Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant DuPont submitted a request to the competent national authority in Ireland to set an import tolerance for the active substance oxathiapiprolin in blueberries in support of an authorised use in the United States. The data submitted in support of the request were found to be sufficient to derive a maximum residue level (MRL) proposal for highbush blueberries by noting that lowbush blueberries (Vaccinium angustifolium) are excluded from the authorised use in the United States. Adequate analytical methods for enforcement are available to control the residues of oxathiapiprolin in plant matrices at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concluded that the long‐term intake of residues resulting from the use of oxathiapiprolin according to the reported agricultural practice is unlikely to present a risk to consumer health.
Keywords: Oxathiapiprolin, blueberries, highbush, import tolerance, pesticide, MRL, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, DuPont submitted an application to the competent national authority in Ireland (evaluating Member State, EMS) to set import tolerances for the active substance oxathiapiprolin in blueberries.
The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 28 February 2022. The EMS proposed to establish a maximum residue level (MRL) for highbush blueberries imported from United States at the level of 0.5 mg/kg, noting that lowbush1 blueberry is specifically excluded from the good agricultural practice (GAP) authorised in the United States for which the import tolerance application is made.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL Regulation. EFSA identified points which needed further clarification, which were requested from the EMS. On 25 March 2022, the EMS submitted a revised evaluation report (Ireland, 2022), which replaced the previously submitted evaluation report.
Based on the conclusions derived by EFSA in the framework of Regulation (EC) No 1107/2009, the data evaluated in previous MRL assessments and the additional data provided by the EMS in the framework of this application, the following conclusions are derived.
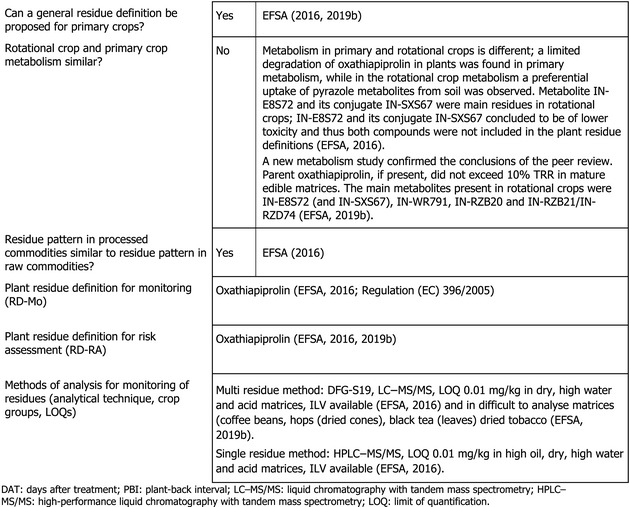
The metabolism of oxathiapiprolin following foliar treatment of primary crops belonging to fruit, leafy and root crop groups has been investigated in the European Union (EU) pesticides peer review and following soil treatment in the framework of a previous EFSA MRL assessment. The main residue in most primary crops following foliar treatment was parent oxathiapiprolin, with exception of mature grapes, where metabolites containing the pyrazole moiety (IN‐E8S72 and IN‐WR791) were major residues. Following soil treatment, parent oxathiapiprolin did not exceed 10% total radioactive residue (TRR) in mature edible matrices; the main components of the TRR in primary crops were metabolites IN‐E8S72, IN‐WR791, IN‐RZB20 and IN‐RZB21/IN‐RZD74. The actual amounts, however, were low, except for metabolite IN‐WR791 in courgettes.
Studies investigating the effect of processing on the nature of oxathiapiprolin (hydrolysis studies) demonstrated that the active substance is stable. As the authorised use of oxathiapiprolin is on imported crops, investigations of residues in rotational crops are not required.
Based on the metabolic pattern identified in the metabolism studies, the hydrolysis studies and the toxicological significance of metabolites, the residue definitions for plant products were proposed by the peer review as ‘oxathiapiprolin’ for enforcement and risk assessment. The same residue definition is implemented in the Regulation (EC) No 396/2005.
EFSA concludes that the residue definitions proposed by the peer review as parent oxathiapiprolin alone are valid also for the crop assessed in the framework of this application.
Sufficiently validated analytical methods based on LC‐MS/MS are available to quantify residues in the crops assessed in this application according to the enforcement residue definition at or above the validated limit of quantification (LOQ) of 0.01 mg/kg.
The available residue trials are sufficient to derive MRL proposals of 0.5 mg/kg for highbush blueberries for the authorised soil application.
Specific processing studies with blueberries are not considered necessary because exposure from the consumption of blueberries is not expected to be significant to consumers.
Processing factors (PF) for blueberries under assessment can be extrapolated for blueberry juice from processing studies on grapes (juice) and can be recommended to be included in Annex VI of Regulation (EC) No 396/2005 as follows:
Grape juice (extrapolated to blueberry juice): 0.16
As blueberries are not fed to livestock, the animal dietary burden and conclusion derived in the previous assessments are considered still applicable.
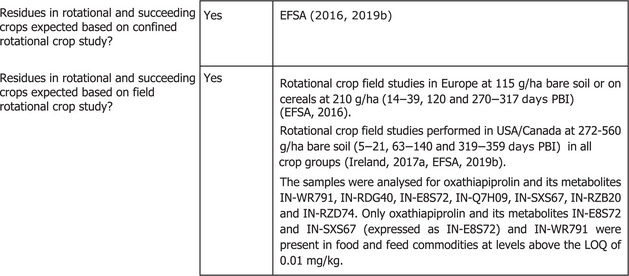
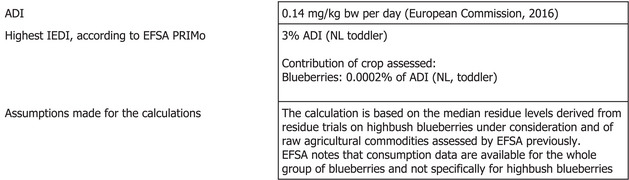
The toxicological profile of oxathiapiprolin was assessed in the framework of the EU pesticides peer review under Regulation (EC) No 1107/2009 and the data were sufficient to derive an acceptable daily intake (ADI) of 0.14 mg/kg body weight (bw) per day. An acute reference dose (ARfD) was not considered necessary and thus was not derived.
The consumer risk assessment was performed with revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo). The estimated long‐term dietary intake accounted for a maximum of 3% of the ADI for NL toddler diet with a contribution of blueberries of 2 × 10−4% of the ADI.
EFSA concluded that the authorised use in the United States of oxathiapiprolin on highbush blueberries and the existing uses of oxathiapiprolin will not result in a consumer exposure exceeding the toxicological reference value and therefore are unlikely to pose a risk to consumers’ health, even when considering a worst‐case scenario, without exclusion of lowbush blueberries from the consumption data which cover the group of blueberries.
EFSA proposes to amend the existing MRLs as reported in the summary table below. Full details of all endpoints and the consumer risk assessment can be found in Appendices Appendix B – List of end points, Appendix C – Pesticide Residue Intake Model (PRIMo)–D.
| Code( a ) | Commodity |
Existing EU MRL (mg/kg) |
Proposed EU MRL (mg/kg) |
Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Oxathiapiprolin | ||||
| 154010 | Blueberries( b ) | 0.01* |
0.5 further risk management considerations |
The submitted data are sufficient to derive an MRL proposal for the import tolerance on highbush blueberries. Risk for consumers unlikely even considering a worst‐case scenario, without exclusion of lowbush blueberries from the consumption data which cover the group of blueberries. It is to be noted that lowbush blueberries( b ) are excluded from the GAP for highbush blueberries authorised in the United States. A distinction between different varieties of a commodity (i.e. highbush and lowbush blueberry) is not possible under the assigned EU commodity code for blueberries (154010) in Part A. Therefore, further risk management considerations are required. |
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
It is noted that lowbush blueberries (Vaccinium angustifolium) are specifically excluded from the GAP authorised in the United States. However, in Part A of the Annex I of Regulation (EC) No 396/2005, no distinction is made between highbush and lowbush blueberries. In Part B, specific EU commodity codes for highbush and lowbush blueberries are also not assigned.
Assessment
The European Food Safety Authority (EFSA) received an application from DuPont to set an import tolerance for the active substance oxathiapiprolin in blueberries. The detailed description of the intended uses of oxathiapiprolin in the United States on highbush blueberries, which are the basis for the current MRL application, is reported in Appendix A.
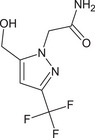
Oxathiapiprolin is the ISO common name for 1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}‐1‐piperidyl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone (IUPAC). The chemical structures of the active substance and its main metabolites are reported in Appendix E.
Oxathiapiprolin was evaluated in the framework of Regulation (EC) No 1107/20092 with Ireland designated as rapporteur Member State (RMS) for the representative uses as a foliar treatment on grapes, potatoes, tomatoes and aubergines. The draft assessment report (DAR) prepared by the RMS has been peer reviewed by EFSA (Ireland, 2015; EFSA, 2016). Oxathiapiprolin was approved3 for the use as fungicide on 3 March 2017.
European MRLs for oxathiapiprolin are established in Annex II of Regulation (EC) No 396/20054. The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) is not foreseen as proposals for setting MRLs covering the representative uses according to good agricultural practices (GAP) in the EU were assessed during the approval of oxathiapiprolin under Regulation (EC) No 1107/2009 and implemented in Regulation in accordance with Article 11(2) of the Regulation (EC)1107/2009. So far EFSA has issued several reasoned opinions on the modification of MRLs for oxathiapiprolin and provided a scientific support for preparing an EU position in the 51st Session of the Codex Committee on Pesticide Residues (CCPR) (EFSA, 2019c). The proposals from these reasoned opinions have been considered in recent MRL Regulations.5
In accordance with Article 6 of Regulation (EC) No 396/2005, DuPont submitted an application to the competent national authority in Ireland (evaluating Member State, EMS) to set an import tolerance for the active substance oxathiapiprolin in blueberries. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the EFSA on 28 February 2022. The EMS proposed to establish a maximum residue level (MRL) for highbush blueberries imported from United States at the level of 0.5 mg/kg. EFSA identified points which needed further clarification, which were requested from the EMS. On 25 March 2022, the EMS submitted a revised evaluation report (Ireland, 2022), which replaced the previously submitted evaluation report.
EFSA based its assessment on the evaluation report submitted by the EMS (Ireland, 2022), the draft assessment report (DAR) and its addendum (Ireland, 2015, 2016) prepared under Regulation (EC) 1107/2009, the Commission review report on oxathiapiprolin (European Commission, 2016), the conclusion on the peer review of the pesticide risk assessment of the active substance oxathiapiprolin (EFSA, 2016), as well as the conclusions from a previous EFSA opinions on oxathiapiprolin (EFSA, 2019b,c, 2020, 2022).
For this application, the data requirements established in Regulation (EU) No 283/20136 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 2010, 2013, 2017, 2020, 2021; OECD, 2007a–d, 2008a,b, 2009a,b, 2011, 2016). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20117.
A selected list of end points of the studies assessed by EFSA in the framework of this MRL application including the end points of relevant studies assessed previously, are presented in Appendix B.
The evaluation report submitted by the EMS (Ireland, 2022) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
In the framework of the EU pesticides peer review, the metabolism of oxathiapiprolin in primary crops belonging to fruit (grapes), leafy (lettuces) and root (potatoes) crops has been investigated following foliar application (EFSA, 2016). Due to the low total radioactive residue (TRR) at harvest, identification of the residues was not attempted in potato tubers. In grapes, lettuces and potato leaves, oxathiapiprolin was observed as the major component of the TRR, accounting for 25–85%.
Additional studies were evaluated in a previous EFSA assessment where the nature of oxathiapiprolin was investigated after soil application (600 g a.s./ha; radiolabelling in pyrazole and isoxazoline moiety) in root (potatoes), leafy (lettuces) and fruit (courgettes) crops (EFSA, 2019b).
The TRR in potato tubers and lettuces decreased over time, whereas in other matrices, an increase of residues was observed. The TRRs from the isoxazoline study in all matrices were generally lower; in mature edible crops, radioactivity was below 0.01 mg eq./kg and thus not further characterised. Parent oxathiapiprolin, if present, did not exceed 10% TRR in mature edible matrices. The main components of the TRR in immature and mature edible matrices (potatoes, lettuces and courgettes) exceeding the trigger value of 10% were metabolites IN‐E8S72, IN‐WR791, IN‐RZB20 and IN‐RZB21/IN‐RZD74. The actual amounts, however, were low, being above 0.01 mg/kg only for metabolite IN‐WR791 in courgettes (0.016 mg/kg) (EFSA, 2019b).
All metabolites identified have been also observed in rotational crops and, to a lesser extent, in primary crops following foliar application (EFSA, 2016, 2019b).
For the use under consideration which is authorised in the United States and intended as an import tolerance in the EU (soil treatment on fruit crop), the metabolic behaviour in primary crops is sufficiently addressed.
1.1.2. Nature of residues in rotational crops
Investigations of residues in rotational crops are not required for imported crops.
It is, however, to be noted that the nature of oxathiapiprolin in rotational crops has been investigated in the EU pesticides peer review in studies where bare soil was treated at an application rate of 210 g/ha, sowing wheat, lettuces and turnips as rotational crops 30, 120 and 365 days after the soil treatment (EFSA, 2016).
In the framework of a recent Art. 10 assessment, the applicant submitted new metabolism studies where the nature of [14C]‐oxathiapiprolin was investigated in turnips, lettuces and wheat grown as rotational crops 30, 120 and 365 days following the soil treatment with oxathiapiprolin at a rate of 600 g/ha. These new studies confirm the conclusions of the peer review (EFSA, 2019b).
A comparison of both studies indicated that there is no significant difference in the magnitude of residues in crops from the low‐ and the high‐dose rate studies. The persistent soil metabolites, which have been identified in the soil degradation studies, were not identified in the rotational crop metabolism studies (EFSA, 2019b).
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of oxathiapiprolin was investigated in the framework of the EU pesticides peer review (EFSA, 2016). These studies showed that oxathiapiprolin is hydrolytically stable under standard processing conditions.
1.1.4. Methods of analysis in plants
Analytical methods for the determination of oxathiapiprolin residues in high oil, dry, high water and high acid content commodities of plant origin were assessed during the EU pesticides peer review (EFSA, 2016).
The multiresidue method using LC‐MS/MS is sufficiently validated for quantifying residues of oxathiapiprolin in the crop under consideration at or above the LOQ of 0.01 mg/kg.
A single residue HPLC‐MS/MS is sufficiently validated quantifying residues of oxathiapiprolin in the crop under consideration at or above the LOQ of 0.01 mg/kg.
Extraction efficiency was demonstrated for both methods (for the multiresidue method being 81–103% and for the single residue method being 98–113% of the incurred residue removed by the metabolism extraction procedure, respectively) (Ireland, 2015, 2016, 2022; EFSA, 2019b; 2020).
Sufficiently validated analytical methods are available for the determination of oxathiapiprolin at the validated LOQ of 0.01 mg/kg in blueberries (high acid matrix).
1.1.5. Storage stability of residues in plants
The storage stability of oxathiapiprolin in plant matrices stored under frozen conditions was investigated in the framework of the EU pesticides peer review (EFSA, 2016) (See Appendix B.1.1.2). It is concluded that in the crop matrix under consideration, the freezer storage stability of oxathiapiprolin has been demonstrated for 18 months when stored at −20°C.
1.1.6. Proposed residue definitions
Based on the metabolic pattern identified in metabolism studies, the results of hydrolysis studies, the toxicological significance of metabolites and the capabilities of enforcement analytical methods, the following residue definitions were proposed during the EU peer review (EFSA, 2016) and confirmed after subsequent MRL assessments (EFSA, 2019b):
residue definition for risk assessment: oxathiapiprolin;
residue definition for enforcement: oxathiapiprolin.
The same residue definitions are applicable to rotational crops and processed products. The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical.
Taking in account the authorised use in USA assessed in this application (soil treatment on fruit crop), EFSA concluded that these residue definitions are appropriate and no modification or further information is required.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
In support of the authorised use in the United States, the applicant submitted residue trials performed on highbush blueberries. The samples were analysed for the parent compound according to the residue definition for enforcement and risk assessment.
According to the assessment of the EMS, the methods used were sufficiently validated and fit for purpose (Ireland, 2022). The samples of these residue trials were stored under conditions for which integrity of the samples has been demonstrated.
Blueberries (highbush) (United States, outdoor soil treatment, 2 × 280 g a.i./ha; interval between applications: 7–30 days; preharvest interval (PHI): 1 day). Low bush blueberries1 for which different use instruction apply in the United States are exempt from the GAP under assessment.
In support of the authorised outdoor soil treatment GAP of oxathiapiprolin on highbush blueberries in the United States, eight GAP compliant field trials were provided. These trials were performed on highbush blueberries in the 2016 growing season in selected trial locations (US EPA, 1996).
The trials were independent and in compliance with the authorised GAP in the United States (two soil‐directed spray applications, each at a nominal rate of 280 g a.s./ha (0.25 lb a.s./A), at a 6‐day (two trials) to 7‐day (six trials) interval, with the last application 1 day prior to harvest). The trials are representative of the most critical conditions of the GAP. No residues of oxathiapiprolin or metabolites at above the LOQ (0.01 mg/kg) were found in any of the untreated samples. Two trials were performed as decline trials with PHIs of 1, 3, 7, 10 and 14 days. These trials did not show any increase of residues at PHI longer than 1 day.
The current residue data are sufficient to derive an MRL proposal of 0.5 mg/kg in support of the authorised GAP for highbush blueberries (according to the product label provided: bushberry subgroup 13‐07B, except blueberry, lowbush1) in the United States, which specifically excludes lowbush1 blueberries, for which oxathiapiprolin has different use instructions in the United States.
For information, the EMS outlined that lowbush blueberry can be grouped in several crop categories in the United States (in the case of oxathiapiprolin, it is 13‐07G (low growing berries) rather than 13‐07B (bushberry subgroup, except blueberry, lowbush1)). The current MRL in the United States for lowbush1 blueberry is 0.4 mg/kg, which is lower than the proposed import tolerance of 0.5 mg/kg for highbush blueberries (Ireland, 2022).
The tolerance established in the United States8 for oxathiapiprolin in highbush blueberries is 0.5 mg/kg.
1.2.2. Magnitude of residues in rotational crops
The investigation of rotational crops is of no relevance for the import tolerance requests considered under the assessment.
1.2.3. Magnitude of residues in processed commodities
The effect of processing on the magnitude of residues was assessed in the evaluation report of the EMS. It was noted that no new studies investigating the effect of processing on the magnitude of residues on blueberries have been submitted (Ireland, 2022).
However, according to the OECD Guidance, the only major (category 1) industrial process using blueberry is juice making for which an extrapolation of a processing factor of grape juice making is applicable (OECD, 2008b). A processing factor for grape juice has been previously derived in the framework of the EU pesticides peer review (EFSA, 2016). This processing factor can therefore be extrapolated to blueberry juice. Available studies on grape juice suffice to derive robust processing factor of 0.16 which is recommended to be included in Annex VI of Regulation (EC) No 396/2005. An overview of available processing factors is presented in Appendix B.1.2.3.
Dehydration is also relevant to blueberries. Drying of blueberries represents a category 2 process, whereby an extrapolation from raisins is not applicable (OECD, 2008b). However, a processing study on drying of blueberries is not available and not required by noting the low overall consumer exposure to oxathiapiprolin residues.
Considering the low overall consumer exposure to oxathiapiprolin residues (see Section 3), the processing factor for blueberry juice is not considered by EFSA in the consumer exposure assessment.
1.2.4. Proposed MRLs
The available data are sufficient to derive an MRL proposal of 0.5 mg/kg as well as risk assessment values for highbush blueberries under evaluation by noting that further risk management considerations are required (see Section 1.2.1 and Appendix B.1.2.1). In Section 3, EFSA assessed whether residues on these crops resulting from the uses authorised for import tolerance requests are likely to pose a consumer health risk.
Notably, it is not possible under the assigned EU commodity code for blueberries to distinguish lowbush blueberries (Vaccinium angustifolium) from highbush blueberries because Commission Regulation (EU) 2018/629 provides only one code (0154010) covering several scientific names of blueberry species including Vaccinium angustifolium. Further risk management considerations are therefore required.
The current tolerance in the United States for oxathiapiprolin in blueberry (bush berry subgroup, 13‐07B except lowbush blueberry) is 0.5 mg/kg.
2. Residues in livestock
Since blueberries are not fed to livestock, the previous dietary burden calculation was not updated (EFSA, 2022). Thus, the nature and magnitude of oxathiapiprolin residues in livestock was not investigated further.
3. Consumer risk assessment
EFSA performed a dietary risk assessment using revision 3.1 of the EFSA PRIMo (EFSA, 2019a). This exposure assessment model contains food consumption data for different subgroups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (EFSA, 2018, 2019a).
The toxicological reference value for oxathiapiprolin used in the risk assessment (i.e. ADI value of 0.14 mg /kg bw per day) was derived in the framework of the EU pesticides peer review (EFSA, 2016; European Commission, 2016). Considering the toxicological profile of the active substance, a short‐term dietary risk assessment was not required.
The long‐term exposure assessment was performed. For this purpose, EFSA updated the previously performed calculation (EFSA, 2022), taking account of the STMR value derived for highbush blueberries assessed in this application. The PRIMo model contains the consumption data for commodities listed in Part A of Annex I of Regulation (EC) 2018/6210, and therefore, consumption figures specific to highbush blueberries only are not available. Thus, the calculations were performed based on consumption of blueberries as reported by Member States.
For the remaining commodities, the existing EU MRLs as established in the Regulation (EU) 2021/180711 were used as input values. For several of these commodities, the STMR values derived in the previous EFSA assessments (EFSA, 2016, 2019b,c, 2020, 2022) were considered as input values by noting that the latest recommendations made in EFSA (EFSA, 2022) are not yet implemented in EU legislation. The complete list of input values is presented in Appendix D.1.
The estimated long‐term dietary intake accounted for a maximum of 3% of the ADI for NL toddler diet. Blueberries contributed with 2 × 10–4% of the ADI (NL, toddlers) which is negligible. Furthermore, because specific data for highbush blueberries only are not available the consumer exposure for the highbush blueberries under considerations may be overestimated. The contribution of residues expected in the commodities assessed in this application to the overall long‐term exposure is presented in detail in Appendix B.3.
EFSA concluded that the long‐term intake of residues of oxathiapiprolin resulting from the existing and the authorised uses is unlikely to present a risk to consumer health. For further details on the exposure calculations, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
4. Conclusion and Recommendations
The data submitted in support of this MRL application are sufficient to derive an MRL proposal for highbush blueberries. EFSA concluded that the authorised use of oxathiapiprolin on the crops under consideration will not result in a consumer exposure exceeding the toxicological reference value and therefore is unlikely to pose a risk to consumers’ health, even when considering a worst‐case scenario, without exclusion of lowbush blueberries from the consumption data which cover the group of blueberries.
It is to be noted that the proposed import tolerance specifically excludes lowbush blueberry (Vaccinium angustifolium), for which oxathiapiprolin has different use instructions and a lower MRL in the United States. A distinction between different varieties of a commodity (i.e. highbush and lowbush blueberry) is not possible in Part A under the assigned EU commodity codes because only one code12 for blueberries (0154010) is available. In Part B, Vaccinium angustifolium is not specifically mentioned and maybe covered by code 0154010‐990 ‘Other species and hybrids of genera Ribes and Vaccinium, not elsewhere mentioned’. Further risk management considerations on how to implement this MRL in the EU legislation are required.
The MRL recommendations are summarised in Appendix B.4.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- Bw
body weight
- CCPR
Codex Committee on Pesticide Residues
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- EC
emulsifiable concentrate
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HPLC‐MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- IEDI
international estimated daily intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LC‐MS/MS
liquid chromatography with tandem mass spectrometry detector
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- STMR
supervised trials median residue
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – Summary of intended GAP triggering the amendment of existing EU MRLs
|
Crop |
NEU, SEU, MS or country |
F G or I( a ) |
Pests or Group of pests controlled |
Preparation | Application | Application rate per treatment |
PHI (days) ( d ) |
Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type( b ) |
Conc. a.s. |
method kind |
range of growth stages & season( c ) |
number min–max |
Interval between application (min) |
g a.s./ L min–max |
Water L/ha min–max |
Rate | Unit | ||||||
|
Blueberries (highbush blueberries, it is noted that lowbush( e ) blueberry is specifically excluded from the GAP) |
US | F | Phytophthora spp. | SC | 200 g a.s./L (equivalent to 1.67 lbs a.s./gal) | Soil treatment – spraying( f ) | New or established plantings | 1–2 | 7–30 |
0.48–2.0 (equivalent to 0.0040–0.017 lb/gal) |
140 (equivalent to a minimum of 15 gal/A) |
67–280 (equivalent to 0.06–0.25 lb a.s./A) | g a.i./ha | 1 |
Annual maximum: 560 g a.s./ha. Established plantings: plants emerged from winter dormancy. A 30‐day minimum interval is recommended on the product label; however, a 7‐day interval with a 1‐day PHI is supported as the most critical condition of the GAP as worst‐case scenario. |
NEU: northern European Union; SEU: southern European Union; MS: Member State; n/a: not applicable.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
Vaccinium angustifolium.
Soil‐directed spray applications were performed.
Appendix B – List of end points
B.1 Residues in plants
B.1.1 Nature of residues and methods of analysis in plants
B.1.1.1 Metabolism studies, methods of analysis and residue definitions in plants
|
Primary crops (available studies) |
Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Grapes | Foliar: 3 × 70 g/ha (BBCH 63–65; BBCH 73 and 77; 14 days interval) |
Foliage: 0 DAT1,2,3, 14 DAT2,3, 76 DALA Berries: 14 DAT2,3, 0 DAT3, 76 DALA |
Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (EFSA, 2016) | |
| Courgettes | Soil: 1 × 600 g/ha (preplanting) | 44 DAT, 79 DAT (maturity) | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (EFSA, 2019b) | ||
| Root crops | Potatoes | Soil: 1 × 600 g/ha (pre‐planting) | Foliage, tubers: 37 DAT, 72 DAT (maturity) | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (EFSA, 2019b) | |
| Foliar: 3 × 70 g/ha (BBCH 53; BBCH 59 and 69; 14 days interval |
Foliage, tubers: 0 DAT2 (foliage only), 14 DAT1,2,3, 28 DAT3 |
Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (EFSA, 2016) | |||
| Leafy crops | Lettuces | Foliar: 3 × 70 g/ha (BBCH 15; BBCH 17 and 19; 10 d interval) | 0 DAT1,2,3, 10 DAT1,2, 0 DAT3, 3, 7, 14 DALA | Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (EFSA, 2016) | |
| Soil: 1 x 600 g/ha (pre‐planting) | 30, 44, 57 DAT | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (EFSA, 2019b) | |||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
| Root/tuber crops | Turnips | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin (EFSA, 2016) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (EFSA, 2019b) | ||||
| Leafy crops | Lettuces | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin. (EFSA, 2016) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (EFSA, 2019b) | ||||
| Cereal (small grain) | Wheat | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin (EFSA, 2016) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (EFSA 2019b) | ||||
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes | Studies performed with pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (EFSA, 2016) | |||
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||||
| Sterilisation (20 min, 120°C, pH 6) | Yes | ||||
| Other processing conditions | – | – | |||
B.1.1.2 Stability of residues in plants
|
Plant products (Available studies) |
Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/ Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
|
High water content |
Tomatoes, wheat forage | –20 | 18 | Months |
Oxathiapiprolin, IN‐Q7H09, IN‐RDG40, IN‐E8S72, IN‐RZB20, IN‐RZD74, IN‐SXS67 and IN‐WR791 |
The stability was established for each compound independently (EFSA, 2016) | |
| High oil content | Soybean seed | ||||||
| High protein content | Dried bean seed | ||||||
| Dry/High starch | Potatoes, wheat grain | ||||||
| High acid content | Grapes | ||||||
| Others | Grape dry pomace | ||||||
| Wheat straw | |||||||
B.1.2 Magnitude of residues in plants
B.1.2.1 Summary of residues data from the supervised residue trials
| Commodity |
Region/ Indoor( a ) |
Residue levels observed in the supervised residue trials (mg/kg) |
Comments/Source |
Calculated MRL (mg/kg) |
HR( b ) (mg/kg) |
STMR( c ) (mg/kg) |
|---|---|---|---|---|---|---|
|
Enforcement residue definition: Oxathiapiprolin Risk assessment residue definition: Oxathiapiprolin | ||||||
| Blueberries, highbush only | USA/outdoor |
Blueberries (highbush): 6 × < 0.01, 0.15, 0.27 |
Sufficient number of GAP compliant trials on highbush blueberries submitted to derive an MRL proposal for highbush blueberries. Residue values represent an average of two analytical replicates. Individual residue values were all below the LOQ for the first six trials and for the last two trials 0.11 and 0.42 mg/kg and 0.1 and 0.2 mg/kg, respectively (Ireland, 2022). |
0.5 | 0.27 | < 0.01 |
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
B.1.2.2 Residues in rotational crops
Investigations of residues in rotational crops are not required for imported crops.
B.1.2.3 Processing factors
| Processed commodity |
Number of valid studies( a ) |
Processing Factor (PF) | CFP ( b ) |
Comment/ Source |
|
|---|---|---|---|---|---|
| Individual values | Median PF | ||||
| No new processing studies were submitted for this application and deem not necessary considering the low consumer exposure (see Appendix B.3). | |||||
| Grape, Juice | 4 | 0.13; 0.14; 0.18; 0.22 | 0.16 | 1 |
EFSA (2016) Extrapolated to blueberries, juice possible |
| Additional processing studies are available (EFSA, 2016, 2019b, 2020) | |||||
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Conversion factor for risk assessment in the processed commodity; median of the individual conversion factors for each processing residues trial.
B.2 Residues in livestock
Dietary burden calculations are not relevant for this assessment.
B.3 Consumer risk assessment
Short‐term exposure is not relevant since no ARfD has been considered necessary.

B.4 Recommended MRLs
| Code( a ) | Commodity |
Existing EU MRL (mg/kg) |
Proposed EU MRL (mg/kg) |
Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Oxathiapiprolin | ||||
| 154010 |
Blueberries( b ) |
0.01* |
0.5 further risk management considerations |
The submitted data are sufficient to derive an MRL proposal for the import tolerance on highbush blueberries. Risk for consumers unlikely even considering a worst‐case scenario, without exclusion of lowbush blueberries from the consumption data which cover the group of blueberries. It is to be noted that lowbush blueberries( b ) are excluded from the GAP for highbush blueberries authorised in the United States. A distinction between different varieties of a commodity (i.e. highbush and lowbush blueberry) is not possible under the assigned EU commodity code for blueberries (154010) in Part A. Therefore, further risk management considerations are required. |
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
It is noted that lowbush blueberries (Vaccinium angustifolium) are specifically excluded from the GAP authorised in the United States. However, in Part A of the Annex I of Regulation (EC) No 396/2005, no distinction is made between highbush and lowbush blueberries. In Part B, specific EU commodity codes for highbush and lowbush blueberries are also not assigned.
Appendix C – Pesticide Residue Intake Model (PRIMo)

Appendix D – Input values for the exposure calculations
D.1 Consumer risk assessment
| Chronic risk assessment | Acute risk assessment | |||||
|---|---|---|---|---|---|---|
| Code | Commodity | Existing/proposed MRL | Source/type of MRL | Input value (mg/kg) | Comment | |
|
154010 |
Blueberries |
0.05 |
Proposed IT in this assessment |
0.01 |
STMR‐RAC |
Not performed because it was not considered necessary to establish an ARfD. |
| 110010 | Grapefruits | 0.05 | EFSA (2020) | 0.01 | STMR‐RAC | |
| 110020 | Oranges | 0.05 | EFSA (2020) | 0.01 | STMR‐RAC | |
| 110030 | Lemons | 0.05 | EFSA (2020) | 0.01 | STMR‐RAC | |
| 110040 | Limes | 0.05 | EFSA (2020) | 0.01 | STMR‐RAC | |
| 110050 | Mandarins | 0.05 | EFSA (2020) | 0.01 | STMR‐RAC | |
| 110990 | Other citrus fruit | 0.05 | EFSA (2020) | 0.01 | STMR‐RAC | |
| 151010 | Table grapes | 0.7 | EFSA (2019b) | 0.12 | STMR‐RAC | |
| 151020 | Wine grapes | 0.7 | EFSA (2019b) | 0.12 | STMR‐RAC | |
| 153010 | Blackberries | 0.5 | EFSA (2020) | 0.01 | STMR‐RAC | |
| 153030 | Raspberries (red and yellow) | 0.5 | EFSA (2020) | 0.01 | STMR‐RAC | |
| 211000 | Potatoes | 0.01 | EFSA (2016) | 0.01 | STMR‐RAC | |
| 220010 | Garlic | 0.04 | EFSA (2019b) | 0.01 | STMR‐RAC | |
| 220020 | Onions | 0.04 | EFSA (2019b) | 0.01 | STMR‐RAC | |
| 220030 | Shallots | 0.04 | EFSA (2019b) | 0.01 | STMR‐RAC | |
| 220040 | Spring onions/green onions and Welsh onions | 2 | EFSA (2019b) | 0.57 | STMR‐RAC | |
| 220990 | Other bulb vegetables | 0.04 | EFSA (2019b) | 0.01 | STMR‐RAC | |
| 231010 | Tomatoes | 0.4 | EFSA (2019b) | 0.04 | STMR‐RAC | |
| 231020 | Sweet peppers/bell peppers | 0.2 | EFSA (2019b) | 0.04 | STMR‐RAC | |
| 231030 | Aubergines/egg plants | 0.4 | EFSA (2019b) | 0.04 | STMR‐RAC | |
| 231040 | Okra/lady’s fingers | 0.2 | EFSA (2019b) | 0.04 | STMR‐RAC | |
| 231990 | Other solanaceae | 0.2 | EFSA (2019b) | 0.04 | STMR‐RAC | |
| 232010 | Cucumbers | 0.2 | EFSA (2019b) | 0.03 | STMR‐RAC | |
| 232020 | Gherkins | 0.2 | EFSA (2019b) | 0.03 | STMR‐RAC | |
| 232030 | Courgettes | 0.2 | EFSA (2019b) | 0.03 | STMR‐RAC | |
| 232990 | Other cucurbits – edible peel | 0.2 | EFSA (2019b) | 0.03 | STMR‐RAC | |
| 233010 | Melons | 0.2 | EFSA (2019b) | 0.05 | STMR‐RAC | |
| 233020 | Pumpkins | 0.2 | EFSA (2019b) | 0.05 | STMR‐RAC | |
| 233030 | Watermelons | 0.2 | EFSA (2019b) | 0.05 | STMR‐RAC | |
| 233990 | Other cucurbits – inedible peel | 0.2 | EFSA (2019b) | 0.05 | STMR‐RAC | |
| 241010 | Broccoli | 1.5 | EFSA (2019b) | 0.12 | STMR‐RAC | |
| 241020 | Cauliflowers | 1.5 | EFSA (2019b) | 0.12 | STMR‐RAC | |
| 241990 | Other flowering brassica | 1.5 | EFSA (2019b) | 0.12 | STMR‐RAC | |
| 242020 | Head cabbages | 0.7 | EFSA (2019b) | 0.14 | STMR‐RAC | |
| 243010 | Chinese cabbages/pe‐tsai | 9 | EFSA (2020) | 2.9 | STMR‐RAC | |
| 243020 | Kales | 1.5 | Proposed by EFSA (2022) | 0.42 | STMR‐RAC | |
| 251010 | Lamb's lettuce/corn salads | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 251020 | Lettuces | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 251030 | Escaroles/broad‐leaved endives | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 251040 | Cress and other sprouts and shoots | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 251050 | Land cress | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 251060 | Roman rocket/rucola | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 251070 | Red mustards | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 251080 | Baby leaf crops (including brassica species) | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 251990 | Other lettuce and other salad plants | 5 | EFSA (2019b) | 1.3 | STMR‐RAC | |
| 252010 | Spinaches | 15 | EFSA (2019b) | 3.35 | STMR‐RAC | |
| 252020 | Purslanes | 15 | EFSA (2019b) | 3.35 | STMR‐RAC | |
| 252030 | Chards/beet leaves | 15 | EFSA (2019b) | 3.35 | STMR‐RAC | |
| 252990 | Other spinach and similar | 15 | EFSA (2019b) | 3.35 | STMR‐RAC | |
| 253000 | Grape leaves and similar species | 40 | EFSA (2016) | 8.8 | STMR‐RAC | |
| 256080 | Basil and edible flowers | 10 | EFSA (2020) | 3.05 | STMR‐RAC | |
| 260030 | Peas (with pods) | 1 | EFSA (2019b) | 0.29 | STMR‐RAC | |
| 270010 | Asparagus | 2 | EFSA (2020) | 0.55 | STMR‐RAC | |
| 270060 | Leeks | 2 | EFSA (2019b) | 0.57 | STMR‐RAC | |
| 401050 | Sunflower seeds | 0.01 | EFSA (2019b) | 0.01 | STMR‐RAC | |
| 633020 | Ginseng root | 0.15 | EFSA (2019b) | 0.05 | STMR‐RAC | |
| 700000 | Hops (dried) | 8 | EFSA (2019b) | 1.6 | STMR‐RAC | |
| … | Other crops/commodities of plant and animal origin | MRL | Reg. (EU) 2021/1807 | |||
STMR‐RAC: standardised median residue in raw agricultural commodities in supervised residue trials
Appendix E – Used compound codes
| Code/trivial name | Chemical name/SMILES notation/InChiKey( a ) | Structural formula( b ) |
|---|---|---|
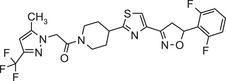
| Oxathiapiprolin |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}‐1‐piperidyl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethenone FC(F)(F)c1cc(C)n(n1)CC(=O)N1CCC(CC1)c1nc(cs1)C=1CC(ON=1)c1c(F)cccc1F IAQLCKZJGNTRDO‐UHFFFAOYSA‐N |
|
| IN‐Q7H09 |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluoro‐4‐hydroxyphenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidin‐1‐yl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone FC(F)(F)c1cc(C)n(n1)CC(=O)N2CCC(CC2)c3nc(cs3)C=4CC(ON=4)c5c(F)cc(O)cc5F XYJWPIOIQYWLNP‐UHFFFAOYSA‐N |
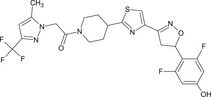
|
| IN‐RDG40 |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluoro‐3‐hydroxyphenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidin‐1‐yl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone FC(F)(F)c1cc(C)n(n1)CC(=O)N2CCC(CC2)c3nc(cs3)C=4CC(ON=4)c5c(F)ccc(O)c5F MCUWVCQCPFWXQQ‐UHFFFAOYSA‐N |
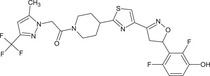
|
| IN‐E8S72 |
3‐(trifluoromethyl)‐1H‐pyrazole‐5‐carboxylic acid FC(F)(F)c1cc(nn1)C(O)=O CIVNBJPTGRMGRS‐UHFFFAOYSA‐N |
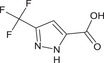
|
| IN‐SXS67 |
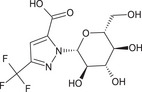
1‐β‐D‐glucopyranosyl‐3‐(trifluoromethyl)‐1H‐pyrazole‐5‐carboxylic acid O = C(O)c2cc(nn2[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C(F)(F)F IYVPJWXJEGAHCP‐DDIGBBAMSA‐N |
|
| IN‐WR791 |
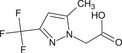
[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]acetic acid OC(=O)Cn1nc(cc1C)C(F)(F)F RBHQAIFXLJIFFM‐UHFFFAOYSA‐N |
|
| IN‐RZB20 |
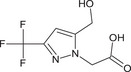
[5‐(hydroxymethyl)‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]acetic acid OC(=O)Cn1nc(cc1CO)C(F)(F)F LGHWWTCDTBCQQI‐UHFFFAOYSA‐N |
|
|
IN‐RZB21 |
2‐[5‐(hydroxymethyl)‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]acetamide O = C(N)Cn1nc(cc1CO)C(F)(F)F LDXIZNIPWOQNPY‐UHFFFAOYSA‐N |
|
| IN‐RZD74 |
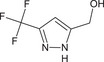
[3‐(trifluoromethyl)‐1H‐pyrazol‐5‐yl]methanol FC(F)(F)c1cc(CO)nn1 KUVPCLYQVMRTPU‐UHFFFAOYSA‐N |
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 June 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 March 2021).
Suggested citation: EFSA (European Food Safety Authority) , Bellisai G, Bernasconi G, Brancato A, Carrasco Cabrera L, Castellan I, Ferreira L, Giner G, Greco L, Jarrah S, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Robinson T, Ruocco S, Santos M, Scarlato AP, Theobald A and Verani A, 2022. Reasoned Opinion on the setting of an import tolerance for oxathiapiprolin in blueberries. EFSA Journal 2022;20(5):7347, 26 pp. 10.2903/j.efsa.2022.7347
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00269
Acknowledgments: EFSA wishes to acknowledge the contribution of Stathis Anagnos, Laszlo Bura, Aija Kazocina, Andrea Mioč, Marta Szot, Aikaterini Vlachou to this opinion
Adopted: 5 May 2022
Notes
Vaccinium angustifolium.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2017/239 of 10 February 2017 approving the active substance oxathiapiprolin in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011 C/2017/0694 OJ L 36, 11.2.2017, p. 39–42
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.03.2005, p. 1–16.
For an overview of all MRL Regulations on this active substance, please consult:https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/active‐substances/?event=search.as
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Commission Regulation (EU) 2018/62 of 17 January 2018 replacing Annex I to Regulation (EC) No 396/2005 of the European Parliament and of the Council. C/2018/0138. OJ L 18, 23.1.2018, p. 1–73
Commission Regulation (EU) 2021/1807 of 13 October 2021 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acibenzolar‐S‐methyl, aqueous extract from the germinated seeds of sweet Lupinus albus, azoxystrobin, clopyralid, cyflufenamid, fludioxonil, fluopyram, fosetyl, metazachlor, oxathiapiprolin, tebufenozide and thiabendazole in or on certain products. C/2021/7292. OJ L 365, 14.10.2021, p. 1–37.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
References
- EFSA (European Food Safety Authority) , 2016. Conclusion on the peer review of the pesticide risk assessment of the active substance oxathiapiprolin. EFSA Journal 2016;14(7):4504, 19 pp. 10.2903/j.efsa.2016.4504 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019a. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1. EFSA supporting publication 2019;16(3):EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2019b. Reasoned opinion on the modification of the existing maximum residue levels and setting of import tolerances for oxathiapiprolin in various commodities. EFSA Journal 2019;17(7):5759, 49 pp. 10.2903/j.efsa.2019.5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2019c. Scientific Report on scientific support for preparing an EU position in the 51st Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal 2019;17(7):5797, 243 pp. 10.2903/j.efsa.2019.5797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Bernasconi G, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2020. Reasoned Opinion on the setting of import tolerances for oxathiapiprolin in various crops. EFSA Journal 2020;18(6):6155, 34 pp. 10.2903/j.efsa.2020.6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Bellisai G, Bernasconi G, Brancato A, Cabrera LC, Ferreira L, Giner G, Greco L, Jarrah S, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Ruocco S, Santos M, Scarlato AP, Theobald A, Vagenende B and Verani A, 2022. Modification of the existing maximum residue level for oxathiapiprolin in kales/radish leaves. EFSA Journal 2022;20(1):7049, 28 pp. 10.2903/j.efsa.2022.7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2010. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2013. Working document on the nature of pesticide residues in fish. SANCO/11187/2013‐rev. 3, 31 January 2013.
- European Commission , 2016. Final review report for the active substance oxathiapiprolin. Finalised in the Standing Committee on Plants, Animal, Food and Feed at its meeting on 7 December 2016 in view of the approval of oxathiapiprolin as active substance in accordance with Regulation (EC) 1107/2009. SANTE/11169/2016 rev 1, 7 December 2016.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.3, 13 June 2017.
- European Commission , 2020. Technical guidelines on data requirements for setting maximum residue levels, comparability of residue trials and extrapolation on residue data on products from plant and animal origin. SANTE/2019/12752, 23 November 2020.
- European Commission , 2021. Guidance Document on Pesticide Analytical Methods for Risk Assessment and Post‐approval Control and Monitoring Purposes. SANTE/2020/12830, Rev.1 24. February 2021.
- Ireland , 2015. Draft Assessment Report (DAR) on the active substance oxathiapiprolin prepared by the rapporteur Member State Ireland in the framework of Regulation (EC). No 1107/2009, February 2015. Available online: www.efsa.europa.eu
- Ireland , 2016. Revised Draft Assessment Report (DAR) on oxathiapiprolin prepared by the rapporteur Member State Ireland in the framework of Regulation (EC). No 1107/2009, March 2016. Available online: www.efsa.europa.eu
- Ireland , 2022. Evaluation report on the MRL application on the setting of Import Tolerances for oxathiapiprolin in blueberries. February 2022, 35 pp.
- OECD (Organisation for Economic Co‐operation and Development) , 2007a. Test No. 501: Metabolism in Crops, Guidelines for the Testing of Chemicals, Section 5, OECD Publishing, Paris, 25 Jan 2007. 10.1787/9789264061835 [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2007b. Guidance Document on Pesticide Residue Analytical Methods. In: Series on Pesticides No 39 / Series on Testing and Assessment No 72. ENV/JM/MONO(2007)17, 13 August 2007. Available online: https://www.oecd.org/chemicalsafety/pesticides‐biocides/publicationsonpesticideresidues
- OECD (Organisation for Economic Co‐operation and Development) , 2007c. Test No 506: Stability of Pesticide Residues in Stored Commodities, OECD Guidelines for the Testing of Chemicals, Section 5, OECD Publishing, Paris, 15 October 2007. 10.1787/9789264061927 [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2007d. Test No. 507: Nature of the Pesticide Residues in Processed Commodities ‐ High Temperature Hydrolysis, OECD Guidelines for the Testing of Chemicals, Section 5, OECD Publishing, Paris, 15 October 2007. 10.1787/9789264067431 [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2008a. Guidance document on the magnitude of pesticide residues in processed commodities. In: Series of Testing and Assessment No 96. ENV/JM/MONO(2008)23, 29 July 2008. Available online: https://www.oecd.org/chemicalsafety/pesticides‐biocides/publicationsonpesticideresidues
- OECD (Organisation for Economic Co‐operation and Development) , 2008b. Test No. 508: Magnitude of the Pesticide Residues in Processed Commodities, OECD Guidelines for the Testing of Chemicals, Section 5, OECD Publishing, Paris, 16 October 2008. 10.1787/9789264067622 [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2009a. Definition of Residue. In: Series on Pesticides, No 31; Series on Testing and Assessment, No. 63. ENV/JM/MONO(2009)30, revision, published 28 July 2009. Available online: https://www.oecd.org/chemicalsafety/pesticides‐biocides/publicationsonpesticideresidues
- OECD (Organisation for Economic Co‐operation and Development) , 2009b, Test No. 509: Crop Field Trial, OECD Guidelines for the Testing of Chemicals, Section 5, OECD Publishing, Paris, 7 September 2009. 10.1787/9789264076457 [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: https://www.oecd.org/chemicalsafety/pesticides‐biocides/publicationsonpesticideresidues [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2016. Guidance Document on Crop Field Trials. In: Series on Pesticides No 66/Series on Testing and Assessment No 164. 2nd Edition. ENV/JM/MONO(2011) 50/REV1, ENV/JM/MONO(2011)50/REV1/ANN, 7 September 2016. Available online: https://www.oecd.org/chemicalsafety/pesticides‐biocides/publicationsonpesticideresidues [Google Scholar]
- US (United States Environmental Protection Agency) , 1996. Residue Chemistry Test Guidelines OPPTS 860.1500 Crop Field Trials, EPA 712–C–96‐183, August 1996.