Abstract
Sclerostin, a protein secreted from osteocytes, negatively regulates the WNT signaling pathway by binding to the LRP5/6 co-receptors and further inhibits bone formation and promotes bone resorption. Sclerostin contributes to musculoskeletal system-related diseases, making it a promising therapeutic target for the treatment of WNT-related bone diseases. Additionally, emerging evidence indicates that sclerostin contributes to the development of cancers, obesity, and diabetes, suggesting that it may be a promising therapeutic target for these diseases. Notably, cardiovascular diseases are related to the protective role of sclerostin. In this review, we summarize three distinct types of inhibitors targeting sclerostin, monoclonal antibodies, aptamers, and small-molecule inhibitors, from which monoclonal antibodies have been developed. As the first-in-class sclerostin inhibitor approved by the U.S. FDA, the monoclonal antibody romosozumab has demonstrated excellent effectiveness in the treatment of postmenopausal osteoporosis; however, it conferred high cardiovascular risk in clinical trials. Furthermore, romosozumab could only be administered by injection, which may cause compliance issues for patients who prefer oral therapy. Considering these above safety and compliance concerns, we therefore present relevant discussion and offer perspectives on the development of next-generation sclerostin inhibitors by following several ways, such as concomitant medication, artificial intelligence-based strategy, druggable modification, and bispecific inhibitors strategy.
KEY WORDS: Sclerostin, WNT signalling pathway, Sclerostin inhibitors, Antibody, Bone diseases, Aptamer, Small molecule inhibitors, Artificial intelligence
Graphical abstract
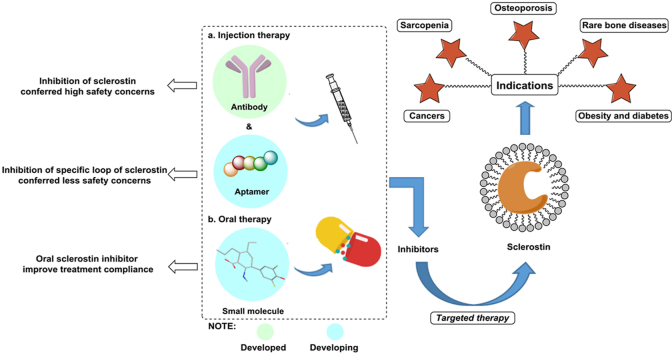
Development of different types of sclerostin inhibitors could resolve safety and compliance concerns caused by romosozumab therapy. Except WNT-related bone diseases, inhibition of sclerostin leads other promising indications including obesity and diabetes, cancers, etc.
1. Introduction
The SOST gene, mapped to human chromosome 17q12–q211 was first discovered as a pathogenic gene in sclerosteosis and Van Buchem disease2,3. Sclerostin is a glycoprotein encoded by the SOST gene in osteocytes. A negative regulator of the WNT signalling pathway, sclerostin binds low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptors, further inhibiting bone formation and promoting bone resorption4,5, making it a promising therapeutic target in bone-related disorders. As the first sclerostin inhibitor approved by the United States Food and Drug Administration (U.S. FDA)6, romosozumab can both promote bone formation and inhibit bone resorption. It has demonstrated excellent effectiveness in the treatment of osteoporosis (OP) in postmenopausal women, suggesting that the development of drugs targeting sclerostin for the treatment of bone diseases is essential.
In addition to OP, rare bone diseases, such as osteogenesis imperfecta (OI) and X-linked hypophosphatemia (XLH), are closely related to sclerostin. An in-depth study of sclerostin revealed the mechanism by which sclerostin regulates bone metabolism is associated with the LRP5/6 co-receptors7. Since mutation in LRP5/6 (G171V) was found to cause metabolic bone diseases, the study of the roles of LRP5/6 and WNT signalling in bone disease has attracted considerable attention8. Additionally, the part of sclerostin in bone formation was closely related to the WNT-β-Catenin signalling pathway upon the discovery that WNT protein binding to LRP5/6 further promotes the expression of an osteoblast-related gene4. In addition, an increasing number of studies have shown that the developments of cancers, obesity, and diabetes are associated with sclerostin9, 10, 11. In addition to the above-mentioned diseases related to the contributional role of sclerostin, some conditions are associated with the protective role of sclerostin, such as rheumatoid arthritis (RA)12 and cardiovascular diseases13. Thus, it is vital to fully understand the structure and functions of sclerostin, as this comprehension will enable the realization of a theoretical basis for the development of sclerostin inhibitors14. In this review, we summarize the following contents: relevant knowledge of sclerostin; the status of anti-sclerostin monoclonal antibody drugs currently in clinical trials; the effectiveness, efficacy, safety, and tortuous road of romosozumab approval; and the progress in the research of other sclerostin inhibitors, such as aptamers and small molecules. Finally, we discuss the safety issues related to romosozumab therapy and compliance concerns raised by injection therapy. Given these issues, the outlook for the discovery of next-generation sclerostin inhibitors is proposed at the end of the manuscript, including the development of concomitant medication, the prospect of artificial intelligence (AI)-based strategies for the discovery of small-molecule sclerostin inhibitors, suggestions for druggable modifications of anti-sclerostin aptamers and the identification of bispecific inhibitors for the treatment of sclerostin-related diseases to improve treatment outcomes.
2. Structure, functions, signaling pathway and diseases related to sclerostin
2.1. Structure and functions of sclerostin
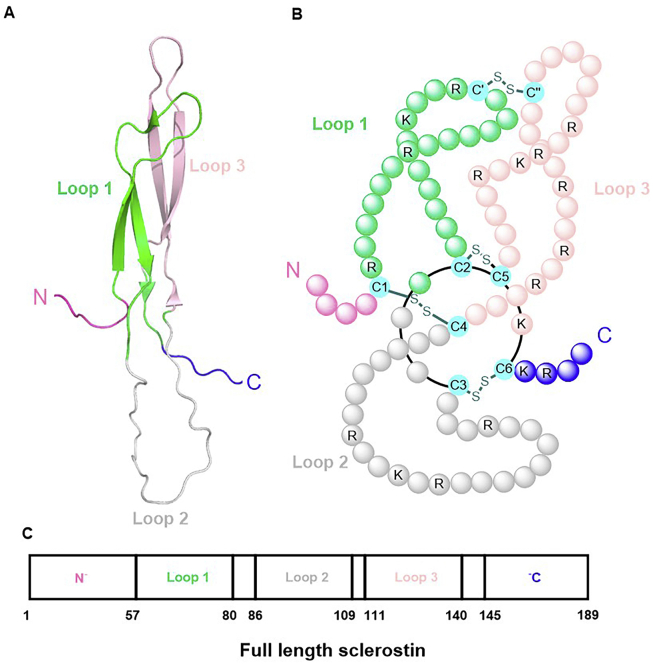
The first investigation of the structure of sclerostin was performed by Veverka et al.15, who used nuclear magnetic resonance spectroscopy (NMR) to analyse the three-dimensional structure of sclerostin. The results indicated that sclerostin has a core cystine-knot structure comprising three specific domains: loop 1, loop 2, and loop 3. Additionally, sclerostin has side chains assembled with a highly flexible N-terminal (amino acid residues 1–55) and C-terminal domains (amino acid residues 145–189). However, according to the crystal structure of sclerostin, the total length of the protein contains 213 amino acid residues16. In general, sclerostin is similar to differential screening-selected gene aberrative in neuroblastoma family proteins, initially identified as bone morphogenetic protein (BMP) antagonists, giving sclerostin the ability to bind BMPs and inhibit BMP signalling and osteoblast mineralization17,18. Four disulfide bonds formed by four pairs of cysteine residues in sclerostin were identified by mass spectrometry. The specific positions of these disulfide bonds are as follows: C1–C4 (Cys56–Cys110), C2–C5 (Cys81–Cys141), C3–C6 (Cys85–Cys143), and C′–C′′ (Cys70–Cys124), which determine the specific pattern of the cystine knot. A cystine ring with eight members is formed by pairs of cysteine residues combined with Cys81–Cys141 (C2–C5) and Cys85–Cys143 (C3–C6). The cystine knot is established by a disulfide bridge between C1 and C4 that passes through the ring. C′–C′′, the terminal pair of cysteine residues, is critical for linking loops 1 and 3 (shown in Fig. 1A–C)15. In contrast to most proteins with cystine knot motifs, sclerostin forms as a monomer, not as a homodimer or heterodimer, containing disulfide bridges19.
Figure 1.
The structure of sclerostin. The residues which were bound with disulfide bonds each other were marked in green. C, cysteine; K, lysine; R, arginine; S, sulfur.
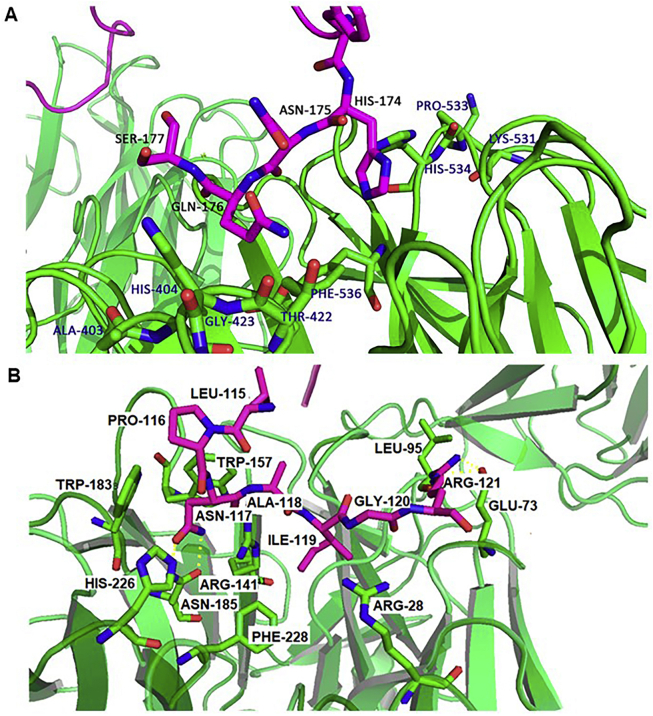
Sclerostin, as a competitor for WNT binding, binds to LRP5/6 co-receptors to negatively regulate bone formation. The LRP5/6 co-receptors are highly homologous proteins involved in the WNT signalling pathway with vital functions. Early study of the LRP6 interaction site in sclerostin showed that the site partially consists of the NXI motif in loop 2, as confirmed by NMR. According to mutational analysis, the proline–asparagine–alanine–isoleucine–glycine (PNAIG) motif on the top of loop 2 was shown to be critical for sclerostin interactions with the LRP5/6 co-receptors20. Very recently, Kim et al.16 presented a crystal structure of the LRP6 E1E2–sclerostin complex with two adjacent interaction sites that discounted the formerly proposed model showing sclerostin loop 2 peptide-bound LRP6 E1. Additionally, performing both structural and functional analyses, Kim et al.16 were the first to report that the four-terminal residues in the C-tail of sclerostin, HNQS, interact with LRP6 E2 (shown in Fig. 2A). The finding of an additional binding site enabled this group to propose a new binding model for sclerostin, showing that the synchronous interaction of sclerostin with LRP6 E1 and E2 causes more efficient suppression of WNT signalling16. Both the HNQS region and a positively charged cluster are crucial for the full suppressive effect of sclerostin on WNT1 signalling, and that they exhibit additive effects on sclerostin activity (shown in Fig. 2A)16.
Figure 2.
Interaction between sclerostin and LRP6. (A) Binding site in the HNQS region of the sclerostin C-tail with LRP6. (B) Binding site in the PNAIG region of the loop2 with LRP6. Red sticks are amino acid residues of sclerostin, green sticks are amino acid residues of LRP6, ILE119, ASN117 correspond to the crystal data of sclerostin, if the NMR results are referred, the residues are ILE95, ASN 93, respectively.
2.2. Signalling pathways related to sclerostin
2.2.1. The WNT signalling pathway
WNT signalling plays an essential role in bone formation, growth, and development21, 22, 23. The WNT-β-Catenin signalling pathway, the canonical WNT signalling pathway, is a critical regulator of bone formation and metabolism. In this pathway, WNT binds to LRP5/6 to promote the further expression of an osteoblast-related gene4. β-Catenin is a positive regulator of bone formation. It not only fosters stem cell osteoblast differentiation but also inhibits osteoclast activity to regulate bone mass. Chen and Long24 found that the decline in mouse bone density caused by reductions in osteoclasts’ number and activity was more significant than that caused by the cutbacks in osteoblast number and activity in β-Catenin knockout (KO) mice. In the WNT/β-Catenin pathway, when a complex is formed by the WNT protein and Frizzled receptor or the LRP5/6 co-receptors25, the serine residue in the cytoplasmic domain of LRP5/6 forms a binding site that recruits AXIN, a protein that can suppress glycogen synthase kinase 3β phosphorylation of β-Catenin25,26. In these cases, the level of β-Catenin in the cytoplasm is sustained, and β-Catenin gradually moves to the nucleus where it interacts with the T cell factor/lymphatic enhancement factor complex27. As a result, the downstream target genes in the inhibitory state are activated, including CD44, Cyclin D1, runt-related transcription factor 2, osteoclastogenesis inhibitory factor, and bone morphogenetic protein 228,29. Sclerostin thus acts as an antagonist of the WNT-β-Catenin pathway to negatively regulate bone formation30. When WNT is inactivated, sclerostin binds to LRP5/6 cell surface receptors, preventing their interaction between the WNT ligand, which prevents the establishment of the WNT-Frizzled-LRP5/6 ternary complex and thus inhibits the canonical WNT signalling pathway31. Cytosolic β-Catenin is phosphorylated and targeted for ubiquitination by the destruction complex and degraded by the proteasome. Cell factor/lymphatic enhancement factor transcription factors in the nucleus associate with Groucho, repressing WNT target gene transcription31. In addition, sclerostin inhibition (by anti-sclerostin antibody (Scl-Ab) administration to ovariectomized (OVX) mice) or SOST deficiency (mutations in the first exon of the SOST gene in patients with sclerosteosis) leads to a compensatory increase in Dickkopf-1 (DKK-1, another WNT antagonist)32, which might confine the effect of sclerostin inhibition on WNT-driven bone formation. Recently, sclerostin neutralization has been consistently found to promote the osteoanabolic effects of DKK-1 inhibition33. DKK-1 deficiency (DKK-1 KO) and Scl-Ab treatment have a synergistic effect34.
2.2.2. NF-κB signaling pathway
Nuclear factor-kappa B (NF-κB), a family of inducible transcription factors (including NF-κB1 (P50), NF-κB2 (P52), RELA (P65), RELB and C-REL) that regulates the expression of various genes involved in inflammation and the immune response35, was recently found to play essential roles in bone metabolism, bone destruction and bone regeneration36. Both canonical and noncanonical NF-κB pathways have been shown to mediate RA pathogenesis and contribute to inflammatory bone loss37,38. In addition, bidirectional and multifunctional crosstalk between WNT/β-Catenin and NF-κB signalling pathway components have been discovered39. WNT/β-Catenin can either negatively regulate NF-κB signaling or positively modulate NF-κB activity40. In osteoblasts and chondrocytes, NF-κB activity can be inhibited by β-Catenin via the physical interaction of β-Catenin with RELA and/or P50 in the presence of a GSK-3 inhibitor or upon WNT3a stimulation. In a study of post-transplant bone disease41,42, it was found that the content of sclerostin in bone increased. In contrast, the expression of receptor activator of NF-κB ligand in both serum and bone was increased, accompanied by WNT pathway inhibition43. In TNF-dependent arthritis models, WNT inhibition by sclerostin blocked tumor necrosis factor alpha (TNF-α)-induced NF-κB activation44. In summary, subsets of NF-κB-induced target genes and their related biological functions are differentially influenced by WNT/β-Catenin in response to different stimuli40. Emerging evidence suggests that sclerostin might play an essential role in NF-κB-associated diseases, providing new opportunities to treat not only WNT-associated diseases but also NF-κB-associated diseases.
2.3. Diseases related to the contributional role of sclerostin
2.3.1. Osteoporosis and age-related muscle weakness related to sclerostin
2.3.1.1. Postmenopausal osteoporosis
OP, a skeletal disorder in which bones become weakened and easily fractured, leading to considerable disability and mortality, is a global healthcare problem45,46. Postmenopausal osteoporosis (POP) is the most common type of OP46. Increasing evidence has shown that a high sclerostin level is a solid and independent risk factor for OP-related fractures in postmenopausal women47,48. As a pivotal regulator of bone formation, sclerostin is considered an attractive target for developing inhibitors for use in anabolic bone and other therapies. Substantial preclinical and clinical evidence supports the use of sclerostin inhibitors in OP (shown in Table 149, 50, 51). In phase III trials, in postmenopausal women with OP, subcutaneous administration of sclerostin antibody (romosozumab) effectively enhanced bone mineral density (BMD), compared with that in the placebo group49, as well as in men with OP50. Anti-sclerostin therapy resulted in enhanced BMD, improved bone structure and greater bone strength4. In 2019, romosozumab (brand name: Evenity®), a humanized therapeutic antibody against sclerostin, was approved by both the U.S. FDA and European Medicines Agency (EMA) for POP with a warning for cardiovascular risk (U.S. FDA Press Announcements, 2019; EMA Documents, 2019). Because it may increase the risk of cardiovascular events, the application of romosozumab is limited. Therefore, the development of new-generation sclerostin inhibitors that do not increase cardiovascular risk is a desired research objective.
Table 1.
The efficacy on bone in human treated with sclerostin antibody.
| Patients | Disease | Age (year) | Treatment | Duration (month) | BMD |
Ref. | ||
|---|---|---|---|---|---|---|---|---|
| LS | F | TH | ||||||
| 6390 women | POP | 55–90, 70.9 | 210 mg, monthly | 12 | 13.3% ↑ | 5.9% ↑ | 6.9% ↑ | 49 |
| 245 men | OP | 55–90, 72 | 210 mg, monthly | 12 | 12.1% ↑ | N/A | TF 2.5% ↑ | 50 |
| 315 women | Low BMD | 55–85, 66 | 70 mg, monthly | 12 | 5.3% ↑ | 0.7% ↑ | 1.2% ↑ | 51 |
| 24 | 6.9% ↑ | 1.2% ↑ | 1.9% ↑ | |||||
| 140 mg, monthly | 12 | 9.0% ↑ | 4.3% ↑ | 3.4% ↑ | ||||
| 24 | 12.5% ↑ | 5.3% ↑ | 4.5% ↑ | |||||
| 210 mg, monthly | 12 | 11.3% ↑ | 3.7% ↑ | 4.1% ↑ | ||||
| 24 | 15.1% ↑ | 5.2% ↑ | 5.4% ↑ | |||||
BMD, bone mineral density; F, femoral neck; LS, lumbar spine; TF, total femur; TH, total hip.
2.3.1.2. Disuse osteoporosis
Disuse OP describes a state of bone loss due to local skeletal unloading, systemic immobilization or a microgravity environment51, 52, 53. Emerging evidence shows that sclerostin plays a vital role in mechanical responses. Mechanical unloading of wild-type mice caused upregulation of sclerostin, while sclerostin-deficient mice were resistant to automatic unloading-induced bone loss54. Mechanical stimulation by enhanced loading of bone (the ulna) in vivo reduced osteocyte expression of sclerostin, while reduced loading (hindlimbs) increased sclerostin expression55. Consistently, although the stimulatory effect of Scl-Ab on bone formation was transient and followed by a downturn in animal models56 and humans49 despite continuous exposure to Scl-Ab, recent reports showed that bone formation induced by Scl-Ab was reactivated upon exposure to mechanical stimuli57. All these data indicated that sclerostin inhibition could be a promising strategy for preventing/rescuing disuse bone loss, especially for those lacking exposure to mechanical stimuli, such as bedridden people, disuse OP patients and long-term aerospace passengers. However, as we mentioned, Scl-Ab has limited application since it might increase the risk of cardiovascular events. Notably, astronauts show higher cardiovascular risks58 and/or higher cardiovascular disease mortality59, suggesting that Scl-Ab may further increase their cardiovascular risk. Therefore, in order to not increase cardiovascular risks and prevent disuse OP in patients with disuse OP and individuals lacking mechanical stimuli, especially those undergoing long-term space flight, the development of new-generation sclerostin inhibitors is warranted.
2.3.1.3. Fracture
Bone fracture is a medical condition in which the continuity of the bone is partially or entirely broken. Genetic evidence has shown that sclerostin deficiency induced by Sost-KO does not inhibit endochondral repair. However, sclerostin enhances fibrocartilage/cartilage callus removal, resulting in united bony calluses with increased bone involvement and increased strength60,61. The effect of sclerostin inhibition induced by monoclonal antibodies on fracture healing has been investigated in several in vivo animal models, including rodent closed fracture models62,63, a rodent open fracture model64, rodent osteotomy models with/without pins/screws65,66 and a primate fibular osteotomy model62. Many non-clinical pharmacological studies have shown that sclerostin inhibition induced by a Scl-Ab can significantly augment bone-specific anabolism and callus formation, promote fracture healing and enhance implant fixation, especially in the early stages of the healing process. Moreover, for fracture healing, dual inhibition of sclerostin and DKK-1 leads to synergistic bone formation in rodents and non-human primates, showing superior bone repair activity compared with monotherapies67. However, two international phase II investigating the effects of romosozumab on fracture healing for patients with fractures showed that short-term treatment with romosozumab did not significantly improve fracture healing-related clinical and/or radiographic outcomes in the studied patient populations68,69. In conclusion, in contrast to evidence obtained with rodents, clinical evidence failed to support the utilization of sclerostin inhibition by administration of romosozumab for accelerating fracture repair.
2.3.1.4. Sarcopenia
Sarcopenia is a condition characterized by loss of skeletal muscle mass and function70. Osteocyte-derived sclerostin was shown to have a significant association with lean muscle mass. Some evidence has shown that a pathological WNT signalling pathway may be a cause of sarcopenia. Serum sclerostin level can be used as a biomarker for identifying the risk of sarcopenia12. In addition, a study by Kim et al.12 revealed that osteocyte-derived sclerostin was significantly associated with skeletal muscle mass. High serum sclerostin levels have been independently correlated with low muscle mass in non-diabetic men and women in Korea. These studies showed that serum sclerostin levels are negatively associated with skeletal muscle mass.
However, since the WNT pathway is engaged in complex interconnections with other ways involved in skeletal muscle regeneration and myogenesis, the implication of the WNT signalling pathway in the regulation of aged skeletal muscle remains ambiguous12. This ambiguity might be associated with the pleiotropic roles of WNT. The physiological relevance of circulating sclerostin to skeletal muscle development needs to be further explored through functional studies.
2.3.2. Rare bone diseases related to sclerostin
2.3.2.1. Osteogenesis imperfecta (OI)
OI is the most common single-gene-inherited bone disorder with skeletal fragility and severe bone mass and architecture defects. To date, no pharmacological treatment has been explicitly developed for OI. Both genetic and pharmacological evidence has indicated that sclerostin inhibition can promote bone anabolism in Col1a2+/G610C mice (the classical phenotype)71,72. Moreover, the efficacy of anti-sclerostin bone anabolic treatment has been validated in Brtl/+ mice (a moderate phenotype) and Col1a1jrt/+ and Crtap–/– mice (severe phenotypes)73. However, the humanized therapeutic Scl-Ab (romosozumab) caused extreme cardiac ischaemic events in the clinic49,50,74. In addition to the primary clinical manifestation related to the skeleton, a series of associated secondary features were observed, including cardiac valve and aortic wall abnormalities75,76. Accordingly, there is also growing cardiovascular concern for OI patients undergoing Scl-Ab treatment, especially those with cardiovascular abnormalities or a history of cardiovascular disease. Thus, a sclerostin inhibitor that can promote bone anabolism but does not increase the cardiovascular risk for patients with OI needs to be developed.
2.3.2.2. X-linked hypophosphatemia
XLH is the most common type of vitamin D resistant rickets. The hypophosphatemia observed in some XLH patients may be due to high levels of bone-derived phosphaturic hormone fibroblast growth factor 23 (FGF23)77. Therapeutic neutralizing FGF23 antibody (FGF23-Ab), which was approved by the U.S. FDA and EMA for the treatment of XLH78, is contraindicated in patients with renal insufficiency, and warnings have been issued to patients with hyperphosphatemia and nephrocalcinosis (U.S. FDA Press Announcements, 2018; EMA Documents, 2018), limiting their use in chronic kidney disease (CKD)79. In CKD rats, FGF23-Ab worsened hyperphosphatemia and led to early death80. Therefore, it is vital to develop novel therapeutic targets for XLH with renal insufficiency.
In both XLH patients and Hyp mice (a widely used model of XLH), sclerostin levels were higher than average81,82. In addition, sclerostin deficiency by Sost-KO elevated the serum phosphate level in Hyp mice83. Administration of a therapeutic Scl-Ab suppressed the circulating levels of intact FGF23 in Hyp mice, which attenuated specific pathologies associated with XLH84. Scl-Ab treatment affected XLH comparable to that of FGF23-Ab therapy (shown in Table 284, 85, 86). Moreover, it was found that Scl-Ab had no significant impact on hyperphosphatemia-related biochemistry in CKD rats87 and that sclerostin deficiency modulated the development of the bone mineral disorder in both CKD mice and normal mice88. All this evidence showed that sclerostin is a potential therapeutic target for XLH patients with or without CKD.
Table 2.
The efficacy on XLH in animal treated with FGF23 antibody (FGF23-Ab) or Sclerostin antibody (Scl-Ab).
| Treatment | FGF23-Ab | FGF23-Ab | Scl-Ab |
|---|---|---|---|
| Animal model | Hyp mice (♂) | Hyp mice (♂) | Hyp mice (♂, ♀) |
| Dose | 16 mg/kg, 1/week, 4 times | 35 mg/kg, 3/week, 30 times | 25 mg/kg, 1/week, 4 times |
| Serum phosphate (%) | WT: 49%↑ | N/A | WT: 56%↑ |
| Hyp: 127%↑ | Hyp: 31%↑ | Hyp: 80%↑ | |
| Bone strength | N/A | ↑ | ↑ |
| BV/TV | ↑ | ↑ | ↑ |
| Trabecular thickness (Tb.Th) | N/A | ↑ | ↑ |
| Ref. | 85 | 86 | 84 |
Currently, inhibition of sclerostin by therapeutic Scl-Ab (romosozumab) has demonstrated bone anabolic potential for clinical use. However, it may increase cardiovascular risk. Notably safety concerns have increased for XLH patients with a history of cardiovascular diseases and who have received romosozumab74. Therefore, the development of next-generation sclerostin inhibitors that do not increase the cardiovascular risk for patients with XLH is needed.
2.3.2.3. Chronic kidney disease mineral bone disorder syndrome related to sclerostin
CKD often causes detrimental bone disturbances in bone turnover, bone mineral balance and develops severe vascular calcification (VC)89. In uremic rats, the plasma levels of sclerostin were significantly higher than those in normal rats89. Clinical data show that, in CKD patients, the circulating levels of sclerostin were significant higher (approximately 3–4 times) than those in normal individuals, and the circulating sclerostin levels decreased during dialysis90,91. Moreover, it was found that sclerostin deficiency modified the development of mineral and bone disorder (MBD) in both CKD mice and normal mice86. Consistently, Scl-Ab treatment could also ameliorate the MBD in CKD rats, although the degree was slight85. All the above evidence showed that sclerostin could be a potential therapeutic target for CKD–MBD. In addition, it was found that the increased cardiovascular risk associated with kidney diseases partly resided in the CKD–MBD syndrome92, in which the circulating sclerostin levels were abnormally higher93,94. The presence of VC, in which sclerostin was identified as one of the secreted factors from the calcified vasculature, directly affected bone metabolism89. In either case, sclerostin was considered a CKD–MBD biomarker and a potential therapeutic target95. However, as mentioned above, sclerostin simultaneously has a protective role in VC and cardiovascular risk in CKD95. In contrast, the utilization of Scl-Ab has cardiovascular risks for patients with CKD–MBD. Therefore, the development of next-generation sclerostin inhibitors that do not increase the cardiovascular risk for patients with CKD–MBD is needed.
2.3.3. Certain cancers related to sclerostin
Clinical studies on a wide range of cancers have revealed elevated levels of sclerostin in patients, such as breast cancer, especially in three negative breast cancer (TNBC)96. With implications for translation into medicinal applications, inhibition of sclerostin expression by a sclerostin-neutralizing antibody suppressed bone metastasis and increased the survival of breast cancer model mice97,98.
At the mechanistic level, two potential bridges can be leveraged to close the gap between cancers and sclerostin expression. One bridge involves the potential role of sclerostin in immunosuppression or by promoting the accumulation of myeloid-derived immunosuppressor cells (MDSCs). It has been found that the population of MDSCs in tumour tissues obtained from TNBC patients was more extensive than that in non-TNBC patients99. In addition, it has been established that the downregulation of the WNT/β-Catenin signalling pathway drives MDSCs recruitment and promotes immunosuppression in the tumour microenvironment100,101. Considering this evidence, one group suggested that sclerostin might participate in the immunomodulation of the tumour microenvironment in TNBC102.
Angiogenesis might be the other bridge connecting sclerostin to cancer. Angiogenesis plays an essential role in tumour growth, progression and metastasis. Interestingly, emerging evidence has indicated that angiogenesis is associated with sclerostin103. It was reported that sclerostin increased not only the proliferation of human umbilical vein endothelial cells but also the formation of anastomosing tubule networks, the percentage of tubules, the total length of tubules and the number of junctions, implying that the role of sclerostin in promoting angiogenesis is similar to that of vascular endothelial growth factor104. Mechanistically, sclerostin was shown to enhance angiogenesis by stimulating the production of two acknowledged proangiogenic cytokines, placental growth factor and vascular endothelial growth factor, in an LRP6-dependent manner104.
2.3.4. Obesity and diabetes related to sclerostin
Emerging evidence has revealed the relationship between sclerostin and obesity. Clinical evidence has demonstrated that circulating sclerostin levels are positively correlated with fat mass in aged men105 and postmenopausal women106. Similar results were confirmed in children, and serum sclerostin levels were slightly altered107. In vivo data demonstrated that in obese mice induced fed a high-fat diet, serum sclerostin levels were significantly higher than those in normal control mice108. In Sost-KO mice, fat mass and adipocyte size were reduced, while the opposite phenotype was observed in mice with sclerostin overexpression109. At the mechanistic level, it was found that sclerostin can induce adipocyte differentiation110, increase beige adipogenesis111 and regulate adipocyte metabolism109, at least in part, through inhibition of WNT signalling in preadipocytes107. With implications for translation into medicinal applications, inhibition of sclerostin by a sclerostin-neutralizing antibody suppressed adipocyte differentiation, inhibited lipid synthesis, and promoted fatty acid oxidation in mice109.
Sclerostin is closely linked with diabetes and glucose metabolism in both children and adolescents. A series of clinical studies demonstrated that circulating sclerostin levels were higher in individuals with prediabetes112, type 1 diabetes and type 2 diabetes113. Moreover, circulating sclerostin levels were positively correlated with adipose insulin resistance in both healthy individuals and people with prediabetes110. A series of in vivo data demonstrated that Sost-KO improved insulin-stimulated glucose uptake and enhanced insulin sensitivity in mice, while sclerostin overexpression resulted in increased insulin resistance109. Inhibiting sclerostin expression by a sclerostin-neutralizing antibody enhanced glucose metabolism and ameliorated insulin resistance in obese mice fed a high-fat diet109.
All this evidence showed that sclerostin is a potential therapeutic target for obesity and diabetes. However, the sclerostin antibody used for treatment might increase the risk of heart attack, stroke and cardiovascular death in humans with a history of cardiovascular disease (U.S. FDA Press Announcements, 2019; EMA Documents, 2019). Moreover, both obese and diabetes patients are typically at high risk of cardiovascular disease114, which further limits the utilization of a sclerostin antibody. The development of new-generation sclerostin inhibitors is desired for the treatment of obesity and diabetes.
2.4. Diseases related to the protective role of sclerotin
2.4.1. Rheumatoid arthritis
In chronic TNF-α-dependent arthritis, most sclerostin is secreted from fibroblast-like synoviocytes. Sclerostin deficiency caused by either gene truncation or antibody-mediated inhibition accelerates the progression of RA-like disease in human TNF-α transgenic mice with enhanced pannus formation and joint destruction12. In a partially TNF-α-dependent glucose-6-phosphate isomerase-induced arthritis mouse model, suppression of sclerostin failed to attenuate clinical signs of joint destruction. However, in K/BxN serum transfer-induced arthritis mouse models, inhibition of sclerostin ameliorated disease severity independently of TNF receptor signalling. These discoveries suggested a particular role for sclerostin in TNF-α signalling. Sclerostin effectively blocked TNF-α but not interleukin-1-induced activation of P38, a crucial step in arthritis development, revealing a previously unknown protective role of sclerostin in TNF-mediated chronic inflammation. The worsening effect of Scl-Ab treatment on the clinical RA outcome in mice under chronic TNF-α-dependent inflammatory conditions indicates that caution should be taken when considering this treatment for either an inflammatory bone loss in RA and/or in patients with TNF-α-dependent comorbidities12.
2.4.2. Cardiovascular diseases related to sclerostin
Atherosclerosis, a common atherosclerotic vascular disease, is a pathology of ischaemic heart diseases such as coronary heart disease, leading to inappropriate proliferation of vascular smooth muscle115. Studies have shown that sclerostin is closely associated with cardiovascular diseases and that sclerostin serum level is related to atherosclerosis13. In a study of 191 African-American men, the levels of sclerostin were positively correlated with coronary artery calcification116. Koos et al.117 found that the level of sclerostin in patients with aortic valve calcification was significantly higher than that in healthy people and was increased with the severity of vascular calcification. A meta-analysis of the effects of romosozumab, a humanized therapeutic Scl-Ab, in phase III randomized controlled trials (BRIDGE and ARCH) indicated a higher risk of cardiac ischaemic events in patients randomized to romosozumab (210 mg per month). Another meta-analysis on BMD-increasing SOST variants (rs7209826 and rs188810925) showed that BMD-increasing SOST variants were associated with higher cardiovascular risk118. Additionally, in CHD patients, sclerostin was expressed at a significantly higher level than that in the non-CHD group and was positively correlated with coronary artery calcification scores. However, contrary conclusions were reported in some studies. For example, in a study of 313 elderly patients, sclerostin in the CHD group was lower than that in the control group, suggesting that the sclerostin level may be a risk factor for CHD119. Another study found that in postmenopausal women with type 2 diabetes, the level of sclerostin was negatively correlated with carotid intima-media thickness113. Additionally, some statistics identified sclerostin as an independent arterial stiffness predictor in hypertensive patients120. These results indicated that cardiovascular diseases are closely associated with sclerostin.
As mentioned above, sclerostin plays different roles in different diseases, summarized in Table 312,68,69,72,73,84,98,109,111,118,121, 122, 123, 124, 125, 126, 127. Inhibition of sclerostin not only promotes bone formation but also inhibits bone resorption, making it a promising therapeutic target for the treatment of WNT-related bone diseases.
Table 3.
A summary of sclerostin-related diseases.
| Sclerostin's role |
Disease | Animal | Therapy |
Ref. | |||
|---|---|---|---|---|---|---|---|
| Role | Change | Signal pathway | Drug | Phase | |||
| Maintain normal function | Sclerostin mutations (loss-of-function mutations in SOST gene) | WNT/β-Catenin | Sclerosteosis | Human | / | / | 121 |
| Sclerostin mutations (a noncoding deletion that removes a SOST-specific regulatory element in bone) | WNT/β-Catenin | van Buchem disease | Human | / | / | 121 | |
| Contributional | Sclerostin (serum) ↑ | WNT/β-Catenin | POP | Mice | Scl-Ab (Romosozumab) | Approved | (FDA Press Announcements, 2019; EMA Documents, 2019) |
| Rats | |||||||
| Human | |||||||
| Sclerostin (serum) ↑ | WNT/β-Catenin | Disuse bone loss | Mice | Scl-Ab | / | 111,122,123 | |
| Rats | |||||||
| Sclerostin (serum) ↑ | WNT/β-Catenin | Combined POP and disuse bone loss | Rats | Scl-Ab | / | 124 | |
| Sclerostin (serum) ns | WNT/β-Catenin | Spinal cord injury (SCI)-induced bone loss | Rats | Scl-Ab | / | 125 | |
| – | WNT/β-Catenin | Fracture | Mice | Scl-Ab | Phase II | 68,69 | |
| Rats | |||||||
| Monkey | |||||||
| Human | |||||||
| / | WNT/β-Catenin | OI | Mice | Scl-Ab (Romosozumab) | Phase I | 72,73,126 | |
| Human | |||||||
| Sclerostin (serum) ↑ | – | XLH | Mice | Scl-Ab (Romosozumab) | / | 84 | |
| Sclerostin (serum) ↑ | WNT/β-Catenin | Age-related muscle weakness | Human | / | / | 12 | |
| Sclerostin (cancer tissue) ↑ | P38/NF-κB | Cancers: triple-negative breast cancer | Mice | Scl-Ab | / | 98 | |
| Sclerostin (cancer tissue) ↑ | P38/NF-κB | Breast cancer-induced muscle weakness | Mice | Scl-Ab | / | 98 | |
| Sclerostin (serum) ↑ | WNT | Obesity | Mice | Scl-Ab | / | 109 | |
| BMP | |||||||
| Sclerostin (serum) ↑ | WNT | Diabetes | Mice | Scl-Ab | / | 109 | |
| Protective | Sclerostin (serum) ↑ | WNT | RA | Mice | Scl-Ab | / | 127 |
| Sclerostin (serum) ↑ | WNT | Cardiovascular diseases | Clinical trials & human genetics | Scl-Ab (Romosozumab) | / | 118 | |
/, Not applicable. –, Not available. ns: no difference.
3. Clinical advancements in the development of anti-sclerostin monoclonal antibody drugs
3.1. The mechanism of action of anti-sclerostin antibodies
Scl-Ab was found to regulate bone metabolism and promote bone formation at multiple levels128. At the gene level, it was reported that Scl-Ab could reduce the level of DNA double-strand-break marker γ-H2AX and increase the level of DNA repair protein KU70, thereby reducing the apoptosis rate of osteoblasts by accelerating DNA repair129. At the cell level, several studies have shown that Scl-Ab can indirectly reduce the activity of osteoclasts by participating in the regulation of osteoblast proliferation and differentiation and by inhibiting osteoblast apoptosis induced during bone metabolism130. It was reported that Scl-Ab inhibits the binding of sclerostin to LRP5/6 and weakens the antagonistic activity of sclerostin on WNT-induced responses131. In addition, Scl-Abs were observed to promote bone formation on remodelled surfaces and resting surfaces in tested gonad-intact female monkeys, Sost-KO mice, and OVX rats. These treated mice also presented with greater bone mass and strength increases than were found in control samples taken from non-OVX rats. The results indicated that Scl-Ab could prolong the lifespan of osteoblasts132, probably because Scl-Ab interacts with both the loop 2 and part of the loop 3 (close to the C-terminal domains) domains in sclerostin by interfering with sclerostin-mediated inhibition of WNT-induced AXIN expression15. Generally, loop 2 is critical for antagonism of the WNT protein19.
3.2. Currently promising anti-sclerostin antibodies
To date, several monoclonal antibodies have been demonstrated to be effective sclerostin inhibitors (shown in Table 471,125,133,134), including romosozumab, which has been approved for clinical use. Some drugs have been in clinical trials, and others aborted during the discovery or preclinical stage because of limited efficacy135,136.
Table 4.
Anti-sclerostin antibodies approved or in clinical research.
Blosozumab, another promising sclerostin inhibitor, passed phase I and II clinical trials. Developed by Eli Lilly Inc. for the treatment of OP, blosozumab acts as a negative regulator of osteoblast activity by binding sclerostin137. In a phase I clinical trial with healthy postmenopausal volunteers, the effects on bone biomarkers, including serum sclerostin, total procollagen type 1 N-terminal propeptide, BMD and so on, were dose-dependent for single- and multiple-dose blosozumab treatments138. These outcomes were corroborated by the results obtained on the 85th day of the trial indicating that bone density at the lumbar spine increased by 3.41% and 7.71%, respectively, from baseline in the single- and multiple-dose groups. In a phase II clinical trial including 120 premenopausal women with low BMD who received different doses of blosozumab or placebo for 12 months, the highest-dose group had a 17.7% increase in lumbar spine bone density and a 6.2% increase in total hip bone density at the end of the trial137.
BPS-804 (setrusumab), a human immunoglobulin G2 monoclonal antibody targeting sclerostin, is used to treat OI, a rare genetic disorder associated with low bone mass and increased bone brittleness, and repeated fracture. This antibody, which can reduce the activity of sclerostin, is in phase II clinical trials. Trial results obtained to date have indicated that treatment with this antibody stimulated bone formation, reduced bone resorption, and increased lumbar spine BMD in adults with moderate OI, showing good tolerance and potential for clinical application71.
SHR-1222, a humanized immunoglobulin G4 monoclonal antibody targeted to soluble sclerostin, is a novel class 1 biological product developed by Jiangsu Hengrui Medicine Co., Ltd. for the treatment of OP and is currently in phase I clinical trials. The preclinical pharmacokinetic and toxicokinetic studies of SHR-1222 indicated its potential for clinical application139. Gao et al.134 developed a powerful detection method for quantifying SHR-1222 in serum samples of cynomolgus monkeys, and the pharmacokinetic results showed that the levels of SHR-1222 after 30 days in vivo were acceptable.
The abovementioned antibody drugs are in the clinical stage, and one previously tested drug romosozumab has been approved by the U.S. FDA.
3.3. Romosozumab: the first universally approved drug targeting sclerostin
3.3.1. Mechanism of action
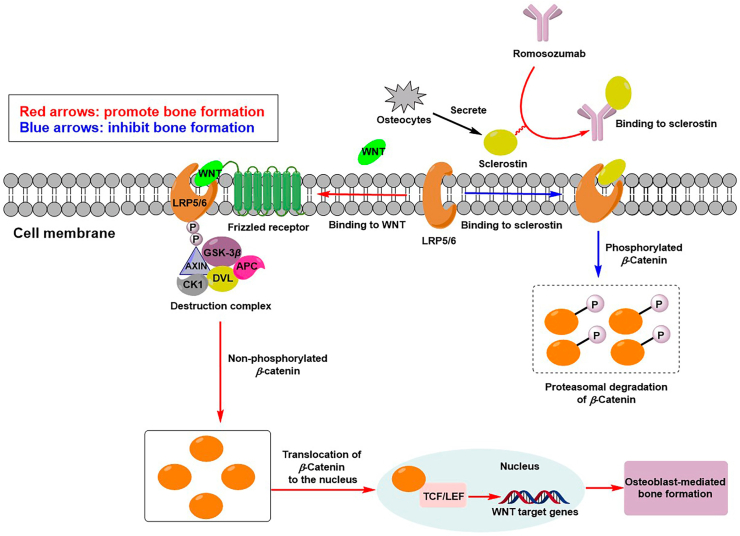
Romosozumab (AMG 785), a humanized monoclonal antibody, is the most mature Scl-Ab. It can both inhibit sclerostin and antagonize its negative regulatory efficacy on bone metabolism, thereby promoting bone formation and inhibiting bone resorption51,140. Romosozumab was successively approved by the U.S. FDA and EMA for the treatment of OP in postmenopausal women with a high risk of fracture in April and October 2019, respectively6,141. The mechanism of action of romosozumab is shown in Fig. 3. Specifically, romosozumab preferentially combines with sclerostin, inhibiting the binding of sclerostin to LRP5/6142. The abovementioned action was beneficial for WNT ligand binding to LRP5/6 co-receptors and the Frizzled143,144, thereby preventing proteasomal degradation of β-Catenin from increasing its concentration.
Figure 3.
The mechanism of action of romosozumab. When WNT bound to LRP-5/6 co-receptors and the Frizzled receptor, the action of AXIN was limited that the β-Catenin destruction complex would not be assembled as lack of free AXIN. P, phosphorylated; GSK3β, glycogen synthase kinase 3β; APC, Adenomatous Polyposis Coli; CK1, casein kinase 1; PVL, protruding-vulva; TCF/LEF, cell factor/lymphatic enhancement factor.
3.3.2. Phase III clinical trials: the effectiveness, efficacy, and safety of romosozumab
U.S. FDA approval of romosozumab was based on data from critical points of phase III clinical trials. The complete clinical assessment of romosozumab was established on 19 clinical studies that enrolled approximately 14,000 patients. The efficacy of therapies including romosozumab, placebo, and other agents in patients with OP was tested. The results showed that romosozumab could effectively reduce the risk of osteoporotic fractures in postmenopausal women and increase BMD values in patients145. As early as 2011, human trials with romosozumab with a cohort of 72 subjects conducted by Padhi et al.146 showed that romosozumab therapy significantly increased the levels of procollagen type 1 N-terminal propeptide and BMD values for the lumbar spine and hip. Nonetheless, some common adverse drug reactions (ADRs), such as injection-site erythema and back pain, persisted146. In the study of the efficacy and safety of romosozumab conducted by Langdahl et al.147, 436 women with postmenopausal OP who received bisphosphonate therapy for more than one year were randomly assigned to 2 groups for circannual treatment of romosozumab or teriparatide. Both romosozumab and teriparatide increased the BMD value of patients significantly, with the former having a more substantial effect than teriparatide which has been approved as an effective anabolic (promoting bone formation) agent that could only stimulate bone formation but don't inhibit bone resorption. The number of adverse events and serious adverse events was balanced in the two groups147.
Cosman et al.49 conducted a study of romosozumab in a phase III clinical trial (FRAME study) that enrolled 7180 postmenopausal women from 55 to 90 years old who presented with a T score of the total hip or femoral neck in a range from −2.5 to −3.5. Participants were randomly assigned to receive a subcutaneous injection of romosozumab or placebo monthly for 12 months. After that, each group of patients received denosumab treatment via a total of two subcutaneous injections taken six months apart. Ultimately, 6390 patients (89%) completed the 12-month trial, and 6026 patients (83.9%) completed the 24-month trial. At 12 months, 0.5% and 1.8% of the patients who had received romosozumab and placebo, respectively, presented with a new incidence of vertebral fracture, and the romosozumab therapy group suffered from a 73% risk of further fracture, which was lower than that of the placebo group (P < 0.001). At 24 months, the chance of a new vertebral fracture incidence increased by 0.6% and 2.5% in the patients of the romosozumab group and placebo group, respectively. The risk of new vertebral fracture was 75% lower in the romosozumab group than in the placebo group (P < 0.001)49. Saag et al.74 carried out another crucial clinical trial (the ARCH study), a randomized, double-blind controlled trial of alendronate. In this study, the researchers recruited 4093 postmenopausal women with OP and fragility fractures. These patients were randomly assigned to 2 groups and received subcutaneous injections of romosozumab or oral alendronate monthly for 12 months. After that, both groups received oral alendronate for another 12 months. At the end of the study, 3654 patients (89.3%) had completed the 12-month trial, and 3150 patients (77.0%) had finished the primary analysis period. The results showed that over the 24 months, 6.2% of the patients in the romosozumab-to-alendronate group and 11.9% in the alendronate-to-alendronate group experienced new vertebral fractures (P < 0.001), and a 48% lower risk of new vertebral fractures was observed in the former group than in the latter group (11.9%) (P < 0.001). Clinical fractures occurred in 198 of the 2046 patients (9.7%) in the romosozumab-to-alendronate group, fewer than in the alendronate-to-alendronate group (13.0%), representing a 27% lower risk upon treatment with this antibody (P < 0.001). Additionally, the risk of nonvertebral fractures and hip fractures in the romosozumab-to-alendronate group was 19% and 38% lower than that in the alendronate-to-alendronate group, respectively, indicating that both romosozumab and alendronate were able to decrease the risk of nonvertebral fractures and hip fractures. Romosozumab owns a more substantial effect than alendronate that has been approved as a class of drugs which inhibit bone resorption but could not promote bone formation. In addition to women, men with OP were subjects in research studies. BRIDGE was a randomized, double-blind, placebo-controlled trial of 245 men between 55 and 90 years old who suffered from OP and had a history of brittle fracture or vertebral fractures, except hip fractures and the total treatment time was 12 months. The results of the BRIDGE trial showed that romosozumab significantly increased total hip and femoral neck BMD50. Overall, the incidence of adverse events and serious adverse events was similar between the two groups74.
In the FRAME study49, the most common ADRs (≥2%) in the romosozumab group were arthralgia, headache, muscle spasm, peripheral oedema, fatigue, insomnia, and sensory abnormalities during the 12-month double-blind study period. In addition, the incidence of cardiovascular deaths and serious cardiovascular events was higher in the romosozumab group49. An imbalance in the number of serious cardiovascular adverse events (SCAEs) also occurred in the ARCH study conducted by Saag et al.74, which were reflected in the number of patients and frequency of SCAEs reported during the double-blind period. In other words, receiving romosozumab therapy increases the risk of patients suffering from an SCAE. In this study, 50 (2.5%) patients in the romosozumab group were reported to have experienced an SCAE, and 38 (1.9%) patients in the alendronate group were reported to have experienced an SCAE. Moreover, a higher frequency of cardiac ischaemic and cerebrovascular events in the romosozumab group contributed to the imbalanced number of patients affected. As mentioned above, sclerostin can confer cardiovascular protection when constitutively expressed in a site related to the vascular system148. For instance, in July 2019, Astellas and Amgen Inc. reported 11 SCAEs, including three deaths within three months following the launch of romosozumab. Specifically, a 71-year-old man died two days after injection because of cardiac failure. Within six months of treatment, 68 drug-related serious cardiovascular events were reported, including 13 deaths149. A meta-analysis performed to evaluate the risk of romosozumab-related cardiovascular events in a clinical trial also showed that romosozumab significantly increased the risk of major SCAEs, such as cardiovascular death, myocardial infarction, stroke, and heart failure150.
3.3.3. The tortuous road to approval
In January 2019, Amgen and UCB Inc. announced that romosozumab for the treatment of OP had been listed on the market by the Ministry of Health, Labour and Welfare, Japan. Remarkably, it was the first endorsement of romosozumab in the world. Compared with the smooth road to approval in Japan, the total review cycle in the United States for romosozumab lasted approximately 33 months. In July 2016, based on FRAME clinical data, Amgen and UCB Inc. submitted an abbreviated new drug application for romosozumab to the U.S. FDA for the first time. However, one year later, the U.S. FDA issued a complete response that requested additional submission of the latest clinical trial data. In July 2018, Amgen and UCB Inc. submitted a new drug application to the U.S. FDA. The U.S. FDA acknowledged the label of Evenity® but limited its approval to postmenopausal women at high risk for fractures and even recommended that the prescription duration should not exceed 12 months.
Furthermore, the approval came with a black box warning indicating that, because it might increase the risk of cardiovascular disease and death, romosozumab should not be used in patients who have had a heart disease or stroke in the past year. Notably, the black box warning in the Japanese approval documentation did not mention cardiovascular events, but these events were highlighted in the U.S. and the European Union versions. Therefore, risks of treatment should be weighed against benefits in patients with other cardiovascular risk factors. During treatment, if patients experience a heart attack or stroke, romosozumab therapy should be discontinued. The U.S. FDA also requested a post-marketing study to assess the cardiovascular safety of romosozumab in postmenopausal women with OP, including a five-year observational feasibility study, followed by a comparative safety study. Although the review cycle of romosozumab in the United States seemed to track a circuitous route, romosozumab was approved by the U.S. FDA in April 2019. However, the European Union directly rejected the listing application of romosozumab in July 2019. On June 27, 2019, Amgen and UCB Inc. announced that the Medicinal Products for Human Use disapproved marketing romosozumab for the treatment of severe OP. The main reason for the refusal was based on the elevated risk of disease to the cardiovascular or circulatory systems, such as a heart attack or stroke upon romosozumab treatment. Although romosozumab was finally approved by the EMA in October 2019, the approval was burdened with a detailed warning about the potential risk of cardiovascular complications, common ADRs such as headaches, allergies, injection-site reactions, osteoarthritis, hypocalcaemia, osteonecrosis of the jaw, and atypical femur fractures150, 151, 152.
As the first sclerostin inhibitor approved by the U.S. FDA, romosozumab shows excellent effectiveness and efficacy. However, in terms of safety, romosozumab is associated with high cardiovascular risk during treatment. Therefore, the development of next-generation sclerostin inhibitors promoting bone formation without increasing cardiovascular risk to solve existing issues caused by romosozumab is a challenging task.
4. Progress in the research of other types of inhibitors targeting sclerostin
4.1. Progress in the research of aptamers targeting sclerostin
In addition to monoclonal antibodies, nucleic acid aptamers are competitive alternatives with high affinities and specificities for their target proteins and potential benefits because of their adaptable chemical synthesis processes and low immunogenicity153. Through a process of screening DNA aptamers targeting sclerostin, Dr. Julian A. Tanner's group154 identified a DNA aptamer that specifically binds sclerostin, showing binding affinities in the nanomolar range, as determined by solid-phase assays and isothermal titration calorimetry. The aptamer was modified and stabilized with a 3′ end thymidine residue, which exhibited robust and specific inhibition of the antagonistic effect of sclerostin on the WNT signalling pathway in a dose-dependent manner154. However, the identified aptamer was mouse-specific and was not experimentally validated in rodents.
Recently, our group identified a new DNA aptamer targeting sclerostin155. As mentioned above, the U.S. FDA-approved sclerostin antibody romosozumab has a boxed warning for potential cardiovascular risk. It was reported that sclerostin had a protective role in the cardiovascular system by inhibiting inflammatory cytokines from preventing aortic aneurysm and atherosclerosis development in Apoe–/– mice induced by angiotensin II156. Before selecting aptamers for sclerostin, Yu et al.155 first investigated the function of the different sclerostin loops participating in cardiovascular protection and bone formation inhibition. They found that loop 2 and/or loop 3 play important roles in inhibiting WNT signalling and osteogenic potential in MC3T3-E1 cells. Either loop 2 and 3 deficiency caused by gene truncation or loop 2 and 3 inhibition induced by an Scl-Ab attenuated the suppressive effects of sclerostin on the expression of inflammatory cytokines and chemokines in macrophages and vascular smooth muscle cells. In contrast, after in vitro angiotensin II infusion, macrophages and vascular smooth muscle cells with loop 3 deficiency caused by gene truncation maintained the suppressive effects of sclerostin described above155. In a tailored aptamer screening, an aptamer was selected by specifically targeting sclerostin loop 3, which showed inhibition of the antagonistic effect of sclerostin on WNT signalling in vitro but had no impact on the protective role of sclerostin in inhibiting the expression of inflammatory cytokines and chemokines in vitro155. The identification of this new aptamer indicated that targeting sclerostin loop 3 is a promising strategy for anti-sclerostin-induced bone anabolic therapy without increasing cardiovascular risk. Excitingly, the aptamer identified for treating OI was granted an orphan drug designation by the U.S. FDA in 2019 (DRU-2019-6966), and its effects were experimentally validated in OI mice. New therapies with aptamers targeting sclerostin loop 3 offer a promising strategy to promote bone formation without increasing cardiovascular risk. However, aptamer or monoclonal antibody drugs can only be administered by injection, which reduces the treatment compliance of patients who prefer oral therapy, thereby reducing the overall treatment outcomes. Oral sclerostin inhibitor drugs may be another treatment option.
4.2. Progress in the research of small-molecule inhibitors targeting sclerostin
4.2.1. Small-molecule inhibitors targeting loop 2 domain in sclerostin
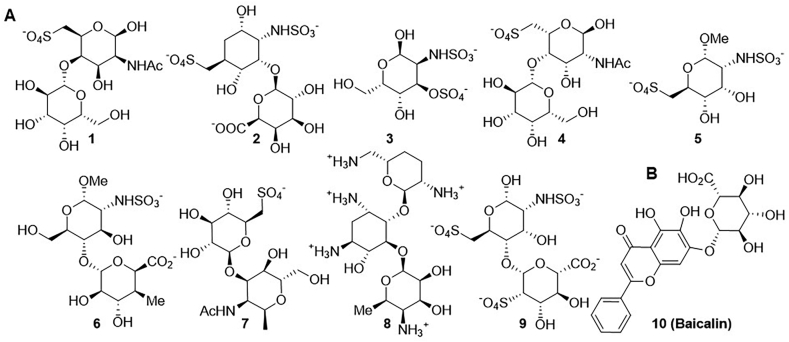
Small-molecule drugs are relatively simple chemical compounds with a molecular weight of less than 1000 Da that can be manufactured by organic synthesis and phytoextraction157. In general, they can be made into readily absorbable tablets or capsules for oral administration to meet the needs of patients who prefer oral therapy. Drug discovery is time-consuming and costly. The typical timeline is 10–20 years, and the budget can be as high as US $2.0 billion158. The discovery of small-molecule drugs can be accelerated through several strategies, such as high-throughput screening, virtual screening159, and AI160. Recently, some initial research progress has been made in the area of small-molecule sclerostin inhibitors via virtual screening performed by several groups161,162. Muthusamy et al.161 identified small molecules targeting the loop 2 domain in sclerostin using in silico virtual screening methods. They used web databases for their virtual screening and found nine small molecules that can potentially bind to loop 2 with a high predicted binding affinity (shown in Fig. 4A)161. Nine compounds in this cluster were equipped with multiple polar groups, such as hydroxyl, amide, sulfonic, carboxyl and quaternary ammonium groups, endowing them with overall polarity and high aqueous solubility. Additionally, it was found that introducing a moderate amount of hydroxyl groups in drugs can enhance or add interactions between receptors and donors163. Eight compounds bearing sulfonic groups or carboxyl groups were polyhydroxylated alkaloid derivatives, which preferentially bind to specific positively charged amino acid residues, including arginine and lysine, by binding to their basic groups. As we mentioned above, sclerostin loop 2 preferentially binds to carboxylic acid derivatives that carry negative charges.
Figure 4.
Ten potential small molecules targeting loop 2 of sclerostin; (A) reported by Muthusamy et al.161; (B) reported by Yooin et al.162.
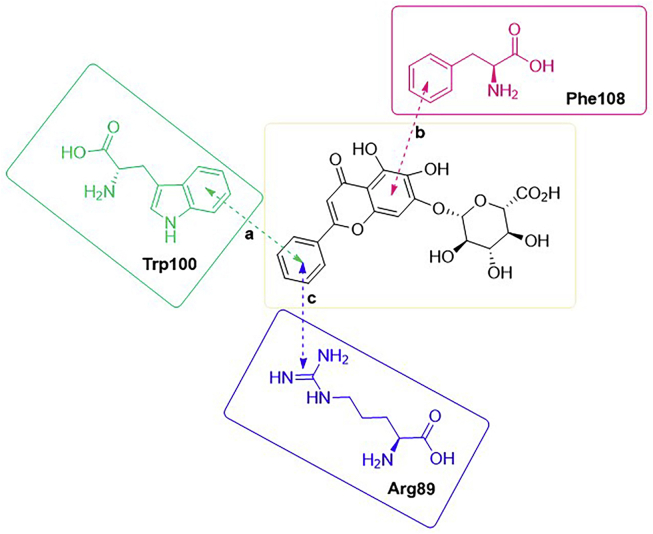
Some amino acid residues, such as isoleucine, asparagine and arginine, in the PNAIG region in the loop2 site of sclerostin have been demonstrated to be inhibitory sites for specific binding LRP6, thereby blocking the WNT signalling pathway (shown in Fig. 2B)15,16,162,164,165. Inspired by their predecessors’ findings, Yooin et al.162 employed computational molecular docking to predict potential herbal compounds targeting loop 2 and function as bone formation stimulators. In their virtual screening experiments, they identified a cluster of some herbal compounds with aryl groups. These compounds showed high predicted binding affinities, indicating that they can strongly and precisely interact with certain aromatic amino acid residues via pi–pi stacking interactions, suggesting that planar structural aromatic groups may increase the number of interactions between small molecules and aromatic amino acid residues. In addition to having pi–pi interactions, Compd. 10 (shown in Fig. 4B), baicalin, having the highest predicted binding affinity for the amino acid residues in loop 2, also exhibited a pi–cation interaction generated through the phenyl group (shown in Fig. 5). The feature indicated that the phenyl group of baicalin plays an essential role in building binding interactions to enhance the binding between the receptor and donor. Additionally, this role was verified by other computational analyses162. However, this study was based on computer simulations and biochemical assays, e.g., determination of the half-maximal inhibitory concentration, to support the computational results are lacking; therefore, it is difficult to determine the actual inhibitory effect of baicalin.
Figure 5.
Interactions related to phenyl groups between baicalin and loop 2 residues. There were three interactions which related to phenyl groups. Interaction (a) between baicalin and Trp100 was pi–pi stacking interaction; interaction (b) between baicalin and Phe108 was pi–pi stacking interaction; interaction (c) between baicalin and Arg89 was pi–cation interaction.
4.2.2. Small-molecule inhibitors targeting the LRP5/6–sclerostin interaction
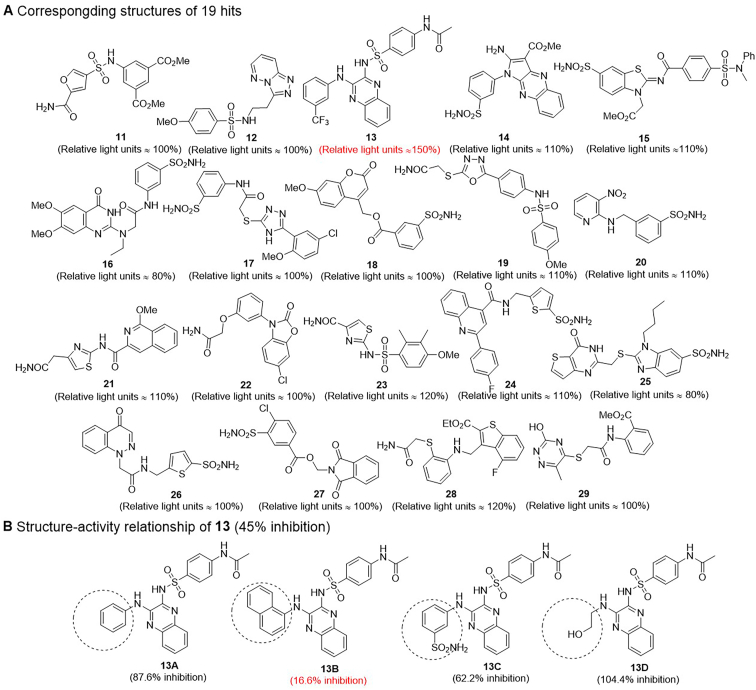
The canonical WNT/β-Catenin signalling pathway is a critical regulator of bone formation and metabolism. Sclerostin is classified as a negative regulatory factor of WNT signaling, which results in reduced bone formation. Therefore, the interaction between LRP5/6 co-receptors and sclerostin is recognized as a worthy study target for the treatment of bone disease. Choi et al.166 set up a process of pharmacophore-based virtual screening to identify small-molecule inhibitors targeting the LRP5/6–sclerostin interaction. Based on screening results (19 candidates) generated from the structure-based virtual screening approach (shown in Fig. 6A)166, this group found that most of the active compounds were heterocyclic sulfonamide derivatives through cluster analysis. Both heterocycles and sulfonamides are critical structural units that have unique pharmacological properties167,168. The introduction of functional groups, including heterocycles, sulfonamides, amides, hydroxyls and amino groups, can add binding sites or enhance the binding affinity of small molecules for acceptors to improve drug activity via hydrogen bond interactions169. Biochemical assays were performed to further investigate the efficacy for the LRP5/6–sclerostin interaction inhibition of the 19 virtual compound candidates; e.g., a luciferase reporter assay was performed in a dose-response experiment. Then, the researchers concluded that Compd. 13 bearing a quinoxaline skeleton showed greater effective inhibition166. The predicted binding mode between Compd. 13 and LRP6 was predicted by docking analysis. The 3-(trifluoromethyl) benzene group interacted with Asn185 of LRP6 and docked into a hydrophobic cavity surrounded by two tryptophan residues, which suggested that the indoles of tryptophan played an essential role in the formation of the LRP6–sclerostin interaction pocket. The quinoxaline group of Compd. 13 is bound to the binding site of isoleucine within the NXI motif. The two nitrogen atoms of the quinoxaline group formed two hydrogen bonds to arginine residues 28 and 141, both carrying positive charges, supporting the prediction of high binding affinity for LRP6-E1166. There was a pi–cation interaction between the benzenesulfonamide group and residue Arg28 of LRP6. Additionally, a hydrogen bond was established between the carbonyl moiety of the acetamide group and Arg27 that affected the stability of the complex formed by LRP6 and the small molecule. As we mentioned above, a specific skeleton cluster obtained by pharmacophore-based virtual screening helped predict and identify predicting and identifying the skeletons of active compounds.
Figure 6.
Hits reported by Choi et al.166. (A) The structures of 19 hits and experimental evaluation on 19 virtual hits by luciferase assay. After WNT3a activation, WNT signaling was inhibited by sclerostin (used as an internal control = 100% inhibition. (B) The structure–activity relationship of Compd. 13. The activity of LRP–sclerostin interaction inhibited by sclerostin was used as internal control in the binding assays (100% inhibition).
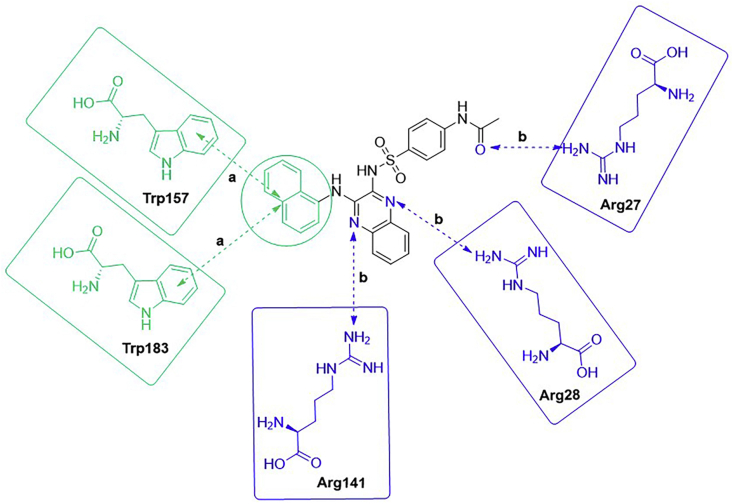
Choi et al.166 selected Compd. 13 as a template to study the structure–activity relationship. Based on the binding mode of this compound and LRP6, they hypothesized that hydrophobic 3-(trifluoromethyl) benzene groups likely form hydrophobic interactions with tryptophan. Therefore, four realizable derivatives were chosen because they bear phenyl, 1-naphthyl, 3-sulfonamidephenyl and 2-hydroxyethyl groups, of which both phenyl and 1-naphthyl are hydrophobic groups, and both 3-sulfonamidephenyl and 2-hydroxyethyl are polar groups. The SAR analysis of Compd. 13 and its four derivatives (shown in Fig. 6B) was performed with a competitive binding ELISA assay and molecular docking experiments. The binding assay contributed to the conclusion that the introduction of a 1-naphthyl group was beneficial for enhancing the hydrophobic interactions between small molecules and surrounding tryptophan residues. While Compd. 13C bearing a 3-sulfonamidephenyl group showed less potent inhibition of the LRP-sclerostin interaction than Compd. 13 or Compd. 13B, suggesting that the compound's bioactivity can be enhanced by introducing suitable residue interaction-oriented groups. The molecular docking analysis showed five interactions, including three hydrogen bond interactions and two pi–pi stacking interactions between Compd. 13B and the target (shown in Fig. 7). Docking was applied to compare the predicted binding modes of the five compounds. The results showed that Compd. 13B is an attractive compound and that further modification is needed to transform these compounds into leads166. In this study, the computer simulation data were verified only by competitive binding assay and molecular docking analysis. The druggability of these compounds was evaluated by neither in vivo nor in vitro experiments.
Figure 7.
Interactions between Compd. 13B and the target. There were five interactions including three hydrogen bonds and two pi–pi stacking interactions. Interaction (a) was pi–pi stacking interaction; Interaction (b) was hydrogen bond.
5. Discussion of concerns caused by romosozumab therapy and future perspectives of developing the next generation of sclerostin inhibitors
5.1. Cardiovascular concerns caused by romosozumab therapy
As the first sclerostin inhibitor approved by the U.S. FDA, romosozumab has demonstrated excellent effectiveness for the treatment of OP in postmenopausal women. In terms of safety, romosozumab therapy increases the cardiovascular risks during treatment, as shown in the ARCH study74. In contrast to alendronate, romosozumab is associated with a 30% increased risk of cardiovascular disease. Although the reason for the elevated cardiovascular risk caused by romosozumab is still unclear, the protective role of sclerostin in the cardiovascular system has been gradually validated by animal experiments170. As we mentioned above, sclerostin bears three specific domains, loop 1, loop 2, and loop 3, and antibodies bind to both loop 2 and part of loop 3. However, it is still unclear whether the deficiency in all or part(s) of these domain(s) regulates bone formation. Relevant functional binding sites of these loops are still under study. Recently, Yu et al.155 found that loop 2 but not loop 3 contributed to the protective role of sclerostin in the cardiovascular system, implying that specific inhibition of loop 3 expression may promote bone formation without increasing cardiovascular risk. The mechanism of how the particular domains of sclerostin participate in cardiovascular protection still needs to be studied in depth, and the results would support the translational significance of developing a new generation of sclerostin inhibitors to circumvent the protective mechanisms. In addition, a combination of computational biology and structural biology methods is essential to investigate the functional binding sites of loop 3. If these studies can be well executed, the results may facilitate the development of the next generation of sclerostin inhibitors that do not increase cardiovascular risk.
5.2. Compliance concerns caused by injection therapy
Patients have preferred routes of therapeutic administration. Some prefer oral administration, while some accept injection. Patients do not receive the required medication because they dislike injection and receive less effective treatment. Monoclonal antibodies and aptamer drugs are administered only by injection, which reduces treatment compliance in patients who prefer oral therapy, thereby decreasing overall treatment effectiveness. The standard treatment of diabetes in patients is daily subcutaneous insulin injections. However, low medication adherence is pervasive in diabetic patients due to frequent, inconvenient, and uncomfortable injections171,172. The emergence of long-acting glucagon-like peptide-1 receptor agonists, such as liraglutide and semaglutide, and the first once-daily oral preparation of semaglutide significantly improved the compliance of patients by meeting their various needs173,174. Therefore, the development of oral small-molecule inhibitors targeting sclerostin will meet the needs of patients who prefer oral therapy by offering them another treatment option.
5.3. Developing concomitant medication for the treatment of osteoporosis to increase treatment outcomes and decrease cardiovascular risks
As we mentioned above, the monoclonal antibody (romosozumab) could increase bone formation and decrease bone resorption for sclerostin inhibitors51,140. A strategy to enhance the efficiency of anabolic effects for anti-OP is to develop concomitant medications of sclerostin inhibitor with another anti-OP drug. One example is bisphosphonates, an anti-resorptive agent. In clinical trials for the treatment of OP patients with fracture, the combination of bisphosphonates with romosozumab has demonstrated promising therapeutic effects. In contrast, the corresponding effects were not observed in the combination bisphosphonates with teriparatide74,147. Another alternative strategy is to develop concomitant medications of sclerostin inhibitor with drugs against cardiovascular disease. Based on the opposing roles of bisphosphonates and the WNT pathway on endothelial dysfunction, vascular wall calcification and lipid accumulation175, the concomitant medications of romosozumab and bisphosphonates could be a candidate therapeutic approach to reduce the adverse cardiovascular risks in romosozumab receivers176.
Taken together, compared to romosozumab administration alone, it is desirable to develop concomitant medications of sclerostin inhibitor with other anti-OP drugs for playing off each other's strengths for the treatment of OP to increase treatment outcomes and decrease risks of SCAEs.
5.4. Developing AI-based strategies for the discovery of small-molecule inhibitors targeting specific loops of sclerostin
Currently, the progress in the research of small-molecule inhibitors targeting the loop 2 domain in sclerostin or LRP5/6–sclerostin interaction is still at an early stage162,166. Most work has been performed at the level of computer simulations without biochemical assays, e.g., the determination of half-maximal inhibitory concentration values; therefore, it is pretty difficult to ensure the actual inhibitory effects of the identified compounds. The small-molecule inhibitors targeting loop 2 or LRP5/6–sclerostin interactions are mainly carbohydrate derivatives or natural products characterized by insufficient skeleton diversity, complex synthesis, and required chemical modifications. Additionally, most known anti-sclerostin compounds have more than 5 hydrogen bond donors162,166, which contradicts Lipinski's rule of five177. This structural feature endows the inhibitors with poor druggability. Too many hydrogen bond donors cause the small molecule to efficiently react with amino acids and DNA with electrophilic addition in vivo. Furthermore, insufficient bioavailability is a fatal drawback for some complex natural products, such as baicalin, due to its poor aqueous solubility.
Notably, the abovementioned inhibitors mainly target loop 2 of sclerostin, which is involved in cardiovascular protection155. If loop 2 were to be set as the target for the development of sclerostin inhibitors, cardiovascular risks to patients would remain, which accordingly cannot prevent the existing issues of romosozumab therapy. Therefore, further research proposed by our group intends to focus on studying the function of loop 3 in sclerostin. To this end, and to address the poor druggability of known compounds, AI-driven approaches will be used to give full play to their advantages and be applied to generate novel molecules targeting only loop 3, especially the positively clustered residues on the surface of loop 3. Unlike traditional virtual screening, which identifies desirable molecules from a structurally known molecular pool, generative AI learns the structural pattern from existing molecules and output varied skeletons. How to wisely choose the molecular representation and the generative model is a broad topic and may largely depend on the specific conditions of the problem we would like to solve. The potential issues include the amount of data we can acquire for training, the chemical representation's ability to capture structure features we want to emphasize, the model's specialization of handling corresponding representation format, and the computing speed and memory limitations. To achieve these goals, a large amount of data needs to be obtained in the preparation stage, mainly common molecular structure data for use as templates for machine learning and generating patent data, which pharmaceutical companies as negative incentives typically claim, to exclude duplicated structures and for experimentally validating data on compounds targeting loop 3 and loop 2, respectively, for determining more-precise mechanisms. Specifically, hundreds of potential target molecules will be selected from a million possibilities through virtual screening, and then cheminformatics, including identification, design, and manipulation of drug-like small-molecule libraries, will be used to perform consistent ranking and clustering analyses, which filters low-scoring undesirable compounds, allowing the remaining compounds to be grouped and characterized by desirable factors such as skeleton diversities and binding modes178,179. High-scoring compounds will be experimentally validated to guarantee the label accuracy of the data input for machine learning; i.e., the compounds that target loop 3, the compounds that target loop 2, and the compounds that target both loop 3 and loop 2 will be labelled accurately.
Based on the available data, a deep learning-based generative model will be employed to generate a series of small molecules with novel skeletons with versatile modifications. Next, a reinforcement learning-based screening will be launched with the specific goals designed for our purposes to ensure that some hits that promote bone formation without increasing cardiovascular risk can be determined among the high number of generated molecules160. The final selection will be further narrowed down through analyses of their structural constraints and ease of synthesis. In addition to generating novel molecules, DL models can also predict absorption, distribution, metabolism, excretion, and toxicity properties, which lead to the finding of compounds with increasing activity. Architectures from simple four-layer fully connected neural networks to specialized deep neural network have been reported effective in a pharmacokinetic study180,181. In general, AI-based strategies will significantly shorten the screening time before final synthesis and verification, which will promote the discovery of next-generation sclerostin inhibitors that do not increase the cardiovascular risk and are suitable for high treatment compliance for the treatment of OP.
5.5. Druggable modification of anti-sclerostin aptamers
Anti-sclerostin aptamers have attracted considerable attention because of their various advantages. One of these drugs has been granted orphan drug designation by the U.S. FDA (DRU-2019-6966). Similar to most aptamers applied in clinical practice, this aptamer was formulated with the high-molecular-weight coupling agent polyethylene glycol (PEG) to increase its molecular weight, which needs to be above the cut-off threshold for glomerular filtration (30–50 kDa) to address renal filtration during extended circulation time182. However, the PEG moiety has a very high molecular weight, such that the active aptamer moiety is produced as a tiny proportion of the PEG-aptamer conjugates. Within a fixed subcutaneous administration volume, there is a severe limitation to the subcutaneous dosage that can be administered to increase the concentration of the aptamer moiety, which dramatically limits the therapeutic potential of the aptamer. In addition, the PEG component can extend the half-life by only 2–3 days in clinical use. Repeated subcutaneous injections in a short interval may profoundly reduce the clinical treatment compliance of patients receiving the candidate aptamer drugs183. In terms of the druggability concerns in conjunction with the abovementioned therapeutic potential and treatment compliance, it is desirable to seek innovative coupling agents to develop long-acting and efficient therapeutic aptamers. Low-molecular-weight coupling agents, which are capable of binding to serum albumin (MW = 67,000), to create a molecular complex with an average mass above the cut-off threshold of glomerular filtration (30–50 kDa), may be novel candidates. If this approach were to be realized, then a general strategy for the druggable modification of anti-sclerostin aptamers may be realized by linking low-molecular-weight coupling agents for developing long-lasting and efficient subcutaneous therapeutic aptamers.
5.6. Developing bispecific inhibitors for the treatment of sclerostin-related diseases to increase treatment outcomes
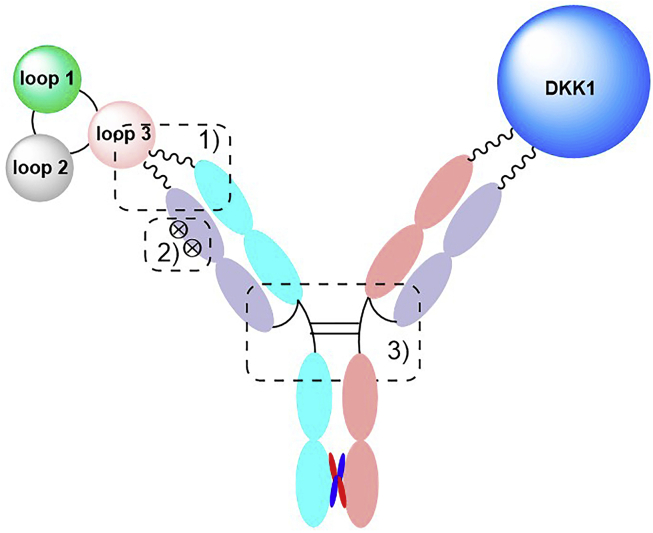
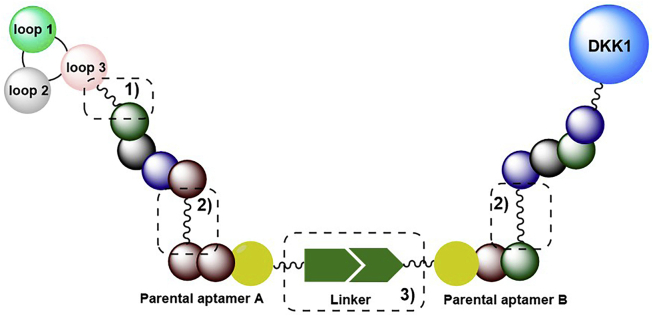
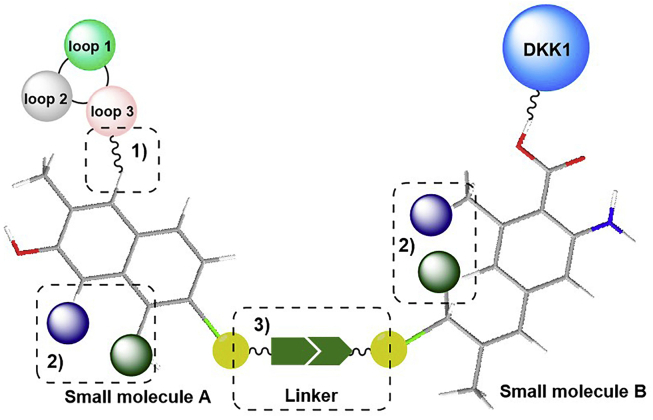
As we mentioned above, sclerostin inhibition or deficiency leads to a compensatory increase in another WNT antagonist, DKK-1, limiting sclerostin inhibition's effect. However, it has been shown that there is synergy between DKK-1 deficiency and Scl-Ab treatment and that dual inhibition of sclerostin and DKK-1 leads to synergistic bone formation, showing superior bone repair activity compared with monotherapies. All the evidence indicates that the compensatory elevation of another WNT inhibitor upon sclerostin inhibition and the subsequent reduction in sclerostin inhibition might occur not only in osteoporotic patients but also in other patients receiving sclerostin antibodies. Therefore, it is desirable to develop bispecific inhibitors, including antibodies, aptamers, and small molecules targeting both sclerostin and DKK-1 for WNT-related bone diseases.
The development of bispecific antibody drugs targeting both sclerostin and DKK-1 is a promising therapy for WNT-related bone diseases. Compared with monoclonal antibodies, bispecific antibodies are more challenging to prepare. The standard synthetic methods for bispecific antibody preparation are chemical coupling and cell fusion, in which two different heavy chains and two different light chains are combined. However, these methods can randomly generate sixteen combinations, making it difficult to purify the target combination. Therefore, introducing different coupling groups to each expected antigen recognition binding site (e.g., a carboxyl group and amino group) by chemical modification strategies to improve the uniformity of the coupling process is a desirable approach to obtain bispecific antibodies targeting both sclerostin and DKK-1. However, the safety concerns raised by romosozumab therapy may also apply to bispecific antibody therapy, suggesting that conjugating romosozumab chains with DKK-1 antibody chains to form a bispecific antibody targeting both sclerostin and DKK-1 accordingly may not prevent the cardiovascular concerns. Therefore, it is desirable to develop an antibody specifically targeting sclerostin loop 3 that conjugate with DKK-1 antibody chains to form a bispecific antibody. Additionally, the design of chemical modification strategies to block certain reactive sites of antibodies that bind to cardiovascular protection-related binding sites of sclerostin is a promising approach for the development of bispecific antibody inhibitors that do not increase cardiovascular risk (Fig. 8).
Figure 8.
Design of bispecific antibody targeting both sclerostin loop 3 and DKK-1. 1) Specifically targeting sclerostin loop 3; 2) Blocking certain reactive sites of antibodies that bind to loop 2; 3) Introduction of different coupling groups to each expected antigen recognition binding site (e.g., a carboxyl group and amino group).
As we mentioned above, aptamers targeting sclerostin loop 3 are promising alternatives for anti-sclerostin bone anabolic therapy that does not increase cardiovascular risk (Fig. 9). Thus, it is desirable to develop bispecific aptamer inhibitors targeting both sclerostin and DKK-1 against WNT-related bone diseases. The linker between two aptamers is a critical bridge for bispecific aptamers. A link that is too weak will cause the decomposition of the bispecific aptamer into its two parental aptamers. Additionally, the pharmacokinetic and pharmacodynamic properties of the aptamer can be regulated by the linker. Thus, the design of linkers should consider both linker stability and the activity of the aptamer conjugate. In addition to the linker, the length of the aptamer also has an impact on the binding affinity of the aptamer for the target. Excessive aptamer length may reduce its affinity for the target, leading to off-target effects. Therefore, reducing some unreactive bases of the two parental aptamers to regulate the size of the bispecific aptamer is an approach for maintaining the binding affinities of bispecific aptamers for the targets.
Figure 9.
Design of bispecific aptamer targeting both sclerostin loop 3 and DKK-1. 1) Specifically targeting sclerostin loop 3; 2) Regulating the length of the bispecific aptamer for maintaining the binding affinities of bispecific aptamers for the targets; 3) The design of linkers should consider both linker stability and the activity of the aptamer conjugate.
In addition to bispecific antibodies and aptamer conjugates, bispecific small-molecule conjugate drugs targeting both sclerostin loop 3 and DKK-1 are other types of bispecific inhibitors (shown in Fig. 10). The linker between two compounds is a vital bridge. The linker of the small-molecule conjugate should be generally cleaved and restabilized within several hours because the conjugate can quickly bind to the receptor and be rapidly cleared by the kidneys. In addition, for conjugates with poor bioavailability, a hydrophilic linker can be introduced to conjugate the two compounds to regulate the polarity of the conjugate and meet the bioavailability objectives, meaning that the design of the linker needs to be very compatible. Compared with its two parental small molecules, the polarity, binding affinity, pharmacodynamics and pharmacokinetics of the small-molecule conjugate may change considerably. Therefore, some functional groups can be introduced or conjugated to two chemistry-modified parental derivatives to enhance the binding affinity of the conjugate and reduce any off-target effect.
Figure 10.
Design of bispecific small-molecule conjugate targeting both sclerostin loop 3 and DKK-1. 1) Specifically targeting sclerostin loop 3; 2) Some functional groups can be introduced to enhance the binding affinity of the conjugate and reduce any off-target effect; 3) The linker of the small-molecule conjugate should be hydrophilic, cleaved and restabilized within several hours.
Acknowledgements
This study was supported by the National Key R&D Program of China (2018YFA0800802), Hong Kong General Research Fund (HKBU 12114416, HKBU 12101117, HKBU 12100918, HKBU 12101018, HKBU 12103519, HKBU 14100218, CUHK 14108816, CUHK 14100218, CUHK 14103420, China), Direct Grant of The Chinese University of Hong Kong (2018.094, China), Interdisciplinary Research Clusters Matching Scheme of Hong Kong Baptist University (RC-IRCs/17-18/02, China), Guangdong Basic and Applied Basic Research Foundation (2019B1515120089, China), and Science and Technology Innovation Commission of Shenzhen Municipality Funds (JCYJ20160229210357960, China). And we thank Simon, WANG for comments and suggestions on the preparation of the manuscript.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Zhenlin Zhang, Email: zhangzl@sjtu.edu.cn.
Baoting Zhang, Email: zhangbaoting@cuhk.edu.hk.
Ge Zhang, Email: zhangge@hkbu.edu.hk.
Author contributions
Sifan Yu, Dijie Li, Ning Zhang wrote the manuscript. Shuaijian Ni, Meiheng Sun, Luyao Wang, Huan Xiao, Jin Liu, and Ge Zhang helped in revising the manuscript. Sifan Yu, Zhenlin Zhang, Baoting Zhang, and Ge Zhang proposed constructive discussions and supervised the manuscript. All authors contributed to the article and approved the submitted version.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Balemans W., Van Den Ende J., Paes-Alves A.F., Dikkers F.G., Willems P.J., Vanhoenackeret F., et al. Localization of the gene for sclerosteosis to the van Buchem disease–gene region on chromosome 17q12–q21. Am J Hum Genet. 1996;64:1661–1669. doi: 10.1086/302416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Buchem F.S.P., Hadders H.N., Ubbens R. An uncommon familial systemic disease of the skeleton: hyperostosis corticalis generalisata familiaris. Acta Radiol. 1995;44:109–120. [PubMed] [Google Scholar]
- 3.Truswell A.S. Osteopetrosis with syndactyly. J Bone Joint Surg Br. 1958;40:208–218. [PubMed] [Google Scholar]
- 4.Li X., Ominsky M.S., Villasenor K.S., Niu Q., Asuncion F.J., Xia X., et al. Sclerostin antibody reverses bone loss by increasing bone formation and decreasing bone resorption in a rat model of male osteoporosis. Endocrinology. 2018;159:260–271. doi: 10.1210/en.2017-00794. [DOI] [PubMed] [Google Scholar]
- 5.ten Dijke P., Krause C., De Gorter D.J.J., Löwik C.W.G.M., van Bezooijen R.L. Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. JBJS. 2008;90 Suppl 1:31–35. doi: 10.2106/JBJS.G.01183. [DOI] [PubMed] [Google Scholar]
- 6.Prather C., Adams E., Zentgraf W. Romosozumab: a first-in-class sclerostin inhibitor for osteoporosis. Am J Health Syst Pharm. 2020;77:1949–1956. doi: 10.1093/ajhp/zxaa285. [DOI] [PubMed] [Google Scholar]
- 7.Khosla S. Pathogenesis of osteoporosis. Transl Endocrinol Metab. 2010;1:55–86. doi: 10.1210/TEAM.9781879225718.ch2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stürznickel J., Rolvien T., Delsmann A., Butscheidt S., Barvencik F., Mundlos S., et al. Clinical phenotype and relevance of LRP5 and LRP6 variants in patients with early-onset osteoporosis (EOOP) J Bone Miner Res. 2021;36:271–282. doi: 10.1002/jbmr.4197. [DOI] [PubMed] [Google Scholar]
- 9.Dai J., Keller J., Zhang J., Lu Y., Yao Z., Keller E.T. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005;65:8274–8285. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y.H.V., Schwartz A.V., Sigurdsson S., Hue T.F., Lang T.F., Harris T.B., et al. Circulating sclerostin associated with vertebral bone marrow fat in older men but not women. J Clin Endocrinol Metab. 2014;99:E2584–E2590. doi: 10.1210/jc.2013-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann T., Hofbauer L.C., Rauner M., Lodes S., Kästner B., Franke S., et al. Clinical and endocrine correlates of circulating sclerostin levels in patients with type 1 diabetes mellitus. Clin Endocrinol. 2014;80:649–655. doi: 10.1111/cen.12364. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.A., Roh E., Hong S., Lee Y., Kim N.H., Yoo H.J., et al. Association of serum sclerostin levels with low skeletal muscle mass: the Korean Sarcopenic Obesity Study (KSOS) Bone. 2019;128:115053. doi: 10.1016/j.bone.2019.115053. [DOI] [PubMed] [Google Scholar]
- 13.De Maré A., D'Haese P.C., Verhulst A. The role of sclerostin in bone and ectopic calcification. Int J Mol Sci. 2020;21:3199. doi: 10.3390/ijms21093199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducy P., Karsenty G. The two faces of serotonin in bone biology. J Cell Biol. 2020;191:7–13. doi: 10.1083/jcb.201006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veverka V., Henry A.J., Slocombe P.M., Ventom A., Mulloy B., Muskett F.W., et al. Characterization of the structural features and interactions of sclerostin. J Biol Chem. 2009;284:10890–10900. doi: 10.1074/jbc.M807994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J., Han W., Park T., Kim E.J., Bang I., Lee H.S., et al. Sclerostin inhibits Wnt signaling through tandem interaction with two LRP6 ectodomains. Nat Commun. 2020;11:5357. doi: 10.1038/s41467-020-19155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler D.G., Sutherland M.K., Geoghegan J.C., Yu C., Hayes T., Skonier J.E., et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jäger A., Götz W., Lossdörfer S. Localization of SOST/sclerostin in cementocytes in vivo and in mineralizing periodontal ligament cells in vitro. J Periodont Res. 2010;45:246–254. doi: 10.1111/j.1600-0765.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 19.Bourhis E., Wang W., Tam C., Hwang J., Zhang Y., Spittler D., et al. Wnt antagonists bind through a short peptide to the first β-propeller domain of LRP5/6. Structure. 2011;19:1433–1442. doi: 10.1016/j.str.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Boschert V., van Dinther M., Weidauer S., van Pee K., Muth E., ten Dijke P., et al. Mutational analysis of sclerostin shows importance of the flexible loop and the cystine-knot for Wnt-signaling inhibition. PLoS One. 2013;8:e81710. doi: 10.1371/journal.pone.0081710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Li Y.P., Paulson C., Shao J., Zhang X., Wu M., et al. Wnt and the Wnt signaling pathway in bone development and disease. Front Biosci (Landmark Ed) 2014;19:379–407. doi: 10.2741/4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manolagas S.C., Almeida M. Gone with the Wnts: β-Catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007;21:2605–2614. doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- 23.Laine C.M., Joeng K.S., Campeau P.M., Kiviranta R., Tarkkonen K., Grover M., et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368:1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Long F. β-Catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. J Bone Miner Res. 2013;28:1160–1169. doi: 10.1002/jbmr.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDonald B.T., He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harbor Perspect Biol. 2012;4:a007880. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald B.T., Semenov M.V., He X. SnapShot: Wnt/β-catenin signaling. Cell. 2007;131:1204. doi: 10.1016/j.cell.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Daniels D.L., Weis W.I. β-Catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 28.Lo Y.C., Chang Y.H., Wei B.L., Huang Y., Chiou W. Betulinic acid stimulates the differentiation and mineralization of osteoblastic MC3T3-E1 cells: involvement of BMP/Runx2 and β-catenin signals. J Agric Food Chem. 2010;58:6643–6649. doi: 10.1021/jf904158k. [DOI] [PubMed] [Google Scholar]
- 29.Rudnicki M.A., Williams B.O. Wnt signaling in bone and muscle. Bone. 2015;80:60–66. doi: 10.1016/j.bone.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado-Calle J., Sato A.Y., Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi: 10.1016/j.bone.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holdsworth G., Roberts S.J., Ke H.Z. Novel actions of sclerostin on bone. J Mol Endocrinol. 2019;62:R167–R185. doi: 10.1530/JME-18-0176. [DOI] [PubMed] [Google Scholar]
- 32.Van Lierop A.H., Moester M.J.C., Hamdy N.A.T., Papapoulos S.E. Serum Dickkopf 1 levels in sclerostin deficiency. J Clin Endocrinol Metab. 2014;99:E252–E256. doi: 10.1210/jc.2013-3278. [DOI] [PubMed] [Google Scholar]
- 33.Witcher P.C., Miner S.E., Horan D.J., Bullock W.A., Lim K., Kang K.S., et al. Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morse A., Cheng T.L., Schindeler A., McDonald M.M., Mohanty S.T., Kneissel M., et al. Dkk1 KO mice treated with sclerostin antibody have additional increases in bone volume. Calcif Tissue Int. 2018;103:298–310. doi: 10.1007/s00223-018-0420-6. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T., Zhang L., Joo D., Sun S. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Z., Xing L., Boyce B.F. NF-κB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest. 2009;119:3024–3034. doi: 10.1172/JCI38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gębura K., Świerkot J., Wysoczańska B., Korman L., Nowak B., Wiland P., et al. Polymorphisms within genes involved in regulation of the NF-κB pathway in patients with rheumatoid arthritis. Int J Mol Sci. 2017;18:1432. doi: 10.3390/ijms18071432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oeckinghaus A., Hayden M.S., Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 40.Ma B., Hottiger M.O. Crosstalk between Wnt/β-catenin and NF-κB signaling pathway during inflammation. Front Immunol. 2016;7:378. doi: 10.3389/fimmu.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma B., van Blitterswijk C.A., Karperien M. A Wnt/β-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheumatol. 2012;64:2589–2600. doi: 10.1002/art.34425. [DOI] [PubMed] [Google Scholar]
- 42.Die L., Yan P., Jiang Z.J., Hua T.M., Cai W., Xing L. Glycogen synthase kinase-3 beta inhibitor suppresses Porphyromonas gingivalis lipopolysaccharide-induced CD40 expression by inhibiting nuclear factor-kappa B activation in mouse osteoblasts. Mol Immunol. 2012;52:38–49. doi: 10.1016/j.molimm.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Arab H.H., Salama S.A., Eid A.H., Kabel A.M., Shahin N.N. Targeting MAPKs, NF-κB, and PI3K/AKT pathways by methyl palmitate ameliorates ethanol-induced gastric mucosal injury in rats. J Cell Physiol. 2019;234:22424–22438. doi: 10.1002/jcp.28807. [DOI] [PubMed] [Google Scholar]
- 44.Wehmeyer C., Frank S., Beckmann D., Bottcher M., Cromme C., Konig U., et al. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci Transl Med. 2016;8:330ra35. doi: 10.1126/scitranslmed.aac4351. [DOI] [PubMed] [Google Scholar]
- 45.Zhang N., Zhang Z., Yu Y., Zhuo Z., Zhang G., Zhang B. Pros and cons of denosumab treatment for osteoporosis and implication for RANKL aptamer therapy. Front Cell Dev Biol. 2020;8:325. doi: 10.3389/fcell.2020.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wade S.W., Strader C., Fitzpatrick L.A., Anthony M.S., O'Malley C.D. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9:182. doi: 10.1007/s11657-014-0182-3. [DOI] [PubMed] [Google Scholar]
- 47.Mödder U.I., Hoey K.A., Amin S., McCready L.K., Achenbach S.J., Riggs B.L., et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–379. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reppe S., Noer A., Grimholt R.M., Halldórsson B.V., Medina-Gomez C., Gautvik V.T., et al. Methylation of bone SOST, its mRNA, and serum sclerostin levels correlate strongly with fracture risk in postmenopausal women. J Bone Miner Res. 2014;30:249–256. doi: 10.1002/jbmr.2342. [DOI] [PubMed] [Google Scholar]
- 49.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 50.Lewiecki E.M., Blicharski T., Goemaere S., Lippuner K., Meisner P.D., Miller P.D., et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103:3183–3193. doi: 10.1210/jc.2017-02163. [DOI] [PubMed] [Google Scholar]
- 51.McClung M.R., Brown J.P., Diez-Perez A., Resch H., Caminis J., Meisner P., et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33:1397–1406. doi: 10.1002/jbmr.3452. [DOI] [PubMed] [Google Scholar]
- 52.Rolvien T., Amling M. Disuse osteoporosis: clinical and mechanistic insights. Calcif Tissue Int. 2022;110:592–604. doi: 10.1007/s00223-021-00836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sibonga J.D. Spaceflight-induced bone loss: is there an osteoporosis risk?. Curr Osteoporos Rep. 2013;11:92–98. doi: 10.1007/s11914-013-0136-5. [DOI] [PubMed] [Google Scholar]
- 54.Lin C., Jiang X., Dai Z., Guo X., Weng T., Wang J., et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 55.Robling A.G., Niziolek P.J., Baldridge L.A., Condon K.W., Allen M.R., Alam I., et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 56.Ma Y.L., Hamang M., Lucchesi J., Bivi N., Zeng Q., Adrian M.D., et al. Time course of disassociation of bone formation signals with bone mass and bone strength in sclerostin antibody treated ovariectomized rats. Bone. 2017;97:20–28. doi: 10.1016/j.bone.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 57.Gerbaix M., Ammann P., Ferrari S. Mechanically driven counter-regulation of cortical bone formation in response to sclerostin-neutralizing antibodies. J Bone Miner Res. 2021;36:385–399. doi: 10.1002/jbmr.4193. [DOI] [PubMed] [Google Scholar]
- 58.Convertino V.A., Cooke W.H. Evaluation of cardiovascular risks of spaceflight does not support the NASA bioastronautics critical path roadmap. Aviat Space Environ Med. 2005;76:869–876. [PubMed] [Google Scholar]
- 59.Delp M.D., Charvat J.M., Limoli C.L., Globus R.K., Ghosh P. Apollo lunar astronauts show higher cardiovascular disease mortality: possible deep space radiation effects on the vascular endothelium. Sci Rep. 2016;6 doi: 10.1038/srep29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morse A., Yu N.Y.C., Peacock L., Mikulec K., Kramer I., Kneissel M., et al. Endochondral fracture healing with external fixation in the Sost knockout mouse results in earlier fibrocartilage callus removal and increased bone volume fraction and strength. Bone. 2015;71:155–163. doi: 10.1016/j.bone.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Alzahrani M.M., Rauch F., Hamdy R.C. Does sclerostin depletion stimulate fracture healing in a mouse model?. Clin Orthop Relat Res. 2016;474:1294–1302. doi: 10.1007/s11999-015-4640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ominsky M.S., Li C., Li X., Tan H.L., Lee E., Barrero M., et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011;26:1012–1021. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- 63.Yee C.S., Xie L.Q., Hatsell S., Hum N., Murugesh D., Economides A.N., et al. Sclerostin antibody treatment improves fracture outcomes in a type I diabetic mouse model. Bone. 2016;82:122–134. doi: 10.1016/j.bone.2015.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morse A., McDonald M.M., Schindeler A., Peacock L., Mikulec K., Cheng T.L., et al. Sclerostin antibody increases callus size and strength but does not improve fracture union in a challenged open rat fracture model. Calcif Tissue Int. 2017;101:217–228. doi: 10.1007/s00223-017-0275-2. [DOI] [PubMed] [Google Scholar]
- 65.Kruck B., Zimmermann E.A., Damerow S., Figge C., Julien C., Wulsten D., et al. Sclerostin neutralizing antibody treatment enhances bone formation but does not rescue mechanically induced delayed healing. J Bone Miner Res. 2018;33:1686–1697. doi: 10.1002/jbmr.3454. [DOI] [PubMed] [Google Scholar]
- 66.Agholme F., Li X., Isaksson H., Ke H.Z., Aspenberg P. Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res. 2010;25:2412–2418. doi: 10.1002/jbmr.135. [DOI] [PubMed] [Google Scholar]
- 67.Florio M., Gunasekaran K., Stolina M., Li X., Liu L., Tipton B., et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:11505. doi: 10.1038/ncomms11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhandari M., Schemitsch E.H., Karachalios T., Sancheti P., Poolman R.W., Caminis J., et al. Romosozumab in skeletally mature adults with a fresh unilateral tibial diaphyseal fracture: a randomized phase-2 study. J Bone Joint Surg Am. 2020;102:1416–1426. doi: 10.2106/JBJS.19.01008. [DOI] [PubMed] [Google Scholar]
- 69.Schemitsch E.H., Miclau T., Karachalios T., Nowak L.L., Sancheti P., Poolman R.W., et al. A randomized, placebo-controlled study of romosozumab for the treatment of hip fractures. J Bone Joint Surg Am. 2020;102:693–702. doi: 10.2106/JBJS.19.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamad B., Basaran S., Benlidayi I.C. Osteosarcopenia among postmenopausal women and handgrip strength as a practical method for predicting the risk. Aging Clin Exp Res. 2020;32:1923–1930. doi: 10.1007/s40520-019-01399-w. [DOI] [PubMed] [Google Scholar]
- 71.Ralston S.H., Gaston M.S. Management of osteogenesis imperfecta. Front Endocrinol. 2019;10:924. doi: 10.3389/fendo.2019.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobsen C.M., Barber L.A., Ayturk U.M., Roberts H.J., Deal L.E., Schwartz M.A., et al. Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J Bone Miner Res. 2014;29:2297–2306. doi: 10.1002/jbmr.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheiber A.L., Barton D.K., Khoury B.M., Marini J.C., Swiderski D.L., Caird M.S., et al. Sclerostin antibody-induced changes in bone mass are site specific in developing crania. J Bone Miner Res. 2019;34:2301–2310. doi: 10.1002/jbmr.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saag K.G., Petersen J., Brandi M.L., Karaplis A.C., Lorentzon M., Thomas T., et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 75.de Vlaming A., Sauls K., Hajdu Z., Visconti R.P., Mehesz A.N., Levine R.A., et al. Atrioventricular valve development: new perspectives on an old theme. Differentiation. 2012;84:103–116. doi: 10.1016/j.diff.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marini J.C., Forlino A., Bächinger H.P., Bishop N.J., Byers P.H., De Paepe A., et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017;3:17052. doi: 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- 77.Yuan B., Takaiwa M., Clemens T.L., Feng J., Kumar R., Rowe P.S., et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamb Y.N. Burosumab: first global approval. Drugs. 2018;78:707–714. doi: 10.1007/s40265-018-0905-7. [DOI] [PubMed] [Google Scholar]
- 79.Imel E.A., Glorieux F.H., Whyte M.P., Munns C.F., Ward L.M., Nilsson O., et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393:2416–2427. doi: 10.1016/S0140-6736(19)30654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shalhoub V., Shatzen E.M., Ward S.C., Davis J., Stevens J., Bi V., et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543–2553. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palomo T., Glorieux F.H., Rauch F. Circulating sclerostin in children and young adults with heritable bone disorders. J Clin Endocrinol Metab. 2014;99:E920–E925. doi: 10.1210/jc.2013-3852. [DOI] [PubMed] [Google Scholar]
- 82.Zelenchuk L.V., Hedge A.M., Rowe P.S.N. PHEX mimetic (SPR4-peptide) corrects and improves HYP and wild type mice energy-metabolism. PLoS One. 2014;9:e97326. doi: 10.1371/journal.pone.0097326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryan Z.C., Ketha H., McNulty M.S., McGee-Lawrence M., Craig T.A., Grande J.P., et al. Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A. 2013;110:6199–6204. doi: 10.1073/pnas.1221255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carpenter K.A., Ross R.D. Sclerostin antibody treatment increases bone mass and normalizes circulating phosphate levels in growing Hyp mice. J Bone Miner Res. 2020;35:596–607. doi: 10.1002/jbmr.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aono Y., Yamazaki Y., Yasutake J.F. Clinical renal pharmacology and therapeutics. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 86.Liu E.S., Martins J.S., Raimann A., Chae B.T., Brooks D.J., Jorgetti V., et al. 1,25-Dihydroxyvitamin D alone improves skeletal growth, microarchitecture, and strength in a murine model of XLH, despite enhanced FGF23 expression. J Bone Miner Res. 2016;31:929–939. doi: 10.1002/jbmr.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moe S.M., Chertow G.M., Parfrey P.S., Kubo Y., Block G.A., Correa-Rotter R., et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation. 2015;132:27–39. doi: 10.1161/CIRCULATIONAHA.114.013876. [DOI] [PubMed] [Google Scholar]
- 88.Kaesler N., Verhulst A., De Maré A., Deck A., Behets G.J., Hyusein A., et al. Sclerostin deficiency modifies the development of CKD–MBD in mice. Bone. 2018;107:115–123. doi: 10.1016/j.bone.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 89.Mace M.L., Gravesen E., Nordholm A., Egstrand S., Morevati M., Nielsen C., et al. Chronic kidney disease-induced vascular calcification impairs bone metabolism. J Bone Miner Res. 2021;36:510–522. doi: 10.1002/jbmr.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asamiya Y., Tsuchiya K., Nitta K. Role of sclerostin in the pathogenesis of chronic kidney disease–mineral bone disorder. Ren Replace Ther. 2016;2:8. [Google Scholar]
- 91.Pelletier S., Dubourg L., Carlier M.C., Hadj-Aissa A., Fouque D. The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol. 2013;8:819–823. doi: 10.2215/CJN.07670712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hruska K.A., Sugatani T., Agapova O., Fang Y. The chronic kidney disease–mineral bone disorder (CKD–MBD): advances in pathophysiology. Bone. 2017;100:80–86. doi: 10.1016/j.bone.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hyder J.A., Allison M.A., Criqui M.H., Wright C.M. Association between systemic calcified atherosclerosis and bone density. Calcif Tissue Int. 2007;80:301–306. doi: 10.1007/s00223-007-9004-6. [DOI] [PubMed] [Google Scholar]
- 94.Kiel D.P., Kauppila L.I., Cupples L.A., Hannan M.T., O'Donnell C.J., Wilson P.W.F. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 95.Figurek A., Rroji M., Spasovski G. Sclerostin: a new biomarker of CKD–MBD. Int Urol Nephrol. 2020;52:107–113. doi: 10.1007/s11255-019-02290-3. [DOI] [PubMed] [Google Scholar]
- 96.Terpos E., Christoulas D., Katodritou E., Bratengeier C., Gkotzamanidou M., Michalis E., et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012;131:1466–1471. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- 97.Zhu M., Liu C., Li S., Zhang S., Yao Q., Song Q. Sclerostin induced tumor growth, bone metastasis and osteolysis in breast cancer. Sci Rep. 2017;7:11399. doi: 10.1038/s41598-017-11913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hesse E., Schröder S., Brandt D., Pamperin J., Saito H., Taipaleenmäki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. 2019;4 doi: 10.1172/jci.insight.125543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar S., Wilkes D.W., Samuel N., Blanco A., Nayak A., Alicea-Torres K., et al. ΔNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J Clin Invest. 2018;128:5095–5109. doi: 10.1172/JCI99673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Capietto A.H., Kim S., Sanford D.E., Linehan D.C., Hikida M., Kumosaki T., et al. Down-regulation of PLCγ2–β-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer. J Exp Med. 2013;210:2257–2271. doi: 10.1084/jem.20130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qian Y., Yuan J., Hu H., Yang Q., Li J., Zhang S., et al. The CUL4B/AKT/β-catenin axis restricts the accumulation of myeloid-derived suppressor cells to prohibit the establishment of a tumor-permissive microenvironment. Cancer Res. 2015;75:5070–5083. doi: 10.1158/0008-5472.CAN-15-0898. [DOI] [PubMed] [Google Scholar]
- 102.Sun M., Chen Z., Wu X., Yu Y., Wang L., Lu A., et al. The roles of sclerostin in immune system and the applications of aptamers in immune-related research. Front Immunol. 2021;12:164. doi: 10.3389/fimmu.2021.602330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borawski J., Zoltko J., Labij-Reduta B., Koc-Zorawska E., Naumnik B. Effects of enoxaparin on intravascular sclerostin release in healthy men. J Cardiovasc Pharmacol Ther. 2018;23:344–349. doi: 10.1177/1074248418770623. [DOI] [PubMed] [Google Scholar]
- 104.Oranger A., Brunetti G., Colaianni G., Tamma R., Claudia C., Luciana L., et al. Sclerostin stimulates angiogenesis in human endothelial cells. Bone. 2017;101:26–36. doi: 10.1016/j.bone.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Sheng Z., Tong D., Ou Y., Zhang H., Zhang Z., Li S., et al. Serum sclerostin levels were positively correlated with fat mass and bone mineral density in central south Chinese postmenopausal women. Clin Endocrinol. 2012;76:797–801. doi: 10.1111/j.1365-2265.2011.04315.x. [DOI] [PubMed] [Google Scholar]
- 106.Urano T., Shiraki M., Ouchi Y., Inoue S. Association of circulating sclerostin levels with fat mass and metabolic disease-related markers in Japanese postmenopausal women. J Clin Endocrinol Metab. 2012;97:E1473–E1477. doi: 10.1210/jc.2012-1218. [DOI] [PubMed] [Google Scholar]
- 107.Stanik J., Kratzsch J., Landgraf K., Vogel M., Thiery J., Kiess W., et al. The bone markers sclerostin, osteoprotegerin, and bone-specific alkaline phosphatase are related to insulin resistance in children and adolescents, independent of their association with growth and obesity. Horm Res Paediatr. 2019;91:1–8. doi: 10.1159/000497113. [DOI] [PubMed] [Google Scholar]
- 108.Baek K., Hwang H.R., Park H.J., Kwon A., Qadir A.S., Ko S., et al. TNF-α upregulates sclerostin expression in obese mice fed a high-fat diet. J Cell Physiol. 2014;229:640–650. doi: 10.1002/jcp.24487. [DOI] [PubMed] [Google Scholar]
- 109.Kim S.P., Frey J.L., Li Z., Kushwaha P., Zoch M.L., Tomlinson R.E., et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017;114:E11238–E11247. doi: 10.1073/pnas.1707876115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ukita M., Yamaguchi T., Ohata N., Tamura M. Sclerostin enhances adipocyte differentiation in 3T3-L1 cells. J Cell Biochem. 2016;117:1419–1428. doi: 10.1002/jcb.25432. [DOI] [PubMed] [Google Scholar]
- 111.Fulzele K., Lai F., Dedic C., Saini V., Uda Y., Shi C., et al. Osteocyte-secreted Wnt signaling inhibitor sclerostin contributes to beige adipogenesis in peripheral fat depots. J Bone Miner Res. 2017;32:373–384. doi: 10.1002/jbmr.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daniele G., Winnier D., Mari A., Bruder J., Fourcaudot M., Pengou Z., et al. Sclerostin and insulin resistance in prediabetes: evidence of a cross talk between bone and glucose metabolism. Diabetes Care. 2015;38:1509–1517. doi: 10.2337/dc14-2989. [DOI] [PubMed] [Google Scholar]
- 113.Gaudio A., Privitera F., Pulvirenti I., Canzonieri E., Rapisarda R., Fiore C.E. The relationship between inhibitors of the Wnt signalling pathway (sclerostin and Dickkopf-1) and carotid intima-media thickness in postmenopausal women with type 2 diabetes mellitus. Diabets Vasc Dis Res. 2014;11:48–52. doi: 10.1177/1479164113510923. [DOI] [PubMed] [Google Scholar]
- 114.Cepeda-Valery B., Pressmane G.S., Figueredo V.M., Romero-Corral A. Impact of obesity on total and cardiovascular mortality-fat or fiction?. Nat Rev Cardiol. 2011;8:233–237. doi: 10.1038/nrcardio.2010.209. [DOI] [PubMed] [Google Scholar]
- 115.Montagnani A. Bone anabolics in osteoporosis: actuality and perspectives. World J Orthop. 2014;5:247. doi: 10.5312/wjo.v5.i3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuipers A.L., Miljkovic I., Carr J.J., Terry J.G., Nestlerode C.S., Ge Y., et al. Association of circulating sclerostin with vascular calcification in Afro-Caribbean men. Atherosclerosis. 2015;239:218–223. doi: 10.1016/j.atherosclerosis.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koos R., Brandenburg V., Mahnken A.H., Schneider R., Dohmen G., Autschbach R., et al. Sclerostin as a potential novel biomarker for aortic valve calcification: an in-vivo and ex-vivo study. J Heart Valve Dis. 2013;22:317. [PubMed] [Google Scholar]
- 118.Bovijn J., Krebs K., Chen C.Y., Boxall R., Censin J.C., Ferreira T., et al. Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics. Sci Transl Med. 2020;12:549. doi: 10.1126/scitranslmed.aay6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He W., Chen Q., Ke D., Li G., Chen J., Do J. The relationship among serum sclerostin, coronary atherosclerotic heart disease, and osteoporosis in the elderly. Chin J Osteoporosis. 2017;23:745–750. [Google Scholar]
- 120.Chang Y.C., Hsu B.G., Liou H.H., Lee C.J., Wang J. Serum levels of sclerostin as a potential biomarker in central arterial stiffness among hypertensive patients. BMC Cardiovasc Disord. 2018;18:214. doi: 10.1186/s12872-018-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sebastian A., Loots G.G. Genetics of Sost/SOST in sclerosteosis and van Buchem disease animal models. Metabolism. 2018;80:38–47. doi: 10.1016/j.metabol.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 122.Spatz J.M., Ellman R., Cloutier A.M., Louis L., van Vliet M., Suva L.J., et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 2013;28:865–874. doi: 10.1002/jbmr.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tian X.Y., Jee W.S.S., Li X., Paszty C., Ke H.Z. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone. 2011;48:197–201. doi: 10.1016/j.bone.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 124.Zhang D., Hu M., Chu T., Lin L., Wang J., Li X., et al. Sclerostin antibody prevented progressive bone loss in combined ovariectomized and concurrent functional disuse. Bone. 2016;87:161–168. doi: 10.1016/j.bone.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beggs L.A., Ye F., Ghosh P., Beck D.T., Conover C.F., Balaez A., et al. Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J Bone Miner Res. 2015;30:681–689. doi: 10.1002/jbmr.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roschger A., Roschger P., Keplingter P., Klaushofer K., Abdullah S., Kneissel M., et al. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone. 2014;66:182–188. doi: 10.1016/j.bone.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 127.Marenzana M., Vugler A., Moore A., Robinson M. Effect of sclerostin-neutralising antibody on periarticular and systemic bone in a murine model of rheumatoid arthritis: a microCT study. Arthritis Res Ther. 2013;15:R125. doi: 10.1186/ar4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rossini M., Gatti D., Adami S. Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93:121–132. doi: 10.1007/s00223-013-9749-z. [DOI] [PubMed] [Google Scholar]
- 129.Chandra A., Lin T., Young T., Tong W., Ma X., Tseng W., et al. Suppression of sclerostin alleviates radiation-induced bone loss by protecting bone-forming cells and their progenitors through distinct mechanisms. J Bone Miner Res. 2017;32:360–372. doi: 10.1002/jbmr.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ren Y., Han X., Jing Y., Yuan B., Ke H., Liu M., et al. Sclerostin antibody (Scl-Ab) improves osteomalacia phenotype in dentin matrix protein 1 (Dmp1) knockout mice with little impact on serum levels of phosphorus and FGF23. Matrix Biol. 2016;52:151–161. doi: 10.1016/j.matbio.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Dinther M., Zhang J., Weidauer S.E., Boschert V., Muth E., Knappik A., et al. Anti-sclerostin antibody inhibits internalization of sclerostin and sclerostin-mediated antagonism of Wnt/LRP6 signaling. PLoS One. 2013;8:e62295. doi: 10.1371/journal.pone.0062295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li X., Ominsky M.S., Warmington K.S., Morony S., Gong J., Cao J., et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 133.MacNabb C., Patton D., Hayes J.S. Sclerostin antibody therapy for the treatment of osteoporosis: clinical prospects and challenges. J Osteoporos. 2016;2016:6217286. doi: 10.1155/2016/6217286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gao Y., Chen Z., Yang C., Zhong D. Liquid chromatography–mass spectrometry method for the quantification of an anti-sclerostin monoclonal antibody in cynomolgus monkey serum. J Pharm Anal. 2021;11:472–479. doi: 10.1016/j.jpha.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sinder B.P., Eddy M.M., Ominsky M.S., Caird M.S., Marini J.C., Kozloff K.M., et al. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res. 2013;28:73–80. doi: 10.1002/jbmr.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boschert V., Frisch C., Back J.W., van Pee K., Weidauer S.E., Muth E.M., et al. The sclerostin-neutralizing antibody AbD09097 recognizes an epitope adjacent to sclerostin's binding site for the Wnt co-receptor LRP6. Open Biol. 2016;6:160120. doi: 10.1098/rsob.160120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fontalis A., Kenanidis E., Kotronias R.A., Papachristou A., Anagnostis P., Potoupnis M., et al. Current and emerging osteoporosis pharmacotherapy for women: state of the art therapies for preventing bone loss. Expert Opin Pharmacother. 2019;20:1123–1134. doi: 10.1080/14656566.2019.1594772. [DOI] [PubMed] [Google Scholar]
- 138.McColm J., Hu L., Womack T., Tang C.C., Chiang A.Y. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2014;29:935–943. doi: 10.1002/jbmr.2092. [DOI] [PubMed] [Google Scholar]
- 139.Zhang D., Yang C., Chen X., Li X., Zhong D. A bridging immunogenicity assay for monoclonal antibody: case study with SHR-1222. Bioanalysis. 2018;10:1115–1127. doi: 10.4155/bio-2017-0289. [DOI] [PubMed] [Google Scholar]
- 140.Rosen C.J. Romosozumab-promising or practice changing. N Engl J Med. 2017;377:1479–1480. doi: 10.1056/NEJMe1711298. [DOI] [PubMed] [Google Scholar]
- 141.Shakeri A., Adanty C. Romosozumab (sclerostin monoclonal antibody) for the treatment of osteoporosis in postmenopausal women: a review. J Popul Ther Clin Pharmacol. 2020;27:e25–e31. doi: 10.15586/jptcp.v27i1.655. [DOI] [PubMed] [Google Scholar]
- 142.Shah A.D., Shoback D., Lewiecki E.M. Sclerostin inhibition: a novel therapeutic approach in the treatment of osteoporosis. Int J Womens Health. 2015;7:565. doi: 10.2147/IJWH.S73244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Semënov M., Tamai K., He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 144.Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol. 2013;9:575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- 145.Kocijan R., Klaushofer K., Misof B.M. Osteoporosis therapeutics 2020. Handb Exp Pharmacol. 2020;262:397–422. doi: 10.1007/164_2020_373. [DOI] [PubMed] [Google Scholar]
- 146.Padhi D., Jang G., Stouch B., Fang L., Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 147.Langdahl B.L., Libanati C., Crittenden D.B., Brown J.P., Daizadeh N.S., Daizadeh E., et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390:1585–1594. doi: 10.1016/S0140-6736(17)31613-6. [DOI] [PubMed] [Google Scholar]
- 148.Brandenburg V.M., Kramann R., Koos R., Krüger T., Schurgers L., Mühlenbruch G., et al. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol. 2013;14:219. doi: 10.1186/1471-2369-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kawaguchi H. Serious adverse events with romosozumab use in Japanese patients: need for clear formulation of contraindications worldwide. J Bone Miner Res. 2020;35:994–995. doi: 10.1002/jbmr.4001. [DOI] [PubMed] [Google Scholar]
- 150.Lv F., Cai X., Yang W., Gao L., Chen L., Wu J., et al. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: systematic review and meta-analysis. Bone. 2020;130:115121. doi: 10.1016/j.bone.2019.115121. [DOI] [PubMed] [Google Scholar]
- 151.Cummings S.R., McCulloch C. Explanations for the difference in rates of cardiovascular events in a trial of alendronate and romosozumab. Osteoporosis Int. 2020;31:1019–1021. doi: 10.1007/s00198-020-05379-z. [DOI] [PubMed] [Google Scholar]
- 152.Blakely K.K., Johnson C. New osteoporosis treatment means new bone formation. Nurs Womens Health. 2020;24:52–57. doi: 10.1016/j.nwh.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 153.Keefe A.D., Pai S., Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shum K.T., Chan C., Leung C.M., Tanner J.A. Identification of a DNA aptamer that inhibits sclerostin's antagonistic effect on Wnt signalling. Biochem J. 2011;434:493–501. doi: 10.1042/BJ20101096. [DOI] [PubMed] [Google Scholar]
- 155.Yu Y., Wang L., Ni S., Zhuo Z., Chu H., Zhang N., et al. American Society of Bone and Mineral Research 2020 annual meeting. 2020 Sep 11-15. Poster: Sclerostin loop3: a potential target for developing a next generation sclerostin inhibitor for bone anabolic therapy with low cardiovascular concern; p. 657.https://drive.google.com/file/d/1NiT6BNDCihsq0unrHyuEkAjVVAHlaF62/view?usp=sharing Available from: [Google Scholar]
- 156.Krishna S.M., Seto S.W., Jose R.J., Li J., Morton S.K., Biros E., et al. Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II-induced aortic aneurysm and atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:553–566. doi: 10.1161/ATVBAHA.116.308723. [DOI] [PubMed] [Google Scholar]
- 157.Srinivasarao M., Low P.S. Ligand-targeted drug delivery. Chem Rev. 2017;117:12133–12164. doi: 10.1021/acs.chemrev.7b00013. [DOI] [PubMed] [Google Scholar]
- 158.Paul S.M., Mytelka D.S., Dunwiddie C.T., Persinger C.C., Munos B.H., Lindborg S.R., et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 159.Zhuo Z., Wan Y., Guan D., Ni S., Wang L., Zhang Z., et al. A loop-based and AGO-incorporated virtual screening model targeting AGO-mediated miRNA–mRNA interactions for drug discovery to rescue bone phenotype in genetically modified mice. Adv Sci. 2020;7:1903451. doi: 10.1002/advs.201903451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhavoronkov A., Ivanenkov Y.A., Aliper A., Veselov M.S., Aladinskiy V.A., Aladinskaya A.V., et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat Biotechnol. 2019;37:1038–1040. doi: 10.1038/s41587-019-0224-x. [DOI] [PubMed] [Google Scholar]
- 161.Muthusamy K., Mohan S., Nagamani S., Kesavan C. Identification of novel small molecules that bind to the Loop2 region of sclerostin—an in silico computational analysis. Physiol Res. 2016;65:871. doi: 10.33549/physiolres.933267. [DOI] [PubMed] [Google Scholar]
- 162.Yooin W., Saenjum C., Ruangsuriya J., Jiranusornkul S. Discovery of potential sclerostin inhibitors from plants with loop2 region of sclerostin inhibition by interacting with residues outside Pro-Asn-Ala-Ile-Gly motif. J Biomol Struct Dyn. 2020;38:1272–1282. doi: 10.1080/07391102.2019.1599427. [DOI] [PubMed] [Google Scholar]
- 163.Cramer J., Sager C.P., Ernst B. Hydroxyl groups in synthetic and natural-product-derived therapeutics: a perspective on a common functional group. J Med Chem. 2019;62:8915–8930. doi: 10.1021/acs.jmedchem.9b00179. [DOI] [PubMed] [Google Scholar]
- 164.Holdsworth G., Slocombe P., Doyle C., Sweeney B., Veverka V., Riche L.K., et al. Characterization of the interaction of sclerostin with the low density lipoprotein receptor-related protein (LRP) family of Wnt co-receptors. J Biol Chem. 2012;287:26464–26477. doi: 10.1074/jbc.M112.350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim J.H., Liu X., Wang J., Chen X., Zhang H., Kim S.H., et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis. 2013;5:13–31. doi: 10.1177/1759720X12466608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Choi J., Lee K., Kang M., Lim S.K., No K.T. In silico discovery of quinoxaline derivatives as novel LRP5/6–sclerostin interaction inhibitors. Bioorg Med Chem Lett. 2018;28:1116–1121. doi: 10.1016/j.bmcl.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 167.Gomtsyan A. Heterocycles in drugs and drug discovery. Chem Heterocycl Compd. 2020;48:7–10. [Google Scholar]
- 168.Yu S., Fu X., Liu G., Qiu H., Hu W. Efficient and facile synthesis of chiral sulfonamides via asymmetric multicomponent reactions. Acta Chim Sin. 2018;76:895–900. [Google Scholar]
- 169.Ertl P., Altmann E., McKenna J.M. The most common functional groups in bioactive molecules and how their popularity has evolved over time. J Med Chem. 2020;63:8408–8418. doi: 10.1021/acs.jmedchem.0c00754. [DOI] [PubMed] [Google Scholar]
- 170.He W., Li C., Chen Q., Xiang T., Wang P., Pang J. Serum sclerostin and adverse outcomes in elderly patients with stable coronary artery disease undergoing percutaneous coronary intervention. Aging Clin Exp Res. 2020;32:2065–2072. doi: 10.1007/s40520-019-01393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wong C.Y., Martinez J., Dass C.R. Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. J Pharm Pharmacol. 2016;68:1093–1108. doi: 10.1111/jphp.12607. [DOI] [PubMed] [Google Scholar]
- 172.Ismail R., Csóka I. Novel strategies in the oral delivery of antidiabetic peptide drugs—insulin, GLP 1 and its analogs. Eur J Pharm Biopharm. 2017;115:257–267. doi: 10.1016/j.ejpb.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 173.Al Musaimi O., Al Shaer D., De la Torre B.G., Albericio F. 2017 FDA peptide harvest. Pharmaceuticals. 2018;11:42. doi: 10.3390/ph11020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao P., Liang Y.L., Belousoff M.J., Deganutti G., Fletcher M.M., Willard F.S., et al. Activation of the GLP-1 receptor by a non-peptidic agonist. Nature. 2020;577:432–436. doi: 10.1038/s41586-019-1902-z. [DOI] [PubMed] [Google Scholar]
- 175.Khosla S., Hopbauer L.C. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907. doi: 10.1016/S2213-8587(17)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Asadipooya K., Weinstock A. Cardiovascular outcomes of romosozumab and protective role of alendronate. Arterioscler Thromb Vasc Biol. 2019;39:1343–1350. doi: 10.1161/ATVBAHA.119.312371. [DOI] [PubMed] [Google Scholar]
- 177.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 178.McInnes C. Virtual screening strategies in drug discovery. Curr Opin Chem Biol. 2007;11:494–502. doi: 10.1016/j.cbpa.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 179.Santana K., do Nascimento L.D., Lima E Lima A., Damasceno V., Nahum C., Braga R.C., et al. Applications of virtual screening in bioprospecting: facts, shifts, and perspectives to explore the chemo-structural diversity of natural products. Front Chem. 2021;9:155. doi: 10.3389/fchem.2021.662688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shin M., Jang D., Nam H., Lee K.H., Lee D. Predicting the absorption potential of chemical compounds through a deep learning approach. IEEE/ACM Trans Comput Biol Bioinf. 2018;15:432–440. doi: 10.1109/TCBB.2016.2535233. [DOI] [PubMed] [Google Scholar]
- 181.Mayr A., Klambauer G., Unterthiner T., Hochreiter S. DeepTox: toxicity prediction using deep learning. Front Environ Sci. 2016;3:80. [Google Scholar]
- 182.Zhou J., Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16:181–202. doi: 10.1038/nrd.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ni S., Zhuo Z., Pan Y., Yu Y., Li F., Liu J., et al. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl Mater Interfaces. 2021;13:9500–9519. doi: 10.1021/acsami.0c05750. [DOI] [PubMed] [Google Scholar]