Abstract
Objectives
There is an urgent need to be able to identify individuals with asymptomatic Leishmania donovani infection, so their risk of progressing to VL and transmitting parasites can be managed. This study examined transcriptional markers expressed by CD4+ T cells that could distinguish asymptomatic individuals from endemic controls and visceral leishmaniasis (VL) patients.
Methods
CD4+ T cells were isolated from individuals with asymptomatic L. donovani infection, endemic controls and VL patients. RNA was extracted and RNAseq employed to identify differentially expressed genes. The expression of one gene and its protein product during asymptomatic infection were evaluated.
Results
Amphiregulin (AREG) was identified as a distinguishing gene product in CD4+ T cells from individuals with asymptomatic L. donovani infection, compared to VL patients and healthy endemic control individuals. AREG levels in plasma and antigen‐stimulated whole‐blood assay cell culture supernatants were significantly elevated in asymptomatic individuals, compared to endemic controls and VL patients. Regulatory T (Treg) cells were identified as an important source of AREG amongst CD4+ T‐cell subsets in asymptomatic individuals.
Conclusion
Increased Treg cell AREG expression was identified in individuals with asymptomatic L. donovani infection, suggesting the presence of an ongoing inflammatory response in these individuals required for controlling infection and that AREG may play an important role in preventing inflammation‐induced tissue damage and subsequent disease in asymptomatic individuals.
Keywords: amphiregulin, CD4+ T cell, Leishmania donovani, regulatory T cell, visceral leishmaniasis
We report the discovery of amphiregulin (AREG) as a distinguishing marker of CD4+ T cells from individuals with asymptomatic Leishmania donovani infection, compared to visceral leishmaniasis (VL) patients and healthy endemic control individuals. Thus, CD4+ T‐cell AREG is elevated during asymptomatic L. donovani infection, suggesting the presence of an ongoing inflammatory response in asymptomatic individuals required for controlling infection and that AREG may play an important role in preventing inflammation‐induced tissue damage and subsequent disease in asymptomatic individuals.

Introduction
The visceral leishmaniasis (VL) elimination initiative in India aims to reduce the incidence of disease to below 1 per 10 000 population per year in VL endemic areas by 2023. One of the major challenges for VL elimination is understanding the risk of disease outbreaks when a significant proportion of healthy individuals living in endemic areas with no history of VL harbour parasites, but remain asymptomatic. 1 , 2 The ratio of incident asymptomatic infections with Leishmania donovani or L. infantum (also known as L. chagasi in South America) to incident clinical cases varies from 1:2.4 in Sudan, 4:1 in Kenya, 5.6:1 in Ethiopia, 18:1 in Brazil, 50:1 in Spain, 4:1 in Bangladesh and 8.9:1 in India and Nepal. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 These data indicate that the majority of individuals infected with Leishmania species develop an effective immune response to contain parasite growth and prevent progression to clinical disease. The role of these asymptomatic individuals in parasite transmission is not well established, but recent evidence indicates they are not a major source of parasite uptake by feeding female sand flies. 10 However, given the important role of acquired immunity, and in particular CD4+ T‐cell responses for controlling parasite growth, changes to the immune status of individuals with asymptomatic infection due to other infections such as human immunodeficiency virus (HIV) or immunosuppressive treatments like steroids can result in progression to VL, and consequently, increased risk of parasite transmission. Therefore, there is a need to be able to identify asymptomatic individuals, so their risk of immune suppression can be managed to reduce the chances of progressing to VL.
The identification of individuals asymptomatically infected with L. donovani is difficult. The precise immune mechanisms underlying protection from VL are still not completely understood in humans. 11 An additional problem is the absence of validated markers for asymptomatic L. donovani infection. Most diagnostic assays are based on anti‐parasite antibodies, with limitations in reliably detecting clinical disease in endemic settings. Therefore, a better test and/or set of biomarkers are needed to make the distinction between acute disease and asymptomatic infection so that individuals in the latter category can be more effectively managed to reduce their risk of progressing to clinical disease. 12
The immune mechanisms responsible for controlling L. donovani growth in asymptomatic individuals are largely unknown. However, CD4+ T cells are likely to play an important role, as indicated by progression of these individuals to VL following HIV infection 13 or immunosuppressive treatments targeting CD4+ T‐cell functions. 14 , 15 Tbet+ IFNγ‐producing CD4+ T (Th1) cells are critical for activating the anti‐microbial machinery in macrophages, the host cell of L. donovani, by inducing the generation of nitric oxide (NO) and reactive oxygen species (ROS) that kill intracellular parasites. 16 There is a marked increase in Th1 cells in some, but not all asymptomatic individuals from Ethiopia, 17 and CD4+ T cells produce IL‐2, IL‐5 and IFNγ in response to stimulation with Leishmania antigens. 18 CD4+ T cells from asymptomatic individuals also produce significantly less IL‐10, compared to CD4+ T cells from VL patients. 19 Furthermore, CD4+ T cells from asymptomatic individuals exhibit enhanced antigen‐specific proliferation, as well as production of cytotoxic molecules, including granzyme B and perforin. 20 Hence, there is a growing body of evidence that functional capacity, and therefore transcriptional activity, of CD4+ T cells from asymptomatic individuals is different from the same cells from VL patients.
In this study, we examined the transcriptional profiles of CD4+ T cells from the blood of asymptomatic individuals, endemic controls and VL patients. We identified a number of differentially expressed genes (DEGs) that could identify CD4+ T cells from asymptomatic individuals. We also noted different distributions of CD4+ T‐cell subsets between asymptomatic individuals, endemic controls and VL patients, and found AREG expression was strongly associated with regulatory T (Treg) cells in the former group. Finally, we measured AREG in plasma and an antigen‐specific whole‐blood assay in asymptomatic individuals infected with L. donovani.
Results
Identifying CD4+ T‐cell molecules that distinguish individuals asymptomatically infected with Leishmania donovani from endemic controls and visceral leishmaniasis patients
CD4+ T cells play critical roles in controlling pathogens such as L. donovani that invade and reside in phagocytic cells. 21 Given that the majority of people infected with this parasite are asymptomatic, 22 , 23 it is likely that CD4+ T cells from asymptomatic individuals exhibit functional properties that distinguish them from uninfected people and those with active disease. As a first step towards understanding these differences, we isolated RNA from peripheral blood CD4+ T cells from asymptomatic, L. donovani‐infected individuals, endemic controls and VL patients and employed RNAseq to identify DEGs (Figure 1). Following correction for false discovery rate (FDR), 66 DEGs were identified in CD4+ T cells from asymptomatic individuals compared to endemic controls with 21 and 45 of these being significantly up‐ and downregulated, respectively (Supplementary table 1; Figure 1). We previously reported on DEGs in CD4+ T cells from VL patients and endemic controls, 24 and next compared DEGs in CD4+ T cells from asymptomatic individuals compared to endemic controls and VL patients, and found 1219 DEGs with 732 and 487 of these significantly up‐ and downregulated, respectively (Supplementary table 2; Figure 1). Therefore, transcriptional differences in CD4+ T cells from asymptomatic L. donovani‐infected individuals, relative to CD4+ T cells from endemic controls and VL patients could be readily detected.
Figure 1.
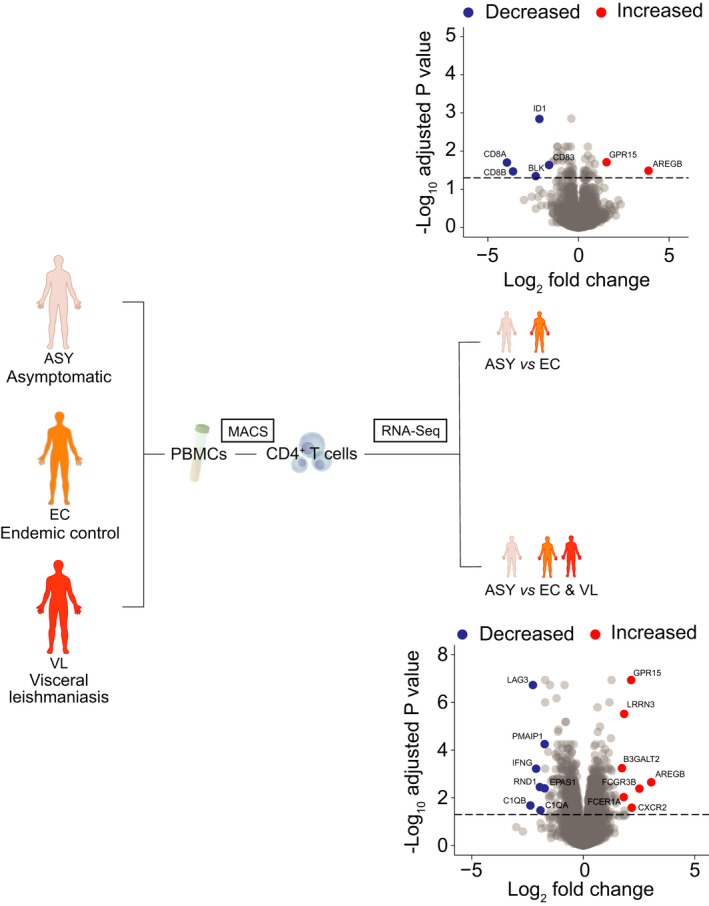
Defining a transcriptomic signature of peripheral blood CD4+ T cells from asymptomatic (ASY) individuals infected with Leishmania donovani. A schematic showing the approach taken to identify transcriptomic signatures of peripheral blood CD4+ T cells from ASY individuals, endemic controls (EC) and visceral leishmaniasis (VL) patients (n = 12 biological replicates in each group). A volcano plot of the differentially expressed genes identified in the comparison of asymptomatic versus endemic controls (upper plot) and ASY versus the average of VL patients and EC. Genes are coloured red (upregulated) or blue (downregulated) according to a false discovery rate < 0.05. Gene labels are shown for selected genes.
CD4+ T‐cell AREG expression marks individuals asymptomatically infected with Leishmania donovani
The top upregulated DEG in CD4+ T cells from asymptomatic individuals compared to the other groups was AREG, which encodes amphiregulin (AREG), a ligand for epidermal receptor growth factor receptor (EGFR) (Figure 1). Notable downregulated DEGs in CD4+ T cells from asymptomatic individuals compared to endemic controls and VL patients were LAG3 and IFNG (Figure 1), both with important functional roles in VL. 25 , 26 The search tool for the retrieval of interacting genes and proteins (STRING): Interaction network analysis was employed to predict protein–protein interactions in DEGs associated with asymptomatic individuals (Supplementary figures 1 and 2). This revealed surprisingly few connected networks, with the exception of a tight network of downregulated DEGs involving IFNG, LAG3, HAVCR2, CD38 and CD83, all molecules, which were previously shown to be significantly upregulated in CD4+ T cells from VL patients compared to endemic controls. 26 , 27 Thus, the increased expression of AREG by CD4+ T cells from asymptomatic individuals was accompanied by reduced expression of genes known to be expressed during active disease.
AREG expression is increased on CD4+ T‐cell subsets from asymptomatic individuals
We next performed a validation study to confirm increased expression of AREG by CD4+ T cells from asymptomatic individuals. We also assessed AREG expression by all peripheral blood mononuclear cells (PBMCs) and found significantly increased AREG expression by PBMCs from asymptomatic individuals, compared to endemic controls, but not VL patients (Figure 2a). Consistent with RNAseq data above, we measured significantly increased expression of AREG by CD4+ T cells from asymptomatic individuals, compared to both endemic controls and VL patients (Figure 2b). Thus, AREG expression by PBMC could distinguish asymptomatic individuals from endemic controls, but not VL patients, while expression of AREG by CD4+ T cells could distinguish asymptomatic individuals from both groups.
Figure 2.
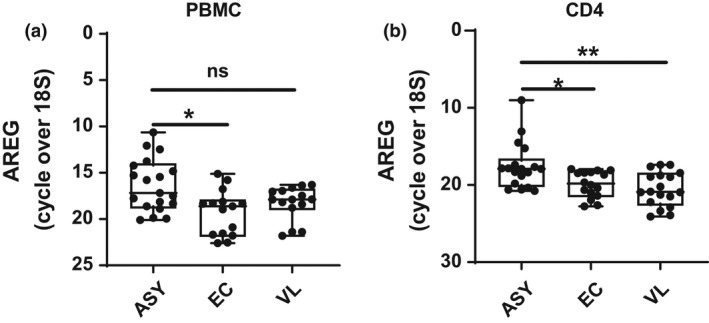
Amphiregulin (AREG) is elevated in CD4+ T cells from asymptomatic individuals infected with Leishmania donovani. RNA was isolated from peripheral blood mononuclear cells (PBMCs) (a) and CD4+ T cells (b) isolated from asymptomatic (ASY; n = 19) individuals, endemic controls (EC; n = 15) and visceral leishmaniasis (VL; n = 15) patients and subjected to qPCR to measure AREG mRNA levels. Median + minimum and maximum are shown; ns = not significant, *P < 0.05, **P < 0.01. Significance was assessed by the Kruskal–Wallis test.
We next assessed the pattern of AREG expression by peripheral blood CD4+ T‐cell subsets using flow cytometry to determine whether changes in CD4+ T‐cell subset distribution and/or AREG expression associate with increased expression of AREG by CD4+ T cells from asymptomatic individuals. We first divided CD4+ T cells into regulatory T (Treg) cells and non‐Treg (conventional) cells (Figure 3a). We further divided CD4+ T cells into Th1, Tfh and Th9 cells (Figure 3b), due to their previous associations with AREG expression, 28 as well as central memory (TCM; CD45RA‐ CCR7+) and effector memory (TEM; CD45RA−/+ CCR7−) cells. The most noticeable differences in CD4+ T‐cell subset frequencies were between asymptomatic individuals and endemic controls and/or VL patients, including decreased frequencies of naïve and TCM CD4+ T cells and increased frequencies of TEM, Tfh, Th1 and Treg cells (Figure 3c). Hence, asymptomatic L. donovani infection is characterised by measurable changes in peripheral blood CD4+ T‐cell subset distribution.
Figure 3.
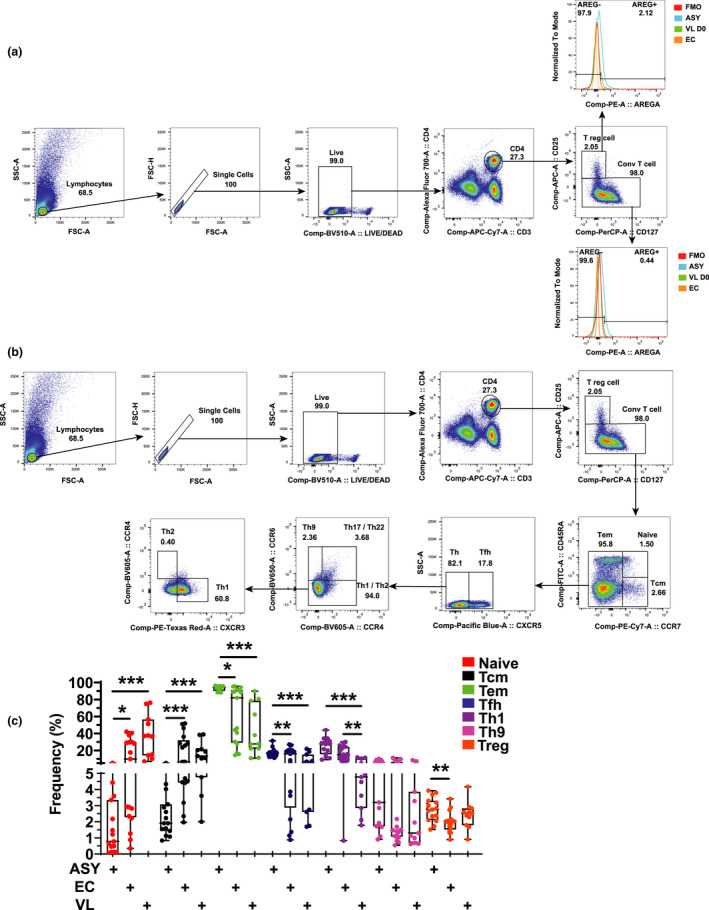
Changes in CD4+ T‐cell subset frequencies and expression of AREG. CD4+ T cells were identified by CD3ε and CD4 expression prior to being divided into regulatory T (Treg) cells and conventional T cells, based on CD25 and CD127 expression, before assessing AREG expression (a). CD4+ T cells were divided into T helper cell subsets based on chemokine receptor expression (b) and frequencies measured in peripheral blood from asymptomatic (ASY; n = 15) individuals, endemic controls (EC; n = 17) visceral leishmaniasis patients (VL; n = 11). The box shows the extent of the lower and upper quartiles plus the median, while the whiskers indicate the minimum and maximum data points (c). *P < 0.05, **P < 0.01 and ***P < 0.001. Significance was assessed by the Kruskal–Wallis test.
Amphiregulin expression by CD4+ T‐cell subsets from asymptomatic individuals, endemic controls and VL patients was next assessed (Figure 4a and Supplementary figure 3a). Increased AREG expression was found when all CD4+ T cells were analysed together, as well as on naïve CD4+ T cells, TCM and Treg cells from asymptomatic individuals, although the difference in AREG expression on naïve CD4+ T cells and TCM cells was only observed with VL patients and not endemic controls. Simplified presentation of incredibly complex evaluations (SPICE) was used to establish overlap in expression of AREG, the CD38 activation marker and chemokine receptors used to identify Th cell subsets (Figure 4b–e and Supplementary figure 3b–e). Again, the increased expression of AREG on Treg cells from asymptomatic individuals was especially pronounced (Figure 4d), but no strong co‐expression with other cell markers was observed. Given the increased frequency of Treg cells and their expression of AREG in asymptomatic individuals, it is likely that Treg cells are an important source of the increased AREG expression associated with CD4+ T cells during asymptomatic L. donovani infection.
Figure 4.
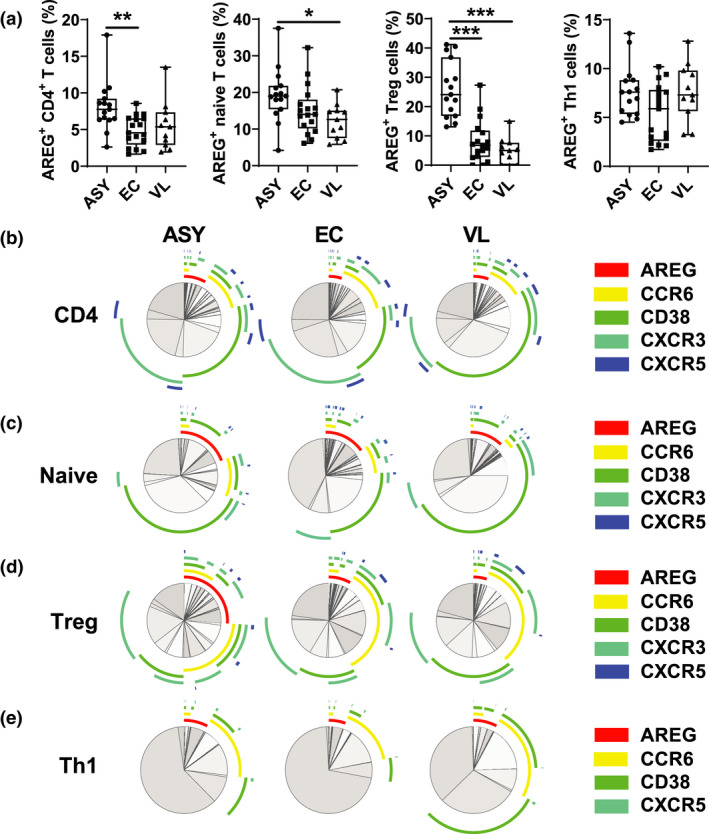
Amphiregulin (AREG) expression by CD4+ T‐cell subsets. The frequency of AREG+ CD4+ T‐cell subsets was measured by FACS. The box shows the extent of the lower and upper quartiles plus the median, while the whiskers indicate the minimum and maximum data points (a). A simplified presentation of incredibly complex evaluations (SPICE) was used to establish overlap in expression of AREG, CD38 and indicated chemokine receptors by all CD4+ T cells (b), naive CD4+ T cells (c), regulatory T (Treg) cells (d) and Th1 cells (e) from asymptomatic (ASY; n = 15) individuals, endemic controls (EC; n = 17) and visceral leishmaniasis (V; n = 11) patients, as indicated. *P < 0.05, **P < 0.01 and ***P < 0.001. Significance was assessed by a one‐way ANOVA with a Tukey’s multiple comparisons test (a).
Increased AREG in plasma and antigen‐stimulated whole‐blood assays from individuals with asymptomatic Leishmania donovani infection
Finally, we sought to determine whether AREG could be readily detected in asymptomatic individuals. We first measured AREG in plasma samples and found AREG levels were significantly elevated in asymptomatic individuals compared to endemic controls and VL patients (Figure 5a). Next, we employed a parasite antigen‐driven whole blood assay 26 , 27 , 28 , 29 and measured significantly elevated levels of AREG in antigen‐stimulated asymptomatic individuals blood samples, but not endemic control and VL patient samples (Figure 5b). The increased antigen‐specific production of AREG correlated with increased IFNγ production in asymptomatic individuals to levels similar or even higher than found in VL patients (Figure 5c). Together, these results indicate that elevated AREG levels could be readily detected in plasma or induced in an antigen‐specific whole‐blood assay from individuals with asymptomatic L. donovani infection.
Figure 5.
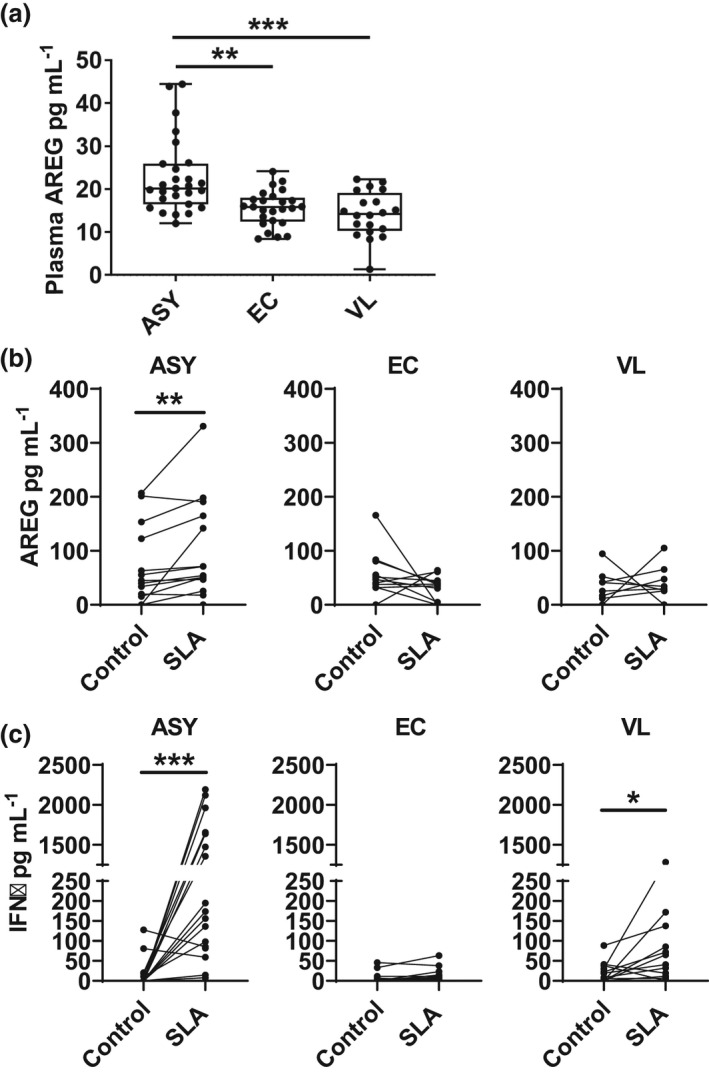
Amphiregulin (AREG) is a marker of asymptomatic L. donovani infection. AREG was measured in plasma from (ASY; n = 27) individuals, endemic controls (EC; n = 25) and visceral leishmaniasis (VL; n = 20) patients (a), as well as from supernatants from whole blood assays stimulated with soluble Leishmania antigen (SLA) or media alone (control), as indicated, from ASY (n = 15), EC (n = 12) and VL (n = 12) (b). IFNγ was measured from the same WBA cell culture supernatants (c). The box shows the extent of the lower and upper quartiles plus the median, while the whiskers indicate the minimum and maximum data points (a). Paired samples are shown in b and c; *P < 0.05, **P < 0.01 and ***P < 0.001. Significance was assessed by the Kruskal–Wallis test (a) or the Wilcoxon matched‐pairs signed rank test (b, c).
Discussion
In this study, we identified a CD4+ T‐cell transcriptional signature for asymptomatic individuals infected with L. donovani. AREG was the most upregulated gene in these cells, while several genes with known roles in VL patients were found to be downregulated in asymptomatic individuals. Accompanying these transcriptional changes were alterations in the distribution of CD4+ T‐cell subsets, including increased frequencies of TEM, Tfh, Th1 and Treg cells in asymptomatic individuals compared to endemic controls and VL patients. However, of these CD4+ T‐cell subsets, Treg cells showed the largest difference in AREG expression between the three groups, indicating they were an important source of AREG in asymptomatic individuals. We also established that elevated AREG protein levels were readily detected in individuals with asymptomatic L. donovani infection.
Amphiregulin, which was first recognised as a Th2 cell cytokine, is a member of epidermal growth factor family. It interacts with EGFR to activate essential intracellular signalling cascades that govern cellular metabolism, inflammation and cell cycle progression. 30 , 31 , 32 It is secreted by various activated immune cells from the innate and adaptive arms of the immune system. 28 Immune‐mediated resistance and tolerance mechanism are critical components of host immune responses. In this context, AREG has been identified as a key regulatory factor, which not only promotes host resistance to pathogens but also assists in tissue repair and wound healing under different inflammatory conditions. 28 Our findings support a similar conclusion, whereby elevated expression of AREG is associated with individuals able to control parasite growth without inducing an inflammatory response that causes disease. Although L. donovani infection stimulates a potent Th1 cell response, 16 similar roles for AREG balancing pathogen‐specific immune responses and pathology have been reported in broad range of infections. For example, in C57BL/6 mice infected with Trichuris muris, AREG provided resistance against this parasite by inducing the proliferation of gut epithelia and facilitated its clearance. 33 Similarly in mice infected with N. brasiliensis, AREG promoted tissue repair after N. brasiliensis‐induced lung injury. 34 In RAG−/− mice infected with influenza virus, AREG not only promoted the regeneration of bronchial epithelium but also enhanced tissue integrity and survival of infected mice. 35 Also, in mice infected with influenza virus and bacterial coinfection, exogenous AREG administration promoted tissue repair and improved survival in co‐infected animals without affecting pathogen burden. 36 In another study, C. albicans infected, dectin‐1 deficient mice had severe damage to pulmonary epithelium compared to wild‐type mice despite similar pathogen burden, and treatment with recombinant AREG not only promoted epithelial repair but also increased survival of the dectin‐1‐deficient mice. 37 The role of AREG in tissue repair appears to be an important aspect of its functions during infection, supported by the observation that mice lacking functional AREG have reduced intestinal regeneration capacity compared to wild‐type mice following radiation‐induced injury. 38 Together, these data suggest that AREG is an important factor that promotes host tolerance by sustaining tissue integrity and homeostasis following infection and inflammation. Thus, it is possible that the pro‐inflammatory environment that develops to control parasite growth in asymptomatic individuals infected with L. donovani is finely balanced to prevent parasite expansion, but also avoid tissue damage, and AREG may be an important component of this process.
CD4+ T cells play an important role in controlling Leishmania parasite replication. 26 Changes in the frequency of CD4+ T cells and their subsets in VL patients before and after treatment, compared to endemic controls and individuals with asymptomatic infection, have been previously reported. 39 , 40 Here, we provide data showing alterations in CD4+ T‐cell subset composition in asymptomatic individuals on a larger scale. Decreased frequencies of naïve and TCM CD4+ T cells and increased frequencies of TEM, Tfh and Th1 cells were observed in the peripheral blood of asymptomatic individuals, compared to VL patients and endemic controls. Naïve and TCM cells re‐circulate through secondary lymphoid organs, while TEM cells are mostly located within non‐lymphoid tissues or remain in circulation and rapidly produce anti‐parasitic effector molecules upon antigen encounter. 41 , 42 Thus, the above‐mentioned increase in the frequency of TEM CD4+ T cells in the peripheral blood of asymptomatic individuals, relative to other groups, suggest it is likely that these CD4+ T cells were actively engaged in anti‐parasitic defence.
Contrary to the findings in VL patients where PBMC Treg cell frequencies were unchanged, compared to endemic controls, 40 we found increased Treg cell frequencies in the blood of asymptomatic individuals compared to endemic controls and/or VL patients (Figure 3c). Tregs cells, which are critical for mediating peripheral tolerance under different inflammatory conditions, also secrete AREG. Accumulation of a transcriptionally distinct Treg cell population overexpressing AREG with enhanced muscle regeneration capacity has been reported in mouse model of acute skeletal muscles injury. 30 AREG expressed by muscle‐resident Treg cells has been shown to improve muscle repair in vivo and also act directly on muscle satellite cells in vitro to mediate tissue repair. 30 , 36 The accumulation of AREG‐producing Treg cells has also been reported in the brains of mice after ischaemic stroke, which suppressed neurotoxic astrogliosis and provided neuronal protection. 43 Since Treg cells are critical in preventing immune dysregulation and we observed increased Treg cell numbers, as well as increased expression of AREG by Treg cells from asymptomatic individuals, it is possible that increased AREG production by Treg cells is a response to prevent tissue damage caused by anti‐parasitic control mechanisms during asymptomatic L. donovani infection. However, it is not clear whether activation of Treg cells in these individuals is mediated by L. donovani infection or other co‐infections prevalent in the VL endemic region under investigation, and this will require further investigation. Because helminth infections are common in the resource‐poor settings of VL endemic areas, it will be important to establish their impact on AREG expression by Treg cells to help resolve this issue. 44 Individuals infected with helminths in endemic areas have a polarised immune responses, 45 and along with the genetic makeup of individuals, co‐infection with L. donovani is likely to have a significant impact on progression to active disease.
Amphiregulin has previously been identified as a biomarker for several types of cancer, 46 , 47 , 48 , 49 , 50 , 51 as well as in other inflammatory conditions such as rheumatoid arthritis, 52 asthma, 53 intestinal acute GVHD 54 and cholesteatoma. 55 It has also been identified as a predictive biomarker for resistance or sensitivity to anti‐EGFR mAb therapy in various cancers. The elevated AREG plasma levels in asymptomatic individuals, compared to those with active diseases and endemic controls, and enhanced AREG production upon Leishmania antigen stimulation in whole‐blood assays support a potential role for AREG as a biomarker for asymptomatic L. donovani infection. However, based on the relative variability of responses between individuals and dynamic range of responses detected in both assays, it is likely that AREG would have to be combined with additional biomarkers to maximise sensitivity and accuracy for a diagnostic assay. This approach has been successful in other infectious diseases, such as leprosy. 56 , 57
In conclusion, we report increased AREG expression associated with increased frequency of Treg cells in seropositive asymptomatic individuals living in a VL endemic area of India. We also observed increased AREG plasma levels, as well as increased secretion of antigen‐specific AREG in the whole blood of these individuals. Taken together, these findings identify AREG as an immunological determinant for asymptomatic L. donovani infection.
Methods
Human subjects and ethical clearance
Blood samples were collected from (n = 46) asymptomatic, (n = 48) symptomatic VL patients and (n = 59) endemic control individuals at the Kala‐azar Medical Research Center (Muzaffarpur, Bihar, India). Active VL cases were confirmed based on clinical signs, including fever (> 2 weeks), splenomegaly, positive serology for rK39 rapid diagnostic test and by microscopic demonstration of Leishmania amastigotes in splenic or bone marrow aspirate smears. Clinical data from these patients are summarised in Table 1. Asymptomatic healthy subjects living in endemic regions were tested for anti‐leishmania antibodies by direct agglutination test (DAT) and rK39 ELISA. A positive and negative control (filter paper pooled eluates from VL patients and non‐endemic healthy controls, NEHC) were run in each rK39‐ELISA, and the positive control was used as a reference to calculate a relative value of positivity of each sample, expressed as percentage positivity (PP). In the first sero‐survey, subjects positive by both DAT and rK39‐ELISA (≥ 1:1600 and ≥ 14PP, respectively), and highly seropositive by one or both assays (≥ 1:25 600 and ≥ 23 PP) and who met the inclusion criteria, were invited to KAMRC within 14 days of identification. All asymptomatic subjects were monitored monthly for 24 months after enrolment in the study to observe any development of active VL. This work was conducted with ethical approval (No. Dean/2017/EC/185 dated 24/10/2017) obtained from Institutional Review Committees of Banaras Hindu University, Varanasi, India. Each study patient was informed both verbally and in writing (in English and Hindi) about the nature of the study, the anticipated risks and benefits, the discomforts to which the patient will be exposed, and their right to discontinue participation at any time of their own free will. Written informed consent was obtained from all participants, and where participants were below 18 years of age, written informed consent was obtained from their legal guardian. We excluded pregnant women or lactating mothers, subjects having a vaccination within 30 days, and hepatitis B or C positive subjects. All subjects were HIV‐negative and above 12 years of age.
Table 1.
Demographic and clinical information on study participants
| Variables | VL Group (n = 48) | ASY Group (n = 47) | EC Group (n = 59) |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 27.94 ± 14.54 | 36.26 ± 16.10 | 34.78 ± 10.29 |
| Median | 24.5 | 38 | 33 |
| Sex, no. | |||
| Male | 31 | 18 | 32 |
| Female | 17 | 29 | 27 |
| Illness duration, days | |||
| Mean ± SD | 26.65 ± 15.23 | NA | NA |
| Median | 28 | NA | NA |
| Haemoglobin level, mg mL−1 | |||
| Before treatment | |||
| Mean ± SD | 9.2 ± 1.60 | ND | ND |
| Median | 9.3 | ND | ND |
| WBC, ×103 cells mm−3 | |||
| D‐0 | |||
| Mean ± SD | 4854.83 ± 3676.85 | ND | ND |
| Median | 4150 | ND | ND |
| Splenic enlargement, cm | |||
| D‐0 | |||
| Mean ± SD | 4.08 ± 2.88 | NA | NA |
| Median | 3 | NA | NA |
ND, Assay not done.
Direct agglutination test
Direct agglutination test (DAT) was performed using finger prick blood collected on Whatman filter paper. Briefly, 100 μL of blood filter paper eluate (1:400 dilutions) was serially diluted up to 1:51 200 with 50 μL DAT buffer in a V‐shaped microtiter plate with one positive and one negative control. A volume of 50 μL of DAT antigen (Institute for Tropical Medicine, Belgium) was then dispensed to every well, and plates were incubated overnight at room temperature for agglutination. The DAT results were observed against a white background. Samples with a titre of 1:1600 or above were considered DAT seropositive, and asymptomatic individuals with a DAT titre ≥1:3200 were enrolled in the study.
rK39 Enzyme‐Linked Immunosorbent Assay
High binding flat‐bottom 96‐well Nunc ELISA plates (Thermo Fisher Scientific, USA) were coated with rK39 (25 ng per well) overnight at 4°C. Plates were blocked with PBS containing 1% (w/v) bovine serum albumin (BSA) (VWR, Life Science, USA) for 2 h at room temperature. A volume of 100 µL of eluted blood from 5‐mm Whatman filter paper was added and incubated for 1 h. The wells were washed four times with PBS‐Tween (PBS‐T) and incubated for 30 min with protein A‐horseradish peroxidase (1:12 000 dilution; Bangalore Genei, India) in PBS containing 0.1% BSA and 1.0% (v/v) Tween‐20. Plates were washed four times in PBS‐T and incubated with 100 μL tetramethylbenzidine (TMB) (Kirkegaard and Perry Laboratories, Gaithersburg, MD) substrate for a further 15 min. The reaction was stopped by addition of 1N H2SO4, and optical density (OD) measurements were undertaken at 450 nm using a microtiter plate ELISA reader (Molecular Devices, San Jose, CA, USA). Samples having ≥ 14PP were considered positive, and individuals with ≥ 20 PP were enrolled in the study.
Human PBMC isolation and RNA extraction
Blood was collected from asymptomatic individuals, VL patients and ECs into 15‐mL Falcon tubes (BD Biosciences) containing 150 U heparin. Blood was layered over Lymphoprep (StemCell Technologies, Vancouver, Canada) to isolate PBMCs. CD4+ T cells were isolated from PBMCs by magnetic‐activated cell sorting (MACS) using MS Columns and anti‐human CD4 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and stored in RLT buffer (Qiagen, cat #79216) at −80°C till further use. Cells were homogenised using the QIAshredder and RNA isolated using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. DNA digestion was performed using either the RNAse‐free DNase set or DNase Max kit (QIAGEN). RNA was quantified using the Qubit RNA HS Assay kit on a Qubit 4 Fluorometer (Thermo Fisher, Waltham, MA, USA). The quality of RNA was determined using the RNA 6000 Nano kit, run on a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), according to the manufacturer’s instructions where an RNA Integrity Number (RIN) value above 8 was optimal.
RNA‐sequencing
Libraries were prepared using the TruSeq Stranded mRNA Library Prep Kit, High Throughput (Illumina, San Diego, CA, USA) with Superscript III Reverse Transcriptase (Life Technologies, Carlsbad, CA, USA) and Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA). Libraries were quantified using the Qubit DNA HS Assay kit (Thermo Fisher), and quality was assessed using the DNA 100 kit (Agilent Technologies), run on a 2100 Bioanalyzer. 75‐bp, paired‐end RNA‐sequencing was performed on the NextSeq 550 using the NextSeq 500/550 High Output Kit v2 (150 cycles) (Illumina). Each flow cell contained 12 libraries.
Differential gene expression and pathway analysis
Cutadapt 58 (version 1.11) was used for trimming adapter sequences from fastq files. STAR 59 (version 2.5.2a) was used for sequence alignment to the GRCh37 assembly with the gene, transcript, and exon features of Ensembl (release 89) gene model. RNA‐SeQC 60 (version 1.1.8) was used to compute quality control metrics, and RSEM 61 (version 1.2.30) was used to quantify reads. Normalisation and differential gene expression analysis were performed using the edgeR package, 62 and pathways analysis was performed using ingenuity pathway analysis (IPA; winter 2018 release; QIAGEN).
RT‐qPCR
Peripheral blood mononuclear cells were isolated from blood layered over Lymphoprep, and CD4+ T cells were enriched by MACS from PBMCs using the anti‐human CD4 MicroBeads (Miltenyi Biotec) according to the manufacturer’s instructions. RNA was then extracted and reverse transcribed to cDNA (both PBMC and CD4+ T cells). RT‐qPCR for amphiregulin was performed on an ABI Prism® 7500 real‐time PCR system (Applied Biosystems, Waltham, MA, USA) using the TaqMan Gene Expression Assay (Assay ID: Hs00950669_m1; Applied Biosystems). Relative quantification was performed using the comparative CT method relative to 18S ribosomal RNA (rRNA) (Assay ID: Hs99999901_s1; Applied Biosystems).
Preparation of soluble Leishmania antigen
Soluble Leishmania antigen (SLA) was prepared as previously described. 63 Briefly, L. donovani amastigotes from clinical isolates (Kala‐azar Medical Research Center, Muzaffarpur, Bihar, India) were grown in Medium 199, Hanks’ Balanced Salts (M199; Thermo Fisher) until transformed into promastigotes, then cultured. 2–3 × 109 stationary‐phase promastigotes were harvested from culture and centrifuged at 3900 g for 20 min to obtain a parasite pellet, which was washed three times with cold 1 x PBS and resuspended in solution (10 mM Trizma hydrochloride solution (TRIS‐HCl; Sigma‐Aldrich), 1 mM pH 8.0 ethylenediaminetetraacetic acid (EDTA; Amresco, ELITechGroup, Puteaux, France), 1.6 mM phenylmethanesulphonyl fluoride (PMSF; HiMedia, Mumbai, India) and 50 mg mL‐1 N‐acetyl‐L‐leucyl‐Lleucyl‐ L‐argininal (leupeptin; Amresco)) at a concentration of 2–3 × 109 parasites m−1. The parasite suspension was sonicated 4–5 times for 15 s at 10 Hz and centrifuged at 2000 g for 30 min at 4°C. The lipid layer was removed from the surface of the supernatant, and the remaining solution was ultracentrifuged at 100 000 g for 4 h at 4°C. The supernatant was removed, dialysed against the PBS overnight and stored at –80°C until used. Protein concentration was measured using a Micro BCA Protein Assay Kit (Thermo Fisher), as per the manufacturer’s instructions.
Ex vivo whole‐blood assay
Heparinised blood was collected from asymptomatic individuals, active VL patients and endemic controls. To remove background plasma cytokine readings, the plasma was removed and the whole blood was washed once with 1 × PBS. The autologous plasma was then replaced with an equal volume of HI‐FBS. The blood was divided equally in 4 polypropylene culture tubes (BD Biosciences; cat #352063) containing 1 mL in each tube. Whole‐blood cells were cultured in the absence or in presence of SLA. A non‐stimulated group (PBS was used as placebo) was included as a negative control and the levels of IFNγ /AREG detected were subtracted from corresponding antigen‐stimulated samples. Whole‐blood cultures will be kept at 37°C and 5% (v/v) CO2 for 24 h, and then, supernatants were collected. AREG levels were measured using a human amphiregulin ELISA kit (Thermo Scientific; Cat# EHAREG), and IFNγ levels were measured using an ELISA kit (BioLegend, San Diego, CA, USA; Cat# 430104) in supernatant according to the manufacturer’s instructions.
Flow cytometry
FACS staining was performed for the phenotypic characterisation of amphiregulin present on CD4+ T cells from different subject groups; asymptomatic individuals (n = 15), VL patients (n = 11) and endemic controls (n = 17). CD4+ T cells and their different subsets were measured by expression of different chemokines/surface markers. Fixable Zombie Aqua™ fixable viability kit (BioLegend; cat #423102) was used to exclude dead cells from the analysis. Briefly, for each sample, 1 × 106 PBMC were dispensed in BD‐polystyrene round‐bottom FACS staining tubes (Corning, Mexico; cat# 352054). Thereafter, cells were incubated in the dark at room temperature for 30 min in 100 µL 1 × PBS with Zombie Aqua™ dye (Detected on BV‐510) for dead cell staining. A volume of 2 mL FACS staining buffer (5% (v/v) FCS in 1 X PBS) was added to each tube and washed twice. Surface staining was performed in FACS staining buffer with antibodies against CD3ε APC‐Cy7 (BD Biosciences; cat #557832), CD4 AF®‐700 (BD Biosciences cat #557922), CD45RA FITC (BD Biosciences cat #555488), CD25 APC (BD Biosciences cat #340939), CD127 PerCP‐Cy5.5 (BD Biosciences cat #8560551), CD197 PE‐Cy7 (BD Biosciences cat #557648), CD194 BV‐605 (BD Biosciences cat #359418), CD196 BV‐650 (BD Biosciences cat #563922), CD185 BV‐421 (BD Biosciences cat #562747), CD183 PE‐CF594 (BD Biosciences cat #562451), CD38 BV‐711 (BD Biosciences cat #563965) and human amphiregulin PE (Invitrogen, cat# 12‐5370‐42) for 30 min in the dark. Finally, stained cells were washed twice with 2‐mL FACS staining buffer and resuspended in 300 µL of FACS staining buffer. Freshly stained cells were acquired by Flow cytometer (BD LSRFortessa flow cytometer, BD Biosciences) using FACS Diva software. FACS data were analysed by FlowJo version 10 software (Tree Star (BD)).
Statistical analysis
Statistical analysis was performed exclusively in GraphPad Prism 7.02 (GraphPad Software, La Jolla, CA, USA). Analysis of human qPCR and cellular assays was performed using a one‐way ANOVA, with either a Kruskal–Wallis or a Tukey’s multiple comparisons test, as appropriate, to assess more than 2 groups within an experiment. A Wilcoxon signed‐rank test was used to compare matched‐pairs. SPICE analysis was performed using SPICE version 5.3 (M. Roeder, Vaccine Research Centre, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, USA; http://exon.niaid.nih.gov). 64 P < 0.05 was considered significant. Graphs depict Box and Whisker plots showing minimum and maximum values or connecting lines when data were paired.
Author contributions
Siddharth Sankar Singh: Data curation; Formal analysis; Investigation; Writing – review & editing. Shashi Bhushan Chauhan: Data curation; Formal analysis; Investigation; Methodology. Susanna SS Ng: Data curation; Formal analysis; Investigation; Methodology; Software; Writing – review & editing. Dillon Corvino: Formal analysis; Investigation; Methodology; Software; Visualization; Writing – review & editing. Fabian de Labastida Rivera: Formal analysis; Investigation; Methodology; Project administration. Jessica A Engel: Formal analysis; Investigation; Methodology. Nic Waddell: Supervision; Data curation; Formal analysis. Pamela Mukhopadhay: Data curation; Investigation; Methodology; Software. Rebecca L Johnston: Data curation; Formal analysis. Lambros T Koufariotis: Data curation; Software. Susanne Nylen: Funding acquisition; Investigation; Project administration; Writing – review & editing. Om Prakesh Singh: Investigation; Project administration. Christian Engwerda: Conceptualization; Investigation; Funding Acquisition; supervision; Writing– review & editing. Rajiv Kumar: Conceptualization; Investigation; Funding Acquisition; supervision; Writing– review & editing. Shyam Sundar: Conceptualization; Funding acquisition; Supervision.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Supplementary figures 1‐3
Supplementary tables 1 and 2
Acknowledgments
We thank the staff at the Kala‐Azar Medical Research Centre (KAMRC), Muzaffarpur, Bihar, India, for their help in collecting clinical samples, as well as patients and volunteers for permission to use their blood samples. This work was made possible through an Indian Council of Medical Research (ICMR) ad hoc grant (no. 2020‐9898) and National Institute of Health Tropical Medicine Research Centre (TMRC) grant (U19 AI074321). The research was supported by grants and fellowships from the Institute of Eminence (IoE) grant of Banaras Hindu University, the National Health and Medical Research Council of Australia (NHMRC; grant numbers 1037304, 1058685, 1132975 and 1154265), as well Senior Research Fellowships from the Indian Council of Medical Research and DST—INSPIRE to Siddharth Singh.
Contributor Information
Christian R Engwerda, Email: christian.engwerda@qimrberhofer.edu.au.
Rajiv Kumar, Email: rajiv082@yahoo.com.
Shyam Sundar, Email: drshyamsundar@hotmail.com.
References
- 1. Stauch A, Sarkar RR, Picado A et al. Visceral leishmaniasis in the Indian subcontinent: modelling epidemiology and control. PLoS Negl Trop Dis 2011; 5: e1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ismael A, Zijlstra EE, Ghalib HW, El‐Hassan AM. Endemic kala‐azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post‐kala‐azar dermal leishmaniasis. Am J Trop Med Hyg 1994; 51: 826–836. [DOI] [PubMed] [Google Scholar]
- 3. Schaefer KU, Kurtzhals JA, Gachihi GS, Muller AS, Kager PA. A prospective sero‐epidemiological study of visceral leishmaniasis in Baringo District, Rift Valley Province, Kenya. Trans R Soc Trop Med Hyg 1995; 89: 471–475. [DOI] [PubMed] [Google Scholar]
- 4. Ali A, Ashford RW. Visceral leishmaniasis in Ethiopia. IV. Prevalence, incidence and relation of infection to disease in an endemic area. Ann Trop Med Parasitol 1994; 88: 289–293. [DOI] [PubMed] [Google Scholar]
- 5. Evans TG, Teixeira MJ, McAuliffe IT et al. Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis 1992; 166: 1124–1132. [DOI] [PubMed] [Google Scholar]
- 6. Moral L, Rubio EM, Moya M. A leishmanin skin test survey in the human population of l'Alacanti region (Spain): implications for the epidemiology of Leishmania infantum infection in southern Europe. Trans R Soc Trop Med Hyg 2002; 96: 129–132. [DOI] [PubMed] [Google Scholar]
- 7. Chowdhury R, Breiman RF, Wahed MA et al. The epidemiology of visceral leishmaniasis and asymptomatic leishmanial infection in a highly endemic Bangladeshi village. Am J Trop Med Hyg 2007; 76: 909–914. [PubMed] [Google Scholar]
- 8. Ostyn B, Gidwani K, Khanal B et al. Incidence of symptomatic and asymptomatic Leishmania donovani infections in high‐endemic foci in India and Nepal: a prospective study. PLoS Negl Trop Dis 2011; 5: e1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Das VNR, Ranjan A, Pandey K et al. Asymptomatic infection with visceral leishmaniasis in a disease‐endemic area in Bihar, India. Am J Trop Med Hyg 2010; 83: 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh OP, Tiwary P, Kushwaha AK et al. Xenodiagnosis to evaluate the infectiousness of humans to sandflies in an area endemic for visceral leishmaniasis in Bihar, India: a transmission‐dynamics study. Lancet Microbe 2021; 2: e23–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gumy A, Louis JA, Launois P. The murine model of infection with Leishmania major and its importance for the deciphering of mechanisms underlying differences in Th cell differentiation in mice from different genetic backgrounds. Int J Parasitol 2004; 34: 433–444. [DOI] [PubMed] [Google Scholar]
- 12. Chappuis F, Sundar S, Hailu A et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 2007; 5: 873–882. [DOI] [PubMed] [Google Scholar]
- 13. Guedes DL, Justo AM, Barbosa Júnior WL et al. Asymptomatic Leishmania infection in HIV‐positive outpatients on antiretroviral therapy in Pernambuco, Brazil. Plos Negl Trop Dis 2021; 15: e0009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abongomera C, Diro E, Vogt F et al. The risk and predictors of visceral leishmaniasis relapse in human immunodeficiency virus‐coinfected patients in ethiopia: a retrospective cohort study. Clin Infect Dis 2017; 65: 1703–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez‐Velez R. The impact of highly active antiretroviral therapy (HAART) on visceral leishmaniasis in Spanish patients who are co‐infected with HIV. Ann Trop Med Parasitol 2003; 97(Suppl 1): 143–147. [DOI] [PubMed] [Google Scholar]
- 16. Kaye P, Scott P. Leishmaniasis: complexity at the host‐pathogen interface. Nat Rev Microbiol 2011; 9: 604–615. [DOI] [PubMed] [Google Scholar]
- 17. Hailu A, van Baarle D, Knol GJ, Berhe N, Miedema F, Kager PA. T cell subset and cytokine profiles in human visceral leishmaniasis during active and asymptomatic or sub‐clinical infection with Leishmania donovani . Clin Immunol 2005; 117: 182–191. [DOI] [PubMed] [Google Scholar]
- 18. Carvalho EM, Barral A, Pedral‐Sampaio D et al. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi . J Infect Dis 1992; 165: 535–540. [DOI] [PubMed] [Google Scholar]
- 19. Mary C, Auriault V, Faugere B, Dessein AJ. Control of Leishmania infantum infection is associated with CD8+ and gamma interferon‐ and interleukin‐5‐producing CD4+ antigen‐specific T cells. Infect Immun 1999; 67: 5559–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egui A, Ledesma D, Pérez‐Antón E et al. Phenotypic and functional profiles of antigen‐specific CD4+ and CD8+ T cells associated with infection control in patients with cutaneous leishmaniasis. Front Cell Infect Microbiol 2018; 8: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tubo NJ, Jenkins MK. CD4+ T Cells: guardians of the phagosome. Clin Microbiol Rev 2014; 27: 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasker E, Kansal S, Malaviya P et al. Latent infection with Leishmania donovani in highly endemic villages in Bihar, India. Plos Negl Trop Dis 2013; 7: e2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh OP, Hasker E, Sacks D, Boelaert M, Sundar S. Asymptomatic Leishmania infection: a new challenge for Leishmania control. Clin Infect Dis 2014; 58: 1424–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar R, Bunn PT, Singh SS et al. Type I interferons suppress anti‐parasitic immunity and can be targeted to improve treatment of visceral leishmaniasis. Cell Rep 2020; 30: 2512–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh B, Bhushan Chauhan S, Kumar R et al. A molecular signature for CD8+ T cells from visceral leishmaniasis patients. Parasite Immunol 2019; 41: e12669. [DOI] [PubMed] [Google Scholar]
- 26. Kumar R, Singh N, Gautam S et al. Leishmania specific CD4 T cells release IFNγ that limits parasite replication in patients with visceral leishmaniasis. PLoS Negl Trop Dis 2014; 8: e3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chauhan SB, Faleiro R, Kumar R et al. Interleukin 2 is an upstream regulator of CD4+ T cells from visceral leishmaniasis patients with therapeutic potential. J Infect Dis 2019; 220: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zaiss DMW, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015; 42: 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ansari NA, Kumar R, Gautam S et al. IL‐27 and IL‐21 are associated with T cell IL‐10 responses in human visceral leishmaniasis. J Immunol 2011; 186: 3977–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burzyn D, Kuswanto W, Kolodin D et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013; 155: 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salomon DS, Normanno N, Ciardiello F, Brandt R, Shoyab M, Todaro GJ. The role of amphiregulin in breast cancer. Breast Cancer Res Treat 1995; 33: 103–114. [DOI] [PubMed] [Google Scholar]
- 32. Trussoni CE, Tabibian JH, Splinter PL, O'Hara SP. Lipopolysaccharide (LPS)‐induced biliary epithelial cell NRas activation requires epidermal growth factor receptor (EGFR). PLoS One 2015; 10: e0125793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turner J‐E, Morrison PJ, Wilhelm C et al. IL‐9‐mediated survival of type 2 innate lymphoid cells promotes damage control in helminth‐induced lung inflammation. J Exp Med 2013; 210: 2951–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science 2006; 314: 1746. [DOI] [PubMed] [Google Scholar]
- 35. Monticelli LA, Sonnenberg GF, Abt MC et al. Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jamieson AM, Pasman L, Yu S et al. Role of tissue protection in lethal respiratory viral‐bacterial coinfection. Science 2013; 340: 1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Branzk N, Lubojemska A, Hardison SE et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 2014; 15: 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shao J, Sheng H. Amphiregulin promotes intestinal epithelial regeneration: roles of intestinal subepithelial myofibroblasts. Endocrinology 2010; 151: 3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cenini P, Berhe N, Hailu A, Mcginnes K, Frommel D. Mononuclear cell subpopulations and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J Infect Dis 1993; 168: 986–993. [DOI] [PubMed] [Google Scholar]
- 40. Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL‐10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med 2007; 204: 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol 2012; 188: 5811–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pepper M, Jenkins MK. Origins of CD4+ effector and central memory T cells. Nat Immunol 2011; 12: 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ito M, Komai K, Mise‐Omata S et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019; 565: 246–250. [DOI] [PubMed] [Google Scholar]
- 44. Greenland K, Dixon R, Khan SA et al. The epidemiology of soil‐transmitted helminths in Bihar State, India. Plos Negl Trop Dis 2015; 9: e0003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maurya R, Alti D, Sambamurthy C, Rani J. A risk of visceral leishmaniasis in case of helminths co‐infection in endemic regions. Innovative J Med Health Sci 2012; 2: 47–50. [Google Scholar]
- 46. Awad AE, Ebrahim MA, Eissa LA, El‐Shishtawy MM. Dickkopf‐1 and amphiregulin as novel biomarkers and potential therapeutic targets in hepatocellular carcinoma. Int J Hematol Oncol Stem Cell Res 2019; 13: 153–163. [PMC free article] [PubMed] [Google Scholar]
- 47. Bostwick DG, Qian J, Maihle NJ. Amphiregulin expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 93 cases. Prostate 2004; 58: 164–168. [DOI] [PubMed] [Google Scholar]
- 48. Yamada M, Ichikawa Y, Yamagishi S et al. Amphiregulin is a promising prognostic marker for liver metastases of colorectal cancer. Clin Cancer Res 2008; 14: 2351–2356. [DOI] [PubMed] [Google Scholar]
- 49. Wang B, Yong H, Zhu H et al. Abnormal amphiregulin expression correlates with gastric cancer prognosis. Oncotarget 2016; 7: 76684–76692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang LI, Wu H, Wang L et al. Expression of amphiregulin predicts poor outcome in patients with pancreatic ductal adenocarcinoma. Diagn Pathol 2016; 11: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McBryan J, Howlin J, Kenny PA, Shioda T, Martin F. ERalpha‐CITED1 co‐regulated genes expressed during pubertal mammary gland development: implications for breast cancer prognosis. Oncogene 2007; 26: 6406–6419. [DOI] [PubMed] [Google Scholar]
- 52. Yamane S, Ishida S, Hanamoto Y et al. Proinflammatory role of amphiregulin, an epidermal growth factor family member whose expression is augmented in rheumatoid arthritis patients. J Inflamm (Lond) 2008; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hachim MY, Elemam NM, Ramakrishnan RK et al. Blood and Salivary Amphiregulin Levels as Biomarkers for Asthma. Front Med (Lausanne) 2020; 7: 561866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Amin K, Yaqoob U, Schultz B et al. Amphiregulin in intestinal acute graft‐versus‐host disease: a possible diagnostic and prognostic aid. Mod Pathol 2019; 32: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Macias MP, Gerkin RD, Macias JD. Increased amphiregulin expression as a biomarker of cholesteatoma activity. Laryngoscope 2010; 120: 2258–2263. [DOI] [PubMed] [Google Scholar]
- 56. van Hooij A, Tjon Kon Fat EM, de Jong D et al. Prototype multi‐biomarker test for point‐of‐care leprosy diagnostics. iScience 2021; 24: 102006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corstjens PLAM, van Hooij A, Tjon Kon Fat EM et al. Fingerstick test quantifying humoral and cellular biomarkers indicative for M. leprae infection. Clin Biochem 2019; 66: 76–82. [DOI] [PubMed] [Google Scholar]
- 58. Martin M. Cutadapt removes adapter sequences from high‐throughput sequencing reads. Embnet J 2011; 17: 10–12. [Google Scholar]
- 59. Dobin A, Davis CA, Schlesinger F et al. STAR: ultrafast universal RNA‐seq aligner. Bioinformatics 2013; 29: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. DeLuca DS, Levin JZ, Sivachenko A et al. RNA‐SeQC: RNA‐seq metrics for quality control and process optimization. Bioinformatics 2012; 28: 1530–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA‐Seq data with or without a reference genome. BMC Bioinformatics 2011; 12: e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010; 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gidwani K, Jones S, Kumar R, Boelaert M, Sundar S. Interferon‐gamma release assay (modified QuantiFERON) as a potential marker of infection for Leishmania donovani, a proof of concept study. PLoS Negl Trop Dis 2011; 5: e1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post‐cytometric complex multivariate datasets. Cytometry A 2011; 79: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures 1‐3
Supplementary tables 1 and 2


