Abstract
Radiation therapy is an effective method to kill cancer cells and shrink tumors using high-energy X-ray or γ-ray. Radiation pneumonitis (RP) is one of the most serious complications of radiation therapy for thoracic cancers, commonly leading to serious respiratory distress and poor prognosis. Here, we prepared curcumin-loaded mesoporous polydopamine nanoparticles (CMPN) for prevention and treatment of RP by pulmonary delivery. Mesoporous polydopamine nanoparticles (MPDA) were successfully synthesized with an emulsion-induced interface polymerization method and curcumin was loaded in MPDA via π‒π stacking and hydrogen bonding interaction. MPDA owned the uniform spherical morphology with numerous mesopores that disappeared after loading curcumin. More than 80% curcumin released from CMPN in 6 h and mesopores recovered. CMPN remarkably protected BEAS-2B cells from γ-ray radiation injury by inhibiting apoptosis. RP rat models were established after a single dose of 15 Gy 60Co γ-ray radiation was performed on the chest area. Effective therapy of RP was achieved by intratracheal administration of CMPN due to free radical scavenging and anti-oxidation ability, and reduced proinflammatory cytokines, high superoxide dismutase, decreased malondialdehyde, and alleviated lung tissue damages were observed. Inhaled CMPN paves a new avenue for the treatment of RP.
KEY WORDS: Radiation pneumonitis, Curcumin, Mesoporous, Polydopamine, Nanoparticle, Pulmonary delivery, Inhalation, Free radical scavenging
Graphical abstract
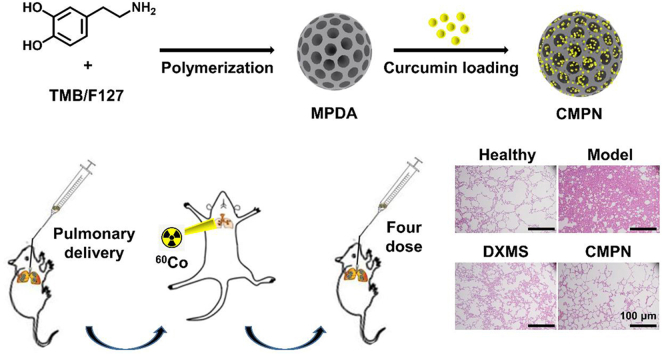
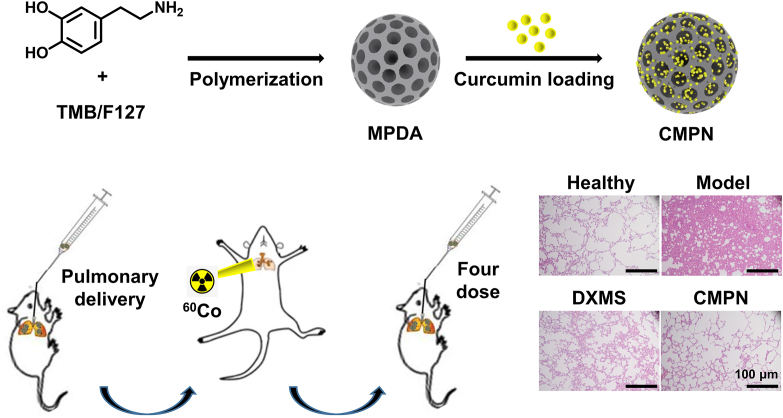
Mesoporous polydopamine (MPDA) was synthesized with self-polymerization and curcumin was encapsulated to get curcumin-loaded MPDA nanoparticles (CMPN). The formulation was intratracheally administrated against radiation pneumonitis by free radical scavenging and anti-oxidation.
1. Introduction
Radiation pneumonitis (RP) is a typical inflammation of the lung following the stereotactic body radiotherapy for thoracic cancers, including lung cancer, breast cancer, esophageal cancer, thymic tumors, mesothelioma, lymphomas, and other malignant tumors on the chest1, 2, 3. RP usually develops in the first few weeks to months from initiation of radiotherapy and shows symptomatic changes such as cough, shortness of breath, and fever, with or without changes in pulmonary function tests2,4. RP affects 15%–40% of people who undergo radiotherapy for lung cancer and these patients have to be tormented by RP besides cancer5, 6, 7. RP is likely to take severe respiratory problems, such as acute respiratory distress syndrome (ARDS) that is a potentially life-threatening condition4,8. Moreover, it would develop to radiation pulmonary fibrosis in the late stage, even leading to respiratory failure9,10. Currently, the clinical treatment of RP aims to decrease inflammation using high-dose of corticosteroids; however, the side effects are obvious, such as weight gain, hyperglycemia, sleep disturbances, mood changes, and edema, so that corticosteroids are not suitable for the prevention of RP or long-term application11,12. Therefore, a safe and effective treatment of RP is urgently needed.
Radiation injury is related to the production of reactive oxygen species (ROS), oxidative stress, and the secretion of proinflammatory cytokines13. Curcumin, a polyphenolic compound, mainly extracted from the rhizome of turmeric, is reported to alleviate radiation injury by anti-inflammation, anti-oxidation, and free radical scavenging14. It would become a non-hormonal anti-inflammatory candidate for the treatment of RP15. However, the clinical application of curcumin is limited for its poor water solubility, instability, low bioavailability, and rapid clearance in the body16. Many efforts have been tried to overcome these problems, such as local delivery of curcumin and nanoparticle formulations17, 18, 19, 20. Local administration of curcumin may be a fundamental solution to its biomedical application because it takes effects in regional area. Although nanoparticle formulations could increase the water dispersing capability and bioavailability of curcumin, the drug loading efficiency and the biocompatibility of vehicles are still challenges21,22.
Polydopamine (PDA) is a bioinspired fascinating polymer with multiple interesting features including the simple preparation protocol, biocompatibility, free radical scavenging23, 24, 25, and photothermal/photoacoustic properties26. Moreover, the free radical scavenging ability of PDA makes it be a suitable candidate for prevention of RP, which has been applied for therapy of inflammations27,28. PDA could be degraded into its monomers and oligomers but no obvious pharmacological effect of dopamine, exhibiting excellent biosafety29. Another advantage of PDA is that it owns the strong molecular adsorption capacity for bioactive chemicals. More importantly, PDA can be easily prepared to mesoporous nanoparticles that are suitable for pulmonary delivery due to high lung deposition28,30. Porous carriers can deliver high doses of drugs depositing in the lung after inhalation and they provide the opportunity of drug controlled release in the lung31.
Pulmonary drug delivery is an attractive noninvasive method by inhalation. Inhalation is commonly regarded as the most efficient treatment strategy for lung diseases such as asthma, pneumonia, chronic obstructive pulmonary disease (COPD) and lung cancer due to direct high-dose drug delivery into the lung19,32, 33, 34. Dry powder inhalers (DPIs) are portable solid powder delivery units35,36. The stability of loaded drugs is usually better in DPIs than in aerosols and nebulizers. DPIs offer good patient convenience and compliance. Therefore, the inhalation of curcumin powder formulations may become an ideal strategy for the prevention of RP.
Here, curcumin-loaded mesoporous PDA nanoparticles (CMPN) were prepared and intratracheally (i.t.) administered to the lung for prevention and treatment of RP (Fig. 1). To our knowledge, this is the first report of pulmonary delivery of curcumin and polydopamine formulations for the prevention and treatment of RP. CMPN can be directly delivered to target the lung tissue by inhalation, enhancing the treatment efficiency of RP by the free radical scavenging and anti-oxidation effect of both drugs and carriers. The characteristics of CMPN were thoroughly investigated and the high therapeutic efficiency and the mechanism were evidenced by the pharmacological study.
Figure 1.
Schematic illustration of inhaled curcumin-loaded mesoporous polydopamine nanoparticles (CMPN) against radiation pneumonitis.
2. Materials and methods
2.1. Materials
Curcumin and 1,3,5-trimethylbenzene (TMB) were purchased from Beijing InnoChem Science &Technology Co., Ltd., Beijing, China. Dopamine hydrochloride was obtained from Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China. Pluronic F127 was purchased from Shandong Yousuo Chemical Technology Co., Ltd., Linyi, China. 1,1-Diphenyl-2-trinitrophenylhydrazine (DPPH) was purchased from Tokyo Chemical Industry, Tokyo, Japan. Dexamethasone (DXMS) was from Tianjin King York Pharmaceutical Co., Ltd., Tianjin, China. HLC-8 media were from Gibco, Grand Island, NY, USA. ELISA kits for rat TNF-α, IL-6, IL-1β and TGF-β1 were obtained from Beijing Neobioscience Technology Co., Ltd., Beijing, China. Purified water was prepared using Heal Force Super NW Water System (Shanghai Canrex Analytic Instrument Co., Ltd., Shanghai, China). All other reagents were of analytical grade without further purification.
2.2. Cells and animals
A normal bronchial epithelium cell line (BEAS-2B) was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Male Sprague–Dawley rats (170–180 g) were provided by the Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China. All experimental procedures were executed according to the protocols approved by the Ethical Committee of Academy of Military Medical Sciences.
2.3. Synthesis of mesoporous polydopamine nanoparticles
Mesoporous polydopamine (PDA) nanoparticles (MPDA) were prepared by emulsion-induced interface polymerization37,38. Firstly, Pluronic F127 (0.5 g) and TMB (0.8 mL) were added into the mixed solvent (water/ethanol, 1:1, v/v, 50 mL). The mixture was magnetically stirred for 30 min to form a white emulsion followed by addition of ammonia (1.875 mL) and dopamine hydrochloride (0.75 g) and then the reaction was continued for 2 h at room temperature. The suspension was centrifuged for 10 min at 14,800×g (H2-16KR, Hunan Kecheng Instrument Equipment Co., Ltd., Changsha, China). The precipitates were collected and washed with water/ethanol (1:1, v/v) twice and the emulsion templates were removed with ethanol/acetone (2:1, v/v) under sonication (30 min, three times). The precipitates were dried at 40 °C for 4 h to obtain black MPDA powders.
2.4. Preparation and characterization of CMPN
Curcumin (100 mg) was dissolved in ethanol (100 mL) and MPDA (100 mg) was added. The mixture was stirred overnight at room temperature and then dried under vacuum to obtain the powders of curcumin mesoporous polydopamine nanoparticles (CMPN). Curcumin was analyzed with a high-performance liquid chromatography (HPLC, Agilent 1260, USA) as the following conditions: a Diamonsil C18 ODS column (250 mm × 4.6 mm, 5 μm), a mobile phase of acetonitrile/water/acetic acid (50:49:1, v/v/v) at a flow rate of 1 mL/min, an injection volume of 20 μL, the detection wavelength of 425 nm, and the column temperature at 30 °C. For the determination of drug loading efficiency, CMPN was thoroughly dispersed in ethanol under ultrasound and centrifuged (14,800×g, 10 min) to separate curcumin, and then the supernatant was filtered for curcumin determination using HPLC. The drug loading efficiency was calculated according to formula as shown in Eq. (1).
| Loading efficiency (%) = Total curcumin/Total CMPN × 100 | (1) |
The particle sizes and zeta potentials of CMPN were measured with the dynamic light scattering (DLS) method on Zetasizer Nano ZS (Malvern, UK) at 25 °C. The morphology of CMPN was inspected using a transmission electron microscope (TEM, H-7650, Hitachi, Tokyo, Japan) after staining with 2% sodium phosphotungstate solutions. The surface structures of MPDA and CMPN were observed under a scanning electron microscope (SEM, S-4800, Hitachi, Tokyo, Japan). In addition, the simulated lung deposition of CMPN powders was measured at 60 L/min using the next generation impactor (NGI, TPK 2000R, Copley, British). The deposited powders in every stage were collected and curcumin was separately determined as above. The fine particle fraction (FPF) of CMPN powders was calculated after the drug contents in Stages 3–7 were divided by the contents in the whole system. The FPF and mass median aerodynamic diameter (MMAD) of CMPN were calculated using the CITDAS software (Version 3.10 Wibu USP32/Ph. Eur. 6.0, the COPLEY company, Nottingham, UK).
The interaction of curcumin and CMPN was identified with the Fourier Transform Infrared spectrometry (FTIR, Spectrum Two, PerkinElmer, USA). An X-ray diffraction (XRD) method was used to investigate the crystalline phase of curcumin in CMPN with the Ni filtered Cu Kα radiation and the XRD instrument (Rigaku Ultiam IV, Japan).
2.5. In vitro drug release study
CMPN (containing ca. 1 mg curcumin) was added to a triangular flask and suspended in 50 mL of the simulated lung fluid containing 0.05% Tween 8039. The flask was placed on a shaker (100 rpm, THZ-D, Taicang Laboratory Instrument Factory, Suzhou, China) at 37 °C for in vitro drug release investigation. At the predetermined time points (0.25, 0.5, 1, 2, 4, 6, 8 and 12 h), an aliquot (1 mL) of the media was withdrawn and replaced with the fresh media of equal volume. The sample was centrifuged at 14,800×g for 10 min to exclude the nanoparticles. The supernatant was filtered through a 0.22-μm filter and analyzed with HPLC to determine released curcumin. Additionally, the morphological changes of CMPN were observed with SEM at different release time points. The experiments were performed in triplicates.
2.6. Assay of free radical scavenging ability
The free radical scavenging ability of MPDA and curcumin were tested using the DPPH method40. Briefly, an ethanol solution of DPPH (0.05 mg/mL) was prepared in the dark. MPDA (3 mg) or curcumin (1 mg) was added to the ethanol solution (1 mL). After incubation for 30 min in the dark, the MPDA-contained solution was centrifuged (14,800×g, 10 min) to separate the particles. The supernatant was tested with an electron spin resonance (ESR) spectrometer (Bruker-A300, Karlsruhe, Germany). The curcumin-contained solution was directly tested without centrifugation. The ESR spectra were recorded with the following conditions: microwave power, 1 mW; modulation amplitude, 1 G; sweep field width, 300 G; sweep time, 180 s. The ethanol solution of DPPH was used as the control.
2.7. Investigation of CMPN cytotoxicity
The cytotoxicities of curcumin and CMPN were investigated on BEAS-2B cells using Cell Counting Kit-8 (CCK-8, Gen-View Sciences Inc., USA). The BEAS-2B cells were seeded in 96-well plates at a density of 104 cells/well and incubated for adherence. Curcumin and CMPN were dissolved or dispersed in dimethyl sulphoxide (DMSO), respectively, and then diluted with the culture media to a series of concentrations. The doped culture media were added to the plates where the final DMSO concentration kept less than 0.1%. The cells were incubated for 24 h and the cell viability was calculated according to the absorbance of cells after 2 h incubation with 10% CCK-8 regents. The absorbance at 450 nm was detected using a microplate reader (ELX800, BioTek Instrument, USA).
2.8. Analysis of BEAS-2B cell apoptosis after irradiation
BEAS-2B cells were seeded in 6-well plates at a density of 2 × 105 cells/well and incubated for 24 h. The cells were treated with curcumin or CMPN and the final concentration of curcumin was maintained at 31.25 μmol/L. After incubation for 24 h, the BEAS-2B cells were irradiated with 15 Gy 60Co γ-ray (Beijing Institute of Radiation Medicine). Twenty-four hours later, cell apoptosis was detected with a flow cytometer (BD II, BD Biosciences, Franklin Lakes, NJ, USA) after V-FITC/PI staining.
2.9. Pharmacodynamic study
Twenty rats were randomly divided into 4 groups: the healthy group (no irradiation), the model group (irradiation without treatment), the DXMS group (irradiation with i.t. dexamethasone), and the CMPN group (irradiation with i.t. CMPN). Irradiation was performed on the area of rat chest with a single dose of 15 Gy 60Co γ-ray at a dose rate of 98.16 cGy/min. DXMS (2.5 mg/kg) or CMPN (containing 2 mg curcumin) was i.t. administered to the rats 5 h pre-irradiation and once a week for consecutive 4 weeks post-irradiation. The CMPN was i.t. administered at 5 h pre-irradiation for consideration of drug release (Fig. 2G). The time period of 5 h would be sufficient to get ready for clinical thoracic radiotherapy. Before the i.t. administration of DXMS or CMPN, the rats were anesthetized with isoflurane using a gas anesthesia system (Shanghai Yuyan Instruments Co., Ltd., Shanghai, China), and then we sprayed the drug formulations into the lung through the trachea with an insufflator (Model DP-4, Penn-Century, Inc., USA). During the experimental period, the rat living states were carefully observed and the body weight was recorded. In addition, the complete blood counting was performed for investigation of the hematopoietic system injury caused by radiation. On Days −1, 1, 3, 6, 10, 14, 21 and 28, an aliquot (20 μL) of tail vein blood was collected and mixed with the diluent (2 mL) for analysis, respectively. The white blood cells (WBC), platelets (PLT), red blood cells (RBC) and neutrophils (NE) in the blood were counted with a CelltacE automatic blood cell analyzer (MEK-7222k, Nihon Kohden, Japan).
Figure 2.
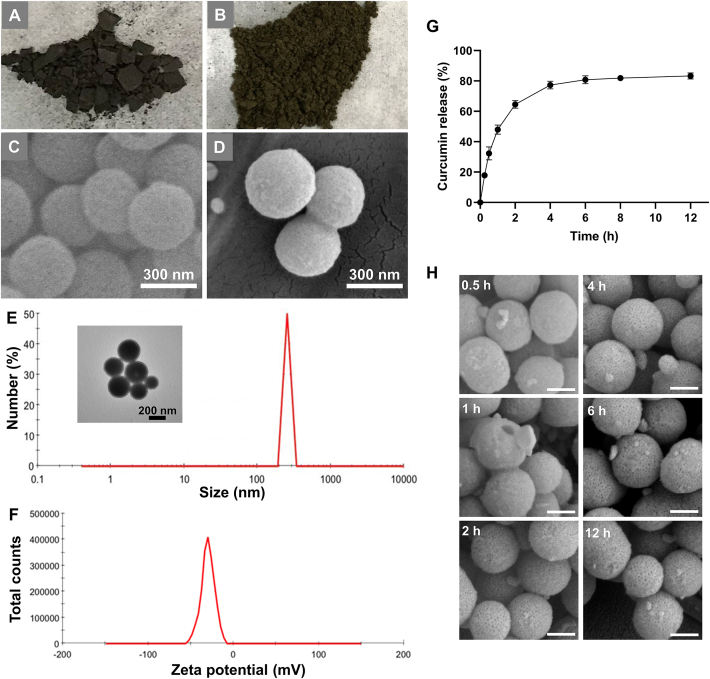
Characteristics of CMPN. Appearance of MPDA powders (A) and CMPN powders (B). SEM images of MPDA (C) and CMPN (D). (E) Size distribution of CMPN (the inserted picture was the TEM image of CMPN). (F) Zeta potential of CMPN. (G) Release profile of curcumin from CMPN, the data are presented as mean ± SD (n = 3). (H) SEM images of residual CMPN after release for different time, Scale bar = 200 nm.
2.10. Histopathological examination
The rats were sacrificed at the end of the pharmacodynamic study and the whole lung tissues were excised. The upper lobe of the right lung was immersed in 10% formalin solutions for 24 h and then embedded in paraffin. After hematoxylin and eosin (H&E) and Masson staining, the pathological sections were observed under a microscope (BDS200-FL, Chongqing Optec Instrument Co., Ltd., Chongqing, China).
2.11. ELISA measurement
The middle lobes of the right lung of rats were homogenized with a homogenizer (KI-II, Servicebio, Wuhan, China) at 60 Hz for 4 min, and then centrifuged at 14,800×g for 20 min. The supernatants were collected for detection of TNF-α, IL-6, IL-1β and TGF-β1 with ELISA kits according to the manufacturer's instructions. The levels of superoxide dismutase (SOD), malondialdehyde (MDA) and total proteins in the lung homogenates were also detected with ELISA kits.
2.12. Statistical analysis
All data were expressed as mean ± standard deviation (SD). The results were calculated statistically using the SPSS 19.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) with the LSD test was used to identify differences (P < 0.05 or P < 0.01) between the data.
3. Results and discussion
3.1. Characteristics of CMPN
MPDA was successfully prepared by dopamine polymerization on the interface of the emulsion templates. TMB acted as the organic phase core to form the emulsion. Thanks to the π-electron rich structure of TMB, dopamine self-assembled on the surface of the emulsion based on the π−π stacking interaction and polymerized to PDA under the alkaline circumstance. After removal of TMB and pluronic-F127, the mesoporous structure, i.e., MPDA, was obtained. We verified the chemical structure of MPDA with FTIR, exhibiting obvious signals of stretching vibration of C—C, C—N and the indole ring (Supporting Information Fig. S1). CMPN was further obtained after curcumin was loaded in MPDA mainly based on π‒π stacking and hydrogen bonding interactions. Interestingly, MPDA was black solid powders (Fig. 2A), while CMPN was yellowish-brown due to the doping of a large amount of curcumin (Fig. 2B). Moreover, many mesopores appeared on the surface of spherical MPDA (Fig. 2C), but the mesopores disappeared in CMPN, which should be attributed to the filling of mesopores with curcumin (Fig. 2D). Thanks to the mesoporous structure and high specific surface area of MPDA, the curcumin loading efficiency was high to about 47%. In recent years, mesoporous materials, characterized with the unique pore size, high surface area and pore volume, are widely explored as drug carriers. The large inner space and many surface pores of mesoporous materials provide the basis of great drug entrapment and adsorption, leading to high drug loading efficiency. The TEM image further revealed that CMPN were uniform spheres (inserted picture in Fig. 2E). The average particle size of CMPN was 290.73 ± 29.73 nm (n = 3) with uniform size distribution (PDI = 0.216 ± 0.017, n = 3) and the zeta potential was −26.4 ± 3.1 mV (n = 3, Fig. 2E and F). The zeta potential value of about −30 mV is considered optimum for nanoparticle stability41.
The release of water-insoluble drugs is a key factor for clinical applications42. Curcumin release from CMPN was relatively stable and rapid with more than 80% accumulative release within 6 h (Fig. 2G), which would improve the biological protection from irradiation. Interestingly, we found that the surface mesopores of the particles gradually reappeared with curcumin release by SEM observation of residual CMPN particles (Fig. 2H).
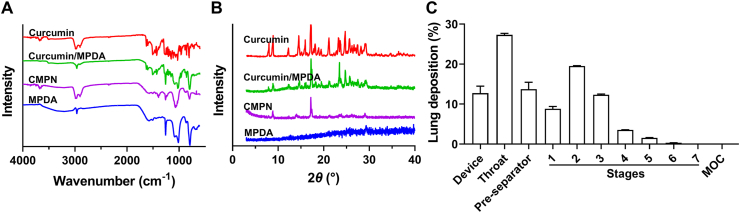
The water solubility of curcumin is often influenced by its state, which would likely affect its release behavior, also in vivo pharmacological effect42,43. In this study, the state of curcumin in CMPN was identified by multiple methods. The FTIR spectra of curcumin showed a characteristic peak of phenolic hydroxyl stretching vibration at 3508 cm−1 (Fig. 3A). The physical mixture of MPDA/curcumin also showed the specific peak of phenolic hydroxyl while the specific peak disappeared in CMPN, which might be attributed to the hydrogen bonding between curcumin and MPDA. Additionally, the XRD spectra showed that MPDA was amorphous, while curcumin had a crystalline structure. The crystalline peak of curcumin still existed in the physical mixture but almost disappeared in CMPN (Fig. 3B). The difference between the mixture and CMPN indicated that the crystalline curcumin in CMPN was little. Amorphous powder inhalers offer the benefits of increased in vivo pharmacological effect for poorly soluble drugs44.
Figure 3.
State of curcumin in CMPN and the simulated drug lung deposition. FTIR spectra (A) and XRD spectra (B) of curcumin, the physical mixture of curcumin/MPDA, CMPN, and MPDA. (C) Simulated drug lung deposition of CMPN powders. Data are presented as mean ± SD (n = 3).
Effective lung deposition is one of the key elements for inhalation drug formulation. The FPF of inhaled powders is an important parameter for evaluation of lung deposition. The in vitro simulated experiment indicated the FPF of CMPN powders was 29.83 ± 0.24%, and the MMAD was 5.17 ± 0.04 μm, indicating that CMPN was appropriate for pulmonary delivery (Fig. 3C). Generally, the droplets or particles of 1–5 μm in aerodynamic diameter are likely to deposit in the small airways and alveoli and more than 50% of the 3 μm diameter particles deposit in the alveolar region33,45. The DLS size of CMPN was several hundred nanometers. So did the electron microscopic size of them. However, PDA usually exhibits adhesive property46, leading to CMPN assembling to microparticles (Fig. 2D and E). The aggregated CMPN powders had suitable MMAD (5.17 μm) for lung deposition. Therefore, CMPN can be inhaled in dry powders. Moreover, porous nanoparticle-aggregate-based particles are recognized as one of the most promising carriers for controlled drug release in the lung31. The mesoporous particles possess the smaller aerodynamic particle size compared to the solid particle with the same size, leading to more lung deposition and avoiding the phagocytosis of alveolar macrophages32.
3.2. Strong free radical scavenging ability of MPDA and curcumin
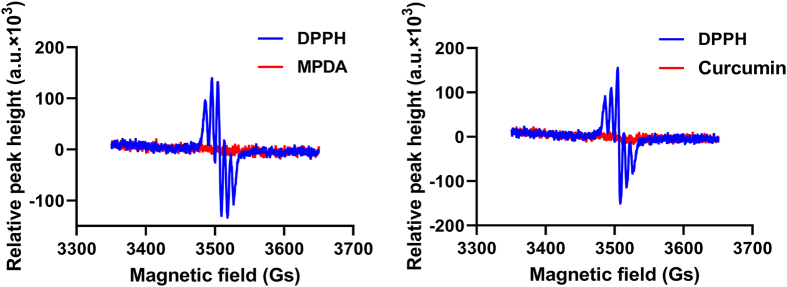
DPPH assay is often used to measure the free radical scavenging ability of compounds, where the reaction can maintain stable for hours in solutions40. Both MPDA and curcumin turned the color of DPPH solutions to light yellow from pink. Moreover, the ESR peaks of DPPH disappeared after incubation with MPDA and curcumin, indicating that the free radicals were scavenged (Fig. 4). The strong free radical scavenging ability of MPDA and curcumin would benefit CMPN scavenging the free radicals in the lung tissues after γ-ray irradiation and alleviating radiation injury.
Figure 4.
ESR spectra of DPPH after incubation with MPDA and curcumin.
3.3. High safety and radiation protection effect of CMPN on BEAS-2B cells
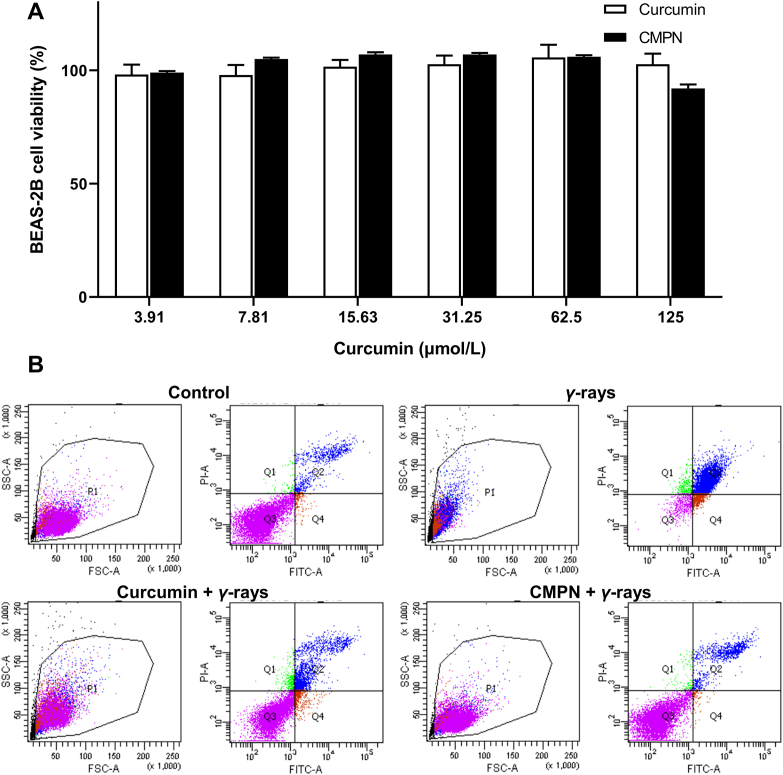
BEAS-2B cells are normal bronchial epithelium cells, which are commonly used as the subjects of inhalation toxicity investigation. Curcumin showed high safety with no cytotoxicity on BEAS-2B cells even at a high concentration of 125 μmol/L (Fig. 5A). The effect of CMPN on BEAS-2B cells was very similar to that of curcumin, indicating the high safety of CMPN (Fig. 5A). Moreover, the in vivo safety of inhaled curcumin had been already confirmed by the previous reports47,48. Inhaled curcumin is safe in animals even when the animals are exposed to the material over long periods. Therefore, CMPN should be safe when inhalation to the lung.
Figure 5.
High safety and radiation protection effect of CMPN on BEAS-2B cells. (A) Cell viability of BEAS-2B cells after incubation with curcumin and CMPN. (B) Flow cytometry plots of BEAS-2B cells after FITC/PI staining. Data are presented as mean ± SD (n = 5).
The aim of radiation therapy is to induce cancer cell apoptosis and death; however, it simultaneously leads to unwanted injury on normal cells49. In this study, BEAS-2B cells were used to explore the protection effect of CMPN. The flow cytometric results remarkably showed the radiation protection effect of curcumin and CMPN with the apoptosis rates of 19.1% and 13.45%, respectively, compared to the high apoptosis rate (>30%) of the control cells after γ-ray irradiation (Fig. 5B). The higher protection effect of CMPN than curcumin could be attributed to the stronger free radical scavenging and anti-oxidation ability of CMPN that rapidly released curcumin to the surroundings. Besides the rapid curcumin release from CMPN, another advantage of CMPN could be enhanced mucus penetration and cell uptake. Recent studies have verified the superior mucus penetrating ability and cell uptake of PDA-modified nanoparticles50,51.
3.4. Protection of hematopoietic system by CMPN
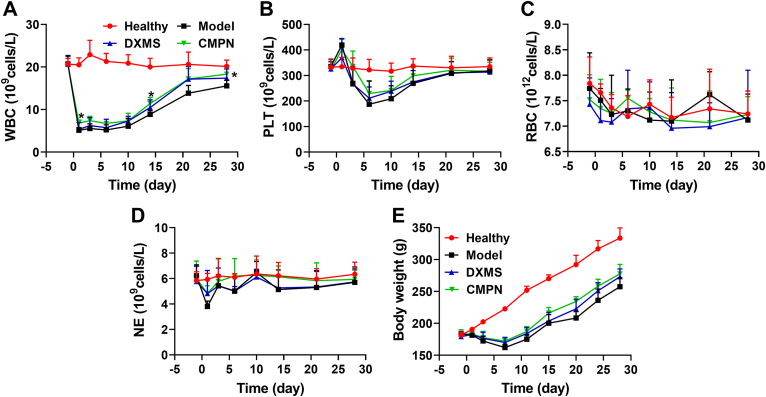
The hematopoietic system is extremely sensitive to ionizing radiation with remarkable decrease of bone marrow cells (BMC) and circulating peripheral blood cells52. Therefore, the counting of blood cells is an important method to evaluate ionizing radiation-induced injury and the protection and treatment efficiency of medicines. All the radiation-exposed rats showed the sharp decrease of WBC compared to the heathy ones, especially in the model group (Fig. 6A). Their WBC began to recover on Day 7, where DXMS and CMPN improved the recovery. The WBC in the CMPN group was significantly higher than that in the model group at some time points, such as Days 1, 14, and 28 (P < 0.05), but there was no significant difference between the CMPN and DXMS groups. PLT exhibited the similar profiles to WBC and no significant difference was shown between the CMPN and DXMS groups too (Fig. 6B). Therefore, the pulmonary delivery of DXMS and CMPN alleviated the hematopoietic damage induced by chest ionizing radiation to some extent. However, RBC and NE did not show significant difference between all groups (Fig. 6C and D), indicating that local ionizing radiation may not hurt the hematopoietic system as seriously as the systemic radiation53,54. The radiation injury is also highly related to the radiation dose. Clinical radiation therapy commonly applies 45–60 Gy for the treatment of breast, head, and neck cancers, although the doses are divided into multiple smaller doses. In the preclinical studies of RP, the radiation conditions are different, although 15 Gy 60Co γ-ray radiation was usually used for establishment of RP animal models16,55. In the preliminary study, we had screened the radiation dose for establishment of RP rat models, involving 10, 15, and 20 Gy. Finally, we found that the dose of 15 Gy was suitable for establishment of moderate RP with little systemic damage.
Figure 6.
Cell counts in the peripheral blood and the body weight of rats. WBC (A), PLT (B), RBC (C), and NE (D) in the peripheral blood. (E) Body weight profiles of rats. The data are presented as mean ± SD (n = 5). ∗P < 0.05 between the CMPN and Model groups.
The body weight of irradiated rats decreased until Day 7 and then recovered with the increasing profiles (Fig. 6E), where the DXMS and CMPN groups recovered faster than the model group. Moreover, the CMPN group maintained the highest body weight among the three irradiation groups, indicating the best prevention and treatment of irradiation injury. The living state of rats represents their whole health states. The rats in the model group exhibited obvious dispiritedness, anorexia, piloerection, and delayed reactions, while DXMS and CMPN alleviated the above adverse states. Additionally, the mental state of the rats in the CMPN group was better than that in the DXMS group and the formers showed significant activity, normal breathing, and smooth fur.
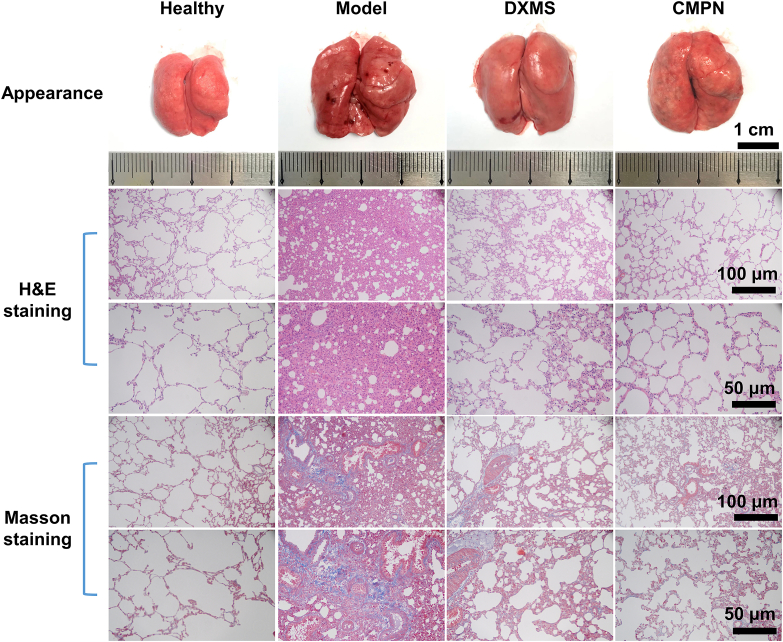
3.5. Highly effective anti-lung injury effect of CMPN
Ionizing radiation seriously damages the lung tissues, leading to RP10. The lung tissues of RP showed obvious hemorrhages, sporadic white nodules, and diffuse alveolar congestion, more importantly, remarkable pulmonary fibrosis, compared to the ruddy and smooth appearance and the clear normal lung alveolar septum and well distributed alveolar walls of the healthy lung tissues (Fig. 7). Radiation-induced pulmonary fibrosis is an emerging disease, which destructs the lung tissue progressively and irreversibly. Both DXMS and CMPN significantly alleviated the hemorrhaging and pulmonary fibrosis in the lung tissues compared to the model group, although a little fibrosis was still present. The CMPN group seemed better than the DXMS group with no hemorrhaging, little infiltrating inflammatory cells and the clear alveolar structure. All the irradiated groups showed enlarged lung tissues compared to the healthy group, probably resulting from the thickened texture and edema. Therefore, CMPN is an effective medicine for prevention of pulmonary fibrosis induced by irradiation.
Figure 7.
Appearance, H&E staining and Masson staining of lung tissues.
3.6. High anti-inflammation effect of CMPN
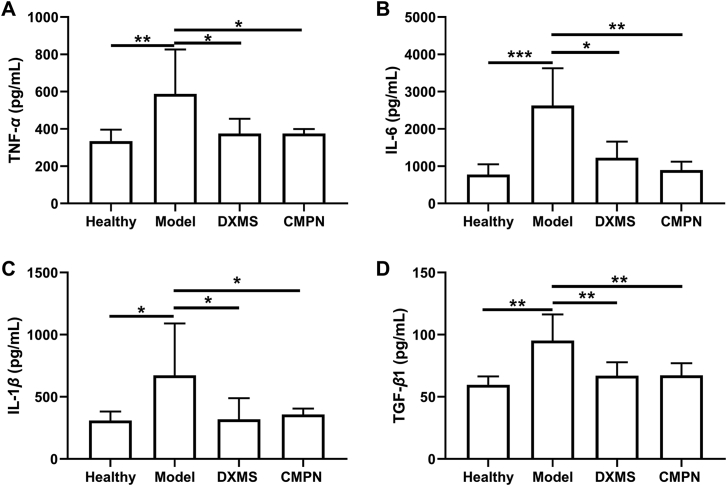
Cytokines are the regulators of host immune responses to infection, inflammation, and trauma. Some cytokines (e.g., TNF-α, IL-6, IL-1β, and TGF-β1) play key roles in the inflammation development process56. TNF-α is an important proinflammatory cytokine in early radiation lung injury, which represents the extent of lung injury57. IL-6, IL-1β, and TGF-β1 are also closely related to the occurrence and development of RP. Moreover, TGF-β1 directly acts on the later stage of RP, promoting the development of radiation fibrosis58. TNF-α, IL-6, IL-1β and TGF-β1 in the model group significantly increased compared to the healthy one (P < 0.01, P < 0.001, P < 0.05, P < 0.01, respectively, Fig. 8), indicating that radiation induced the occurrence of pneumonia. By contrast, the inflammatory cytokines decreased remarkably after treatment with DXMS or CMPN, even close to the normal levels (Fig. 9). Therefore, both of CMPN and DXMS had strong anti-inflammatory effects to alleviate the lung injury induced by irradiation.
Figure 8.
Cytokines in the lung tissues. (A) TNF-α, (B) IL-6, (C) IL-1β, and (D) TGF-β1 in the lung tissues. Data are presented as mean ± SD (n = 4). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Figure 9.
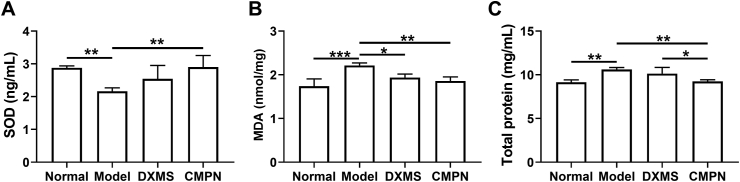
Levels of SOD (A), MDA (B) and total proteins (C) in the rat lung tissues. Data are presented as mean ± SD (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
3.7. High anti-oxidation effect of CMPN
SOD is found to be predominantly and highly expressed in the extracellular matrix of lung and helps to break down potentially harmful oxygen molecules59. Due to its strong oxygen radical scavenging ability, SOD can reduce acute radiation toxicity caused by over-oxidation60. MDA is one of the main lipid peroxides, which can trigger a chain reaction of radicals61. Additionally, the alveolar epithelial cell damage would lead to an increase of total protein content in the lung homogenate62. In this study, the SOD in the model group decreased sharply compared to that in the healthy group (P < 0.01, Fig. 9A), because it was greatly consumed by the irradiation-induced oxygen radicals. Meanwhile, the MDA and total proteins in the model group increased remarkably (Fig. 9B and C), indicating the lipid peroxidation reaction and alveolar epithelial cell damage. By contrast, after treatment with DXMS or CMPN, SOD increased while MDA and total proteins decreased, indicating their effectiveness against RP. Moreover, CMPN exhibited better therapeutic effect on RP than DXMS in term of total proteins in the lung tissues, although no statistical difference of SOD and MDA was shown between them. Therefore, CMPN improved SOD activity for anti-oxidation, inhibited lipid peroxidation, and protected alveolar epithelial cells, favoring the treatment of RP.
Here, we demonstrate that both of CMPN and DXMS had significant therapeutic efficiency for RP, involving reduced proinflammatory cytokines, high SOD, decreased MDA and total proteins, and alleviated lung tissue damage. However, the therapeutic effect of CMPN was better than that of DXMS in some aspects, such as lower total proteins in the lung tissues, prevention of pulmonary fibrosis, and alleviated lung tissue damage. Glucocorticoids are widely used for therapy of pneumonitis, achieving positive clinical outcome to some extent63. However, the side effects of glucocorticoids are obvious, such as body weight gain, hyperglycemia, sleep disturbances, mood changes and edema, indicating that these medicines are not suitable for prevention of RP or long-term application12. Moreover, glucocorticoids have inhibitory effects on a broad range of immune responses, leading to immunosuppressive effect. Consistently, glucocorticoids strongly suppress cell-mediated immunity and cause viral infection and tumor development64,65. Therefore, inhaled CMPN becomes a promising alternative strategy for prevention or treatment of RP due to its safety and high effectiveness.
4. Conclusions
RP is one type of severe common clinical diseases but effective and safe treatments are absent. In this study, curcumin and PDA were proved to have great anti-oxidation and free radical scavenging ability, suitable as the active drug and carrier for prevention and treatment of RP. The mesoporous structure of MPDA provided the large inner space and numerous surface pores to obtain high drug loading efficiency. Moreover, the amorphous state of curcumin in CMPN improved its dissolution and release to make the prevention and treatment very quickly after pulmonary delivery. Besides the anti-oxidation and free radical scavenging ability, inhaled CMPN guaranteed the highly effective prevention and treatment of RP by reduced proinflammatory cytokines, enhanced SOD, decreased MDA, and alleviated lung tissue damage. The combination of curcumin and MPDA and the unique administration route, i.e., inhalation of CMPN, are the keys to the prevention and treatment of RP.
Acknowledgments
This study is partially supported by the National Key New Drug Creation and Manufacturing Program, Ministry of Science and Technology (CN) (No. 2018ZX09J18104-001).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.10.027.
Author contributions
Ting Chen, Bo Zhuang and Yiguang Jin designed the research, carried out the experiments and performed data analysis. Yueqi Huang, Yan Liu, Wanmei Wang, and Tianyu Yuan participated part of the experiments. Bochuan Yuan, Lina Du and Yiguang Jin supervised the experiments and performed data analysis. Ting Chen, Bo Zhuang and Yiguang Jin wrote and revised the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bledsoe T.J., Nath S.K., Decker R.H. Radiation pneumonitis. Clin Chest Med. 2017;38:201–208. doi: 10.1016/j.ccm.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Hanania A.N., Mainwaring W., Ghebre Y.T., Hanania N.A., Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156:150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aso S., Navarro-Martin A., Castillo R., Padrones S., Castillo E., Montes A., et al. Severity of radiation pneumonitis, from clinical, dosimetric and biological features: a pilot study. Radiat Oncol. 2020;15:246. doi: 10.1186/s13014-020-01694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo-Hernández M., Maldonado F., Lozano-Ruiz F., Muñoz-Montaño W., Nuñez-Baez M., Arrieta O. Radiation-induced lung injury: current evidence. BMC Pulm Med. 2021;21:9. doi: 10.1186/s12890-020-01376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain V., Berman A.T. Radiation pneumonitis: old problem, new tricks. Cancers (Basel) 2018;10:222. doi: 10.3390/cancers10070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ullah T., Patel H., Pena G.M., Shah R., Fein A.M. A contemporary review of radiation pneumonitis. Curr Opin Pulm Med. 2020;26:321–325. doi: 10.1097/MCP.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 7.Palma D.A., Senan S., Tsujino K., Barriger R.B., Rengan R., Moreno M., et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas R., Chen Y.H., Hatabu H., Mak R.H., Nishino M. Radiographic patterns of symptomatic radiation pneumonitis in lung cancer patients: imaging predictors for clinical severity and outcome. Lung Cancer. 2020;145:132–139. doi: 10.1016/j.lungcan.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azmoonfar R., Amini P., Saffar H., Rezapoor S., Motevaseli E., Cheki M., et al. Metformin protects against radiation-induced pneumonitis and fibrosis and attenuates upregulation of dual oxidase genes expression. Adv Pharm Bull. 2018;8:697–704. doi: 10.15171/apb.2018.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Lu L., Liu B., Qin S. Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed Pharmacother. 2020;132:110826. doi: 10.1016/j.biopha.2020.110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia C., Shi W., Zhang Y., Ding L., Gao L., Wang Q., et al. Prevention and treatment of radiation-induced lung injury. Future Med Chem. 2020;12:2161–2173. doi: 10.4155/fmc-2019-0162. [DOI] [PubMed] [Google Scholar]
- 12.Khan N.A., Donatelli C.V., Tonelli A.R., Wiesen J., Ribeiro Neto M.L., Sahoo D., et al. Toxicity risk from glucocorticoids in sarcoidosis patients. Respir Med. 2017;132:9–14. doi: 10.1016/j.rmed.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Simone C.B. Thoracic radiation normal tissue injury. Semin Radiat Oncol. 2017;27:370–377. doi: 10.1016/j.semradonc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Farhood B., Mortezaee K., Goradel N.H., Khanlarkhani N., Salehi E., Nashtaei M.S., et al. Curcumin as an anti-inflammatory agent: implications to radiotherapy and chemotherapy. J Cell Physiol. 2019;234:5728–5740. doi: 10.1002/jcp.27442. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz K., Dobiasch S., Nguyen L., Schilling D., Combs S.E. Modification of radiosensitivity by curcumin in human pancreatic cancer cell lines. Sci Rep. 2020;10:3815. doi: 10.1038/s41598-020-60765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amini P., Saffar H., Nourani M.R., Motevaseli E., Najafi M., Taheri R.A., et al. Curcumin mitigates radiation-induced lung pneumonitis and fibrosis in rats. Int J Mol Cell Med. 2018;7:212–219. doi: 10.22088/IJMCM.BUMS.7.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du L., Feng X., Xiang X., Jin Y. Wound healing effect of an in situ forming hydrogel loading curcumin-phospholipid complex. Curr Drug Deliv. 2016;13:76–82. doi: 10.2174/1567201813666151202195437. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y., Du L., Liu Y., Li X., Li M., Jin Y., et al. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-β-cyclodextrin for melanoma treatment. Int J Pharm. 2014;469:31–39. doi: 10.1016/j.ijpharm.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T., Chen Y., Ge Y., Hu Y., Li M., Jin Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm Sin B. 2018;8:440–448. doi: 10.1016/j.apsb.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y., Li M., Zhang M., Jin Y. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. Int J Pharm. 2018;551:212–222. doi: 10.1016/j.ijpharm.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Yang G., Jin S., Xu L., Zhao C.X. Development of high-drug-loading nanoparticles. Chempluschem. 2020;85:2143–2157. doi: 10.1002/cplu.202000496. [DOI] [PubMed] [Google Scholar]
- 22.Hossen S., Hossain M.K., Basher M.K., Mia M.N.H., Rahman M.T., Uddin M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15:1–18. doi: 10.1016/j.jare.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jing Y., Deng Z., Yang X., Li L., Gao Y., Li W. Ultrathin two-dimensional polydopamine nanosheets for multiple free radical scavenging and wound healing. Chem Commun (Camb) 2020;56:10875–10878. doi: 10.1039/d0cc02888f. [DOI] [PubMed] [Google Scholar]
- 24.Liu H., Qu X., Tan H., Song J., Lei M., Kim E., et al. Role of polydopamine's redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta Biomater. 2019;88:181–196. doi: 10.1016/j.actbio.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 25.Hu J., Yang L., Yang P., Jiang S., Liu X., Li Y. Polydopamine free radical scavengers. Biomater Sci. 2020;8:4940–4950. doi: 10.1039/d0bm01070g. [DOI] [PubMed] [Google Scholar]
- 26.Guan Q., Guo R., Huang S., Zhang F., Liu J., Wang Z., et al. Mesoporous polydopamine carrying sorafenib and SPIO nanoparticles for MRI-guided ferroptosis cancer therapy. J Control Release. 2020;320:392–403. doi: 10.1016/j.jconrel.2020.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Jin A., Wang Y., Lin K., Jiang L. Nanoparticles modified by polydopamine: working as “drug” carriers. Bioact Mater. 2020;5:522–541. doi: 10.1016/j.bioactmat.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao H., Zeng Z., Liu L., Chen J., Zhou H., Huang L., et al. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale. 2018;10:6981–6991. doi: 10.1039/c8nr00838h. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Yang W., Zhang J., Zhang L., Shen H., Shi D. Alkalinity triggered the degradation of polydopamine nanoparticles. Polym Bull. 2020;78:4439–4452. [Google Scholar]
- 30.Garcia-Mouton C., Hidalgo A., Cruz A., Pérez-Gil J. The lord of the lungs: the essential role of pulmonary surfactant upon inhalation of nanoparticles. Eur J Pharm Biopharm. 2019;144:230–243. doi: 10.1016/j.ejpb.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Liang Z., Ni R., Zhou J., Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discov Today. 2015;20:380–389. doi: 10.1016/j.drudis.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L., Li M., Liu X., Du L., Jin Y. Inhalable oridonin-loaded poly(lactic-co-glycolic)acid large porous microparticles for in situ treatment of primary non-small cell lung cancer. Acta Pharm Sin B. 2017;7:80–90. doi: 10.1016/j.apsb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M.Y., Chan J.G., Chan H.K. Pulmonary drug delivery by powder aerosols. J Control Release. 2014;193:228–240. doi: 10.1016/j.jconrel.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 34.Kuzmov A., Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release. 2015;219:500–518. doi: 10.1016/j.jconrel.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Debnath S.K., Saisivam S., Omri A. PLGA ethionamide nanoparticles for pulmonary delivery: development and in vivo evaluation of dry powder inhaler. J Pharm Biomed Anal. 2017;145:854–859. doi: 10.1016/j.jpba.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 36.Rudén J., Frenning G., Bramer T., Thalberg K., An J., Alderborn G. Linking carrier morphology to the powder mechanics of adhesive mixtures for dry powder inhalers via a blend-state model. Int J Pharm. 2019;561:148–160. doi: 10.1016/j.ijpharm.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Chen F., Xing Y., Wang Z., Zheng X., Zhang J., Cai K. Nanoscale polydopamine (PDA) meets pi–pi interactions: an interface-directed coassembly approach for mesoporous nanoparticles. Langmuir. 2016;32:12119–12128. doi: 10.1021/acs.langmuir.6b03294. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z., Wang L., Prabhakar N., Xing Y., Rosenholm J.M., Zhang J., et al. CaP coated mesoporous polydopamine nanoparticles with responsive membrane permeation ability for combined photothermal and siRNA therapy. Acta Biomater. 2019;86:416–428. doi: 10.1016/j.actbio.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Radivojev S., Luschin-Ebengreuth G., Pinto J.T., Laggner P., Cavecchi A., Cesari N., et al. Impact of simulated lung fluid components on the solubility of inhaled drugs and predicted in vivo performance. Int J Pharm. 2021;606:120893. doi: 10.1016/j.ijpharm.2021.120893. [DOI] [PubMed] [Google Scholar]
- 40.Sridhar K., Charles A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: estimation methods for EC50 using advanced statistical programs. Food Chem. 2019;275:41–49. doi: 10.1016/j.foodchem.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 41.Cacua K., Ordoñez F., Zapata C., Herrera B., Pabón E., Buitrago-Sierra R. Surfactant concentration and pH effects on the zeta potential values of alumina nanofluids to inspect stability. Colloids Surf A. 2019;583:123960. [Google Scholar]
- 42.Liu Y., Wu C., Hao Y., Xu J., Zhao Y., Qiu Y., et al. Preparation of a novel starch-derived three-dimensional ordered macroporous carbon for improving the dissolution rate and oral bioavailability of water-insoluble drugs. J Pharm Biomed Anal. 2016;118:267–275. doi: 10.1016/j.jpba.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Kalepu S., Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L., Okuda T., Lu X.Y., Chan H.K. Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev. 2016;100:102–115. doi: 10.1016/j.addr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Woo M.W., Mansouri S., Chen X.D. Antisolvent vapor precipitation: the future of pulmonary drug delivery particle production? Expert Opin Drug Deliv. 2014;11:307–311. doi: 10.1517/17425247.2014.875527. [DOI] [PubMed] [Google Scholar]
- 46.Yang H., Le Q.V., Shim G., Oh Y.K., Shin Y.K. Molecular engineering of antibodies for site-specific conjugation to lipid polydopamine hybrid nanoparticles. Acta Pharm Sin B. 2020;10:2212–2226. doi: 10.1016/j.apsb.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClure R., Ong H., Janve V., Barton S., Zhu M., Li B., et al. Aerosol delivery of curcumin reduced amyloid-beta deposition and improved cognitive performance in a transgenic model of Alzheimer's disease. J Alzheimers Dis. 2017;55:797–811. doi: 10.3233/JAD-160289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taki M., Tagami T., Fukushige K., Ozeki T. Fabrication of nanocomposite particles using a two-solution mixing-type spray nozzle for use in an inhaled curcumin formulation. Int J Pharm. 2016;511:104–110. doi: 10.1016/j.ijpharm.2016.06.134. [DOI] [PubMed] [Google Scholar]
- 49.Adjemian S., Oltean T., Martens S., Wiernicki B., Goossens V., Vanden Berghe T., et al. Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis. 2020;11:1003. doi: 10.1038/s41419-020-03209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poinard B., Lam S.A.E., Neoh K.G., Kah J.C.Y. Mucopenetration and biocompatibility of polydopamine surfaces for delivery in an ex vivo porcine bladder. J Control Release. 2019;300:161–173. doi: 10.1016/j.jconrel.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 51.Hu S., Yang Z., Wang S., Wang L., He Q., Tang H., et al. Zwitterionic polydopamine modified nanoparticles as an efficient nanoplatform to overcome both the mucus and epithelial barriers. Chem Eng J. 2022;428:132107. [Google Scholar]
- 52.Kiang J.G., Olabisi A.O. Radiation: a poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019;9:25. doi: 10.1186/s13578-019-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu X., Li M., Zhu L., Li J., Zhang G., Fang R., et al. Amifostine-loaded armored dissolving microneedles for long-term prevention of ionizing radiation-induced injury. Acta Biomater. 2020;112:87–100. doi: 10.1016/j.actbio.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Li H., Liang X., Duan J., Chen Y., Tian X., Wang J., et al. ROS-responsive EPO nanoparticles ameliorate ionizing radiation-induced hematopoietic injury. Biomater Sci. 2021;9:6474–6485. doi: 10.1039/d1bm00919b. [DOI] [PubMed] [Google Scholar]
- 55.Qin M., Chen W., Cui J., Li W., Liu D., Zhang W. Protective efficacy of inhaled quercetin for radiation pneumonitis. Exp Ther Med. 2017;14:5773–5778. doi: 10.3892/etm.2017.5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnes P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2018;18:454–466. doi: 10.1038/s41577-018-0006-6. [DOI] [PubMed] [Google Scholar]
- 57.Jang S.S., Kim H.G., Lee J.S., Han J.M., Park H.J., Huh G.J., et al. Melatonin reduces X-ray radiation-induced lung injury in mice by modulating oxidative stress and cytokine expression. Int J Radiat Biol. 2013;89:97–105. doi: 10.3109/09553002.2013.734943. [DOI] [PubMed] [Google Scholar]
- 58.Lierova A., Jelicova M., Nemcova M., Proksova M., Pejchal J., Zarybnicka L., et al. Cytokines and radiation-induced pulmonary injuries. J Radiat Res. 2018;59:709–753. doi: 10.1093/jrr/rry067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei L., Zhang J., Yang Z.L., You H. Extracellular superoxide dismutase increased the therapeutic potential of human mesenchymal stromal cells in radiation pulmonary fibrosis. Cytotherapy. 2017;19:586–602. doi: 10.1016/j.jcyt.2017.02.359. [DOI] [PubMed] [Google Scholar]
- 60.Rabbani Z.N., Anscher M.S., Folz R.J., Archer E., Huang H., Chen L., et al. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer. 2005;5:59. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao K., Lei X., Liu H., Zhao H., Guo J., Chen Y., et al. Polydatin alleviated radiation-induced lung injury through activation of Sirt3 and inhibition of epithelial–mesenchymal transition. J Cell Mol Med. 2017;21:3264–3276. doi: 10.1111/jcmm.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao D.Y., Qu H.J., Guo J.M., Zhao H.N., Yang Y.Y., Zhang P., et al. Protective effects of myrtol standardized against radiation-induced lung injury. Cell Physiol Biochem. 2016;38:619–634. doi: 10.1159/000438655. [DOI] [PubMed] [Google Scholar]
- 63.Rahi M.S., Parekh J., Pednekar P., Parmar G., Abraham S., Nasir S., et al. Radiation-induced lung injury-current perspectives and management. Clin Pract. 2021;11:410–429. doi: 10.3390/clinpract11030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cain D.W., Cidlowski J.A. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.