Abstract
Oral human papillomavirus genotype 16 (HPV16) infection causes oropharyngeal squamous cell carcinoma (SCC), and the prevalence of oropharyngeal SCC is higher among men than women in the United States. In a cohort study of oral HPV infection among 409 individuals aged 18–25 years, the risk among men but not among women significantly increased as the number of recent (ie, within the prior 3 months) oral sex partners increased (Pinteraction = .05). In contrast, the risk among women but not among men significantly decreased as the lifetime number of vaginal sex partners increased (Pinteraction = .037). Men were also significantly less likely than women to clear oral HPV infection. Our data contribute to understanding sex differences in risk for HPV-positive oropharyngeal SCC.
Clinical Trials Registration. NCT00994019.
Keywords: oral HPV, young adults, STD clinic, sex, epidemiology
Oral human papillomavirus genotype 16 (HPV16) infection causes a majority of cases of oropharyngeal squamous cell carcinoma (SCC) in the United States, and incidence rates among men are higher and rising more rapidly than among women [ 1, 2]. The higher risk of oropharyngeal SCC among US men is explained in part by a 5-fold higher oral HPV16 prevalence [ 3–5], but the factors contributing to this sex difference in prevalence are unknown.
Currently, it is unknown whether the higher oral HPV prevalence among men is attributable to increased incidence, decreased clearance, or a combination of both factors. Previous studies indicate that the natural history of oral HPV infection may differ by sex, but inferences were limited by small sample size, short follow-up, inclusion of a single sex, or a focus on specific populations (eg, human immunodeficiency virus (HIV)–infected individuals) [ 6–11]. We investigated differences in the natural history of oral HPV infection among young adult men and women, a population known to be at high risk for sexually transmitted infections.
METHODS
To gain insight into sex differences, we conducted a prospective study of the natural history of oral HPV infection among young adult men and women aged 18–25 years attending 2 Baltimore County Health Department sexually transmitted diseases clinics for routine care. Participants were enrolled into the Study of Papillomavirus in Teens and Twenties (SPITT; ClinicalTrials.gov number NCT00994019) between April 2010 and November 2012, as previously described [ 12]. Individuals who attended a second visit were considered enrolled into a prospective cohort study and were followed every 3 months for 18 months. Participants with persistent infection at their 18-month visit had extended follow-up up to 30 months. This allowed us to observe the time to clearance of many incident infections acquired later in the study during this extended follow-up.
At each visit, participants provided a 30-second oral rinse and gargle sample, as previously described [ 12], and completed a computer-assisted self-interview containing questions regarding behavioral information (including number of lifetime and recent [ie, during the past 3 months] number of partners for open-mouthed kissing and oral and vaginal sex), demographic characteristics, and tobacco, alcohol, and drug use. Participants were provided $15 incentives and a gift with a value of $2–$5 (ie, a water bottle, snack, or cosmetic/cologne) at each follow-up visit. Oral rinse samples were processed and tested for 36 HPV genotypes by use of PGMY09/11 PCR primer pools and primers for β-globin, followed by reverse line blot hybridization to the Roche linear array [ 13]. Oncogenic HPV types included HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and 73.
Incident infection was defined as a newly detected HPV genotype, and clearance was defined as detection of a type-specific HPV infection followed by 2 consecutive visits with negative test results (eg, visit A, positive test result; visit B, negative test result; and visit C, negative test result). The time to clearance of HPV infection was defined as the time from the first positive test result to the time of the first negative test result and is visualized using Kaplan–Meier curves. Risk factors for incidence and clearance of type-specific oral HPV infection were explored using WLW models with robust variance (these are Cox models with the Wei-Lin-Weisfeld extension), including time-updated covariates in each model. Differences in risk factors between males and females were tested for interaction between sex and each sexual behavior.
The median follow-up time was 18 months (interquartile range [IQR], 10–18 months), with a median of 6 oral rinse samples collected per participant (IQR, 3–7 samples). Loss to follow-up was due to relocation (for 56 participants), entry into a drug treatment facility (for 28), incarceration (for 10), or death (for 2).
RESULTS
The median age of the 409 participants was 21 years (IQR, 20–23 years), 52% were men, 73% were black, and 39% had a history of sexually transmitted disease. Men and women were similar with regard to age, race, recent cigarette smoking, and lifetime number of oral sex partners. However, men were more likely than women to report >10 lifetime vaginal sex partner (51% vs 32%), >1 recent vaginal sex partner (50% vs 36%), and >1 recent oral sex partner (28% vs 22%; P < .05 for all comparisons). Recent performance of cunnilingus was reported by 70% of men and 9% of women, and recent performance of fellatio was reported by 8% of men and 71% of women.
Men had a higher prevalence and incidence of oral HPV infection than women. At baseline, 68 prevalent, type-specific oral HPV infections were detected among 44 participants (11%; 15.4% of men vs 5.6% of women; P = .001). A total of 134 incident, type-specific oral HPV infections were detected during follow-up, for an estimated incidence rate of 22.0 infections per 1000 person-months. The incidence of HPV16 infection (2.5 vs 0.7 infections per 1000 person-months; P = .08) and any oncogenic HPV infection (15.5 vs 8.9 infections per 1000 person-months; P = .04) was higher among men than among women.
In univariate analysis, in addition to male sex, other risk factors for oral HPV infection included younger age and sexual behavior. In the entire cohort, the risk of oral infection increased as the number of recent oral sex partners (Ptrend = .007) and recent vaginal sex partners (Ptrend < .001) increased, but associations between risk and lifetime number of oral sex partners or lifetime number of vaginal sex partners were not observed. Recent cigarette, alcohol, or marijuana use and recent open-mouthed kissing were not associated with risk (data not shown).
As shown in Table 1, we observed significant interactions between sex, oral sexual behavior, and the risk of incident oral HPV infection. Notably, recent performance of oral sex significantly increased the risk of oral HPV infection among men but not women (hazard ratio [HR], 3.15 vs 0.66; Pinteraction = .01). Further, the risk among men but not among women increased significantly as the number of recent oral sex partners increased (Pinteraction = .052). In contrast, the risk among women but not among men significantly declined with higher lifetime number of vaginal sexual partners (Pinteraction = .037; Table 1).
Table 1.
Risk of Oral Human Papillomavirus (HPV) Infection Associated With Each Sexual Behavior, by Participant Sex
| Characteristic | Univariate HR (95% CI) |
P interaction | |
|---|---|---|---|
| Men | Women | ||
| Recently a performed any oral sex | |||
| No | 1.00 | 1.00 | .010 |
| Yes | 3.15 (1.42–6.98) | 0.66 (.27–1.64) | |
| Recent a no. of people performed oral sex on | |||
| 0 | 1.00 | 1.00 | .052 |
| 1 | 3.02 (1.34–6.80) | 0.56 (.22–1.43) | |
| 2 | 3.07 (1.06–8.87) | 1.13 (.39–3.23) | |
| ≥3 | 4.54 (1.39–14.83) | 0.95 (.19–4.80) | |
| Ptrend | .001 | .86 | |
| Lifetime no. of people performed oral sex on | |||
| ≤2 | 1.00 | 1.00 | .071 |
| 3–5 | 1.46 (.68–3.12) | 0.44 (.21–.94) | |
| ≥6 | 1.00 (.45–2.21) | 0.57 (.25–1.28) | |
| Ptrend | .88 | .037 | |
| Lifetime no. of vaginal sex partners | |||
| ≤2 | 1.00 | 1.00 | .037 |
| 3–5 | 0.52 (.17–1.55) | 0.37 (.14–.95) | |
| ≥6 | 1.15 (.54–2.44) | 0.31 (.13–.71) | |
| Ptrend | .22 | .003 | |
| Recent a performance of cunnilingus | |||
| No | 1.00 | 1.00 | .042 |
| Yes | 2.52 (1.27–5.02) | 0.77 (.31–1.95) | |
| Recent a performance of fellatio | |||
| No | 1.00 | 1.00 | .333 |
| Yes | 0.85 (.29–2.54) | 0.43 (.17–1.08) | |
| Sexual orientation | |||
| Heterosexual | 1.00 | 1.00 | .494 |
| Homosexual/bisexual | 0.75 (.25–2.30) | 1.17 (.53–2.54) | |
P values were considered statistically significance at an α of ≤0.05. Additional details abut participant enrollment, characteristics and eligibility are available elsewhere [ 12 ].
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Defined as within the past 3 months.
To better understand reasons behind the interaction of participant sex and behavior (performing oral sex), we evaluated the risk of oral HPV infection associated with recent performance of cunnilingus versus the risk associated with recent performance of fellatio. This analysis also revealed differences by sex (Table 1). Recent performance of cunnilingus increased the risk of oral HPV infection among men but not among women (HR, 2.52 vs 0.77; Pinteraction = .042), whereas recent performance of fellatio was not associated with risk among men or women.
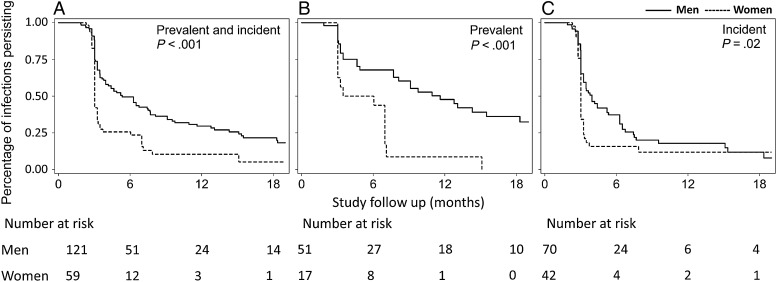
Given the sex differences in oral HPV infection incidence, we next evaluated the effect of sex on oral HPV infection clearance. There were 202 type-specific oral HPV infections in men and women in this study, including 68 prevalent and 134 incident infections. The median follow-up time was 12.2 months (IQR, 6.3–18.1 months) after first detection. The median time to clearance was 3.7 months, with 24% of infections persisting to 12 months. The median time to clearance was significantly longer in men than in women (5.3 vs 3.0 months; P < .001; Figure 1). When considering only the 16 oral HPV16 infections, the median time to clearance remained higher among men than among women (7.5 vs 3.5 months; P = .34; Supplementary Figure 1), although this difference was not statistically significant.
Figure 1.
Kaplan–Meier curves of time to clearance of type-specific oral human papillomavirus (HPV) infections, stratified by sex. Shown is the natural history of all 180 prevalent and incident oral HPV infections (A), 68 prevalent infections (B), and 112 incident infections (of 134 total incident infections detected) that had follow-up after detection (C). Overall 12-month clearance was 70.4% in men, compared with 89.6% in women (P < .001). The median time to clearance was significantly shorter for incident versus prevalent infections (3.3 vs 8.1 months; P < .001). Log-rank P values for a comparison of time to clearance of oral HPV infections in men and women are shown.
Factors significantly associated with reduced oral HPV infection clearance in unadjusted analysis included male sex (HR, 0.54; 95% confidence interval [CI], .37–.78), infection category (prevalent vs incident; HR, 0.47; 95% CI, .32–.68), and recent alcohol use (HR, 0.62; 95% CI, .42–.91). In adjusted analysis, men remained 37% less likely than women (adjusted HR, 0.63; 95% CI, .42–.95) to clear an oral HPV infection (Supplementary Table 1).
DISCUSSION
We conclude that the risk factors and natural history of oral HPV infection are significantly different among men and women. The sex of an individual who recently performed oral sex appears to influence their risk of oral HPV infection. Recent performance of cunnilingus by men but not recent performance of cunnilingus by women or recent performance of fellatio by women or men was associated with an increased risk of oral HPV infection, and that risk increased as the number of oral sex partners increased. This is consistent with findings from a recent population-based study, which showed that the higher oral HPV infection prevalence among men, relative that among women, was not explained by differences in the number of oral sex partners [ 5] but instead was explained by stronger associations between oral sexual behavior and infection among men.
Notably, the inverse association between lifetime number of vaginal sex partners and risk of oral HPV infection among women is consistent with the leading hypothesis that women are more likely to mount a vigorous immune response to genital HPV infection, conferring subsequent protection from oral HPV infection [ 5]. Importantly, our data also indicate that men are at greater risk of oral HPV infection persistence than women. Given the observed rapid clearance of incident infections, it is possible that some of the detected HPV DNA represents viral deposition without establishment of epithelial infection, thus attenuating sex differences. The differences in risk factors and natural history of oral HPV infection among men and women observed here likely contribute to the higher risk of HPV-related head and neck cancer among men.
The rapid oral HPV clearance observed among most of the young adults in this study is comparable to observations in some recent studies, which reported a median time to clearance of around 6 months among adults [ 9], but more rapid than findings in other studies, which reported a median time to clearance of 18–48 months [ 14, 15]. As expected, the incidence rate observed among high-risk young adults recruited from a sexually transmitted diseases clinic in this study (16.5 infections per 1000 person-months) was higher than that in 2 studies composed of university students with similar ages (5.6 [ 8] and 7.5 [ 6] infections per 1000 person-months). Studies of adults reported lower oral HPV incidence rates than those observed among these college students/young adults [ 9].
This study had several strengths and limitations. Samples were collected every 3 months, allowing delineation of the rapid viral clearance. Behavioral risk factors were collected prospectively at every visit, using a computer-assisted self-interview. Men and women were enrolled in the same clinic within a small age range (18–25 years), representing the same source population. The study may be vulnerable to bias due to losses of follow-up. Additionally, this study population was selected as a higher-risk population and was therefore expected to have a higher incidence of oral HPV infection than the general population; while risk factors identified in this study would be expected to be the same in the general population, the actual incidence of infection in this study does not reflect that in the general population. There were also a limited number of infections persisting after 6 months, limiting the power to evaluate differences in persistence after 6 months.
This study describes the natural history of oral HPV infection over 18 months among high-risk young adults, a group known to be at increased risk for sexually transmitted infections, including HPV infection. We are the first to describe a difference in the natural history of oral HPV infection between men and women. Indeed, our results suggest that an individual's sex influences their risk of oral HPV infection when performing oral sex and that performing cunnilingus confers a higher risk of oral HPV acquisition than fellatio. These natural history differences suggest that the increased prevalence of oral HPV16 infection and the higher rates of HPV-related oropharyngeal cancer observed in men as compared to women in the United States are explained both by higher oral HPV acquisition rates, as well as by slower clearance of HPV once infected.
Supplementary Material
Notes
Disclaimer. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp and Dohme.
Financial support. This work was supported by the Investigator-Initiated Studies Program of Merck Sharp and Dohme (grant 36205 to G. D.) and by the Oral Cancer Foundation.
Potential conflicts of interest. G. D. received research support from Merck Sharp and Dohme for this study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chaturvedi A, Engels E, Pfeiffer Ret al. Human papillomavirus (HPV) and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemal A, Simard EP, Dorell Cet al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013; 105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gillison ML, Broutian T, Pickard RKet al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012; 307:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One 2014; 9:e86023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaturvedi AK, Graubard BI, Broutian Tet al. NHANES 2009–2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res 2015; 75:2468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edelstein ZR, Schwartz SM, Hawes Set al. Rates and determinants of oral human papillomavirus infection in young men. Sex Transm Dis 2012; 39:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kero K, Rautava J, Syrjanen K, Willberg J, Grenman S, Syrjanen S. Smoking increases oral HPV persistence among men: 7-year follow-up study. Eur J Clin Microbiol Infect Dis 2014; 33:123–33. [DOI] [PubMed] [Google Scholar]
- 8. Pickard RK, Xiao W, Broutian TR, He X, Gillison ML. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sex Transm Dis 2012; 39:559–66. [DOI] [PubMed] [Google Scholar]
- 9. Kreimer AR, Pierce Campbell CM, Lin HYet al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet 2013; 382:877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beachler DC, Sugar EA, Margolick JBet al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol 2015; 181:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darwich L, Canadas MP, Videla Set al. Oral human papillomavirus type-specific infection in HIV-infected men: a prospective cohort study among men who have sex with men and heterosexual men. Clin Microbiol Infect 2014; 20:O585–9. [DOI] [PubMed] [Google Scholar]
- 12. D'Souza G, Kluz N, Wentz Aet al. Oral human papillomavirus (HPV) infection among unvaccinated high-risk young adults. Cancers 2014; 6:1691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Broutian TR, He X, Gillison ML. Automated high throughput DNA isolation for detection of human papillomavirus in oral rinse samples. J Clin Virol 2011; 50:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Louvanto K, Rautava J, Willberg Jet al. Genotype-specific incidence and clearance of human papillomavirus in oral mucosa of women: a six-year follow-up study. PLoS One 2013; 8:e53413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Videla S, Darwich L, Canadas MPet al. Natural history of human papillomavirus infections involving anal, penile, and oral sites among HIV-positive men. Sex Transm Dis 2013; 40:3–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.