Abstract
Background:
Coronavirus disease 2019 (COVID-19) is associated with increased thrombosis prevalence. However, there are insufficient data supporting the appropriate anticoagulation dose in COVID-19.
Objective:
We aim to systematically assess the currently available data regarding the effects of different dosing regimens of low molecular weight heparin and/or fondaparinux (LMWH/F) on mortality risk as well as the risk of arterial/venous thrombotic events and hemorrhagic complications in confirmed COVID-19 cases.
Design:
We conducted a living systematic review and meta-analysis on the effects of different LMWH/F doses on mortality, thrombotic and hemorrhagic events in COVID-19 patients.
Data Sources and Methods:
MEDLINE, Scopus, Embase, Cochrane Library, Cochrane COVID-19 study register, European Union Drug Regulating Authorities Clinical Trials Database, and ClinicalTrials.gov were searched to detect observational cohort studies and randomized-controlled clinical trials (RCTs) comparing difference doses of LMWH/F among confirmed COVID-19 cases.
Results:
Thirty-one eligible studies (6 RCTs and 25 cohort studies) with 11,430 hospitalized patients were included. No association was found between LMWH/F and mortality during the following comparisons: (1) no LMWH/F versus any LMWH/F; (2) prophylactic versus higher than prophylactic LMWH/F; (3) prophylactic versus therapeutic LMWH/F; (4) intermediate versus therapeutic LMWH/F; and (5) lower than therapeutic versus therapeutic LMWH/F. Mortality was higher in patients receiving prophylactic versus intermediate LMWH/F (OR = 2.01; 95% CI: 1.19–3.39). However, this effect was mostly driven by observational data. No associations were detected between the intensity of LMWH/F and the risk of thrombotic and hemorrhagic events, except the lower risk for hemorrhage in patients on prophylactic compared to higher LMWH/F doses.
Conclusion:
The risk for all-cause mortality was higher in patients receiving prophylactic LMWH/F compared to those on an intermediate dose of LMWH/F, based on observational data. These results should be interpreted in light of the moderate quality and heterogeneity of the included studies.
Registration:
The study protocol has been registered in the International Prospective Register of Ongoing Systematic Reviews PROSPERO (Registration number: CRD42021229771).
Keywords: COVID-19, fondaparinux, low molecular weight heparin, mortality, SARS-CoV-2
Introduction
As the coronavirus disease 2019 (COVID-19) pandemic continues to evolve globally, our understanding of the underlying pathogenetic mechanisms still remains largely obscure. However, an increasing body of data supports that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and—most importantly—its progression to severe and critical COVID-19 disease, may be attributed to the widespread endothelial cell damage either directly by the virus itself, or indirectly by the burst of proinflammatory cytokines, as well as the activation of complement and the development of systemic microangiopathy that leads to multi-organ damage. 1
The loss of endothelial integrity (endotheliopathy) and, consequently, the destruction of the vascular wall homeostasis are associated with a procoagulant state that activates and propagates intravascular coagulation, and leads to an increased prevalence of arterial and venous thrombotic complications in severely and critically ill COVID-19 patients. 1 The procoagulant state in COVID-19 patients is evident not only through abnormal coagulation profiles 2 —that are routinely used in most laboratories worldwide—but also through more sophisticated tests. Indeed, a study by Ranucci et al. 3 in patients with COVID-19-induced acute respiratory distress syndrome demonstrated an increased clot strength with platelet and fibrinogen contribution, using point-of-care viscoelastic tests, thus strengthening the hypothesis of activation of intravascular coagulation mechanism and verifying the hypercoagulability seen in COVID-19 infection. The prevalence of both arterial and venous thrombotic events in COVID-19 cases has been reported to range between 5.2% and 30% among different studies,4–7 although the overall risk of stroke among hospitalized patients with SARS-CoV-2 infection was found as low as 0.5% in a large multi-center observational study. 7
Taking these observations into consideration, the international stakeholders and organizations have issued guidelines on the use of antithrombotic agents in hospitalized COVID-19 patients; the National Institutes of Health of the USA and the American Society of Hematology recommend the use of prophylactic anticoagulation in all non-pregnant hospitalized adults (unless contraindicated) and suggest the administration of therapeutic doses when thrombosis is proved or highly suspected on a clinical basis.8,9 However, there are currently insufficient data supporting the use of intermediate or high (therapeutic) anticoagulation dose outside this context. 8 In addition, the effects of no anticoagulation versus anticoagulation and the different antithrombotic dosing schemes on mortality remain unknown.
To address these questions, we conducted a living systematic review and meta-analysis of studies comparing the mortality of patients with laboratory-confirmed COVID-19 disease not receiving any anticoagulation versus those who were anticoagulated with low molecular weight heparin or fondaparinux (LMWH/F); furthermore, we compared the mortality of patients under LMWH/F at a standard thromboprophylaxis dose versus intermediate or therapeutic dose. Finally, we explored the risk of hemorrhagic and thrombotic events among the aforementioned COVID-19 patient subgroups.
Methods
Search strategy, study selection, and data extraction
The meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines 2020. 10 The study protocol was established a priori and has been registered in the International Prospective Register of Ongoing Systematic Reviews PROSPERO (Registration number: CRD42021229771). Our study did not require an ethical board approval or written informed consent by the patients according to the study design (systematic review and meta-analysis).
A systematic literature search was conducted to identify eligible studies published in MEDLINE, Scopus, Embase, Cochrane Library, Cochrane COVID-19 study register, EudraCT (European Union Drug Regulating Authorities Clinical Trials Database), and ClinicalTrials.gov, between 30 December 2019 (the day of declaration of the first COVID-19 case) to 28 November 2021. The combination of search strings applied to query all databases included the following: ‘low molecular weight heparin’, ‘dalteparin’, ‘enoxaparin’, ‘nadroparin’, ‘tinzaparin’, ‘fondaparinux’, ‘heparin’, ‘antithrombotic’, ‘anticoagulant’, and ‘COVID-19’, ‘SARS-CoV-2’, or ‘coronavirus’. The respective algorithms for each database search are available in the online Supplement. Database interrogation was performed by three independent researchers (PCF, LP, and MIS), who additionally searched manually conference abstracts and reference lists of published articles to ensure the comprehensiveness of bibliography.
All observational studies (prospective or retrospective) and randomized-controlled clinical trials (RCTs) that provided data on mortality, thrombotic and/or hemorrhagic events in COVID-19 patients undergoing anticoagulation with LMWH/F at any dose (as standard thromboprophylaxis, intermediate or therapeutic dose) were identified.
Eligible studies were included using the following criteria: (1) studies including patients of any age, (2) with COVID-19 diagnosis confirmed by a positive molecular test of any severity (the criteria for confirmed COVID-19 are shown in Supplementary Table 1), (3) who received LMWH/F at any dose and contemporary COVID-19 controls who underwent a different dosing scheme of LMWH/F (including no anticoagulation), and (4) reported data on the outcomes of interest.
We excluded studies: (1) including suspected or probable COVID-19 cases (case definitions are shown in Supplemental Table 1); (2) without control population; and (3) reporting interventions not aligned with our pre-defined inclusion criteria, including treatment with classic (unfractionated) heparin, other parenteral or oral anticoagulants. Non-English publications, case reports, case series with <10 patients, commentaries, narrative and systematic reviews, non-peer reviewed studies, and pre-prints were also excluded from further analyses.
In case of overlapping data between studies, the study with the largest dataset was retained. Independent assessment of retrieved studies was performed based on the previous inclusion/exclusion criteria by three reviewers (PCF, LP, and MIS), and any disagreements were resolved by the senior author (GT).
Data extraction was performed by three independent reviewers (PCF, LP, and MIS). We extracted data regarding study details (type of study, dates of recruitment, location, publication year, etc.), baseline characteristics of each study’s population (mean age, number of males, COVID-19 severity and setting of treatment, co-morbidities), and details on the outcomes of interest (type and dose of anticoagulation, mortality rate number and type of thrombotic and hemorrhagic events for each group of different treatments). Potential disagreements in data abstraction were resolved by the senior author (GT).
An aggregate data meta-analysis was performed including observational studies and RCTs reporting on rates of all-cause mortality, thrombotic or hemorrhagic events in COVID-19 patients undergoing anticoagulation with LMWH/F versus contemporary COVID-19 controls as previously defined.
Plan for establishing living evidence
We plan to update our results with emerging evidence arising from new observational studies or RCTs, by following the same search method as described in our protocol every four months. The reviewers who did the initial search (PCF, LP, and MIS) will evaluate the new evidence according to the pre-defined inclusion and exclusion criteria, and we will meta-analyze the new data according to our pre-defined methods.
Study quality control and risk of bias assessment
Eligible studies were subjected to quality control and bias assessment employing the Cochrane Collaboration toll (RoB 2) 11 for RCTs and the Newcastle-Ottawa Scale 12 for cohort studies. The quality control and risk of bias assessment was conducted independently by three reviewers (PCF, LP, and MIS), and disagreements were resolved via consensus after discussion with the senior author (GT).
Outcomes
Our pre-defined primary outcome measure was all-cause mortality of COVID-19 patients under no anticoagulation versus LMWH/F at any dose. Secondary outcomes comprised: (1) thrombotic events (including venous thromboembolism, pulmonary thromboembolism, deep vein thrombosis, ischemic stroke, and myocardial infarction), and (2) hemorrhagic events (including intracerebral hemorrhage and hemorrhagic complications of any type) in COVID-19 patients under no anticoagulation versus those under different doses of LMWH/F. The same outcomes were also assessed in COVID-19 patients receiving: (1) prophylactic versus higher than prophylactic LMWH/F dose (i.e., intermediate or therapeutic); (2) prophylactic versus therapeutic LMWH/F; (3) prophylactic versus intermediate LMWH/F; (4) intermediate versus therapeutic LMWH/F; and (5) lower than therapeutic LMWH/F (i.e., intermediate or prophylactic) versus therapeutic LMWH/F.
We evaluated for potential differences in demographics between groups of COVID-19 patients stratified by anticoagulation status. We also assessed the previous outcomes of interest in subgroup analyses after stratification according to the treatment setting of COVID-19 (i.e., outpatient, inpatient medical ward or ICU). Finally, we performed a secondary analysis to assess for potential differences between study designs (RCTs versus observational studies).
Statistical analysis and measures of effects
In the current meta-analysis, the aforementioned outcomes of interest were dichotomous variables. The random-effects model of meta-analysis (DerSimonian and Laird) 13 was used to estimate individual study effects for each association. The random-effect model, since a different underlying true effect was assumed for each study and we aimed to provide more generalizable hypotheses for the population.13,14 Pairwise comparisons between COVID-19 patients with LMWH/F and COVID-19 controls (according to the pre-defined between-group comparisons based on LMWH/F dose) are reported using odds ratios (ORs) and corresponding 95% confidence intervals (95% CI), as measures of effects.
As per the Cochrane Handbook for Systematic Reviews of Interventions, 15 heterogeneity between included studies was assessed using the Cochran Q and I² statistics. For the qualitative interpretation of heterogeneity, I2 values > 50% and values >75% were considered to represent substantial and considerable heterogeneity, respectively. 15 The significance level for the Q statistic was set at 0.1.
Publication bias across individual studies was graphically assessed for the primary outcome, when more than five studies were included in each analysis, using both funnel plot inspection and the Egger’s linear regression test, 16 and the equivalent z-test for each pooled estimate with a two-tailed p value < 0.05 was considered statistically significant.
All statistical analyses were performed using the Cochrane Collaboration’s Review Manager (RevMan 5.3) Software Package (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014), the Open MetaAnalyst 17 and R software version 3.5.0 (package: metafor).
Results
Literature search and included studies
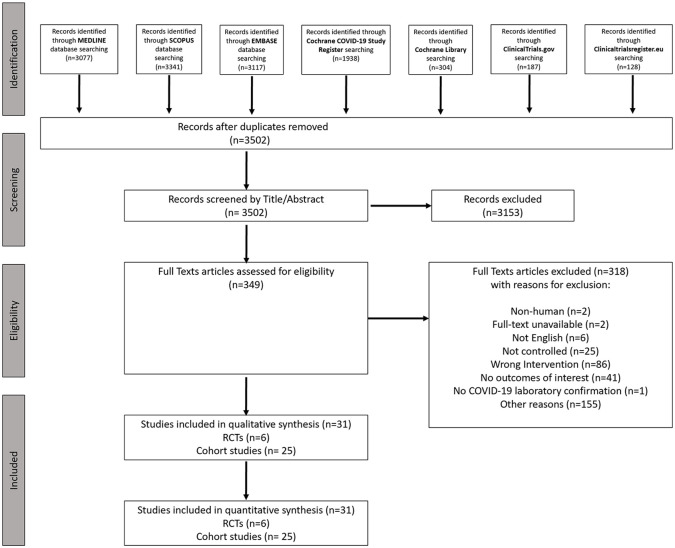
The records retrieved by the systematic search in each electronic database are shown in Figure 1. After excluding duplicates and out-of-scope articles based on title/abstract, 349 potentially eligible records for inclusion were selected for full-text reading. After full-text reading, 318 articles were excluded, leading to the inclusion of 31 eligible studies18–48 (6 RCTs and 25 cohort studies) with a total of 11,430 COVID-19 patients (Table 1). Only hospitalized patients were included in these studies.
Figure 1.
Systematic review flow chart.
Table 1.
Characteristics of included studies.
| First author (Ref.) | Study location | Study period | Study design | Setting of population of interest | N of COVID-19 patients | Outcomes of interest | Type of anticoagulation | LMWH/F dose | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Prophylactic | Intermediate | Therapeutic | ||||||||
| Randomized-controlled trials | ||||||||||
| ACTION 18 | Brazil | 24 June 2020 to 26 February 2021 | RCT | ICU or medical ward | 285 | All Hemorrhagic Events | LMWH | ⊕ | ⊖ | ⊕ |
| BEMICOP 21 | Spain | October 2020 to May 2021 | RCT | Medical ward | 65 | Mortality | LMWH | ⊕ | ⊖ | ⊕ |
| HEP-COVID 30 | US | 8 May 2020 to 14 May 2021 | RCT | ICU or medical ward | 253 | Mortality; VTE; PE; DVT; IS; MI; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊕ |
| INSPIRATION 31 | Iran | 29 July to 19 November 2020 | RCT | ICU | 562 | Mortality; VTE; IS; MI; ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊖ |
| Oliynyk et al. 38 | Ukraine | 1 July 2020 to 1 March 2021 | RCT | ICU | 84 | Mortality | LMWH | ⊕ | ⊖ | ⊕ |
| Perepu et al. 42 | US | 26 April 2020 to 6 January 2021 | RCT | ICU or medical ward | 173 | Mortality; VTE; IS; MI; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊖ |
| Observational studies | ||||||||||
| Albani et al. 19 | Italy | 20 February to 10 May 2020 | Retrospective cohort | ICU or medical ward | 1403 | Mortality: VTE; PE; IS; MI; All Hemorrhagic Events | LMWH | ⊕ | ⊖ | ⊕ |
| Avruscio et al. 20 | Italy | 4 March to 30 April 2020 | Prospective cohort | ICU or medical ward | 85 | VTE | LMWH/F | ⊕ | ⊖ | ⊕ |
| Canoglu et al. 22 | Turkey | 11 March to 30 April 2020 | Retrospective cohort | ICU or medical ward | 154 | Mortality | LMWH | ⊕ | ⊖ | ⊕ |
| Copur et al. 23 | Turkey | 11 March to 11 April 2020 | Retrospective cohort | Medical ward | 115 | Mortality | LMWH | ⊕ | ⊖ | ⊕ |
| Elmelhat et al. 24 | Dubai | March–June 2020 | Retrospective cohort | ICU or medical ward | 59 | Mortality; ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊖ | ⊕ |
| Espallargas et al. 25 | Spain | 18 March to 11 April 2020 | Retrospective cohort | ICU or medical ward | 47 | PE | LMWH | ⊕ | ⊕ | ⊕ |
| Falcone et al. 26 | Italy | 4 March to 30 April 2020 | Prospective cohort | ICU or medical ward | 315 | Mortality: ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊖ | ⊕ |
| Gonzalez-Porras et al. 27 | Spain | 1 March to 7 April 2020 | Retrospective cohort | Medical ward | 690 | Mortality; PE; DVT; IS; MI; ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊕ |
| Grandone et al. 28 | Italy | 03 March to 30 August 2020 | Retrospective cohort | ICU or medical ward | 264 | All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊕ |
| Hamilton et al. 29 | UK | 31 March to 16 November 2021 | Retrospective cohort | ICU | 58 | PE | LMWH | ⊕ | ⊕ | ⊖ |
| Jimenez-Guiu et al. 32 | Spain | April 2020 | Prospective cohort | Medical ward | 57 | DVT; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊕ |
| Jiménez-Soto et al. 33 | Mexico | 12 March to 15 July 2020 | Retrospective cohort | ICU or medical ward | 321 | Mortality; PE; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊕ |
| Jonmarker et al. 34 | Sweden | March to April 2020 | Retrospective cohort | ICU | 152 | Mortality; VTE; PE; DVT; IS; ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊕ |
| Martinelli et al. 35 | Italy | 9 March to 7 April 2020 | Retrospective cohort | ICU or medical ward | 278 | Mortality; VTE: PE; DVT; ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊖ | ⊖ |
| Mennuni et al. 36 | Italy | 20 February to 12 May 2020 | Retrospective cohort | ICU or medical ward | 436 | Mortality: VTE; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊕ |
| Nadkarni et al. 37 | USA | 1 March to 30 April 2020 | Retrospective cohort | ICU or medical ward | 2202 | All Hemorrhagic Events | LMWH | ⊕ | ⊖ | ⊕ |
| Paolisso et al. 39 | Italy | 1 March to 10 April 2020 | Retrospective cohort | ICU or medical ward | 450 | Mortality; IS; MI; ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊖ |
| Pavoni et al. 40 | Italy | NR | Retrospective cohort | ICU | 42 | Mortality; PE; DVT | LMWH | ⊖ | ⊕ | ⊕ |
| Perazzo et al. 41 | Italy | March to April 2020 | Retrospective cohort | Medical ward | 16 | Mortality | LMWH | ⊕ | ⊕ | ⊖ |
| Pieralli et al. 43 | Italy | 21 March to 25 May 2020 | Prospective cohort | Medical ward | 222 | DVT | LMWH/F | ⊕ | ⊕ | ⊕ |
| Qin et al. 44 | China | 10 Jan to 28 Feb 2020 | Retrospective cohort | ICU or medical ward | 749 | Mortality; All Hemorrhagic Events | LMWH | ⊕ | ⊖ | ⊕ |
| Shen et al. 45 | China | 26 January to 26 March 2020 | Retrospective cohort | ICU or medical ward | 525 | Mortality: ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊖ | ⊕ |
| Stessel et al. 46 | Belgium | 13 March to 20 April 2020 | Retrospective cohort | ICU | 72 | Mortality; VTE; ICH; All Hemorrhagic Events | LMWH | ⊕ | ⊕ | ⊖ |
| Trigonis et al. 47 | USA | 23 March to 8 April 2020 | Retrospective cohort | ICU | 45 | DVT | LMWH | ⊕ | ⊕ | ⊖ |
| Ugur et al. 48 | Turkey | 12 March to 17 April 2020 | Retrospective cohort | Medical ward | 1251 | Mortality | LMWH | ⊕ | ⊕ | ⊕ |
DVT: deep vein thrombosis; ICH: intracranial hemorrhage; ICU: intensive care unit; IS: ischemic stroke; LMWH: low molecular weight heparin; LMWH/F: low molecular weight heparin or fondaparinux; MI: myocardial infarction; NR: not reported; PE: pulmonary embolism; and VTE: venous thromboembolism.
Quality control of included studies and risk of bias assessment
The risk of bias in included RCTs was assessed by the Cochrane Collaboration tool (RoB 2) 11 and is shown in Supplementary Figures S1 and S2. Overall, the included RCTs presented low risk of bias in most individual domains, with the exception of high risk of performance and detection bias, since blinding was not achieved in the majority of the studies. The risk of bias of the included observational studies was assessed by the Newcastle-Ottawa scale 12 (Supplementary Table S2). The overall score was 194 of 225 (86%), which is indicative of moderate quality. Funnel plot inspection revealed low risk of publication bias, with the exception of the comparison of prophylactic versus intermediate LMWH/F, which presented evidence of publication bias, possibly due to small study effects (Supplementary Figures S3–S7).
Demographics of study population
The mean age of included COVID-19 patients was 63.40 years (95% CI: 59.78–67.03; 23 studies; I2 = 99.1%; p for Cochran Q < 0.0001; Supplementary Figure S8). No clinically significant difference in mean age was identified between COVID-19 patients receiving: a) no anticoagulation versus any LMWH/F (mean difference (MD) = –0.49; 95% CI: –4.02, 3.05; 4 studies; I2 = 87%; p for Cochran Q < 0.0001; Supplementary Figure S9); b) prophylactic versus higher than prophylactic LMWH/F (MD = 0.22; 95% CI: –0.96, 1.39; 14 studies; I2 = 30%; p for Cochran Q = 0.14; Supplementary Figure S10); c) prophylactic versus therapeutic LMWH/F (MD = –2.16; 95% CI: –4.18, –0.15); 6 studies; I2 = 21%; p for Cochran Q = 0.27; Supplementary Figure S11); d) prophylactic versus intermediate LMWH/F (MD = 1.50; 95% CI:–0.43, 3.42; 9 studies; I2 = 49%; p for Cochran Q = 0.05; Supplementary Figure-S12); e) intermediate versus therapeutic LMWH/F (MD = –4.40; 95% CI: –8.43,0.37; 4 studies; I2 = 68%; p for Cochran Q = 0.02; Supplementary Figure S13); and f) lower than therapeutic versus therapeutic LMWH/F (MD = –1.69; 95% CI: –4.11,0.73; 7 studies; I2 = 58%; p for Cochran Q = 0.03; Supplementary Figure S14). The pooled proportion of men out of the total population included in this meta-analysis was 60.6% (95% CI: 56.7–64.5%), with no sex differences noted, stratified by different LMWH/F dosing schemes (Supplementary Figures S15–S21).
Effects on mortality
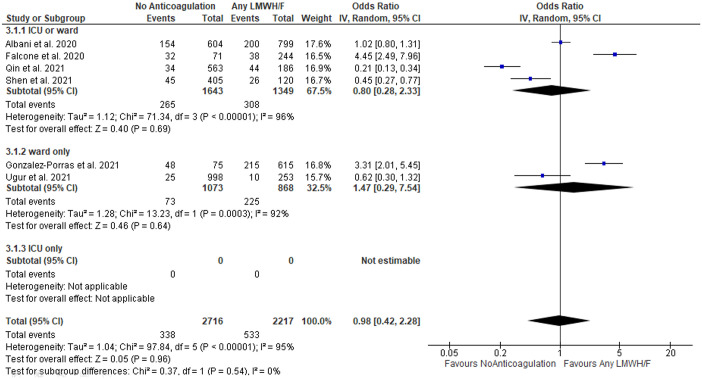
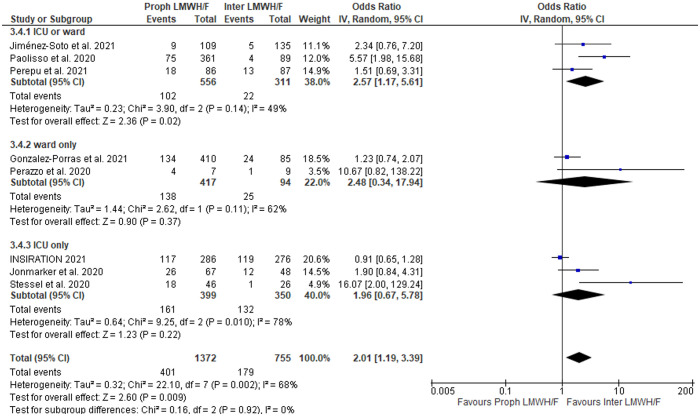
A summary of findings is shown in Table 2. No association between LMHW/F administration and mortality was detected in COVID-19 patients without anticoagulation versus any LMWH/F (OR = 0.98; 95% CI: 0.42–2.28; 6 studies; I2 = 95%; p for Cochran Q: < 0.00001; Figure 2), with the limitation that only 6 studies with significant heterogeneity between reported outcomes were identified. There was also no difference in mortality among patients treated with prophylactic LMWH/F versus higher than prophylactic LMWH/F (OR = 1.28; 95% CI: 0.84–1.94; 16 studies; I2 = 78%; p for Cochran Q < 0.00001; Supplementary Figure S22) or among patients treated with prophylactic versus therapeutic LMWH/F (OR = 0.82; 95% CI: 0.39–1.76; 9 studies; I2 = 83%; p for Cochran Q < 0.00001; Supplementary Figure S23). Conversely, the risk for all-cause mortality was higher in patients receiving prophylactic versus intermediate LMWH/F (OR = 2.01; 95% CI: 1.19–3.39; 8 studies; I2 = 68%; p for Cochran Q = 0.002; Figure 3). The corresponding mortality rates were comparable between patients undergoing intermediate compared with therapeutic LMWH/F (OR = 0.60; 95% CI: 0.26–1.41; 4 studies; I2 = 55%; p for Cochran Q = 0.08; Supplementary Figure S24), as well as between patients treated with lower than therapeutic versus therapeutic LMWH/F (OR = 0.79; 95% CI: 0.42–1.47; 11 studies; I2 = 80%; p for Cochran Q < 0.00001; Supplementary Figure S25).
Table 2.
Summary of findings on mortality, thrombotic and hemorrhagic events analyses.
| Outcome | No Anticoagulation versus
Any LMWH/F |
Prophylactic LMWH/F versus Higher than Prophylactic LMWH/F | Prophylactic LMWH/F versus
Therapeutic LMWH/F |
Prophylactic LMWH/F versus
Intermediate LMWH/F |
Intermediate LMWH/F versus
Therapeutic LMWH/F |
Lower than Therapeutic versus
Therapeutic LMWH/F |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N of studies | Odds ratio (95% CI) | I2, p (CQT) | N of studies | Odds ratio (95% CI) | I2, p (CQT) | N of studies | Odds ratio (95% CI) | I2, p (CQT) | N of studies | Odds ratio (95% CI) | I2, p (CQT) | N of studies | Odds ratio (95% CI) | I2, p (CQT) | N of studies | Odds ratio (95% CI) | I2, p (CQT) | |
| Mortality | 6 | 0.98 (0.42–2.28) | 95%, < 0.00001 | 16 | 1.28 (0.84–1.94) | 78%, <0.00001 | 9 | 0.82 (0.39–1.76) | 83%, <0.00001 | 8 | 2.01 (1.19–3.39) | 68%, 0.002 | 4 | 0.60 (0.26–1.41) | 55%, 0.08 | 11 | 0.79 (0.42–1.47) | 80%, < 0.00001 |
| Thrombotic events | ||||||||||||||||||
| Venous thromboembolism | – | – | – | 8 | 0.67 (0.30–1.47) | 78%, <0.0001 | 3 | 83%, 0.003 | 4 | 1.24 (0.66–2.35) | 32%, 0.22 | – | – | – | 3 | 1.07 (0.08–14.21) | 88%, 0.0003 | |
| Pulmonary embolism | – | – | – | 7 | 0.55 (0.18–1.70) | 73%, 0.001 | 5 | 0.62 (0.11–3.68) | 77%, 0.002 | 5 | 0.79 (0.31–2.00) | 38%, 0.17 | 5 | 1.49 (0.49–4.54) | 15%, 0.32 | 7 | 0.78 (0.20–3.10) | 77%, 0.0002 |
| Deep vein thrombosis | – | – | – | 6 | 1.24 (0.73–2.11) | 0%, 0.48 | 3 | 2.09 (0.35–12.36) | 0%, 0.91 | 5 | 1.14 (0.42–3.07) | 29%, 0.23 | 3 | 1.01 (0.05–22.19) | 76%, 0.01 | 4 | 2.77 (1.32–5.80) | 0%, 0.99 |
| Ischemic stroke | – | – | – | 6 | 1.03 (0.37–2.91) | 0%, 0.44 | 3 | 1.22 (0.33–4.50) | 0%, 0.37 | 5 | 0.87 (0.23–3.33) | 0%, 0.49 | – | – | – | 4 | 1.05 (0.32–3.41) | 0%, 0.65 |
| Myocardial infarction | – | – | – | 5 | 0.59 (0.24–1.45) | 0%, 0.41 | – | – | – | 4 | 0.54 (0.08–3.67) | 52%, 0.12 | – | – | – | 3 | 1.02 (0.19–5.31) | 37%, 0.21 |
| Hemorrhagic events | ||||||||||||||||||
| Intracranial hemorrhage | – | – | – | 9 | 0.74 (0.08–6.98) | 34%, 0.22 | – | – | – | 5 | 0.45 (0.05–4.51) | 37%, 0.20 | – | – | – | 3 | 1.12 (0.12–10.25) | 0%, 0.72 |
| Any hemorrhage | 7 | 0.57 (0.25–1.28) | 53%, 0.05 | 16 | 0.37 (0.21–0.64) | 54%, 0.005 | 10 | 0.30 (0.14–0.64) | 56%, 0.01 | 8 | 0.63 (0.32–1.24) | 49%, 0.06 | 2 | 0.50 (0.03–8.20) | 88%, 0.003 | 11 | 0.30 (0.15–0.62) | 57%, 0.01 |
CI: confidence interval; CQT: Cochran’s Q test; and LMWH/F, low molecular weight heparin or fondaparinux.
Figure 2.
Forest plot presenting the odds ratio for all-cause mortality between patients treated with no versus any anticoagulation with low molecular weight heparin and/or fondaparinux.
Figure 3.
Forest plot presenting the odds ratio for all-cause mortality between patients who received prophylactic versus intermediate anticoagulation with low molecular weight heparin and/or fondaparinux.
Effects on thrombotic events
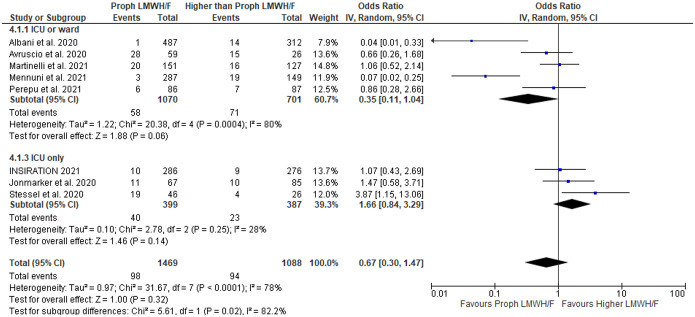
Concerning thrombotic events in COVID-19 patients, no association was found between intensity of LMWH/F and risk of venous thromboembolism (VTE), defined as the composite of deep vein thrombosis or pulmonary embolism, in patients receiving: a) prophylactic compared to higher than prophylactic LMWH/F (OR = 0.67; 95% CI: 0.30–1.47; 8 studies; I2 = 78%; p for Cochran Q < 0.0001; Figure 4); b) prophylactic compared to therapeutic LMWH/F (OR = 0.59; 95% CI: 0.06–6.01; 3 studies; I2 = 83%; p for Cochran Q = 0.003; Supplementary Figure S26); c) prophylactic compared to intermediate LMWH/F (OR = 1.24; 95% CI: 0.66–2.35; 4 studies; I2 = 32%; p for Cochran Q = 0.22; Supplementary Figure S27); and d) lower than therapeutic versus therapeutic LMWH/F (OR = 1.07; 95% CI: 0.08–14.21; 3 studies; I2 = 88%; p for Cochran Q = 0.0003; Supplementary Figure S28).
Figure 4.
Forest plot presenting the odds ratio for venous thromboembolism between patients who received prophylactic versus higher than prophylactic anticoagulation with low molecular weight heparin and/or fondaparinux.
Similarly, the odds for pulmonary embolism (PE) were comparable between patients receiving: a) prophylactic compared to higher than prophylactic LMWH/F (OR = 0.55; 95% CI: 0.18–1.70; 7 studies; I2 = 73%; p for Cochran Q = 0.001; Supplementary Figure S29); b) prophylactic compared to therapeutic LMWH/F (OR = 0.62; 95% CI: 0.11–3.68; 5 studies; I2 = 77%; p for Cochran Q = 0.002; Supplementary Figure S30); c) prophylactic compared to intermediate LMWH/F (OR = 0.79; 95% CI: 0.31–2.00; 5 studies; I2 = 38%; p for Cochran Q = 0.17; Supplementary Figure S31); d) intermediate versus therapeutic LMWH/F (OR = 1.49; 95% CI: 0.49–4.54; 5 studies; I2 = 15%; p for Cochran Q = 0.32; Supplementary Figure S32); and e) lower than therapeutic compared to therapeutic LMWH/F (OR = 0.78; 95% CI: 0.20–3.10; 7 studies; I2 = 77%; p for Cochran Q = 0.0002; Supplementary Figure S33).
Regarding deep vein thrombosis (DVT), we detected no associations between the intensity of anticoagulation and the risk for DVT in patients receiving: a) prophylactic versus higher than prophylactic LMWH/F (OR: 1.24; 95% CI 0.73–2.11; 6 studies; I2 0%; p for Cochran Q = 0.48; Supplementary Figure S34); b) prophylactic versus therapeutic LMWH/F (OR: 2.09; 95% CI 0.35–12.36; 3 studies; I2 0%; p for Cochran Q = 0.91; Supplementary Figure S35); c) prophylactic versus intermediate LMWH/F (OR 1.14; 95% CI 0.42–3.07; 5 studies; I2 29%; p for Cochran Q = 0.23; Supplementary Figure S36); and d) intermediate versus therapeutic LMWH/F (OR: 1.01; 95% CI 0.05–22.19; 3 studies; I2 76%; p for Cochran Q = 0.01; Supplementary Figure S37). When lower than therapeutic LMWH/F was compared to therapeutic LMWH/F, a higher rate of DVT was recorded in the lower-than-therapeutic LMWH/F group (OR = 2.77; 95% CI: 1.32–5.80; 4 studies; I2 = 0%; p for Cochran Q = 0.99; Supplementary Figure S38), which was mostly driven by the results of HEP-COVID. 30
No increased risk of ischemic stroke was detected in patients receiving: a) prophylactic versus higher than prophylactic LMWH/F (OR = 1.03; 95% CI: 0.37–2.91; 6 studies; I2 = 0%; p for Cochran Q = 0.44; Supplementary Figure S39); b) prophylactic versus therapeutic LMWH/F (OR = 1.22; 95% CI: 0.33–4.50; 3 studies; I2 = 0%; p for Cochran Q = 0.37; Supplementary Figure S40); c) prophylactic versus intermediate LMWH/F (OR = 0.87; 95% CI: 0.23–3.33; 5 studies; I2 = 0%; p for Cochran Q = 0.49; Supplementary Figure S41); and d) lower than therapeutic versus therapeutic LMWH/F (OR = 1.05; 95% CI: 0.32–3.41; 4 studies; I2 = 0%; p for Cochran Q = 0.65; Supplementary Figure S42).
We detected no associations between the intensity of LMWH/F and the risk for myocardial infarction in patients receiving a) prophylactic versus higher than prophylactic LMWH/F (OR: 0.59; 95% CI 0.24–1.45; 5 studies; I2 0%; p for Cochran Q = 0.41; Supplementary Figure S43); b) prophylactic versus intermediate LMWH/F (OR: 0.54; 95% CI 0.08–3.67; 4 studies; I2 52%; p for Cochran Q = 0.12; Supplementary Figure S44); and c) lower than therapeutic versus therapeutic LMWH/F (OR: 1.02; 95% CI 0.19–5.31; 3 studies; I2 37%; p for Cochran Q = 0.21; Supplementary Figure S45).
Effects on hemorrhagic events
Concerning hemorrhagic events in COVID-19 patients, no associations were detected between intensity of LMWH/F and the risk for intracerebral hemorrhage (ICH) when comparing: a) prophylactic versus higher than prophylactic LMWH/F (OR = 0.74; 95% CI: 0.08–6.98; 9 studies; I2 = 34%; p for Cochran Q = 0.22; Supplementary Figure S46); b) prophylactic versus intermediate LMWH/F (OR = 0.45; 95% CI: 0.05–4.51; 5 studies; I2 = 37%; p for Cochran Q = 0.20; Supplementary Figure S47); and c) lower than therapeutic versus therapeutic LMWH/F (OR = 1.12; 95% CI: 0.12–10.25; 3 studies; I2 = 0%; p for Cochran Q = 0.72; Supplementary Figure S48).
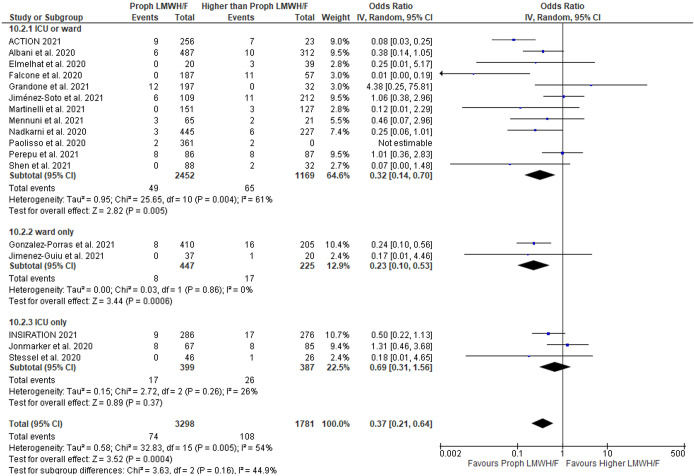
With respect to the composite outcome of hemorrhagic complications of any type, no differences were noted between patients receiving no anticoagulation versus any LMWH/F (OR = 0.57; 95% CI: 0.25–1.28; 7 studies; I2 = 53%; p for Cochran Q = 0.05; Supplementary Figure S49). However, we found a lower risk for hemorrhagic complications in patients undergoing prophylactic LMWH/F compared to higher doses of LMWH/F (OR = 0.37; 95% CI: 0.21–0.64; 16 studies; I2 = 54%; p for Cochran Q = 0.005; Figure 5). Similarly, the risk for any hemorrhagic complications was lower in patients treated with prophylactic versus therapeutic LMWH/F (OR = 0.30; 95% CI: 0.14–0.64; 10 studies; I2 = 56%; p for Cochran Q = 0.01; Supplementary Figure S50), but not in patients treated with prophylactic versus intermediate LMWH/F (OR = 0.63; 95% CI: 0.32–1.24; 8 studies; I2 = 49%; p for Cochran Q = 0.06; Supplementary Figure S51) or in patients treated with intermediate versus therapeutic LMWH/F (OR = 0.50; 95% CI: 0.03–8.20; 3 studies; I2 = 88%; p for Cochran Q = 0.003; Supplementary Figure S52). Patients treated with lower than therapeutic compared to therapeutic LMWH/F had a lower likelihood of presenting hemorrhagic complications (OR = 0.30; 95% CI: 0.15–0.62; 11 studies; I2 = 57%; p for Cochran Q = 0.01; Supplementary Figure S53).
Figure 5.
Forest plot presenting the odds ratio for hemorrhagic events between patients who received prophylactic versus higher than prophylactic anticoagulation with low molecular weight heparin and/or fondaparinux.
Secondary analysis
A secondary analysis of the same outcomes of interest (mortality, thrombotic events, and hemorrhagic events) was performed stratifying by different study design (RCTs versus observational studies) to assess for potential differences. The results of this analysis are shown in Supplementary Table S3. Significant subgroup differences between study designs were disclosed in the analysis of mortality among patients receiving prophylactic versus intermediate LMWH/F (p for subgroup differences = 0.01; Supplementary Figure S54). The effect of prophylactic compared to intermediate LMWH/F on mortality was mostly driven by observational studies (OR = 2.85; 95% CI: 1.41–5.76; 6 studies; I2 = 61%; p for Cochran Q = 0.03), whereas no difference was disclosed when RCTs were evaluated (OR = 1.03; 95% CI: 0.68–1.56; 2 studies; I2 = 24%; p for Cochran Q = 0.25). Significant differences also emerged in the analysis of all hemorrhagic events between patients on prophylactic versus therapeutic LMWH/F; however, both subgroups pointed to the same direction (for RCTs: OR = 0.08; 95% CI: 0.03–0.25; 1 study; for observational studies: OR = 0.37; 95% CI: 0.17–0.80; 9 studies; I2 = 45%; p for Cochran Q = 0.07; p for subgroup differences = 0.03; Supplementary Figure S55). No other significant differences between the two subgroups were disclosed.
Discussion
Our living systematic review and meta-analysis provides an overview of the currently available data regarding the effects of different dosing regimens of LMWH/F on mortality risk as well as the risk of arterial/venous thrombotic events and hemorrhagic complications in confirmed COVID-19 cases.
The risk of all-cause mortality was higher in patients receiving prophylactic compared to intermediate doses of LMWH/F. However, this effect was mostly driven by observational data. Similar mortality rates were observed during the comparisons of (a) prophylactic versus higher than prophylactic LMWH/F; (b) prophylactic versus therapeutic LMWH/F; (c) intermediate versus therapeutic LMWH/F; and (d) lower than therapeutic versus therapeutic LMWH/F. These results may be partly explained by the higher rates of hemorrhagic complications observed in patients treated with higher doses of LMWH/F, whereas the prophylactic doses seem to be equally safe when compared with intermediate LMWH/F.
In addition, no significant difference on mortality risk was identified when we compared populations under no anticoagulation versus any dose of LMWH/F. This paradox could be possibly explained by the fact that the majority of the eligible studies included hospitalized patients who often require anticoagulation. 8 In addition, type-II errors cannot be excluded, since in our review, only 6 studies including patients not on anticoagulation were meta-analyzed, while these studies suffered from significant heterogeneity in reported results.26,45 Thus, no robust conclusions can be drawn for our primary outcome given the paucity of available studies examining the benefits of LMWH/F versus no anticoagulation, especially in mild COVID-19 outpatients.
Interestingly, we demonstrated that the risk for all thrombotic events remained similar among the subgroups of different LMWH/F dosing schemes. This phenomenon may indicate that anticoagulants, and especially heparin-derived agents, avail the course of COVID-19 disease through pathophysiologic mechanisms that lie beyond their antithrombotic properties. Indeed, preclinical studies have shown that unfractionated heparin exerts both anti-inflammatory and direct antiviral effects against SARS-CoV-2. 49 Heparin binds irreversibly to the spike-protein (S-protein) as a competitive inhibitor and abrogates the viral entry into the host cells.49–51 However, shorter-length heparins like the LMWHs have shown lower affinity to S-protein, and therefore, possibly exert little or maybe no direct antiviral effects against SARS-CoV-2, compared to unfractionated heparin. 50 Further studies are needed to explore the antiviral properties of heparin-derived antithrombotics in COVID-19 patients. Besides antiviral activity, it has been repeatedly demonstrated that unfractionated heparin dampens the inflammation in the vasculature and/or respiratory tract by (1) interacting with proinflammatory molecules, (2) neutralizing the extracellular cytotoxic histones, (3) inhibiting the heparanase activity (thus reducing endothelial leakage), and (4) abolishing the adhesion and trafficking of inflammatory cells.49,52 Therefore, unfractionated heparin and heparin-derived drugs may potentially have pleiotropic beneficial effects on COVID-19 disease, by targeting the activation of coagulation cascade, the hyper-inflammatory response, and the virus itself. 53 This phenomenon, might also explain that effects of anticoagulation were similar in patients hospitalized in the ICU and in non-ICU wards, as observed in our analysis.
With regard to thrombotic events, we found no associations between VTE, DVT, PE, stroke, and myocardial infarction risk with the use or the intensity of LMWH/F administered. This could be possibly explained by the small number and the design of the included studies, leading to substantial heterogeneity. Future analyses including well-designed RCTs are needed before we can draw robust conclusions on the risk of thrombotic events among different dosing schemes of LMWH/F.
Regarding hemorrhagic complications, we demonstrated a lower risk among patients undergoing prophylactic LMWH/F compared to therapeutic doses, as reasonably anticipated. However, there was no association between the risk of any hemorrhagic complications and the administration of no anticoagulation versus any LMWH/F, prophylactic versus intermediate LMWH/F, and intermediate versus therapeutic LMWH/F. Similarly, we found no associations between the LMWH/F dosing (including no administration of anticoagulation) and the risk of ICH. Despite that intermediate doses of LMWH/F seem equally safe with prophylactic LMWH/F regarding hemorrhagic complications, future RCTs are warranted for the assessment of the true impact of different LMWH/F dosing regimens on the hemorrhagic complications and the cost-benefit balance in patients with COVID-19 infection.
Our meta-analysis focuses only on the effects of LMWH/F on all-cause mortality, arterial and venous thromboembolic events and hemorrhagic phenomena in patients with COVID-19. Results from previously published systematic reviews and meta-analyses examining the mortality risk among COVID-19 receiving different doses of anticoagulation are in line with our results, although they have also included other types of antithrombotics. Indeed, Kamel et al. 54 in their meta-analysis reported that in-hospital anticoagulation was associated with a beneficial effect on mortality, while Wijaya et al. 55 found a tendency toward reduced mortality among mechanically ventilated patients who received therapeutic anticoagulation. Anticoagulation was also associated with lower mortality rate in the systematic review and meta-analysis conducted by Parisi et al. 56 However, the aforementioned meta-analyses had analyzed all types of antithrombotic agents, including oral anticoagulants and unfractionated heparin, while we aimed to exclusively evaluate the effectiveness and safety of LMWH/F. In addition, our meta-analysis included a larger number of studies.
The main strength of the current meta-analysis is the fact that it has been conducted by a multidisciplinary team, using robust methodological pipeline according to an a priori established, PRISMA-based protocol. In addition, we utilized a robust and thorough literature search that was performed by three independent reviewers. Compared to previously published meta-analyses, that included studies with any type of antithrombotic agents, our study focuses only on the effects of LMWH/F, which are the most commonly and widely applied anticoagulants in clinical practice, especially among hospitalized patients due to their ease of use (once or twice daily) and minimal monitoring (compared to unfractionated heparin). Finally, as COVID-19 research is a continuously changing landscape, the living nature of this meta-analysis will allow emerging evidence to shape new results on the effects of LMWH and fondaparinux on mortality risk, thrombotic events, and hemorrhagic complications on a regular basis.
Certain limitations of this report need to be acknowledged. First, there is a lack of a standardized definition of the ‘intermediate dose’ of LMWH/F; although clinicians use this term empirically for doses that are higher than prophylactic and lower than therapeutic, it is important to follow a universal definition provided by international stakeholders and specified further for each agent by the manufacturers themselves. Second, there are insufficient data on the risks and benefits of anticoagulation administration in mild COVID-19 who do not require hospitalization. As the majority of COVID-19 patients remain outpatients, it will be useful to conclude on the role of LMWH/F in this patient population, especially in the context of pleiotropic effects of unfractionated heparin and heparin-derived drugs. Furthermore, our results should be interpreted cautiously due to the moderate quality and the heterogeneity of the included studies. Pooling together data from RCTs and observational studies and recruiting diverse COVID-19 patients of different disease severity who were treated in different settings are acknowledged as potential limitations that led to further analyses exploring for potential differences between subgroups. We expect that with the emergence of data from well-designed large-scale RCTs, these methodological limitations will soon be mitigated.
Conclusion
Our meta-analysis shows that the risk of all-cause mortality may be reduced in patients who receive intermediate doses of LMWH/F compared to prophylactic doses, based on observational data. Although anticoagulation constitutes a common clinical practice among patients with COVID-19 disease globally, especially among those who require hospitalization, robust evidence from ongoing RCTs defining their risks and benefits as well as the appropriate dosing scheme is warranted. We expect that our living systematic review and meta-analysis will shed light on the role and impact of LMWH/F in COVID-19 disease, as new data emerge from well-designed, high-quality trials.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221099472 for Effects of low molecular weight heparin and fondaparinux on mortality, hemorrhagic and thrombotic complications in COVID-19 patients by Paraskevi C. Fragkou, Lina Palaiodimou, Maria Ioanna Stefanou, Aristeidis H. Katsanos, Vaia Lambadiari, Dimitrios Paraskevis, Elisabeth Andreadou, Dimitra Dimopoulou, Christina Zompola, Panagiotis Ferentinos, Theodoros I. Vassilakopoulos, Anastasia Kotanidou, Petros P. Sfikakis, Sotirios Tsiodras and Georgios Tsivgoulis in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
Ethics approval and consent to participate: This study did not require an ethical board approval or written informed consent by the patients according to the study design (systematic review and meta-analysis).
Consent for publication: Not applicable.
Author contributions: Paraskevi Fragkou: Conceptualization; Data curation; Investigation; Methodology; Writing – original draft.
Lina Palaiodimou: Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft.
Maria Ioanna Stefanou: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Aristeidis Katsanos: Writing – review & editing.
Christina Zompola: Writing – review & editing.
Dimitra Dimopoulou: Writing – review & editing.
Elisabeth Andreadou: Writing – review & editing.
Dimitrios Paraskevis: Writing – review & editing.
Anastasia Kotanidou: Writing – review & editing.
Theodoros Vassilakopoulos: Writing – review & editing.
Panagiotis Ferentinos: Writing – review & editing.
Vaia Lambadiari: Writing – review & editing.
Petros Sfikakis: Writing – review & editing.
Sotirios Tsiodras: Conceptualization; Writing – review & editing.
Georgios Tsivgoulis: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
ORCID iDs: Lina Palaiodimou  https://orcid.org/0000-0001-7757-609X
https://orcid.org/0000-0001-7757-609X
Maria Ioanna Stefanou  https://orcid.org/0000-0002-2305-6627
https://orcid.org/0000-0002-2305-6627
Georgios Tsivgoulis  https://orcid.org/0000-0002-0640-3797
https://orcid.org/0000-0002-0640-3797
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest.
Protocol registration: The protocol of this systematic review and meta-analysis has been registered to the International Prospective Register of Ongoing Systematic Reviews PROSPERO (Registration number: CRD42021229771)
Availability of data and materials: The data that support the findings of this study are available from the corresponding author (GT), upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Paraskevi C. Fragkou, First Department of Critical Care Medicine and Pulmonary Services, School of Medicine, National and Kapodistrian University of Athens, Evangelismos Hospital, Athens, Greece.
Lina Palaiodimou, Second Department of Neurology, School of Medicine, National and Kapodistrian University of Athens, ‘Attikon’ University Hospital, Athens, Greece.
Maria Ioanna Stefanou, Second Department of Neurology, School of Medicine, National and Kapodistrian University of Athens, ‘Attikon’ University Hospital, Athens, Greece.
Aristeidis H. Katsanos, Division of Neurology, Population Health Research Institute, McMaster University, Hamilton, ON, Canada
Vaia Lambadiari, Second Department of Internal Medicine Research Unit and Diabetes Center, School of Medicine, National and Kapodistrian University of Athens, ‘Attikon’ University Hospital, Athens, Greece.
Dimitrios Paraskevis, Department of Hygiene, Epidemiology and Medical Statistics, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Elisabeth Andreadou, First Department of Neurology, School of Medicine, National and Kapodistrian University of Athens, Eginition Hospital, Athens, Greece.
Dimitra Dimopoulou, Second Department of Pediatrics, School of Medicine, National and Kapodistrian University of Athens, ‘Panagiotis and Aglaia Kyriakou’ Children’s Hospital, Athens, Greece.
Christina Zompola, Second Department of Neurology, School of Medicine, National and Kapodistrian University of Athens, ‘Attikon’ University Hospital, Athens, Greece.
Panagiotis Ferentinos, Second Department of Psychiatry, School of Medicine, National and Kapodistrian University of Athens, ‘Attikon’ University Hospital, Athens, Greece.
Theodoros I. Vassilakopoulos, Third Department of Critical Care Medicine, School of Medicine, National and Kapodistrian University of Athens, Evgenideio Hospital, Athens, Greece
Anastasia Kotanidou, First Department of Critical Care Medicine and Pulmonary Services, School of Medicine, National and Kapodistrian University of Athens, Evangelismos Hospital, Athens, Greece.
Petros P. Sfikakis, First Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Sotirios Tsiodras, Fourth Department of Internal Medicine, School of Medicine, National and Kapodistrian University of Athens, ‘Attikon’ University Hospital, Athens, Greece.
Georgios Tsivgoulis, Second Department of Neurology, School of Medicine, National and Kapodistrian University of Athens, ‘Attikon’ University Hospital, Rimini 1, Chaidari, Athens 12462, Greece; Department of Neurology, University of Tennessee Health Science Center, Memphis, TN, USA.
References
- 1. Perico L, Benigni A, Casiraghi F, et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol 2021; 17: 46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 2020; 120: 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020; 18: 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elbadawi A, Elgendy IY, Sahai A, et al. Incidence and outcomes of thrombotic events in symptomatic patients with COVID-19. Arterioscler Thromb Vasc Biol 2021; 41: 545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020; 324: 799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shahjouei S, Naderi S, Li J, et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine 2020; 59: 102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institutes of Health (NIH). Antithrombotic therapy| COVID-19 treatment guidelines, 2021, https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/ (accessed 28 November 2021). [PubMed]
- 9. Americal Society of Hematology. COVID-19 and VTE/anticoagulation: frequently asked questions, 2021, https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation (accessed 28 November 2021).
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the prisma 2020 statement. J Clin Epidemiol 2021; 134: 103–112. [DOI] [PubMed] [Google Scholar]
- 11. Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.Ohri.Ca/programs/clinical_epidemiology/oxford.Asp (accessed 28 November 2021).
- 13. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary Clinical Trials 2015; 45: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–114. [DOI] [PubMed] [Google Scholar]
- 15. Deeks JJ, Higgins JPT, Altman DG, et al. Analysing data and undertaking meta-analyses. In: Cochrane handbook for systematic reviews of interventions. Wiley, 2019, pp. 241–284, https://onlinelibrary.Wiley.Com/doi/abs/10.1002/9781119536604.Ch10 (accessed 28 March 2021). [Google Scholar]
- 16. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the gap between methodologists and end-users: R as a computational back-end, https://www.jstatsoft.org/article/view/v049i05
- 18. Lopes RD, de Barros E, Silva PGM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet 2021; 397: 2253–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albani F, Sepe L, Fusina F, et al. Thromboprophylaxis with enoxaparin is associated with a lower death rate in patients hospitalized with SARS-CoV-2 infection. A cohort study. EClinicalMedicine 2020; 27: 100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avruscio G, Camporese G, Campello E, et al. COVID-19 and venous thromboembolism in intensive care or medical ward. Clin Transl Sci 2020; 13: 1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marcos M, Carmona-Torre F, Vidal Laso R, et al. Therapeutic versus prophylactic bemiparin in hospitalized patients with nonsevere COVID-19 pneumonia (BEMICOP Study): an open-label, multicenter, randomized, controlled trial. Thromb Haemost 2022; 122: 295–299. [DOI] [PubMed] [Google Scholar]
- 22. Canoglu K, Saylan B. Therapeutic dosing of low-molecular-weight heparin may decrease mortality in patients with severe COVID-19 infection. Ann Saudi Med 2020; 40: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Copur B, Surme S, Sayili U, et al. Comparison of standard prophylactic and preemptive therapeutic low molecular weight heparin treatments in hospitalized patients with COVID-19. Bratisl Lek Listy 2021; 122: 626–630. [DOI] [PubMed] [Google Scholar]
- 24. Elmelhat A, Elbourai E, Dewedar H, et al. Comparison between prophylactic versus therapeutic doses of low-molecular-weight heparin in severely ill coronavirus disease 2019 patients in relation to disease progression and outcome. Dubai Med J 2020; 3: 162–169. [Google Scholar]
- 25. Espallargas I, Rodríguez Sevilla JJ, Rodríguez Chiaradía DA, et al. CT imaging of pulmonary embolism in patients with COVID-19 pneumonia: a retrospective analysis. Eur Radiol 2021; 31: 1915–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falcone M, Tiseo G, Barbieri G, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis 2020; 7: ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonzalez-Porras JR, Belhassen-Garcia M, Lopez-Bernus A, et al. Low molecular weight heparin is useful in adult COVID-19 inpatients. Experience during the first Spanish wave: observational study. Sao Paulo Med J 2022; 140: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grandone E, Tiscia G, Pesavento R, et al. Use of low-molecular weight heparin, transfusion and mortality in COVID-19 patients not requiring ventilation. J Thromb Thrombolysis 2021; 52: 772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamilton DO, Main-Ian A, Tebbutt J, et al. Standard- versus intermediate-dose enoxaparin for anti-factor xa guided thromboprophylaxis in critically ill patients with COVID-19. Thromb J 2021; 19: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med 2021; 181: 1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with covid-19 admitted to the intensive care unit: the inspiration randomized clinical trial. JAMA 2021; 325: 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jimenez-Guiu X, Huici-Sánchez M, Rmera-Villegas A, et al. Deep vein thrombosis in noncritically ill patients with coronavirus disease 2019 pneumonia: deep vein thrombosis in nonintensive care unit patients. J Vasc Surg Venous Lymphat Disord 2021; 9: 592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiménez-Soto R, Aguilar-Soto M, Rodríguez-Toledo CA, et al. The impact of different prophylactic anticoagulation doses on the outcomes of patients with COVID-19. Thromb Res 2021; 202: 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jonmarker S, Hollenberg J, Dahlberg M, et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Critical Care 2020; 24: 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinelli I, Ciavarella A, Abbattista M, et al. Increasing dosages of low-molecular-weight heparin in hospitalized patients with Covid-19. Intern Emerg Med 2021; 16: 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mennuni MG, Renda G, Grisafi L, et al. Clinical outcome with different doses of low-molecular-weight heparin in patients hospitalized for COVID-19. J Thromb Thrombolysis 2021; 52: 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol 2020; 76: 1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oliynyk O, Barg W, Slifirczyk A, et al. Comparison of the effect of unfractionated heparin and enoxaparin sodium at different doses on the course of COVID-19-associated coagulopathy. Life (Basel) 2021; 11: 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paolisso P, Bergamaschi L, D’Angelo EC, et al. Preliminary experience with low molecular weight heparin strategy in COVID-19 patients. Front Pharmacol 2020; 11: 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pavoni V, Gianesello L, Pazzi M, et al. Venous thromboembolism and bleeding in critically ill COVID-19 patients treated with higher than standard low molecular weight heparin doses and aspirin: a call to action. Thromb Res 2020; 196: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perazzo P, Giorgino R, Briguglio M, et al. From standard to escalated anticoagulant prophylaxis in fractured older adults with SARS-CoV-2 undergoing accelerated orthopedic surgery. Front Med (Lausanne) 2020; 7: 566770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perepu US, Chambers I, Wahab A, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost: 2021; 19: 2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pieralli F, Pomero F, Giampieri M, et al. Incidence of deep vein thrombosis through an ultrasound surveillance protocol in patients with COVID-19 pneumonia in non-ICU setting: a multicenter prospective study. PLoS ONE 2021; 16: e0251966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qin W, Dong F, Zhang Z, et al. Low molecular weight heparin and 28-day mortality among patients with coronavirus disease 2019: a cohort study in the early epidemic era. Thromb Res 2021; 198: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen L, Qiu L, Liu D, et al. The association of low molecular weight heparin use and in-hospital mortality among patients hospitalized with COVID-19. Cardiovasc Drugs Ther 2022; 36: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stessel B, Vanvuchelen C, Bruckers L, et al. Impact of implementation of an individualised thromboprophylaxis protocol in critically ill ICU patients with COVID-19: a longitudinal controlled before-after study. Thromb Res 2020; 194: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trigonis RA, Holt DB, Yuan R, et al. Incidence of venous thromboembolism in critically ill coronavirus disease 2019 patients receiving prophylactic anticoagulation. Crit Care Med 2020; 48: e805–e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ugur M, Adiyeke E, Recep E, et al. Aggressive thromboprophylaxis improves clinical process and decreases the need of intensive care unit in COVID-19. Pak J Med Sci 2021; 37: 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hippensteel JA, LaRiviere WB, Colbert JF, et al. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol 2020; 319: L211–L217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim SY, Jin W, Sood A, et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res 2020; 181: 104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Conzelmann C, Müller JA, Perkhofer L, et al. Inhaled and systemic heparin as a repurposed direct antiviral drug for prevention and treatment of COVID-19. Clin Med (Lond) 2020; 20: e218–e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buijsers B, Yanginlar C, Maciej-Hulme ML, et al. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine 2020; 59: 102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gozzo L, Viale P, Longo L, et al. The potential role of heparin in patients with COVID-19: beyond the anticoagulant effect. A review. Front Pharmacol 2020; 11: 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kamel AM, Sobhy M, Magdy N, et al. Anticoagulation outcomes in hospitalized Covid-19 patients: a systematic review and meta-analysis of case-control and cohort studies. Rev Med Virol 2021; 31: e2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wijaya I, Andhika R, Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin Appl Thromb Hemost 2020; 26: 1076029620960797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parisi R, Costanzo S, Di Castelnuovo A, et al. Different anticoagulant regimens, mortality, and bleeding in hospitalized patients with COVID-19: a systematic review and an updated meta-analysis. Semin Thromb Hemost 2021; 47: 372–391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221099472 for Effects of low molecular weight heparin and fondaparinux on mortality, hemorrhagic and thrombotic complications in COVID-19 patients by Paraskevi C. Fragkou, Lina Palaiodimou, Maria Ioanna Stefanou, Aristeidis H. Katsanos, Vaia Lambadiari, Dimitrios Paraskevis, Elisabeth Andreadou, Dimitra Dimopoulou, Christina Zompola, Panagiotis Ferentinos, Theodoros I. Vassilakopoulos, Anastasia Kotanidou, Petros P. Sfikakis, Sotirios Tsiodras and Georgios Tsivgoulis in Therapeutic Advances in Neurological Disorders