Key Points
Monocyte count and monocyte/lymphocyte ratio were associated with greater risk of cardiovascular disease (CVD), CVD death, and all-cause death in the CKD population.
These findings provide evidence to enhance our understanding of the association between monocytes and CVD risk in patients with CKD.
Monocyte count and monocyte/lymphocyte ratio have the potential to be clinically available indicators of cardiovascular risk in CKD.
Keywords: chronic kidney disease, cardiovascular disease, inflammation, lymphocytes, monocyte, mortality
Visual Abstract
Abstract
Background
Emerging evidence suggests an association of higher monocyte count and monocyte/lymphocyte ratio (MLR) with the risk of cardiovascular disease (CVD) in individuals without chronic kidney disease (CKD); however, limited studies have examined if this association translates to the CKD population. This study examined whether monocyte count and MLR are associated with the risk of CVD, CVD death, and all-cause death in patients with nondialysis CKD who participated in the Chronic Renal Insufficiency Cohort observational study.
Methods
Baseline monocyte count and MLR were categorized into tertiles and also modeled continuously. Cox proportional hazards models were used to examine the association between monocyte count (primary predictor) and MLR (secondary predictor) at baseline and time to a composite of CVD events, including heart failure, myocardial infarction, ischemic stroke, and peripheral artery disease (primary outcome). Secondary outcomes were time to CVD death and all-cause death.
Results
The median follow-up time was 9 years for CVD events and 11.7 years for death. In the fully adjusted model, participants with a higher monocyte count and MLR had a greater risk of CVD events (hazard ratio [HR] per doubling of monocyte count=1.2 [95% CI, 1.1 to 1.31]; HR per doubling of MLR=1.26 [95% CI, 1.16 to 1.36]), CVD death (HR=1.18 [95% CI, 0.99 to 1.41]; HR=1.27 [95% CI, 1.1 to 1.48]), and all-cause death (HR=1.17 [95% CI, 1.06 to 1.3]; HR=1.18 [95% CI, 1.09 to 1.29]).
Conclusions
These results suggest that monocyte count and MLR may have the potential to be cost-effective, clinically available indicators of CVD risk in the CKD population.
Introduction
Monocytes are key innate immune cells involved in the pathophysiology of cardiovascular disease (CVD), including atherosclerotic CVD (1) and heart failure (2). As one of the first responders of the innate immune system, monocytes are recruited to the site of vascular injury and trigger cytokine secretion from the vascular endothelium (2,3). Cytokines secreted from both monocytes and vascular endothelium attract additional monocytes to the injured vasculature, resulting in a vicious cycle of monocyte recruitment and subsequent vascular inflammation, thus contributing to the development of CVD (2,3).
Monocyte count is associated with a higher risk of cardiovascular events and mortality in individuals without CKD (4,5). In addition, monocyte/lymphocyte ratio (MLR; the absolute monocyte count divided by the absolute lymphocyte count) has been demonstrated to be a novel inflammatory parameter that is associated with cardiovascular risk in non-CKD populations (6–8). A uremic environment induces a proinflammatory monocyte phenotype, contributing to vascular inflammation and an increased CVD risk in the CKD population (9,10). Although it is well documented that both monocyte count and MLR are associated with an elevated risk of CVD in individuals without CKD, limited studies with small samples sizes have examined whether this association translates to the CKD population (11–14).
Accordingly, the current study aimed to examine if monocyte count or MLR at baseline can be used as a cost-effective, clinically available indicator of cardiovascular risk in the CKD population by evaluating the association of monocyte count and MLR with the risk of a composite of CVD events, CVD death, and all-cause death in patients with nondialysis dependent stages 2–4 CKD who participated in the Chronic Renal Insufficiency Cohort (CRIC) observational study.
Materials and Methods
Study Design
The CRIC study is a multicenter, prospective, observational study of risk factors for the progression of CKD and its complications. A total of 3939 participants with mild to moderate CKD were recruited from 13 clinical sites in the United States between May 2003 through March 2008. Data (including follow-up data through 2018) for the analysis were obtained through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) repository on June 25, 2021. The major inclusion criteria were participants with CKD aged 21–74 years old with an eGFR of 20–70 ml/min per 1.73 m2 at enrollment. Other detailed eligibility criteria, study design, methods, and baseline characteristics have been described previously (15–17). The study was approved by the Institutional Review Board of all participating centers, and all participants provided written informed consent.
In this study, 548 participants were excluded from the analysis due to missing data for the predictor variables (monocyte count, n=55; lymphocyte count, n=56) and/or covariates (body mass index, n=10; smoking status, n=327; number of antihypertensives, n=28; statin use, n=28; systolic blood pressure, n=1; total cholesterol, n=12; HDL cholesterol, n=12; and urinary albumin, n=180), resulting in a final analytic sample of 3391 participants (Supplemental Figure 1).
Study Variables
The primary predictor was monocyte count at baseline. The secondary predictor was MLR at baseline. The primary outcome was time to a composite of CVD events (heart failure, myocardial infarction, ischemic stroke, and peripheral artery disease, as originally defined by adjudication in CRIC). CVD events were confirmed every 6 months by phone or in person during follow-up visits and adjudicated by medical record review as possible, probable, or definite events. Adjudicated heart failure events were defined as hospital admission for signs and symptoms of poor cardiac output, and our analysis included probable and definite adjudicated heart failure events. Adjudicated atherosclerotic events were defined as possible, probable, or definite myocardial infarction; probable or definite stroke; and probable or definite peripheral artery disease. Secondary outcomes were time to CVD death and all-cause death. Deaths were confirmed by death certificate (18).
Confounders potentially related to CVD and CKD, all measured at baseline, were selected a priori as covariates for the analysis. Demographic characteristics (age, sex, race, and smoking status) and medical conditions (prevalent diabetes and CVD) were self-reported (17). Current medications (antihypertensives and statin use) were reviewed and documented (17). Anthropometric measures (height, weight, and body mass index) were measured by trained study personnel (15–17). Blood pressure was measured using a standard and validated protocol (15–17). A complete blood count was performed in the CRIC Central Laboratory (19), and plasma and urine samples were collected for initial study measures (15). Concentrations of total and HDL cholesterol were determined in the plasma. Urinary albumin excretion was determined from a 24-hour urine collection (17). eGFR was evaluated in the CRIC Central Laboratory calculated using the CKD Epidemiology Collaboration (CKD-EPI) estimating equation (17,19).
Statistical Analyses
Covariates were summarized by mean±SD or median (interquartile range [IQR]) for continuous variables, and number (n) and proportion (%) for categorical variables. Number of events and event rates per 100 person-years were calculated by tertile for CVD events, CVD death, and all-cause death. Event-free survival was examined by survival analysis, including Kaplan–Meier and statistical comparison by the log rank test. Cox proportional hazard regression models were used to evaluate the longitudinal association of monocyte count and MLR with time to first CVD event, CVD death, and all-cause death. Participants were censored at death or loss for follow-up. All analyses evaluated monocyte count or MLR using log base 2-transformed continuous variable and tertile, with the lowest tertile serving as the reference category. Hazard ratios (HR) of the continuous predictor variables were interpreted as HR per doubling of monocyte count or MLR. Model 1 was adjusted for demographics, including age, race, and clinic site. Model 2 was additionally adjusted for traditional CVD risk factors, including systolic blood pressure, number of antihypertensives, total cholesterol, HDL cholesterol, statin use, smoking status, prevalent CVD, and prevalent diabetes. Model 3 was further adjusted for markers of kidney disease, including eGFR and urinary albumin (log-transformed). Model 4 was further adjusted for high-sensitivity C-reactive protein (hs-CRP; log-transformed). In addition, using the Fine and Gray method, a sensitivity analysis was conducted where all-cause death was evaluated as a competing risk for the CVD composite end point. A two-tailed P value of ≤0.05 was considered statistically significant for all analyses. All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Among the 3939 CRIC participants, 3391 participants were included in the final analytic cohort. The mean eGFR was 45 ml/min per 1.73 m2 (SD=15 ml/min per 1.73 m2), the mean age was 58 years (SD=11 years), 46% (n=1553) were women, and 45% (n=1530) were Black (Table 1). Participants in the highest tertile of monocyte count and MLR were more likely to be men and have prevalent CVD compared with the lowest tertile.
Table 1.
Demographic characteristics and laboratory measurements by tertile of monocyte count and monocyte/lymphocyte ratio
| Characteristics | Overall cohort (N=3391) | Monocyte Count | Monocyte/Lymphocyte Ratio | ||||
|---|---|---|---|---|---|---|---|
| Tertile 1, <400/mm3 (N=1003) | Tertile 2, ≥400 and <523/mm3 (N=1225) | Tertile 3, ≥523/mm3 (N=1133) | Tertile 1, <0.23 (N=1107) | Tertile 2, ≥0.23 and <0.33 (N=1068) | Tertile 3, ≥0.33 (N=1216) | ||
| Monocyte count, mm3, median (IQR) | 470 (365–600) | 300 (260–346) | 455 (400–500) | 640 (600–750) | — | — | — |
| MLR, median (IQR) | 0.28 (0.21–0.38) | — | — | — | 0.18 (0.15–0.2) | 0.27 (0.25–0.3) | 0.42 (0.36–0.5) |
| Age, yr, mean±SD | 58±11 | 58±11 | 58±11 | 59±11 | 57±11 | 58±11 | 60±11 |
| Women, n (%) | 1553 (46) | 589 (59) | 554 (44) | 410 (36) | 704 (64) | 473 (44) | 376 (31) |
| Race, n (%) | |||||||
| White | 1686 (50) | 447 (45) | 642 (51) | 597 (53) | 413 (37) | 528 (49) | 745 (61) |
| Black | 1530 (45) | 493 (49) | 551 (44) | 486 (43) | 673 (58) | 471 (44) | 422 (35) |
| Other | 175 (5) | 63 (6) | 62 (5) | 50 (4) | 57 (5) | 69 (7) | 49 (4) |
| BMI, kg/m2, mean±SD | 32.1±8 | 31.2±7.9 | 32.1±7.7 | 33±8.2 | 32.4±8.3 | 32.1±7.8 | 31.9±7.7 |
| Current smoking, n (%) | 456 (13) | 118 (12) | 163 (13) | 175 (15) | 195 (18) | 127 (12) | 134 (11) |
| CVD, n (%) | 1152 (34) | 278 (28) | 414 (33) | 460 (41) | 317 (29) | 364 (34) | 471 (39) |
| Diabetes, n (%) | 1573 (46) | 410 (41) | 592 (47) | 571 (50) | 485 (44) | 491 (46) | 597 (49) |
| # Antihypertensives, mean±SD | 2.6±1.5 | 2.4±1.6 | 2.6±1.5 | 2.9±1.5 | 2.4±1.5 | 2.6±1.5 | 2.8±1.5 |
| Statin use, n (%) | 1870 (55) | 495 (49) | 713 (57) | 662 (58) | 576 (52) | 588 (55) | 706 (58) |
| SBP, mm Hg, mean±SD | 127±22 | 126±22 | 128±21 | 128±22 | 126±21 | 128±22 | 129±22 |
| Cholesterol, mg/dl, mean±SD | 183±44 | 188±45 | 183±46 | 178±41 | 190±47 | 182±43 | 176±41 |
| HDL cholesterol, mean±SD | 48±16 | 52±17 | 47±14 | 46±14 | 50±16 | 48±15 | 47±15 |
| eGFR, ml/min per 1.73 m2, mean±SD | 45±15 | 46±16 | 45±15 | 43±14 | 47±16 | 45±15 | 43±14 |
| Urinary albumin, g/24 h, median (IQR) | 0.06 (0.01–0.49) | 0.03 (0.01–0.37) | 0.06 (0.01–0.51) | 0.08 (0.01–0.55) | 0.04 (0.01–0.41) | 0.06 (0.01–0.49) | 0.08 (0.01–0.54) |
| hs-CRP, mg/L, median (IQR) | 2.57 (1.05–6.52) | 1.87 (0.82–4.87) | 2.42 (1.07–5.81) | 3.58 (1.35–8.5) | 2.5 (0.96–6.61) | 2.22 (0.96–5.71) | 3.01 (1.22–7.27) |
BMI, body mass index; CVD, cardiovascular disease; hs-CRP, high-sensitivity C-reactive protein; IQR, interquartile range; MLR, monocyte/lymphocyte ratio; SBP, systolic blood pressure.
Risk of CVD, CVD Death, and All-Cause Death
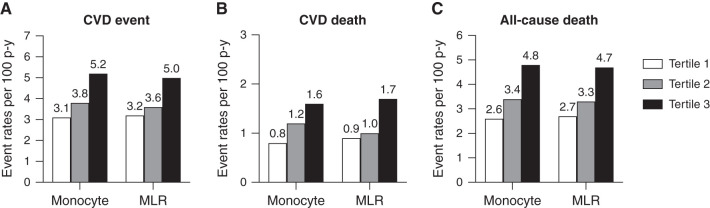
There were 1088 first composite CVD events over a median follow-up of 9 years (IQR 3.6–12.5 years). During the median follow-up of 11.7 years (IQR 7.4–13.1 years), there were 1220 all-cause deaths, including 411 CVD deaths. The rates of first CVD composite events, CVD death, and all-cause death per 100 person-years were greater in the highest tertile of monocyte count and MLR compared with the lowest tertile (Figure 1). During the period of observation, participants with a higher monocyte count and MLR experienced lower CVD-, CVD death-, and all-cause death-free survival (log rank P<0.001; Figure 2).
Figure 1.
Unadjusted event rates of CVD, CVD death, and all-cause death per 100 person-years of follow-up in CRIC participants. Event rates of CVD (A), CVD death (B), and all-cause death (C) per 100 person-years of follow-up are presented in tertile of monocyte count and MLR. CRIC, Chronic Renal Insufficiency Cohort; CVD, cardiovascular disease; MLR, monocyte/lymphocyte ratio.
Figure 2.
CVD-, CVD death-, and all-cause death-free survivals (Kaplan–Meir curve) in CRIC participants. CVD-, CVD death-, and all-cause death-free survivals presented in tertile of monocyte count (A–C) and MLR (D–F).
Association of Monocyte Count and MLR with the Risk of CVD, CVD Death, and All-Cause Death
In unadjusted analyses, the highest tertile of monocyte count and MLR were associated with a higher risk of first CVD event compared with the lowest tertile (Tables 2 and 3). This association was attenuated but remained significant after further adjustment for demographics (model 1), traditional CVD risk factors (model 2), and markers of kidney disease (model 3). After additional adjustment for hs-CRP, the highest tertile of monocyte count and MLR (compared with the lowest tertile) were associated with a 31% (95% CI, 14% to 49%) and a 41% (95% CI, 23% to 61%) greater risk of CVD, respectively. Similar results were obtained when monocyte count and MLR were modeled continuously (HR per doubling of monocyte count=1.2 [95% CI, 1.1 to 1.31]; HR per doubling of MLR=1.26 [95% CI, 1.16 to 1.36], both model 4).
Table 2.
Associations of monocyte count with cardiovascular disease, cardiovascular disease death, and all-cause death
| Tertile 1, <400/mm3 (N=1003) | Tertile 2, ≥400 and <523/mm3 (N=1225) | Tertile 3, ≥523/mm3 (N=1133) | Per Doubling of Monocyte Count | |
|---|---|---|---|---|
| CVD | ||||
| Unadjusted | Ref. | 1.25 (1.1 to 1.42) | 1.77 (1.57 to 2.01) | 1.52 (1.39 to 1.65) |
| Model 1 | Ref. | 1.21 (1.06 to 1.38) | 1.64 (1.44 to 1.86) | 1.42 (1.3 to 1.54) |
| Model 2 | Ref. | 1.15 (1.01 to 1.32) | 1.43 (1.25 to 1.63) | 1.28 (1.17 to 1.4) |
| Model 3 | Ref. | 1.13 (0.99 to 1.29) | 1.37 (1.2 to 1.56) | 1.25 (1.14 to 1.36) |
| Model 4 | Ref. | 1.11 (0.97 to 1.27) | 1.31 (1.14 to 1.49) | 1.2 (1.1 to 1.31) |
| CVD death | ||||
| Unadjusted | Ref. | 1.38 (1.06 to 1.79) | 1.87 (1.45 to 2.41) | 1.59 (1.34 to 1.89) |
| Model 1 | Ref. | 1.23 (0.94 to 1.61) | 1.61 (1.23 to 2.09) | 1.45 (1.21 to 1.72) |
| Model 2 | Ref. | 1.13 (0.87 to 1.48) | 1.33 (1.02 to 1.73) | 1.27 (1.07 to 1.51) |
| Model 3 | Ref. | 1.13 (0.86 to 1.47) | 1.26 (0.97 to 1.64) | 1.22 (1.03 to 1.46) |
| Model 4 | Ref. | 1.11 (0.85 to 1.45) | 1.2 (0.92 to 1.58) | 1.18 (0.99 to 1.41) |
| All-cause death | ||||
| Unadjusted | Ref. | 1.32 (1.13 to 1.53) | 1.89 (1.63 to 2.19) | 1.55 (1.41 to 1.71) |
| Model 1 | Ref. | 1.2 (1.03 to 1.41) | 1.68 (1.45 to 1.96) | 1.43 (1.29 to 1.58) |
| Model 2 | Ref. | 1.14 (0.98 to 1.33) | 1.47 (1.26 to 1.71) | 1.29 (1.16 to 1.42) |
| Model 3 | Ref. | 1.12 (0.96 to 1.31) | 1.38 (1.18 to 1.61) | 1.23 (1.11 to 1.36) |
| Model 4 | Ref. | 1.1 (0.94 to 1.29) | 1.3 (1.11 to 1.52) | 1.17 (1.06 to 1.3) |
Data are shown as hazard ratios (95% CI). Model 1: Unadjusted+demographics (age, sex, race, and clinic site). Model 2: Model 1+traditional CVD risk factors (systolic blood pressure, number of antihypertensives, total cholesterol, HDL cholesterol, statin use, smoking status, prevalent CVD, and prevalent diabetes). Model 3: Model 2+markers of kidney disease (eGFR and urinary albumin). Model 4: Model 3+hs-CRP. CI, confidence interval; CVD, cardiovascular disease; Ref., reference; hs-CRP, high-sensitivity C-reactive protein.
Table 3.
Associations of monocyte/lymphocyte ratio with cardiovascular disease event, cardiovascular disease death, and all-cause death
| Tertile 1, <0.23 (N=1107) | Tertile 2, ≥0.23 and <0.33 (N=1068) | Tertile 3, ≥0.33 (N=1216) | Per Doubling of MLR | |
|---|---|---|---|---|
| CVD | ||||
| Unadjusted | Ref. | 1.18 (1.03 to 1.34) | 1.66 (1.48 to 1.87) | 1.39 (1.29 to 1.48) |
| Model 1 | Ref. | 1.14 (1 to 1.31) | 1.6 (1.41 to 1.82) | 1.34 (1.25 to 1.44) |
| Model 2 | Ref. | 1.11 (0.97 to 1.27) | 1.54 (1.35 to 1.75) | 1.32 (1.23 to 1.43) |
| Model 3 | Ref. | 1.1 (0.96 to 1.25) | 1.46 (1.28 to 1.67) | 1.29 (1.19 to 1.39) |
| Model 4 | Ref. | 1.09 (0.95 to 1.24) | 1.41 (1.23 to 1.61) | 1.26 (1.16 to 1.36) |
| CVD death | ||||
| Unadjusted | Ref. | 1.18 (0.9 to 1.54) | 1.97 (1.55 to 2.5) | 1.56 (1.37 to 1.78) |
| Model 1 | Ref. | 1.04 (0.79 to 1.38) | 1.67 (1.29 to 2.16) | 1.42 (1.23 to 1.63) |
| Model 2 | Ref. | 1.03 (0.78 to 1.37) | 1.55 (1.19 to 2.02) | 1.36 (1.18 to 1.58) |
| Model 3 | Ref. | 1.01 (0.76 to 1.33) | 1.43 (1.09 to 1.86) | 1.3 (1.12 to 1.51) |
| Model 4 | Ref. | 1 (0.76 to 1.32) | 1.38 (1.06 to 1.81) | 1.27 (1.1 to 1.48) |
| All-cause death | ||||
| Unadjusted | Ref. | 1.23 (1.06 to 1.43) | 1.77 (1.54 to 2.03) | 1.43 (1.32 to 1.54) |
| Model 1 | Ref. | 1.14 (0.98 to 1.33) | 1.58 (1.36 to 1.84) | 1.33 (1.22 to 1.45) |
| Model 2 | Ref. | 1.14 (0.97 to 1.33) | 1.52 (1.3 to 1.77) | 1.3 (1.19 to 1.41) |
| Model 3 | Ref. | 1.1 (0.94 to 1.28) | 1.38 (1.18 to 1.61) | 1.23 (1.13 to 1.34) |
| Model 4 | Ref. | 1.08 (0.93 to 1.27) | 1.31 (1.12 to 1.53) | 1.18 (1.09 to 1.29) |
Data are shown as hazard ratios (95% CI). Model 1: Unadjusted+demographics (age, sex, race, and clinic site). Model 2: Model 1+traditional CVD risk factors (systolic blood pressure, number of antihypertensives, total cholesterol, HDL cholesterol, statin use, smoking status, prevalent CVD, and prevalent diabetes). Model 3: Model 2+markers of kidney disease (eGFR and urinary albumin). Model 4: Model 3+hs-CRP. CVD, cardiovascular disease; Ref., reference; MLR, monocyte/lymphocyte ratio.
In sensitivity analyses evaluating all-cause mortality as a competing risk factor for CVD, the HRs were similar to those obtained from Cox proportional hazard models. Although monocyte count was not associated with CVD risk after adjustment for markers of kidney disease (model 3) and CRP (model 4), MLR remained associated with a higher CVD risk in unadjusted analysis and all adjusted analyses (models 1–4; Supplemental Tables 1 and 2).
In unadjusted analysis and analyses adjusted for demographics (model 1) and traditional CVD risk factors (model 2), the highest tertile of monocyte count and MLR were significantly associated with a higher risk of CVD death compared with the lowest tertile (Tables 2 and 3). After further adjustment for markers of kidney disease (model 3) and hs-CRP (model 4), this association was no longer statistically significant for monocyte count. However, the highest tertile of MLR (compared with the lowest tertile) was associated with a 38% (95% CI, 6% to 81%) greater risk of CVD death in the fully adjusted model (model 4). When monocyte count and MLR were modeled continuously, monocyte count was not associated with the risk of CVD death (model 4), but MLR remained associated with a greater risk of CVD death (HR per doubling of MLR=1.27 [95% CI, 1.1 to 1.48], model 4).
Finally, in unadjusted analysis and analyses adjusted for demographics (model 1), traditional CVD risk factors (model 2), and markers of kidney disease (model 3), the highest tertile of monocyte count and MLR were associated with a higher risk of all-cause death compared with the lowest tertile (Tables 2 and 3). In the fully adjusted model (model 4), this association was attenuated but remained statistically significant. Compared with the lowest tertile, the highest tertile of monocyte count and MLR were associated with a 30% (95% CI, 11% to 52%) and a 31% (95% CI, 12% to 53%) higher risk of all-cause death, respectively. Similarly, in the continuous model, monocyte count and MLR were associated with a higher risk of all-cause death (HR per doubling monocyte count=1.17 [95% CI, 1.06 to 1.3]; HR per doubling MLR=1.18 [95% CI, 1.09 to 1.29], both model 4).
Discussion
We demonstrated a graded association of monocyte count with a higher risk of CVD, CVD death, and all-cause death in patients with nondialysis-dependent CKD, during a median follow-up of 9 years for CVD and 11.7 years for death. MLR also was associated with a higher risk of CVD, CVD death, and all-cause death. These findings suggest that a higher monocyte count at baseline may be associated with the risk of CVD, CVD death, and all-cause death in patients with nondialysis-dependent CKD.
Chronic low-grade inflammation, characterized by elevated circulating concentrations of proinflammatory mediators, has been identified as a key mechanism partially mediating vascular dysfunction and elevated risk of CVD in the general population and in patients with CKD (20). Given that monocyte count is associated with circulating levels of proinflammatory mediators (21), monocyte count may serve as a surrogate marker of inflammation that is involved with the increased risk of CVD. Additionally, MLR has attracted attention for its application to predict the risk of CVD (6–8). Whereas monocytes are involved in the inflammatory reaction process, lymphocytes, which consist of T cells, B cells, and natural killer cells, represent the regulatory pathway of the immune system; thus, an elevated MLR may indicate an increased inflammatory reaction and impaired immune response (22).
In clinical practice, complete blood count is easily and inexpensively measured and often performed as part of a routine checkup, in contrast to other circulating inflammatory markers (e.g., ILs and TNF-α). Previous studies demonstrated monocyte/HDL ratio as a potential prognostic cardiovascular marker in the non-CKD population (23); however, sensitivity and specificity of CVD diagnosis are similar between MLR and monocyte/HDL ratio (24). Thus, we hypothesized that monocyte count and MLR could serve as simple, cost-effective, clinically available surrogate markers of inflammation associated with CVD risk in the CKD population.
Monocytes play a key role in vascular inflammation and further contribute to CVD development (2,3). In individuals without CKD, numerous studies demonstrated a strong association between monocyte count and cardiovascular events and/or mortality. In adults without a history of CVD, monocyte count is associated with a higher risk of incident CVD (5). Monocyte count is also associated with a higher risk of CVD mortality in older adults (25). Furthermore, previous studies report an association of monocyte count and MLR with a higher CVD mortality in patients with prevalent CVD, including myocardial infarction (8) and heart failure (26). In the current study, monocyte count and MLR were independently associated with the risk of CVD and CVD death in patients with nondialysis-dependent CKD, suggesting this previously observed association in a population without CKD may translate to the CKD population.
In the current study, the association between monocyte count and MLR and the risk of CVD and CVD death remained significant, even after adjustment for traditional CVD risk factors and markers of kidney disease. We also demonstrated that monocyte count and MLR were associated with a higher risk of CVD, even after further adjustment for hs-CRP, a well-known marker of systemic inflammation (27). Thus, our findings suggest that monocytes may be at least in part associated with the development of CVD in patients with CKD, at least in part independent from inflammation reflected by hs-CRP. Indeed, a growing body of literature suggests that the uremic environment in CKD predisposes to vascular inflammation via inducing a proinflammatory phenotype of the monocytes (9,10). Monocytes derived from the patients with CKD are more likely to react to inflammatory cytokines (i.e., increased expression of TNF receptor), to adhere and potentially transmigrate to vascular endothelium (i.e., elevated expression of CD11b), and to secrete proinflammatory cytokines, including TNF-α, IL-1β, and IL-6 (10).
Human monocytes are a heterogeneous cell population in the circulation and are classified as classical (CD14++CD16–), intermediate (CD14++CD16+), and nonclassical (CD14+CD16++) monocytes (28), which contribute differently to inflammatory responses and CVD development (29). Notably, a systematic review and meta-analysis reported there may be a shift in the distribution of monocyte subsets from classical toward intermediate and/or nonclassical monocytes in individuals at cardiovascular risk compared with healthy controls (30). In individuals with CKD, the proportion of the intermediate monocytes, known to be highly proinflammatory (31), are expanded compared with those without CKD (32,33). Notably, Rogacev et al. demonstrated intermediate monocyte count was independently associated with incident CVD events (HR=1.26 [95% CI, 1.04 to 1.52]) in patients with nondialysis-dependent CKD (n=119) (34). This suggests that not only increased monocyte count but also an expansion in the circulating intermediate monocytes in patients with CKD may contribute to vascular inflammation and further development of CVD. Monocyte subsets were not available in this study, but it is biologically plausible that patients with a higher monocyte count may have an increased proportion of the intermediate monocyte population.
Four other prospective analyses in small cohorts investigated the association between monocytes and the risk of CVD in CKD populations (three studies in CKD cohorts undergoing dialysis and one study in a nondialysis-dependent CKD cohort). Xiang et al. demonstrated a higher risk of cardiovascular mortality (HR=6.99; 95% CI, 1.94 to 25.12]) with a higher MLR in patients who had been undergoing hemodialysis for at least 6 months (n=355; 62% men) during a median follow-up of 51 months (14). Kato et al. demonstrated that the highest tertile of monocyte count (compared with the lowest tertile) was associated with a higher risk of CVD death (HR for CVD death=1.98 [95% CI, 1.1 to 3.57]) in patients undergoing chronic hemodialysis (n=333; 65% men) during a follow-up of 40 months (12). Wen et al. reported that the highest tertile of MLR (compared with the lowest tertile) was associated with a high risk of CVD death (HR for CVD death=1.45; 95% CI, 1.13 to 2.51]) in patients undergoing peritoneal dialysis (n=1753; 57% men) during a median follow-up of 31 months (13). Kanbay et al. reported that monocyte/HDL ratio was associated with a higher risk of CVD (HR for fatal CVD event=1.59 [95% CI, 1.44 to 1.75]) in patients with stages 1–5 CKD (n=340; 51% men) during a mean follow-up of 33 months (11). Our results strongly support the previous findings, with a larger sample size and a longer follow-up duration.
We also demonstrated an association between monocyte count and MLR and all-cause mortality in patients with CKD. Numerous prospective analyses highlight the association between monocytes and the risk of all-cause mortality in diverse populations, including the general population (35), individuals with prevalent CVD (myocardial infarction and heart failure) (8,26), and patients with CKD (11,36). We hypothesize that a high monocyte count and MLR may be implicated in or even promote impaired immune function, increased inflammation, and/or poor overall health, which partially mediate the risk of all-cause mortality.
There are several limitations to our study. The results are observational rather than causational due to the nature of the analysis. We adjusted for numerous important covariates, but the results may be subject to residual confounding factors, either unmeasured or unknown, which may explain the higher CVD risk in those with higher monocyte count and MLR. Adjusted models for CVD death may be overfitted; thus, results need to be interpreted with caution. Moreover, the associations of monocyte count and MLR with the primary and secondary end points (CVD events, CVD death, and all-cause death) were relatively small on the basis of doubling of continuous predictors. However, monocyte count and MLR as categorical predictors were also significantly associated with the end points, which supports our overall conclusion. Monocytes are a heterogenous cell population, and intermediate monocytes, in particular, play an important role in the development of CVD. However, our data lack information on the proportion of the monocyte subsets. An important strength of this study is the large sample size with similar proportions of men and women and racial diversity; thus, results may be generalizable in the CKD population.
In summary, we demonstrated higher monocyte count and MLR were significantly associated with increased risk of CVD, CVD death, and all-cause death, suggesting that monocyte count and MLR may have the potential to be simple, cost-effective, clinically available indicators of cardiovascular risk in patients with nondialysis-dependent CKD.
Disclosures
All authors have nothing to disclose.
Funding
This study was supported by NIH, NIDDK (5T32DK007135-46) to E.S. Oh, Veterans Affairs Merit Award (I01CX001985) to A.J. Jovanovich, and NIH, NIDDK (R01 DK130255) to K.L. Nowak.
Acknowledgments
Data for the analysis were obtained through the NIDDK repository.
Author Contributions
E.S. Oh, A.J. Jovanovich, and K.L. Nowak conceptualized the study; A.J. Jovanovich and K.L. Nowak supervised the study; A.J. Jovanovich, K.L. Nowak, and E.S. Oh were responsible for validation; E.S. Oh and Z. You was responsible for the methodology; E.S. Oh wrote the original draft of the manuscript; Z. You was responsible for the investigation; and all authors reviewed and edited the manuscript and approved the final version of the submitted manuscript.
Data Sharing Statement
Original data analyzed in this study are available in a persistent repository upon request: Observational Data, CRIC, https://repository.niddk.nih.gov/studies/cric/.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0007922021/-/DCSupplemental.
Study flow chart. Download Supplemental Figure 1, PDF file, 65 KB (216.8KB, pdf)
Sensitivity analysis of the association (hazard ratios; 95%CI) between monocyte count and cardiovascular disease (CVD) event evaluating all-cause death as a competing risk. Download Supplemental Table 1, PDF file, 65 KB (216.8KB, pdf)
Sensitivity analysis of the association (hazard ratios; 95% CI) between monocyte/lymphocyte ratio and CVD event evaluating all-cause death as a competing risk. Download Supplemental Table 2, PDF file, 65 KB (216.8KB, pdf)
References
- 1.Fayad ZA, Swirski FK, Calcagno C, Robbins CS, Mulder W, Kovacic JC: Monocyte and macrophage dynamics in the cardiovascular system: JACC macrophage in CVD series (Part 3). J Am Coll Cardiol 72: 2198–2212, 2018. 10.1016/j.jacc.2018.08.2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahid F, Lip GYH, Shantsila E: Role of monocytes in heart failure and atrial fibrillation. J Am Heart Assoc 7: e007849, 2018. 10.1161/JAHA.117.007849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Čejková S, Králová-Lesná I, Poledne R: Monocyte adhesion to the endothelium is an initial stage of atherosclerosis development. Cor Vasa 58: e419–e425, 2016. 10.1016/j.crvasa.2015.08.002 [DOI] [Google Scholar]
- 4.Waterhouse DF, Cahill RA, Sheehan F, McCreery C: Prediction of calculated future cardiovascular disease by monocyte count in an asymptomatic population. Vasc Health Risk Manag 4: 177–187, 2008. 10.2147/vhrm.2008.04.01.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassale C, Curtis A, Abete I, van der Schouw YT, Verschuren WMM, Lu Y, Bueno-de-Mesquita HBA: Elements of the complete blood count associated with cardiovascular disease incidence: Findings from the EPIC-NL cohort study. Sci Rep 8: 3290, 2018. 10.1038/s41598-018-21661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji H, Li Y, Fan Z, Zuo B, Jian X, Li L, Liu T: Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: A syntax score assessment. BMC Cardiovasc Disord 17: 90, 2017. 10.1186/s12872-017-0507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gijsberts CM, Ellenbroek GHJM, Ten Berg MJ, Huisman A, van Solinge WW, Lam CS, Asselbergs FW, den Ruijter HM, Pasterkamp G, Hoefer IE, de Kleijn DP: Effect of monocyte-to-lymphocyte ratio on heart failure characteristics and hospitalizations in a coronary angiography cohort. Am J Cardiol 120: 911–916, 2017. 10.1016/j.amjcard.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Ma J, Jiang Z, Wu F, Ping J, Ming L: Association of lymphocyte-to-monocyte ratio with in-hospital and long-term major adverse cardiac and cerebrovascular events in patients with ST-elevated myocardial infarction. Medicine (Baltimore) 96: e7897, 2017. 10.1097/MD.0000000000007897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hénaut L, Candellier A, Boudot C, Grissi M, Mentaverri R, Choukroun G, Brazier M, Kamel S, Massy ZA: New insights into the roles of monocytes/macrophages in cardiovascular calcification associated with chronic kidney disease. Toxins (Basel) 11: 529, 2019. 10.3390/toxins11090529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girndt M, Trojanowicz B, Ulrich C: Monocytes in Uremia. Toxins (Basel) 12: 340, 2020. 10.3390/toxins12050340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanbay M, Solak Y, Unal HU, Kurt YG, Gok M, Cetinkaya H, Karaman M, Oguz Y, Eyileten T, Vural A, Covic A, Goldsmith D, Turak O, Yilmaz MI: Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 46: 1619–1625, 2014. 10.1007/s11255-014-0730-1 [DOI] [PubMed] [Google Scholar]
- 12.Kato A, Takita T, Furuhashi M, Maruyama Y, Kumagai H, Hishida A: Blood monocyte count is a predictor of total and cardiovascular mortality in hemodialysis patients. Nephron Clin Pract 110: c235–c243, 2008. 10.1159/000167871 [DOI] [PubMed] [Google Scholar]
- 13.Wen Y, Zhan X, Wang N, Peng F, Feng X, Wu X: Monocyte/lymphocyte ratio and cardiovascular disease mortality in peritoneal dialysis patients. Mediators Inflamm 2020: 9852507, 2020. 10.1155/2020/9852507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang F, Chen R, Cao X, Shen B, Liu Z, Tan X, Ding X, Zou J: Monocyte/lymphocyte ratio as a better predictor of cardiovascular and all-cause mortality in hemodialysis patients: A prospective cohort study. Hemodial Int 22: 82–92, 2018. 10.1111/hdi.12549 [DOI] [PubMed] [Google Scholar]
- 15.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009. 10.2215/CJN.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003. 10.1097/01.asn.0000070149.78399.ce [DOI] [PubMed] [Google Scholar]
- 17.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP; CRIC and H-CRIC Study Groups : CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis 58: 214–227, 2011. 10.1053/j.ajkd.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bundy JD, Cai X, Mehta RC, Scialla JJ, de Boer IH, Hsu CY, Go AS, Dobre MA, Chen J, Rao PS, Leonard MB, Lash JP, Block GA, Townsend RR, Feldman HI, Smith ER, Pasch A, Isakova T; CRIC Study Investigators : Serum calcification propensity and clinical events in CKD. Clin J Am Soc Nephrol 14: 1562–1571, 2019. 10.2215/CJN.04710419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NIDDKH : Chronic Renal Insufficiency Cohort Study (CRIC) Protocol Version 3.0. Available at: https://repository.niddk.nih.gov/studies/cric/cric%20protocols/. Accessed October 6, 2021
- 20.Zanoli L, Lentini P, Briet M, Castellino P, House AA, London GM, Malatino L, McCullough PA, Mikhailidis DP, Boutouyrie P: Arterial stiffness in the heart disease of CKD. J Am Soc Nephrol 30: 918–928, 2019. 10.1681/ASN.2019020117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J: Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke 35: 1619–1624, 2004. 10.1161/01.STR.0000130857.19423.ad [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Li M, Liu L, Dang X, Zhu D, Tian G: Monocyte/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients with non-ST-elevation myocardial infarction. Medicine (Baltimore) 98: e16267, 2019. 10.1097/MD.0000000000016267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganjali S, Gotto AM Jr, Ruscica M, Atkin SL, Butler AE, Banach M, Sahebkar A: Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol 233: 9237–9246, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Zhan F, Wang Y: Evaluation of monocyte-to-high-density lipoprotein cholesterol ratio and monocyte-to-lymphocyte ratio in ischemic stroke. J Int Med Res 48: 300060520933806, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi SH, Kim JH, Lim S, Lim JY, Kim KW, Park KS, Jang HC: Monocyte count as a predictor of cardiovascular mortality in older Korean people. Age Ageing 46: 433–438, 2017. 10.1093/ageing/afw226 [DOI] [PubMed] [Google Scholar]
- 26.Silva N, Bettencourt P, Guimarães JT: The lymphocyte-to-monocyte ratio: An added value for death prediction in heart failure. Nutr Metab Cardiovasc Dis 25: 1033–1040, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Sproston NR, Ashworth JJ: Role of C-reactive protein at sites of inflammation and infection. Front Immunol 9: 754, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB: Nomenclature of monocytes and dendritic cells in blood. Blood 116: e74–e80, 2010. 10.1182/blood-2010-02-258558 [DOI] [PubMed] [Google Scholar]
- 29.Heine GH, Ortiz A, Massy ZA, Lindholm B, Wiecek A, Martínez-Castelao A, Covic A, Goldsmith D, Süleymanlar G, London GM, Parati G, Sicari R, Zoccali C, Fliser D; European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) : Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol 8: 362–369, 2012. 10.1038/nrneph.2012.41 [DOI] [PubMed] [Google Scholar]
- 30.Oh ES, Na M, Rogers CJ: The association between monocyte subsets and cardiometabolic disorders/cardiovascular disease: A systematic review and meta-analysis. Front Cardiovasc Med 8: 640124, 2021. 10.3389/fcvm.2021.640124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honold L, Nahrendorf M: Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res 122: 113–127, 2018. 10.1161/CIRCRESAHA.117.311071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naicker SD, Cormican S, Griffin TP, Maretto S, Martin WP, Ferguson JP, Cotter D, Connaughton EP, Dennedy MC, Griffin MD: Chronic kidney disease severity is associated with selective expansion of a distinctive intermediate monocyte subpopulation. Front Immunol 9: 2845, 2018. 10.3389/fimmu.2018.02845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borges Bonan N, Schepers E, Pecoits-Filho R, Dhondt A, Pletinck A, De Somer F, Vanholder R, Van Biesen W, Moreno-Amaral A, Glorieux G: Contribution of the uremic milieu to an increased pro-inflammatory monocytic phenotype in chronic kidney disease. Sci Rep 9: 10236, 2019. 10.1038/s41598-019-46724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH: CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J 32: 84–92, 2011. 10.1093/eurheartj/ehq371 [DOI] [PubMed] [Google Scholar]
- 35.Welsh C, Welsh P, Mark PB, Celis-Morales CA, Lewsey J, Gray SR, Lyall DM, Iliodromiti S, Gill JMR, Pell J, Jhund PS, Sattar N: Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK Biobank. Arterioscler Thromb Vasc Biol 38: 1415–1423, 2018. 10.1161/ATVBAHA.118.310945 [DOI] [PubMed] [Google Scholar]
- 36.Agarwal R, Light RP: Patterns and prognostic value of total and differential leukocyte count in chronic kidney disease. Clin J Am Soc Nephrol 6: 1393–1399, 2011. 10.2215/CJN.10521110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study flow chart. Download Supplemental Figure 1, PDF file, 65 KB (216.8KB, pdf)
Sensitivity analysis of the association (hazard ratios; 95%CI) between monocyte count and cardiovascular disease (CVD) event evaluating all-cause death as a competing risk. Download Supplemental Table 1, PDF file, 65 KB (216.8KB, pdf)
Sensitivity analysis of the association (hazard ratios; 95% CI) between monocyte/lymphocyte ratio and CVD event evaluating all-cause death as a competing risk. Download Supplemental Table 2, PDF file, 65 KB (216.8KB, pdf)