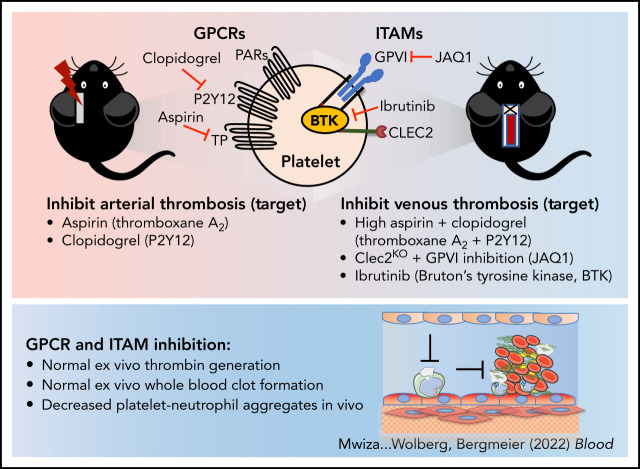
Platelets are known to contribute to arterial thrombosis and have been hypothesized to also play a role in venous thrombosis (VT). Mwiza and colleagues studied the role of platelets in VT and demonstrated that platelet activation is required for VT in mice. Thrombosis is decreased by combining aspirin and clopidogrel together, suggesting that signaling through G protein–coupled receptors and immunoreceptor tyrosine-based activation motif receptors is required for clot formation.
Key Points
Platelet activation via GPCRs and ITAM receptors is critical for platelet–neutrophil aggregate formation during VT initiation in mice.
Inhibitors of these pathways, especially of ITAM receptor signaling, may provide a unique strategy to more safely prevent VT.
Visual Abstract
Abstract
Platelets are critical in hemostasis and a major contributor to arterial thrombosis (AT). (Pre)clinical studies suggest platelets also contribute to venous thrombosis (VT), but the mechanisms are largely unknown. We hypothesized that in VT, platelets use signaling machinery distinct from AT. Here we aimed to characterize the contributions of platelet G protein–coupled (GPCR) and immunoreceptor tyrosine-based activation motif (ITAM) receptor signaling to VT. Wild-type (WT) and transgenic mice were treated with inhibitors to selectively inhibit platelet-signaling pathways: ITAM-CLEC2 (Clec2mKO), glycoprotein VI (JAQ1 antibody), and Bruton’s tyrosine kinase (ibrutinib); GPCR-cyclooxygenase 1 (aspirin); and P2Y12 (clopidogrel). VT was induced by inferior vena cava stenosis. Thrombin generation in platelet-rich plasma and whole-blood clot formation were studied ex vivo. Intravital microscopy was used to study platelet–leukocyte interactions after flow restriction. Thrombus weights were reduced in WT mice treated with high-dose aspirin + clopidogrel (dual antiplatelet therapy [DAPT]) but not in mice treated with either inhibitor alone or low-dose DAPT. Similarly, thrombus weights were reduced in mice with impaired ITAM signaling (Clec2mKO + JAQ1; WT + ibrutinib) but not in Clec2mKO or WT + JAQ1 mice. Both aspirin and clopidogrel, but not ibrutinib, protected mice from FeCl3-induced AT. Thrombin generation and clot formation were normal in blood from high-dose DAPT- or ibrutinib-treated mice; however, platelet adhesion and platelet–neutrophil aggregate formation at the vein wall were reduced in mice treated with high-dose DAPT or ibrutinib. In summary, VT initiation requires platelet activation via GPCRs and ITAM receptors. Strong inhibition of either signaling pathway reduces VT in mice.
Introduction
Thrombotic occlusion of arteries (arterial thrombosis [AT], including myocardial infarction and stroke) or veins (venous thrombosis [VT], with or without pulmonary embolism) are leading causes of death in the United States and Europe. Strategies to reduce the incidence of AT and VT and therefore mitigate the health burden are limited, in part, by the increased bleeding risk that often accompanies antithrombotic strategies.1
Studies in both humans and animal models indicate that AT and VT have different etiology. AT occurs in high shear stress conditions and is usually triggered by plaque rupture, leading to the formation of a platelet-rich thrombus (so-called “white thrombus”).1 In contrast, VT is thought to be initiated by reduced blood flow and endothelial activation, leading to the formation of a thrombus enriched in fibrin and erythrocytes (so-called “red thrombus”).1,2 Accordingly, therapeutic management to prevent AT and VT differ. Whereas platelet antagonists are used to prevent AT, these have been largely ineffective at preventing primary VT.1,3 Instead, anticoagulants that inhibit thrombin generation and/or activity are used to prevent VT.2 Interestingly, however, observations from clinical, preclinical, and in vitro studies implicate platelets in VT pathogenesis.4-13 In particular, thrombocytosis is a risk factor for VT in patients with cancer,4-7 and thrombocytopenia is protective against experimental VT in mice.8 Notably, large clinical trials suggest that aspirin, the platelet inhibitor most widely used to prevent AT, may reduce VT in certain patients.9-11 However, a head-to-head comparison showed superior efficacy for the anticoagulant rivaroxaban over aspirin in preventing VT recurrence,14 and studies have failed to consistently show a role for aspirin in preventing primary VT.15 Collectively, these data suggest that platelets contribute to VT but also raise questions about whether using aspirin to inhibit the cyclooxygenase 1 pathway is the optimal therapeutic strategy to target platelets and reduce VT.
Platelet activation and function are mediated through surface receptors that are organized into 2 major classes. G protein–coupled receptors (GPCRs) respond to soluble agonists released by activated platelets or generated at sites of vascular injury, whereas immunoreceptor tyrosine-based activation motif (ITAM) receptors are activated by insoluble agonists found in the vascular wall.16,17 The GPCRs include the thrombin receptors protease-activated receptor (PAR) 1, 3, and 4; the thromboxane A2 receptor; and the adenosine 5′-diphosphate (ADP) receptors P2Y1 and P2Y12.18 ITAM receptors expressed by platelets include the glycoprotein VI (GPVI) receptor for collagen, laminin, and polymerized fibrin19; C‐type lectin‐like receptor 2 (CLEC2), which is a receptor for podoplanin20 and heme21; and FcγRIIA, which mediates the response to immune complexes.22 Studies in rodent models suggest that targeting platelet surface receptors, including platelet receptor glycoprotein Ibα (GPIbα)8 and its main ligand, von Willebrand factor,12 CLEC2,23 and the ADP receptors P2Y12 and P2Y124-27 may reduce VT. However, VT models used in these prior studies differed in their method of initiation (eg, FeCl3 vs flow restriction), with important downstream consequences for both the rate of thrombus growth and thrombus composition and, therefore, the operant pathophysiology being targeted. For example, FeCl3 application triggers radical-induced endothelial cell damage and rapid formation of platelet-rich thrombi, whereas inferior vena cava (IVC) flow restriction produces slower forming thrombi that resemble fibrin- and erythrocyte-rich red thrombi.28 Thus, a systematic evaluation of key platelet receptors/signaling pathways in an experimental model that produces thrombi enriched in fibrin and erythrocytes is required to define the role of platelets in VT pathogenesis and determine whether these pathways are viable therapeutic targets for VT management.
Here, we used genetic and pharmacologic strategies combined with state-of-the-art in vivo flow restriction VT models to show that both GPCR and ITAM receptors are required during VT initiation and that strong inhibition of either pathway may provide a viable strategy to mitigate VT.
Methods
Mice
All animal procedures were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee. Experiments were performed in 10- to 12-week-old, 25 to 30 g male mice. All mice were on a C57BL/6 background.29,30 The mice were housed in an environment with controlled temperature and a 12-hour/12-hour light–dark cycle.
Platelet depletion
Depletion of endogenous platelets in wild-type (WT) mice was achieved by intravenous injection of anti-GPIbα antibodies (2 mg/kg; Emfret Analytics, Würzburg, Germany) 30 minutes before surgery.31
Platelet function analyses ex vivo
Platelet counts, aggregometry, and the activated integrin αIIbβ3 binding assay were performed as described previously.32
IVC stenosis
Experimental VT was induced in the IVC using a modified partial flow restriction (stenosis) procedure.33 Briefly, mice were anesthetized by using isoflurane (3.5% for induction and maintained at 2% during surgery). Only male mice were used for this model, as ligation in female mice may result in necrosis of the reproductive organs due to drainage of the right uterine vein into the IVC.28 The IVC was exposed via sterile laparotomy. After securing the intestines and covering with sterile gauze soaked in warm saline, the side branches were ligated if they joined the IVC below the left renal branch. All visible lumbar branches located above the lumbar lymph node were closed by cauterization. The IVC was separated from the abdominal aorta just below the left renal branch, and a 30-gauge blunt needle was positioned on the outside of the IVC to serve as a temporary spacer during ligation. The spacer was then removed, the intestines replaced, and the abdominal wall closed with a simple continuous suture. The mice were administered 0.5 mL of warm saline subcutaneously to replace fluids lost during surgery, placed in a warmed cage, and monitored for signs of distress until recovery. The mice were administered buprenorphine (0.1 mg/kg) 30 minutes before surgery and every 12 hours thereafter until euthanasia to achieve analgesia. After 48 hours of flow restriction, mice were euthanized, and thrombi were separated from the IVC, weighed, snap-frozen, and stored at −80°C for further analysis.
To inhibit GPCRs, 2 dosing regimens were used that model dual antiplatelet therapy (DAPT): low-dose DAPT (15 mg/kg of cyclooxygenase-1 inhibitor [aspirin] and 3 mg/kg of P2Y12 inhibitor [clopidogrel]) and high-dose DAPT (20 mg/kg aspirin and 25 mg/kg clopidogrel). We also treated mice with individual inhibitors at a high dose. Mice were treated by oral gavage once daily until euthanasia.
To selectively inhibit ITAM receptors, we used the Clec2mKO (Clec2fl/fl;Pf4Cre+) transgenic mouse line with documented defects in platelet signaling30 and an established GPVI-blocking antibody.34 WT or Clec2mKO mice were intravenously administered a GPVI-blocking antibody (JAQ1, 50 μg) 5 days before surgery to deplete GPVI from the platelet surface. C57BL/6 mice were administered a Bruton’s tyrosine kinase (BTK) inhibitor (ibrutinib [12.5 mg/kg]) via intraperitoneal injection 4 hours before and 24 hours after IVC stenosis.
FeCl3-induced carotid artery occlusion
As previously described,35 mice were anesthetized with isoflurane (3.5% for induction and maintained at 2% during surgery) and placed in a supine position. After the right common carotid artery was isolated, a 1 mm2 piece of filter paper soaked in 8% FeCl3 was applied to the ventral surface of the exposed carotid artery for 3 minutes. A microvascular ultrasonic flow probe (MA0.5PSB, Transonic Systems Inc, Ithaca, NY) was used to detect blood flow for 30 minutes after removal of the filter paper. The occlusion time was defined as a cessation of blood flow (0 mL/min) for ≥2 minutes.
Thrombin generation
Thrombin generation was measured in platelet-rich plasma (PRP). Briefly, blood was collected from the IVC into 10% sodium citrate (vol/vol). PRP was prepared by 2 rounds of centrifugation of whole blood at 130g for 5 minutes. Platelet counts were adjusted to 3 × 108 platelets/mL with platelet-poor plasma. Thrombin generation was measured with calibrated automated thrombography, as described previously,36 using 1 pM tissue factor in PRP and 1 pM tissue factor/4 µM lipids in platelet-poor plasma. Data were calculated using Thrombinoscope software (Thrombinoscope BV, Maastricht, The Netherlands).
Whole-blood clot formation
Whole-blood clot formation was analyzed in undiluted blood as described previously.33
Great saphenous vein flow restriction
To study VT initiation, we induced complete flow restriction (stasis) in the great saphenous vein. Anesthesia was achieved using isoflurane (3.5% for induction and maintained at 2% during surgery and intravital imaging). The saphenous vein was exposed as described previously.37 Briefly, after aseptically preparing the left hind leg, a 5-mm skin incision was made just proximal to the knee on the medial side. The great saphenous vein was separated from the saphenous artery using thumb forceps with atraumatic movements. A narrowing ligature (5-0 silk) was tied around the saphenous vein to stop blood flow. Warm sterile saline was used to keep the area moist. After ensuring there was no bleeding, the incision was closed by using 5-0 silk with a continuous suture. Isoflurane was discontinued, and mouse was given buprenorphine (0.1 mg/kg), placed in a warmed cage with food and water, and observed every 15 minutes for 2 hours for signs of distress.
Two hours after flow restriction, the mouse was re-anesthetized with isoflurane and given a retro-orbital injection of 2.5 μg Alexa 488–labeled antibody against Ly-6G and Alexa 647–labeled antibody against GPIX. The skin was removed (1-2 cm2) around the flow-restricted area to visualize the saphenous vein distal to the suture location. Mice were maintained on 2% isoflurane and 100% oxygen and moved to a warming pad for intravital imaging by spinning disk confocal microscopy using the Zeiss Axio Examiner Z1 microscope (Intelligent Imaging Innovations, Denver, CO) focused through a 20× water immersion objective lens. During imaging, a constant perfusion of warm (37°C) saphenous vein buffer (32 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 2 mM CaCl2, and 18 mM NaHCO3 pH 7.3, bubbled with 5% carbon dioxide/95% N2 for 15 minutes before the start of the experiment) was dripped on the exposed saphenous vein as described previously.37 Videos were acquired for 5 to 6 minutes and were analyzed with ImageTank software version 1.0.38 Neutrophil “roundness” was analyzed by randomly selecting regions of images from the videos and having 5 independent, blinded assessors score the cells as round or not round; results from this analysis are expressed as percent round for each condition.
Statistical analysis
Normality was assessed by using the Shapiro-Wilk test and analyzed by using parametric (paired t-test or one-way analysis of variance) or nonparametric (Kruskal-Wallis or Mann-Whitney) tests as appropriate in GraphPad Prism 9.3.0 (GraphPad Software, Inc, La Jolla, CA). Differences in the incidence of thrombus formation between groups were assessed by Fisher’s exact test. P values ≤.05 were considered significant.
Results
Thrombocytopenia protects mice from VT
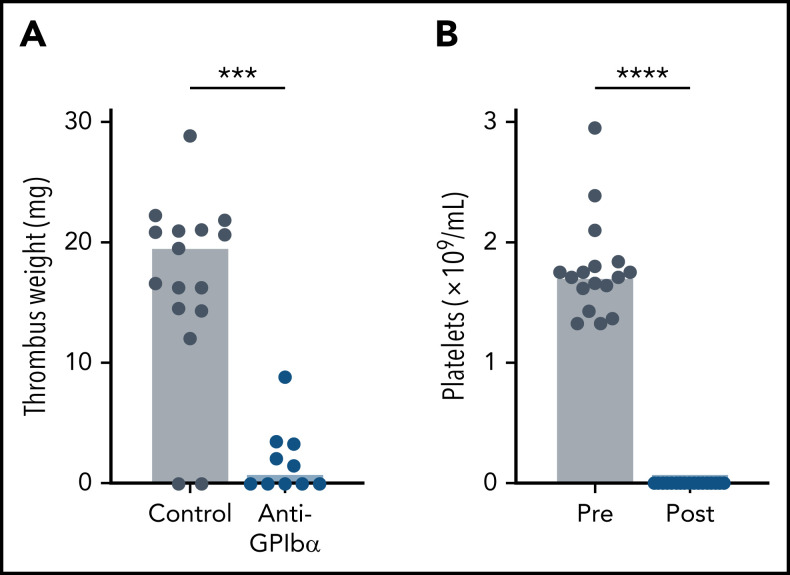
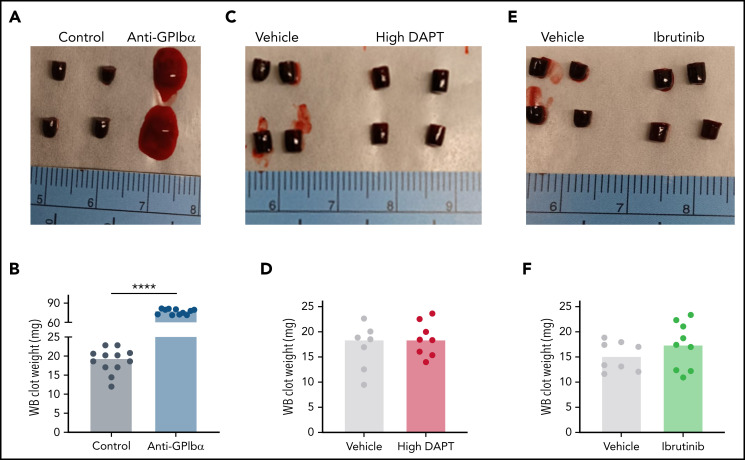
The IVC stenosis model with its ∼90% reduction in blood flow is the gold standard mouse model to mimic the conditions of disturbed flow leading to VT in humans.28 A limitation of this model is the low VT incidence and variability in thrombus weight observed by some investigators. To obtain more consistent results, we ligated side branches and cauterized lumbar branches.39 Venous thrombi were observed in 88% of WT mice after IVC stenosis, with an average clot weight of 17.5 ± 8.1 mg (Figure 1A). Previous work found that thrombocytopenic mice are strongly protected in the IVC stenosis model.8 To confirm this finding and to assess the relative contribution of platelets to VT induced by our surgical procedure, we induced thrombocytopenia in WT mice by treatment with antibodies to GPIbα, which led to depletion of >99% of circulating platelets (Figure 1B). Consistent with the earlier study,8 we observed strong protection from IVC stenosis-induced VT in thrombocytopenic mice (15 of 17 vs 5 of 10; P < .0001; mean ± standard deviation, 17.5 ± 8.1 mg vs 1.9 ± 2.8 mg, respectively, P < .0001). Together, these studies showed the platelet dependence of our IVC stenosis model, enabling mechanistic studies on the contribution of select platelet signaling pathways to experimental VT.
Figure 1.
Thrombocytopenia protects mice from VT. WT mice were treated with anti-GPIbα (platelet-depleting) antibody. (A) Thrombus weight after 48 hours of IVC flow restriction. (B) Flow cytometric analysis of the peripheral platelet count before (Pre) and after (Post) anti-GPIbα treatment. Only mice with peripheral platelet counts ≤1% of control mice were subjected to the stenosis model. Dots represent individual mice, bars indicate medians. ***P < .001; ****P < .0001.
Inhibition of GPCR signaling is less protective in VT than in AT
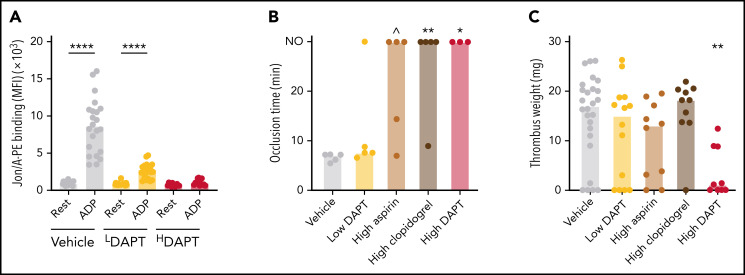
Aspirin and P2Y12 inhibitors target cyclooxygenase 1 (thromboxane A2 formation) and the P2Y12 receptor, respectively. These drugs are highly effective at preventing AT in humans and mice.1,40 We first treated WT mice with different doses of aspirin and/or clopidogrel and monitored drug efficacy by ex vivo determination of the integrin activation response. Treatment of mice with concentrations of aspirin and clopidogrel corresponding to those given to humans (15 mg/kg aspirin plus 3 mg/kg clopidogrel, “low DAPT”), led to significant but incomplete inhibition of integrin activation in response to ADP stimulation (Figure 2A). Consistently, mice treated with low DAPT were not protected from FeCl3-induced carotid artery thrombosis (Figure 2B). In contrast, treatment of mice with high-dose aspirin (20 mg/kg) plus clopidogrel (25 mg/kg) (high DAPT) or either inhibitor alone led to complete inhibition of arachidonic acid–induced and/or ADP-induced integrin activation (Figure 2A; supplemental Figure 1A-B, available on the Blood Web site) and, in the case of clopidogrel alone or high DAPT, significant impairment of AT. Prolonged occlusion times were also observed in 3 of 5 mice treated with aspirin, but this difference was not statistically significant. When subjected to stenosis-induced VT, thrombosis incidence and thrombus weights in mice treated with low-dose DAPT, high-dose aspirin, or high-dose clopidogrel were not different from those in control mice (Figure 2C). However, both incidence (22 of 26 vs 5 of 9; P < .0001) and thrombus weights were lower in mice treated with high-dose DAPT. These findings indicate that GPCR signaling contributes to VT pathogenesis, but compared with AT stronger inhibition of these receptors is required to reduce VT.
Figure 2.
Inhibitors of platelet ADP and thromboxane A2 signaling provide limited protection against VT. WT mice were treated with aspirin and/or clopidogrel to inhibit Cox1 and P2Y12, respectively, and subjected to FeCl3-induced carotid artery thrombosis or to IVC stenosis for 48 hours. (A) Flow cytometric analysis of integrin activation response (JON/A-PE binding) to ADP in blood from mice treated with low (LDAPT) or high (HDAPT) doses of DAPT. (B) Vessel occlusion times after FeCl3 treatment for 3 minutes; the experiment was terminated at 30 minutes. NO indicates no occlusion. (C) Thrombus weights after 48 hours of IVC flow restriction. Dots represent individual mice, bars indicate medians. ∧P < .07; *P < .05; **P < .01; ****P < .0001. MFI, mean fluorescence intensity.
Platelet ITAM receptor signaling is important for VT pathogenesis in mice
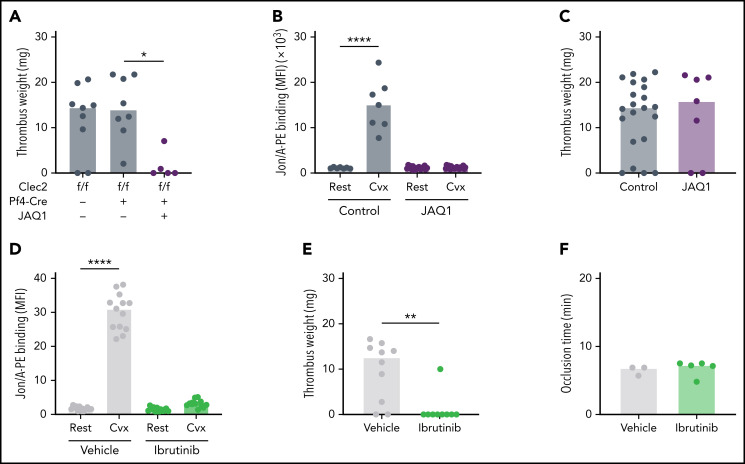
We previously showed that ITAM receptor signaling is essential for platelet function and vascular integrity at sites of inflammation.31 Because VT is considered a thromboinflammatory condition,2,8 we hypothesized that platelet ITAM signaling is also required for VT. Mouse platelets express two ITAM receptors, CLEC2 and GPVI, and a previous study suggested that CLEC2 contributes to experimental VT in mice.23 Surprisingly, we did not observe protection from VT in mice carrying the CLEC2 deficiency in megakaryocytes and platelets (Clec2mKO) (Figure 3A), even though Clec2mKO platelets were unresponsive to stimulation with the CLEC2 agonist rhodocytin with platelets from Cre-negative controls (supplemental Figure 1C). To determine whether GPVI contributes to VT, we treated WT mice with an anti-GPVI antibody (JAQ1, 50 µg per mouse), which depletes GPVI from circulating platelets.34 Ex vivo studies confirmed loss of GPVI function 6 hours after antibody injection, an effect that persisted for >14 days (Figure 3B; supplemental Table 1). We induced IVC stenosis in mice 5 days after JAQ1 administration, a time point when the platelet count and activation response to non-GPVI agonists were indistinguishable from untreated controls. Interestingly, incidence and thrombus weights were not different between JAQ1-treated and untreated WT mice (Figure 3C). However, treatment of Clec2mKO mice with JAQ1 to simultaneously block functional responses of both ITAM receptors reduced the incidence (8 of 8 vs 2 of 5; P < .0001) and thrombus weights after IVC stenosis.
Figure 3.
Platelet ITAM receptor signaling is critical for VT but not AT. (A) WT or Clec2mKO mice were treated with JAQ1 antibody to deplete GPVI from circulating platelets. Thrombus weight was determined after 48 hours of IVC flow restriction. (B) Integrin activation response (JON/A-PE binding) after convulxin (Cvx) stimulation of platelets isolated from untreated or JAQ1-treated WT mice. (C) Thrombus weights after 48 hours of IVC stenosis in untreated (control) or JAQ1-treated WT mice. (D) JON/A-PE binding following Cvx stimulation of platelets isolated from dimethyl sulfoxide (vehicle)-treated or ibrutinib-treated WT mice. (E) Thrombus weights after 48 hours of IVC stenosis in vehicle- or ibrutinib-treated WT mice. (F) Vessel occlusion times after FeCl3 treatment for 3 minutes in vehicle- or ibrutinib-treated mice. Dots represent individual mice, bars indicate medians. *P < .05; **P < .01; ****P < .0001.
BTK is an important component of the ITAM signaling pathway in platelets. To inhibit ITAM signaling downstream of GPVI and Clec2, we treated mice with the BTK inhibitor ibrutinib (12.5 mg/kg), which is approved for the treatment of B-cell malignancies.41,42 Ex vivo platelet function testing confirmed loss of ITAM signaling in ibrutinib-treated mice (Figure 3D). Consistent with our findings in JAQ1-treated Clec2mKO mice, ibrutinib-treated mice were strongly protected against VT (Figure 3E). Interestingly, ibrutinib-treated mice were not protected from FeCl3-induced AT (Figure 3F). Collectively, these findings suggest: (1) ITAM receptor signaling strongly contributes to the pathophysiology of VT; and (2) redundancy between the platelet ITAM receptors GPVI and CLEC2 during VT formation.
Inhibition of GPCR or ITAM signaling in mouse platelets does not impair thrombin generation or whole-blood clot formation ex vivo
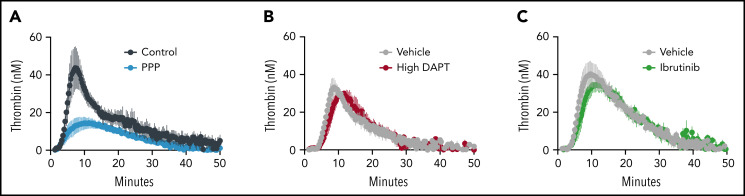
Conventional therapies to reduce primary and recurrent VT act by inhibiting the generation or activity of thrombin. Platelet ITAM and GPCR signaling are important for the conversion of proadhesive to procoagulant platelets.43 We thus used calibrated automated thrombography to determine whether inhibitors of GPCR or ITAM signaling reduce platelet-mediated thrombin generation in a tissue factor–initiated setting. Compared with WT controls, thrombin generation in platelet-poor plasma was reduced (Figure 4A; Table 1). In contrast, thrombin generation was not different in PRP from mice treated with high DAPT (Figure 4B; Table 1) or ibrutinib (Figure 4C; Table 1).
Figure 4.
Inhibition of GPCR or ITAM signaling in mouse platelets does not impair thrombin generation. Thrombin generation was measured by calibrated automated thrombography in PRP (Control) and platelet-poor plasma (PPP) (A), and in plasma from mice treated with high DAPT (B) or ibrutinib (C). Curves show mean ± standard error of the mean for all mice for each condition; the number of mice studied is indicated in Table 1.
Table 1.
Ex vivo thrombin generation parameters for mice treated with platelet inhibitors
| Parameter | Lag time (min) | Time to peak (min) | Velocity index (nM/min) | Peak (nM) | ETP (nM/min) |
|---|---|---|---|---|---|
| Control (untreated littermates, n = 7) | 4.4 ± 1.2 | 9.5 ± 3.2 | 12.8 ± 9.4 | 48.6 ± 26.6 | 541.3 ± 187.5 |
| Platelet-poor plasma (n = 5) | 4.3 ± 1.6 | 13.3 ± 2.8 | 1.5 ± 1.1* | 12.1 ± 5.3* | 264.6 ± 95.0* |
| Vehicle (water) (n = 3) | 4.8 ± 0.6 | 9.1 ± 1.2 | 14.0 ± 10.5 | 52.5 ± 27.9 | 569.1 ± 123.8 |
| High DAPT (n = 3) | 5.3 ± 0.6 | 10.4 ± 2.0 | 8.0 ± 2.8 | 38.3 ± 6.8 | 390.9 ± 84.5 |
| Vehicle (dimethyl sulfoxide) (n = 6) | 5.2 ± 1.1 | 10.4 ± 3.0 | 10.9 ± 5.8 | 46.6 ± 15.2 | 708.4 ± 158.1 |
| Ibrutinib (n = 7) | 6.1 ± 1.7 | 12.3 ± 3.7 | 8.1 ± 5.2 | 39.5 ± 11.3 | 667.4 ± 189.9 |
Values indicate mean ± standard deviation; comparisons are by unpaired t test within each group. ETP, endogenous thrombin potential.
P < .05 compared with appropriately matched control, indicated by groups in the table.
We also characterized the effects of GPCR and ITAM signaling on clot formation and consolidation in whole blood. Complete blood counts confirmed that thrombocytopenic mice had normal red blood cell counts, and that mice treated with high DAPT or ibrutinib had normal red blood cell and platelet counts compared with untreated or vehicle-treated mice (supplemental Table 2). Compared with findings in control mice, clots generated ex vivo from thrombocytopenic mice were poorly formed and heavier (Figure 5A-B), likely due to the reduced procoagulant activity (Figure 4A; Table 1) and loss of platelet-mediated clot contraction. In contrast, whole-blood clots from mice treated with high DAPT (Figure 5C-D) or ibrutinib (Figure 5E-F) did not differ from those in control mice. These findings suggest that inhibition of platelet GPCR or ITAM signaling does not interfere with clot propagation or consolidation and, therefore, that the antithrombotic mechanisms invoked by GPCR and ITAM receptor inhibition are fundamentally different from that of established anticoagulants.
Figure 5.
Inhibition of GPCR or ITAM signaling in mouse platelets does not impair whole-blood (WB) clot formation in vitro. Recalcified WB was clotted with tissue factor. After 2 hours, clots were visualized and weighed. Representative images and clot weights of WB clots from mice treated with anti-GPIbα antibodies (A-B), high DAPT (C-D), or ibrutinib (E-F). Dots represent individual mice, bars indicate medians. ****P < .0001.
Both GPCR and ITAM signaling contribute to platelet–neutrophil aggregate formation during VT initiation
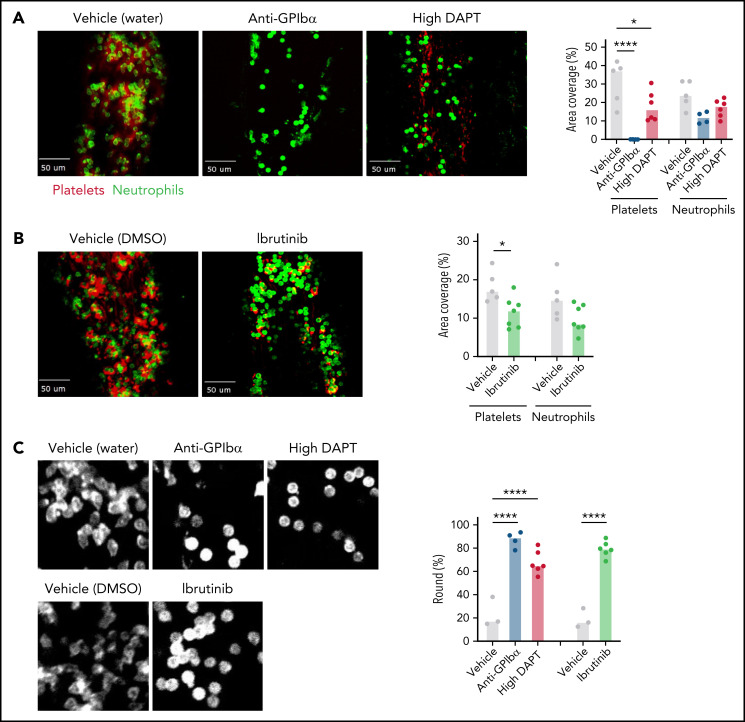
Our findings from ex vivo whole-blood clot formation studies suggested that the contribution of platelet GPCR and ITAM signaling arises before the thrombus propagation and consolidation phases of VT. It has been proposed that VT is initiated by platelet-mediated recruitment and activation of leukocytes on intact but dysregulated endothelium.2,8 To visualize the interplay between platelets and neutrophils during the VT initiation phase, intravital microscopy was used to image the saphenous vein surface 2 hours after flow restriction by ligation. Specificity of platelet detection was confirmed in thrombocytopenic mice, in which platelet accumulation was not observed (Figure 6A). Interestingly, in these thrombocytopenic mice, neutrophil adhesion appeared more transient than that seen in control mice. Furthermore, compared with control mice, a significantly higher number of adherent neutrophils in thrombocytopenic mice remained in a round, less activated state (Figure 6A,C; supplemental Videos 1 and 2). We also observed significantly reduced platelet adhesion in WT mice treated with high DAPT or ibrutinib (Figure 6A-B). In addition, similar to thrombocytopenic mice, neutrophil adhesion in mice treated with high DAPT or ibrutinib was more transient than in control mice, and cells maintained a round, less activated state (Figure 6; supplemental Videos 1, 3, 4, and 5). Collectively, these data suggest that platelets contribute to VT via GPCR- and ITAM receptor–mediated formation of platelet–neutrophil aggregate (PNA) and neutrophil activation at the endothelial surface during VT initiation.
Figure 6.
Both platelet GPCR and ITAM receptor signaling contribute to PNA formation on the endothelium during VT initiation. WT mice were treated with anti-GPIbα antibodies to deplete circulating platelets, or with high DAPT (A), or ibrutinib (B) and then subjected to saphenous vein ligation. Spinning disk confocal intravital microscopy was used to visualize platelet (anti-GPIX antibody, red) and neutrophil (anti-Ly6G antibody, green) adhesion to the saphenous vein wall. Representative still frames from the videos taken after 2 hours of flow restriction and quantification of the area covered by platelets or neutrophils are shown. Dots represent individual mice, bars indicate medians. (C) Left panel: higher magnification images illustrating altered morphology of adherent neutrophils in mice treated with anti-GPIbα antibodies, high DAPT, or ibrutinib. Right panel: Quantification of percent round cells in selected fields of view. Dots represent individual mice, bars indicate medians. *P < .05, ****P < .0001.
Discussion
Platelets are best known for their role in hemostatic plug formation and pathologic contribution to AT, but data from both human clinical trials and animal models suggest that platelets also contribute to VT pathogenesis. To date, however, the molecular pathways by which platelets participate in VT initiation and propagation have not been clarified. Knowledge of these pathways may reveal novel targets to effectively and safely reduce VT formation. To fill this gap, we performed a systematic analysis of platelet GPCR and ITAM signaling pathways in the clinically relevant experimental model of stenosis-induced VT. We also developed a new mouse model to visualize and quantify platelet–neutrophil interactions during the initiation phase of VT. We used these models as well as established in vitro methods to quantify platelet procoagulant activity and clot formation and to define mechanisms by which inhibiting these pathways alters the course of VT. Our studies show the contributions of both receptor families to VT and suggest that platelet GPCR or ITAM inhibition predominantly reduces the initiation phase of VT. These data suggest that GPCR or ITAM inhibition has entirely different mechanisms of antithrombotic activity than current anticoagulants, and they identify novel pharmacologic strategies that may reduce VT.
VT is a thromboinflammatory disease; that is, disease progression is triggered by a proinflammatory/prothrombotic shift in endothelial cells and subsequent recruitment of platelets and leukocytes to the endothelium. The interaction between platelets and leukocytes, particularly neutrophils, seems central in these events. Multiple receptor pairs, including P-selectin-PSGL1, GPIbα-(kininogen)-Mac-1, αIIbβ3-SLC44A2, and αIIbβ3-fibrinogen-Mac-1, mediate PNA formation and are required to fully activate neutrophils and induce release of neutrophil extracellular traps (NETs).44-49 von Brühl et al showed that platelet depletion compromises both neutrophil adhesion and NET formation, causing a marked reduction in VT formation.8 We confirmed these findings, as we observed significantly reduced neutrophil adherence to the endothelium and altered morphology of adherent neutrophils (ie, more round) in platelet-depleted mice. Importantly, pharmacologic inhibition of GPCR (high DAPT) or ITAM signaling (ibrutinib) impaired stenosis-induced thrombus formation, and this action occurred in parallel with significantly reduced platelet adhesion to, and impaired neutrophil activation at, the inflamed vascular wall. Our attempts to quantify NET formation in areas of flow restriction in vivo were unsuccessful, but studies by others have shown that platelets are a powerful modulator of NET formation and that platelets activated by bacteria, viruses, or classical platelet agonists can induce NET formation.44
Within the ITAM pathway, both GPVI and CLEC2 are important for thrombus formation in the IVC stenosis model, as in vivo thrombus weight was significantly reduced in JAQ1-treated Clec2mKO mice, but not when these surface receptors were targeted individually (Clec2mKO- or JAQ1-treated WT mice). Our findings partially contradict a study reporting significantly smaller thrombi in Clec2mKO mice subjected to the IVC stenosis model.23 Differences in the surgical procedure may, at least in part, account for these discrepant results. ITAM receptors are best known for their role in platelet functions outside of classical hemostasis.20 One such example is inflammatory hemostasis, in which single platelets plug small openings in the endothelial monolayer that are introduced by transmigrating inflammatory cells.50 Using pharmacologic inhibitors and transgenic mice, we and others have shown that platelet ITAM, but not GPCR, signaling is required to prevent hemorrhage during inflammation.31,50,51 Based on our findings presented here, we propose that inflammation at sites of flow restriction also causes endothelial weakening and ITAM-dependent platelet activation. However, flow restriction also leads to platelet–leukocyte aggregate formation, inflammatory cell activation, and ultimately thrombin generation. Thrombin promotes fibrin formation, a key component of venous thrombi. Thrombin also activates platelets via PAR receptors, and we recently reported that PAR4 receptor knockout mice are protected from thrombus formation in the IVC stenosis model.35 Here we extend these observations to show that: (1) other GPCRs, such as P2Y12 and thromboxane A2 receptors, also contribute to VT formation in mice; and (2) high-dose DAPT or ibrutinib treatment impairs PNA formation and neutrophil activation (ie, they inhibit VT even before thrombin generation).
A key finding of the current study is that inhibition of platelet ITAM signaling may be ineffective in preventing AT but a powerful approach for preventing VT. Targeting this pathway could be performed on the receptor level, an approach that is appealing because expression of GPVI and CLEC2 are restricted to few cell types (megakaryocytes and platelets for GPVI; megakaryocytes, platelets, and neutrophils for CLEC2). Given the functional redundancy of these receptors suggested by the IVC stenosis model, both receptors would likely need to be inhibited to prevent VT. However, only GPVI inhibitors are currently being evaluated for clinical use.1 Alternatively, downstream molecules within the ITAM signaling pathway may be useful targets. BTK is a central component of immune cell signaling, and ibrutinib is approved for the treatment of B-cell malignancies. Consistent with the role of BTK in platelet ITAM signaling, patients occasionally experience mild bleeding complications. However, bleeding is a less frequent complication of the newer BTK inhibitors such as zanubrutinib.52 Thus, targeting ITAM signaling molecules such as BTK may provide an opportunity to treat VT with a fundamentally different approach than conventional anticoagulants. Importantly, this approach may result in a lower bleeding risk, a major adverse event that limits anticoagulant use in certain situations. Whether BTK inhibitors could be safely used in combination with anticoagulants must still be determined, and their negative effects on the immune system would need to be considered.
In summary, our studies suggest that both GPCRs and ITAM receptors in platelets are involved in VT pathogenesis. Although inhibition of single receptors did not reduce VT in mice, we observed significant protection in mice treated with high-dose DAPT and in mice in which both ITAM receptors or their downstream signaling were inhibited. Finally, our findings provide new evidence that platelets play a vital role in the initiation of VT, likely by recruiting and activating leukocytes at sites of endothelial inflammation. Continued efforts to define these mechanisms and develop platelet-targeted therapeutics may yield new strategies for the management of VT.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Abigail Ballard and Katie Poe for their help with using ImageTank software and mouse husbandry, respectively. The authors also thank Mark Kahn for providing the Clec2(flox) mice.
This study was supported by the American Heart Association (20PRE35080130, J.M.N.M.), the American Society of Hematology and National Blood Foundation (ASH Scholar Award and NBF Early Career Grant, R.H.L.), National Institutes of Health, National Heart, Lung, and Blood Institute (F31HL154562, J.M.N.M.; R25GM055336, University of North Carolina at Chapel Hill/J.M.N.M. [University of North Carolina at Chapel Hill Initiative for Maximizing Student Development]; Integrative Biology Training Program [T32HL69768, University of North Carolina at Chapel Hill /J.M.N.M], R35HL144976 to W.B., R01HL126974 to A.S.W., and R35HL155657 to N.M.).
Footnotes
Requests for original data may be submitted to the corresponding authors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.M.N.M. designed and performed experiments, analyzed and interpreted the data, and wrote the manuscript; A.S.W. and W.B. designed the research, analyzed and interpreted the data, and wrote the manuscript; R.H.L., D.S.P., L.A.H., T.K., W.J.S., N.M., and B.C.C. performed experiments and/or analyzed data; B.N. contributed vital reagents; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 8018A Mary Ellen Jones Building, Chapel Hill, NC 27599; e-mail: alisa_wolberg@med.unc.edu; and Wolfgang Bergmeier, Department of Biochemistry and Biophysics, University of North Carolina at Chapel Hill, 8212A Mary Ellen Jones Building, Chapel Hill, NC 27599; e-mail: bergmeie@email.unc.edu.
REFERENCES
- 1.Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discov. 2020;19(5):333-352. [DOI] [PubMed] [Google Scholar]
- 2.Wolberg AS, Rosendaal FR, Weitz JI, et al. Venous thrombosis. Nat Rev Dis Primers. 2015;1(1):15006. [DOI] [PubMed] [Google Scholar]
- 3.Steinhubl SR, Eikelboom JW, Hylek EM, Dauerman HL, Smyth SS, Becker RC. Antiplatelet therapy in prevention of cardio- and venous thromboembolic events. J Thromb Thrombolysis. 2014;37(3):362-371. [DOI] [PubMed] [Google Scholar]
- 4.Simanek R, Vormittag R, Ay C, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost. 2010;8(1):114-120. [DOI] [PubMed] [Google Scholar]
- 5.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822-2829. [DOI] [PubMed] [Google Scholar]
- 6.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score. J Thromb Haemost. 2004;2(12):2156-2161. [DOI] [PubMed] [Google Scholar]
- 7.Jensvoll H, Blix K, Braekkan SK, Hansen JB. Platelet count measured prior to cancer development is a risk factor for future symptomatic venous thromboembolism: the Tromsø Study. PLoS One. 2014;9(3):e92011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Brühl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brighton TA, Eikelboom JW, Mann K, et al. ; ASPIRE Investigators . Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367 (21):1979-1987. [DOI] [PubMed] [Google Scholar]
- 10.Simes J, Becattini C, Agnelli G, et al. ; INSPIRE Study Investigators (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism) . Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130(13):1062-1071. [DOI] [PubMed] [Google Scholar]
- 11.Cavallari I, Morrow DA, Creager MA, et al. Frequency, predictors, and impact of combined antiplatelet therapy on venous thromboembolism in patients with symptomatic atherosclerosis. Circulation. 2018;137(7):684-692. [DOI] [PubMed] [Google Scholar]
- 12.Brill A, Fuchs TA, Chauhan AK, et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117(4): 1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann M, Schoeman RM, Krohl PJ, et al. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI-dependent manner in an in vitro venous thrombosis model. Arterioscler Thromb Vasc Biol. 2018;38(5):1052-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weitz JI, Lensing AWA, Prins MH, et al. ; EINSTEIN CHOICE Investigators . Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211-1222. [DOI] [PubMed] [Google Scholar]
- 15.Gelbenegger G, Postula M, Pecen L, et al. Aspirin for primary prevention of cardiovascular disease: a meta-analysis with a particular focus on subgroups. BMC Med. 2019;17(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25(4):155-167. [DOI] [PubMed] [Google Scholar]
- 17.Bergmeier W, Stefanini L. Platelets at the vascular interface. Res Pract Thromb Haemost. 2018;2(1):27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99(12):1293-1304. [DOI] [PubMed] [Google Scholar]
- 19.Perrella G, Nagy M, Watson SP, Heemskerk JWM. Platelet GPVI (Glycoprotein VI) and thrombotic complications in the venous system. Arterioscler Thromb Vasc Biol. 2021;41(11):2681-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129(1):12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oishi S, Tsukiji N, Otake S, et al. Heme activates platelets and exacerbates rhabdomyolysis-induced acute kidney injury via CLEC-2 and GPVI/FcRγ. Blood Adv. 2021;5(7):2017-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arman M, Krauel K. Human platelet IgG Fc receptor FcγRIIA in immunity and thrombosis. J Thromb Haemost. 2015;13(6):893-908. [DOI] [PubMed] [Google Scholar]
- 23.Payne H, Ponomaryov T, Watson SP, Brill A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood. 2017;129(14):2013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YX, Vincelette J, da Cunha V, et al. A novel P2Y(12) adenosine diphosphate receptor antagonist that inhibits platelet aggregation and thrombus formation in rat and dog models. Thromb Haemost. 2007;97(5):847-855. [PubMed] [Google Scholar]
- 25.Lenain N, Freund M, Léon C, Cazenave JP, Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J Thromb Haemost. 2003;1(6):1144-1149. [DOI] [PubMed] [Google Scholar]
- 26.Bird JE, Wang X, Smith PL, Barbera F, Huang C, Schumacher WA. A platelet target for venous thrombosis? P2Y1 deletion or antagonism protects mice from vena cava thrombosis. J Thromb Thrombolysis. 2012;34(2):199-207. [DOI] [PubMed] [Google Scholar]
- 27.Mezouar S, Darbousset R, Dignat-George F, Panicot-Dubois L, Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer. 2015;136(2):462-475. [DOI] [PubMed] [Google Scholar]
- 28.Diaz JA, Saha P, Cooley B, et al. Choosing a mouse model of venous thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(3):311-318. [DOI] [PubMed] [Google Scholar]
- 29.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109(4):1503-1506. [DOI] [PubMed] [Google Scholar]
- 30.Herzog BH, Fu J, Wilson SJ, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502(7469):105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulaftali Y, Hess PR, Getz TM, et al. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest. 2013;123(2):908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanini L, Paul DS, Robledo RF, et al. RASA3 is a critical inhibitor of RAP1-dependent platelet activation. J Clin Invest. 2015;125(4):1419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aleman MM, Byrnes JR, Wang JG, et al. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124(8):3590-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieswandt B, Schulte V, Bergmeier W, et al. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001;193(4):459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee RH, Kawano T, Grover SP, et al. Genetic deletion of platelet PAR4 results in reduced thrombosis and impaired hemostatic plug stability. J Thromb Haemost. 2022;20(2):422-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miszta A, Kopec AK, Pant A, et al. A high-fat diet delays plasmin generation in a thrombomodulin-dependent manner in mice. Blood. 2020;135(19):1704-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Getz TM, Piatt R, Petrich BG, Monroe D, Mackman N, Bergmeier W. Novel mouse hemostasis model for real-time determination of bleeding time and hemostatic plug composition. J Thromb Haemost. 2015;13(3):417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Shaughnessy EC, Stone OJ, LaFosse PK, et al. Software for lattice light-sheet imaging of FRET biosensors, illustrated with a new Rap1 biosensor. J Cell Biol. 2019;218(9):3153-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz JA, Farris DM, Wrobleski SK, Myers DD, Wakefield TW. Inferior vena cava branch variations in C57BL/6 mice have an impact on thrombus size in an IVC ligation (stasis) model. J Thromb Haemost. 2015;13(4):660-664. [DOI] [PubMed] [Google Scholar]
- 40.Cooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31(6):1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee RH, Piatt R, Conley PB, Bergmeier W. Effects of ibrutinib treatment on murine platelet function during inflammation and in primary hemostasis. Haematologica. 2017;102(3):e89-e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Kinoshita T, Gururaja T, et al. The effect of Bruton’s tyrosine kinase (BTK) inhibitors on collagen-induced platelet aggregation, BTK, and tyrosine kinase expressed in hepatocellular carcinoma (TEC). Eur J Haematol. 2018;101(5):604-612. [DOI] [PubMed] [Google Scholar]
- 43.Reddy EC, Rand ML. Procoagulant phosphatidylserine-exposing platelets in vitro and in vivo. Front Cardiovasc Med. 2020;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carestia A, Kaufman T, Schattner M. Platelets: new bricks in the building of neutrophil extracellular traps. Front Immunol. 2016;7(271):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Constantinescu-Bercu A, Grassi L, Frontini M, Salles-Crawley II, Woollard K, Crawley JT. Activated αIIbβ3 on platelets mediates flow-dependent NETosis via SLC44A2. eLife. 2020;9:e53353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evangelista V, Manarini S, Sideri R, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93(3):876-885. [PubMed] [Google Scholar]
- 47.Pitchford S, Pan D, Welch HC. Platelets in neutrophil recruitment to sites of inflammation. Curr Opin Hematol. 2017;24(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossaint J, Margraf A, Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol. 2018;9:2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Gao H, Shi C, et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα [published correction appears in Nat Commun. 2017;8:16124]. Nat Commun. 2017;8:15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gros A, Syvannarath V, Lamrani L, et al. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood. 2015;126(8):1017-1026. [DOI] [PubMed] [Google Scholar]
- 51.Rayes J, Jadoui S, Lax S, et al. The contribution of platelet glycoprotein receptors to inflammatory bleeding prevention is stimulus and organ dependent. Haematologica. 2018;103(6):e256-e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tam CS, Opat S, D’Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.