Abstract
Background
Vessels that encapsulate tumor cluster (VETC) is associated with poor prognosis in hepatocellular carcinoma (HCC). Vessels that encapsulate tumor cluster estimation before initial treatment is helpful for clinical doctors. We aimed to construct a novel predictive model for VETC, using preoperatively accessible clinical parameters and imagine features.
Methods
Totally, 365 HCC patients who received curative hepatectomy in the Sun Yat-Sen University Cancer Center from 2013 to 2014 were enrolled in this study. Vessels that encapsulate tumor cluster pattern was confirmed by immunochemistry staining. 243 were randomly assigned to the training cohort while the rest was assigned to the validation cohort. Independent predictive factors for VETC estimation were determined by univariate and multivariate logistic analysis. We further constructed a predictive nomogram for VETC in HCC. The performance of the nomogram was evaluated by C-index, receiver operating characteristic (ROC) curve, and calibration curve. Besides, the decision curve was plotted to evaluate the clinical usefulness. Ultimately, Kaplan–Meier survival curves were utilized to confirm the association between the nomogram and survival.
Results
Immunochemistry staining revealed VETC in 87 patients (23.8%). lymphocyte to monocyte ratio (>7.75, OR = 4.06), neutrophil (>7, OR = 4.48), AST to ALT ratio (AAR > .86, OR = 2.16), ALT to lymphocyte ratio index (BLRI > 21.73, OR = 2.57), alpha-fetoprotein (OR = 1.1), and tumor diameter (OR = 2.65) were independent predictive factors. The nomogram incorporating these predictive factors performed well with an area under the curve (AUC) of .746 and .707 in training and validation cohorts, respectively. Calibration curves indicated the predicted probabilities closely corresponded with the actual VETC status. Moreover, the decision curve proved our nomogram could provide clinical benefits with patients. Finally, low probability of VETC group had significantly longer recurrence free survival (RFS) and overall survival (OS) than the high probability of the VETC group (all P < .001).
Conclusion
A novel predictive nomogram integrating clinical indicators and image characteristics shows strong predictive VETC performance and might provide standardized net clinical benefits.
Keywords: hepatocellular carcinoma, vessels that encapsulate tumor cluster, preoperative prediction, early diagnosis, nomogram
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. According to Global Cancer Statistics 2020, HCC is the third leading cause of cancer death and it is estimated that there are approximately 905 677 new cases and 830 180 new deaths of HCC. 1 The clinical symptoms of early-stage HCC are not often obvious, and most patients are already in advanced stage HCC when they go to the hospital for medical consultation with obvious discomfort. Although some HCC patients have undergone radical surgery, their prognosis is still poor with a 5-year recurrence rate of more than 50%. 2 Intrahepatic metastasis accounts for most recurrent cases.
In recent years, a novel risk factor contributing to HCC metastasis, vessels that encapsulate tumor cluster (VETC) is identified, which is different from microvascular invasion (MVI). Tumor vascular endothelial cells (CD34+) encapsulate HCC cell clusters, forming cobweblike networks, which is defined as VETC. 3 Hepatocellular carcinoma with VETC can metastasize in an EMT-independent manner. The expression of EMT marker proteins including Snail and Twist is not up-regulated in HCC metastatic lesions with VETC pattern, whereas E-cadherin is dramatically up-regulated. 3 Previous research has shown that VTEC enters the circulatory system as a whole, then moves to the target organ with blood flow and proliferates to produce new metastatic lesions. 4 A growing body of research suggests that VETC is linked to liver cancer recurrence and a bad prognosis. Recently, Masaki Mori, et al. found that VETC expression can be a prognostic biomarker for mortality after living-donor liver transplantation. 5 A large multi-institutional study confirms that VETC can accurately predict aggressive HCC. 6 Lu et al. have shown that VETC status provides additional discriminative information for patients with either MVI− or MVI+ and a combination of VETC and MVI may help classify subtypes and predict the prognosis of HCC patients. 7 In addition, VETC pattern may represent a reliable marker for selecting recurrent early-stage HCC patients who may benefit from repeat hepatic resection. 8 Notably, it is suggested that the VETC pattern is associated with the effect of sorafenib treatment for HCC patients. Vessels that encapsulate tumor cluster-positive HCC patients may have a better prognosis than that of VETC-negative HCC patients when treated with sorafenib. 9 Furthermore, VETC can prevent lymphocytes from interacting with HCC cells, limiting the effectiveness of immunotherapy. 10 Thus, anatomical liver resection, vigorous postoperative adjuvant therapy, and frequent countercheck may lower the probability of recurrence and enhance the prognosis of VETC-positive HCC patients. However, the diagnosis of VETC is dependent on postoperative pathology. Although previous studies have shown that preoperative Gd-EOB-DTPA-Enhanced MRI has a certain value in predicting VETC,11,12 Gd-EOB-DTPA-Enhanced MRI has not yet been widely used. Therefore, exploring a simple and practical approach for predicting VETC before HCC surgery is indeed imperative.
In the real world, the pathological specimens of many HCC patients are absent before treatment. In the present study, we aimed to establish a novel prediction for VETC pattern in HCC using preoperatively accessible clinical parameters and imagine features to provide a strategy for surgical planning, predicting the prognosis, and formulating preoperative neoadjuvant therapy plans, to further improve the prognosis of HCC patients.
Materials and Methods
Patients
Hepatocellular carcinoma patients who underwent hepatectomy at Sun Yat-Sen University Cancer Center between 2013 and 2014 were retrospectively analyzed in this study. The following criteria were used to determine inclusion: (I) HCC pathologically proven; (II) patients undergoing curative hepatectomy with a resection margin more than 1 cm from tumor borders; (III) Child-Pugh grade A or B; and (IV) no macrovascular invasion or distant metastases. The following were the exclusion criteria: (I) patients who have had other preoperative adjuvant therapy; (II) patients who have been diagnosed with additional malignant diseases; and (III) patients who have incomplete clinical data. Ultimately, 365 HCC patients were enrolled in the present analysis.
Clinical Parameters and Imagine Features
Clinical parameters and imagine features were retrieved from medical records and imaging examinations, including preoperative ultrasound, CT, and MRI. Clinical parameters included age, gender, HBV infection history, α-fetoprotein (AFP), neutrophil count (109/L), prothrombin time (PT), total bilirubin (TB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), lymphocyte to monocyte ratio (LMR), platelet-lymphocyte ratio (PLR), γ-glutamyl transpeptidase to platelet ratio (GPR), AST to ALT ratio (AAR), AST to lymphocyte ratio index (ALRI), AST to neutrophil ratio index (ANRI), AST to platelet ratio index (APRI), and ALT to lymphocyte ratio index (BLRI). The foremost survival outcomes of interest in our study were overall survival (OS), and recurrence-free survival (RFS). Imagine features mainly included splenomegaly, liver cirrhosis, tumor number, ascites, tumor diameter, and tumor envelope.
Vessels that encapsulate tumor cluster
We performed immunochemistry staining to confirm the pathological characteristics of VETC. The 4-µm paraffin tissue sections used in this study were deparaffinized in xylene and rehydrated using graded ethanol washes. Then, endogenous peroxidase activity of the paraffin tissue sections was quenched by hydrogen peroxide (.3%) and sequentially antigen retrieval was performed by pressure cooking in 10 mM citrate buffer (pH 6.0). Next, HCC tissue was incubated with a primary antibody at 4°C overnight. Finally, we utilized the Envision system (Dako Cytomation, Denmark) to perform immunochemistry staining and then sections were counterstained with hematoxylin. Histological data on HCC tissue specimens were evaluated by 2 experimented pathologists who were blinded by clinical information. In terms of CD34 (ZSGB-BIO, ZM-0046, 1:100) assessment, VETC was characterized as unambiguous immunoreactivity of a continuous lining surrounding tumor clusters. The area of VETC was analyzed in 5% of the units using semi-quantitative methods. The VETC-positive region was graded on a scale of 0 to 100% of the tumor surface. Consistent with the large multi-institutional study, HCC patients were further divided into 2 groups: the VETC-negative group and the VETC-positive group with a cut-off value of 55%. 6
Statistical Analysis
The normal value range of laboratory parameters in our hospital was regarded as the cut-off value. For those clinical parameters that do not have standard cut-off values, we plotted receiver operating characteristic (ROC) curves and determine the best cut-off value. We performed a logarithmic transformation of AFP and tumor diameter. Based on “car” package, all included HCC patients were randomly divided into 2 groups: training cohort and validation cohort. The continuous variables were analyzed by using an unpaired Student’s t-test for parametric data and Mann–Whitney rank sum test for non-parametric data. Categorical variables were compared using Pearson’s chi-square test or Fisher exact test. Then, univariate logistic regression analysis was used to identify diagnostic-associated variables (P < .10), which were sequentially subjected to multivariate logistic regression analysis to investigate independent prognostic factors. The significant diagnostic factors (P < .05) identified in the logistic regression model were used to establish a nomogram. The predictive ability of the nomogram was assessed via the computer consistency coefficient (C-index) and the area under the curve (AUC) of the ROC curve. A calibration curve was plotted to evaluate the consistency between the observed rates and predicted diagnosis. Decision curve analysis (DCA) was conducted to determine the clinical usefulness of the nomogram by quantifying the net benefits along with the increase of threshold probabilities. 13 Further, we divided all included HCC patients into a high probability of VETC group and a low probability of VETC group according to the mean value of the probability scores calculated from our nomogram. We conducted a survival analysis between different probabilities of VETC groups by plotting Kaplan–Meier curves and verified their differences by the log-rank test. Data analysis was performed by using Medcalc software (version 20.018), SPSS software (version 20.0) and R software (version 4.1.1, https://www.r-project.org/). In our study, “survival,” “survminer,” “car,” “rms,” “Proc,” and “DecisionCurve” packages were utilized to construct and validate the predictive nomogram. Unless stated otherwise, a two-sided P < .05 was considered statistically significant.
Results
Baseline Characteristics
A total of 365 HCC patients, including 319 males and 46 females were included after being chosen based on our inclusion and exclusion criteria. The average age was 50.58 ± 11.21 years old. About 23.84% of HCC patients were confirmed with VETC according to postoperative immunochemistry staining reports (Figure 1). Three hundred and twenty-two HCC patients (88.22%) were with HBV infection history. All enrolled patients were with well-preserved liver function.Supplementary Table 1summarizes the baseline characteristics of patients in this retrospective study. We further investigated the relationship between VETC and clinical characteristics. As listed in Table 1, VETC was significantly associated with LMR, PLR, neutrophil, AAR, ALRI, ANRI, APRI, BLRI, SII, AFP, and tumor diameter (all P < .05).
Figure 1.
Representative IHC images of VETC pattern in HCC tissues. VETC-positive (A) and VETC-negative (B). Tumor vascular endothelial cells (CD34+) encapsulate HCC cell clusters, forming cobweblike networks, which is defined as VETC.
Table 1.
The Correlation between VETC and Clinical Parameters and Imagine Features.
| Parameters | VETC | χ2 | P-Value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Age | 51.21 ± 10.80 | 48.60 ± 12.31 | .058 | |
| Gender | ||||
| Female | 38 (82.61%) | 8 (17.39%) | 1.2040 | .2725 a |
| Male | 240 (75.24%) | 79 (24.76%) | ||
| LMR | ||||
| ≤7.75 | 267 (77.39%) | 78 (22.61%) | 5.2208 | .0223 a |
| >7.75 | 11 (55.00%) | 9 (45.00%) | ||
| PLR | ||||
| ≤40 | 108 (82.44%) | 23 (17.56%) | 4.4367 | .0352 a |
| >40 | 170 (72.65%) | 64 (27.35%) | ||
| Neutrophil | ||||
| ≤7 | 267 (77.39%) | 78 (22.61%) | 5.2208 | .0352 a |
| >7 | 11 (55.00%) | 9 (45.00%) | ||
| GPR | ||||
| ≤.17 | 67 (83.75%) | 13 (16.25%) | 3.2474 | .0715 a |
| >.17 | 211 (74.04%) | 74 (25.96%) | ||
| AAR | ||||
| ≤.86 | 128 (82.05%) | 28 (17.95%) | 5.2007 | .0226 a |
| >.86 | 150 (71.77%) | 59 (28.23%) | ||
| ALRI | ||||
| ≤29.75 | 213 (80.08%) | 53 (19.92%) | 8.2622 | .0040 a |
| >29.75 | 65 (65.66%) | 34 (34.34%) | ||
| ANRI | ||||
| ≤12.43 | 187 (79.91%) | 47 (20.09%) | 5.0507 | .0246 a |
| >12.43 | 91 (69.47%) | 40 (30.53%) | ||
| APRI | ||||
| ≤.21 | 149 (80.98%) | 35 (19.02%) | 4.7364 | .0295 a |
| >.21 | 129 (71.27%) | 52 (28.73%) | ||
| BLRI | ||||
| ≤21.73 | 149 (81.42%) | 34 (18.58%) | 5.5856 | .0181 a |
| >21.73 | 129 (70.88%) | 53 (29.12%) | ||
| SII | ||||
| ≤545.6 | 212 (78.81%) | 57 (21.19%) | 3.9444 | .0470 a |
| >545.6 | 66 (68.75%) | 30 (31.25%) | ||
| SIRI | ||||
| ≤.64 | 79 (71.82%) | 31 (28.18%) | 1.6383 | .2006 a |
| >.64 | 199 (78.04%) | 56 (21.96%) | ||
| ALT | ||||
| ≤40 | 145 (77.96%) | 41 (22.04%) | .6713 | .4126 a |
| >40 | 133 (74.30%) | 46 (25.70%) | ||
| AST | ||||
| ≤45 | 201 (78.82%) | 54 (21.18%) | 3.2957 | .0695 a |
| >45 | 77 (70.00%) | 33 (30.00%) | ||
| TB | ||||
| ≤17.1 | 221 (76.21%) | 69 (23.79%) | .0014 | .9701 a |
| >17.1 | 57 (76.00%) | 18 (24.00%) | ||
| PT | ||||
| ≤13.5 | 269 (76.64%) | 82 (23.36%) | 1.1315 | .2874 a |
| >13.5 | 9 (64.29%) | 5 (35.71%) | ||
| HBV infection | ||||
| Negative | 37 (86.05%) | 6 (13.95%) | 2.6220 | .1054 a |
| Positive | 241 (74.84%) | 81 (25.16%) | ||
| Splenomegaly | ||||
| Negative | 231 (76.24%) | 72 (23.76%) | .0053 | .9421 a |
| Positive | 47 (75.81%) | 15 (24.19%) | ||
| Liver cirrhosis | ||||
| Negative | 215 (75.17%) | 71 (24.83%) | .7127 | .3985 a |
| Positive | 63 (79.75%) | 16 (20.25%) | ||
| Tumor number | ||||
| Solitary | 243 (76.66%) | 74 (23.34%) | .3211 | .5709 a |
| Multiple | 35 (72.92%) | 13 (27.08%) | ||
| Ascites | ||||
| Negative | 274 (75.90%) | 87 (24.10%) | .0000 | .5764 b |
| Positive | 4 (100.00%) | 0 (.00%) | ||
| Tumor envelope | ||||
| Complete | 179 (74.27%) | 62 (25.73%) | 1.3966 | .2373 a |
| Incomplete | 99 (79.84%) | 25 (20.16%) | ||
| TNM_stage | ||||
| I-II | 260 (94%) | 77 (89%) | 1.702 | .1921 a |
| III-IV | 18 (6%) | 10 (11%) | ||
| Tumor diameter (ln) | 1.46 (1.06,1.80) | 1.78 (1.41,2.19) | <.0001 | |
| AFP(ln) | 4.77±3.32 | 4.5415±3.15 | <.0001 | |
aPearson chi-square test.
bFisher’s exact test.
VETC: Vessels that encapsulate tumor cluster; LMR: lymphocyte to monocyte ratio; PLR: platelet-lymphocyte ratio; GPR: γ-glutamyl transpeptidase to platelet ratio; AAR: AST to ALT ratio; ALRI: AST to lymphocyte ratio index; ANRI: AST to neutrophil ratio index; APRI: AST to platelet ratio index; BLRI: ALT to lymphocyte ratio index; SII: systemic immune-inflammation index; SIRI: systemic inflammation response index.
Univariate and Multivariate Logistic Regression Analysis of VETC-Related Factors
Based on seed number 1234 in a 2:1 ratio, two hundred and forty-three and one hundred and sixteen HCC patients were categorized into the training cohort and validation cohort, respectively. There were fifty-eight and twenty-nine VETC-positive HCC patients in the training cohort and validation cohort, respectively. As we can see in Table 2, there were no significantly different baseline characteristics between the training cohort and validation cohort. The result of univariate logistic regression analysis indicated that LMR (>7.75, Odds ratio (OR) = 3.490, P = .003), PLR (>40, OR = 2.003, P = .042), neutrophil (>7, OR = 2.684, P = .062), AAR (>.86, OR = 1.769, P = .075), ALRI (>29.75, OR = 1.874, P = .052), ANRI (>12.43, OR = 1.735, P = .073), BLRI (>21.73, OR = 1.779, P = .059), AFP (OR = 1.139, P = .004), and tumor diameter (OR = 3.770, P < .0001) were potentially predictive biomarkers (Table 3). We incorporated the potentially predictive biomarkers into the multivariate logistic regression analysis. As a result, we confirmed LMR (>7.75, OR = 4.060, P = .031), neutrophil (>7, OR = 4.482, P = .025), AAR (>.86, OR = 2.158, P = .049), BLRI (>21.73, OR = 2.567, P = .042), AFP (OR = 1.103, P = .046), and tumor diameter (OR = 2.649, P = .004) as independent predictive factors for VETC (Table 3).
Table 2.
Clinical Parameters and Imagine Features of the Training Cohort and Validation Cohort.
| Parameters | Training Cohort (n = 243) | Validation Cohort (n = 122) | P-Value |
|---|---|---|---|
| VETC | |||
| Negative | 185 (76.1%) | 93 (76.2%) | .999 |
| Positive | 58 (23.9%) | 29 (23.8%) | |
| LMR | |||
| ≤7.75 | 229 (94.2%) | 116 (95.1%) | .928 |
| >7.75 | 14 (5.8%) | 6 (4.9%) | |
| PLR | |||
| ≤40 | 86 (35.4%) | 45 (36.9%) | .869 |
| >40 | 157 (64.6%) | 77 (63.1%) | |
| Neutrophil | |||
| ≤7 | 227 (93.4%) | 118 (96.7%) | .287 |
| >7 | 16 (6.6%) | 4 (3.3%) | |
| AAR | |||
| ≤.86 | 100 (41.2%) | 56 (45.9%) | .451 |
| >.86 | 143 (58.8%) | 66 (54.1%) | |
| ALRI | |||
| ≤29.75 | 179 (73.7%) | 87 (71.3%) | .725 |
| >29.75 | 64 (26.3%) | 35 (28.7%) | |
| ANRI | |||
| ≤12.43 | 158 (65%) | 76 (62.3%) | .692 |
| >12.43 | 85 (35% | 46 (37.7%) | |
| APRI | |||
| ≤.21 | 128 (52.7%) | 56 (45.9%) | .267 |
| >.21 | 115 (47.3%) | 66 (54.1%) | |
| BLRI | |||
| ≤21.73 | 127 (52.3%) | 56 (45.9%) | .3 |
| >21.73 | 116 (47.7%) | 66 (54.1%) | |
| SII | |||
| ≤545.6 | 175 (72%) | 94 (77%) | .366 |
| >545.6 | 68 (28%) | 28 (23%) | |
| AFP(ln) | 4.76 ± 3.36 | 4.64 ± 3.12 | .924 |
| Tumor diameter (ln) | 1.53 (1.13, 1.87) | 1.62 (1.13,2.03) | .21 |
VETC: Vessels that encapsulate tumor cluster; LMR: lymphocyte to monocyte ratio; PLR: platelet-lymphocyte ratio; AAR: AST to ALT ratio; ALRI: AST to lymphocyte ratio index; ANRI: AST to neutrophil ratio index; APRI: AST to platelet ratio index; BLRI: ALT to lymphocyte ratio index; SII: systemic immune-inflammation index.
Table 3.
Univariate and Multivariable Logistic Regression Analysis of Predictive Factors for VETC in the Training Cohort.
| Parameter | Univariate | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| LMR | ||||
| ≤7.75 | Reference | |||
| >7.75 | 3.49 (1.17–10.41) | .025 | 4.06 (1.14–14.48) | .031 |
| PLR | ||||
| ≤40 | Reference | |||
| >40 | 2.003 (1.02–3.91) | .042 | 2.32 (.98–5.48) | .056 |
| Neutrophil | ||||
| ≤7 | Reference | |||
| >7 | 2.684 (.95–7.56) | .062 | 4.48 (1.20–16.70) | .025 |
| AAR | ||||
| ≤.86 | Reference | |||
| >.86 | 1.77 (.95–3.31) | .075 | 2.16 (1.00–4.64) | .049 |
| ALRI | ||||
| ≤29.75 | Reference | |||
| >29.75 | 1.87 (.99–3.54) | .052 | .71 (.25–2.00) | .513 |
| ANRI | ||||
| ≤12.43 | Reference | |||
| >12.43 | 1.74 (.95–3.17) | .073 | 1.18 (.51–2.70) | .698 |
| APRI | ||||
| ≤.21 | Reference | |||
| >.21 | 1.51 (.84–2.74) | .171 | ||
| BLRI | ||||
| ≤21.73 | Reference | |||
| >21.73 | 1.78 (.98–3.24) | .059 | 2.58 (1.04–6.37) | .042 |
| SII | ||||
| ≤545.6 | Reference | |||
| >545.6 | 1.50 (.80–2.83) | .208 | ||
| AFP(ln) | 1.14 (1.04–1.24) | .004 | 1.10 (1.00–1.22) | .046 |
| Tumor diameter (ln) | 3.77 (2.05–6.92) | <.0001 | 2.65 (1.36–5.16) | .004 |
OR: Odds ratio; LMR: lymphocyte to monocyte ratio; PLR: platelet-lymphocyte ratio; AAR: AST to ALT ratio; ALRI: AST to lymphocyte ratio index; ANRI: AST to neutrophil ratio index; APRI: AST to platelet ratio index; BLRI: ALT to lymphocyte ratio index; SII: systemic immune-inflammation index.
Construction and Validation of a Novel Predictive Nomogram for VETC
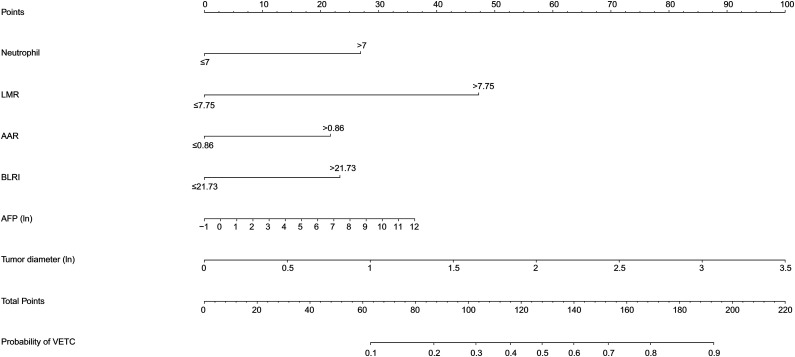
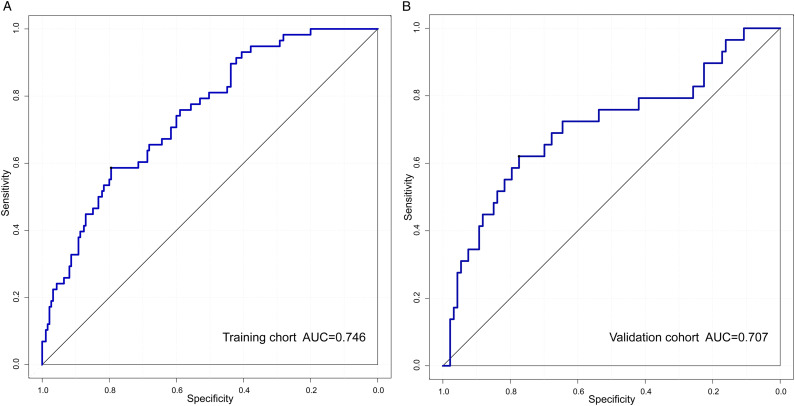
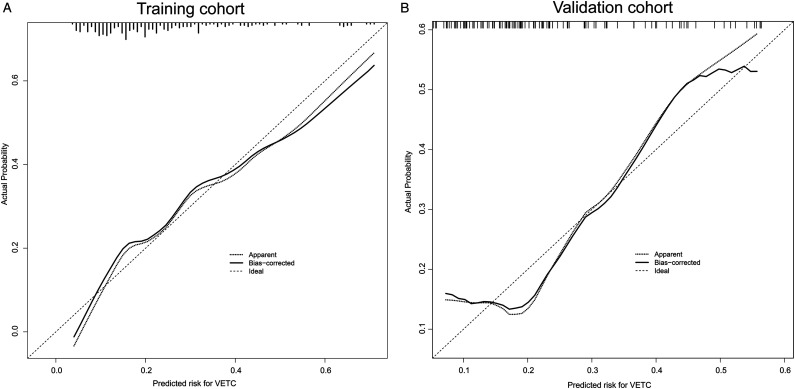
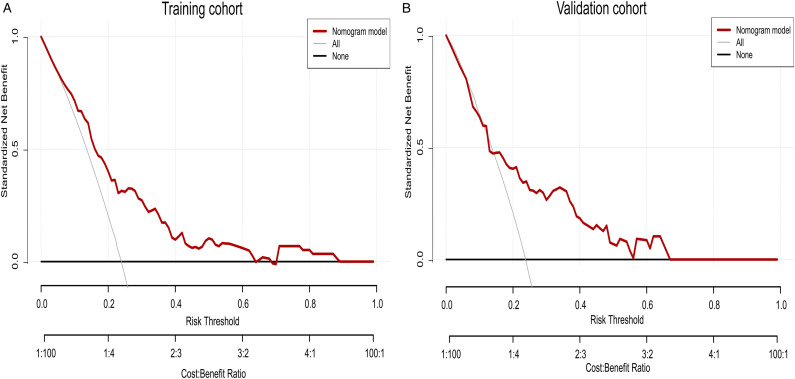
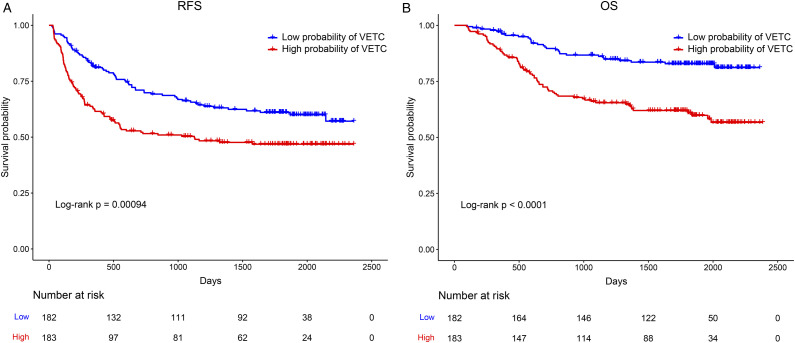
The independent predictive factors mentioned above were used to construct a novel predictive nomogram for preoperative VETC estimation (Figure 2). The nomogram showed good accuracy in preoperative VETC prediction, with a C-index of .746 and .707 in the training cohort and the validation cohort, respectively. At the same time, we also plotted the ROC curve and calculated the AUC to assess the predictive ability of the nomogram (Figure 3A). Interestingly, the AUC of the nomogram in the training cohort and the validation cohort were .746 (95%CI: .586–.795) and .707 (95%CI: .621–.774), respectively (Figure 3B). The AUC value indicated our prediction model has good sensitivity and specificity. As shown in Figure 4A and B, calibration curves indicated that the predicted probabilities of the nomogram were closely corresponded with the actual VETC status in the training [mean absolute error = .028] and validation cohorts (mean absolute error = .037). Moreover, our nomogram model could provide standardized net clinical benefits to HCC patients when the risk threshold ranged approximately from .3 to .6 in both cohorts (Figure 5A and B). We further compared the survival time of 2 different probabilities of VETC groups. As we can see in Figure 6A and B, the RFS was significantly longer in the low probability of the VETC group compared to the high probability of the VETC group (HR = 1.6779, 95%CI: 1.2323–2.2848, P = .0009). A similar result was also found in OS (HR = 2.7347, 95%CI: 1.8456–4.0522, P < .0001).
Figure 2.
Nomogram to preoperatively estimate the risk of VETC pattern in hepatocellular carcinoma. A vertical line was drawn upward to get points received for LMR, AAR, BLRI, AFP, tumor diameter, and neutrophil. The sum of six factors was presented on the total point axis, and a vertical line was also drawn downward to the probability of MVI. VETC: Vessels that encapsulate tumor cluster; LMR : lymphocyte to monocyte ratio; AAR: AST to ALT ratio; BLRI: ALT to lymphocyte ratio index.
Figure 3.
The ROC curves of the training cohort (A) and the validation cohort (B).
Figure 4.
The calibration curves for predicting VETC pattern in the training cohort (A) and the validation cohort (B), respectively.
Figure 5.
Decision curve analysis (DCA) for the nomogram in the training cohort (A) and the validation cohort (B), respectively.
Figure 6.
Kaplan–Meier curves for RFS (A) and OS (B) of patients in the low probability of VETC group and the high probability of VETC group. RFS, recurrence free survival; OS, overall survival; VETC, vessels that encapsulate tumor cluster.
Discussion
Previous studies have demonstrated that the VETC pattern is associated with recurrence and prognosis of HCC patients. In present study, LMR (>7.75, OR = 4.060, P = .031), neutrophil (>7, OR = 4.482, P = .025), AAR (>.86, OR = 2.158, P = .049), BLRI (>21.73, OR = 2.567, P = .042), AFP (OR = 1.103, P = .046), and tumor diameter (OR = 2.649, P = .004) were identified as independent predictive factors for VETC. A predictive nomogram model consisting of these independent factors was further constructed and validated for predicting VETC. Besides, the results of the C-index, AUC of ROC, calibration curves, DCA, and survival analysis confirmed that our predictive nomogram model had a good performance of predictive VETC and could bright standardized net clinical benefits to HCC patients.
In recent years, some scholars have developed predictive models to predict the occurrence of VETC. But these models were mostly based on special imaging examinations, such as Gd-EOB-DTPA-Enhanced MRI. For example, Hu established a model to predict the occurrence of VETC based on non-invasive parameters, including serum AST level (>40 Ul−1), non-rim diffuse and heterogeneous arterial phase hyperenhancement, tumor-to-liver SI ratio of 1.135 or more on AP images, and tumor-to-liver SI ratio of .585 or less on HBP images. 14 However, the results require specific equipment to achieve, which is inconvenient for the clinical doctor. Thus, we tried to construct a simple predictive model which has the characteristic of high specificity and high sensitivity, fairly suit to be widely used in the clinical diagnosis of VETC patterns. We discovered that LMR, PLR, neutrophil, AAR, ALRI, ANRI, BLRI, AFP, and tumor diameter were potentially predictive indicators for preoperative VETC estimation by evaluating the 365 HCC patients treated in our department between 2013 and 2014. LMR, neutrophil, AAR, BLRI, AFP, and tumor diameter were revealed as independent predictors of VETC in a multivariate logistic regression analysis.
Tumor promotion inflammation, tissue invasion and metastasis, and avoiding immune destruction are hallmarks of cancer. 15 Furthermore, there is no question that chronic inflammation is a major contributor to the development of HCC, let alone HBV infection in China. A variety of clinical indexes based on blood cell counts, serum biochemical tests and other laboratory tests are related to the prognosis of HCC.16,17 Moreover, increasing evidence has demonstrated the development of MVI is inseparable from the stimulation of serum inflammatory indicators. As a result, we hypothesized that the balance of serum inflammatory and immunological indexes was related to the VETC pattern. Notably, we revealed that patients with LMR, neutrophils, and BLRI were more prone to VETC. Studies have found that neutrophils secrete related proteases which degrade the extracellular matrix to promote tumor cell invasion and distant metastasis. 18 Neutrophils also release high levels of matrix metallopeptidase 9 to regulate the activities of other proteases and cytokines. For example, matrix metallopeptidase 9 promotes the release of vascular endothelial growth factor and participates in angiogenesis, which is beneficial to tumor growth and promotes distant metastasis of tumors. 19 Circulating tumor cells are cancer cells that are shed from the primary site of a malignant tumor and enter the blood circulation through the blood vessel or lymphatic system. They are usually considered as precursors for cancer metastasis. And it is believed that neutrophils might escort circulating tumor cells to enable cell cycle progression in a VCAM1-dependent manner. 20 As for LMR, it is associated with a poor prognosis for many tumors, such as distal bile duct cancer, pancreatic cancer, penile cancer, and colorectal cancer.21-24 Monocytes play a key role in the tumor microenvironment. They frequently accumulate in the stroma and differentiate into tumor-associated macrophages, which are classified as type 1 macrophages (M1) and type 2 macrophages (M2). M2 macrophages can promote tissue remodeling, tumor invasion, and metastasis. 25 Lymphocytes, including T lymphocytes cells, B lymphocytes cells, and natural killer cells, play a vital role in the anti-tumor immune response. A growing body of data suggests that CD4+Th1 cells can eradicate malignant tumor cells, and that activated CD8+ cytotoxic T lymphocytes can either directly kill cancer cells or impede angiogenesis by secreting cytotoxins; natural killer cells can directly destroy cancer cells without the need for antigen activation or antibody generation. 26 Collectively, the reason why LMR, neutrophils, and BLRI might be considered as independent predictors of VETC may be related to inflammatory and immunological cells. However, the potential mechanism needs further investigation.
ALT and AST are liver function indicators. AAR may be associated with the presence of HCV-associated liver fibrosis. 27 However, splenomegaly and liver cirrhosis were not the potential predictive markers for VETC in our study. The discrepancy is most likely since HBV is the primary cause of splenomegaly and liver cirrhosis in the present study. Interestingly, elevated AAR was associated with liver inflammatory necrosis, which in turn promoted HCC invasion and recurrence. 28 Thus, the association between VETC and AAR needs further investigation. We also identified patients with AFP and tumor diameter were more prone to VETC. AFP is a glycoprotein derived from embryonic endothelial cells currently and it is the most widely used classic molecular marker for early diagnosis of HCC. 29 The concentration of AFP in the healthy adult blood is so negligible that it cannot even be detected. When normal liver cells transfer to HCC cells, they restore the capacity to synthesize AFP and secrete it into the circulatory system. The higher the AFP level, the more active the tumor cells are. What’s more, a high level of AFP can refrain immune system and promote HCC invasiveness. 30 Consistent with earlier researches, we discovered that a high level of AFP was an independent predictor of VETC.31,32 HCC with a bigger diameter has more invasive biological properties than HCC with a small diameter. The explanation for this might be because big HCC has an irregular border, an abundant blood supply, and frequently invades adjacent vasculature, which may lead to the development of VETC. Pengfei Rong et al. discovered that tumor size of more than 5 cm was an independent predictor of VETC pattern, and we obtained consistent outcomes in our investigation. 31
After the integration of endothelial cells which encapsulate the tumor clusters with metastatic vessels, the tumor cluster can migrate to the metastatic vessels as a whole and subsequently develop distant metastasis. This biological feature predisposes VETC-positive HCC to intrahepatic and distant metastasis, as well as postoperative recurrence. Nowadays, the diagnosis of VETC pattern depends only on postoperative histology examination. Compared with previous studies, the present study had some differences. Our model incorporates several serological inflammatory and immunological indications that are readily accessible before the surgery. The emergence of VETC suggests a more aggressive biological behavior of HCC cells. Both surgical resection and radiofrequency thermal ablation are the treatment for patients with single nodular HCC ≤5 cm, but Ueno M, et al. found HCC patients with high invasiveness treated with surgical resection had a significant survival advantage over those treated with radiofrequency thermal ablation. 33 In addition, anatomical resection is more likely to achieve a negative surgical margin than non-anatomical resection. A previous study has demonstrated that for patients with VETC-positive HCC, the recurrence rate at 2 years after anatomic liver resection was significantly lower than that of non-anatomical liver resection patients (53.33% vs 36.90%). 34 Besides, preoperative prediction of VETC helps screen the best liver transplant recipients. Liver transplantation is a radical surgical modality for HCC patients who meet the Milan criteria. However, organs should be allocated to patients with the best long-term survival, due to the limited number of liver transplant donors and the presence of post-transplant recurrence. 35 The latest study confirmed that for those patients who underwent living-donor liver transplantation, the OS of VETC-positive patients was significantly lower than that of VETC-negative patients (72.0% vs 87.1%). 5 Therefore, anatomical resection rather than liver transplantation should be considered first for VETC-positive patients. Thus, the preoperative VETC pattern may help surgeons guide surgical management.
This novel prediction for the VETC pattern could also provide a strategy for formulating preoperative neoadjuvant therapy plans. Systemic therapy is the predominant therapeutic modality for unresectable HCC, including molecular targeted therapy. Tyrosine kinase inhibitors block the activity of tyrosine kinases, inhibit cell proliferation, and have been developed as several antitumor drugs. 36 Sorafenib was approved for the first-treatment of patients with unresectable HCC in 2007. 37 A study published in 2019 confirmed that sorafenib could effectively reduce the risk of death and prolong the OS of VETC-positive HCC patients. 9 Moreover, VETC-positive HCC patients were likely to be more sensitive to sorafenib.9,38 The specific mechanism was that sorafenib blocked the Raf/MEK/ERK signaling pathway and inhibited the process of endothelial cells wrapping tumor cells, thereby inhibiting the formation of VETC. Therefore, if patients with unresectable intermediate and advanced liver cancer are predicted to be VETC-positive, it is recommended to receive TKIs treatment such as sorafenib.
One limitation of the current study is that it is a single-center retrospective analysis. Although internal validation has been performed, multi-center studies with large sample sizes are required to confirm the dependability of our model. Another restriction refers to HBV-related HCC patients; consequently, we must test the universality of our model in other HCC patients with varied liver disease histories.
Conclusion
We constructed and validated a novel predictive nomogram for preoperative VETC estimation. The nomogram incorporated clinical parameters and imagine features had a good performance of predictive VETC and could bring standardized net clinical benefits to HCC patients.
Supplemental Material
Supplemetary Material for Development and Validation of a Novel Nomogram for Predicting Vessels that Encapsulate Tumor Cluster in Hepatocellular Carcinoma by Renguo Guan, Wenping Lin, Jingwen Zou, Jie Mei, Yuhua Wen, Lianghe Lu, and Rongping Guo in Cancer Control
Acknowledgments
Thank Lijie Gan for providing a lot of statistical suggestions.
Appendix.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- AAR
AST to ALT ratio
- ALRI
AST to lymphocyte ratio index
- ANRI
AST to neutrophil ratio index
- APRI
AST to platelet ratio index
- AFP
α-fetoprotein
- AUC
The area under the curve
- BLRI
ALT to lymphocyte ratio index
- C-index
Computer consistency coefficient
- DCA
Decision curve analysis
- GPR
γ-glutamyl transpeptidase to platelet ratio
- HCC
Hepatocellular carcinoma
- LMR
Lymphocyte to monocyte ratio
- MVI
Microvascular invasion
- M1
Type 1 macrophages
- M2
Type 2 macrophages
- PLR
Platelet-lymphocyte ratio
- PT
Prothrombin time;
- ROC
Receiver operating characteristic
- SII
Systemic immune-inflammation index
- SIRI
Systemic inflammation response index
- TB
Total bilirubin
- VETC
Vessels that encapsulate tumor cluster
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No. 82172579, No. 81871985); Natural Science Foundation of Guangdong Province (No. 2018A0303130098, No. 2017A030310203); Science and Technology Planning Project of Guangdong Province (No. 2017A020215112); Medical Scientific Research Foundation of Guangdong Province (No. A2017477); Science and Technology Planning Project of Guangzhou (No. 201903010017, No. 201904010479); Clinical Trials Project (5010 Project) of Sun Yat-sen University (No. 5010-2017009).
Ethics Statement: The Institutional Review Board of Sun Yat-Sen University Cancer Center approved this study (B2019-057-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Data Availability: The datasets used during the current study are available from the corresponding author on reasonable request.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Renguo Guan https://orcid.org/0000-0002-9487-7369
Rongping Guo https://orcid.org/0000-0003-2799-3463
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358-380. [DOI] [PubMed] [Google Scholar]
- 3.Fang JH, Zhou HC, Zhang C, et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015;62(2):452-465. [DOI] [PubMed] [Google Scholar]
- 4.Cheung KJ, Ewald AJ. A collective route to metastasis: Seeding by tumor cell clusters. Science. 2016;352(6282):167-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawasaki J, Toshima T, Yoshizumi T, et al. Prognostic impact of vessels that encapsulate tumor cluster (VETC) in patients who underwent liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2021;28(13):8186-8195. [DOI] [PubMed] [Google Scholar]
- 6.Renne SL, Woo HY, Allegra S, et al. Vessels encapsulating tumor clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology. 2020;71(1):183-195. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Wei W, Huang C, et al. A new horizon in risk stratification of hepatocellular carcinoma by integrating vessels that encapsulate tumor clusters and microvascular invasion. Hepatol Int. 2021;15(3):651-662. [DOI] [PubMed] [Google Scholar]
- 8.Chen ZY, Guo ZX, Lu LH, et al. The predictive value of vessels encapsulating tumor clusters in treatment optimization for recurrent early-stage hepatocellular carcinoma. Cancer Med. 2021;10(16):5466-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang JH, Xu L, Shang LR, et al. Vessels that encapsulate tumor clusters (VETC) pattern is a predictor of sorafenib benefit in patients with hepatocellular carcinoma. Hepatology. 2019;70(3):824-839. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Wu X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. 2020;9(21):8086-8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Fan Y, Wang X, et al. Gd-EOB-DTPA-enhanced MRI radiomics to predict vessels encapsulating tumor clusters (VETC) and patient prognosis in hepatocellular carcinoma. Eur Radiol. 2021;32:959-970. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y, Yu Y, Wang X, et al. Texture analysis based on Gd-EOB-DTPA-enhanced MRI for identifying vessels encapsulating tumor clusters (VETC)-positive hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:349-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Calster B, Wynants L, Verbeek J, et al. Reporting and interpreting decision curve analysis: A guide for investigators. Eur Urol. 2018;74(6):796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Y, Yu Y, Hu M, et al. Imaging features based on Gd-EOB-DTPA-enhanced MRI for predicting vessels encapsulating tumor clusters (VETC) in patients with hepatocellular carcinoma. Br J Radiol. 2021;94(1119):20200950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646-674. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Hu X, Xiao L, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. 2021;25(2):421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23(3):141-148. [DOI] [PubMed] [Google Scholar]
- 19.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553-557. [DOI] [PubMed] [Google Scholar]
- 21.Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg. 2018;55:128-138. [DOI] [PubMed] [Google Scholar]
- 22.Hu C, Bai Y, Li J, et al. Prognostic value of systemic inflammatory factors NLR, LMR, PLR and LDH in penile cancer. BMC Urol. 2020;20(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis. Clin Chim Acta. 2018;481:142-146. [DOI] [PubMed] [Google Scholar]
- 24.Miyahara Y, Takashi S, Shimizu Y, Ohtsuka M. The prognostic impact of neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) in patients with distal bile duct cancer. World J Surg Oncol. 2020;18(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: Radiotherapy versus chemo- and immunotherapies. Front Immunol. 2017;8:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santori FR. The immune system as a self-centered network of lymphocytes. Immunol Lett. 2015;166(2):109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lok AS, Ghany MG, Goodman ZD, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: Results of the HALT-C cohort. Hepatology. 2005;42(2):282-292. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZX, Jiang CP, Cao Y, Zhang G, Chen WB, Ding YT. Preoperative serum liver enzyme markers for predicting early recurrence after curative resection of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14(2):178-185. [DOI] [PubMed] [Google Scholar]
- 29.Nault JC, Villanueva A. Biomarkers for hepatobiliary cancers. Hepatology. 2021;73(suppl 1):115-127. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Liu Y, Yan Z, et al. A nomogram predicting pulmonary metastasis of hepatocellular carcinoma following partial hepatectomy. Br J Cancer. 2014;110(5):1110-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Z, Li H, Zhao H, et al. Preoperative CT for characterization of aggressive macrotrabecular-massive subtype and vessels that encapsulate tumor clusters pattern in hepatocellular carcinoma. Radiology. 2021;300(1):219-229. [DOI] [PubMed] [Google Scholar]
- 32.Ziol M, Pote N, Amaddeo G, et al. Macrotrabecular-massive hepatocellular carcinoma: A distinctive histological subtype with clinical relevance. Hepatology. 2018;68(1):103-112. [DOI] [PubMed] [Google Scholar]
- 33.Ueno M, Hayami S, Shigekawa Y, et al. Prognostic impact of surgery and radiofrequency ablation on single nodular HCC 5 cm: Cohort study based on serum HCC markers. J Hepatol. 2015;63(6):1352-1359. [DOI] [PubMed] [Google Scholar]
- 34.Yang JR, Zhong JT, Liu TQ, et al. The diagnostic value of preoperative contrast-enhanced ultrasound and intraoperative fast frozen pathological sections for VETC carcinoma nest-type hepatocellular carcinoma and the best surgical approach. Chinese Journal of New Clinical Medicine. 2021;14(03):262-266. [Google Scholar]
- 35.Pavel MC, Fuster J. Expansion of the hepatocellular carcinoma Milan criteria in liver transplantation: Future directions. World J Gastroenterol. 2018;24(32):3626-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36(7):422-439. [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Liang J, Meng YM, et al. Vascular CXCR4 expression promotes vessel sprouting and sensitivity to sorafenib treatment in hepatocellular carcinoma. Clin Cancer Res. 2017;23(15):4482-4492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemetary Material for Development and Validation of a Novel Nomogram for Predicting Vessels that Encapsulate Tumor Cluster in Hepatocellular Carcinoma by Renguo Guan, Wenping Lin, Jingwen Zou, Jie Mei, Yuhua Wen, Lianghe Lu, and Rongping Guo in Cancer Control