 1 and Table 2). Allylsulfide concentrations of approximately 500 μM were necessary for 50% inhibition of NH4+ oxidation in the slurry, compared to the concentration of 1 μM required for the same level of inhibition of the nitrifier enrichment culture. We suggest the following three possible explanations for this insensitivity. (i) Heterotrophic nitrification may contribute to humisol nitrification; since allylsulfide is known to be an irreversible, mechanism-based inhibitor of nitrifier AMO (22), heterotrophic nitrification may be less affected than autotrophic nitrification is. (ii) Allylsulfide may be abiologically sequestered or adsorbed to soil components and thus unavailable for inhibiting humisol nitrification. (iii) Nitrifiers may be present in an immobilized state in aggregates of soil and/or microorganisms that provide protection from allylsulfide.
Heterotrophic nitrification.
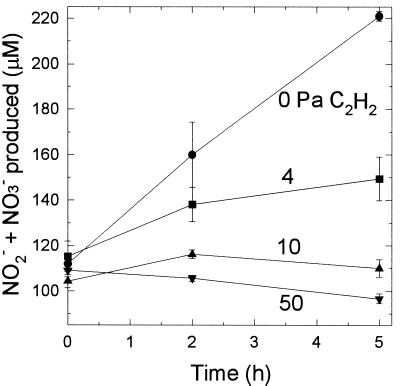
Acetylene is an effective inhibitor of autotrophic nitrification; this compound causes suicidal inactivation of AMO (17, 18) but does not affect heterotrophic nitrification in an Arthrobacter sp. (18). Acetylene at a pressure of 10 Pa completely inhibits nitrification in pure cultures of N. europaea (18) and in humisol (9). In this study, acetylene completely inhibited NH4+ oxidation by humisol slurries at a pressure of 10 Pa (Fig. 2), suggesting that the nitrification observed in the humisol was not heterotrophic nitrification.
FIG. 2.
Acetylene inhibition of nitrification (NO2− and NO3− production) by humisol slurries. Acetylene pressures (in pascals) are indicated next to the lines. The data are averages ± standard errors of the means based on data from three flasks. Error bars that are not visible are smaller than the symbols.
Abiological adsorption of allylsulfide.
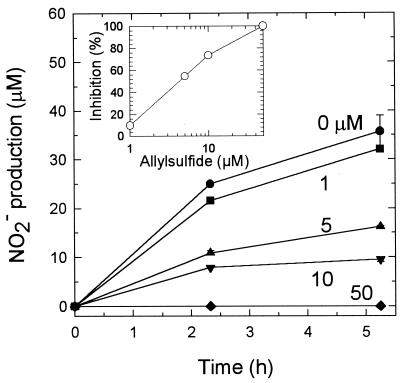
The ability of nitrapyrin to inhibit nitrification can be reduced by increasing the organic matter content of soils, and it has been suggested that the reduced ability is due to sorption of nitrapyrin to the soil organic matter (4). In addition, we observed decreases in NO3− concentrations in blended or autoclaved humisol samples compared to untreated slurries. In sterilized slurries, the NO3− concentrations decreased with time and shaking (unpublished data). Anion binding sites may become exposed so that they can adsorb NO3− when soil is blended or sterilized. To determine whether allylsulfide was adsorbed by the organic fraction of the humisol, soil samples were sterilized and supplemented with suspensions of the nitrifier enrichment culture and various concentrations of allylsulfide. These slurries were 50% inhibited by allylsulfide at a concentration of 4 μM (Fig. 3 and Table 2); this result was similar to the result obtained in the enrichment culture study. This finding indicated that allylsulfide was present in the aqueous phases of the humisol slurries and was available to inhibit nitrifiers that were added. It also suggested that no compounds which interfered or competed with the allylsulfide inhibitory effect were present.
FIG. 3.
Effect of allylsulfide on activity of a nitrifier enrichment culture added to autoclaved humisol (measured by determining NO2− production in the presence of 10 mM KClO3). The allylsulfide concentrations (micromolar) are indicated next to the lines. The data are averages ± standard errors of the means based on data from three flasks. Error bars that are not visible are smaller than the symbols. (Inset) Percent inhibition as a function of allylsulfide concentration.
Protection by microenvironment.
Nitrifiers are known to colonize soil aggregates (11) and to occur as colonial aggregates (6). Furthermore, several model systems have shown that nitrifiers attached to artificial surfaces are more resistant to inhibitors (25, 27). Therefore, we attempted to disrupt microenvironments by homogenization. Slurries were blended for 10 min, and although this decreased the NH4+ oxidation activity, the activity that remained was inhibited like the activity in unblended slurries (Fig. 1 and 4). It is likely that nitrifiers are active only when they are protected by microenvironments, and the NH4+ oxidation that is observed may be attributed to nitrifiers that are active within undisturbed aggregates. To confirm that microenvironments of nitrifiers offer resistance to inhibitors, we tested picolinate, another potential differential inhibitor of nitrifiers and methanotrophs (23, 30). NH4+ oxidation by our nitrifier enrichment culture was inhibited 50% by 25 μM picolinate (data not shown); previously, a similar concentration (51 μM) was found to inhibit N. europaea by 50% (2). However, in our study, 800 μM picolinate was required to inhibit humisol nitrification by 50% (data not shown), and in the humisol study of Megraw and Knowles (23) nitrification was not affected by 2 mM picolinate. Therefore, it appears that at least in the two humisols studied, the microenvironment plays a significant role in protecting nitrifiers from normally potent inhibitors. This effect has also been observed previously with nitrapyrin (26), but this study is the first study to demonstrate that inhibition of NH4+ oxidation in soils by allylsulfide is significantly less than the inhibition observed in liquid cultures. It is not clear why the microenvironment protects nitrifiers from picolinate, nitrapyrin, and allylsulfide but not from acetylene. It is likely that smaller gas molecules, such as acetylene molecules, are less susceptible to mass transfer limitations in soil microenvironments. Our results also demonstrate that methanotrophic activities in CH4-enriched humisol slurries are as sensitive to allylsulfide as are the activities of at least two pure cultures of methanotrophs. Methane-oxidizing bacteria are probably not protected in the same way that nitrifiers are protected in the humisol which we tested.
FIG. 4.
Effect of allylsulfide (AS) on blended humisol slurry nitrification (measured by determining NO2− production in the presence of 10 mM KClO3). An unblended control (□) was included for comparison. The allylsulfide concentrations (micromolar) are indicated next to the lines. The NO2− production data are averages ± standard errors of the means based on data from three flasks. Error bars that are not visible are smaller than the symbols. (Inset) Percent inhibition of a blended slurry as a function of allylsulfide concentration.
Although we found that humisol nitrification was rather insensitive to allylsulfide, lake sediment slurry nitrification has been found to be sensitive to allylsulfide at concentrations that inhibit pure cultures (Table 2). This suggests that inhibition of nitrifiers by allylsulfide depends on the nature of the sample being examined. The humisol and the sediment slurry differed considerably in their organic matter contents, and this may have been related to the different sensitivities of the nitrifier populations in these two systems to allylsulfide. To what extent allylsulfide differentially inhibits endogenous populations of nitrifiers and methanotrophs in a variety of soil and sediment systems has not been assessed yet.
Conclusion.
We confirmed that allylsulfide is a differential inhibitor of nitrifiers and methanotrophs by performing liquid culture studies. However, we also found that in the humisol, nitrifiers are protected from this potent inhibitor by what appears to be immobilization within microenvironments. Thus, it should be realized that some inhibitors of nitrifiers may not be effective in some soil environments. Differential inhibitors of nitrifiers and methanotrophs, such as allylsulfide and picolinate, should be assessed to determine their inhibitory effects in a soil or sediment system before they are used in studies of N and C cycling activities.
ACKNOWLEDGMENTS
This research was funded by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to R.K.
We are thankful for the helpful suggestions of R. Roy and F. Archibald during preparation of the manuscript.
REFERENCES
- 1.Bédard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bédard C, Knowles R. Some properties of methane oxidation in a thermally stratified lake. Can J Fish Aquat Sci. 1997;54:1639–1645. [Google Scholar]
- 3.Belser L W, Mays E L. Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl Environ Microbiol. 1980;39:505–510. doi: 10.1128/aem.39.3.505-510.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chancy H F, Kamprath E J. Effect of nitrapyrin rate on nitrification in soils having different organic matter contents. Soil Sci. 1987;144:29–35. [Google Scholar]
- 5.Dalton H. Ammonia oxidation by the methane-oxidizing bacterium Methylococcus capsulatus strain Bath. Arch Microbiol. 1977;114:273–279. [Google Scholar]
- 6.De Boer W, Gunnewick P J A K, Keenhuis M, Bock E, Laanbroek H J. Nitrification at low pH by aggregated chemolithotrophic bacteria. Appl Environ Microbiol. 1991;57:3600–3604. doi: 10.1128/aem.57.12.3600-3604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dua R D, Bhandari B, Nicholas D J D. Stable isotope studies on the oxidation of ammonia to hydroxylamine by Nitrosomonas europaea. FEBS Lett. 1979;106:401–404. doi: 10.1016/0014-5793(79)80541-4. [DOI] [PubMed] [Google Scholar]
- 8.Dunfield P F, Knowles R. Kinetics of inhibition of methane oxidation by nitrate, nitrite, and ammonium in a humisol. Appl Environ Microbiol. 1995;61:3129–3135. doi: 10.1128/aem.61.8.3129-3135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunfield P F, Knowles R. Biological oxidation of nitric oxide in a humisol. Biol Fertil Soils. 1997;24:294–300. [Google Scholar]
- 10.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattori T. Microbial life in the soil. New York, N.Y: Marcel Dekker; 1973. [Google Scholar]
- 12.Henckel, T. Personal communication.
- 13.Hollocher T C, Tate M E, Nicholas D J D. Oxidation of ammonia by Nitrosomonas europaea. Definitive 18O-tracer evidence that hydroxylamine formation involves a monooxygenase. J Biol Chem. 1981;256:10834–10836. [PubMed] [Google Scholar]
- 14.Hooper A, Vannelli T, Bergmann D J, Arciero D M. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie van Leeuwenhoek. 1997;71:59–67. doi: 10.1023/a:1000133919203. [DOI] [PubMed] [Google Scholar]
- 15.Hutton W E, Zobell C E. Production of nitrite from ammonia by methane-oxidizing bacteria. J Bacteriol. 1953;65:216–219. doi: 10.1128/jb.65.2.216-219.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyman M R, Wood P M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983;212:31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman M R, Wood P M. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J. 1985;227:719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes R K, Knowles R. Effect of acetylene on autotrophic and heterotrophic nitrification. Can J Microbiol. 1982;28:334–340. [Google Scholar]
- 19.Jones R D, Morita R Y. Methane oxidation by Nitrosomonas oceanus and Nitrosomonas europaea. Appl Environ Microbiol. 1983;45:401–410. doi: 10.1128/aem.45.2.401-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones R D, Morita R Y. Effect of several nitrification inhibitors on carbon monoxide and methane oxidation by ammonium oxidizers. Can J Microbiol. 1984;30:1276–1279. [Google Scholar]
- 21.Juliette L Y, Hyman M R, Arp D J. Inhibition of ammonia oxidation in Nitrosomonas europaea by sulfur compounds: thioethers are oxidized to sulfoxides by ammonia monooxygenase. Appl Environ Microbiol. 1993;59:3718–3727. doi: 10.1128/aem.59.11.3718-3727.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juliette L Y, Hyman M R, Arp D J. Mechanism-based inactivation of ammonia monooxygenase in Nitrosomonas europaea by allylsulfide. Appl Environ Microbiol. 1993;59:3728–3735. doi: 10.1128/aem.59.11.3728-3735.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Megraw S R, Knowles R. Effect of picolinic acid (2-pyridine carboxylic acid) on the oxidation of methane and ammonia in soil and in liquid culture. Soil Biol Biochem. 1990;22:635–641. [Google Scholar]
- 24.O’Neill J G, Wilkinson J F. Oxidation of ammonia by methane-oxidizing bacteria and the effects of ammonia on methane oxidation. J Gen Microbiol. 1977;100:407–412. [Google Scholar]
- 25.Powell S J. Laboratory studies of inhibition of nitrification. In: Prosser J I, editor. Nitrification. Washington, D.C: IRL Press; 1986. pp. 79–97. [Google Scholar]
- 26.Powell S J, Prosser J I. Inhibition of ammonium oxidation by nitrapyrin in soil and liquid culture. Appl Environ Microbiol. 1986;52:782–787. doi: 10.1128/aem.52.4.782-787.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell S J, Prosser J I. Protection of Nitrosomonas europaea colonizing clay minerals from inhibition by nitrapyrin. J Gen Microbiol. 1991;137:1923–1929. doi: 10.1099/00221287-137-8-1923. [DOI] [PubMed] [Google Scholar]
- 28.Roy R, Knowles R. Effects of methane metabolism on nitrification and nitrous oxide production in polluted freshwater sediment. Appl Environ Microbiol. 1994;60:3307–3314. doi: 10.1128/aem.60.9.3307-3314.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy R, Knowles R. Differential inhibition by allylsulfide of nitrification and methane oxidation in freshwater sediment. Appl Environ Microbiol. 1995;61:4278–4283. doi: 10.1128/aem.61.12.4278-4283.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvas P L, Taylor B F. Effect of pyridine compounds on ammonia oxidation by autotrophic nitrifying bacteria and Methylosinus trichosporium OB3b. Curr Microbiol. 1984;10:53–56. [Google Scholar]
- 31.Schmidt E L, Belser L W. Autotrophic nitrifying bacteria. In: Weaver R W, Angle S, Bottomley P, Bezdicek D, Smith S, Tabatabai A, Wollum A, editors. Methods of soil analysis, part 2. Microbiological and biochemical properties. Madison, Wis: Soil Science Society of America; 1994. pp. 159–177. [Google Scholar]
- 32.Voysey P A, Wood P M. Methanol and formaldehyde oxidation by an autotrophic nitrifying bacterium. J Gen Microbiol. 1987;133:283–290. [Google Scholar]
- 33.Ward B B. Kinetic studies on ammonia and methane oxidation by Nitrosomonas oceanus. Arch Microbiol. 1987;147:126–133. [Google Scholar]