Abstract
Context
For decades, there has been epidemiologic evidence linking chronic toxic metal exposure with cardiovascular disease, suggesting a therapeutic role for metal chelation. Given the lack of compelling scientific evidence, however, the indications for metal chelation were never clearly defined. To determine the safety and efficacy of chelation therapy, the National Institutes of Health funded the Trial to Assess Chelation Therapy (TACT). TACT was the first double-blind, randomized, controlled trial to demonstrate an improvement in cardiovascular outcomes with edetate disodium therapy in patients with prior myocardial infarction. The therapeutic benefit was striking among the prespecified subgroup of patients with diabetes.
Design
We review the published literature focusing on the atherogenic nature of diabetes, as well as available evidence from clinical trials, complete and in progress, of metal chelation with edetate disodium therapy in patients with diabetes.
Results
The TACT results support the concept that ubiquitous toxic metals such as lead and cadmium may be modifiable risk factors for cardiovascular disease, particularly in patients with diabetes.
Conclusions
The purpose of this review is to discuss the potential mechanisms unifying the pathogenesis of atherogenic factors in diabetes with toxic metal exposure, and the potential role of metal chelation.
This review summarizes the epidemiological and basic evidence linking toxic metal accumulation and diabetes-related cardiovascular disease, supported by the salutary effects of chelation in TACT.
On 4 November 2012, the results of the 10-year National Institutes of Health–funded Trial to Assess Chelation Therapy (TACT) were presented at the American Heart Association (1). Remarkably, infusions of an edetate disodium–based infusion reduced recurrent cardiovascular events in the study cohort composed of nondiabetic patients (63%) and patients with type 1 and 2 diabetes (37%) with prior myocardial infarction (MI). Subsequent analyses identified the largest effects [41% reduction in major cardiovascular events (P = 0.0002), and a 43% reduction in total mortality (P = 0.011)] in patients with diabetes (2). The salutary effect of edetate disodium–based chelation in the prevention of cardiovascular disease appears to be substantially larger than with other interventions in recent cardiovascular outcome studies (EMPA-REG, CANVAS, SUSTAIN-6); however, the TACT findings have received relatively little attention. Although metals may contribute to atherosclerotic cardiovascular disease in all types of diabetes, most data to date, including TACT, have addressed the role of chelation in a population that by far predominates in type 2 diabetes. Therefore, we think that our discussion is most relevant to type 2 diabetes. Despite current advances in cardiovascular disease prevention and care, patients with diabetes still develop atherosclerosis at an accelerated rate despite optimal therapy (3), a phenomenon known as residual risk. Numerous atherogenic risks coexist in the diabetic population, perhaps accounting for the residual risk. First, endothelial dysfunction is potentiated in diabetes. This is a multifactorial phenomenon, with a decline in nitric oxide–mediated vascular reactivity as well as enhanced endothelial expression of adhesion molecules in the initial phases of plaque formation (3–6). Second, diabetic dyslipidemia also plays a role owing to the combination of impaired lipid influx/efflux equilibrium, a distinct diabetes atherogenic lipid phenotype, and a resulting increased lipid core of atherogenic plaque in patients with type 2 diabetes. Part of the diabetic lipid phenotype is incremental formation and uptake of oxidized low-density lipoprotein (LDL). Oxidized LDL is a modified lipoprotein toxic to endothelium and a potent chemoattractant for macrophages. It is normally degraded by paraoxonase (PON), a high-density lipoprotein (HDL)–associated esterase/lactonase found mainly in serum, but the degradation may be diminished in diabetes as a result of a lower and/or modified HDL cholesterol (3, 6, 7). This phenomenon along with the higher macrophage apoptosis may explain the observed larger necrotic cores in lesions from individuals with diabetes vs nondiabetic patients (3, 7, 8). Fourth, to further complicate the situation, thrombogenicity might be enhanced, probably through elevation of plasminogen activator inhibitor levels, prothrombotic substances, and increased platelet aggregation. Increased protease activity has also been reported, provoking instability in the fibrous cap of the lesion and greater intraplaque hemorrhage as a result (3, 4, 7–9). The underlying and unifying pathogenic basis for many of these atherogenic factors in diabetes may be directly related to hyperglycemia, insulin resistance, oxidative stress, inflammation, or a combination of all of these.
The purpose of this review is to discuss the potential mechanisms of the beneficial effects of chelation, and to put these findings in context by focusing on the atherogenic nature of diabetes, as well as the role that oxidant metals, both essential and toxic, may play in the pathobiology of complications of diabetes. We analyze the available evidence from clinical trials, complete and in progress, of metal chelation with edetate disodium therapy in the prevention of diabetic-associated cardiovascular disease.
Design and Results of TACT With Emphasis on the Diabetes Population
A chelating drug is an organic molecule that has a pocket with a negative charge and as such is able to capture metal ions, bind them, and permit renal or biliary excretion, thus removing the metal from the body. Natural chelators, such as the heme molecule, are essential for vital processes, binding iron within the Hb molecule. Edetate disodium, a synthetic amino acid, has a high affinity for ions with +2 to +6 valences. Edetate and its salts (disodium, calcium disodium, and others) may bind toxic and essential metals, including divalent cations such as calcium and magnesium, and enhance their renal excretion. Transition metals are elements with a partially filled electron subshell. Their interchangeable prevalent ionic form, the catalytic metal ion, makes them useful cofactors in biochemical redox reactions. However, these same chemical properties can also be responsible for uncontrolled redox reactions in the cell, leading to cell poisoning. Nonessential metals such as cadmium, lead, and mercury are toxic metals generally at lower levels of exposure. Toxicity depends on chemical species, absorbed dose, route of exposure, and duration of exposure. The edetates were first patented by Munz in 1938 and were used as lead chelators for industrial poisoning and as treatment of hypercalcemia (10). Serendipitously, at a time when there were no effective treatments for atherosclerosis and diffuse coronary calcification was common in autopsy specimens, Clarke et al. (11, 12) reasoned that decalcification of these arteries might be beneficial. The first publication reporting edetate disodium infusions for cardiovascular disease was in 1956 (11). Clarke et al. used edetate disodium in 20 patients with atherosclerotic disease and reported improvement of angina symptomatic relief in 19. Starting in the 1990s, small clinical studies followed with mixed results (13). These enrolled, in aggregate, <400 patients, had surrogate endpoints such as angina or claudication and short follow-up periods (14). In 2002, the Cochrane Collaborative reviewed the literature to date and concluded that there was insufficient evidence to declare whether EDTA chelation was beneficial or harmful for patients with atherosclerotic disease (15).
In the clinical arena, chelation persisted in alternative medicine practice (16) although persistent questions regarding safety and efficacy remained (17). This uneasy equipoise prompted the National Center for Complementary and Alternative Medicine (now the National Center for Complementary and Integrative Health) to release a $30 million request for applications cofunded by the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Design of TACT and overall results
TACT was designed to test the most prevalent chelation solution in use. As published, it included not only up to 3 g of edetate disodium, but also 7 g of ascorbic acid, B vitamins, procaine, 500 U of unfractionated heparin, magnesium, and other electrolytes. Moreover, most patients receiving chelation also received high doses of oral multivitamins and multiminerals (OMVM). Therefore, a factorial design was selected for TACT, in which eligible patients were randomly assigned to one of four groups (1): (i) active IV chelation infusions plus active OMVM; (ii) active IV chelation infusions plus placebo OMVM; (iii) placebo IV chelation infusions plus active OMVM; and (iv) placebo IV chelation infusions plus placebo OMVM.
Based on discussions with experts in the chelation community, TACT included 30 weekly infusions followed by 10 maintenance infusions up to 8 weeks apart. OMVM or placebo was to be taken daily throughout the duration of the trial. Eligible patients were 50 years of age or older, had a prior MI >6 weeks before the enrollment date, and had a serum creatinine level ≤2.0 mg/dL.
The primary endpoint for the study was a composite of all-cause mortality, myocardial infarction, stroke, coronary revascularization, and hospitalization for angina. The major secondary endpoint was cardiovascular mortality, stroke, or recurrent MI.
TACT enrolled 1708 participants (839 randomly assigned to chelation and 869 to placebo) at 134 sites in the United States and Canada, of which 37% had diabetes. Study patients had a median age of 65 years, 18% were female, 9% were nonwhite, and 83% had either a prior coronary bypass or percutaneous coronary intervention. At baseline, 92% were taking antithrombotic therapy (aspirin, clopidogrel, or warfarin). The median LDL was 89 mg/dL, with 73% of patients taking statins at baseline.
A total of 55,222 placebo or active infusions were administered. Seventy-six percent of participants completed at least 30 (of a total of 40 possible) infusions. All 40 infusions were completed by 65% of participants. The median follow-up was 55 months. Active infusions of edetate chelation reduced the primary composite endpoint by 18%: hazard ratio (HR), 0.82; 95% CI, 0.69 to 0.99; P = 0.035; 5-year number needed to treat (NNT) to prevent one event was 18.
Results in patients with diabetes
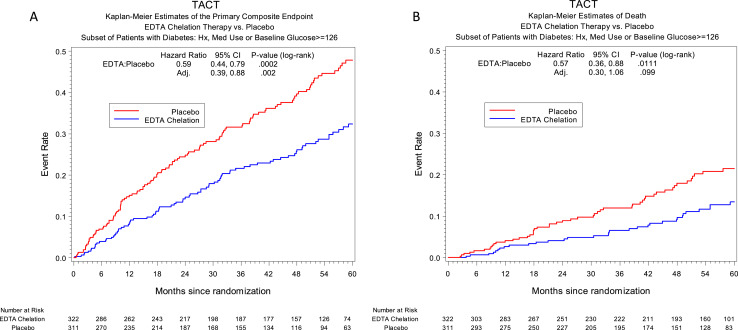
The results of active infusion of edetate disodium chelation in the 37% of patients with diabetes (n = 633) showed a prespecified analysis of a 41% reduction (HR, 0.59; 95% CI, 0.44 to 0.79; P = 0.0002) in the primary endpoint during 5 years compared with placebo (Fig. 1A). The 5-year NNT to reduce one primary event during 5 years in this prespecified population was 6.5. All individual components of the primary endpoint were consistently lower in the active IV chelation group (Table 1). All-cause mortality was reduced by 43% (10% vs 16%; HR, 0.57; 95% CI, 0.36 to 0.88; P = 0.011; 5-year NNT to prevent one death was 12; Fig. 1B), as was the principal secondary endpoint (cardiovascular death, reinfarction, or stroke; 11% vs 17%; HR, 0.60; 95% CI, 0.39 to 0.91; P = 0.017; Table 1) (2). There was a 52% relative reduction in the risk of recurrent MI among patients with diabetes (HR, 0.48; 95% CI, 0.26 to 0.88; P = 0.015). There was no difference between groups in control of glucose as defined by fasting glycemia during the course of the initial 30 infusions, suggesting that glycemic control was not an explanation for these findings. These findings ultimately led to the funding of the second TACT (TACT2) by the National Center for Complementary and Integrative Health, the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Environmental Health Sciences.
Figure 1.
TACT Kaplan–Meier estimates of the primary composite endpoint. EDTA chelation therapy vs placebo: (A) subset of patients with diabetes mellitus; (B) mortality in patients with diabetes mellitus by infusion group.
Table 1.
Clinical Endpoints by Infusion Arms for Patients With Diabetes Mellitus
| Endpoint | EDTA Chelation (n = 322) | Placebo (n = 311) | HR (95% CI) | P Value |
|---|---|---|---|---|
| Primary endpoint | 80 (25%) | 117 (38%) | 0.59 (0.44–0.79) | <0.001 |
| Death | 32 (10%) | 50 (16%) | 0.57 (0.36–0.88) | 0.011 |
| MI | 16 (5%) | 30 (10%) | 0.48 (0.26–0.88) | 0.015 |
| Stroke | 4 (1%) | 3 (1%) | 1.19 (0.27–5.30) | 0.829 |
| Coronary revascularization | 48 (15%) | 62 (20%) | 0.68 (0.48–0.99) | 0.042 |
| Hospitalization for angina | 5 (2%) | 6 (2%) | 0.72 (0.22–2.36) | 0.588 |
| Secondary endpoint | 35 (11%) | 52 (17%) | 0.60 (0.39–0.91) | 0.017 |
| Cardiovascular death | 19 (6%) | 27 (9%) | 0.63 (0.35–1.13) | 0.118 |
What is the mechanism underlying the benefit seen?
If edetate can reduce the clinical consequences of atherosclerotic coronary artery disease, questions arise as to what possible mechanisms might be responsible. Additionally, edetate seems to be limited in the ways it might affect pathophysiology by its confinement to the vascular space and its rapid excretion in the urine. The “chemical claw” is thought to do one thing in the body: bind cations and facilitate their excretion. To investigate whether edetate disodium chelated toxic metals, Waters et al. (18) collected 24-hour urine for 2 days prior to and 2 days after an edetate disodium–based infusion and analyzed the samples for various toxic and essential metals. Following the infusion, the excretion of lead during 2 days increased by 3830%, and cadmium increased by 514%. Arenas et al. (19) performed a similar experiment in 26 TACT-eligible patients, 13 (50%) with diabetes. Toxic metals were detected in the baseline urine of >80% of patients. There was no increase in urine metal excretion following a TACT placebo infusion. Following an active infusion, however, levels of urine metals increased (lead by 3835%, P < 0.0001; cadmium by 633%, P < 0.0001; nickel by 373%, P < 0.0001; and aluminum by 275%, P < 0.001). These findings demonstrate that edetate disodium mobilizes lead and cadmium, the most vasculotoxic of the abovementioned metals, from their chronic storage compartments and facilitates their excretion. Whether these effects of chelation are the mechanism for its therapeutic action depends, of course, on whether accumulation of these metals is important in the atherosclerotic disease process. The major findings of TACT, particularly in patients with diabetes, merit a brief review of the atherogenicity of diabetes, with special emphasis of the role that edetate-chelatable metals may play.
Diabetes, Oxidative Stress, and Metals
Glycoxidation
There is substantial evidence suggesting that diabetic complications may arise, at least partially, as a result of uncompensated oxidative stress. Hyperglycemia leads to an increased production of reactive oxygen species (ROS) and to nonenzymatic glycoxidation of proteins, which alters their structure and function (4, 5). This process is initiated by the oxidation of an aldose or ketose to a more reactive dicarbonyl sugar (glucosone), which will then react with a protein to form a ketoimine adduct. The resulting reduced oxygen products, such as superoxide and hydrogen peroxide, may cause oxidative damage to neighboring molecules (7, 20). Therefore, autoxidative glycosylation requires, or at least is enhanced by, metal catalyzed oxygen chemistry to produce oxygen free radicals. The products of these reactions have been termed advanced glycation end-products (AGEs) owing to the interplay between glycation and oxidation in their formation (21, 22).
The effects of AGEs can be direct or receptor-dependent. Given that advanced glycation occurs during a prolonged period of time, structural components of connective tissue matrix and basement membrane components (i.e., collagen IV) as well as other long-lived proteins such as myelin, tubulin, plasminogen activator 1, and fibrinogen may be targeted (21, 23). Alternatively, the receptor-dependent effects of AGEs can be mediated by different proteins or receptors that bind these components, with receptor of the advanced glycation end-products (RAGE) being the best characterized. RAGE, once activated, triggers secondary messenger pathways such as protein kinase C. Nuclear factor κB is a key target of the RAGE pathway and results in the increased transcription of intercellular adhesion molecule-1, E-selectin, endothelin-1, tissue factor, vascular endothelial growth factor, and proinflammatory cytokines as well as upregulation of RAGE itself. In endothelial cells, RAGE activation may activate additional damaging pathways involving nicotinamide dinucleotide phosphate oxidase p21RAS and MAPKs, further enhancing the production of ROS (21).
The impact of AGEs in diabetic complications was studied in type 1 diabetes in the DCCT Skin Collagen Ancillary Study Group, which compared intensive vs conventional glucose management and performed skin biopsies in a subset of the DCCT cohort. There was an association of AGEs with microvascular complications of diabetes. The association of microvascular complications with AGE measures persisted after adjustment for chronic HbA1c levels, and glycated collagen (furosine) had the strongest correlation with complications. This suggested that AGEs were more proximal to the cause of diabetic complications. This implicated tissue catalytic and inhibitory factors such as metals and oxidative stress that determine their formation rate (24). In another study, serum levels of AGEs were elevated in patients with type 2 diabetes when compared with nondiabetic controls and patients with diabetes and coronary heart disease demonstrated higher levels of AGEs in general (25).
Glycation products formed on intact proteins might bind transition metals, possibly allowing them to participate in reduction/oxidation reactions (1, 23). Of note, redox-active metals are sequestered in nonreactive forms (chelates) within proteins in vivo, being transported this way through compartments. However, metal homeostasis may become dysregulated during hyperglycemia, as glycated proteins may have lower capacity to form strong complexes with metals, potentially increasing their activation tendency in redox processes (7, 23, 26). This “glycochelates” hypothesis was tested in vitro by Qian et al. (23) by comparing the iron and copper binding capacity of three proteins (albumin, gelatin, and elastin) in their natural vs glycated state. Binding capacity was enhanced in the glycated forms, as was the redox activity of the bound metals. If this mechanism were active in vivo, it might produce redox-active glycochelates in areas such as the elastic laminae of arteries and elsewhere, potentially causing long-term consequences and contributing to the genesis of many diabetes complications. Thus, there is some evidence suggesting that metals may play an important role in the complications of diabetes, and the results of TACT suggest that macrovascular complications are responsive to metal chelation. Qian et al. (23) concluded that perhaps these oxidation reactions that take place in diabetes are not just limited to copper and iron but to transition metals as well. The chelation of metals in TACT2 may be providing a direct means of testing this hypothesis.
Lipoxidation
Oxidative modification of LDL cholesterol is considered a crucial step in the pathogenesis of atherosclerosis and might be enhanced in patients with diabetes, suggesting a relationship between hyperglycemia and accelerated oxidation (27). Cell-mediated oxidation of LDL depends on the balance between cellular pro-oxidants [i.e., reduced form of NAD phosphate (NADPH) oxidase, lipoxygenase, myeloperoxidase] and antioxidants (glutathione system, superoxide dismutase) as well as the balance between pro-oxidants and antioxidants in lipoproteins themselves (7). Traces of transition metal ions in free form or in redox-active complexes are generally agreed to be essential for the production of oxidized LDL. LDL oxidation starts with the consumption of its antioxidants, the role of which is to scavenge lipid peroxyl radicals. After this depletion occurs, an initiating free radical extracts a hydrogen atom from one of the polyunsaturated fatty acids contained in the LDL lipids. Then, metal ions catalyze a series of propagation reactions, which include the breakdown of lipid hydroperoxides and the formation of aldehydes. As a result, the apolipoprotein B100 molecule undergoes oxidative modification, affecting its charge and dimensional configuration, which renders it unable to bind to the LDL receptor, binding to the CD36, scavenger receptor A, and lectin-like receptor family instead (7, 28–30).
LDL oxidation by macrophages has a major role during the early stage of atherosclerosis. Macrophages have the ability to reduce iron or copper ions, which then rapidly react with lipid hydroperoxides, leading to the formation of reactive lipid radicals and the conversion of the metal back to its oxidized form. Superoxide ions are required for the LDL oxidation initiation, and in macrophages their main source is the NADPH oxidase system (7, 31).
In diabetes, LDL particles are characteristically small and dense and are considered more atherogenic owing to their capacity to penetrate the intima, poor affinity to the LDL receptor, and increased susceptibility to oxidation and glycation (32). At the same time, glycated LDL or AGE-LDL also has proinflammatory activity, mediated by its interactions with MCP-1, RAGE and the scavenger receptors, and Toll-like 4 receptor, triggering signaling pathways to produce proinflammatory cytokines (32, 33). In this context, in vivo studies have observed an increased level of plasma lipid hydroperoxides in patients with diabetes, with a decrease in antioxidant capacity (measured by the total radical-trapping antioxidant potential) not associated with HbA1c levels (25). Furthermore, glycation of HDL in diabetes may inversely impact its vasoprotective functions such as reverse cholesterol transport, antioxidant, anti-inflammatory, and vasodilatory effects (7, 29–33). For example, and as mentioned previously, PON protective action against lipid peroxidation in lipoproteins, macrophages, and atherosclerotic lesions might be impaired in diabetes (7, 34). It has been shown in vitro that glucose inactivates PON-1 and glycated PON-1 has reduced ability to hydrolyze membrane hydroperoxides (7). Also, several studies have found an inverse association between chronic exposure to different metals (such as cadmium, lead, and mercury) and PON-1 activity (7, 35, 36). Therefore diabetes can be considered as a state of increased free radical production and compromised total plasma antioxidant capacity, resulting in enhanced oxidative stress. Metal-catalyzed oxygen chemistry, therefore, may be important in establishing and enhancing oxidative state.
Effect of Metals on Atherosclerotic Disease and Diabetes Mellitus
Lead
Lead has also been extensively linked to cardiovascular outcomes of atherosclerotic origin, including coronary disease, hypertension, stroke, and peripheral vascular disease.
Animal studies have shown fatty degeneration and sclerotic changes on arterial walls of rats exposed to lead (37). In humans, there is a direct relationship of blood lead levels with cardiovascular events and cardiovascular mortality (37, 38). The National Health and Nutrition Examination Survey (NHANES), a large prospective, representative cohort of the US population, showed a significant association between blood lead level and mortality from both myocardial infarction and stroke at lead levels <10 µg/dL (39). Several epidemiological studies have also shown a significant association between blood lead level and systemic blood pressure (40, 41). In the Normative Aging Study, longitudinal analysis of bone lead levels was associated with increased systolic blood pressure and increased rate of all-cause mortality driven largely by cardiovascular causes, particularly ischemic heart disease (41, 42). A recent analysis of NHANES data estimated ∼250,000 deaths from cardiovascular disease attributable to low level lead exposure with concentration <5 µg/dL (43). In this study, participants who had a high blood lead level were also more likely to have elevated levels of serum cholesterol and higher rates of hypertension and diabetes. Although not a transition metal, lead shares the role of transition metals in potentiating oxidative stress by favoring the formation of ROS and increasing lipid peroxidation. As previously explained, experimental studies have shown an association between lead exposure and decrease in major antioxidant enzymes such as glutathione peroxidase, glutathione reductase, superoxide dismutase, and catalase (44, 45). It has been observed that lead exposure in lead-acid battery factory workers decreased serum PON-1 activity, further contributing to the atherosclerosis process (38). Chen et al. (46) found that patients with type 2 diabetes and higher lead body burden treated with EDTA chelation therapy showed a significant delay in the progression of diabetic nephropathy. However, no specific study has analyzed the relationship of lead exposure and diabetic cardiovascular disease and atherosclerosis. The design of TACT2, with metal levels assayed several times during the trial, should clarify this relationship.
Cadmium
Cadmium is a toxic metal with widespread population exposure and a long intracorporeal half-life (47–49). Besides its known toxic effects, cadmium has been associated with numerous diseases such as hypertension, kidney disease, diabetes, and atherosclerosis, particularly peripheral artery disease (47). For example, cadmium was associated with increased vascular endothelial permeability by inhibition of endothelial cell proliferation and induction of cell death in vitro (50). Also, Ferramola et al. (36) demonstrated that rats exposed for different intervals of time to cadmium showed an increased level of lipid peroxides, a product of oxidative stress (measured by thiobarbituric acid reactive substances as byproducts of lipid peroxidation), vs controls, which increased even further with longer exposure. At the same time, an initial increase in PON-1 activity was observed in early stages, followed by decreased levels with longer exposure time. In NHANES, urine cadmium concentration, a marker of cumulative exposure, was associated with cardiovascular mortality in men, but not in women (51). Messner et al. (50) measured the carotid intima media thickness of young healthy women and serum metal concentrations. The study showed an independent association between high cadmium levels and increased intima media thickness, and thus a marker of early atherosclerotic vessel wall thickening. Epidemiological studies have also shown an association between cadmium and hypertension similar to the effect of lead (52, 53). Navas-Acien et al. (54) also showed that blood lead and cadmium levels were strongly associated with increased prevalence of peripheral vascular disease in the NHANES population (55). In the Strong Heart Study, a positive correlation between cadmium, cardiovascular mortality, and coronary heart disease was observed, even after adjusting for traditional cardiovascular risks factors (56). Although the association was nominally stronger in the diabetes subpopulation, albeit not statistically significant presumably owing to sample size, the diabetic population might be more susceptible to the effect of toxic metals. In this context, the NHANES III cross-sectional study found a strong positive correlation between urinary cadmium levels, prediabetes, and diabetes, although cigarette smoking, a confounder, also showed a strong correlation with impaired glucose metabolism (57).
Copper and iron
The edetates enhance excretion of copper, another active participant in oxidative reactions that may be particularly damaging in diabetes. Copper is an essential metal. Free copper ions are highly redox active and might contribute to tissue damage by the generation of ROS (7). Copper exists in biological systems primarily as Cu+, which is mostly found intracellularly, and Cu2+, the main copper form in extracellular matrix. The main copper-binding proteins in plasma are ceruloplasmin (95%) and albumin. However, regulation of copper metabolism might be abnormal in diabetes (58, 59). In vitro studies have shown that chronic hyperglycemia can damage the copper-binding properties of ceruloplasmin and albumin. When incubated with glucose, ceruloplasmin is fragmented and there is a time-dependent release of its bound copper, which then might participate in a Fenton reaction to produce hydroxyl radicals (60). This and the fact that AGEs can bind and localize catalytically active Cu2+ might explain some of the increased oxidative stress seen in diabetes and its ensuing cardiovascular complications (7, 58–60). Thus, another mechanism for edetate benefit may be chelation of free copper.
Iron is also intimately linked to oxidative stress, despite its other important functions. Similar to other transition metals, it can also participate in the Fenton reaction on its ferrous form (Fe2+) to create highly toxic free radicals and produce lipid peroxides. In diabetes, glycation of transferrin decreases its ability to bind ferrous iron and, owing to the now increased pool of free iron, indirectly stimulates ferritin synthesis (61). Glycated holotransferrin is additionally known to facilitate the production of free oxygen radicals that further amplify iron’s oxidative effects. The toxic iron fraction is usually stored as ferritin. It can be released by the action of reducing agents that convert Fe3+ into Fe2+, which in turn increases ferritin translation. This is consistent with the observation of increased ferritin levels in patients with diabetes, although, as an acute phase reactant, ferritin levels may also reflect underlying inflammation. Additionally, its protein apoferritin exerts ferroxidase activity, catalyzing the oxidation of Fe2+ to Fe3+, allowing iron storage and preventing oxidative stress propagation. However, when antioxidant concentrations are low, there is a rapid release of iron from ferritin and a downregulation of ferroxidase activity in this setting, resulting in an enhancement in free radical generation. In this context, animal and human studies in diabetes showed that selective iron chelators ameliorate endothelial dysfunction (7, 61–63). Edetate disodium increases iron excretion. Whether edetate disodium reduces catalytically active iron (Fe3+) or copper, however, has yet to be determined.
Arsenic and mercury
Although the impact of other transition metals and metalloids on atherosclerosis has been studied, evidence in the population with diabetes is limited. For example, arsenic, a metalloid, has been shown to have dose- and time-dependent inhibitory effects on proliferation and viability of endothelial cells and may cause endothelial dysfunction, leading to apoptosis via induction of p21 (47–49, 64). Also, arsenic induces oxidative stress by increased production of ROS (especially superoxide), depletion of antioxidant factors such as glutathione, and indirectly on its methylated form by releasing redox-active iron from ferritin. Therefore, there is an increase in lipid peroxides, enhancing further arsenic-induced endothelial dysfunction and atherosclerosis (64). Arsenic, however, is not effectively chelated by the edetates.
There have been numerous cohort studies on the association of mercury with cardiovascular disease, given this metal’s ample distribution and exposure, especially in marine products. These observations are confounded. Mercury is found in many forms of seafood, but the consumption of seafood is associated with a lower cardiovascular event rate. Moreover, mercury is also not effectively removed from the body by the edetates, so we will not cover the extensive literature on this very interesting metal, other than to point out that the potential atherosclerotic mechanism of mercury, besides the shared pathobiology of transition metals, might involve its effect on selenium and formation of mercury selenide, impairing selenium function as a cofactor for glutathione peroxidase. Also, methylmercury may inactivate catalase, glutathione, and superoxide dismutase, causing further oxidative burden (65).
Chelating Properties of Common Drugs Used in Diabetes
Several medications that are routinely used in patients with diabetes, such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), metformin, and carvedilol, among others, have chelating properties (22, 66–68) [Table 2 (53, 57, 59, 62, 67–69)]. These chelating properties should be viewed anew in the post-TACT era. As previously mentioned, chelators may inhibit the metal catalyzed oxidation reactions that form AGEs. In 1986, Brownlee et al. (69) introduced aminoguanidine as the first AGE inhibitor that trapped chemical intermediates in the formation of AGEs. It was a weak chelator that required high doses to achieve efficacy in vivo, which led to toxic effects related to vitamin B6 deficiency, thus hampering its clinical use (70). Subsequently, in search for other drugs with chelating characteristics, Miyata et al. (71) found that olmesartan, an ARB, and temocaprilat, an ACEI, significantly reduced the in vitro production of two AGEs, pentosidine and N-carboxymethyllysine, acting primarily by inhibiting their oxidative formation. They analyzed the effect on metal ions and reported that olmesartan chelated copper, inhibiting the catalyzed auto-oxidation of ascorbic acid. A total of six additional ARBs and four ACEIs were tested in their study with similar AGE-lowering potential and similar mechanisms.
Table 2.
Oral Medications and Their Chelating Properties
| Drug | Target | Effects |
|---|---|---|
| ACEIs | Inhibit in vitro the metal-catalyzed oxidation of ascorbic acid, increased activities of superoxide dismutase | Decrease AGE formation (57, 62) |
| ARBs | Inhibit in vitro the metal-catalyzed oxidation of ascorbic acid | Decrease AGE formation (62) |
| Metformin | Interaction with mitochondrial copper to exert its effects in AMP-activated protein kinase, traps α-dicarbonyl | Decrease in gluconeogenesis (67, 68) |
| Carvedilol | Inhibition of electron adduction, inhibiting lipid peroxidation in myocardial cell membranes, inhibiting neutrophil release of O2, scavenging peroxychlorous and hypochlorous radicals | Possible role in cardioprotection (69) |
| Deferoxamine | Chelates iron; intercepts ROS | Helps decrease diabetes-induced endothelial dysfunction (53) |
| Vitamin C | Intercepts ROS | Decrease in fasting insulin levels (59) |
Other studies have shown that in addition to renal protection by ACEIs or ARBs such as ramipril, benzapril, and valsartan in hypertensive and diabetic rat models there was also a decrease in AGE accumulation and expression of RAGE in the kidney, as well as decreased expression of NADPH oxidase and other biomarkers of oxidative stress (72–74). Forbes et al. (72) randomized diabetic rats to ramipril (3 mg/L), aminoguanidine (1 g/L), and placebo and followed them for 12 weeks. In rats treated with ramipril and aminoguanidine, renal AGE accumulation was significantly reduced along with renal nitrotyrosine, a marker of protein oxidation, suggesting that both compounds inhibit AGE through effects on ROS production. Thus, these two series of experiments may be interpreted to suggest that the renoprotective effects of ACEIs and ARBs might be secondary to their inhibition of metal-mediated oxidative stress. In another study in diabetic-induced rats, captopril and enalapril were shown to increase activities of the antioxidant enzyme, superoxide dismutase, another mechanism for reducing the cytotoxic effects of enhanced oxidative stress (75).
A prime example of this hypothesis is metformin’s chelating properties, which are possibly key to its metabolic effects in diabetes (76, 77). Several studies have also shown that metformin reduced diabetes-associated vascular risk, demonstrating benefits beyond the expected glucose-lowering effect. Metformin acts as a ligand for several divalent transition metals but has greater affinity for copper (76). This drug might interact with mitochondrial copper to exert its effects in AMP-activated protein kinase and other pathways to inhibit glucose production (76). Additional evidence suggests that metformin also reacts with α-dicarbonyls, reducing toxic dicarbonyls and AGEs (77). Carvedilol, widely used for its cardioprotective properties, also contains an antioxidant (carbazol) compound in its molecular structure. Adding to its potential role as a chelator, this includes: (i) inhibiting electron adduction, (ii) inhibiting lipid peroxidation in myocardial cell membranes, and (iii) inhibiting neutrophil release of O2 and other antioxidant properties (78). Additionally, iron chelators such as deferoxamine have both shown beneficial effects on endothelial dysfunction in diabetes animal models by interrupting the ROS generating cycle (62). Mild chelating activity might be the basis for the health effects in diabetes of citrus foods and some nutraceuticals (79). Nagai et al. (80) examined the effect of citrate, a natural chelator found in citrus fruits, on diabetic mice observing an inhibition of nephropathy (albuminuria), accumulation of AGEs in lens, and delayed cataract development. This evidence showed that even a common natural dietary chelator can inhibit AGE formation and protect against diabetic complications. Thus, the underlying role of catalytic metal ions in oxidative stress may serve as a target for AGE inhibition and potential mechanism of many of these drugs.
TACT2
Based on the results of TACT, the National Institutes of Health has funded TACT2, a replicative placebo-controlled, factorial clinical trial that will assess the effects of edetate disodium–based chelation therapy and high-dose oral multivitamins and multiminerals on cardiovascular outcomes in patients with diabetes (type 1 or 2) with a prior myocardial infarction. Based on conservative estimations of event rates and effect size, TACT2 will have excellent power to detect a clinically important difference between treatment groups. Additionally, and different from TACT, TACT2 has a strong mechanistic component focusing on toxic metals, and it is funded to maintain a biorepository. Thus, the questions of mechanisms that have arisen following TACT should be answered. The trial is in its early phases of enrollment, and additional clinical sites are being recruited.
Summary
The results of TACT strongly suggest that heavy metals, either as catalysts of oxidative stress or some other mechanism, are an emerging therapeutic target in cardiovascular disease, especially in patients with diabetes. Professionals specializing in the care of patients with diabetes, atherosclerosis, and cardiovascular diseases should be familiar with the potential role that metal chelation with edetate disodium may have as a strategy to reduce residual atherosclerotic risk in optimally treated patients.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACEI
angiotensin-converting enzyme inhibitor
- AGE
advanced glycation end-product
- ARB
angiotensin II receptor blocker
- HDL
high-density lipoprotein
- HR
hazard ratio
- LDL
low-density lipoprotein
- MI
myocardial infarction
- NADPH
reduced form of NAD phosphate
- NHANES
National Health and Nutrition Examination Survey
- NNT
number needed to treat
- OMVM
oral multivitamin and minerals
- PON
paraoxonase
- RAGE
receptor for advanced glycation end-products
- ROS
reactive oxygen species
- TACT
Trial to Assess Chelation Therapy
- TACT2
second TACT
References
- 1. Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J, Lee KL; TACT Investigators. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309(12):1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Escolar E, Lamas GA, Mark DB, Boineau R, Goertz C, Rosenberg Y, Nahin RL, Ouyang P, Rozema T, Magaziner A, Nahas R, Lewis EF, Lindblad L, Lee KL. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurst RT, Lee RW. Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: mechanisms and management. Ann Intern Med. 2003;139(10):824–834. [DOI] [PubMed] [Google Scholar]
- 4. Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108(12):1527–1532. [DOI] [PubMed] [Google Scholar]
- 6. Kanter JE, Averill MM, Leboeuf RC, Bornfeldt KE. Diabetes-accelerated atherosclerosis and inflammation. Circ Res. 2008;103(8):e116–e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jenkins AJ, Toth PP, Lyons TL, eds. Lipoproteins in Diabetes Mellitus. Totowa, NJ: Humana Press; 2014. [Google Scholar]
- 8. Kanter JE, Johansson F, LeBoeuf RC, Bornfeldt KE. Do glucose and lipids exert independent effects on atherosclerotic lesion initiation or progression to advanced plaques? Circ Res. 2007;100(6):769–781. [DOI] [PubMed] [Google Scholar]
- 9. Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, Lopes-Virella M, Reusch J, Ruderman N, Steiner G, Vlassara H. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation. 2002;105(18):e138–e143. [DOI] [PubMed] [Google Scholar]
- 10. Grier MT, Meyers DG. So much writing, so little science: a review of 37 years of literature on edetate sodium chelation therapy. Ann Pharmacother. 1993;27(12):1504–1509. [DOI] [PubMed] [Google Scholar]
- 11. Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;232(6):654–666. [DOI] [PubMed] [Google Scholar]
- 12. Clarke NE, Clarke CN, Mosher RE. The in vivo dissolution of metastatic calcium; an approach to atherosclerosis. Am J Med Sci. 1955;229(2):142–149. [DOI] [PubMed] [Google Scholar]
- 13. Kitchell JR, Palmon F Jr, Aytan N, Meltzer LE. The treatment of coronary artery disease with disodium EDTA. A reappraisal. Am J Cardiol. 1963;11(4):501–506. [DOI] [PubMed] [Google Scholar]
- 14. Guldager B, Jelnes R, Jørgensen SJ, Nielsen JS, Klaerke A, Mogensen K, Larsen KE, Reimer E, Holm J, Ottesen S. EDTA treatment of intermittent claudication—a double-blind, placebo-controlled study. J Intern Med. 1992;231(3):261–267. [DOI] [PubMed] [Google Scholar]
- 15. Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev. 2002; (4):CD002785. [DOI] [PubMed] [Google Scholar]
- 16. Olszewer E, Carter JP. EDTA chelation therapy in chronic degenerative disease. Med Hypotheses. 1988;27(1):41–49. [DOI] [PubMed] [Google Scholar]
- 17. Lewin MR. Chelation therapy for cardiovascular disease. Review and commentary. Tex Heart Inst J. 1997;24(2):81–89. [PMC free article] [PubMed] [Google Scholar]
- 18. Waters RS, Bryden NA, Patterson KY, Veillon C, Anderson RA. EDTA chelation effects on urinary losses of cadmium, calcium, chromium, cobalt, copper, lead, magnesium, and zinc. Biol Trace Elem Res. 2001;83(3):207–221. [DOI] [PubMed] [Google Scholar]
- 19. Arenas IA, Navas-Acien A, Ergui I, Lamas GA. Enhanced vasculotoxic metal excretion in post-myocardial infarction patients following a single edetate disodium-based infusion. Environ Res. 2017;158:443–449. [DOI] [PubMed] [Google Scholar]
- 20. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. [DOI] [PubMed] [Google Scholar]
- 21. Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93(4):1143–1152. [DOI] [PubMed] [Google Scholar]
- 22. Nagai R, Murray DB, Metz TO, Baynes JW. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61(3):549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qian M, Liu M, Eaton JW. Transition metals bind to glycated proteins forming redox active “glycochelates”: implications for the pathogenesis of certain diabetic complications. Biochem Biophys Res Commun. 1998;250(2):385–389. [DOI] [PubMed] [Google Scholar]
- 24. Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, Cleary PA, Lachin J, Genuth S. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes. 1999;48(4):870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kilhovd BK, Berg TJ, Birkeland KI, Thorsby P, Hanssen KF. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care. 1999;22(9):1543–1548. [DOI] [PubMed] [Google Scholar]
- 26. Monnier VM. Transition metals redox: reviving an old plot for diabetic vascular disease. J Clin Invest. 2001;107(7):799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Esterbauer H, Wäg G, Puhl H. Lipid peroxidation and its role in atherosclerosis. Br Med Bull. 1993;49(3):566–576. [DOI] [PubMed] [Google Scholar]
- 28. Santini SA, Marra G, Giardina B, Cotroneo P, Mordente A, Martorana GE, Manto A, Ghirlanda G. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes. 1997;46(11):1853–1858. [DOI] [PubMed] [Google Scholar]
- 29. Stringer MD, Görög PG, Freeman A, Kakkar VV. Lipid peroxides and atherosclerosis. BMJ. 1989;298(6669):281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26(8):1702–1711. [DOI] [PubMed] [Google Scholar]
- 31. Chisolm GM, Irwin KC, Penn MS. Lipoprotein oxidation and lipoprotein-induced cell injury in diabetes. Diabetes. 1992;41(Suppl 2):61–66. [DOI] [PubMed] [Google Scholar]
- 32. Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46(6):733–749. [DOI] [PubMed] [Google Scholar]
- 33. Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the Toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(12):2275–2281. [DOI] [PubMed] [Google Scholar]
- 34. Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299(11):1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pollack AZ, Sjaarda L, Ahrens KA, Mumford SL, Browne RW, Wactawski-Wende J, Schisterman EF. Association of cadmium, lead and mercury with paraoxonase 1 activity in women. PLoS One. 2014;9(3):e92152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferramola ML, Antón RI, Anzulovich AC, Giménez MS. Myocardial oxidative stress following sub-chronic and chronic oral cadmium exposure in rats. Environ Toxicol Pharmacol. 2011;32(1):17–26. [DOI] [PubMed] [Google Scholar]
- 37. Skoczyńska A, Smolik R, Jeleń M. Lipid abnormalities in rats given small doses of lead. Arch Toxicol. 1993;67(3):200–204. [DOI] [PubMed] [Google Scholar]
- 38. Li WF, Pan MH, Chung MC, Ho CK, Chuang HY. Lead exposure is associated with decreased serum paraoxonase 1 (PON1) activity and genotypes. Environ Health Perspect. 2006;114(8):1233–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 μmol/L (10 μg/dL) and mortality among US adults. Circulation. 2006;114(13):1388–1394. [DOI] [PubMed] [Google Scholar]
- 40. Glenn BS, Stewart WF, Links JM, Todd AC, Schwartz BS. The longitudinal association of lead with blood pressure. Epidemiology. 2003;14(1):30–36. [DOI] [PubMed] [Google Scholar]
- 41. Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the Normative Aging Study. Am J Epidemiol. 2001;153(2):164–171. [DOI] [PubMed] [Google Scholar]
- 42. Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, Hu H. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the Department of Veterans Affairs Normative Aging Study (published correction appears in Circulation. 2014;130(5):e43). Circulation. 2009;120(12):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health. 2018;3(4):e177–e184. [DOI] [PubMed] [Google Scholar]
- 44. Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18(2):321–336. [DOI] [PubMed] [Google Scholar]
- 45. Mabrouk A, Cheikh HB. Thymoquinone ameliorates lead-induced suppression of the antioxidant system in rat kidneys. Libyan J Med. 2016;11(1):31018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen KH, Lin JL, Lin-Tan DT, Hsu HH, Hsu CW, Hsu KH, Yen TH. Effect of chelation therapy on progressive diabetic nephropathy in patients with type 2 diabetes and high-normal body lead burdens. Am J Kidney Dis. 2012;60(4):530–538. [DOI] [PubMed] [Google Scholar]
- 47. Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. 2014;168(6):812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lippman M. Enviromental Toxicants. Human Exposures and Their Health Effects. 3rd ed.New York, NY: Wiley-Interscience; 2009. [Google Scholar]
- 49. Hutchinson TC. Lead, Mercury, Cadmium and Arsenic in the Environment. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 50. Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, Shen YH, Zeller I, Willeit J, Laufer G, Wick G, Kiechl S, Bernhard D. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol. 2009;29(9):1392–1398. [DOI] [PubMed] [Google Scholar]
- 51. Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect. 2009;117(2):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Franceschini N, Fry RC, Balakrishnan P, Navas-Acien A, Oliver-Williams C, Howard AG, Cole SA, Haack K, Lange EM, Howard BV, Best LG, Francesconi KA, Goessler W, Umans JG, Tellez-Plaza M. Cadmium body burden and increased blood pressure in middle-aged American Indians: the Strong Heart Study. J Hum Hypertens. 2017;31(3):225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 2010;118(12):1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109(25):3196–3201. [DOI] [PubMed] [Google Scholar]
- 55. Zhuang X, Ni A, Liao L, Guo Y, Dai W, Jiang Y, Zhou H, Hu X, Du Z, Wang X, Liao X. Environment-wide association study to identify novel factors associated with peripheral arterial disease: Evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis. 2018;269:172–177. [DOI] [PubMed] [Google Scholar]
- 56. Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB, Navas-Acien A. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24(3):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26(2):468–470. [DOI] [PubMed] [Google Scholar]
- 58. Cooper GJ, Chan YK, Dissanayake AM, Leahy FE, Keogh GF, Frampton CM, Gamble GD, Brunton DH, Baker JR, Poppitt SD. Demonstration of a hyperglycemia-driven pathogenic abnormality of copper homeostasis in diabetes and its reversibility by selective chelation: quantitative comparisons between the biology of copper and eight other nutritionally essential elements in normal and diabetic individuals. Diabetes. 2005;54(5):1468–1476. [DOI] [PubMed] [Google Scholar]
- 59. Cooper GJ. Selective divalent copper chelation for the treatment of diabetes mellitus. Curr Med Chem. 2012;19(17):2828–2860. [DOI] [PubMed] [Google Scholar]
- 60. Argirova MD, Ortwerth BJ. Activation of protein-bound copper ions during early glycation: study on two proteins. Arch Biochem Biophys. 2003;420(1):176–184. [DOI] [PubMed] [Google Scholar]
- 61. Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51(8):2348–2354. [DOI] [PubMed] [Google Scholar]
- 62. Pieper GM, Siebeneich W. Diabetes-induced endothelial dysfunction is prevented by long-term treatment with the modified iron chelator, hydroxyethyl starch conjugated-deferoxamine. J Cardiovasc Pharmacol. 1997;30(6):734–738. [DOI] [PubMed] [Google Scholar]
- 63. Nitenberg A, Ledoux S, Valensi P, Sachs R, Antony I. Coronary microvascular adaptation to myocardial metabolic demand can be restored by inhibition of iron-catalyzed formation of oxygen free radicals in type 2 diabetic patients. Diabetes. 2002;51(3):813–818. [DOI] [PubMed] [Google Scholar]
- 64. Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51(2):257–281. [DOI] [PubMed] [Google Scholar]
- 65. Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gómez-Aracena J, Kark JD, Riemersma RA, Martín-Moreno JM, Kok FJ; Heavy Metals and Myocardial Infarction Study Group. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347(22):1747–1754. [DOI] [PubMed] [Google Scholar]
- 66. Wihler C, Schäfer S, Schmid K, Deemer EK, Münch G, Bleich M, Busch AE, Dingermann T, Somoza V, Baynes JW, Huber J. Renal accumulation and clearance of advanced glycation end-products in type 2 diabetic nephropathy: effect of angiotensin-converting enzyme and vasopeptidase inhibition. Diabetologia. 2005;48(8):1645–1653. [DOI] [PubMed] [Google Scholar]
- 67. Logie L, Harthill J, Patel K, Bacon S, Hamilton DL, Macrae K, McDougall G, Wang HH, Xue L, Jiang H, Sakamoto K, Prescott AR, Rena G. Cellular responses to the metal-binding properties of metformin. Diabetes. 2012;61(6):1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Frizzell N, Baynes JW. Chelation therapy: overlooked in the treatment and prevention of diabetes complications? Future Med Chem. 2013;5(10):1075–1078. [DOI] [PubMed] [Google Scholar]
- 69. Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232(4758):1629–1632. [DOI] [PubMed] [Google Scholar]
- 70. Freedman BI, Wuerth JP, Cartwright K, Bain RP, Dippe S, Hershon K, Mooradian AD, Spinowitz BS. Design and baseline characteristics for the Aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control Clin Trials. 1999;20(5):493–510. [DOI] [PubMed] [Google Scholar]
- 71. Miyata T, van Ypersele de Strihou C, Ueda Y, Ichimori K, Inagi R, Onogi H, Ishikawa N, Nangaku M, Kurokawa K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol. 2002;13(10):2478–2487. [DOI] [PubMed] [Google Scholar]
- 72. Forbes JM, Cooper ME, Thallas V, Burns WC, Thomas MC, Brammar GC, Lee F, Grant SL, Burrell LM, Jerums G, Osicka TM. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy (published corrections appear in Diabetes. 2002;51(12):3592 and Diabetes. 2010;59(4):1113). Diabetes. 2002;51(11):3274–3282. [DOI] [PubMed] [Google Scholar]
- 73. Liu XP, Pang YJ, Zhu WW, Zhao TT, Zheng M, Wang YB, Sun ZJ, Sun SJ. Benazepril, an angiotensin-converting enzyme inhibitor, alleviates renal injury in spontaneously hypertensive rats by inhibiting advanced glycation end-product-mediated pathways. Clin Exp Pharmacol Physiol. 2009;36(3):287–296. [DOI] [PubMed] [Google Scholar]
- 74. Forbes JM, Thomas MC, Thorpe SR, Alderson NL, Cooper ME. The effects of valsartan on the accumulation of circulating and renal advanced glycation end products in experimental diabetes. Kidney Int Suppl. 2004;66(92):S105–S107. [DOI] [PubMed] [Google Scholar]
- 75. Kedziora-Kornatowska KZ, Luciak M, Paszkowski J. Lipid peroxidation and activities of antioxidant enzymes in the diabetic kidney: effect of treatment with angiotensin convertase inhibitors. IUBMB Life. 2000;49(4):303–307. [DOI] [PubMed] [Google Scholar]
- 76. Repiščák P, Erhardt S, Rena G, Paterson MJ. Biomolecular mode of action of metformin in relation to its copper binding properties. Biochemistry. 2014;53(4):787–795. [DOI] [PubMed] [Google Scholar]
- 77. Ruggiero-Lopez D, Lecomte M, Moinet G, Patereau G, Lagarde M, Wiernsperger N. Reaction of metformin with dicarbonyl compounds. Possible implication in the inhibition of advanced glycation end product formation. Biochem Pharmacol. 1999;58(11):1765–1773. [DOI] [PubMed] [Google Scholar]
- 78. Dulin B, Abraham WT. Pharmacology of carvedilol. Am J Cardiol. 2004;93(9A):3B–6B. [DOI] [PubMed] [Google Scholar]
- 79. Karpen CW, Pritchard KA Jr, Arnold JH, Comwell DG, Panganamala RV. Restoration of the prostacyclin/thromboxane A2 balance in the diabetic rat: influence of vitamin E. Diabetes. 1982;31(11):947–951. [Google Scholar]
- 80. Nagai R, Nagai M, Shimasaki S, Baynes JW, Fujiwara Y. Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochem Biophys Res Commun. 2010;393(1):118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]