Abstract
Tumorigenesis is a multi-step process marked by variations in numerous metabolic pathways that affect cellular architectures and functions. Cancer cells reprogram their energy metabolism to enable several basic molecular functions, including membrane biosynthesis, receptor regulations, bioenergetics, and redox stress. In recent years, cancer diagnosis and treatment strategies have targeted these specific metabolic changes and the tumor’s interactions with its microenvironment. Positron emission tomography (PET) captures all molecular alterations leading to abnormal function and cancer progression. As a result, the development of PET radiotracers increasingly focuses on irregular biological pathways or cells that over-express receptors that have the potential to function as biomarkers for early diagnosis and treatment measurements as well as research. This chapter reviews both established and evolving PET radiotracers used to image tumor biology. We have also included a few advantages and disadvantages of the routinely used PET radiotracers in cancer imaging.
Keywords: PET, cancer, radiotracers, biomarkers, metabolism
Introduction
Nuclear medicine-molecular imaging is used to visualize, characterize, and measure biological pathways and processes at both the cellular and molecular levels, in particular the workings of cancer in vivo. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) are two powerful and commonly used nuclear medicine imaging tools often utilized in the field of cancer biology [1]. While both play a critical role in cancer research, PET is especially effective in (a) differentiating malignant and benign tumors based on accurate identification of their site; (b) detecting primary small or unknown tumors in patients with metastatic disease; and (c) grading tumor malignancy based on the quantification of tracer uptake [2,3]. With this reliable information, oncologists can make treatment judgments more efficiently.
Typically, a PET scanner needs millions of cells in extremely close proximity to accumulate enough radiotracer to accurately visualize the tumor and location of activity against the background. The specific number of cells that can be imaged at one time depends on several factors, including the exact level of tracer uptake by non-targeted surrounding tissues, the type of target tissue under study, and the positron energy of the ligand. Its results are more accurate than those from computed tomography (CT) or magnetic resonance (MR) images and are better at detecting metastatic tumors than other imaging modalities. Nevertheless, it has some disadvantages [2]. First, the imaging itself due to the radiotracer productions are expensive and second significant inter-observer variability is associated with defining tumor margins and the thresholds of the imaging software [4]. From the subject’s perspective, while PET-CT can acquire anatomical and functional information simultaneously, it is slightly slower than other image analyses due to several critical parameters to be considered from the radiotracer and scanner to interpret the results [5]. Recently advances in camera technology have tremendously facilitated molecular imaging assays in rodents [6,7]. These approaches allow rapid testing of human cell targets implanted into mice before being tested in humans and/or large animals. The imaging and pharmacokinetic parameters of the tracer can be well-optimized before clinical translations [8]. These systems have a spatial resolution of ~23 mm3 and are being upgraded to ~13 mm3 [5]. MicroPET imaging requires radiotracers with high specificity due to the limited sensitivity of small-animal scanners and the smaller mass of tracer injected [8,9]. The development of novel in vitro cell-based molecular assays and in vivo small-animal models increased PET’s ability to validate the efficacy of radiotracers early in the translational pipeline [10]. Following validation, the same radiotracer can be used in multiple established PET research facilities globally. Figure 1 shows how radiotracers are translated from cell culture to preclinical models to real-world clinical imaging [4]. Using positron-emitting radionuclides to capture an image is minimally invasive [3]. PET is often compared to a camera that requires only a few seconds to a few minutes’ exposure to photograph/image the subject [5]. However, unlike a camera, it cannot image visible light but instead captures the high-energy γ-rays the subject emits. Molecules are first labeled with a radioisotope that produces two γ-rays after ejecting a positron from the nucleus [1]. The positron gradually loses energy as it travels a short distance from its site of origin to the biological tissue. In an annihilation reaction, it reacts with an electron to generate two 511keV photons, which are ejected in two different, anti-parallel directions, 180° apart. They interact with the detector rings at the opposite sides within the PET scanner, drawing a visible line along the site of the annihilation reaction to allow accurate localization. The common term used to describe this simultaneous detection event is a coincidence, [11] and several coincidences form the final PET image. Most PET scanners are relatively sensitive in the range of 10−11 to 10−12 moles/L, independent of the location and depth of the tumor and the accuracy of the tracer utilized [5].
Figure 1.

Important steps in a typical PET radiotracer development lab, starting from design of tumor imaging targets to PET imaging sessions in cancer patients.
Various well-established radionuclides produce radiotracers specifically tailored to precursor compounds [1]. These neutron-deficient positron radionuclides, helpful in diagnosing abnormal cell growth, are routinely made in cyclotrons [11]. Automated radiochemistry systems installed in lead-shielded hot cells then introduce these positron emitters into organic compounds, such as peptides, proteins, drugs, small molecules, or receptor ligands, via rapid, reliable synthetic methods. Certain characteristics determine a given PET imaging agent’s effectiveness. High positron energy dictates the distance the emission can travel through tissue to capture the final target area [1]. In addition, the isotope must have a physical half-life compatible with the biological half-life of the imaging target [3]. Frequently used positron-emitting isotopes include fluorine-18, carbon-11, oxygen-15, and nitogen-13 [12]. Others include copper-64, copper-62, iodine-124, bromine-76, rubidium-82, and gallium-68 [11]. Fluorine-18 ([18F]) with an optimal half-life of 109.7 min, 511 KeV of positron energy, and decay caused most directly by positron emission (>90%) has a wide-stream application among all the isotopes. [1,12] more recently, gallium-68 ([68Ga]) with a half-life of 67.7 min is gaining a lot of attention in both preclinical and clinical settings; it can be generated by cyclotron or generator, so an on-site cyclotron is not strictly necessary [1,2,4].
PET radiotracers (see Figure 2) are routinely used in oncology to study metabolism, vascularization and perfusion, transporters, receptors, metabolic processes, even gene and/or proteins, enzymes and antigen expression [5,13]. They play a critical role in staging/restaging, predicting potential risk, initial screening, diagnosis, and follow-up or prognosis. Thus, researchers are investigating various strategies for imaging target cancer cells with PET due to its high accuracy and sensitivity [2,4] [5,13] [14,15].
Figure 2.
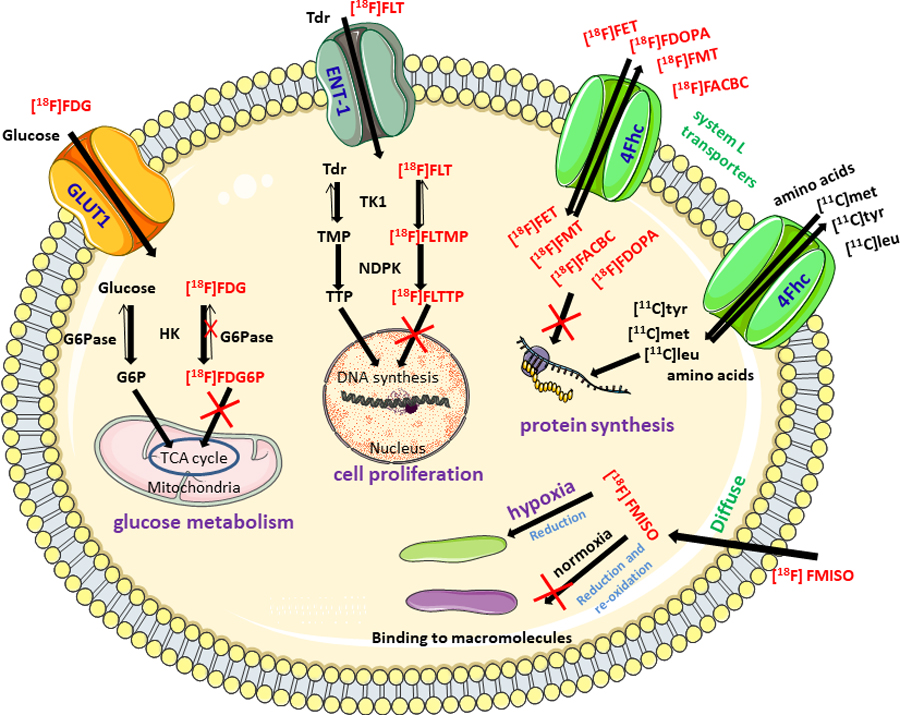
Key molecular mechanisms effected in cancer with corresponding routinely used PET radiotracers
Tumor-linked metabolisms
Mitochondrial oxidative phosphorylation provides most of the energy required for normal cellular processes. It is manufactured through cellular respiration [16]. In contrast, cancer cells rely on a metabolic reprogramming called the Warburg effect, among several other altered metabolic activities and cell cycles [17]. They upregulate anabolism levels, sharply increasing their ability to produce nucleic acids, proteins, and lipids, and altering metabolites and enzyme functions. Aerobic glycolysis may be an inefficient way to generate adenosine 5′-triphosphate (ATP), but for reasons not clearly understood, it offers an advantage to cancer cells. These alterations modify cell-cycle regulation, gene/trait expression, cellular metabolism, and apoptosis [16]. Fortunately, researchers can image these features to study cancer mechanisms [17]; some of the important ones are listed below.
Carbohydrate metabolism
2-deoxy-D-[14C]glucose (DG) is produced by modifying the hydroxyl group in the C2 position of D glucose. It was created for use as a drug to slow down or entirely block glycolysis in cancerous cells. However, it also blocks glycolysis in essential regions like the brain. It was then used to image glycolysis using autoradiography: DG was labeled with carbon-14, and subject animals have to be euthanized. The application was then modified to use the PET radiopharmaceutical, 2-[18F]fluoro-2-deoxy-D-glucose (FDG), to image living subjects non-invasively with specificity [14,17,18]. [18F]FDG exploits the process of aerobic glycolysis in cancer cells [19]. Automatic radiotracer synthesizers replace the tosyl precursor with the [18F]fluoride isotope in a bimolecular nucleophilic substitution (SN2) reaction. Acetyl protection groups are then cleaved by an acidic or a basic hydrolysis variant to yield [18F]FDG. Following purification via several cartridges, [18F]FDG is diluted in a physiological buffer for injection into the subject. Currently, [18F]FDG is widely used for imaging glucose metabolism in the brain, breast, lung, prostate, pancreatic, bladder, and other cancers [20]. It is important in tumor imaging, particularly for estimating the extent of glucose overuse in malignant lesions, where anaerobic and inefficient glycolysis impairs energy storage and ATP synthesis. In the cell, [18F]FDG uptake mechanism is similar to that observed in natural D-glucose and enters cancer cells through glucose transporters, such as (GLUT) −1 and −3, and is then phosphorylated by hexokinase to become [18F]FDG-6-phosphate. While glucose- 6-phosphate undergoes further structural shifts to become the isomer fructose-6-phosphate in the glycolytic pathway or oxidizes to become 6-phosphogluconolactone in the pentose phosphate pathway (PPP), [18F]FDG-6-phosphate cannot be catabolized because it lacks an oxygen atom at C-2 and cannot diffuse out of cells. In a process termed metabolic trapping, it accumulates due to its slow dephosphorylation rate. Thus, [18F]FDG uptake provides a way to assess the excessive glucose uptake rates in tumors [14] compared to surrounding tissues. Since the accumulated [18F]FDG is eliminated via the renal system, low levels are seen in the blood [16,17]. The pharmacokinetics of glucose uptake, trapping, and clearance in blood last a half to a full hour, enabling [18F]FDG to be used in imaging several cancers, including gliomas, prostate cancer (PCa), lung cancer (LC), and hepatocellular carcinomas (HCC) [21,4,22].
Gliomas are the most common and malignant type of brain tumor; patients median survival span is less than one year [3] [23,20]. Recent clinical studies have investigated a variety of PET radiotracers for imaging gliomas [21], and since [18F]FDG exploits the upregulation of glycolysis in brain cancer cells, evidence supports a link between its uptake and prognosis. However, high physiological uptake of [18F]FDG diminishes contrast, occasionally to the point where tumors and normal brain cells become indistinguishable [12,24]. Neuro-oncological applications to grade glioma in immunocompromised patients are restricted by inconclusive anatomical imaging and lymphoma assessment, cerebral tumors cannot be differentiated from infections [23,25]. Prostate cancer (PCa), prevalent in men [22,26] is diagnosed and tracked by quantifying the serum prostate-specific antigen (PSA). However, in the beginning stages, PSA levels are low and therefore imaging techniques may be better than other commonly used techniques at providing information for clinical management [27]. However, the low glucose metabolism of PCa cells limits the utility of [18F]FDG [28,29]. It is only recommended for high Gleason score tumors and/or patients with high PSA values, based on its ability to image bone metastases at advanced stages of PCa [22]. With improved sensitivity, PCa-specific PET radiotracers can now detect early-stage PCa and localize recurrence by detecting small lymph nodes and bone metastases even when PSA values are low [22,30]. Lung cancer, the most incident cancer is classified as either as non-small cell lung carcinoma (NSCLC) or small cell lung carcinoma (SCLC) [31] based on various characteristics and response to conventional therapies. Histologic characterization plays a crucial role in the ‘personalized medicine’ approach to diagnosis and treatment [32]. NSCLC is more common than SCLC and includes adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, which are generally characterized by slower growth and metastasis [33]. SCLC can be grouped with other tumors exhibiting neuroendocrine differentiation and more aggression, resulting in rapid metastasis of surrounding tissue [31]. [18F]FDG imaging plays an important role in the staging, treatment planning, and prognostic assessment of lung carcinomas. It provides metabolic and morphological information that improves accuracy in diagnosing malignant lung nodules assessment of lymph node involvement, and detection of both local and distant metastatic disease over CT imaging alone. Additionally, it is often used to interpret lesions misidentified by outdated imaging modalities. Its advantages are especially important in characterizing pulmonary solitary masses, where a correct diagnosis affects treatment drastically [34]. PET-CT also provides predictive and prognostic information following neo-adjuvant therapy. A major concern about [18F]FDG is associated with its uptake mechanisms. For example, association with inflammatory and/or infectious processes can result in false-positive results [35], affecting diagnoses of sarcoidosis, tuberculosis, and other granulomatous pathologies involving the lung. False-negative results occasionally occur in well-differentiated malignancies with a small tumor-to-background ratio. Nevertheless, use of [18F]FDG is now established for diagnosis of indeterminate pulmonary nodules and lung cancer staging [34]. Hepatocellular carcinoma (HCC) is a primary neoplasm of the liver, and accounts for ~75 percent of cancer cases worldwide [36,26]. Since, the liver is a common metastatic site, hepatic metastases are more prevalent than primary liver cancer [37]. [18F]FDG is effective in distinguishing malignant and benign liver lesions but has low sensitivity for HCC detection since the rate of gluconeogenesis in well-differentiated HCC and surrounding liver parallels the [18F]FDG uptake, and displays a low tumor-to-background ratio [38]. [18F]FDG is still useful in the detection of extrahepatic disease and distant metastases, adding incremental value in predicting survival [39].
DNA synthesis and cell proliferation
Malignant transformation increases cell proliferation and the number of cells undergoing deoxyribonucleic acid (DNA) replication. Thymidine analogs provide a method to target DNA replication in all cells, including cancer cells, because thymidine is required for DNA synthesis and agents such as [11C]thymidine and 3′- deoxy-3′-[18F]fluorothymidine ([18F]FLT) have been extensively studied [40,41]. Imaging probes are incorporated into DNA but not ribonucleic acid (RNA). Upregulation of thymidine transport and mammalian thymidine kinases then provides the needed molecular targets. Once inside the cell, they are phosphorylated by thymidine kinase (TK)-1 into [18F]FLT-monophosphate, which is already present in the cell [4,22,41]. DNA polymerases can be also incorporate molecules that, like thymidine can be phosphorylated by TKs and labeled with [18F], for example, [18F](1-(2′-deoxy-2′-fluoro-β-arabino furanosyl) thymine) ([18F]FMAU) and 1-(2′-deoxy-2′-fluoro-1-β- D-arabinofuranosyl)-5-bromouracil ([18F]FBAU) [3]. [18F]FLT imaging may be more useful than [18F]FDG imaging during therapy for three reasons. First, [18F]FLT uptake will be lower than that of [18F]FDG following an inflammatory response to disease. Second, cytostatic chemotherapeutics, which often affect cell division more they do glucose metabolism, can be easily monitored with [18F]FLT. Third, [18F]FLT can better image brain tumors due to its low background brain-cell binding [22,41]. Proliferation radiotracers are also very useful in breast cancer imaging [42]. In addition to imaging glucose metabolism in gliomas, [18F]FLT has also been used to study protein synthesis, proliferation, and hypoxia mechanisms [3,23,12]. As a radiolabeled-pyrimidine used to track DNA synthesis [4], [18F]FLT permeates the cell membrane via diffusion promoted by blood-brain barrier breakdown. It is then phosphorylated by the upregulated S-phase-specific enzyme thymidine kinase 1 to 3′-flurothymidine monophosphate in the proliferating cell and is trapped. It cannot cross the blood-brain barrier through transporters, so its uptake depends completely on blood-brain barrier permeability [23,4], which hampers its clinical value and limits its use in low-grade gliomas with the blood-brain barrier intact. However, it can image high-grade gliomas with very high sensitivity [4,24,43]. Recent developments in molecular imaging have improved the accuracy of breast cancer diagnosis, staging, and prognosis –advancing ‘precision medicine’ strategies early on. PET is commonly used to identify breast cancer subtypes and to monitor treatment response [42]. Radiotracers may also be useful for imaging different biological processes among the various types of breast cancers. While [18F]FLT is not commonly used in breast cancer imaging practice under clinical pipeline, it may still play a key role in the staging, and prediction of response to novel therapeutic regimens [44].
Cellular respiration
Oxygenation is another crucial parameter in successfully treating cancers [16]. Hypoxia is a significant prognostic variable, and using radiotracers to monitor it before, during, and after treatment will probably become more widespread [45]. [18F]fluoromisonidazole (FMISO), an analog of 2- nitroimidazole, has been used to image hypoxia in tumors [46], especially in the brain and lung. It diffuses into all cells and is reduced and re-oxidized in normal cells, but in hypoxic cells, it continues to be reduced and binds to cell components [40,41]. Its relatively poor cellular uptake and slow clearance from normal tissues deter its use [45]. In contrast, copperbis(thiosemicarbazones), such as Cu(II)-diacetylbis (N4-methylthiosemicarabaone) ([64Cu]ATSM), washes out of normal tissues quickly but is still retained in hypoxic tissues due to its reduction by oxygen-depleted mitochondria. The extent and nature of tumor vasculature are crucial in tracking tumor growth and the oxygen status of the surrounding tissue. Hypoxia sites can induce changes that enable tumor cells to remain viable and grow in unfavorable environments [45]. Cancer cells are able to counteract the effects of a stressful environment by upregulating the production of angiogenic factors, such as the vascular endothelial growth factor (VEGF), to continue proliferation [47]. Angiogenic markers, such as αβ integrins and tumor expression of VEGF are also reliable PET radiotracers [40,47,48]. Hypoxia may also be linked to resistance to therapy [23]. This effect depends on the formation and interaction of reactive oxygen species (ROS) to cause DNA damage and apoptosis [3], so identifying hypoxic areas is crucial to predict the tumor’s treatment response. Significant research interest is being vested on PET imaging of intact levels of ROS in tumor cells using novel [18F]-based small molecule ligands including [18F]KS1, an ascorbate-based radiotracer to image ROS in murine models of PCa and head and neck tumors [49]. [18F]FMISO still has a great prognostic value because it freely crosses the blood-brain barrier, is enzymatically reduced and trapped within viable cells, and its uptake by tumor cells is proportional to their hypoxic level [46]. Thus, in cases of high-grade gliomas, its uptake correlates with predicted adverse prognosis and patient mortality. It can also detect hypoxic tumor areas resistant to therapy. Its main limitation is lipophilicity [46], which is responsible for its slow clearance from normal brain tissue and, consequently, a low tumor-to-background ratio, which can be problematic in imaging [1,50,23].
Protein synthesis
Due to their accelerated growth, tumors have increased protein synthesis and demand for amino acids [16]. Cancer cells’ elevated consumption and upregulation of transporters require more than the regular intracellular supply of amino acids [14]. An array of amino acids have been radiolabeled to evaluate protein synthesis in tumor cells including [11C]methionine ([11C]met), [11C]leucine ([11C]leu), and [11C]tyrosine ([11C]tyr) [40]. They can be used to measure amino-acid transporter activity—not discerned by aminoacyl transfer RNA (tRNA) synthetases and, therefore, no role in protein incorporation mechanisms [14,40]. Some examples that can be diagnose cancer and monitor treatment response are [18F]fluoroethyltyrosine ([18F]FET), [18F]dihydroxy-phenyl-alanine ([18F]FDOPA), [18F]fluoromethyl-tyrosine ([18F]FMT), and trans-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid ([18F]FACBC) [13,40,15,51–53]. Besides leucine, amino acids like glutathione and cysteine present opportunities to have more radiotracer derivatives including [18F]5-fluoro-L-aminosuberic acid ([18F]FASu), [18F]-(2S,4S)-4-fluoroglutamate and [18F](2S,4S)-4-(3-fluoropropyl)glutamate ([18F]FSPG) [12,54]. Just as glutamine is essential for anabolic metabolism in normal cells, glutaminolysis is important in the anaplerosis that occurs in cancer cells [14], many cannot proliferate without it because it provides precursors to produce critical biomolecules and ATP (glutamine addiction). PET radiotracers like L-5-[11C]glutamine, [18F](2S,4R)-4-fluoroglutamine ([18F]FGln), and [18F](2S,4S)-4-(3-fluoropropyl)glutamine ([18F]FPGln) have been called as glutaminolysis imaging agents [12,54]. Methionine plays an essential role in protein synthesis. Overexpression of L-type amino acid transporters 1 and 2 (LAT-1 and LAT-2) in central nervous system (CNS) tumor cells appears to cause a high tumor-to-normal brain background ratio [15]. LAT transports [11C]met across tumor cell membranes, and it is incorporated into protein synthesis and metabolized by alternative pathways [25]. [11C]met can detect low-grade brain tumors with higher sensitivity and specificity than [18F]FDG [23]. Its high radiochemical yield and low uptake in normal brain are significant advantages [24]. Amino acids and their derivatives, including [11C]met and L-3-[18F]fluoro-α-methyltyrosine ([18F]FAMT) are also being increasingly used to detect lung carcinomas [55]. Both [11C]met and [18F]FAMT have proven more efficient in imaging malignant thoracic nodules with high specificity than [18F]FDG [56]. However, more clinical research is needed to used them in imaging lung carcinomas at different stages. L-3,4-dihydroxy-6-[18F]fluorophenylalanine ([18]FDOPA) is a radiolabeled amino acid analogue originally developed to assess the presynaptic dopaminergic function in patients with neurodegenerative and movement disorders [15]. Its uptake occurs by LAT1 and high uptake explains its accuracy in imaging primary, metastatic, and recurrent brain tumors [23]. It is frequently considered a surrogate of [11C]met, since with both, high uptake reveals hot spots and they both match well in detecting hot spots with higher uptake and revealing a low uptake, contralateral normal brain tissue [25,51], but [18F]FDOPA is more widely used due to the longer half-life of [18F] (1.83 h) over [11C] (20 min), and its more favorable pharmacological properties than methionine’s [15]. Despite these advantages, it proved inefficacious in the clinical diagnosis of residual disease after first-line radio- or chemotherapy. Recently, dopaminergic pathway radiotracers are being tested for diagnosing pulmonary nodules and evaluate treatment responses [23,57].
Conclusions
PET radiotracer development is accelerating due to the precise mapping of novel molecular tumor targets and rapid in vitro and preclinical in vivo testing of lead compounds in the early stages of development. Although [18F]FDG is still considered the gold standard for imaging metabolic processes in all cancers, its ability to differentiate tumor from infection or acute inflammation and tumor growth and recurrence in both low-grade and high-grade tumors is poor. Several tumor target-specific radiotracers are now in the translational pipeline to meet the growing demand for imaging technology that can help in managing complex aspects of cancer diagnosis, monitoring, and prognosis.
Acknowledgment
NIH: R01AG0658389-01 (KKSS), Wake Forest CRBM pilot: P30CA012197(KKSS)
References
- 1.Mettler FA Jr, Guiberteau MJ (2012) Essentials of Nuclear Medicine Imaging: Expert Consult-Online and Print. Elsevier Health Sciences [Google Scholar]
- 2.Lewis DY, Soloviev D, Brindle KM (2015) Imaging tumor metabolism using positron emission tomography. Cancer J 21 (2):129–136. doi: 10.1097/PPO.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrantes AM, Pires AS, Monteiro L, Teixo R, Neves AR, Tavares NT, Marques IA, Botelho MF (2020) Tumour functional imaging by PET. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1866 (6):165717. doi: 10.1016/j.bbadis.2020.165717 [DOI] [PubMed] [Google Scholar]
- 4.Doot RK, McDonald ES, Mankoff DA (2014) Role of PET quantitation in the monitoring of cancer response to treatment: Review of approaches and human clinical trials. Clin Transl Imaging 2 (4):295–303. doi: 10.1007/s40336-014-0071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadrmas DJ, Hoffman JM (2013) Methodology for Quantitative Rapid Multi-Tracer PET Tumor Characterizations. Theranostics 3 (10):757–773. doi: 10.7150/thno.5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurdziel K, Ravizzini G, Croft B, Tatum J, Choyke P, Kobayashi H (2008) The evolving role of nuclear molecular imaging in cancer. Expert opinion on medical diagnostics 2 (7):829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang B-S (2013) MicroSPECT and MicroPET Imaging of Small Animals for Drug Development. Toxicol Res 29 (1):1–6. doi: 10.5487/TR.2013.29.1.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YY, Wang K, Xu ZY, Song Y, Wang CN, Zhang CQ, Sun XL, Shen BZ (2017) High-resolution dynamic imaging and quantitative analysis of lung cancer xenografts in nude mice using clinical PET/CT. Oncotarget 8 (32):52802–52812. doi: 10.18632/oncotarget.17263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koba W, Jelicks LA, Fine EJ (2013) MicroPET/SPECT/CT Imaging of Small Animal Models of Disease. The American Journal of Pathology 182 (2):319–324. doi: 10.1016/j.ajpath.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 10.Kumar Solingapuram Sai K, Sattiraju A, Almaguel FG, Xuan A, Rideout S, Krishnaswamy RS, Zhang J, Herpai DM, Debinski W, Mintz A (2017) Peptide-based PET imaging of the tumor restricted IL13RA2 biomarker. Oncotarget 8 (31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla AK, Kumar U (2006) Positron emission tomography: An overview. Journal of medical physics/Association of Medical Physicists of India 31 (1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallabhajosula S 18F-labeled positron emission tomographic radiopharmaceuticals in oncology: an overview of radiochemistry and mechanisms of tumor localization. In: Seminars in nuclear medicine, 2007. vol 6. Elsevier, pp 400–419 [DOI] [PubMed] [Google Scholar]
- 13.Chen Z-Y, Wang Y-X, Lin Y, Zhang J-S, Yang F, Zhou Q-L, Liao Y-Y (2014) Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. BioMed research international 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambhir SS (2002) Molecular imaging of cancer with positron emission tomography. Nature Reviews Cancer 2 (9):683–693. doi: 10.1038/nrc882 [DOI] [PubMed] [Google Scholar]
- 15.Lukey MJ, Katt WP, Cerione RA (2017) Targeting amino acid metabolism for cancer therapy. Drug discovery today 22 (5):796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challapalli A, Aboagye EO (2016) Positron Emission Tomography Imaging of Tumor Cell Metabolism and Application to Therapy Response Monitoring. Front Oncol 6:44–44. doi: 10.3389/fonc.2016.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bensinger SJ, Christofk HR New aspects of the Warburg effect in cancer cell biology. In: Seminars in cell & developmental biology, 2012. vol 4. Elsevier, pp 352–361 [DOI] [PubMed] [Google Scholar]
- 18.Toyama H, Ichise M, Liow J-S, Modell KJ, Vines DC, Esaki T, Cook M, Seidel J, Sokoloff L, Green MV, Innis RB (2004) Absolute Quantification of Regional Cerebral Glucose Utilization in Mice by 18F-FDG Small Animal PET Scanning and 2–14C-DG Autoradiography. Journal of Nuclear Medicine 45 (8):1398–1405 [PubMed] [Google Scholar]
- 19.Almuhaideb A, Papathanasiou N, Bomanji J (2011) 18F-FDG PET/CT imaging in oncology. Ann Saudi Med 31 (1):3–13. doi: 10.4103/0256-4947.75771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeill KA (2016) Epidemiology of brain tumors. Neurologic clinics 34 (4):981–998 [DOI] [PubMed] [Google Scholar]
- 21.Giammarile F, Castellucci P, Dierckx R, Estrada Lobato E, Farsad M, Hustinx R, Jalilian A, Pellet O, Rossi S, Paez D (2019) Non-FDG PET/CT in Diagnostic Oncology: a pictorial review. European Journal of Hybrid Imaging 3 (1):20. doi: 10.1186/s41824-019-0066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadvar H (2016) PET of glucose metabolism and cellular proliferation in prostate cancer. Journal of Nuclear Medicine 57 (Suppl 3):25S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansoor NM, Thust S, Militano V, Fraioli F (2018) PET imaging in glioma: techniques and current evidence. Nuclear Medicine Communications 39 (12) [DOI] [PubMed] [Google Scholar]
- 24.Salmon E, Ir CB, Hustinx R Pitfalls and limitations of PET/CT in brain imaging. In: Seminars in nuclear medicine, 2015. vol 6. Elsevier, pp 541–551 [DOI] [PubMed] [Google Scholar]
- 25.Mullen KM, Huang RY (2017) An update on the approach to the imaging of brain tumors. Current neurology and neuroscience reports 17 (7):53. [DOI] [PubMed] [Google Scholar]
- 26.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68 (6):394–424 [DOI] [PubMed] [Google Scholar]
- 27.Xu KM, Chen RC, Schuster DM, Jani AB Role of novel imaging in the management of prostate cancer. In: Urologic Oncology: Seminars and Original Investigations, 2019. Elsevier; [DOI] [PubMed] [Google Scholar]
- 28.Wallitt KL, Khan SR, Dubash S, Tam HH, Khan S, Barwick TD (2017) Clinical PET imaging in prostate cancer. Radiographics 37 (5):1512–1536 [DOI] [PubMed] [Google Scholar]
- 29.Jadvar H (2009) FDG PET in prostate cancer. PET clinics 4 (2):155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans JD, Jethwa KR, Ost P, Williams S, Kwon ED, Lowe VJ, Davis BJ (2018) Prostate cancer–specific PET radiotracers: A review on the clinical utility in recurrent disease. Practical radiation oncology 8 (1):28–39 [DOI] [PubMed] [Google Scholar]
- 31.Lemjabbar-Alaoui H, Hassan OU, Yang Y-W, Buchanan P (2015) Lung cancer: Biology and treatment options. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1856 (2):189–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postmus P, Kerr K, Oudkerk M, Senan S, Waller D, Vansteenkiste J, Escriu C, Peters S (2017) Early and locally advanced non-small-cell lung cancer (NSCLC) [DOI] [PubMed] [Google Scholar]
- 33.Postmus P, Kerr K, Oudkerk M, Senan S, Waller D, Vansteenkiste J, Escriu C, Peters S (2017) Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 28 (suppl_4):iv1–iv21 [DOI] [PubMed] [Google Scholar]
- 34.Greenspan BS (2017) Role of PET/CT for precision medicine in lung cancer: perspective of the Society of Nuclear Medicine and Molecular Imaging. Translational lung cancer research 6 (6):617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpi S, Ali JM, Tasker A, Peryt A, Aresu G, Coonar AS (2018) The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Annals of translational medicine 6 (5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massarweh NN, El-Serag HB (2017) Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control 24 (3):1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolthammer JA, Corn DJ, Tenley N, Wu C, Tian H, Wang Y, Lee Z (2011) PET imaging of hepatocellular carcinoma with 18 F-fluoroethylcholine and 11 C-choline. European journal of nuclear medicine and molecular imaging 38 (7):1248–1256 [DOI] [PubMed] [Google Scholar]
- 38.Lee SM, Kim HS, Lee S, Lee JW (2019) Emerging role of 18F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma. World journal of gastroenterology 25 (11):1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubash SR, Idowu OA, Sharma R (2015) The emerging role of positron emission tomography in hepatocellular carcinoma. Hepatic oncology 2 (2):191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith G, Carroll L, Aboagye EO (2012) New frontiers in the design and synthesis of imaging probes for PET oncology: current challenges and future directions. Molecular imaging and biology 14 (6):653–666 [DOI] [PubMed] [Google Scholar]
- 41.Kiran Kumar Solingapuram S, Lynne AJ, Robert HM (2013) Development of 18F-Labeled PET Probes for Imaging Cell Proliferation. Current Topics in Medicinal Chemistry 13 (8):892–908. doi: 10.2174/1568026611313080003 [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Chen Y, Wu S, Song F, Zhang H, Tian M (2016) Molecular imaging using PET and SPECT for identification of breast cancer subtypes. Nuclear medicine communications 37 (11):1116–1124 [DOI] [PubMed] [Google Scholar]
- 43.Jung J-h, Ahn B-C (2018) Current radiopharmaceuticals for positron emission tomography of brain tumors. Brain tumor research and treatment 6 (2):47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solingapuram sai Kk, Jones L, Mach R (2013) Development of F-18-Labeled PET Probes for Imaging Cell Proliferation. Current Topics in Medicinal Chemistry 13:892–908 [DOI] [PubMed] [Google Scholar]
- 45.Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR (2019) Hypoxia-modified cancer cell metabolism. Frontiers in cell and developmental biology 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Z, Li X-F, Zou H, Sun X, Shen B (2017) (18)F-Fluoromisonidazole in tumor hypoxia imaging. Oncotarget 8 (55):94969–94979. doi: 10.18632/oncotarget.21662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haubner R, Wester H-J, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M (2001) Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer research 61 (5):1781–1785 [PubMed] [Google Scholar]
- 48.Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL, Schnell O, Niemeyer M, Kessler H, Wester H-J (2006) Positron emission tomography using [18F] Galacto-RGD identifies the level of integrin αvβ3 expression in man. Clinical Cancer Research 12 (13):3942–3949 [DOI] [PubMed] [Google Scholar]
- 49.Solingapuram Sai KK, Bashetti N, Chen X, Norman S, Hines JW, Meka O, Kumar JVS, Devanathan S, Deep G, Furdui CM, Mintz A (2019) Initial biological evaluations of 18F-KS1, a novel ascorbate derivative to image oxidative stress in cancer. EJNMMI Research 9 (1):43. doi: 10.1186/s13550-019-0513-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.TACHIBANA I, NISHIMURA Y, HANAOKA K, INADA M, FUKUDA K, TATEBE H, ISHIKAWA K, NAKAMATSU K, KANAMORI S, HOSONO M (2018) Tumor Hypoxia Detected by 18F-fluoromisonidazole Positron Emission Tomography (FMISO PET) as a Prognostic Indicator of Radiotherapy (RT). Anticancer Research 38 (3):1775–1781 [DOI] [PubMed] [Google Scholar]
- 51.Calabria F, Cascini GL (2015) Current status of 18F-DOPA PET imaging in the detection of brain tumor recurrence. Hellenic journal of nuclear medicine 18 (2):152–156 [DOI] [PubMed] [Google Scholar]
- 52.Peyraga G, Robaine N, Khalifa J, Cohen-Jonathan-Moyal E, Payoux P, Laprie A (2018) Molecular PET imaging in adaptive radiotherapy: brain. The quarterly journal of nuclear medicine and molecular imaging: official publication of the Italian Association of Nuclear Medicine (AIMN)[and] the International Association of Radiopharmacology (IAR),[and] Section of the Society of 62 (4):337–348 [DOI] [PubMed] [Google Scholar]
- 53.Fink JR, Muzi M, Peck M, Krohn KA (2015) Multimodality brain tumor imaging: MR imaging, PET, and PET/MR imaging. Journal of Nuclear Medicine 56 (10):1554–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends in biochemical sciences 35 (8):427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubota K, Yamada K, Fukada H, Endo S, Ito M, Abe Y, Yamaguchi T, Fujiwara T, Sato T, Ito K (1984) Tumor detection with carbon-11-labelled amino acids. European journal of nuclear medicine 9 (3):136–140 [DOI] [PubMed] [Google Scholar]
- 56.Hsieh H-J, Lin S-H, Lin K-H, Lee C-Y, Chang C-P, Wang S-J (2008) The feasibility of 11 C-methionine-PET in diagnosis of solitary lung nodules/masses when compared with 18 F-FDG-PET. Annals of nuclear medicine 22 (6):533. [DOI] [PubMed] [Google Scholar]
- 57.Humbert O, Bourg V, Mondot L, Gal J, Bondiau P-Y, Fontaine D, Saada-Bouzid E, Paquet M, Chardin D, Almairac F (2019) 18 F-DOPA PET/CT in brain tumors: impact on multidisciplinary brain tumor board decisions. European journal of nuclear medicine and molecular imaging 46 (3):558–568 [DOI] [PubMed] [Google Scholar]


