Key Points
Question
Do adverse events and health care expenditures differ in children given inappropriate vs appropriate oral antibiotic prescriptions for common outpatient infections?
Findings
In this cohort study of more than 2.8 million children with commercial insurance, inappropriate antibiotics were associated with increased risk of several adverse drug events (eg, Clostridioides difficile infection, severe allergic reaction) and generally higher 30-day all-cause attributable expenditures. National annual expenditure estimates associated with inappropriate antibiotic treatment in the pediatric commercially insured population were highest for suppurative otitis media, pharyngitis, and viral upper respiratory infection.
Meaning
Inappropriate antibiotic prescriptions were associated with avoidable adverse drug events and substantial individual- and national-level health care expenditures.
This cohort study evaluates the comparative safety and health care expenditures of inappropriate vs appropriate oral antibiotic prescriptions for common outpatient pediatric infections.
Abstract
Importance
Nonguideline antibiotic prescribing for the treatment of pediatric infections is common, but the consequences of inappropriate antibiotics are not well described.
Objective
To evaluate the comparative safety and health care expenditures of inappropriate vs appropriate oral antibiotic prescriptions for common outpatient pediatric infections.
Design, Setting, and Participants
This cohort study included children aged 6 months to 17 years diagnosed with a bacterial infection (suppurative otitis media [OM], pharyngitis, sinusitis) or viral infection (influenza, viral upper respiratory infection [URI], bronchiolitis, bronchitis, nonsuppurative OM) as an outpatient from April 1, 2016, to September 30, 2018, in the IBM MarketScan Commercial Database. Data were analyzed from August to November 2021.
Exposures
Inappropriate (ie, non–guideline-recommended) vs appropriate (ie, guideline-recommended) oral antibiotic agents dispensed from an outpatient pharmacy on the date of infection.
Main Outcomes and Measures
Propensity score–weighted Cox proportional hazards models were used to estimate hazards ratios (HRs) and 95% CIs for the association between inappropriate antibiotic prescriptions and adverse drug events. Two-part models were used to calculate 30-day all-cause attributable health care expenditures by infection type. National-level annual attributable expenditures were calculated by scaling attributable expenditures in the study cohort to the national employer-sponsored insurance population.
Results
The cohort included 2 804 245 eligible children (52% male; median [IQR] age, 8 [4-12] years). Overall, 31% to 36% received inappropriate antibiotics for bacterial infections and 4% to 70% for viral infections. Inappropriate antibiotics were associated with increased risk of several adverse drug events, including Clostridioides difficile infection and severe allergic reaction among children treated with a nonrecommended antibiotic agent for a bacterial infection (among patients with suppurative OM, C. difficile infection: HR, 6.23; 95% CI, 2.24-17.32; allergic reaction: HR, 4.14; 95% CI, 2.48-6.92). Thirty-day attributable health care expenditures were generally higher among children who received inappropriate antibiotics, ranging from $21 to $56 for bacterial infections and from −$96 to $97 for viral infections. National annual attributable expenditure estimates were highest for suppurative OM ($25.3 million), pharyngitis ($21.3 million), and viral URI ($19.1 million).
Conclusions and Relevance
In this cohort study of children with common infections treated in an outpatient setting, inappropriate antibiotic prescriptions were common and associated with increased risks of adverse drug events and higher attributable health care expenditures. These findings highlight the individual- and national-level consequences of inappropriate antibiotic prescribing and further support implementation of outpatient antibiotic stewardship programs.
Introduction
Approximately 29% of outpatient antibiotics prescribed to children in the United States are inappropriate.1 These include a large proportion of children inappropriately prescribed any antibiotic agent for a viral infection (eg, 21% of viral upper respiratory infection [URI] diagnoses)1 or inappropriately prescribed a non–first-line antibiotic agent for a bacterial infection (eg, 33% of treated otitis media [OM] diagnoses, 40% of treated pharyngitis diagnoses, and 48% of treated sinusitis diagnoses), ie, non–guideline-concordant antibiotic use.2,3 Inappropriate antibiotic prescriptions are harmful on a societal level because they propel the spread of antimicrobial resistance4 and harmful on an individual level because they are associated with adverse drug events (ADEs), such as allergic reactions (eg, anaphylaxis, skin rash) and microbiome disruption-related conditions (eg, Clostridioides difficile infection).5,6,7 The clinical management of antibiotic-resistant infections and antibiotic-related ADEs require costly health care use,4 much of which is likely avoidable.6,7
Despite inappropriate antibiotic prescribing for the treatment of pediatric infections in the outpatient setting,1,8 evidence is limited on the risks related to inappropriate antibiotic prescriptions. Additional study is needed in large, infection-specific cohorts to estimate the comparative risk of individual ADEs among recipients of inappropriate vs appropriate antibiotic prescriptions. Furthermore, comprehensive estimates of attributable health care utilization and expenditures associated with inappropriate antibiotic prescriptions for common outpatient conditions are generally unavailable.9,10
The objectives of this study were to evaluate the comparative safety and attributable health care expenditures associated with inappropriate outpatient antibiotic prescriptions for several common bacterial and viral infections, in a cohort of children with commercial insurance in the United States. We also sought to estimate the national-level annual attributable expenditures of inappropriate antibiotic prescriptions for the pediatric commercially-insured population.
Methods
Data Source
We used the IBM MarketScan Commercial Database (2015-2018), which contains longitudinal, patient-level data on enrollment and adjudicated inpatient and outpatient insurance claims as well as outpatient pharmacy-dispensed medications for individuals with primarily employer-sponsored commercial insurance and their spouses and dependents.11 The institutional review board at Washington University School of Medicine deemed this study exempt from human participant review. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study Design and Population
We identified children aged 6 months to 17 years diagnosed in an outpatient setting with a common bacterial infection (suppurative OM, pharyngitis, sinusitis) or viral infection (influenza, viral URI, bronchiolitis [age 6 months to 3 years], bronchitis [age 5 to 17 years], nonsuppurative OM) from April 1, 2016, to September 30, 2018. We constructed cohorts for each infection type based on categories developed by Fleming-Dutra et al1; we adapted definitions from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes to ICD-10-CM codes per the Centers for Medicare & Medicaid Services general equivalence mappings12 (eTable 1 in the Supplement). The index date was defined as the date of diagnosis (ignoring diagnostic and/or rule-out claims).
Children were required to have continuous health insurance enrollment and prescription drug coverage during the 180-day baseline period before the index date. To restrict the study population to otherwise healthy children with minimal antibiotic exposure, we excluded index events with inpatient or skilled nursing facility admission within 90 days before index, hospice care or mechanical ventilation within 180 days before index, serious underlying medical conditions within 180 days before index (eTables 2 and 3 in the Supplement), a previous diagnosis for the condition of interest within 180 days prior to the index date (eg, a diagnosis that was not eligible as an index event due to other inclusion and exclusion criteria), or antibiotic use (intravenous, intramuscular, oral) within 90 days before index (eTable 2 in the Supplement). We excluded index events with multiple oral antibiotic prescription dispensings or unusual treatment durations (ie, <5 days or >14 days) at index. We applied a tiered approach to study inclusion and exclusion for index events with multiple, simultaneous infection-related diagnoses of interest (eTable 4 in the Supplement).13 For study inclusion for viral infection index events, we allowed multiple index diagnoses for which antibiotics are not warranted (ie, other viral index infection). For bacterial infection index events, we allowed multiple diagnoses for which antibiotics are not warranted (ie, index viral infections) and allowed other bacterial index infections (eg, index suppurative OM and sinusitis, assuming patients received first-line antibiotics). For study exclusion, we excluded index events with other diagnoses for which antibiotics are warranted (eTable 4 in the Supplement), irrespective of documentation for a dispensed antibiotic prescription. For example, we excluded bacterial and viral infection index events on the same day as any condition in eTable 4 in the Supplement regardless of antibiotic prescriptions (eg, sinusitis index event also coded for sepsis). We excluded viral infection index events simultaneously coded for a bacterial infection index condition (ie, suppurative OM, sinusitis, or pharyngitis). For nonsuppurative OM, we excluded index events with an antibiotic eardrop prescription at index for expenditure analyses (eTable 2 in the Supplement). Finally, we restricted the population to the first qualifying event per diagnosis per child (eFigure 1 in the Supplement).
Antibiotic Exposure
An oral antibiotic prescription was linked to an outpatient infection if it occurred on the day of the index diagnosis. We defined 36 index oral antibiotics based on the 2016 antibiotic utilization quality measure in the Healthcare Effectiveness Data and Information Set (eTable 5 in the Supplement).14 For bacterial infections, we categorized antibiotic prescriptions by agent as appropriate (ie, first-line antibiotic agent) or inappropriate (ie, non–first-line antibiotic agent) based on treatment guidelines. First-line antibiotic agents included amoxicillin for suppurative OM15; amoxicillin or penicillin for pharyngitis16; and amoxicillin or amoxicillin-clavulanate for sinusitis.17 For viral infections, we categorized antibiotic prescriptions as appropriate (ie, no antibiotic prescription) or inappropriate (ie, antibiotic prescription). Primary analyses focused on bacterial infections because of the use of an active comparator (ie, all children prescribed an antibiotic), which reduces measured and unmeasured confounding in observational studies.18,19,20 Secondary analyses focused on viral infections.
Safety Outcomes
We identified individual ADEs using ICD-10-CM diagnosis codes on all medical claims during follow-up (eTable 6 in the Supplement).21,22,23 The duration of outcome-specific follow-up periods ranged from 2 to 90 days. To ensure identification of new-onset outcomes, we excluded children diagnosed with the outcome of interest within 30 days prior to the index for each respective ADE. Analyses for the C. difficile outcome were restricted to children aged 2 to 17 years.
Health Care Expenditure Outcomes
Health care expenditures were computed as the sum of out-of-pocket patient expenditures (copayments, coinsurance, deductible) and health plan expenditures (negotiated fees paid to providers [defined in the data source as individual clinicians and facilities] for services including coordination of benefits). We used 2 outcome definitions to compute 30-day expenditures recorded on medical and pharmacy claims: (1) all-cause health care expenditures represented an upper bound by including expenditures recorded on all claims and (2) ADE-associated health care expenditures represented a lower bound by only including expenditures recorded on claims with antibiotic-related ADEs of interest. We included all claims billed with diagnosis codes for select ADEs, provided that the initial ADE-related code occurred within the specified follow-up window. We examined total expenditures and expenditures by setting (inpatient medical, emergency department medical, outpatient medical, outpatient pharmacy). Expenditures were inflation adjusted to 2018 US dollars using the medical care component of the Consumer Price Index.24
Covariates
Baseline covariates were assessed during a 180-day baseline period before the index antibiotic prescription. Potential confounders of the association between antibiotic exposure and ADE outcomes were identified a priori based on clinical knowledge, and included age, sex, health insurance plan type, urban vs rural residence, geographic region, month and year of index, provider specialty, provider location, number of emergency department encounters, and number of unique medication therapeutic groups.25 Additional potential confounders incorporated into the expenditure analyses included mean monthly medical and prescription expenditures, number of office visits, frailty markers (eTable 7 in the Supplement), and comorbid conditions defined using the Elixhauser classification (eTable 8 in the Supplement).26,27
Statistical Analysis
Data analyses were performed from August to November 2021. We used stabilized inverse probability of treatment (IPT) weights to balance treatment groups within each cohort with respect to potential confounding factors. We used logistic regression to estimate the propensity of appropriate (vs inappropriate) antibiotic agent, conditional on baseline covariates. Propensity scores were used to create weighted cohorts to estimate the treatment effects in the total population, ie, the average treatment effect (eMethods in the Supplement).28,29 We assessed the balance of observed covariates between treatment groups; absolute standardized mean differences of less than 10% in the weighted population were considered adequate.30
To examine the association between inappropriate antibiotic agents and each ADE outcome, we used Cox proportional hazards models to estimate unadjusted and weighted hazard ratios (HRs). We used robust variance estimators to calculate 95% CIs.31 Children were censored at the end of the outcome-specific follow-up period, end of continuous coverage, subsequent antibiotic prescription for a different agent (eTable 5 in the Supplement), hospitalization, or end of study (December 31, 2018). We selected tendinopathy (including tendon rupture) as a negative control outcome because it is known to be causally unrelated to the exposure (ie, nonfluoroquinolone antibiotics commonly prescribed to children to treat the infections of interest). Although fluoroquinolone antibiotics are associated with tendinopathy,32 fluoroquinolones are rarely prescribed to treat the pediatric conditions under study. Given the absence of a biologically plausible mechanism for nonfluoroquinolone antibiotics to cause tendinopathy, estimates of tendinopathy should be null in the absence of confounding.33
To estimate attributable expenditures, we used 2-part models. Part 1 was a logistic regression of any vs no expenditures, and part 2 was a flexible model of the level of health care expenditures from a generalized linear model with a log-link and gamma distribution.34,35 The modified Park test was used to guide selection of the appropriate distribution.36,37 The attributable expenditure was then estimated as the marginal effect (in dollars) that combines both parts. We computed 95% CIs using a nonparametric bootstrap based on 250 resamples.38,39 These analyses were restricted to children with continuous health insurance coverage for 30 days of follow-up after index.
To estimate the financial burden of inappropriate antibiotic prescriptions on the US health care system, we scaled the attributable expenditure estimates in the study cohort to the national employer-sponsored insurance population. We standardized and scaled all index events in 2017 to the national employer-sponsored insurance population using MarketScan weights constructed from the American Community Survey with respect to census division, age group, sex, and relationship to the insurance policy holder. We used the calculated IPT-weighted all-cause attributable expenditures to estimate total national-level expenditures for inappropriate antibiotics.
We performed a priori analyses for asthma and allergy, a noninfectious clinical condition frequently treated contrary to guidelines with antibiotic prescriptions, and a subset analyses for asthma exacerbation, applying study inclusion and exclusion criteria as per viral infections. For the all-cause expenditure analyses, we (1) redefined inappropriate antibiotic exposure as inappropriate agent or duration for bacterial infections (eMethods in the Supplement); (2) extended follow-up to 90 days; and (3) excluded beneficiaries with health maintenance organization and point of service with capitation plans.
Analysis was conducted with SAS version 9.4 (SAS Institute). Statistical significance was defined as the absence of the null value within the 95% CIs.
Results
The study sample included 1 601 019 bacterial infection index events (601 711 [38%] suppurative OM, 617 215 [39%] pharyngitis, and 382 093 [24%] sinusitis) and 1 203 226 viral infection index events (180 996 [15%] influenza, 772 040 [64%] viral URI, 23 931 [2%] bronchiolitis, 72 407 [6%] bronchitis, and 153 852 [13%] nonsuppurative OM) (eFigure 1 in the Supplement). The study sample had a median (IQR) age of 8 (4-12) years, 52% were male, and 48% resided in the South. The proportion of children who received inappropriate antibiotics differed by cohort (bacterial infections: sinusitis, 137 065 [36%]; pharyngitis, 208 705 [34%]; and suppurative OM, 186 832 [31%]; viral infections: bronchitis, 50 806 [70%], nonsuppurative OM, 73 368 [48%]; viral URI, 93 013 [12%]; bronchiolitis, 2120 [9%]; and influenza, 6817 [4%]) (Table 1). The distribution of antibiotic agents differed by infection type (eTable 9 in the Supplement). For example, children with pharyngitis were inappropriately treated with azithromycin (13%), cefdinir (8%), amoxicillin-clavulanate (6%), cephalexin (5%), and other agents (2%). Table 1 and eTable 10 in the Supplement summarize baseline characteristics by exposure group.
Table 1. Selected Baseline Characteristics of Infections of Interest Among Childrena.
| Characteristic | Participants, No. (%) (N = 2 804 245) | |||
|---|---|---|---|---|
| Bacterial infections (primary analysis)b | Viral infections (secondary analysis)c | |||
| Appropriate antibiotic (n = 1 068 417) | Inappropriate antibiotic (n = 532 602) | Appropriate antibiotic (n = 977 102) | Inappropriate antibiotic (n = 226 124) | |
| Demographic characteristics | ||||
| Age, mean (SD), yd | 7 (5) | 9 (5) | 7 (5) | 8 (5) |
| Sex | ||||
| Male | 531 360 (49.7) | 267 618 (50.3) | 505 091 (51.8) | 115 988 (51.3) |
| Female | 537 057 (50.3) | 264 984 (49.8) | 472 011 (48.3) | 110 136 (48.7) |
| Urban residence | 860 296 (80.5) | 405 581 (76.2) | 801 688 (82.1) | 175 341 (77.5) |
| Geographic region | ||||
| Midwest | 239 621 (22.4) | 99 628 (18.7) | 182 162 (18.6) | 47 279 (20.9) |
| Northeast | 192 453 (18.0) | 79 496 (14.9) | 183 343 (18.8) | 34 893 (15.4) |
| South | 498 727 (46.7) | 295 115 (55.4) | 449 866 (46.0) | 112 327 (49.7) |
| West | 137 616 (12.9) | 58 363 (11.0) | 161 731 (16.6) | 31 625 (14.0) |
| Health insurance plan type | ||||
| Basic, comprehensive | 155 208 (14.5) | 68 225 (12.8) | 141 964 (14.5) | 30 539 (13.5) |
| CDHP | 146 332 (13.7) | 72 333 (13.6) | 125 526 (12.9) | 30 391 (13.4) |
| EPO or PPO | 586 972 (54.9) | 302 112 (56.7) | 533 125 (54.6) | 127 185 (56.3) |
| HMO | 95 896 (9.0) | 42 401 (8.0) | 91 203 (9.3) | 18 511 (8.2) |
| POS or POS with capitation | 65 335 (6.1) | 38 486 (7.2) | 64 807 (6.6) | 15 410 (6.8) |
| Unknown | 18 674 (1.8) | 9045 (1.7) | 20 477 (2.1) | 4088 (1.8) |
| Index characteristics | ||||
| Index diagnosis | ||||
| Suppurative OM | 414 879 (38.8) | 186 832 (35.1) | NA | NA |
| Pharyngitis | 408 510 (38.2) | 208 705 (39.2) | NA | NA |
| Sinusitis | 245 028 (22.9) | 137 065 (25.7) | NA | NA |
| Influenza | NA | NA | 174 179 (17.8) | 6817 (3.0) |
| Viral URI | NA | NA | 679 027 (69.5) | 93 013 (41.1) |
| Bronchiolitis | NA | NA | 21 811 (2.2) | 2120 (0.9) |
| Bronchitis | NA | NA | 21 601 (2.2) | 50 806 (22.5) |
| Nonsuppurative OM | NA | NA | 80 484 (8.2) | 73 368 (32.5) |
| Index provider specialty | ||||
| Emergency medicine | 22 312 (2.1) | 11 891 (2.2) | 36 677 (3.8) | 5891 (2.6) |
| Internal medicine | 23 261 (2.2) | 15 911 (3.0) | 20 679 (2.1) | 7587 (3.4) |
| Other or unknown | 304 565 (28.5) | 14 6359 (27.5) | 26 7952 (27.4) | 64 306 (28.4) |
| Pediatrics or family medicine | 718 279 (67.2) | 358 441 (67.3) | 651 794 (66.7) | 148 340 (65.6) |
| Index provider location | ||||
| Emergency department | 5748 (0.5) | 3087 (0.6) | 42 252 (4.3) | 1884 (0.8) |
| Office | 947 102 (88.7) | 474 315 (89.1) | 844 585 (86.4) | 196 686 (87.0 |
| Other or unknown | 17 713 (1.7) | 7856 (1.5) | 20 411 (2.1) | 4029 (1.8 |
| Retail clinic | 3649 (0.3) | 1145 (0.2) | 1869 (0.2) | 414 (0.2 |
| Urgent care center | 94 205 (8.8) | 46 199 (8.7) | 67 985 (7.0) | 23 111 (10.2) |
| Prior health care utilization | ||||
| Prior emergency department visit | 73 220 (6.9) | 36 947 (6.9) | 61 648 (6.3) | 14 019 (6.2) |
| Prior No. of office visits, median (IQR) | 1 (0-3) | 1 (0-3) | 1 (0-2) | 1 (0-2) |
| Prior No. of unique medication classes, median (IQR) | 0 (0-1) | 1 (0-1) | 0 (0-1) | 0 (0-1) |
Abbreviations: CDHP, consumer directed health plans; EPO, exclusive provider organization; HMO, health maintenance organization; NA, not applicable; OM, otitis media; POS, point-of-service; PPO, preferred provider organization; URI, upper respiratory infection.
Demographic and index characteristics were assessed on the index date. Prior health care utilization was assessed in the 180-day baseline period before the index date (days −180 to −1).
For children diagnosed with bacterial infections (ie, suppurative OM, pharyngitis, or sinusitis), antibiotic prescriptions were categorized as appropriate (ie, first-line antibiotic agent) or inappropriate (ie, non-first-line antibiotic agent); patients without an antibiotic prescription were excluded. First-line antibiotic agents were defined as amoxicillin for suppurative OM; amoxicillin or penicillin for pharyngitis; and amoxicillin or amoxicillin-clavulanate for sinusitis.
For children diagnosed with viral infections (ie, influenza, viral URI, bronchiolitis, bronchitis, or non-suppurative OM), antibiotic prescriptions were categorized as appropriate (no antibiotic) or inappropriate (antibiotic).
Bronchiolitis cohort was restricted to ages 6 months to 3 years; bronchitis cohort was restricted to ages 5 to 17 years.
ADEs
After propensity score weighting and outcome-specific exclusions (eTable 11 in the Supplement), exposure groups were similar with respect to baseline characteristics, except for provider specialty and month of index in some cohorts (eFigure 2 in the Supplement). For each infection-specific cohort, case counts, rates, and unadjusted and weighted HR estimates of each ADE outcome following appropriate vs inappropriate antibiotic prescriptions are presented in Figure 1 and eFigure 3 and eTable 12 in the Supplement. Rates of adverse events varied widely, ranging from 0.00 to 0.01 cases per 10 000 person-days for Stevens-Johnson syndrome or toxic epidermal necrolysis to 1.49 to 9.55 cases per 10 000 person-days for skin rash or urticaria.
Figure 1. Hazard Ratio (HR) Estimates of Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Pediatric Patients.
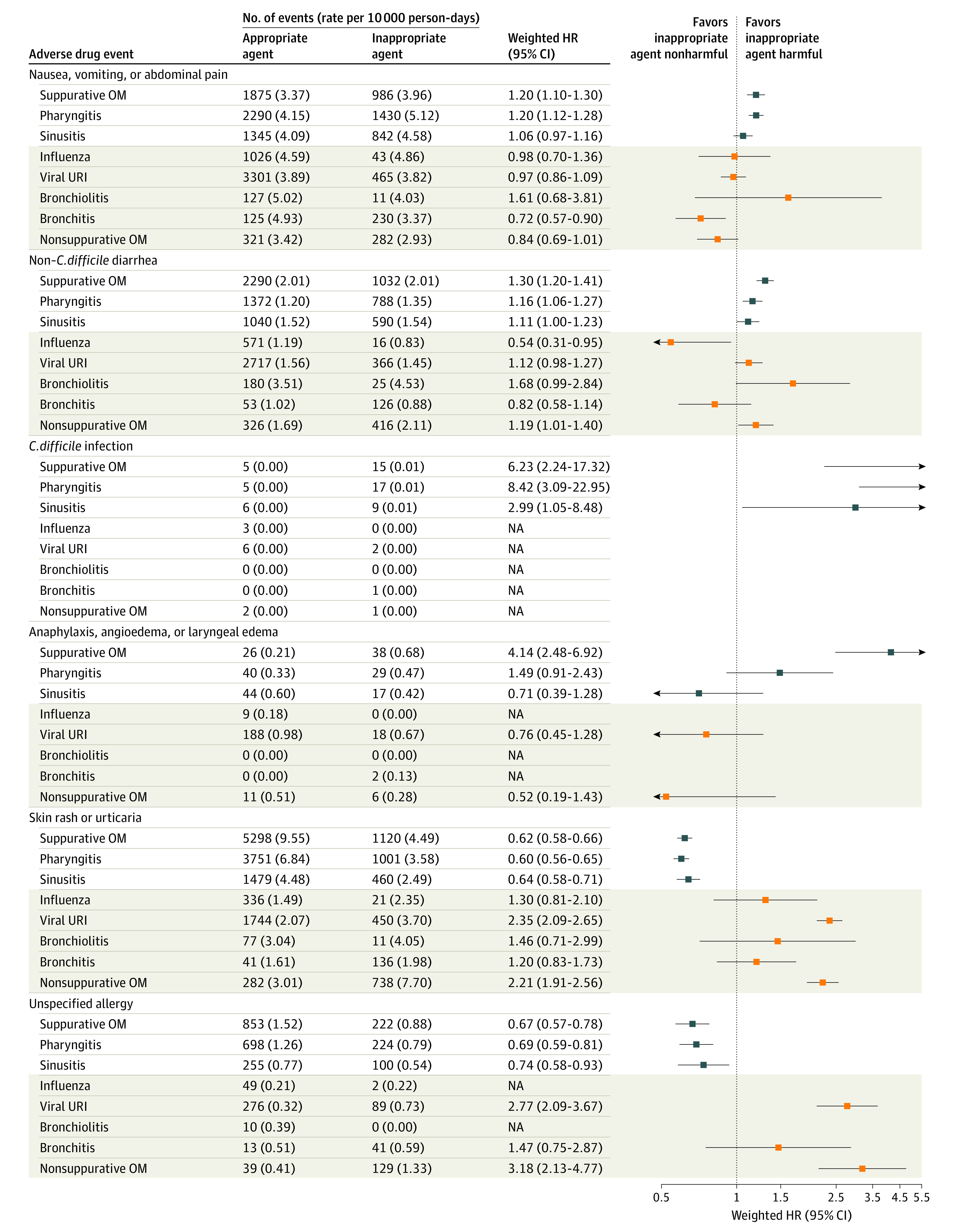
Between 0.0% and 1.8% patients were excluded for 30-day safety outcomes (eTable 11 in the Supplement). Definitions of appropriate and inappropriate agents for bacterial and viral infections are provided in the Methods section. For HR estimation, at least 5 adverse event cases were required in both the reference category (ie, appropriate antibiotic prescription) and the comparator group (ie, inappropriate antibiotic prescription) to ensure stability of the effect estimate. Results for bacterial infections are denoted by a white background with blue boxes; viral infections, brown background with orange boxes. OM indicates otitis media; URI, upper respiratory infection.
For children with bacterial infections, inappropriate antibiotic prescriptions were usually associated with higher risk of C. difficile infection (eg, children with suppurative OM: HR, 6.23; 95% CI, 2.24-17.32); non–C. difficile diarrhea (eg, children with suppurative OM: HR, 1.30; 95% CI, 1.20-1.41); and nausea, vomiting, or abdominal pain (eg, children with suppurative OM: HR, 1.20; 95% CI, 1.10-1.30) and lower risk of skin rash or urticaria (eg, children with suppurative OM: HR, 0.62; 95% CI, 0.58-0.66) as well as unspecified allergy (eg, children with suppurative OM: HR, 0.67; 95% CI, 0.57-0.78) (Figure 1). For children with viral infections, inappropriate antibiotic prescriptions were associated with higher risk of skin rash or urticaria as well as unspecified allergy for viral URI and nonsuppurative OM (Figure 1). Case counts were too rare to estimate some effects (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis and acute kidney failure) (eFigure 3 in the Supplement). In the negative control outcome analysis, we observed similar risks of tendinopathy among children who received appropriate vs inappropriate antibiotic prescriptions, as indicated by 95% CIs that included the null value of 1, for all infection cohorts except pharyngitis (eFigure 3 in the Supplement).
Attributable Expenditures and National Burden
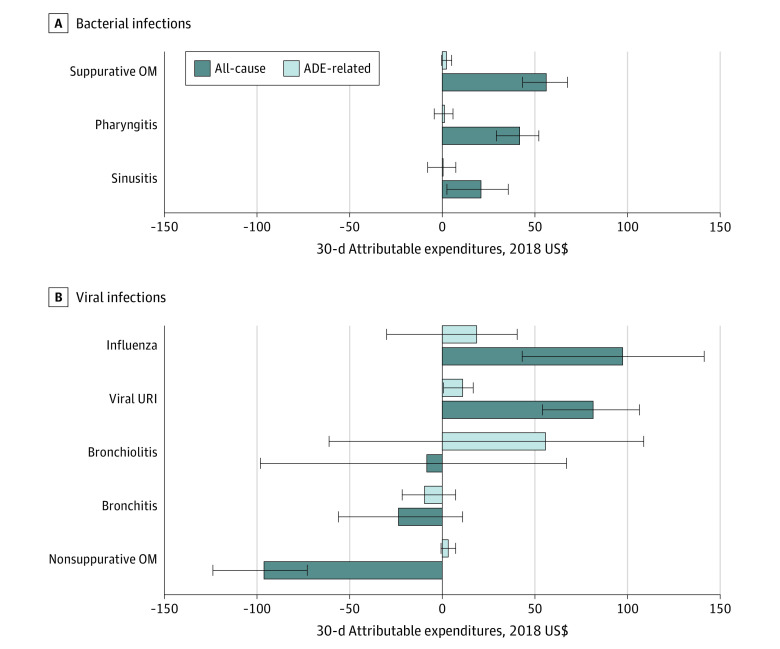
After weighting, the exposure groups were similar with respect to baseline characteristics, with few exceptions (eTable 13 in the Supplement). Health care utilization and total per-patient expenditure estimates are presented by infection type for all-cause expenditures (Table 2) and ADE-associated expenditures (eTable 14 in the Supplement). Utilization of inpatient medical care was rare in the 30 days following infection; 0.2% to 0.3% of patients in the bacterial cohort and 0.2% to 1.1% of patients in the viral cohort received inpatient care. For bacterial infections, the mean total attributable expenditure of an inappropriate antibiotic prescription ranged from $21 (95% CI, $3 to $36) for sinusitis to $56 (95% CI, $43 to $68) for suppurative OM; thus, inappropriate vs appropriate antibiotic prescriptions were associated with higher expenditures for suppurative OM, pharyngitis, and sinusitis (Figure 2; eTable 13 in the Supplement). For viral infections, the estimates ranged from −$96 (95% CI, −$124 to −$73) for nonsuppurative OM to $97 (95% CI, $43 to $141) for influenza; thus, inappropriate vs appropriate antibiotic prescriptions were associated with expenditures that were higher for influenza and viral URI, similar for bronchiolitis and bronchitis, and lower for nonsuppurative OM. The ADE-associated attributable expenditure estimates followed a similar pattern but were much closer to the null. The total attributable expenditure differences were largely driven by outpatient pharmacy and outpatient medical utilization and expenditures (eTable 13 in the Supplement).
Table 2. Inverse Probability of Treatment–Weighted 30-Day All-Cause Health Care Utilization and Total Per-Patient Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children by Setting.
| Expenditure category | Health care utilization, No. (%) | Total per-patient expenditure estimates, mean (SD), $ | ||
|---|---|---|---|---|
| Appropriate antibiotic | Inappropriate antibiotic | Appropriate antibiotic | Inappropriate antibiotic | |
| Bacterial infections (primary analysis) | ||||
| Suppurative OM | ||||
| Total | 402 815 (100.0) | 181 486 (100.0) | 426 (1985) | 498 (2362) |
| Inpatient medical | 954 (0.2) | 449 (0.2) | 42 (1639) | 44 (1869) |
| Emergency department | 10 743 (2.7) | 49,12 (2.7) | 34 (354) | 36 (351) |
| Outpatient medical | 401 128 (99.6) | 180 777 (99.6) | 300 (815) | 315 (988) |
| Outpatient pharmacy | 402 810 (100.0) | 181 486 (100.0) | 50 (501) | 103 (678) |
| Pharyngitis | ||||
| Total | 398 702 (100.0) | 203 231 (100.0) | 392 (2164) | 471 (2439) |
| Inpatient medical | 913 (0.2) | 477 (0.2) | 44 (1837) | 50 (2085) |
| Emergency department | 9093 (2.3) | 4926 (2.4) | 32 (397) | 41 (388) |
| Outpatient medical | 397 324 (99.7) | 202 460 (99.6) | 265 (793) | 290 (795) |
| Outpatient pharmacy | 398 697 (100.0) | 203 230 (100.0) | 51 (539) | 90 (639) |
| Sinusitis | ||||
| Total | 238 337 (100.0) | 133 133 (100.0) | 476 (2409) | 507 (2684) |
| Inpatient medical | 584 (0.2) | 335 (0.3) | 53 (1975) | 49 (2013) |
| Emergency department | 5217 (2.2) | 2956 (2.2) | 38 (392) | 39 (429) |
| Outpatient medical | 237 976 (99.8) | 132 917 (99.8) | 307 (997) | 311 (1372) |
| Outpatient pharmacy | 238 330 (100.0) | 133 131 (100.0) | 78 (669) | 109 (704) |
| Viral infections (secondary analysis) | ||||
| Influenza | ||||
| Total | 162 779 (100.0) | 6366 (100.0) | 549 (2254) | 644 (1935) |
| Inpatient medical | 382 (0.2) | 11 (0.2) | 43 (1989) | 43 (1537) |
| Emergency department | 8237 (5.1) | 324 (5.1) | 78 (492) | 47 (411) |
| Outpatient medical | 159 233 (97.8) | 6201 (97.4) | 270 (639) | 341 (715) |
| Outpatient pharmacy | 115 157 (70.7) | 6366 (100.0) | 158 (527) | 213 (556) |
| Viral URI | ||||
| Total | 630 381 (100.0) | 87 985 (100.0) | 480 (2605) | 531 (4251) |
| Inpatient medical | 1546 (0.2) | 267 (0.3) | 53 (2239) | 74 (3976) |
| Emergency department | 22 367 (3.5) | 2947 (3.3) | 56 (463) | 42 (437) |
| Outpatient medical | 624 443 (99.1) | 87 103 (99.0) | 310 (946) | 322 (1039) |
| Outpatient pharmacy | 252 277 (40.0) | 87 981 (100.0) | 61 (589) | 93 (817) |
| Bronchiolitis | ||||
| Total | 19 222 (100.0) | 1994 (100.0) | 783 (2362) | 704 (1506) |
| Inpatient medical | 214 (1.1) | 13 (0.6) | 147 (1967) | 64 (1039) |
| Emergency department | 1367 (7.1) | 136 (6.8) | 114 (529) | 74 (515) |
| Outpatient medical | 19 006 (98.9) | 1970 (98.8) | 476 (1002) | 481 (817) |
| Outpatient pharmacy | 10 119 (52.6) | 1994 (100.0) | 45 (360) | 85 (358) |
| Bronchitis | ||||
| Total | 20 886 (100.0) | 49 378 (100.0) | 651 (2327) | 515 (2406) |
| Inpatient medical | 52 (0.2) | 120 (0.2) | 59 (1786) | 57 (1968) |
| Emergency department | 1022 (4.9) | 2312 (4.7) | 177 (837) | 47 (456) |
| Outpatient medical | 20 491 (98.1) | 48 350 (97.9) | 331 (1007) | 299 (932) |
| Outpatient pharmacy | 11 521 (55.2) | 49 375 (100.0) | 83 (374) | 112 (719) |
| Nonsuppurative OM | ||||
| Total | 61 563 (100.0) | 62 160 (100.0) | 643 (2231) | 480 (1989) |
| Inpatient medical | 143 (0.2) | 155 (0.2) | 46 (1449) | 41 (1508) |
| Emergency department | 2106 (3.4) | 2227 (3.6) | 55 (438) | 38 (384) |
| Outpatient medical | 61 036 (99.1) | 61 528 (99.0) | 495 (1436) | 334 (821) |
| Outpatient pharmacy | 20 426 (33.2) | 62 158 (100.0) | 47 (506) | 66 (634) |
Abbreviations: OM, otitis media; URI, upper respiratory infection.
Figure 2. Inverse Probability of Treatment–Weighted 30-Day Patient-Level Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children by Infection Type.
Black lines indicate 95% CIs. ADE indicates adverse drug event; OM, otitis media; and URI, upper respiratory infection.
The sum of attributable expenditures of inappropriate prescriptions in the MarketScan study population is presented by infection type and setting in eTable 15 in the Supplement. Table 3 and eTable 16 in the Supplement present the national annual expenditure estimates of inappropriate antibiotic treatment in the pediatric commercially insured population, which were highest for suppurative OM ($25.3 million), pharyngitis ($21.3 million), and viral URI ($19.1 million).
Table 3. Annual National Attributable 30-Day Expenditures of Inappropriate Antibiotic Prescriptions Among the US Commercially Insured Population, Aged 6 Months to 17 Yearsa.
| Index diagnoses | Attributable expenditures, 2018 US $ | ||||
|---|---|---|---|---|---|
| Inpatient medical | Emergency department | Outpatient medical | Outpatient pharmacy | Total | |
| Bacterial infections (primary analysis) | |||||
| Suppurative OM | 1 235 313 | 777 904 | 7 846 200 | 15 441 487 | 25 300 317 |
| Pharyngitis | −750 188 | 2 564 653 | 6 706 388 | 12 752 577 | 21 271 338 |
| Sinusitis | −503 873 | 277 773 | 407 416 | 6 899 358 | 7 078 513 |
| Viral infections (secondary analysis) | |||||
| Influenza | −98 806 | −53 888 | 1 132 300 | 615 754 | 1 594 541 |
| Viral URI | 5 430 897 | 439 555 | 8 243 074 | 5 023 360 | 19 132 099 |
| Bronchiolitis | −334 451 | 48 984 | 88 877 | 159 028 | −37 871 |
| Bronchitis | 1 059 296 | −2 593 873 | −4 624 124 | 2 988 452 | −3 173 797 |
| Non-suppurative OM | −16 270 | −962 935 | −17 980 659 | 3 569 023 | −15 395 644 |
Abbreviations: OM, otitis media; URI, upper respiratory infection.
Bronchiolitis cohort was restricted to ages 6 months to 3 years; bronchitis cohort was restricted to ages 5 to 17 years. The 95% confidence intervals are presented in eTable 16 in the Supplement.
Subgroup and Sensitivity Analyses
Results of the safety analyses for asthma and allergy and the asthma exacerbation subset were consistent with results for viral conditions, for which appropriate treatment was defined as the absence of an antibiotic prescription (eTables 17-22 and eFigures 4-6 in the Supplement). An antibiotic prescription to treat asthma and allergy was associated with increased expenditures (weighted mean total attributable expenditure, $246 [95% CI, $147-$327]); results were null and imprecise for asthma exacerbation (eTable 21 and eFigure 6 in the Supplement). We did not observe meaningful differences in calculated expenditures in sensitivity analyses that accounted for inappropriate antibiotic duration; extended follow-up from 30 to 90 days; or excluded HMO and POS with capitation plans (eTable 23 in the Supplement).
Discussion
We conducted a national study of the safety and attributable expenditures associated with inappropriate outpatient antibiotic prescriptions for the treatment of several common bacterial and viral infections among children with commercial insurance. Inappropriate antibiotic prescriptions were associated with increased risk of ADEs, including C. difficile infection (suppurative OM, pharyngitis, and sinusitis cohorts), severe allergic reaction (suppurative OM cohort), and skin rash (viral URI and nonsuppurative OM cohorts). The 30-day all-cause attributable expenditures associated with inappropriate prescriptions were substantial on both the individual and national levels (eg, $56 per patient and $25.3 million nationally for suppurative OM).
The present study also broadens the evidence on pediatric antibiotic safety by quantifying the risks of individual ADEs associated with inappropriate antibiotics. Gerber and colleagues40 found that broad- vs narrow-spectrum antibiotics were associated with higher risk of a composite ADE outcome in children diagnosed with acute OM and similar risk in smaller cohorts of children diagnosed with sinusitis or pharyngitis. Our work builds on this study by estimating the risk of individual ADEs among children with bacterial infections as well as among children with viral infections, for whom antibiotics are inappropriate.
Our study fills a critical evidence gap by quantifying the increased expenditures associated with inappropriate antibiotic prescriptions for several common pediatric infections. Previous studies have calculated overall national antibiotic-related expenditures41,42 as well as antibiotic expenditures for influenza9 and upper respiratory infection.10 Our comparative expenditure analyses extend beyond the index prescription and incorporate downstream expenditures. Notably, inappropriate prescriptions were associated with higher health care expenditures for all 3 bacterial infections under study, higher or similar expenditures for 4 of 5 viral infections, and higher expenditures for noninfectious asthma and allergy.
One possible explanation for the association between inappropriate antibiotic prescriptions and larger expenditures is the higher ADE risk among recipients of inappropriate antibiotic prescriptions, which may lead to avoidable health care encounters. These encounters present additional opportunities for testing, treatment, and referrals, cascades of care that may not lead to clinically meaningful outcomes yet are associated with patient harms and monetary costs.43,44 Our estimates of ADE-associated expenditures represented only a small proportion of all-cause expenditures, possibly because of patients with milder ADEs choosing not to seek care, and thus, having no billable medical encounter. This phenomenon was demonstrated by a pediatric study that identified 10 times more ADEs via telephone calls to families vs manual review of electronic health record data.40 In the event of a cascade of care, it is possible that a minor ADE may not be recorded as a diagnosis on the claim and thus would be excluded from the ADE-attributable expenditures. Even in the absence of a billable encounter, health care providers commonly prescribe treatments for ADEs after telephone or telemedicine consultation, which may explain the higher all-cause outpatient pharmacy expenditures observed in our study.45
We observed widespread use of inappropriate antibiotics, consistent with previous studies1,8 and contrary to guidance by the US Centers for Disease Control and Prevention to reduce inappropriate antibiotic prescriptions in outpatient settings.46,47 Given our findings on increased patient harms and expenditures, these results warrant a call to action to key stakeholders for widespread adoption of outpatient antibiotic stewardship programs. Our study identifies suppurative OM, pharyngitis, sinusitis, and viral URIs as likely high yield targets for stewardship efforts, which could generate meaningful reductions in inappropriate antibiotic prescribing practices. Future reductions in inappropriate antibiotic prescribing will require engagement with payers, policy makers, quality improvement organizations, and patient advocacy groups. From the payer perspective, inappropriate antibiotics are a prime target for reducing health care expenditures and wasted resources,48,49 as antibiotics are the most commonly prescribed medication among children.50
Limitations
Our findings are subject to limitations. First, owing to the nonrandomized nature of the exposure, the results may be susceptible to bias due to residual confounding. We attempted to reduce potential confounding using several established epidemiologic methods, including an active comparator new-user design,18,19,20 restriction of study population to otherwise healthy children,18 and propensity score methods.51,52,53,54,55,56 Furthermore, the null findings in the negative control safety analyses suggest that residual confounding was minimal.33 Second, cohort eligibility was based on diagnosis codes and the presence or absence of a same-day antibiotic prescription dispensing, but we cannot rule out potential misclassification of viral infections as bacterial infections, or vice versa, because of misdiagnosis by health care providers. For example, children with nonsuppurative OM, a viral condition for which antibiotics are not indicated, had lower health care expenditures if they inappropriately received an antibiotic. This finding is likely because of misidentification or incorrect coding by health care professionals of suppurative OM as nonsuppurative OM. Third, we did not account for history of antibiotic allergies or intolerances; therefore, some antibiotics deemed inappropriate may have been misclassified. Fourth, our short-term individual-level estimates of attributable health care expenditures are conservative since they do not incorporate over-the-counter treatments for ADEs or downstream medical consequences of antibiotic exposure (eg, antibiotic-resistant infections, eczema).4,57,58 Fifth, our national expenditure results are underestimates because they only account for children with commercial insurance (approximately 55% of the national pediatric population)59 and are further limited to recipients of same-day antibiotics (ie, not delayed antibiotic prescriptions). Furthermore, MarketScan is limited to commercially insured children and also overrepresents residents from the South and underrepresents residents of the West; thus, results may not be generalizable to other populations.60
Conclusions
This national study underscores the negative health and financial consequences associated with inappropriate antibiotic prescriptions to treat common outpatient bacterial and viral infections in children. These findings are critical to inform decisions by health care stakeholders—including patient advocacy groups, public and private payers, and health care administrators—to implement widespread antimicrobial stewardship activities in outpatient settings to reduce antibiotic-related harms and expenditures.
eMethods. Definition of Inappropriate Antibiotic Duration for Bacterial Infections, Statistical Analysis
eTable 1. Diagnosis Codes to Identify Eligible Patients for Pediatric Cohorts
eTable 2. Medications to Identify Pediatric Patients for Exclusion
eTable 3. Codes to Identify Pregnancy, Mechanical Ventilation, Hematologic or Solid Organ Malignant Neoplasms, and Hematologic or Immunologic Conditions for Exclusion
eTable 4. Codes to Identify Pediatric Patients with Viral or Bacterial Infections for Exclusion
eTable 5. Medications to Identify Index Oral Antibiotic Treatment
eTable 6. Codes and Timing to Identify Adverse Drug Events for Comparative Safety Analyses
eTable 7. Codes to Identify Baseline Characteristics
eTable 8. Diagnosis Codes to Identify Elixhauser Comorbidities
eTable 9. Distribution of Index Antibiotic Agents Prescribed to Children by Infection Type
eTable 10. Additional Selected Baseline Characteristics of Children Diagnosed with Infections of Interest
eTable 11. Number of Exclusions For Adverse Drug Event Outcomes That Occurred Within 30 Days Prior to the Index Date
eTable 12. Unadjusted and Propensity Score–Weighted Hazard Ratio Estimates of Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Pediatric Patients
eTable 13. Inverse Probability Of Treatment–Weighted 30-Day All Cause and Adverse Drug Event–Related Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children by Setting
eTable 14. Inverse Probability of Treatment–Weighted 30-Day Adverse Drug Event–Related Health Care Utilization and Total Per-Patient Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children
eTable 15. Total 30-Day Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions in 2017 Pediatric MarketScan Study Population, Age 6 Months to 17 Years
eTable 16. Confidence Intervals for Annual National Attributable 30-Day Expenditures of Inappropriate Antibiotic Prescriptions Among the US Commercially Insured Population, Age 6 Months to 17 Years
eTable 17. Baseline Characteristics of Children Diagnosed with a Noninfectious Clinical Condition
eTable 18. Distribution of Index Antibiotic Agents Prescribed to Children by Noninfectious Clinical Condition
eTable 19. Number of Exclusions For Adverse Drug Event Outcomes That Occurred Within 30 Days Prior to the Index Date by Noninfectious Clinical Condition
eTable 20. Unadjusted and Propensity Score–Weighted Hazard Ratio Estimates of Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Pediatric Patients by Noninfectious Clinical Condition
eTable 21. Inverse Probability of Treatment–Weighted 30-Day Health Care Utilization and All-Cause and Adverse Drug Event–Related Total Per-Patient and Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children by Noninfectious Clinical Condition
eTable 22. Total Attributable Expenditures of Inappropriate Antibiotic Prescriptions Among Children by Noninfectious Clinical Condition
eTable 23. Sensitivity Analyses for Inverse Probability of Treatment–Weighted All-Cause Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children by Condition
eFigure 1. Derivation of Pediatric Infection Cohort in MarketScan Commercial Database (Index Events April 1, 2016, to September 30, 2018)
eFigure 2. Standardized Mean Differences of Patient- and Provider-Level Characteristics Between Treatment Groups, in the Unweighted and Weighted Pediatric Populations, for Acute Kidney Failure Outcome Cohort
eFigure 3. Propensity Score–Weighted Hazard Ratio Estimates of Additional Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Pediatric Patients
eFigure 4. Standardized Mean Differences of Patient- and Provider-Level Characteristics Between Treatment Groups, in the Unweighted and Weighted Populations of Children with Asthma and Allergy or Asthma Exacerbation, for Acute Kidney Failure Safety Outcome Cohort
eFigure 5. Propensity Score–Weighted Hazard Ratio Estimates of Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Asthma or Allergy and Asthma Exacerbation Pediatric Cohorts
eFigure 6. Weighted 30-Day Attributable Expenditures of Inappropriate Antibiotic Prescriptions for Asthma or Allergy and Asthma Exacerbation Pediatric Cohorts
eReferences.
References
- 1.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi: 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 2.The Pew Charitable Trusts. Health experts establish national targets to improve outpatient antibiotic selection. October 24, 2016. Accessed November 15, 2021. https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2016/10/health-experts-establish-national-targets-to-improve-outpatient-antibiotic-selection
- 3.Hersh AL, Fleming-Dutra KE, Shapiro DJ, Hyun DY, Hicks LA; Outpatient Antibiotic Use Target-Setting Workgroup . Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176(12):1870-1872. doi: 10.1001/jamainternmed.2016.6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States: 2019. Revised December 2019. Accessed April 19, 2022. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- 5.Dantes R, Mu Y, Hicks LA, et al. Association between outpatient antibiotic prescribing practices and community-associated Clostridium difficile infection. Open Forum Infect Dis. 2015;2(3):ofv113. doi: 10.1093/ofid/ofv113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovegrove MC, Geller AI, Fleming-Dutra KE, Shehab N, Sapiano MRP, Budnitz DS. US emergency department visits for adverse drug events from antibiotics in children, 2011-2015. J Pediatric Infect Dis Soc. 2019;8(5):384-391. doi: 10.1093/jpids/piy066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA. 2016;316(20):2115-2125. doi: 10.1001/jama.2016.16201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua KP, Fischer MA, Linder JA. Appropriateness of outpatient antibiotic prescribing among privately insured US patients: ICD-10-CM based cross sectional study. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misurski DA, Lipson DA, Changolkar AK. Inappropriate antibiotic prescribing in managed care subjects with influenza. Am J Manag Care. 2011;17(9):601-608. [PubMed] [Google Scholar]
- 10.Tsuzuki S, Kimura Y, Ishikane M, Kusama Y, Ohmagari N. Cost of inappropriate antimicrobial use for upper respiratory infection in Japan. BMC Health Serv Res. 2020;20(1):153. doi: 10.1186/s12913-020-5021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IBM Watson Health . IBM MarketScan Research Databases for life sciences researchers. Accessed April 22, 2022. https://www.ibm.com/downloads/cas/0NKLE57Y
- 12.Centers for Medicare & Medicaid Services . ICD-10-CM and ICD-10 PCS and GEMs Archive. Updated May 17, 2018. Accessed September 16, 2021. https://www.cms.gov/Medicare/Coding/ICD10/Archive-ICD-10-CM-ICD-10-PCS-GEMs
- 13.Dubberke ER, Olsen MA, Stwalley D, et al. Identification of Medicare recipients at highest risk for Clostridium difficile infection in the US by population attributable risk analysis. PLoS One. 2016;11(2):e0146822. doi: 10.1371/journal.pone.0146822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee on Quality Assurance. Antibiotic utilization (ABX). Accessed April 22, 2022. https://www.ncqa.org/hedis/measures/antibiotic-utilization/
- 15.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964-e999. doi: 10.1542/peds.2012-3488 [DOI] [PubMed] [Google Scholar]
- 16.Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):1279-1282. doi: 10.1093/cid/cis847 [DOI] [PubMed] [Google Scholar]
- 17.Chow AW, Benninger MS, Brook I, et al. ; Infectious Diseases Society of America . IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72-e112. doi: 10.1093/cid/cis370 [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss S, Patrick AR, Stürmer T, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10)(suppl 2):S131-S142. doi: 10.1097/MLR.0b013e318070c08e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221-228. doi: 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Arcy M, Stürmer T, Lund JL. The importance and implications of comparator selection in pharmacoepidemiologic research. Curr Epidemiol Rep. 2018;5(3):272-283. doi: 10.1007/s40471-018-0155-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones G, Taright N, Boelle PY, et al. Accuracy of ICD-10 codes for surveillance of Clostridium difficile infections, France. Emerg Infect Dis. 2012;18(6):979-981. doi: 10.3201/eid1806.111188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bann MA, Carrell DS, Gruber S, et al. Identification and validation of anaphylaxis using electronic health data in a population-based setting. Epidemiology. 2021;32(3):439-443. doi: 10.1097/EDE.0000000000001330 [DOI] [PubMed] [Google Scholar]
- 23.Butler AM, Durkin MJ, Keller MR, Ma Y, Powderly WG, Olsen MA. Association of adverse events with antibiotic treatment for urinary tract infection. Clin Infect Dis. Published online July 19, 2021. doi: 10.1093/cid/ciab637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Labor, Bureau of Labor Statistics . Consumer Price Index. Accessed April 21, 2020. https://beta.bls.gov/dataQuery/find?fq=survey:%5bcu%5d&s=popularity:D&q=medical+care
- 25.IBM Watson Health . IBM Micromedex RED BOOK(R) Flat File. Accessed August 1, 2021. https://www.ibm.com/products/micromedex-red-book
- 26.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 27.Agency for Healthcare Research and Quality . Elixhauser comorbidity software, version 3.7. Healthcare Cost and Utilization Project (HCUP). Accessed December 21, 2020. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp
- 28.Stürmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution—a simulation study. Am J Epidemiol. 2010;172(7):843-854. doi: 10.1093/aje/kwq198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stürmer T, Webster-Clark M, Lund JL, et al. Propensity score weighting and trimming strategies for reducing variance and bias of treatment effect estimates: a simulation study. Am J Epidemiol. 2021;190(8):1659-1670. doi: 10.1093/aje/kwab041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074-1078. doi: 10.1080/01621459.1989.10478874 [DOI] [Google Scholar]
- 32.van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HG, Stricker BH. Fluoroquinolones and risk of Achilles tendon disorders: case-control study. BMJ. 2002;324(7349):1306-1307. doi: 10.1136/bmj.324.7349.1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipsitch M, Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383-388. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deb P, Norton EC. Modeling health care expenditures and use. Annu Rev Public Health. 2018;39:489-505. doi: 10.1146/annurev-publhealth-040617-013517 [DOI] [PubMed] [Google Scholar]
- 35.Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897-916. doi: 10.1002/hec.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465-488. doi: 10.1016/j.jhealeco.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 37.Park RE. Estimation with heteroscedastic error terms. Econometrica. 1966;34(4):888. doi: 10.2307/1910108 [DOI] [Google Scholar]
- 38.Efron B, Tibshirani R. An Introduction to the Bootstrap. Chapman & Hall; 1994. doi: 10.1201/9780429246593 [DOI] [Google Scholar]
- 39.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? comparing methods of modeling Medicare expenditures. J Health Econ. 2004;23(3):525-542. doi: 10.1016/j.jhealeco.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 40.Gerber JS, Ross RK, Bryan M, et al. Association of broad- vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318(23):2325-2336. doi: 10.1001/jama.2017.18715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Matusiak LM, Schumock GT. Antibiotic expenditures by medication, class, and healthcare setting in the United States, 2010-2015. Clin Infect Dis. 2018;66(2):185-190. doi: 10.1093/cid/cix773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Danziger LH. A national evaluation of antibiotic expenditures by healthcare setting in the United States, 2009. J Antimicrob Chemother. 2013;68(3):715-718. doi: 10.1093/jac/dks445 [DOI] [PubMed] [Google Scholar]
- 43.Mold JW, Stein HF. The cascade effect in the clinical care of patients. N Engl J Med. 1986;314(8):512-514. doi: 10.1056/NEJM198602203140809 [DOI] [PubMed] [Google Scholar]
- 44.Ganguli I, Simpkin AL, Lupo C, et al. Cascades of care after incidental findings in a US national survey of physicians. JAMA Netw Open. 2019;2(10):e1913325-e1913325. doi: 10.1001/jamanetworkopen.2019.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riedle BN, Polgreen LA, Cavanaugh JE, Schroeder MC, Polgreen PM. Phantom prescribing: examining the frequency of antimicrobial prescriptions without a patient visit. Infect Control Hosp Epidemiol. 2017;38(3):273-280. doi: 10.1017/ice.2016.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Healthcare Infection Control Practices Advisory Committee . Antibiotic Stewardship Statement for Antibiotic Guidelines—The Recommendations of the Healthcare Infection Control Practices Advisory Committee. HICPAC; 2016. [Google Scholar]
- 47.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. doi: 10.15585/mmwr.rr6506a1 [DOI] [PubMed] [Google Scholar]
- 48.Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. doi: 10.1001/jama.2019.13978 [DOI] [PubMed] [Google Scholar]
- 49.Speer M, McCullough JM, Fielding JE, Faustino E, Teutsch SM. Excess medical care spending: the categories, magnitude, and opportunity costs of wasteful spending in the United States. Am J Public Health. 2020;110(12):1743-1748. doi: 10.2105/AJPH.2020.305865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002-2010. Pediatrics. 2012;130(1):23-31. doi: 10.1542/peds.2011-2879 [DOI] [PubMed] [Google Scholar]
- 51.Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006;35(2):345-352. doi: 10.1093/ije/dyi275 [DOI] [PubMed] [Google Scholar]
- 52.Nelson JC, Jackson ML, Weiss NS, Jackson LA. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among seniors. J Clin Epidemiol. 2009;62(7):687-694. doi: 10.1016/j.jclinepi.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 53.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348-354. doi: 10.1093/aje/kwm070 [DOI] [PubMed] [Google Scholar]
- 54.Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59-66. doi: 10.1002/pds.3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512-522. doi: 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604-611. doi: 10.1161/CIRCOUTCOMES.113.000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsakok T, McKeever TM, Yeo L, Flohr C. Does early life exposure to antibiotics increase the risk of eczema? a systematic review. Br J Dermatol. 2013;169(5):983-991. doi: 10.1111/bjd.12476 [DOI] [PubMed] [Google Scholar]
- 58.Aversa Z, Atkinson EJ, Schafer MJ, et al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin Proc. 2021;96(1):66-77. doi: 10.1016/j.mayocp.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaiser Family Foundation . Health insurance coverage of children 0-18. Accessed September 24, 2021. https://www.kff.org/other/state-indicator/children-0-18/
- 60.Butler AM, Nickel KB, Overman RA, Brookhart MA. IBM MarketScan Research Databases. In: Sturkenboom MC, Schink T, eds. Databases for Pharmacoepidemiological Research. Springer; 2021:243-251. doi: 10.1007/978-3-030-51455-6_20 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Definition of Inappropriate Antibiotic Duration for Bacterial Infections, Statistical Analysis
eTable 1. Diagnosis Codes to Identify Eligible Patients for Pediatric Cohorts
eTable 2. Medications to Identify Pediatric Patients for Exclusion
eTable 3. Codes to Identify Pregnancy, Mechanical Ventilation, Hematologic or Solid Organ Malignant Neoplasms, and Hematologic or Immunologic Conditions for Exclusion
eTable 4. Codes to Identify Pediatric Patients with Viral or Bacterial Infections for Exclusion
eTable 5. Medications to Identify Index Oral Antibiotic Treatment
eTable 6. Codes and Timing to Identify Adverse Drug Events for Comparative Safety Analyses
eTable 7. Codes to Identify Baseline Characteristics
eTable 8. Diagnosis Codes to Identify Elixhauser Comorbidities
eTable 9. Distribution of Index Antibiotic Agents Prescribed to Children by Infection Type
eTable 10. Additional Selected Baseline Characteristics of Children Diagnosed with Infections of Interest
eTable 11. Number of Exclusions For Adverse Drug Event Outcomes That Occurred Within 30 Days Prior to the Index Date
eTable 12. Unadjusted and Propensity Score–Weighted Hazard Ratio Estimates of Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Pediatric Patients
eTable 13. Inverse Probability Of Treatment–Weighted 30-Day All Cause and Adverse Drug Event–Related Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children by Setting
eTable 14. Inverse Probability of Treatment–Weighted 30-Day Adverse Drug Event–Related Health Care Utilization and Total Per-Patient Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children
eTable 15. Total 30-Day Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions in 2017 Pediatric MarketScan Study Population, Age 6 Months to 17 Years
eTable 16. Confidence Intervals for Annual National Attributable 30-Day Expenditures of Inappropriate Antibiotic Prescriptions Among the US Commercially Insured Population, Age 6 Months to 17 Years
eTable 17. Baseline Characteristics of Children Diagnosed with a Noninfectious Clinical Condition
eTable 18. Distribution of Index Antibiotic Agents Prescribed to Children by Noninfectious Clinical Condition
eTable 19. Number of Exclusions For Adverse Drug Event Outcomes That Occurred Within 30 Days Prior to the Index Date by Noninfectious Clinical Condition
eTable 20. Unadjusted and Propensity Score–Weighted Hazard Ratio Estimates of Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Pediatric Patients by Noninfectious Clinical Condition
eTable 21. Inverse Probability of Treatment–Weighted 30-Day Health Care Utilization and All-Cause and Adverse Drug Event–Related Total Per-Patient and Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children by Noninfectious Clinical Condition
eTable 22. Total Attributable Expenditures of Inappropriate Antibiotic Prescriptions Among Children by Noninfectious Clinical Condition
eTable 23. Sensitivity Analyses for Inverse Probability of Treatment–Weighted All-Cause Attributable Expenditure Estimates of Inappropriate Antibiotic Prescriptions Among Children by Condition
eFigure 1. Derivation of Pediatric Infection Cohort in MarketScan Commercial Database (Index Events April 1, 2016, to September 30, 2018)
eFigure 2. Standardized Mean Differences of Patient- and Provider-Level Characteristics Between Treatment Groups, in the Unweighted and Weighted Pediatric Populations, for Acute Kidney Failure Outcome Cohort
eFigure 3. Propensity Score–Weighted Hazard Ratio Estimates of Additional Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Pediatric Patients
eFigure 4. Standardized Mean Differences of Patient- and Provider-Level Characteristics Between Treatment Groups, in the Unweighted and Weighted Populations of Children with Asthma and Allergy or Asthma Exacerbation, for Acute Kidney Failure Safety Outcome Cohort
eFigure 5. Propensity Score–Weighted Hazard Ratio Estimates of Adverse Drug Events Following Inappropriate vs Appropriate Antibiotic Prescriptions Among Asthma or Allergy and Asthma Exacerbation Pediatric Cohorts
eFigure 6. Weighted 30-Day Attributable Expenditures of Inappropriate Antibiotic Prescriptions for Asthma or Allergy and Asthma Exacerbation Pediatric Cohorts
eReferences.