Abstract
Every day we march closer to finding the cure for multiple myeloma. The myeloma cells inflict their damage through specialized cellular meshwork and cytokines system. Implicit in these interactions are cellular adhesion molecules and their regulators which include but are not limited to integrins and syndecan-1/CD138, immunoglobulin superfamily cell adhesion molecules, such as CD44, cadherins such as N-cadherin, and selectins, such as E-selectin. Several adhesion molecules are respectively involved in myelomagenesis such as in the transition from the precursor disorder monoclonal gammopathy of undetermined significance to indolent asymptomatic multiple myeloma (smoldering myeloma) then to active multiple myeloma or primary plasma cell leukemia, and in the pathological manifestations of multiple myeloma.
Keywords: : bone marrow microenvironment, cellular adhesion molecules, drug resistance, monoclonal antibody, multiple myeloma, targeted therapies
Practice points.
Cell adhesion molecules (CAMs) are a subcategory of cell adhesion proteins located on the cell surface involved in cell–matrix interactions and cell–cell interactions.
CAMs have been shown to play a vital role in the interactions between multiple myeloma (MM) cells and bone marrow (BM) cells; this interaction promotes proliferation and the survival of malignant plasma cells (MPC).
MM cells can circulate, extravasate and then migrate back to the BM, a process mediated by numerous chemotactic factors and adhesion molecules.
CAMs are strongly expressed by BM plasma cells (PC), but some adhesion molecules were found to be more significantly expressed in MPC in comparison to normal PC.
Adhesion molecules like VLA-4 are involved in myeloma bone disease (MBD) through interactions with osteoblasts and osteoclasts which makes them interesting therapeutic targets in that case.
CAM's expression is critical in the mechanisms underlying the adherence and detachment of PC from the bone marrow micro-environment (BMM), thus in the extramedullary spread of MPC; loss of CD56 expression on MPC is correlated with their capacity to disseminate and thrive outside of the BMM.
MM remains incurable in most patients, with drug resistance driving relapse. Adhesion of MM cells to the BM stromal cells and the composition of the BM micro-environment contribute to MM drug resistance.
CAMs play a role in the downregulation of the immune system by upregulating myeloid-derived suppressor cells and down-regulating dendritic cells and T cells.
Daratumumab (a monoclonal anti-CD38 antibody), Lenalidomide, Bortezomib and Natalizumab are important examples of drugs interfering with CAMs that have been developed for the treatment of MM.
More than 40 years have passed since the first definitive demonstrations of molecular activity facilitating cell-cell adhesion [1–3]. Cell adhesion molecules (CAMs) are a subcategory of cell adhesion proteins located on the cell surface involved in binding with other cells or with the extracellular matrix (ECM) [4]. Adhesion plays an integral role in cell communication, regulation and development and maintenance of tissues. Some of the stimulating signals CAMs are involved with include but are not limited to cell differentiation, cell cycle maintenance, cell migration and cell survival. According to the cell adhesion model, the greater the number of chemical bonds a cell has on its surface the greater the cell-to-cell interaction. This model is also referred to as the point attachment model since it reflects the contact area between the cell and the surface to be a small, homogeneous region that mediates the initial attachment of the cell surface [5]. In addition to their function as a ‘molecular glue,’ CAMs are integral in affecting cellular mechanisms such as growth, contact inhibition and apoptosis. Changes in cell adhesion can be the defining event in a wide range of diseases including arthritis [6,7], atherosclerosis [8,9], osteoporosis [10,11] and cancer [12–14]. Cell adhesiveness is greatly reduced in malignancies. Reduced intercellular adhesiveness allows cancer cells to disobey social order which leads to the destruction of histologic architecture, a benchmark of malignant tumors [14]. In malignancies, variations in cell-matrix and cell–cell interactions are cell type- and oncogene-specific [8]. In multiple myeloma (MM), not only do CAMs regulate the interaction between MM cells and osteoblasts, osteoclasts, but they also mediate the interaction between MM and bone marrow stromal cells (BMSC), lymphocytes and endothelial cells. A deeper knowledge of CAMs can also play a role in stratifying patients, developing and employing targeted therapies, and in advancing research into understanding and overcoming drug resistance [15].
Structure & families of CAMs
Structure
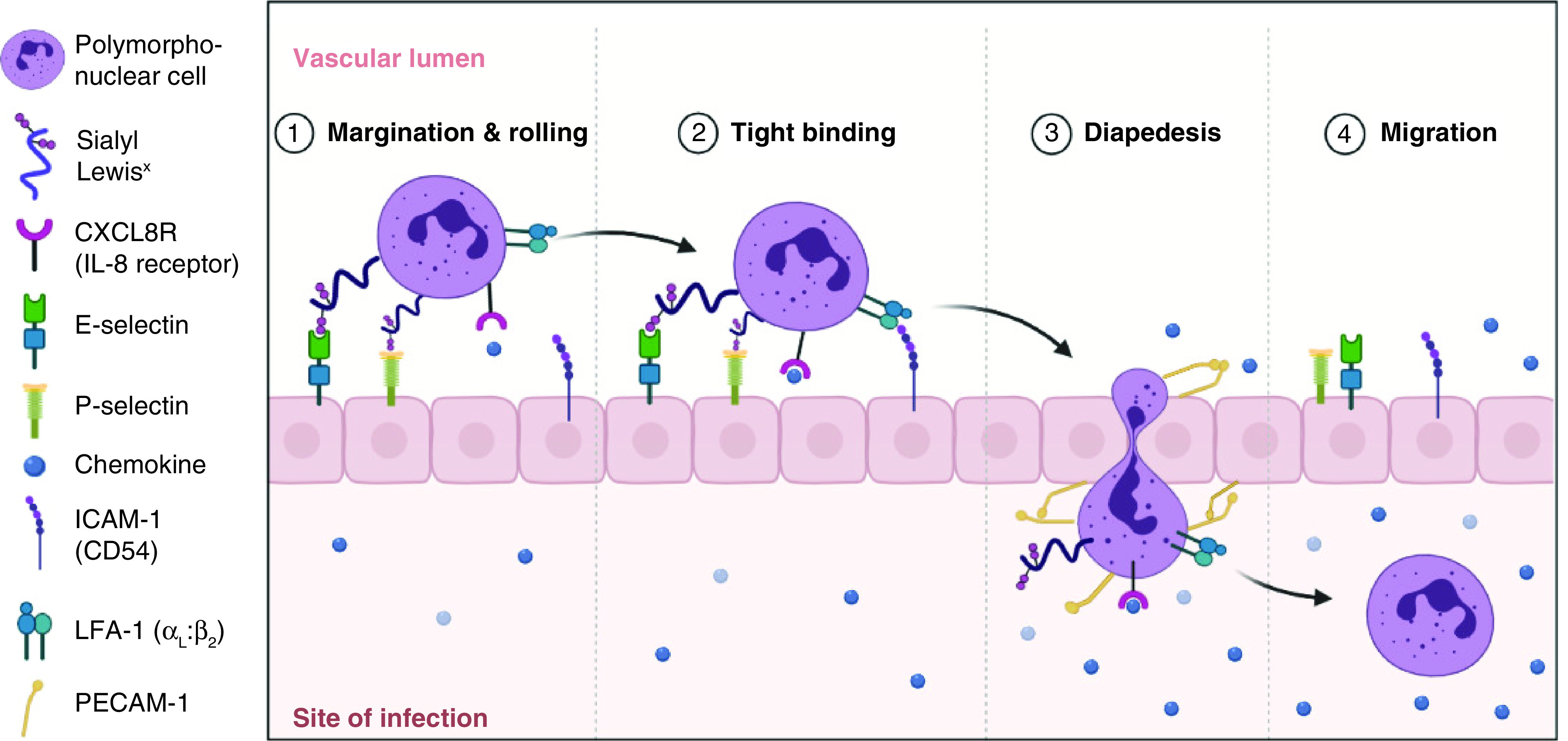
Composed of three conserved domains, CAMs are typically single-pass transmembrane receptors [16,17]. The first domain represents an intracellular portion that interacts with the cytoskeleton, the second is a transmembrane domain, and the third is an extracellular portion. When CAMs bind with the same CAMs, the binding is known as homophilic binding. On the other hand, when cell adhesion occurs between molecules of two neighboring cells that are not identical, this binding is known as heterophilic binding. In some cases, a heterophilic binding can occur between CAMs and the ECM meshwork such as collagen fibers, fibronectin protein dimers and polysaccharides. CAMs have broad functions. One of which is depicted in Figure 1.
Figure 1. . The process of leukocyte extravasation and the role of cell adhesion molecules.
Families of CAMs
There are four major superfamilies or groups of CAMs: Immunoglobulin super family CAMs (IgCAMs), cadherins, integrins and the superfamily of C-type lectin-like domains' proteins (CTLDs). According to Brackenbury et al., three criteria have been utilized to distinguish among systems of embryonic cell adhesion: dependence on Ca2+, binding specificity, and the involvement of cell-surface molecules. CAMs can be divided into calcium-independent or calcium-dependent molecules [18]. Integrins and the IgCAMs belong to the latter while CTLDs and selectins belong to the former. The integrins play a role in cell–matrix interactions, while the rest of the CAMs participate in cell–cell interactions [16].
CAMs in myelomagenesis
The proliferation of myeloma cancer cells and the acquisition of an invasive profile is related to integrin clustering. This is mediated by the rearrangement of cytoskeletal proteins and the recruitment of molecules involved in the signaling cascade, as well as the phosphorylation of pp60 Src and focal adhesion kinase [19]. The role of CAMs in MM pathophysiology is primarily related to the homing of tumor cells in the BM, which in turn aids in the recirculation of the malignant plasma cells (PC) and stimulates growth factor production [20]. Moreover, in patients with MM, drastic changes in the stroma of the BM have been observed. CAMs have been shown to play a vital role in the interactions between MM cells and BM cells; this interaction promotes proliferation and the survival of malignant plasma cells (MPC) [21,22]. Table 1 summarizes the expression of CAMs on normal cells compared with their expression on MM cells.
Table 1. . Cell adhesion molecules phenotypic expression on normal versus myeloma plasma cells.
| Plasma cell CAMs expressed | Normal plasma cell expression | Myeloma plasma cell expression |
|---|---|---|
| H-CAM (CD44) | Weak express + | Weak express + |
| VLA-4 (CD49d/CD29) | Weak expression + | Weak expression + |
| ICAM-1 (CD54) | Strong expression ++ | Strong expression ++ |
| N-CAM (CD56) | Weak/absent expression +/- | • Strong expression ++ • Absent expression in extramedullary involvement- |
| VLA-5- immature (CD49e/Cd29)† | Weak expression + | Absent/weak expression-/+ |
| VLA-5+ mature (CD49e/CD29)† | Weak expression + | Absent/weak expression |
| CD38 | Very strong expression +++ | Very strong expression +++ |
| CD19 | Strong expression ++ | Absent/weak expression-/+ |
| LFA-1 (CD11a) | Strong expression ++ | Weak expression + |
| CD40 | Weak expression + | Weak expression + |
| LFA-3 (CD58) | Absent expression - | Strong expression ++ |
| Syndecan | Strong presentation ++ | Expression on small fraction |
| VLA-2 (CD49b) | Absent expression | Weak/moderate expression +/++ |
| E-Selectin | Absent expression | Increased expression in progressing MM +++ |
| CXCR4 | Weak expression + | Strong expression ++ |
| MAC-1 | Absent expression | Strong expression ++ |
| N-Cadherin | Strong expression ++ | Strong expression in newly diagnosed patients ++ |
| E- Cadherin | Strong expression + | Strong expression ++ |
| PSGL-1 | Strong expression ++ | Very strong expression ++ |
Integrins
Integrins are heterodimeric membrane glycoproteins that are usually found on surfaces of diverse cell types. They act as the primary receptors that mediate the interaction between the extracellular matter and cell-cell adhesion molecules. Very late antigens such as the β1 integrin and in particular Very Late Appearing Antigen (VLA)-4 (CD49d) and VLA-5 (CD49e) have been prominent in MM cell lines and patient-derived MPCs [20]. The latter suggests that VLA-4 is one of the main integrins allowing MPCs to adhere to fibronectin through Arg-Gly-Asp dependent mechanisms in monoclonal gammopathy of undetermined significance [20,24]. Integrins are composed of α and β units. The α4 subunit was observed to be twice the level of β1 subunit in normal peripheral blood B lymphocytes which inherently express VLA-4. This suggests that the α4 subunits are not all necessarily functionally associated with the β1 subunit [20,25]. In contrast, MPCs express an equal amount of α4 and β1 subunits; inferring that MPC will possess a higher functional ability than normal peripheral blood B lymphocytes [20,23]. In addition, VLA-4 has been shown to play a role in facilitating migration and homing of MPCs to the BM stroma triggering an Interleukin (IL-6) secretion by the BM stroma [20,26–30]. VLA-5 (CD49e) expression by MPCs has been observed to vary as regards to fresh MPCs and human myeloma cell lines [20,31]. VLA-5 expression has been found to be upregulated upon contact with BM stroma [20]. A study by Kawano et al. recognized two subpopulations of MPCs in relation to VLA-5 expression. The first subpopulation is VLA-5 negative immature MPCs and the second subpopulation VLA-5+ mature MPCs [20,32]. VLA-negative immature MPCs have been observed to have a higher proliferative potential in addition to an increased responsiveness to IL-6, while VLA-5+ mature MPCs have a higher production of M protein [20,33].
The expression of other integrins in/by MPCs has also been reported. They include LFA-1, also known as lymphocyte function associated antigen, (CD11a/CD18) which was seen to be expressed on both human myeloma cell lines and MPCs. LFA-1 has been shown to be correlated with tumor cell growth [34]. In a study conducted by Ahsman et al., LFA-1 expression was seen to be upregulated in fulminant disease states and to be scarcely expressed in patients with a stable disease. The latter suggests that LFA-1 expression correlates with plasma cell labeling index. Recent evidence proved that blocking LFA-1 by monoclonal antibodies can drastically reduce IL-6 production from the BM stroma. With that being said, LFA-1 may be implicated in important MPCs proliferation and cell adhesion interactions [20,35]. One possible explanation is probably through induction of IL-6 secretion by the BM stroma [33,35]. Similarly to VLA-4, LFA-1 is a ligand involved in B lymphocyte adhesion to dendritic cells (DCs) [20]. In a study conducted by Asosingh et al., LFA-1 negative cells were not able to cause disease in vivo, but LFA-1 cells expressing 5T33MMvt were able to induce disease in vivo [21,36]. Drug resistance in MM patients was associated with MPCs expressing LFA-1 and VLA-4 [21,37]. MAC-1 (CD11b/CD18) which is also known as Membrane Attack Complex Type 1 has also been shown to be expressed on MPCs. MAC-1 mediates leukocyte adhesion to endothelial cell which in turn permits extravasation [20,24]. MAC-1 expression has been observed on MPCs, yet VLA-5 negative immature MPCs do not express MAC-1. Riet et al., found a weak expression of VLA-6 which is a laminin receptor and CD51 which is a vitronectin receptor in malignant myeloma cells [23,38].
Immunoglobulin superfamily CAMs
IgCAMs have different numbers of immunoglobulin domains. A large part of the IgCAMs members control cell behavior by acting as signal transducing receptors.
Pellat-Deceunynck et al. found that NCAM, neural cell adhesion molecule, (CD56) is strongly expressed on MPCs of patients with MM compared with normal PCs [20,38–44]. Research has suggested that the surface expression of NCAM in MPCs is controlled at the transcriptional level. This is due to the fact that some human myeloma cell lines have shown the absence on NCAM on their surface yet tested positive for the presence of its mRNA [20]. CD56 is an important marker because its increased expression promotes adhesion of neoplastic cells to osteoblastic cells [40]. A downregulation in the expression of NCAM, VLA-5 and CD11a with an increase in HCAM, homing cell adhesion molecule, (CD44) has been seen in patients with extramedullary spread of MPCs. The former features portray that NCAM could possibly aid in the localization of MM tumor cells to the BM stroma. HCAM may play a role in the passage of cells to the peripheral circulation [45,46]. Moreover, the down regulation of NCAM has been shown to facilitate the progression of MPC to extramedullary sites [20]. HCAM (CD44) and RHAMM, receptor for HA mediated motility, are hyaluronan receptors of MM cells [47]. HCAM (CD44) allows the interaction between malignant cells and the BM stroma, which increases IL-6 production and cancer proliferation. The use of monoclonal antibodies to CD44 has been shown to decrease IL-6 production by stromal cells, demonstrating the importance of the CD44/hyaluronate interaction [48].
MAC-1 is an integrin involved in leukocyte adhesion to the endothelium. It is not found in normal PCs, but rather in myeloma cells. Its functional role is not well understood [24]. LFA-3 (CD58) is expressed on MPCs while it is absent on normal PCs [20,32,41,44]. ICAM-1, Intracellular adhesion molecule-1, (CD54) is strongly expressed on MPCs, but it is also present on normal PCs. This highlights CD54 involvement in the physiological process of homing normal PCs to the BM [32,38,41,42,49,50]. Xu et al., coined ALCAM, activated leukocyte cell adhesion molecule, (CD166) as a possible CAM which helps in malignant progression of MM [39]. CD166 is highly expressed in MM cell lines and primary BM cells from MM patients [39]. Moreover, myeloma cells homed more efficiently to the BM in immunodeficient mice expressing CD166 compared with those without it [39]. Last, a co-expression of ICAM-1 and LFA-1 was observed on 50% of the MPCs by Kawano et al., and this was associated with the presence of in vitro homotypic aggregates [23,51].
Cadherins
N-Cadherin plays an important role in regulating MM cell proliferation and ensuring its viability [52]. A study conducted by Sadler et al., showed that when N-Cadherin is inhibited, MM cells were seen to increasingly proliferate; yet the addition of N-Cadherin antagonist peptide led to the death of MM adherent cells [52]. Studies conducted by Vandyke et al. and Groen et al., showed that in 50% of newly diagnosed MM patients N-Cadherin expression is upregulated on PCs [53]. Moreover, the previously mentioned studies have shown that circulating N-Cadherin, which has a direct correlation with expressed cadherin on MPCs, are upregulated in 30% of newly diagnosed MM patients [53]. N-cadherin-mediated-cell-substrate or homotypic cell-cell adhesion has shown no contribution to myeloma growth in vitro [54]. In-vivo N-cadherin showed direct mediation of the bone marrow (BM) retention and localization of myeloma cells [54]. To add, N-cadherin in vivo facilitated an interaction between N-cadherin positive osteoblasts and myeloma cells [54]. Moreover, plasmacytoid dendritic cells (pDCs) have been shown to promote tumor growth and aid in immune suppression in MM [55]. When CpG was added to human pDCs they were activated and resulted in apoptosis of myeloma cells, yet direct contact of CpG with the myeloma cells resulted in the conversion of pDCs into tumor promoting cells [55]. E-Cadherin, which is expressed on myeloma cells and pDC cells was seen to help in the mediation of the interactions [55].
Selectins
Selectins play a role in extravasation and homing of leukocytes to target organs. PSGL-1, p-selectin glycoprotein ligand-1, is the major ligand of P-selectin in neoplastic PCs and is expressed in high levels on normal and MPCs [56]. PSGL-1 was shown to have a role in the growth regulation, dissemination and contributed to the development of drug resistant in MM in relation to the bone marrow microenvironment (BMM) [57]. PSGL-1 is highly expressed on MM cells and regulates the homing and adhesion of MM cells to the microenvironment [57]. Moreover, activation of some integrins and downstream signaling was found to be regulated by PSGL-1. The latter interaction helps in regulation the proliferation of MM cell in the BMM and confers drug resistance [57].
E-Selectin has shown a role in homing and retention of MM cells in the BM [31,58]. Structures such as Sialyl Lewis A which are recognized by E-Selectin have been shown the help MM cells escape cytotoxic effects of bortezomib [58]. In a study conducted by Alexandrakis et al., E-selectin was seen to be significantly increased with progression MM to advanced stages [59]. Moreover, a positive correlation was observed between IL-6, ICAM-1 and E-selectin [59]. The majority of MM patients relapse and become refractory to therapy since the tumor microenvironment plays a supportive role in development of drug resistance and spread [60]. A study which tested the effect of blocking E-selectin and CXCR4 using GMI-127 and GMI-1359 in order to sensitize MM cells to chemotherapy, found that decreasing the expression of E-selectin, and thus CXCR-4 mediated adhesion and MM cells chemotaxis, made the tumor cells more sensitive to treatment in vivo [60].
Role of CAMs in MM
CAMs in disease dissemination
Recent data has shown promising results in the role of CAMs for MM treatments, and possible cure. Understanding CAMs expression in MM at the molecular level is the first step toward devising a potential cure. MPCs have the ability to home in the bone BMM, supplementing myeloma cells with the optimal entourage for mediation of clonal proliferation and differentiation [20]. During the disease, the survival and proliferation of MM cells mainly rely on the BMM. The reciprocal interactions between the cancerous cells and the BM allow not only these cells' growth but also facilitate inhibition of apoptosis, angiogenesis and disease progression [61]. One of the particularities of MM is the presence of a significant amount of MPC within the BM. However, small amounts of MM cells can also be detected in the bloodstream called circulating plasma cells [61]. This implicates that MM cells can circulate, extravagate, and then migrate back to the BM. This multistep process is mediated by numerous chemotactic factors and adhesion molecules and implicates adhesion to the endothelium, followed by invasion of the subendothelial membrane and finally migration within the stroma [62]. At later stages of the disease, MM cells start to become stroma-independent and proliferate even in the absence of BMM due to the development of autocrine growth supporting loops [63,64]. Thus, an increasing number of extramedullary cells is observed, and extramedullary spread is common. This process is the result of molecular alterations involving adhesion molecules and chemokine receptor expression. The following summarizes the recent progress in the role of adhesion molecules in MM BM homing and extramedullary spread.
With the use of murine 5T MM model, Vanderkerken K et al. demonstrated that the specific localization of MM cells in the BM derives from a combination of selective homing and appropriate BMM [65]. Intravenously injected 5T2 MM cells (5T2MM) and 5T33 MM cells (5T33MM) migrate selectively to the BM, the liver, and the spleen. Interestingly, these cells only survive in the BM and the spleen [65,66]. This multistep homing process implies that appropriate cell surface receptors are found on MM cells allowing them to adhere and then traverse the endothelium as a first step [62]. To gain more insight into the subject, the expression of several adhesion molecules on human myeloma cell lines was analyzed [41,67–71]. These studies demonstrated that CAMs are strongly expressed by BM PC. The most relevant ones to note being the intercellular adhesion molecule ICAM-1 (CD54), the collagen-1 binding proteoglycan, the B cell differentiation antigen CD21, the lymphocyte homing receptor H-CAM (CD44), the fibronectin (FN) receptors called very late activation antigen-4 (VLA-4) (CD49d) and VLA-5 (CD49e), syndecan-1 (CD 138), and the plasma cell antigen MPC-1. Interestingly, a very similar PC phenotype was characterized in normal MM-free individuals, suggesting that these expressed adhesion molecules may also play a significant role in the normal BM homing process of PC. Nonetheless, some adhesion molecules were found to be more significantly expressed in MPC in comparison to normal PC. These include, the leukocyte adhesion molecule LFA-3 (CD58) [41] and the neural cell adhesion molecule N-CAM (CD56) [40]. Ahsmann EJM et al. also found that MPC can also express the lymphocyte function-associated antigen LFA-1 (CD11a/CD18) which was associated with tumor growth and homotypic tumor cell adhesion/aggregation [33]. The diverse expression of these different CAMs may suggest their direct implication in MPC homing and biology. However, to be biologically relevant, ligands of these receptors must be available within the tumor cell microenvironment. Therefore, Faid L et al. analyzed stromal layers of BM of MM patients and normal donors to find out which ligands are expressed. Results demonstrated that for most receptors found on the MPC surface, the corresponding ligands are actually found in the BM stroma [72]. As an example, it has been shown that MM cells strongly adhere to fibronectin using the very late activation antigen-4 (VLA-4) (CD49d) and to collagen-1 using syndecan-1 [71]. CD44 for its part, interacts with hyaluronic acid expressed on BM endothelium [73].
Furthermore, receptor-blocking antibodies against most of these CAMs (VLA-4, CD56, MPC-1, CD21) were found to block, but only partially, the adhesion of MM cells to the BM stroma, shedding the light on the presence of additional adhesion mechanism yet to be discovered [69,70]. Particular attention should also be given to the fibronectin receptor VLA-4. This CAM is functionally involved in the pre-B cell-stroma interactions which suggests a similar role in myeloma homing in the BM [27]. Moreover, it has been observed that VLA-4 – Fibronectin binding is an essential step that supports the IL6-mediated induction of PCs in normal BM since antibodies against VLA-4 were found to inhibit the secretion of IL6 in co-cultures of MM cells and stromal cells [27,35]. Finally, it is worth noting that some homing molecules could not be detected on myeloma PCs: the selectin molecule L-selectin and the collagen receptor VLA-2.
Myeloma bone disease
MM is a hematologic malignancy associated with poorer overall survival than other B neoplasia. This is partly explained by the presence of myeloma bone disease (MBD). MBD affects about two-thirds of patients and is characterized by the occurrence of pathological bone events such as hypercalcemia, pain, pathological fractures, and spinal cord compression. This involvement of the skeletal system increases the morbidity and mortality of the disease, significantly altering the patients' quality of life in the process [74–80]. Adhesion molecules are involved in MBD through interactions with osteoblasts and osteoclasts. This makes adhesion molecules interesting therapeutic targets for the alleviation of bone damage associated with MM [81].
Osteoclastic cell precursors carry the RANK (receptor activator of nuclear factor kappa-B) receptor on their surface. The binding of RANKL (receptor activator of nuclear factor kappa-B ligand) expressed by osteoblasts is necessary for RANK-RANKL interaction to allow osteoclast differentiation and the initiation of bone resorption [82]. RANKL is not the only ligand of RANK. BMSC produce osteoprotegerin (OPG) which binds to RANK and inhibits osteoclast differentiation and thus protects against osteolysis. In multiple myeloma, there is an increase in RANKL and a decrease in OPG, Leading to a consequent increase in osteoclastic activity and promotes osteolytic bone lesions and pathological bone events [83]. It is thought that interactions between MPCs and stromal cells through adhesion molecules represent the process underlying these changes in multiple myeloma patients [84,85]. The integrin VLA-4 present on the surface of myeloma cells binds to VCAM-1, vascular cell adhesion molecule-1, and fibronectin, facilitating the proliferation of these tumor cells [86]. Antibodies to the alpha 4 portion of VLA-4 prevent binding of myeloma cells to fibronectin and intact stroma [87]. Osteoclastic activity is stimulated by VLA-4 interaction with VCAM-1. This binding leads to the production of MIP-1 alpha and MIP-1 beta by MM cells. These chemokines are responsible for an increase in RANK-L production leading to osteoclast activation and bone resorption [54]. VLA-4 plays a crucial role in the occurrence of MBD. This has been demonstrated in studies blocking VLA-4 adhesion where a decrease in osteolytic events was noted [87]. Notch-1, a single-pass transmembrane receptor, is strongly present on the surface of B-malignancy cells and interacts with the cell-surface protein, Jagged-1, of BMSC. This stimulates cell survival and growth [88,89]. The resultant stimulation of the Notch signaling pathway leads to an increase in osteoclastic activity. This results in an increase in the number and size of osteoclasts, potentiating their resorptive effect on bone, and leading to MBD [90]. Cadherins play a role in the localization of myeloma cells in the BM in the vicinity of N-cadherin expressing osteoblasts. The presence of this cadherin is associated with a worse prognosis [54]. N-cadherin binds Lrp5, which blocks the Wnt signaling pathway and thus decreases osteoblastic bone formation. This increases the number of osteoblasts expressing N-cadherin, and a vicious cycle of pathological bone degradation is initiated [91]. Xu et al. demonstrated in their work that the adhesion molecule CD166 leads to cancer progression and the occurrence of bone damage in patients with multiple myeloma. CD166 acts in a dual manner in the genesis of MBD. This is related to a decrease in osteoblastogenesis. The expression of CD166 by MM cells leads to a suppression of RUNX2 the primary transcription factor in the differentiation of mesenchymal stem cells into osteoblasts in the BM. CD166 also increases RANK-L, potentiating osteoclastic differentiation and osteolytic events [39].
Extramedullary spread
During the course of MM, PC remains essentially located within the BM, and hardly ever spreads to other parts of the body, at the exception of the terminal stage of the disease during which patients develop spread at different extramedullary sites such as lung, liver, pleural fluid and ascites fluid [68,92]. Both the qualitative and quantitative expression of CAMs during the different stages of MM is critical in the mechanisms underlying the adherence and detachment of PC from the BMM [93]. Pellat-Deceunynck C et al. demonstrated that N-CAM (CD56) expression was normally seen on noncancerous BM PC, up-regulated on myeloma BM PC and, interestingly, down-regulated on PC of patients with extramedullary spread independently of their location (whether it was in the PB or in the BM). These findings seemingly indicate that the loss of CD56 expression on myeloma PC is correlated with their capacity to disseminate and thrive outside of the BMM. Furthermore, Pellat-Deceunynck C et al. also proved that osteoblastic cell lines interact with myeloma cells using CD56. Therefore, it is reasonable to say that the overexpression of CD56 on myeloma cells favors their adherence capacity within the BM while its downregulation favors the migration of myeloma cells in the PB [94]. On another note, many authors showed that the loss of VLA-4 (CD49d), VLA-5 (CD49e) and CD138 was statistically significant when comparing PC in disseminated disease and PC in BM localized disease [94–96]. Finally, Drucker L et al. and Tohami T et al., recently demonstrated that the expression of tetraspanins, a family of ubiquitously expressed cell membrane proteins with many fundamental biological functions such as adhesion, migration and proliferation, also plays a role in the invasive capacity of MM cells [96,97]. Generally speaking, tetraspanins are usually down-regulated in malignant diseases and the metastatic potential of numerous cancers has been inversely linked to the expression of tetraspanins [98]. In the case of MM, CD81 and CD82 tetraspanins are down-regulated in PC especially during end stage disease [96]. Additionally, the induction of CD81 and CD82 overexpression in these PC decreased cell motility and invasion potential [97]. Therefore, it is likely that the deregulation of tetraspanin expression on MPC at later stages of the disease also plays a role in the dissemination of the neoplastic cells and thus the development of extramedullary spread. To sum up, it can clearly be affirmed that adhesion molecules showed lower expression on PC of patients with disseminated disease when compared with PC of patients with BM localized disease.
Hypercoagulability in MM
Like all other cancers, MM is associated with a hypercoagulable state. There are several explanations for this. One explanation is related to adhesion molecules. Indeed, the endothelial damage induced by cancer cells or by chemotherapy causes an increase in the expression of adhesion molecules. This allows blood elements and tumor cells to adhere to the site of damage, leading to thrombosis through the secretion of thrombogenic substances [99]. IL-6 triggers the coagulation cascade and increases fibrinogen production. As previously mentioned, IL-6 production is partially mediated by adhesive interactions between malignant PCs and BM stromal cells [49,100].
CAMs in myeloma drug resistance
Although there is constant development of new and improved therapeutics, MM remains incurable in most patients, with drug resistance driving relapse. There is a crucial need for the elucidation and resolution of intrinsic and acquired drug resistance pathways. These pathways vary widely and involve apoptosis pathways, DNA damage and repair mechanisms, modifications in gene expression, epigenetic events, and more [101].
Moreover, cell adhesion-mediated drug resistance is a well described phenomenon wherein adhesion of MM cells to the BM stromal cells and the composition of the BM micro-environment contribute to MM drug resistance. The adhesion can be to the BM stroma, hematopoietic niche constituents, endothelial cells, and the adjacent extra-cellular matrix [86]. This can reduce the sensitivity to chemotherapy agents, for instance, by upregulation of members of the anti-apoptotic Bcl-2 family and of ABC drug transporters, considered in the multidrug resistance proteins family, among other mechanisms [102].
As previously mentioned, the chemokine receptors CXCR4, CD49d (ITGA4) and CD44, highly expressed on PCs, are the main agents of adhesion of MM cells to the BM. CXCR4 has been linked to bortezomib resistance and poor patient outcomes with bortezomib. CXCR4 may be an interesting diagnostic biomarker of response to treatment. In addition, reduced CXCR4 expression was linked with extramedullary disease in murine specimens. CXCR4 mRNA expression was directly observed in bortezomib-resistant MM cell lines but not bortezomib-sensitive MM cell lines [103]. AMD3100 is a CXCR4 inhibitor that was shown to enhanced multiple therapeutic agents' action on MM cell lines in vitro by disrupting the adhesion to BM stromal cells in a murine myeloma model [104].
Role of CAMs in Immune-microenvironment regulation
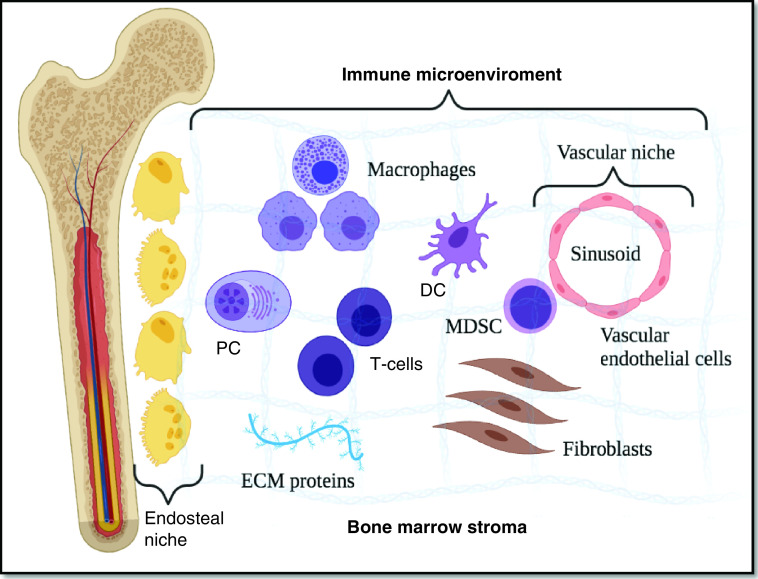
As previously mentioned, the neoplastic PC in MM vary from other B-cell malignancies by an almost exclusive homing to the BMM. The latter provides the adequate biological and physical support to induce clonal proliferation and differentiation. Anatomical and functional compartments, or niches, within the BM support different cellular populations, which together make up the BMM responsible for a sustained hematopoiesis. Classically, the BMM has been divided into endosteal and vascular niches set within a stroma of differentiated accessory or “stromal” cells, such as fibroblasts, osteoclasts, osteoblasts, adipocytes, endothelial cells, macrophages, and mast cells as well as ECM proteins consisting of a mixture of collagens, proteoglycans, and glycoproteins. As for the term ‘immune microenvironment’, it refers more to a functional compartment of differentiated immune cells located throughout the marrow stroma, rather than an anatomically distinct niche [105,106]. (Figure 2) Numerous fundamental changes and alterations in the BMM of MM patients are thought to promote growth of neoplastic progenitors. Focusing on the immune-microenvironment, it is now known that the evidence for the role of the immune system in the pathogenesis of MM is derived from a genetically humanized mouse model that demonstrated the microenvironment-dependent growth of malignant PCs [107]. The adhesion process occurring between MPC and both BM stromal cells and ECM proteins has been well-established. These adhesions can influence the growth, invasion, immune evasion and drug resistance of MM cells and are involved in lytic bone lesions and angiogenesis [102,108]. The following summarizes the recent progress in the role of adhesion molecules in MM tumor microenvironment and in particular, myeloma immune microenvironment.
Figure 2. . Overview of the bone marrow's immune microenvironment.
The bone marrow microenvironment is divided into endosteal, and vascular niches set within a stroma of differentiated accessory or ‘stromal’ cells, such as fibroblasts, osteoclasts, osteoblasts, adipocytes, endothelial cells, macrophages and mast cells as well as ECM proteins. As for the term ‘immune microenvironment’, it refers to a functional compartment of differentiated immune cells located throughout the marrow stroma.
DC: Dendritic cell; ECM: Extracellular matrix; MDSC: Myeloid-derived suppressor cell; PC: Plasma cell.
Adhesion molecules & T cells
T cells are crucial for the identification of tumor-specific antigens and for the killing of neoplastic cells [102]. CD8+ T cells are essential in protecting the host from malignant tumor growth [109]. High CD8+ T cell/Treg ratio in the tumor immune microenvironment has been associated with favorable prognosis in different human malignancies including MM [110,111]. Defects in T cell distribution and function have been reported in MM, including the decrease of CD4+ and CD8+T cell frequency, and abnormal Th1/Th2 ratio with impaired T cell responses [112]. As for immune checkpoint signaling it is upregulated in MM patients. Indeed, PD-1,programmed cell death protein-1, is overexpressed on T cells after activation and direct interaction with its ligand (PD-L1) on myeloma cells inhibits the T cell function by impairing proliferation and cytokine secretion [113]. Murine studies have shown that expression levels of integrin ligands on T cells directly impact T cell tumor infiltration. Fisher et al. demonstrated that ICAM-1 deficiency or blockade decreased CD8+ T cell infiltration into cancerous cells [114]. Furthermore, high expression of ICAM-1 and VCAM-1 on human studies, has been shown to correlate with higher density of CD8+ T cells also with prolonged disease free survival [115]. Also, Schmits et al. proved that LFA-1 deficiency in mice induced defects in CD8+ T cell and failure to reject immunogenic tumors [116]. In summary, integrins, integrin ligands and other cell adhesion molecules expressed on T cells mediate CD8+ T cell trafficking into tumors but more studies regarding their specific role in MM ought to be conducted.
Adhesion molecules & DCs
DCs, act as professional antigen-presenting cells and as a link between innate and adaptive immunity [117]. DCs modulate immune responses and it is now widely accepted that DCs also play an important role in regulating the host immune responses to cancer [118]. Within the tumor microenvironment, many cancerous cells die naturally or because of anti-cancerous treatments. Consequently, DCs interact with dying tumor cells enabling them to acquire tumor antigens and amplifying immune response [119]. It is now presumed that MM patients' BM DCs are functionally defective. In fact, several authors already proved that some immunological properties – like the expression of HLA-DR, CD40, CD80 and CD86 - of DCs are altered during myelomagenesis, thus decreasing anti-tumor immune responses and leading to myeloma escape [120]. Adhesion receptors on DCs are involved in many of the processes involved in DC-mediated anti-tumor responses. Several receptors such as αVβ5-integrin expressed on immature DCs are involved in the interaction with and phagocytosis of dying cells. In addition, given that dying cells often become opsonized by complement component iC3b, DCs can also interact with dying tumor cells via the β2-integrins MAC-1 and αXβ2. However, β2-integrins often have anti-inflammatory effects in myeloid cells such as DCs, and these interactions lead to suppression of DC activation and, thus, tolerance [121]. Further, since inflammation of various levels has often been associated with tumor development, and ICAM-1 expression is up-regulated in lymphatic vessels during inflammation, interaction between MAC-1 and ICAM-1 expressed on DCs and inflamed lymphatic endothelium, respectively, may lead to decreased ability of DCs to activate T cells. Therefore, integrins on DCs may be involved in the uptake of dying tumor cells via adhesion receptors such as αVβ5-integrin, and in the subsequent initiation of DC-mediated anti-tumor responses. However, β2-integrins expressed on DCs may instead be involved in suppressing DC function [122]. In conclusion, although integrins play a key role in DC biology, the role of cell adhesion molecules in DC-mediated anti-tumor responses and DC-mediated tumorigenesis is still unclear and clearly requires further study, especially in human patients.
Adhesion molecules & myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are a diverse population of immature myeloid cells. They are known to be negative regulators in infectious processes, autoimmune diseases, and cancer [123]. Their role in the MM BM microenvironment is just starting to be untangled. MDSCs accumulate in the BM of MM patients and their increasing levels correlate with advanced disease stage and worsening prognosis [124]. Indeed, after reaching the tumor site, MDSCs successfully repress the anti-tumor immunity by numerous mechanisms such as by depleting T cell nutrients, by inducing the formation of reactive oxygen species and nitric oxide and by favoring the development of Tregs [125]. Focusing on CAMs, Jin et al. demonstrated that VLA-4, a highly expressed integrin in MM, is responsible for recruitment of MDSCs into the tumor site [126]. Later on, it was also found that tumor growth was significantly reduced in mice lacking activated form of VLA-4 [127]. Also, it is known that the integrin MAC-1 is highly found on myeloid cells and plays an essential role in numerous myeloid cell functions [128]. In addition, Palmen MJ et al. showed that anti-MAC-1 monoclonal antibodies not only reduced myeloid cell tissue infiltration and inflammation but also decreased the recruitment of MDSCs into the tumor site [129]. Joined together, these studies prove that MDSCs act in favor of myeloma PCs and promote disease evolution. Therefore, MDSC-targeted therapies might overcome BM MM niche immunosuppression and increase the anti-tumor effect of additional therapies for MM patients.
Adhesion molecules & tumor-associated vessels
The levels of CAMs such as ICAM-1, E-selectin, P-selectin, and T cell infiltration levels have also been shown to be correlated with malignancies such as in melanoma, glioblastoma, Merkel cell carcinoma and squamous cell carcinoma [130–133]. When squamous cell carcinoma samples were treated with a toll-like-receptor-7 agonist, imiquimod, up-regulated tumor vessels E-selectin expression was found on previously E-selectin negative tumor-associated vessels. This caused an increase in CLA+ CD8+ T cell influx into tumors, and a decrease in Treg frequency and tumor regression [134]. Integrins and VAP-1 on T cells and integrin ligands on endothelial cells are of crucial importance for T cell infiltration into tumors [132].
CAM-targeted therapies in MM
CD38
CD38 is a type II transmembrane glycoprotein of 46 kiloDaltons that is highly expressed on the surface of MM [135]. It functions as an ectoenzyme as well as a receptor involved in regulation of cell adhesion, cell migration, and signal transduction [136]. The very elevated cell surface density of CD38 on MM cells has made it a prime candidate for monoclonal antibody (mAb) targeting. Daratumumab (fully human; Janssen Pharmaceuticals) is the first therapeutic monoclonal anti-CD38 antibody approved by the Federal Drug Agency (FDA) for the treatment of MM both as a single agent and in combination with lenalidomide or bortezomib [137]. The mechanism of action of anti-CD38 mAb on MM cells is mediated by Fc-dependent immune mechanisms. Figure 3 illustrates the 4 main pathways of anti-CD38 action: complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) and direct apoptosis after secondary cross-linking [138].
Figure 3. . Anti-CD38 monoclonal antibodies mechanisms of action.
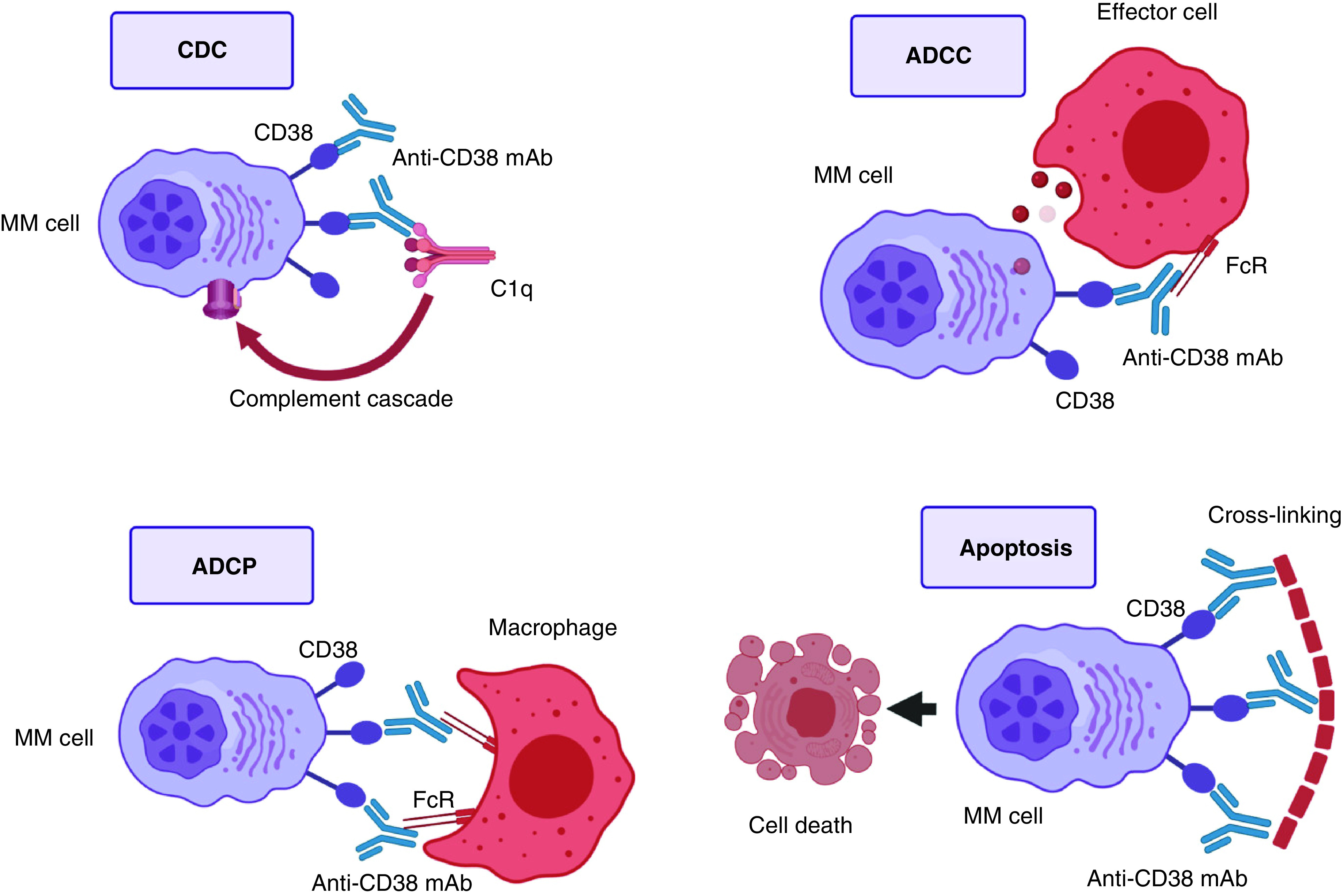
Top left: mAbs bind CD38. The Fc fragment is bound by C1q, initiating the complement cascade, and resulting in a membrane attack complex, leading to cell lysis and death. Top right: mAbs bind CD38. The Fc fragment is then bound by an FcR-bearing effector cell, such as a natural killer cell, leading to activation of cytotoxic processes. Bottom left: mAbs bind CD38, and its Fc fragment is then bound by an FcR-bearing macrophage, inducing phagocytosis. Bottom right: FcR-mediated crosslinking of mAbs induces direct cellular apoptosis.
ADCC: Antibody-dependent cell-mediated cytotoxicity; ADCP: Antibody-dependent cellular phagocytosis; CDC: Complement-dependent cytotoxicity; MAC: Membrane attack complex; MM: Multiple myeloma.
Lenalidomide
Lenalidomide (and its predecessor Thalidomide), a potent immunomodulatory (IMiDs), is highly active agents in the treatment of MM [139]. IMiDs amplify the cytotoxic T-lymphocyte and natural killer (NK) activity against multiple myeloma cells [140]. Nonetheless, in addition to these immunomodulatory effects, recent studies have indicated that its mechanisms of action are highly complex and involve diverse molecular targets in the BM microenvironment. Studies showed that lenalidomide/thalidomide decrease gelatinase, matrix metalloproteinase 2 and 9, production by interfering with multiple integrin-mediated signaling pathways involved in the regulation of cell motility, invasion and survival [141]. Furthermore, treatment with lenalidomide decreases v3-integrin in tartrate-resistant acid phosphatase positive cells, attenuates RANKL secretion in bone marrow stromal cells (BMSCs) [142]. This IMiDs-mediated disruption of MM-BMSCs signaling may then disrupt multiple contact-dependent tumor cell interactions with the bone marrow microenvironment which are crucial for the survival of MM cells.
Bortezomib
Similar to IMiDs, the proteasome inhibitor bortezomib also has its place in the treatment of patients with newly diagnosed or relapsed MM [143]. Treatment of MM cells with bortezomib leads to the induction of apoptosis by interfering with NF-KB signaling [144], leading to the accumulation of misfolded proteins within ER with cellular death [145]. Interestingly, in addition to these mechanisms, proteasome inhibition downregulates the expression of a4b1 and a5b3 integrins, important mediators of the MM-BMSCs signaling, which enhances the survival of MM cells [146].
Natalizumab
In contrast to the IMiDs and proteasome inhibitors which indirectly affect MM cells adhesion properties, many classes of drugs are directly aimed at disrupting the myeloma cell-stromal cell interaction and may have a promising role in the management of MM. To date, targeting of integrins in multiple myeloma have mainly focused on VLA-4. A monoclonal antibody (PS/2), which binds to the a4 chain of murine integrin VLA-4, showed important anti-MM activity in mice injected with 5TGM1 MM cells as well as significant decrease in tumor burden in the bone marrow, spleen, liver, of treated animals [147]. Natalizumab, a recombinant humanized IgG4 monoclonal antibody that binds integrin-a4, has demonstrated ability to inhibit adhesion of MM cells to noncellular and cellular components of the BM microenvironment [148].
Other
N-cadherin antagonist peptide (ADH-1) has shown to act by increasing the permeability of tumor-associated vasculature and hence leading to an increase in therapeutic drug delivery, which subsequently leads to an enhanced response [149]. LCRF-006 is a mimetic of the classical cadherin His-Ala-Val region of ADH-1 [149]. It was found that LCRF-0006 worked by disrupting in a rapid transient and reversible manner the endothelial cell junctions which in turn lead to the increase in the vascular permeability at sited of Multiple Myeloma tumor in vivo [149]. It was also found that LCRF-0006 increased the in vivo anti-MM tumor response to low dose bortezomib [149].
Table 2 depicts some of the agents discussed in this section along with others.
Table 2. . Mechanism of actions of various molecules in multiple myeloma.
| Drugs | Mechanism of action |
|---|---|
| Non-mAb | |
| Thalidomide†, Lenalidomide†, Pomalidomide†, Iberdomide | Immunomodulator |
| Panobinostat†, Vorinostat, Abexinostat, Belinostat, Givinostat, Romidepsin, Entinostat, Tacedinaline | Histone deacetylase inhibitors |
| Sotatercept | Activin inhibitor |
| Evofosfamide | Hypoxia-activated prodrug |
| Plerixafor | CXCR4 antagonist |
| Anti-CD19, anti-CD138, anti-BCMA, anti-SLAM7 CAR—T cells | Chimeric antigen receptor (CAR) T cells |
| Venetoclax | BCL-2 inhibitor |
| DANFIN | NF-κB inhibitor |
| Sorafenib, Vemurafenib, Cobimetinib, Selumetinib | RAS/RAF/MEK/ERK inhibitors |
| Palbociclib | CDK4/6 inhibitor |
| Dovitinib, BGJ398, MFGR1877S, AZD4547 | FGFR inhibitors |
| Clioquinol, SC-06, BEZ235, BAY80-6946, MK-2206 | PI3K/AKT/mTOR inhibitors |
| Selinexor† | Selective inhibitor of nuclear export |
| mAb | |
| Blinatumomab, AMG 701, REGN5458 | Bispecific T cell engagers |
| Ulocuplumab | Anti-CXCR4 mAb |
| Nivolumab, Pembrolizumab, Cemiplimab, Cetrelimab | Anti-PD-1 mAbs |
| Atezolizumab, Avelumab, Pidilizumab | Anti-PD-L1 mAbs |
| Ipilimumab | Anti-CTLA-4 mAb |
| Alemtuzumab | Anti-CD52 mAb |
| Siltuximab | Anti-IL-6 mAb |
| BI-505 | Anti-ICAM-1 mAb |
| Daclizumab | Anti-CD25 mAb |
| AVE1642 | Anti-IGF1R mAb |
| BHQ880 | Anti-DKK1 mAb |
| Bevacizumab | Anti-VEGF mAb |
| Tabalumab | Anti-BAFF mAb |
| Daratumumab†, Isatuximab† | Anti-CD38 mAb |
| Elotuzumab† | Anti-SLAMF7 mAb |
| Denosumab† | Anti-RANKL mAb |
| Belantamab mafodotin† | Anti-BCMA conjugated mAb |
mAb-approved drugs.
mAb: monoclonal antibody.
Conclusion
Progress in our comprehension of the pathophysiology of MM and the advent of novel and targeted therapies have heralded a remarkable decade of progress in myeloma. This has in turn translated into better patient outcomes. Yet, despite these advances, MM remains an incurable hematologic malignancy. CAMs have emerged as important players in this disease and their domain structures, superfamily groupings, and interactions have been heavily studied. CAMs play a remarkable role in the development of targeted therapies, the understanding of MM pathogenesis/dissemination and the emergence of drug resistance. They are involved in numerous cellular mechanisms such as notably epigenetic stability, BM homing, immune microenvironment regulation and more. CAMs have been shown to play a vital role in the interactions between MM cells and BM cells, promoting proliferation and survival of the former. Some involved mechanisms include but are not limited to, inhibition of apoptosis, induction of angiogenesis and alteration of cell–cell interaction. Clinically, this translates into myeloma bone disease – mainly VLA-4 mediated, extramedullary spread – mainly by N-CAM (CD56) downregulation, and the emergence of drug resistance, whether directly or indirectly. The quasi-omnipresence of CAMs has allowed their use as biomarkers of disease as well as targets for therapies that will push the boundaries of the care available to MM patients. Actual treatments of note interacting with CAMs include anti-CD38 monoclonal antibodies (ie. daratumumab), immunomodulators IMiDs (i.e., lenalidomide), proteasome inhibitors downregulating a4b1 and a5b3 integrins (ie. Bortezomib) and a4-VLA-4 integrin inhibitors (ie. Natalizumab). Anti-SLAMF7 drug, CAR T cells, and anti-BCMA drugs are also emerging treatment options. Finally, it is worth noting that the successful incorporation of CAM-targeting drugs into the clinical arsenal in MM may pave the way to their use in other hematological malignancies.
Future perspective
Whether it is liquid biopsies, immunotherapy, CAR T cells or FoundationOne, the field of cancer therapy has been revolutionized during the last decade. CAMs seem to be promising targets for cancer therapy. Clinical trials directly targeting integrins on malignant cells have not been promising so far. A more efficient approach to consider may be to increase the expression or function of β2-integrins on immune cells or their ligands on tumor-associated blood vessels. This would enhance anti-tumor responses in a more efficient approach. Enhancing β2-integrin function could open a chance to overcome immunotherapy's inability to access the tumor microenvironment. In addition, β2-integrins functions and expression regulation needs to be addressed so that harmful-treatment related adverse events are avoided.
Footnotes
Author contributions
MB Zerdan and L Nasr drafted the manuscript. All authors contributed to the discussion section. J Kassab and L Saba contributed to the figures. MB Zerdan conceived the idea for the paper. All authors have read and approved the final manuscript.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
- 1.Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 75(2), 464–474 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brackenbury R, Thiery J, Rutishauser U, Edelman G. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J. Biol. Chem. 252(19), 6835–6840 (1977). [PubMed] [Google Scholar]
- 3.Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science 236(4803), 799–806 (1987). [DOI] [PubMed] [Google Scholar]
- 4.Edelman GM. Cell adhesion molecules. Science 219(4584), 450–457 (1983). [DOI] [PubMed] [Google Scholar]
- 5.Hammer DA, Lauffenburger DA. A dynamical model for receptor-mediated cell adhesion to surfaces. Biophys. J. 52(3), 475–487 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasky LA, Singer MS, Dowbenko D et al. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell 69(6), 927–938 (1992). [DOI] [PubMed] [Google Scholar]
- 7.Szekanecz Z, Koch AE. Endothelial cells and immune cell migration. Arthritis Res. Ther. 2(5), 1–6 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nature Immunol. 6(12), 1191–1197 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu. Rev. Biomed. Eng. 7, 151–185 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Khalili AA, Ahmad MR. A review of cell adhesion studies for biomedical and biological applications. International J. Mol. Sci. 16(8), 18149–18184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho P, Schneider GB, Kellogg B, Zaharias R, Keller JC. Effect of glucocorticoid-induced osteoporotic-like conditions on osteoblast cell attachment to implant surface microtopographies. Implant dentistry 15(4), 377–385 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nature Cell Biol. 1(5), E131–E138 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Okegawa T, Pong R-C, Li Y, Hsieh J-T. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim. Pol. 51(2), 445–457 (2004). [PubMed] [Google Scholar]
- 14.Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 94(7), 575–581 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terpos E, Migkou M, Christoulas D et al. Increased circulating VCAM-1 correlates with advanced disease and poor survival in patients with multiple myeloma: reduction by post-bortezomib and lenalidomide treatment. Blood Cancer J. 6(5), e428–e428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Cell-cell adhesion and communication. In: Molecular Cell Biology (4th edition). WH Freeman, New York, NY, USA: (2000). [Google Scholar]
- 17.Ribeiro M, Levay K, Yon B, Ayupe AC, Salgueiro Y, Park KK. Neural Cadherin Plays Distinct Roles for Neuronal Survival and Axon Growth under Different Regenerative Conditions. Eneuro 7(6), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brackenbury R, Rutishauser U, Edelman GM. Distinct calcium-independent and calcium-dependent adhesion systems of chicken embryo cells. Proc. Natl Acad. Sci. 78(1), 387–391 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ria R, Vacca A, Ribatti D, Di Raimondo F, Merchionne F, Dammacco F. Alpha (v) beta (3) integrin engagement enhances cell invasiveness in human multiple myeloma. Haematologica 87(8), 836–845 (2002). [PubMed] [Google Scholar]
- 20.Cook G, Dumbar M, Franklin IM. The role of adhesion molecules in multiple myeloma. Acta Haematol. 97(1–2), 81–89 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Fronti. Immunol. 10, 1078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guillerey C, Nakamura K, Vuckovic S, Hill GR, Smyth MJ. Immune responses in multiple myeloma: role of the natural immune surveillance and potential of immunotherapies. Cell. Mol. Life Sci. 73(8), 1569–1589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchiyama H, Barut BA, Chauhan D, Cannistra SA, Anderson KC. Characterization of adhesion molecules on human myeloma cell lines. Blood 80(9), 2306–2314 (1992). [PubMed] [Google Scholar]
- 24.Larson RS, Springer TA. Structure and function of leukocyte integrins. Immunol. Rev. 114(1), 181–217 (1990). [DOI] [PubMed] [Google Scholar]
- 25.Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu. Rev. Immunol. 8(1), 365–400 (1990). [DOI] [PubMed] [Google Scholar]
- 26.Anderson KC, Jones RM, Morimoto C, Leavitt P, Barut BA. Response patterns of purified myeloma cells to hematopoietic growth factors. Blood 73(7), 1915–1924 (1989). [PubMed] [Google Scholar]
- 27.Roldan E, Garcia-Pardo A, Brieva J. VLA-4-fibronectin interaction is required for the terminal differentiation of human bone marrow cells capable of spontaneous and high rate immunoglobulin secretion. J. Exp. Med. 175(6), 1739–1747 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawano MM, Mihara K, Huang N, Tsujimoto T, Kuramoto A. Differentiation of early plasma cells on bone marrow stromal cells requires interleukin-6 for escaping from apoptosis. Blood 85(2), 487–494 (1995). [PubMed] [Google Scholar]
- 29.Holzmann B, Mcintyre BW, Weissman IL. Identification of a murine Peyer's patch—specific lymphocyte homing receptor as an integrin molecule with an α chain homologous to human VLA-4α. Cell 56(1), 37–46 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Koopman G, Parmentier HK, Schuurman H, Newman W, Meijer C, Pals ST. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J. Exp. Med. 173(6), 1297–1304 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drew M, Barker HF, Ball J, Pearson C, Cook G, Franklin I. Very late antigen (VLA) expression by normal and neoplastic human plasma cells; including an assessment of antibodies submitted to the Vth International Workshop on Leucocyte Differentiation Antigens using human myeloma cell lines. Leuk. Res. 20(7), 619–624 (1996). [DOI] [PubMed] [Google Scholar]
- 32.Kawano MM, Huang N, Harada H et al. Identification of immature and mature myeloma cells in the bone marrow of human myelomas. Blood 82(2), 564–570 (1993). [PubMed] [Google Scholar]
- 33.Ahsmann E, Lokhorst HM, Dekker AW, Bloem AC. Lymphocyte function-associated antigen-1 expression on plasma cells correlates with tumor growth in multiple myeloma. Blood 79(8), 2068–2075 (1992). [PubMed] [Google Scholar]
- 34.Teoh G, Anderson KC. Interaction of tumor and host cells with adhesion and extracellular matrix molecules in the development of multiple myeloma. Hematol. Oncol. Clin. North Am. 11(1), 27–42 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Lokhorst HM, Lamme T, De Smet M et al. Primary tumor cells of myeloma patients induce interleukin-6 secretion in long-term bone marrow cultures. Blood 84(7), 2269–2277 (1994). [PubMed] [Google Scholar]
- 36.Asosingh K, Vankerkhove V, Van Riet I, Van Camp B, Vanderkerken K. Selective in vivo growth of lymphocyte function-associated antigen-1–positive murine myeloma cells: involvement of function-associated antigen-1–mediated homotypic cell-cell adhesion. Exp. Hematol. 31(1), 48–55 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Li C, Zhao Z, Zhou J, Liu Y, Wang H, Zhao X. Relationship between the TERT, TNIP1 and OBFC1 genetic polymorphisms and susceptibility to colorectal cancer in Chinese Han population. Oncotarget 8(34), 56932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riet IV, Camp BV. The involvement of adhesion molecules in the biology of multiple myeloma. Leukemia Lymphoma 9(6), 441–452 (1993). [DOI] [PubMed] [Google Scholar]
- 39.Xu L, Mohammad KS, Wu H et al. Cell adhesion molecule CD166 drives malignant progression and osteolytic disease in multiple myeloma. Cancer Res. 76(23), 6901–6910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Camp B, Durie B, Spier C et al. Plasma cells in multiple myeloma express a natural killer cell-associated antigen: CD56 (NKH-1; Leu-19). Blood 76(2), 377–382 (1990). [PubMed] [Google Scholar]
- 41.Barker H, Hamilton M, Ball J, Drew M, Franklin I. Expression of adhesion molecules LFA-3 and N-CAM on normal and malignant human plasma cells. Br. J. Haematol. 81(3), 331–335 (1992). [DOI] [PubMed] [Google Scholar]
- 42.Leo R, Boeker M, Peest D et al. Multiparameter analyses of normal and malignant human plasma cells: CD38++, CD56+, CD54+, cIg+ is the common phenotype of myeloma cells. Ann. Hematol. 64(3), 132–139 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Drach J, Gattringer C, Huber H. Expression of the neural cell adhesion molecule (CD56) by human myeloma cells. Clinical Experimental Immunol. 83(3), 418–422 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellat-Deceunynck C, Bataille R, Robillard N et al. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood 84(8), 2597–2603 (1994). [PubMed] [Google Scholar]
- 45.Jalkanen S, Joensuu H, Söderström K, Klemi P. Lymphocyte homing and clinical behavior of non-Hodgkin's lymphoma. J. Clinical Investigation 87(5), 1835–1840 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Rossi G, Zarcone D, Mauro F et al. Adhesion molecule expression on B-cell chronic lymphocytic leukemia cells: malignant cell phenotypes define distinct disease subsets. Blood 81(10), 2679–2687 (1993). [PubMed] [Google Scholar]
- 47.Katz B-Z. Adhesion molecules—the lifelines of multiple myeloma cells. Presented at: Seminars in Cancer Biology. Hematology Institute, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel: (2010). [DOI] [PubMed] [Google Scholar]
- 48.Kim I, Uchiyama H, Chauhan D, Anderson KC. Cell surface expression and functional significance of adhesion molecules on human myeloma-derived cell lines. Br. J. Haematol. 87(3), 483–493 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Van Riet I, Waele MD, Remels L, Lacor P, Schots R, Camp BV. Expression of cytoadhesion molecules (CD56, CD54, CD18 and CD29) by myeloma plasma cells. Br. J. Haematol. 79(3), 421–427 (1991). [DOI] [PubMed] [Google Scholar]
- 50.Springer TA. Adhesion receptors of the immune system. Nature 346(6283), 425–434 (1990). [DOI] [PubMed] [Google Scholar]
- 51.Kawano MM, Huang N, Tanaka H et al. Homotypic cell aggregations of human myeloma cells with ICAM-1 and LFA-1 molecules. Br. J. Haematol. 79(4), 583–588 (1991). [DOI] [PubMed] [Google Scholar]
- 52.Sadler NM, Harris BR, Metzger BA, Kirshner J. N-cadherin impedes proliferation of the multiple myeloma cancer stem cells. Am. J. Blood Res. 3(4), 271 (2013). [PMC free article] [PubMed] [Google Scholar]
- 53.Mrozik KM, Cheong CM, Hewett D et al. Therapeutic targeting of N-cadherin is an effective treatment for multiple myeloma. Br. J. Haematol. 171(3), 387–399 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Groen RW, De Rooij MF, Kocemba KA et al. N-cadherin-mediated interaction with multiple myeloma cells inhibits osteoblast differentiation. Haematologica 96(11), 1653 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bi E, Li R, Bover LC et al. E-cadherin expression on multiple myeloma cells activates tumor-promoting properties in plasmacytoid DCs. J. Clinical Investigation 128(11), 4821–4831 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tripodo C, Florena A, Macor P et al. P-selectin glycoprotein ligand-1 as a potential target for humoral immunotherapy of multiple myeloma. Curr. Cancer Drug Targets 9(5), 617–625 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Azab AK, Quang P, Azab F et al. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood J. Am. Soc. Hematol. 119(6), 1468–1478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Natoni A, Farrell ML, Harris S et al. Sialyltransferase inhibition leads to inhibition of tumor cell interactions with E-selectin, VCAM1, and MADCAM1, and improves survival in a human multiple myeloma mouse model. Haematologica 105(2), 457 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alexandrakis MG, Passam FH, Sfiridaki K et al. Clinical significance of circulating endothelial adhesion molecules (sE-selectin and sICAM) in untreated multiple myeloma patients. Clin. Chim. Acta 349(1–2), 39–43 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Muz B, Azab F, Fiala M et al. Inhibition of E-Selectin (GMI-1271) or E-selectin together with CXCR4 (GMI-1359) re-sensitizes multiple myeloma to therapy. Blood Cancer J. 9(9), 1–6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witzig TE, Kimlinger TK, Ahmann GJ, Katzmann JA, Greipp PR. Detection of myeloma cells in the peripheral blood by flow cytometry. Cytometry 26(2), 113–120 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 272(5258), 60–67 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Podar K, Tai Y-T, Davies FE et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood, The Journal of the American Society of Hematology 98(2), 428–435 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Derksen P, De Gorter D, Meijer H et al. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia 17(4), 764–774 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Vanderkerken K, De Greef C, Asosingh K et al. Selective initial in vivo homing pattern of 5T2 multiple myeloma cells in the C57BL/KalwRij mouse. Br. J. Cancer 82(4), 953–959 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alici E, Konstantinidis KV, Aints A, Dilber MS, Abedi-Valugerdi M. Visualization of 5T33 myeloma cells in the C57BL/KaLwRij mouse: establishment of a new syngeneic murine model of multiple myeloma. Exp. Hematol. 32(11), 1064–1072 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Van Riet I, Vanderkerken K, De Greef C, Van Camp B. Homing behaviour of the malignant cell clone in multiple myeloma. Med. Oncol. 15(3), 154–164 (1998). [DOI] [PubMed] [Google Scholar]
- 68.Pellat-Deceunynck C, Barillé S, Puthier D et al. Adhesion molecules on human myeloma cells: significant changes in expression related to malignancy, tumor spreading, and immortalization. Cancer Res. 55(16), 3647–3653 (1995). [PubMed] [Google Scholar]
- 69.Huang N, Kawano MM, Harada H et al. Heterogeneous expression of a novel MPC-1 antigen on myeloma cells: possible involvement of MPC-1 antigen in the adhesion of mature myeloma cells to bone marrow stromal cells. (1993). [PubMed] [Google Scholar]
- 70.Huang N, Kawano MM, Mahmoud MS et al. Expression of CD21 antigen on myeloma cells and its involvement in their adhesion to bone marrow stromal cells. Blood 85(12), 3704–3712 (1995). [PubMed] [Google Scholar]
- 71.Ridley RC, Xiao H, Hata H, Woodliff J, Epstein J, Sanderson RD. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood 81(3), 767–774 (1993). [PubMed] [Google Scholar]
- 72.Faid L, Van Riet I, De Waele M et al. Adhesive interactions between tumour cells and bone marrow stromal elements in human multiple myeloma. Eur. J. Haematol. 57(5), 349–358 (1996). [DOI] [PubMed] [Google Scholar]
- 73.Riet IV, Greef CD, Favero HD, Demanet C, Camp BV. Production of fibronectin and adherence to fibronectin by human myeloma cell lines. Br. J. Haematol. 87(2), 258–265 (1994). [DOI] [PubMed] [Google Scholar]
- 74.Klauber-Demore N, Tan LK, Liberman L et al. Sentinel lymph node biopsy: is it indicated in patients with high-risk ductal carcinoma-in-situ and ductal carcinoma-in-situ with microinvasion? Ann. Surg. Oncol. 7(9), 636–642 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia 23(3), 449–456 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coleman RE. Skeletal complications of malignancy. Cancer 80(S8), 1588–1594 (1997). [DOI] [PubMed] [Google Scholar]
- 77.Kariyawasan C, Hughes D, Jayatillake M, Mehta A. Multiple myeloma: causes and consequences of delay in diagnosis. QJM 100(10), 635–640 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Croucher PI, Apperley JF. Bone disease in multiple myeloma. Br. J. Haematol. 103(4), 902–910 (1998). [DOI] [PubMed] [Google Scholar]
- 79.Terpos E, Mihou D, Szydlo R et al. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia 19(11), 1969–1976 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Terpos E, Politou M, Rahemtulla A. New insights into the pathophysiology and management of bone disease in multiple myeloma. Br. J. Haematol. 123(5), 758–769 (2003). [DOI] [PubMed] [Google Scholar]
- 81.De Raeve H, Vanderkerken K. The role of the bone marrow microenvironment in multiple myeloma. Histol. Histopathol. (2005). [DOI] [PubMed] [Google Scholar]
- 82.Terpos E, Dimopoulos M-A. Myeloma bone disease: pathophysiology and management. Ann. Oncol. 16(8), 1223–1231 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Roux S, Mariette X. The high rate of bone resorption in multiple myeloma is due to RANK (receptor activator of nuclear factor-κB) and RANK ligand expression. Leukemia & Lymphoma 45(6), 1111–1118 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Roodman G. Treatment strategies for bone disease. Bone Marrow Transplant. 40(12), 1139–1146 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Reddy SV, Takahashi S, Dallas M, Williams RE, Neckers L, Roodman GD. Interleukin-6 antisense deoxyoligonucleotides inhibit bone resorption by giant cells from human giant cell tumors of bone. J. Bone Miner. Res. 9(5), 753–757 (1994). [DOI] [PubMed] [Google Scholar]
- 86.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 93(5), 1658–1667 (1999). [PMC free article] [PubMed] [Google Scholar]
- 87.Mori Y, Shimizu N, Dallas M et al. Anti-α4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood 104(7), 2149–2154 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Jundt F, PröBsting KS, Anagnostopoulos I et al. Jagged1-induced Notch signaling drives proliferation of multiple myeloma cells. Blood 103(9), 3511–3515 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Houde C, Li Y, Song L et al. Overexpression of the NOTCH ligand JAG2 in malignant plasma cells from multiple myeloma patients and cell lines. Blood 104(12), 3697–3704 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Ashley JW, Ahn J, Hankenson KD. Notch signaling promotes osteoclast maturation and resorptive activity. J. Cell. Biochem. 116(11), 2598–2609 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haÿ E, Laplantine E, Geoffroy V et al. N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/β-catenin signaling, osteoblast function, and bone formation. Mol. Cell. Biol. 29(4), 953–964 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klimiené I, Radzevičius M, Matuzevičiené R, Sinkevič-Belliot K, Kučinskiené ZA, Pečeliūnas V. Adhesion molecule immunophenotype of bone marrow multiple myeloma plasma cells impacts the presence of malignant circulating plasma cells in peripheral blood. Int. J. Lab. Hematol. 43(3), 403–408 (2021). [DOI] [PubMed] [Google Scholar]
- 93.Arana P, Paiva B, Cedena M-T et al. Prognostic value of antigen expression in multiple myeloma: a PETHEMA/GEM study on 1265 patients enrolled in four consecutive clinical trials. Leukemia 32(4), 971–978 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Paiva B, Paino T, Sayagues J-M et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 122(22), 3591–3598 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Kraj M, Kopeć-Szlęzak J, Pogłód R, Kruk B. Flow cytometric immunophenotypic characteristics of 36 cases of plasma cell leukemia. Leuk. Res. 35(2), 169–176 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Drucker L, Tohami T, Tartakover-Matalon S et al. Promoter hypermethylation of tetraspanin members contributes to their silencing in myeloma cell lines. Carcinogenesis 27(2), 197–204 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Tohami T, Drucker L, Shapiro H, Radnay J, Lishner M. Overexpression of tetraspanins affects multiple myeloma cell survival and invasive potential. FASEB J. 21(3), 691–699 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Uchida S, Shimada Y, Watanabe G et al. Motility-related protein (MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph node metastasis in oesophageal squamous cell carcinoma. Br. J. Cancer 79(7), 1168–1173 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zangari M, Saghafifar F, Mehta P, Barlogie B, Fink L, Tricot G. The blood coagulation mechanism in multiple myeloma. Presented at: Seminars in thrombosis and hemostasis. University of Arkansas for Medical Sciences, Myeloma Institute for Research and Therapy, Little Rock, AK, USA: (2003). [DOI] [PubMed] [Google Scholar]
- 100.Bianchi G, Kumar S, Ghobrial IM, Roccaro AM. Cell trafficking in multiple myeloma. Open journal of hematology 3(Suppl. 1), (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robak P, Drozdz I, Szemraj J, Robak T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 70, 199–208 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Abdi J, Chen G, Chang H. Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget 4(12), 2186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stessman H, Mansoor A, Zhan F et al. Reduced CXCR4 expression is associated with extramedullary disease in a mouse model of myeloma and predicts poor survival in multiple myeloma patients treated with bortezomib. Leukemia 27(10), 2075–2077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stessman HA, Baughn LB, Sarver A et al. Profiling bortezomib resistance identifies secondary therapies in a mouse myeloma model. Mol. Cancer Ther. 12(6), 1140–1150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lomas OC, Tahri S, Ghobrial IM. The microenvironment in myeloma. Curr. Opin. Oncol. 32(2), 170–175 (2020). [DOI] [PubMed] [Google Scholar]
- 106.Caligaris-Cappio F, Bergui L, Gregoretti MG et al. Role of bone marrow stromal cells in the growth of human multiple myeloma. (1991). [PubMed] [Google Scholar]
- 107.Das R, Strowig T, Verma R et al. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat. Med. 22(11), 1351–1357 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kawano Y, Moschetta M, Manier S et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol. Rev. 263(1), 160–172 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Lee N-R, Ban J, Lee N-J et al. Activation of RIG-I-mediated antiviral signaling triggers autophagy through the MAVS-TRAF6-Beclin-1 signaling axis. Front. Immunol. 9, 2096 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sato E, Olson SH, Ahn J et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl Acad. Sci. 102(51), 18538–18543 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer 112(11), 1782–1790 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cohen AD, Raje N, Fowler JA, Mezzi K, Scott EC, Dhodapkar MV. How to train your T cells: overcoming immune dysfunction in multiple myeloma. Clin. Cancer Res. 26(7), 1541–1554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang Y, Jiang Y, Chen Y et al. Bone marrow PD-1 positive T cells reflect tumor mass and prognosis in multiple myeloma. Int. J. Clin. Exp. Pathol. 11(1), 304 (2018). [PMC free article] [PubMed] [Google Scholar]
- 114.Fisher DT, Chen Q, Skitzki JJ et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J. Clinical Investigation 121(10), 3846–3859 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mlecnik B, Tosolini M, Charoentong P et al. Biomolecular network reconstruction identifies T cell homing factors associated with survival in colorectal cancer. Gastroenterology 138(4), 1429–1440 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Schmits R, Kündig TM, Baker DM et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J. Exp. Med. 183(4), 1415–1426 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boer MC, Joosten SA, Ottenhoff TH. Regulatory T cells at the interface between human host and pathogens in infectious diseases and vaccination. Front. Immunol. 6, 217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Loschke F, Homberg M, Magin TM. Keratin isotypes control desmosome stability and dynamics through PKCα. J. Invest. Dermatol. 136(1), 202–213 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Diff. 15(1), 39–50 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown RD, Pope B, Murray A et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-β1 and interleukin-10. Blood 98(10), 2992–2998 (2001). [DOI] [PubMed] [Google Scholar]
- 121.Rennert P, Schneider P, Cachero TG et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J. Exp. Med. 192(11), 1677–1684 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verbovetski I, Bychkov H, Trahtemberg U et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J. Experimental Med. 196(12), 1553–1561 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9(3), 162–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Görgün GT, Whitehill G, Anderson JL et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 121(15), 2975–2987 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang G, Lu X, Dey P et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Disc. 6(1), 80–95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin α4β1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 66(4), 2146–2152 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Foubert P, Kaneda MM, Varner JA. PI3Kγ activates integrin α4 and promotes immune suppressive myeloid cell polarization during tumor progression. Cancer Immunol. Res. 5(11), 957–968 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. (1990). [PubMed] [Google Scholar]
- 129.Palmen M, Dijkstra C, Van Der Ende M, Pena A, Van Rees E. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin. Exp. Immunol. 101(2), 351–356 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weishaupt C, Munoz KN, Buzney E, Kupper TS, Fuhlbrigge RC. T cell distribution and adhesion receptor expression in metastatic melanoma. Clin. Cancer Res. 13(9), 2549–2556 (2007). [DOI] [PubMed] [Google Scholar]
- 131.Afanasiev OK, Nagase K, Simonson W et al. Vascular E-selectin expression correlates with CD8 lymphocyte infiltration and improved outcome in Merkel cell carcinoma. J. Invest. Dermatol. 133(8), 2065–2073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clark RA, Huang SJ, Murphy GF et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J. Experimental Med. 205(10), 2221–2234 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lohr J, Ratliff T, Huppertz A et al. Effector T cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin. Cancer Res. 17(13), 4296–4308 (2011). [DOI] [PubMed] [Google Scholar]
- 134.Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giuliani N, Accardi F, Marchica V et al. Novel targets for the treatment of relapsing multiple myeloma. Expert Rev. Hematol. 12(7), 481–496 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Malavasi F, Deaglio S, Funaro A et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 88(3), 841–886 (2008). [DOI] [PubMed] [Google Scholar]
- 137.Sanchez L, Wang Y, Siegel DS, Wang ML. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J. Hematol. Oncol. 9(1), 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mustafa N, Nee HFA, Lee XTJ et al. Daratumumab efficiently targets NK/T cell lymphoma with high CD38 expression. Blood 130, 2814 (2017).29284611 [Google Scholar]
- 139.Richardson P. Management of the relapsed/refractory myeloma patient: strategies incorporating lenalidomide. Presented at: Semin. Hematol. Harvard Medical School, Boston, MA, USA: (2005). [DOI] [PubMed] [Google Scholar]
- 140.Davies FE, Raje N, Hideshima T et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98(1), 210–216 (2001). [DOI] [PubMed] [Google Scholar]
- 141.Segarra M, Lozano E, Corbera-Bellalta M et al. Thalidomide decreases gelatinase production by malignant B lymphoid cell lines through disruption of multiple integrin-mediated signaling pathways. Haematologica 95(3), 456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Breitkreutz I, Raab M-S, Vallet S et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 22(10), 1925–1932 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Ito S. Proteasome inhibitors for the treatment of multiple myeloma. Cancers (Basel) 12(2), 265 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boccadoro M, Morgan G, Cavenagh J. Preclinical evaluation of the proteasome inhibitor bortezomib in cancer therapy. Cancer Cell Int. 5(1), 1–9 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reddy N, Czuczman M. Enhancing activity and overcoming chemoresistance in hematologic malignancies with bortezomib: preclinical mechanistic studies. Ann. Oncol. 21(9), 1756–1764 (2010). [DOI] [PubMed] [Google Scholar]
- 146.Hatano K, Kikuchi J, Takatoku M et al. Bortezomib overcomes cell adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma. Oncogene 28(2), 231–242 (2009). [DOI] [PubMed] [Google Scholar]
- 147.Olson DL, Burkly LC, Leone DR, Dolinski BM, Lobb RR. Anti-α4 integrin monoclonal antibody inhibits multiple myeloma growth in a murine model. Mol. Cancer Ther. 4(1), 91–99 (2005). [PubMed] [Google Scholar]
- 148.Podar K, Zimmerhackl A, Fulciniti M et al. The selective adhesion molecule inhibitor Natalizumab decreases multiple myeloma cell growth in the bone marrow microenvironment: therapeutic implications. Br. J. Haematol. 155(4), 438–448 (2011). [DOI] [PubMed] [Google Scholar]
- 149.Mrozik KM, Cheong CM, Hewett DR et al. LCRF-0006, a small molecule mimetic of the N-cadherin antagonist peptide ADH-1, synergistically increases multiple myeloma response to bortezomib. FASEB BioAdvances 2(6), 339–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]