Abstract
Background:
There is little evidence of endovascular therapy (EVT) being performed in acute ischemic stroke beyond 24 h, and that evidence is limited to anterior circulation stroke.
Objective:
To extend evidence of efficacy and safety of EVT after more than 24 h in both anterior and posterior circulation stroke.
Methods:
Local, prospectively collected registries were screened for patients with acute ischemic stroke and large-vessel occlusion who had received either EVT > 24 h after last-seen-well but <24 h after symptom recognition (EVT>24LSW) or EVT > 24 h since first (definitive) symptom recognition (EVT>24DEF). Patients treated <24 h served as a group for comparison. Favorable outcome was defined as modified Rankin scale (mRS) 0–2 or return to prestroke mRS at 3 months.
Results:
Between January 2014 and August 2021, N = 2347 were treated with EVT at our comprehensive stroke center, of whom n = 43 met the inclusion criteria (EVT>24LSW, n = 16, EVT>24DEF, n = 27). EVT>24LSW patients were treated at a median of 28.7 h [interquartile range (IQR) = 27.3–32.8] after last-seen-well and 7.3 h (IQR = 2.8–14.3) after symptom recognition; EVT>24DEF patients were treated 52.5 h (IQR = 26.5–94.2) after first symptoms. Favorable outcome was achieved by 23.3% (10/43) in the EVT > 24 compared with 39.4% (886/2250) in the EVT < 24 group (p = 0.04). Bleeding rates were similar across groups. Mortality was also similar [EVT > 24, 27.9% (12/43) versus EVT < 24, 25.7% (584/2264), p = 0.727; posterior circulation, EVT > 24, 41.7% (5/12) versus EVT < 24, 36.5% (92/252) p = 0.764].
Conclusion:
In selected patients, EVT seems effective and safe beyond 24 h for both anterior and posterior circulation stroke.
Keywords: endovascular therapy, ischemic stroke, thrombectomy
Introduction
Randomized clinical trials have shown beneficial effects of endovascular therapy (EVT) in selected patients with anterior circulation stroke presenting more than 6 h and up to a maximum of 24 h after last being seen well (last-seen-well).1–6 According to a pooled analysis, the adjusted common odds ratio for an increase of 1 point on the modified Rankin scale (mRS) was found to be higher in patients enrolled in a later time window of 12–24 h than for the 6- to 12-h period. 7 Importantly, outcome was worse for patients in the later time window when randomized to the control group than for those in the earlier time window, increasing the relative beneficial effect of EVT. However, a detailed subgroup analysis that has been adjusted for use of intravenous thrombolysis (known to be more frequently applied in the earlier groups), 7 baseline Alberta stroke program early CT score (ASPECTS), perfusion-based infarct core, collateral status, and occlusion site is not available. Nevertheless, the available data point toward beneficial effects of EVT in later time windows up to 24 h. However, patients may present after more than 24 h after last-seen-well or even after symptoms have been definitively recognized, for various reasons. For patients with progressive stroke or relevant tissue-at-risk 8 and large-vessel occlusion (LVO), causal treatment options despite an increase in blood pressure have not been established in prolonged time windows of more than 24 h. Small studies advocate for emergency extracranial-intracranial bypass surgery,9,10 but other studies, including one randomized controlled trial (RCT) enrolling patients beyond 24 h of symptom onset, raised safety and futility concerns.11,12 Recently, case series and observational studies reported that EVT was effective in patients more than 24 h after last-seen-well or definitive symptom onset, but treatment was limited to highly selected patients with anterior circulation stroke.13–18 We therefore aimed to extend the evidence for the efficacy and safety of EVT performed after more than 24 h in both acute anterior and posterior circulation stroke.
Methods
Study design, setting, and patients
Two local prospective recanalization databases were screened for consecutive patients with acute ischemic stroke and LVO presenting directly or who were referred to our comprehensive stroke center (CSC) for EVT between January 2014 and August 2021. Patients were included in the analysis if LVO was present in the anterior [internal carotid artery (ICA), carotid T, middle cerebral artery (MCA, segments M1–M3), or anterior cerebral artery (ACA)] or posterior circulation [posterior cerebral artery (PCA), vertebral artery (VA), or basilar artery (BA)], and EVT (groin or brachial-artery puncture) had been started after more than 24 h (EVT>24). Patients treated at our CSC during the same time period within 24 h served as a control group (EVT<24).
Presence of LVO in the anterior or posterior circulation was determined by computed tomography (CT) or magnetic resonance imaging (MRI), including CT or MR angiography (CTA, MRA). Registries and analyses used in this study have been approved by the ethics committee of the Medical Faculty of Heidelberg (S-247/2009; S-325/2015). The need for informed consent of individual participants was waived due to the observational retrospective design of this study according to local and European Union (EU) data regulations.
Data acquisition and definitions
The decision to perform EVT was left to the discretion of the treating physicians. In principle, the decision to perform EVT is based on a local standard operating procedure providing decision algorithms for patients presenting within the 24-h time window [briefly, for patients presenting with supratentorial ischemia, National Institutes of Health Stroke Scale score (NIHSS) ⩾ 6 and last-seen-well between 9 and 24 h, we aim for performance of multimodal CT-imaging including CT-perfusion; while for infratentorial ischemia, MRI is preferred; see Ringleb et al. 19 for the latest version]. The standard operating procedure is adapted in a continuous process according to available evidence and guideline recommendations and has been updated five times during the period covered by this study. The standard operating procedure does not yet specifically cover the case of patients presenting in a ⩾24-h time window. Patients or legal representatives, if available, were informed about the emergency EVT and the scarce data regarding its use in prolonged time windows. Information about stroke severity and clinical course and treatment modalities as well as time metrics and the medical history were extracted from the database and the hospital information system (source data). Etiology of stroke was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST). 20 Grade of reperfusion after EVT was determined using the modified thrombolysis in cerebral infarction (mTICI) score. 21 Collateral status according to the Tan score 22 (range = 0–3, with 0 indicating absent collateral supply to the occluded MCA territory and 3 full collateral supply of the occluded MCA territory) was assessed by an experienced neuroradiological reader (C.W.) in patients with anterior circulation stroke and available CTA for patients in the EVT>24 group. Regarding ASPECTS, electronically calculated e-ASPECTS (Brainomix®) was used if appropriate, and if there was disagreement about visual readings, the visual ASPECTS or pc-ASPECTS 23 was applied. The intraclass correlation co-efficient was calculated to evaluate the reliability of the visual and electronically ASPECTS scorings (two-way mixed, single-measure, absolute agreement). Intraclass correlation coefficient showed excellent reliability [0.930, 95% confidence interval (CI), 0.822–0.973]. Functional outcome before and after stroke was assessed using the mRS [score range of 0 (no symptoms) to 5 (severe disability, bedridden), or 6 (death)]. The follow-up outcome at 3 months after stroke was obtained through rehabilitation reports, outpatient assessments, or standardized interviews, and the assessment was part of the prospective database. Patients with a pre-existing disability (i.e. mRS ⩾ 2) were not excluded, and favorable functional outcome was defined as mRS 0–2 or return to prestroke mRS.
Statistics
Descriptive statistics were used to characterize demographic, clinical, and radiological data. For categorical data, absolute and relative frequencies (count and percentage) are reported, and the distribution of continuous data is described as mean (SD) or median [interquartile ranges (IQRs)]. The Kolmogorov–Smirnov test was used to ascertain the distribution of data. Subgroups were established for patients treated more than 24 h after last-seen-well but less than 24 h after definitive symptom recognition (EVT>24LSW) or EVT after more than 24 h since definitive symptom recognition (EVT>24DEF). A sensitivity analysis, including patients with anterior circulation stroke only, was performed. To compare proportions of demographic, clinical, and radiological characteristics between the EVT > 24 and < 24 groups, as well as the subgroups, the χ2 test or Fisher exact test was used, as appropriate. To compare continuous variables, the t-test or the Mann–Whitney test was employed, according to the skewness of the data. We refrained from propensity score matching because in-depth radiological characteristics in the EVT < 24 group were not available and, otherwise, the patient characteristics were balanced. All statistical tests were two-sided, and p values of <0.05 were considered statistically significant. Owing to the limited number of patients in the EVT > 24 group, no multivariable analyses were performed. Analyses were conducted using IBM® SPSS® Statistics, V.28.0.1. This study was performed according to the Strengthening of the Reporting of Observational Studies in Epidemiology guidelines for observational studies.
Results
Group characteristics
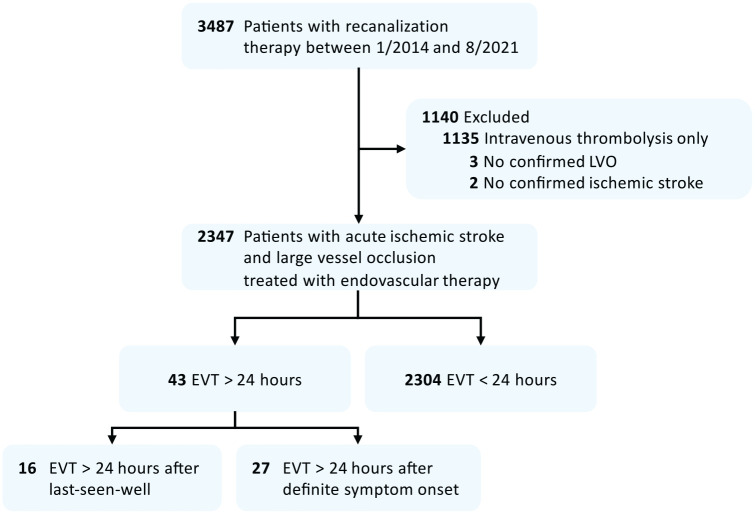
Of N = 2347 patients treated with EVT, n = 43 (1.8%) matched screening criteria (EVT>24; see Figure 1 for flow diagram). These patients had received either EVT more than 24 h after last-seen-well but less than 24 h after definitive symptom recognition (EVT>24LSW, n = 16) or EVT after more than 24 h since symptoms were first recognized (EVT>24DEF, n = 27). Patients treated within a 24-h time window served as a group for comparison (n = 2304, EVT<24). Patients in the EVT>24LSW subgroup received EVT at a median of 28.7 h (IQR = 27.3–32.8) after last-seen-well and 7.3 h (IQR = 2.8–14.3) after symptom recognition, while patients in the EVT>24DEF subgroup were treated 52.5 h (IQR = 26.5–94.2) after definite symptom recognition. The most frequent patient-related reason for delayed EVT was delayed presentation, whereas for hospital-related factors, a primary watchful-waiting strategy followed by a secondary deterioration was identified here (see Figure 2 for a matrix of reasons). In 78% (21/27) of patients in the EVT>24DEF subgroup, stepwise neurological worsening was observed. None of the patients had a complete disappearance of symptoms after the stroke onset. Of the three patients treated with thrombolysis in the EVT > 24 group (Table 1), thrombolysis was started in the 4.5 h time window at referring hospitals in two patients, followed by a watchful-waiting strategy despite early detection of LVO. One patient in the EVT>24LSW group received thrombolysis based on MR-imaging criteria.
Figure 1.
Flow diagram of patient screening process.
EVT: endovascular therapy; LVO: large-vessel occlusion.
Figure 2.
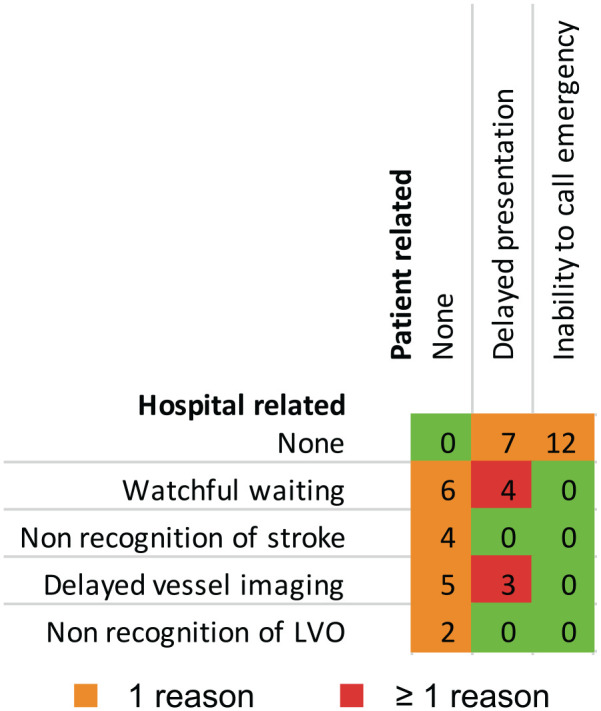
Matrix of key reasons leading to late endovascular therapy. Numbers are affected patients; total sample size, n = 43.
Table 1.
Demographic and clinical characteristics and functional outcome.
| EVT>24 (n = 43) | EVT<24 (n = 2304) | p value | |
|---|---|---|---|
| Age, years, mean (SD) | 75.5 (10.1) | 73.9 (12.7) | 0.422 |
| Female sex, n (%) | 23 (53.5) | 1193 (51.8) | 0.878 |
| Referral, n (%) | 28 (65.1) | 1210/2250 (53.8) | 0.165 |
| Comorbidities, n (%) | |||
| Prior stroke/TIA | 5/42 (11.9) | 497/2295 (21.7) | 0.182 |
| Atrial fibrillation | 13 (30.2) | 1075/2294 (46.9) | 0.031 |
| Arterial hypertension | 32/42 (76.2) | 1749/2301 (76) | > 0.99 |
| Diabetes mellitus | 12/42 (28.6) | 540/2301 (23.5) | 0.463 |
| Hyperlipidemia | 14 (32.6) | 822/2285 (36) | 0.749 |
| Ischemic heart disease | 10/42 (23.8) | 626/2296 (27.3) | 0.728 |
| Peripheral artery disease | 2/42 (4.8) | 159/2271 (7) | 0.766 |
| Prior medication, n (%) | |||
| Antiplatelet | 15/41 (36.6) | 738/2262 (32.6) | 0.616 |
| Oral anticoagulation | 4/42 (9.5) | 454/2279 (19.9) | 0.116 |
| NIHSS before EVT, median (IQR) a | 13 (8–21) | 15 (9–21) | 0.493 |
| Intravenous thrombolysis, n (%) | 3 (7) | 1141 (49.5) | < 0.001 |
| Functional status b | |||
| Prestroke mRS, median (IQR) | 0 (0–2) | 1 (0–2) | 0.55 |
| mRS at day 90, median (IQR) | 4 (3–6) | 3 (2–6) | 0.03 |
| Favorable outcome, n (%) | 10 (23.3%) | 886/2250 (39.4) | 0.04 |
| Death, n (%) | 12 (27.9) | 584/2264 (25.8) | 0.727 |
EVT: endovascular therapy; IQR: interquartile range; mRS: modified Rankin scale; NIHSS: National Institutes of Health Stroke Scale score; TIA: transient ischemic attack.
Missing data in 15/2304 (0.7%) and 1/43 (2.3%).
Prestroke mRS missing in 14/2304 (0.6%), mRS d90 missing in 40/2304 (1.7%).
Clinical characteristics
Patient characteristics despite time window were similar between the EVT>24 and EVT<24 groups (Table 1; see Supplementary Table S1 for subgroup analysis of EVT>24LSW versus EVT>24DEF). Briefly, more patients in the EVT>24 group presented with posterior circulation stroke (12/43 (27.9%) versus 256/2304 (11.1%) p = 0.002), 25.6% versus 8.9% of patients showing BA occlusions. In EVT>24 patients, a pre-existing stenosis was detected in 16/43 (37.2%) during EVT.
Comorbidities were mainly balanced between groups. Atrial fibrillation was more frequent in the EVT<24 [1077/2299 (46.8%) than in the EVT>24 group (13/43 (30.2%), p = 0.031]. Stroke etiology differed among groups, large-artery atherosclerosis and cardioembolic stroke being most frequent. Large-artery atherosclerosis was diagnosed in 23/43 (53.5%) of the EVT>24 versus 586/2303 (25.4%) in the EVT<24 patients (p < 0.001), while cardioembolic stroke was diagnosed in 13/43 (30.2%) versus 1157/2303 (50.2%; p = 0.013). Large-artery atherosclerosis was also found more frequently in the EVT>24hDEF (20/27 (74.1%) than in the EVT>24hLSW (3/16, (18.8%) p < 0.001) subgroup, whereas, in contrast, cardioembolic stroke was less frequent in the EVT>24hDEF [4/27 (14.8%)] compared with the EVT>24hLSW group [9/16 (56.3%)].
Radiological outcome
While excellent (mTICI 2c–3) and good to excellent (mTICI 2b–3) reperfusion grades were commonly achieved in all groups (Table 2), no reperfusion was observed in more patients in the EVT>24 group [9/43 (20.9%)] than in the EVT<24 group [222/2283 (9.7%)] (see Supplementary Table S2 for subgroup analysis of EVT>24LSW versus EVT>24DEF). Excellent reperfusion was achieved in 8 of 12 (66.7%) patients in the EVT>24 group with posterior circulation compared with 17 of 31 (54.8%) with anterior circulation stroke (p = 0.731), similar to rates achieved in EVT<24 [posterior circulation, 170/251 (67.7%) and anterior circulation, 1170/2037 (57.4%), respectively; see Supplementary Table S3].
Table 2.
Radiological characteristics.
| EVT>24 (n = 43) | EVT<24 (n = 2304) | p value | |
|---|---|---|---|
| ASPECTS, median (IQR) a | 9 (7–10) | 9 (8–10) | 0.52 |
| Collateral status, median (IQR) b | 3 (1–3) | – | – |
| Occlusion site, n (%) | <0.001 | ||
| ICA | 2 (4.7) | 113 (4.9) | |
| ICA plus MCA | 6 (14) | 248 (10.8) | |
| ACA | 0 (0) | 20 (0.9) | |
| Carotid T | 7 (16.3) | 326 (14.1) | |
| MCA, M1 | 10 (23.3) | 887 (38.5) | |
| MCA, M2 | 5 (11.6) | 449 (19.5) | |
| MCA, M3 | 1 (2.3) | 5 (0.2) | |
| PCA | 0 (0) | 38 (1.6) | |
| BA | 11 (25.6) | 206 (8.9) | |
| VA | 1 (2.3) | 12 (0.5) | |
| Stenosis, detected, n (%) | 16 (37.2) | – | – |
| Reperfusion, n (%) | |||
| mTICI 2c–3 | 25 (58.1) | 1336/2283 (58.5) | > 0.99 |
| mTICI 2b–3 | 33 (76.7) | 1945/2283 (85.2) | 0.13 |
| mTICI 0 | 9 (20.9) | 222/2283 (9.7) | 0.033 |
ACA: anterior cerebral artery; ASPECTS: Alberta stroke program early CT score; BA: basilar artery; ICA: internal carotid artery; IQR: interquartile range; MCA: middle cerebral artery; mTICI: modified thrombolysis in cerebral infarction score; PCA: posterior circulation artery; VA: vertebral artery.
Assessable in 27/43 and 1878/2304 cases.
Collateral status according to Tan score, assessable in n = 27/43 cases (anterior circulation only).
Functional outcome and bleedings
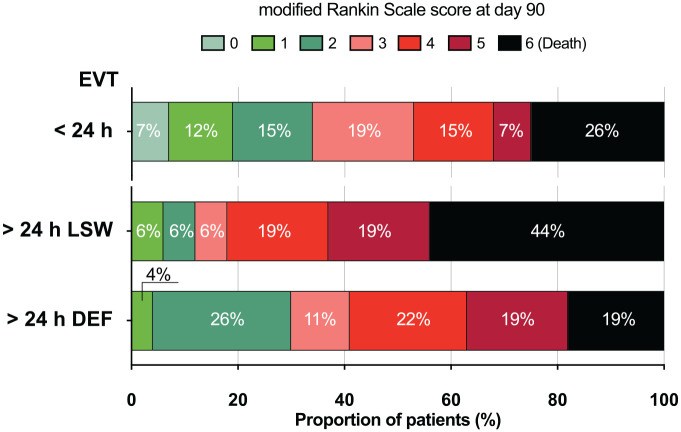
Functional outcomes are summarized in Table 1 and visualized in Figure 3. Briefly, 3 months after stroke, 10/43 (23.3%) of patients of the EVT>24 group had achieved a favorable outcome compared with 886/2250 (39.4%) in the EVT<24 group (p = 0.04). Of the EVT>24 patients in whom no reperfusion could be achieved, none recovered to a favorable outcome. Mortality was similar for the EVT>24 and EVT<24 groups. Early symptomatic bleedings developed in 1 of 43 (2.3%) patients in the EVT>24 group versus 88 of 2294 (3.8%) in EVT<24. No fatal intracranial hemorrhage (ICH) occurred in any of the EVT>24 group versus 38/2303 (1.7%; p > 0.99) in the EVT<24 group (see Table 3 for all intracranial bleeding types and rates).
Figure 3.
Distribution of modified Rankin scale scores after 3 months.
EVT: endovascular thrombectomy; LSW: EVT after last-seen-well but less than 24 h after definitive symptom recognition; DEF: EVT after more than 24 h since symptoms were first recognized. For distribution of prestroke mRS, see Online Supplementary Figure SF1.
Table 3.
Intracranial hemorrhages according to the Heidelberg Bleeding Classification.
| Class | Type | EVT>24 (n = 43) | EVT<24 (n = 2304) |
|---|---|---|---|
| 0 | None | 32 (74.4) | 1710/2287 (74.8) |
| 1a | HI1 | 5 (11.6) | 241/2287 (10.5) |
| 1b | HI2 | 2 (4.7) | 66/2887(2.9) |
| 1c | PH1 | 0 (0) | 56/2287 (2.4) |
| 2 | PH2 | 0 (0) | 86/2287 (3.8) |
| 3a | Remote PH | 0 (0) | 20/2287 (0.9) |
| 3b | IVH | 0 (0) | 4/2287 (0.2) |
| 3c | SAH | 4 (9.3) | 101 (4.4) |
| 3d | SDH | 0 (0) | 1 (0.09) |
| Other | Nonclassified | 0 (0) | 2 (0.1) |
Data are n (%) or n/N (%). HI: hemorrhagic infarction; IVH: intraventricular hemorrhage; PH: parenchymal hemorrhage; SAH: subarachnoid hemorrhage; SDH: subdural hematoma.
Characteristics of patients with favorable outcomes
In univariate analysis of the clinical and radiological characteristics, collaterals were better among patients with a favorable outcome than in patients with an unfavorable outcome [median (IQR) Tan score, 3 (3–3) versus 2 (1–3), p = 0.043; Supplementary Table S3]. Patients with a favorable outcome in the subgroup with known, definite symptom onset were treated at a median of 72 h (IQR = 53–107), with a maximum time window of 288 h (12 days): In a 79-year-old patient who presented with stepwise progressive stroke, reaching a NIHSS of 8 before transfer to our CSC, stent-retriever thrombectomy of a M1 occlusion was successful, but additional intraprocedural intracranial stenting had to be performed because a new thrombus developed. The patient is capable of living independently, with an mRS of 1 at the last control examination 2.7 years after successful EVT.
Sensitivity analysis
The characteristics of patients with anterior circulation stroke only were similar in the EVT>24 and EVT<24 groups (Supplementary Table S4). Reperfusion could not be achieved in more anterior circulation stroke patients in the EVT>24 than in the EVT<24 group [7/31 (22.6%) versus 200/2032 (9.8%), p = 0.03].
Posterior stroke patients treated after more than 24 h had mainly been referred to our CSC [11/12 (91.7%) versus 126/256 (49.8%), p = 0.006]. In both anterior and posterior circulation stroke patients, functional outcome and mortality did not differ between the extended time window treatment group and EVT<24.
Discussion
EVT performed more than 24 h after last-seen-well or definitive symptom onset was feasible and safe, and one-fifth of the treated patients had a favorable outcome at 3 months. Excellent or good reperfusion rates could be achieved at similar rates in anterior and posterior circulation stroke patients who were treated beyond 24 h.
Fortunately, the vast majority of the 2347 patients treated with EVT at our CSC in the 7.7-year observational period could be treated within the 24-h time window, and thus within the time window for which evidence from RCTs exists – at least for the anterior circulation. However, approximately 2% of the ultimately EVT-treated patients were admitted or transferred to our CSC beyond 24 h for patient-related reasons (delayed presentation or inability to call emergency), hospital-related reasons (mainly due to not recognizing ischemic stroke, delayed performance of vessel imaging, or by choosing a watchful-waiting strategy in initially mildly affected patients), or a mixture of reasons.
For those 43 patients in whom EVT was considered after 24 h, the rate of favorable outcome in 23.3% in our study is lower than reported in two of the largest previous observational studies,13,17 despite comparable rates of good to excellent reperfusion. Table 4 summarizes previous studies. The most probable reason for outcome differences is the stricter preselection of patients in the previous cohorts. Desai et al. reported on patients with ICA or M1 occlusion who presented more than 24 h after last-seen-well and matched DAWN criteria, including a prestroke mRS limited to 0–1, and found favorable outcome in 9 out of 21 patients (43%). In an analysis of data from the Italian Registry of Endovascular Thrombectomy in Acute Stroke, patients with acute ICA or MCA occlusion were treated 29 h (IQR = 26–31) after symptom onset, and 14 of the 34 (41%) patients achieved an mRS of 0–2 after 3 months. Prestroke mRS was limited to 0–2, and patients had to have good to excellent collaterals and a relatively small infarct core. 17 Despite not limiting treatment to those being independent before stroke, 24 we also included patients at even later time windows at a median of 53 h (IQR = 26.5–94.2). The rate of favorable outcome was also numerically lower in posterior circulation stroke patients who were included in our analysis, and mortality higher than for anterior circulation stroke, although differences did not reach statistical significance due to the limited sample size for those treated in the extended time window. Safety was excellent, with no fatal ICH in the group treated after more than 24 h, and symptomatic ICH only occurred in 1 of the 43 patients.
Table 4.
Summary of past studies on EVT after more than 24 h cited within the main text.
| Reference | Type | Time period | n | Main selection criteria | LVO | Time to EVT | TICI ⩾ 2b | mRS 0–2 |
|---|---|---|---|---|---|---|---|---|
| Desai et al. 13 | Retrospective 3 centers |
2010–2018 | 21 | >24 h after LSW pmRS 0–1 Age < 80: NIHSS ⩾ 10 + core < 31 ml or NIHSS ⩾ 20 + core < 51 ml; age ⩾ 80: NIHSS ⩾ 10 + core < 21 ml |
ICA (52%) MCA M1 (48%) |
48 (30–72) h after LSW | 17/21 (81%) | 9/21 (43%) |
| Manning et al. 14 | Retrospective analysis of prospective registry at 2 CSCs | 2016–2017 | 5 | >24 h onset | ICA + MCA (40%) MCA M1 (60%) |
45 (25–90) h after onset | TICI 3 (4/5; TICI 2a 1/5) | 4/5 (80%) |
| Mokin et al. 15 | Retrospective registry analysis | Not reported | 3 | >24 LSW or onset, anterior circulation |
M1/M2 (33%) M3 (67%) |
26 (26–32) h (not specified in how many cases LSW) | 1/3 (33%) | 1/3 (33%) |
| Beharry et al. 16 | Case report | Not reported | 1 | MRI perfusion with favorable profile | MCA M2 (100%) | N/A/; TICI3b after 31 h after LSW | n = 1/1 | n = 1/1 |
| Casetta et al. 17 | Retrospective analysis of prospective national registry | Not reported | 34 | >24 h after onset NIHSS ⩾ 6, collateral-score 2–3, CTP core (CBV) ⩽ 50% (MTT) or <1/3 of MCA territory; pmRS 0–2 |
ICA (32%) MCA M1 (68%) |
29 (26–31) h after onset | 26/34 (77%) | 14/34 (41%) |
| Kim et al. 18 | Retrospective analysis of prospectively acquired data, single center | 2012–2018 | 13 | (Subgroup) >24 h after LSW; NIHSS ⩾ 6; anterior circulation |
ICA or ICA + M1 (23%) distal ICA (8%), MCA M1 (54%) MCA M2 (15%) |
46 (36–37) h after LSW | Not reported | Not reported |
|
This study
Purrucker et al. 2022 |
Retrospective analysis of prospectively acquired data, single center | January 2014–August 2021 | 43 | >24 h after LSW or >24 h after definite onset, progressive stroke or tissue at risk | ICA (5%), ICA + MCA (14%), Carotid T (16%) MCA M1 (23%) MCA M2 (12%) MCA M3 (2%) BA (26%) VA (2%) |
28.7 h (27.3–32.8) after LSW or 52.5 h (26.5–94.2) after definitive symptom onset |
33/43 (77%) | 10/43 (23%) (mRS 0–2 or return to pmRS) |
BA: basilar artery; CSC, comprehensive stroke center; ICA: internal carotid artery; LVO: large-vessel occlusion; MCA: middle cerebral artery; mTICI: modified thrombolysis in cerebral infarction score; mRS: modified Rankin scale score; PCA: posterior circulation artery; pmRS: prestroke mRS; VA: vertebral artery.
Our data extend the upper time window in which EVT might be considered, with technical and clinically successful thrombectomy in a patient 288 h after stroke onset. We do not know whether a maximum time window for attempting EVT can be determined. As the numbers of patients presenting after more than 24 h is small even at high-volume CSCs, international, pooled data might help to better define not only a potential, maximum reachable time window, but also ‘inclusive-enough’ selection criteria for EVT for those patients with remaining tissue-at-risk at late time windows and progressive symptoms.
Patients with LVO based on pre-existing large-artery stenosis may have better developed collaterals, and stroke progression may be slower than patients with cardioembolic stroke, leading to sudden occlusion and rapidly growing infarcts. Indeed, stroke etiology differed among patients treated later than 24 h compared with those treated within 24 h. Large-artery atherosclerosis was considered the cause of stroke more often in patients treated after more than 24 h, while cardioembolism was more often diagnosed in the earlier time windows. Likewise, patients treated more than 24 h after last-seen-well, but less than 24 h after definitive symptom recognition more often had cardioembolic stroke than large-artery atherosclerosis, while patients treated more than 24 h after definite symptom recognition more often had larger-artery atherosclerosis. In approximately two-fifths of the EVT > 24 patients, a pre-existing stenosis was detected during EVT, mainly in the group of patients treated more than 24 h after definite symptom recognition. BA occlusion was particularly frequent in our population treated beyond 24 h. Such occlusions based on pre-existing stenosis can be clinically challenging, as patients often present with unspecific dizziness or vertigo or gait imbalance and might initially be only mildly affected. 25 Therefore, physicians might refrain from direct vessel imaging, or, if BA occlusion is detected, consider the risk of treatment greater than the benefit. In our patients with posterior circulation stroke treated after more than 24 h (41.7%), despite good to excellent reperfusions achieved in more than 80%, only 16.7% of the patients reached favorable outcome, and mortality was high (41.7%). As stepwise neurological worsening over hours/days was observed in most of these patients, we should aim for early detection of LVO and, consequently, earlier EVT.
Our study has limitations. It was an observational retrospective study of prospectively collected data without a predefined control group. In the group treated within <24 h, fewer patients experienced a favorable outcome (mRS 0–2) at 3 months than in RCTs investigating the benefit of thrombectomy in patients within 12 h after symptom onset. 26 Less favorable outcomes might have been caused by extending treatment to patients with more premorbid disability (22% with prestroke mRS ⩾ 3). Another reason could be the naturally very low rate of intravenous thrombolysis preceding EVT in the more than 24 h group compared with patients treated within 24 h. Although this study comprises the so far largest reported cohort of acute ischemic stroke patients treated with EVT after more than 24 h and it includes patients with posterior circulation stroke, the sample size is still small, impeding more in-depth statistical analyses. Some of the observed group and subgroup differences may simply have been due to chance. We did not use a predefined decision algorithm to select patients for thrombectomy after more than 24 h after last-seen-well or definite symptom recognition. Instead, considering the limited utility of perfusion imaging especially in the posterior circulation, we individualized our decision-making process by integrating both clinical considerations and additional radiological characteristics.
Conclusion
EVT performed more than 24 h after last-seen-well or definite symptom recognition seems safe and resulted in a favorable functional outcome in one out of five patients with anterior or posterior circulation stroke. As none of the patients treated after more than 24 h and in whom no reperfusion could be achieved had a favorable outcome, and the expected prognosis in patients with progressive stroke and LVO is known to be unfavorable, 8 our data encourage offering EVT at a time window beyond 24 h for both anterior and posterior circulation stroke.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_17562864221101083 for Leaving the day behind: endovascular therapy beyond 24 h in acute stroke of the anterior and posterior circulation by Jan C. Purrucker, Peter A. Ringleb, Fatih Seker, Arne Potreck, Simon Nagel, Silvia Schönenberger, Anne Berberich, Ulf Neuberger, Markus Möhlenbruch and Charlotte Weyland in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors acknowledge Sherryl Sundell for language editing.
Footnotes
Ethics statement and consent to participate: Registries and analyses used in this study have been approved by the ethics committee of the Medical Faculty of Heidelberg (S-247/2009; S-325/2015). The need for informed consent of individual participants was waived due to the observational retrospective design of this study according to local and EU data regulations.
Author contribution(s): Jan C. Purrucker: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft.
Peter A. Ringleb: Investigation; Methodology; Resources; Software; Writing – review & editing.
Fatih Seker: Writing – review & editing.
Arne Potreck: Writing – review & editing.
Simon Nagel: Writing – review & editing.
Silvia Schönenberger: Writing – review & editing.
Anne Berberich: Writing – review & editing.
Ulf Neuberger: Writing – review & editing.
Markus Möhlenbruch: Investigation; Supervision; Writing – review & editing.
Charlotte Weyland: Conceptualization; Data curation; Investigation; Validation; Writing – review & editing.
ORCID iD: Jan C. Purrucker  https://orcid.org/0000-0003-2978-4972
https://orcid.org/0000-0003-2978-4972
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge financial support by the Open Access Publishing Fund of Ruprecht-Karls-University Heidelberg.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JCP has received consultation fees and travel expenses from Akcea, Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer. PAR received speaker fees from Bayer, Boehringer Ingelheim, Daiichi Sanyko, and Pfizer. SN reports consulting fees from Brainomix and lecture fees from Boehringer Ingelheim and BMS Pfizer. MM has received consulting honoraria, speaker honoraria, and travel support outside this work from Covidien/Medtronic, MicroVention, and Stryker. The other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Data are available upon reasonable request from the corresponding author.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jan C. Purrucker, Department of Neurology, Heidelberg University Hospital, Im Neuenheimer Feld 400, 69120 Heidelberg, Germany.
Peter A. Ringleb, Department of Neurology, Heidelberg University Hospital, Heidelberg, Germany
Fatih Seker, Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany.
Arne Potreck, Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany.
Simon Nagel, Department of Neurology, Heidelberg University Hospital, Heidelberg, Germany.
Silvia Schönenberger, Department of Neurology, Heidelberg University Hospital, Heidelberg, Germany.
Anne Berberich, Department of Neurology, Heidelberg University Hospital, Heidelberg, Germany.
Ulf Neuberger, Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany.
Markus Möhlenbruch, Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany.
Charlotte Weyland, Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany.
References
- 1. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 2. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 5. Martins SO, Mont’ Alverne F, Rebello LC, et al. Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med 2020; 382: 2316–2326. [DOI] [PubMed] [Google Scholar]
- 6. Mocco J, Siddiqui AH, Fiorella D, et al. POSITIVE: Perfusion imaging selection of ischemic stroke patients for endovascular therapy. J Neurointerv Surg 2022; 14(2): 126–132. [DOI] [PubMed] [Google Scholar]
- 7. Jovin TG, Nogueira RG, Lansberg MG, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet 2022; 399: 249–258. [DOI] [PubMed] [Google Scholar]
- 8. Sarraj A, Mlynash M, Heit J, et al. Clinical outcomes and identification of patients with persistent penumbral profiles beyond 24 hours from last known well: analysis from DEFUSE 3. Stroke 2021; 52(3): 838–849. [DOI] [PubMed] [Google Scholar]
- 9. Noh YH, Chung JW, Ko JH, et al. Efficacy and safety of emergency extracranial-intracranial bypass for revascularization within 24 hours in resolving large artery occlusion with intracranial stenosis. World Neurosurg 2021; 155: e9–e18. [DOI] [PubMed] [Google Scholar]
- 10. Otani N, Yoshino A. Ischemic stroke revascularization. Adv Tech Stand Neurosurg 2022; 44: 79–96. [DOI] [PubMed] [Google Scholar]
- 11. Powers WJ, Clarke WR, Grubb RL, Jr, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 2011; 306: 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rice CJ, Cho SM, Taqui A, et al. Early versus delayed extracranial-intracranial bypass surgery in symptomatic atherosclerotic occlusion. Neurosurgery 2019; 85: 656–663. [DOI] [PubMed] [Google Scholar]
- 13. Desai SM, Haussen DC, Aghaebrahim A, et al. Thrombectomy 24 hours after stroke: beyond DAWN. J Neurointerv Surg 2018; 10(11): 1039–1042. [DOI] [PubMed] [Google Scholar]
- 14. Manning NW, Wenderoth J, Alsahli K, et al. Endovascular thrombectomy > 24-hr from stroke symptom onset. Front Neurol 2018; 9: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mokin M, Abou-Chebl A, Castonguay AC, et al. Real-world stent retriever thrombectomy for acute ischemic stroke beyond 6 hours of onset: analysis of the NASA and TRACK registries. J Neurointerv Surg 2019; 11(4): 334–337. [DOI] [PubMed] [Google Scholar]
- 16. Beharry J, Duncan R, Krauss M, et al. Endovascular thrombectomy: 31 hours from symptom onset. Pract Neurol 2020; 20(1): 80–81. [DOI] [PubMed] [Google Scholar]
- 17. Casetta I, Fainardi E, Saia V, et al. Endovascular thrombectomy for acute ischemic stroke beyond 6 hours from onset: a real-world experience. Stroke 2020; 51(7): 2051–2057. [DOI] [PubMed] [Google Scholar]
- 18. Kim BJ, Menon BK, Kim JY, et al. Endovascular treatment after stroke due to large vessel occlusion for patients presenting very late from time last known well. JAMA Neurol 2020; 78: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ringleb P, Nagel S, Gumbinger C, et al. Standard operating procedure (SOP) recanalization therapy, https://www.klinikum.uni-heidelberg.de/fileadmin/neurologie/pdf_downloads/Standard_Rekanalisationstherapie_20201124.pdf (2020, accessed 3 February 2022).
- 20. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial – TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 21. Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the interventional management of stroke II trial. AJNR Am J Neuroradiol 2008; 29(3): 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009; 30(3): 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puetz V, Sylaja PN, Coutts SB, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008; 39(9): 2485–2490. [DOI] [PubMed] [Google Scholar]
- 24. Saver JL, Chaisinanunkul N, Campbell BCV, et al. Standardized nomenclature for Modified Rankin Scale global disability outcomes: consensus recommendations from stroke therapy academic industry roundtable XI. Stroke 2021; 52(9): 3054–3062. [DOI] [PubMed] [Google Scholar]
- 25. Novakovic-White R, Corona JM, White JA. Posterior circulation ischemia in the endovascular era. Neurology 2021; 97: S158–S169. [DOI] [PubMed] [Google Scholar]
- 26. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_17562864221101083 for Leaving the day behind: endovascular therapy beyond 24 h in acute stroke of the anterior and posterior circulation by Jan C. Purrucker, Peter A. Ringleb, Fatih Seker, Arne Potreck, Simon Nagel, Silvia Schönenberger, Anne Berberich, Ulf Neuberger, Markus Möhlenbruch and Charlotte Weyland in Therapeutic Advances in Neurological Disorders