Abstract
Background:
Monitoring the longevity of immunoglobulin G (IgG) responses following severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections is vital to understanding the role of antibodies in preventing infection.
Aims:
To determine the quantitative IgG responses specific to the Spike-S1 (S1) receptor-binding domain (S1/RBD) region of the virus in serum samples taken between 4 weeks and 7 months after polymerase chain reaction (PCR) positivity in patients who are diagnosed with coronavirus disease-2019 (COVID-19).
Study Design:
A longitudinal study.
Methods:
This study included 113 patients with a clinical and molecular diagnosis of COVID-19. The first and second serum samples were taken 1 and 7 months, respectively, after the PCR positivity. S1/RBD-specific IgG antibody response was assayed using anti-SARS-CoV- 2 QuantiVac ELISA (IgG) kit (Euroimmun, Lübeck, Germany). The neutralizing antibodies were investigated in 57 patients whose IgG test results were above the cut-off value.
Results:
In 57 patients with SARS-CoV-2 IgG, the anti-SARS-CoV-2 IgG quantitative antibody levels significantly decreased after 7 months (Z = −2.197, p = 0.028). A correlation was detected between the anti-SARS-CoV-2 IgG and nAb percent inhibition (IH%) levels detected in 1 month (rs = 0.496, p < 0.001), but without significant correlation in serum samples taken on 7 months. The nAb IH% levels of the first and second were compared for COVID-19 severity and revealed no statistical difference (p = 0.256). In the second serum sample, the nAb IH%s of patients with moderate COVID-19 showed a statistically significant difference from patients with mild COVID-19 (p = 0.018), but without significant differences between severe and moderate or mild COVID-19.
Conclusion:
SARS-CoV-2 quantitative IgG antibody titers are significantly reduced at long-term follow-up (> 6 months). Due to the limited information on seroconversion, comprehensive studies should be conducted for long-term follow-up of the immune response against SARS-CoV-2.
INTRODUCTION
The coronavirus disease-2019 (COVID-19) outbreak had claimed the lives of over 4.4 million people globally, and over 209.8 million infections were recorded as of August 20, 2021.1 Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), causing COVID-19, is a betacoronavirus that binds to the angiotensin-converting enzyme 2 (ACE2) receptor by its receptor-binding domain (RBD) in human cells.2 The immune system generates immunoglobulins M (IgM), IgA, and IgG antibodies against the virus’s spike (S) and nucleocapsid proteins (N). IgM antibodies are detected in the serum 5–10 days after the symptom, IgA on 10–12 days, and IgG on 12–14 days.3 Additionally, antibodies against Spike-S1 (S1) protein are shown to have neutralizing activity.4 The IgG antibody response to SARS-CoV-2 infection varies depending on disease severity; however, its immunity in recovered patients remains controversial. Monitoring and learning about the longevity of IgG responses following SARS-CoV-2 infection is pivotal to public health and understanding the role of antibodies in preventing infection.The present study aimed to determine the quantitative S1/RBD-specific IgG responses in serum samples that were taken 4 weeks and 7 months after PCR positivity from patients with COVID-19 who applied to our center for evaluation of the time-dependent change of these antibodies.
MATERIALS AND METHODS
One-hundred-thirteen patients who applied to the Istanbul University-Cerrahpaşa, Cerrahpasa Medicine Faculty, COVID-19 First Application Outpatient Clinic between 01.05.2020 and 01.07.2020, with the suspicion of COVID-19 and received a clinical and molecular diagnosis were included. The clinical classification of patients for COVID-19 severity was made according to the Infectious Diseases Society of America Guidelines.5 The first serum samples were taken 1 month after the first PCR positivity and the second at 7 months after the first PCR positivity from patients with COVID-19. Demographic and laboratory data of patients were retrospectively obtained. Serum samples were immediately delivered to the laboratory under appropriate transport conditions.Serum samples were stored at -80 °C until assayed. Quantitative IgG antibody response specific to the S1/RBD region of the virus in both serum samples was assayed using Anti-SARS-CoV-2 QuantiVac enzyme-linked immunoassay (ELISA) (IgG) kit (Euroimmun, Lübeck, Germany) using the ELISA method. The evaluated results with the calibration curve are expressed as Relative Unit/ml (RU/ml), wherein <8 RU/ml were considered negative, 8–11 RU/ml as borderline, and ≥11 RU/ml as positive. The obtained results in RU/ml were multiplied by 3.2 and calculated in Binding Antibody Unit/ml (BAU/ml), which was the first International Standard of the World Health Organization to detect SARS-CoV-2 neutralizing antibodies. The presence of neutralizing antibodies was investigated in 57 patients whose quantitative IgG test results were above the cut-off value in the first serum samples.
In both serum samples, neutralizing antibodies that inhibit the binding of the viral SARS-CoV-2 S1-RBD to ACE2 receptors of human cells were detected by surrogate neutralization assay with the competitive ELISA method (SARS-CoV-2 NeutraLISA, Euroimmun, Lübeck, Germany). The neutralization capacity was calculated as percent inhibition (IH%) by subtracting the optical density of the patient sample from the ratio of the optical density of the blank. Test results were interpreted following the manufacturer’s instructions; IH of < 20% were evaluated as negative, IH of 20%–35% as a breakpoint, and IH of ≥35% as positive.
The Statistical Package for the Social Sciences software (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) was used for the statistical analysis, and data are presented as median (interquartile range [IQR]) or n (%). Categorical variables were represented as numbers (percentage) and continuous variables as median (IQR); values are presented as mean ± standard deviation. The Shapiro-Wilk test was used to test the conformity of the obtained variables to the normal distribution. The test results revealed that the variables did not comply with the normal distribution assumption (p < 0.05). Therefore, the Kruskal-Wallis and Dunn posthoc test and the Wilcoxon signed-rank test were used. The Spearman Correlation analysis was performed to examine the correlation analysis of different variables. Informed consent was obtained from all patients.
Ethics
This study was conducted under the approval of the Turkey Ministry of Health General Directorate of Health Services Scientific Research Studies Commission (date: December 14, 2020), Istanbul University-Cerrahpaşa, Cerrahpaşa Faculty of Medicine, Scientific Research and Evaluation Commission (date: December 21, 2021, and no: 165738) and by the ethical committee of Istanbul University-Cerrahpaşa, Cerrahpaşa Medical Faculty (February 9th, 2021 decision no: 27402).
RESULTS
The age distribution analyses of patients with COVID-19 according to disease severity revealed a significant difference (p < 0.001). The median age in mild, moderate, and severe COVID-19 was 36, 39, and 52 years, respectively (Table 1). The examination of the effect of gender on disease severity revealed no significant difference. The SARS-CoV-2 IgG antibody level analyses using the QuantiVac ELISA kit (Euroimmune) from the serum samples taken at 1 month according to disease severity revealed that the SARS-CoV-2 IgG levels were significantly low in patients with mild compared to patients with moderate and severe COVID-19.
Table 1. The Demographical and Laboratory Characteristics of patients According to the Severity of COVID-19.
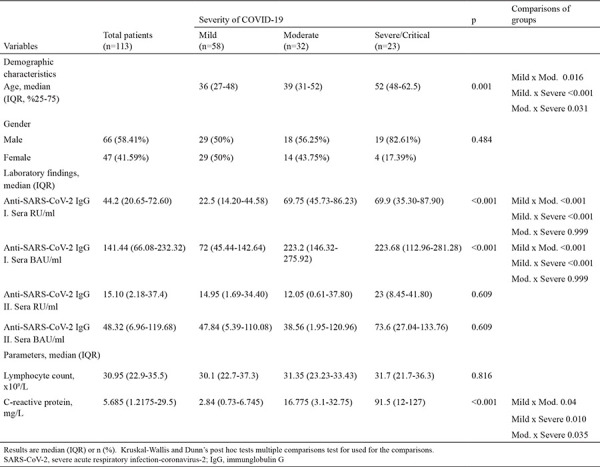
No statistically significant difference was found in the SARS-CoV-2 IgG levels between patients with moderate and severe COVID-19. The SARS-CoV-2 IgG antibody levels of patients with COVID-19 in their second serum samples taken after 7 months were similar in all three groups and decreased compared to the IgG levels at 1 month, without statistical difference between the three groups (p < 0.609). The analyses of the relationship between the disease severity and lymphocyte counts and C-reactive protein (CRP) levels were examined revealed that disease severity has no significant effect on the lymphocyte counts, while CRP levels significantly increase with disease severity (p < 0.05) (Table 1).
The changes in IgG antibody results in patients with COVID-19 were significantly lower in 7 months compared to 1 month (Z = −5.204, p < 0.001). The mean SARS-CoV-2 IgG antibody titers detected in the serum samples taken after 7 months of PCR positivity were significantly lower than that in 1 month (p < 0.001) (Table 2).
Table 2. The Changes in the Anti-SARS-CoV-2 IgG QuantiVac ELISA Test Results of the Patients with COVID-19 According to the First- and Seventh-Month Tests.

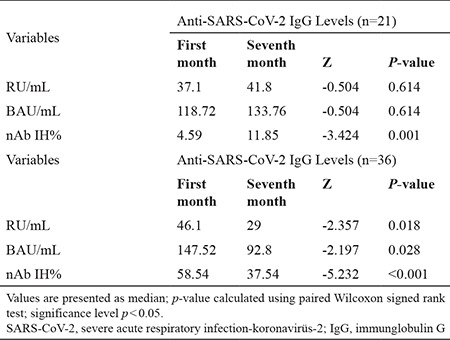
Among the 113 patients, the data of 57 patients with SARS-CoV-2 IgG results above the cut-off value were analyzed. Similar to the results in Table 2, the anti-SARS-CoV-2 IgG quantitative antibody levels of these 57 patients also significantly decreased, as seen in Table 3 (Z = −2.197, p = 0.028). In 36 of these patients, the first IgG antibody levels were higher than the second IgG antibody levels, and the anti-SARS-COV-2 IgG antibody levels detected in the second sera were higher than the first sera IgG antibody levels in 21 patients. Of these 21 patients, nAb IH% results were increased by 4.59–11.85 after 7 months of PCR positivity (p < 0.001) (Table 3).
Table 3. The Comparison of the time-Dependent Changes of Neutralizing Anti-SARS-CoV-2 IgG Antibodies Detected by Quantivac ELISA and SARS-CoV-2 Neutralizing Antibodies Detected by NeutraLISA.
Based on the SARS-CoV-2 NeutraLISA (Euroimmun, Lübeck, Germany) surrogate neutralization assay study in the serum samples of 57 patients taken in 1 and 7 months after PCR positivity, the changes in the nAb IH% results of patients with COVID-19 between 1 and 7 months tests were statistically significant (Z = -2.793, p = 0.005). Therefore, the nAb IH% results measured in the second serum samples of patients with COVID-19 without family contact were significantly lower than the nAb IH% results measured in the first serum samples but higher in the second serum samples of patients with COVID-19 with family contact (Figure 1).
Figure 1.
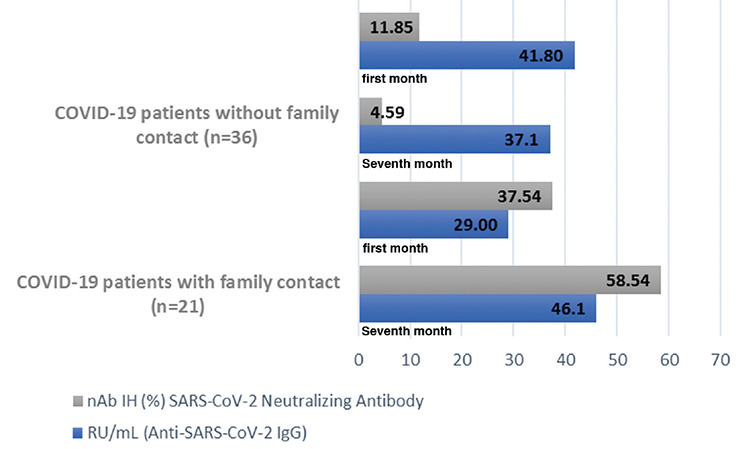
The time-dependent changes of neutralizing anti-SARS-CoV-2 IgG and SARS-CoV-2 neutralizing antibodies in the first and second blood samples of patients with COVID-19 with and without family contact
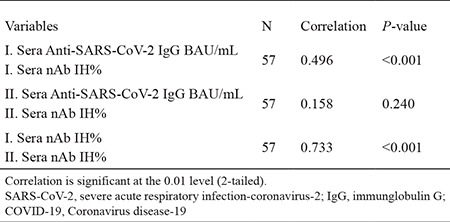
A positive and significant relationship was detected between the SARS-CoV-2 IgG quantitative antibody levels and the nAb IH% levels detected in the first serum samples of patients with COVID-19 according to Spearman’s Rank Correlation Coefficient analysis (rs = 0.496, p < 0.001), without significant correlation between the SARS-CoV-2 IgG quantitative antibody and nAb IH% level in the second serum samples taken on 7 months (rs = 0.158, p > 0.05). The nAb IH% levels in the first serum samples are higher than that in the second serum samples; however, a positive and significant relationship was found between the two test results (rs: 0.733, p < 0.001) (Table 4).
Table 4. The Spearman Correlation Analyses between the Anti-SARS-CoV-2 IgG and nAb IH% Levels Detected in the First Month or the Seventh-Month Serum Samples of COVID-19 Patients (57 patients).
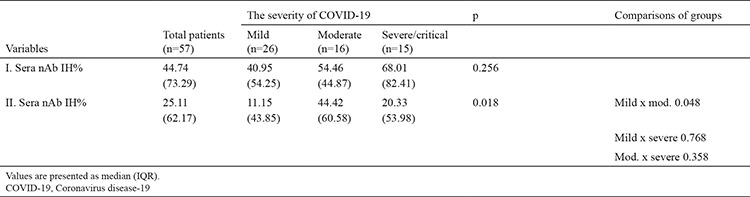
The nAb IH% levels detected in the first serum samples taken at 1 month and the second serum samples were taken at 7 months were evaluated according to COVID-19 severity revealed no difference in the nAb IH% levels in terms of the first serum samples (p = 0.256). The results of the second serum samples revealed that the nAb IH%s of patients with moderate COVID-19 was statistically significantly different than that of patients with mild COVID-19 (p = 0.018) but without significant differences between the nAb IH% of patients with severe and moderate or mild COVID-19. A significant decrease was found in nAb IH% between all patients’ first and second serum samples, and this decrease was observed at the lowest level in patients with moderate COVID-19 (Table 5).
Table 5. The Determination of nAb IH% Levels in COVID-19 Patients Due to the Severity of Disease.
DISCUSSION
Long-term follow-up of the natural and acquired immunity against SARS-CoV-2 in patients with COVID-19 is critical for early infection detection and prediction.6 However, our knowledge of the immune response to SARS-CoV-2 is still limited.7 The outcomes of patients’ demographic data analysis reveal that COVID-19 severity is statistically significant with the increase in age and CRP level. However, the gender difference and lymphocyte count do not alter the disease severity. A population-based case-control study by McKeigue et al.8 revealed that age increase and male gender were effective risk factors for COVID-19 severity. A meta-analysis by Romero Starke et al.9 revealed that an increase in age was a 2.7-fold risk factor of COVID-19. Another study10 revealed that high CRP levels were an important marker for COVID-19 severity as CRP induced the overproduction of inflammatory cytokines and caused tissue destruction. Lu et al.11 reported increased CRP levels in patients with severe COVID-19 but observed a decrease in lymphocyte count. The severity of the disease improved upon corticosteroid administration to these patients.11 Yamasaki et al.12 revealed similar results to that of Lu et al.11, revealing a decrease in lymphocyte count with the disease severity, which could be used as a marker to determine severe infection in patients with COVID-19.12
Our results revealed that COVID-19 severity, CRP, and age are consistent with previous studies; however, no relationship is noted between the disease severity, lymphocyte count, and gender. The differences might be associated with the low number of patients and its single-center nature. Regarding the long-term serological analysis and neutralizing antibody levels of patients with COVID-19, Legros et al.7 revealed no statistical difference between anti-SARS-CoV-2 IgG (AU/ml) levels in the serum samples of patients with mild, moderate, and severe disease, and without changes in antibody titer with the disease severity. However, neutralizing antibody responses were correlated with the disease severity. The same study7 examines the nAb responses of 11 patients after 60 days and revealed that nAb response could not be detected in 7 patients. However, nAb response decreased 4-fold in four patients.7 Muecksch et al.6 studied the serum samples of patients with COVID-19 at hospital re-visits at 2, 4, and 8 weeks using in vitro diagnostic serological systems. The IgG antibody levels decreased in the Abbott Nucleocapsid-targeted test, increased in the Diasorin spike-targeted test, but remained stable in the Roche and Siemens total antibody-targeted tests at second and third re-visits of patients with COVID-19. The same study performed a pseudotype-based neutralization assay. The above study found a correlation of the quantitative serological results of Siemens and Diasorin from S protein-based analysis, showing a stronger correlation than Abbott and Roche systems, performed with N protein-based serological analysis.6 Wang et al.13 revealed that anti-SARS-CoV-2 IgG titers against the S or N regions were moderately correlated with nAb titers. In this study, we used the Euroimmune brand kit to present the quantitative S1/RBD-specific SARS-CoV-2 IgG results. The correlation of anti-SARS-CoV-2 IgG titers with the nAb IH% was similar to the data in the literature[AME5]. The plaque reduction neutralization test was used in most studies and the use of surrogate neutralization tests was uncommon; however, our study showed a correlation between the nAb titers in the first serum samples and the quantitative S1/RBD IgG titer results. However, this correlation was not found in the second serum samples. We believed that the decrease in IgG titers at 7 months might be associated with the decreased nAb IH%.Zhang et al.14 demonstrated that SARS-CoV-2-specific humoral immunity was present in 95% of convalescents and T-cell memory of patients at 12-month post-infection against at least one viral antigen in ~90% of patients. Moreover, researchers revealed that from 6 to 12 months post-infection, anti-SARS-CoV-2 IgG and IgM levels have a declining trend, but the levels of NAb and CD8+ and CD4+ T cells against SARS-CoV-2 were sustained. Another study revealed that most patients with COVID-19 at 6 months post-symptom onset were still positive for neutralizing antibodies (N = 44/52, 84.6%).15
Hou et al.16 revealed that IgG levels remained at certain levels up to 48 days after examining the anti-SARS-CoV-2 IgG and IgM antibody titers in patients with mild, severe, and critical COVID-19. They reported a relationship between the disease severity and the antibody levels.16 Wang et al.13 followed the levels of IgG antibody titers against different proteins of SARS-CoV-2, such as S1, S2, RBD, and N, in patients with mild and severe COVID-19 at 2, 3, and 4 weeks and revealed that the 4-week level of IgG antibody titers against the S1 protein was higher in patients with severe COVID-19 than that of mild COVID-19.Ren et al.17 revealed that the level of IgG antibody titers against the S protein was lower in patients who died on 22–28 days compared to those who recovered.
The virus-specific neutralizing antibodies developed after infection are pivotal for protective immunity. The plaque reduction neutralization test (PRNT) is the reference standard method; however, it could not be used in biosafety level 3 laboratories and by experienced specialists. The competitive QuantiVac ELISA surrogate neutralization test was studied and was 98.6% compatible with PRNT.18 A low number of patients and the single-center nature are the limitations of our study. Therefore, we suggest a long-term follow-up of >7 months in patients. We believe that the mean of SARS-CoV-2 quantitative IgG antibody titers decrease by approximately 24% and nAb IH% titers decreased by 19% 7 months after PCR positivity.
Moreover, nAb IH% decreased as SARS-CoV-2 IgG quantitative antibody levels decreased. The absence of correlation is noted between the mean SARS-CoV-2 quantitative IgG antibody titer and the average nAb IH% results in the serum samples taken 7 months after the PCR positivity although a decrease in the quantitative antibody results is recorded. Additionally, the increase in nAb IH% and SARS-CoV-2 quantitative IgG antibody titers of 21 patients after 7 months of PCR positivity may be attributed to a new contact with the virus, since we have the information that these 21 patients had a history of family contact after the first serum sampling. Due to limited information in the literature regarding seroconversion, comprehensive studies for the long-term follow-up of the immune response against SARS-CoV-2 should be conducted.
Acknowledgments
The authors thank patients for their participation to the study.
Footnotes
Ethics Committee Approval: İstanbul University-Cerrahpaşa, Cerrahpaşa Faculty of Medicine, Clinical Studies Etihical Committee date: 09.02.2021 number: 27402.
Patient Consent for Publication: Informed consent was obtained from the patients. Datasharing Statement: Data available on request from the authors. The data that support the findings of this study are available from the corresponding author.
Author Contributions: Concept - H.Ö.D.; Design – M.A.K.; Data Collection or Processing – S.S., A.N.A., R.K., Y.T.T, D.Ö., R.A., N.G.; Analysis or Interpretation – M.D., K.M.; , G.A.;Literature Search - Y.E.Ö., S.S.; Writing - H.Ö.D., S.S.; Supervision – B.K.
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest: The authors have no conflicts of interest to declare.
Funding: The financial expenses have covered by the authors in this study.
References
- 1.Word Health Organization (WHO). Coronavirus disease (COVID-2019) situation reports. [Internet] https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 2.Zhu N, Zhang D, Wang W, et al. A novel Coronavirus from Patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 5.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis. 2020;ciaa478. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muecksch F, Wise H, Batchelor B, et al. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J Infect Dis. 2021;223:389–398. doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKeigue PM, Weir A, Bishop J, et al. Rapid Epidemiological Analysis of Comorbidities and Treatments as risk factors for COVID-19 in Scotland (REACT-SCOT): A population-based case-control study. PLoS Med. 2020;17:e1003374. doi: 10.1371/journal.pmed.1003374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero Starke K, Petereit-Haack G, Schubert M, et al. The Age-Related Risk of Severe Outcomes Due to COVID-19 Infection: A Rapid Review, Meta-Analysis, and Meta-Regression. Int J Environ Res Public Health. 2020;17:5974. doi: 10.3390/ijerph17165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92:2409–2411. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu C, Liu Y, Chen B, et al. Prognostic value of lymphocyte count in severe COVID-19 patients with corticosteroid treatment. Signal Transduct Target Ther. 2021;6:106. doi: 10.1038/s41392-021-00517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamasaki Y, Ooka S, Tsuchida T, et al. The peripheral lymphocyte count as a predictor of severe COVID-19 and the effect of treatment with ciclesonide. Virus Res. 2020;290:198089. doi: 10.1016/j.virusres.2020.198089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020;130:5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Lin H, Ye B, et al. One-year sustained cellular and humoral immunities of COVID-19 convalescents. Clin Infect Dis. 2021;iab884. doi: 10.1093/cid/ciab884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillot C, Favresse J, Maloteau V, Dogné JM, Douxfils J. Dynamics of Neutralizing Antibody Responses Following Natural SARS-CoV-2 Infection and Correlation with Commercial Serologic Tests. A Reappraisal and Indirect Comparison with Vaccinated Subjects. Viruses. 2021;13:2329. doi: 10.3390/v13112329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou H, Wang T, Zhang B, et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunology. 2020;9:e01136. doi: 10.1002/cti2.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren L, Fan G, Wu W, et al. Antibody Responses and Clinical Outcomes in Adults Hospitalized With Severe Coronavirus Disease 2019 (COVID-19): A Post hoc Analysis of LOTUS China Trial. Clin Infect Dis. 2021;72:e545–e551. doi: 10.1093/cid/ciaa1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]


